Biological Rhythms in the Skin
Abstract
:1. Fundamental Concepts
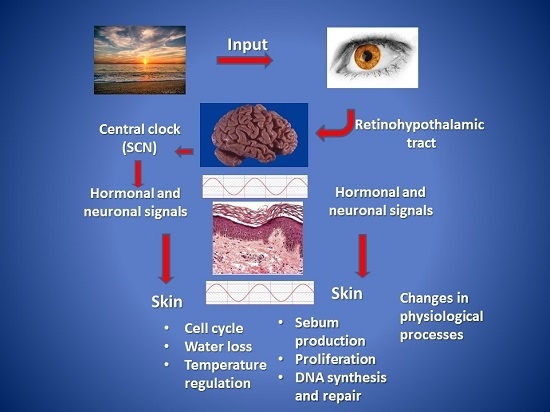
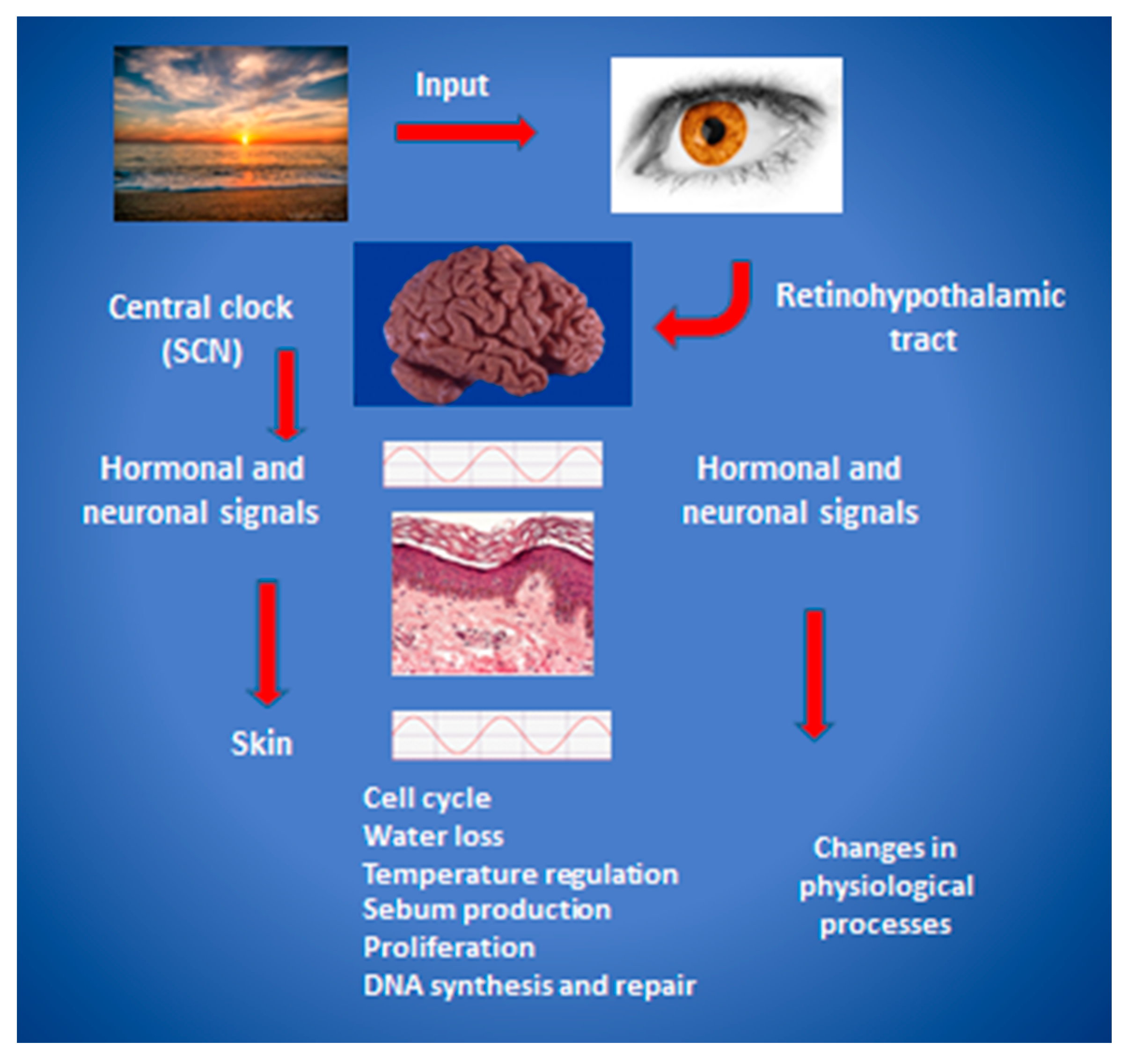
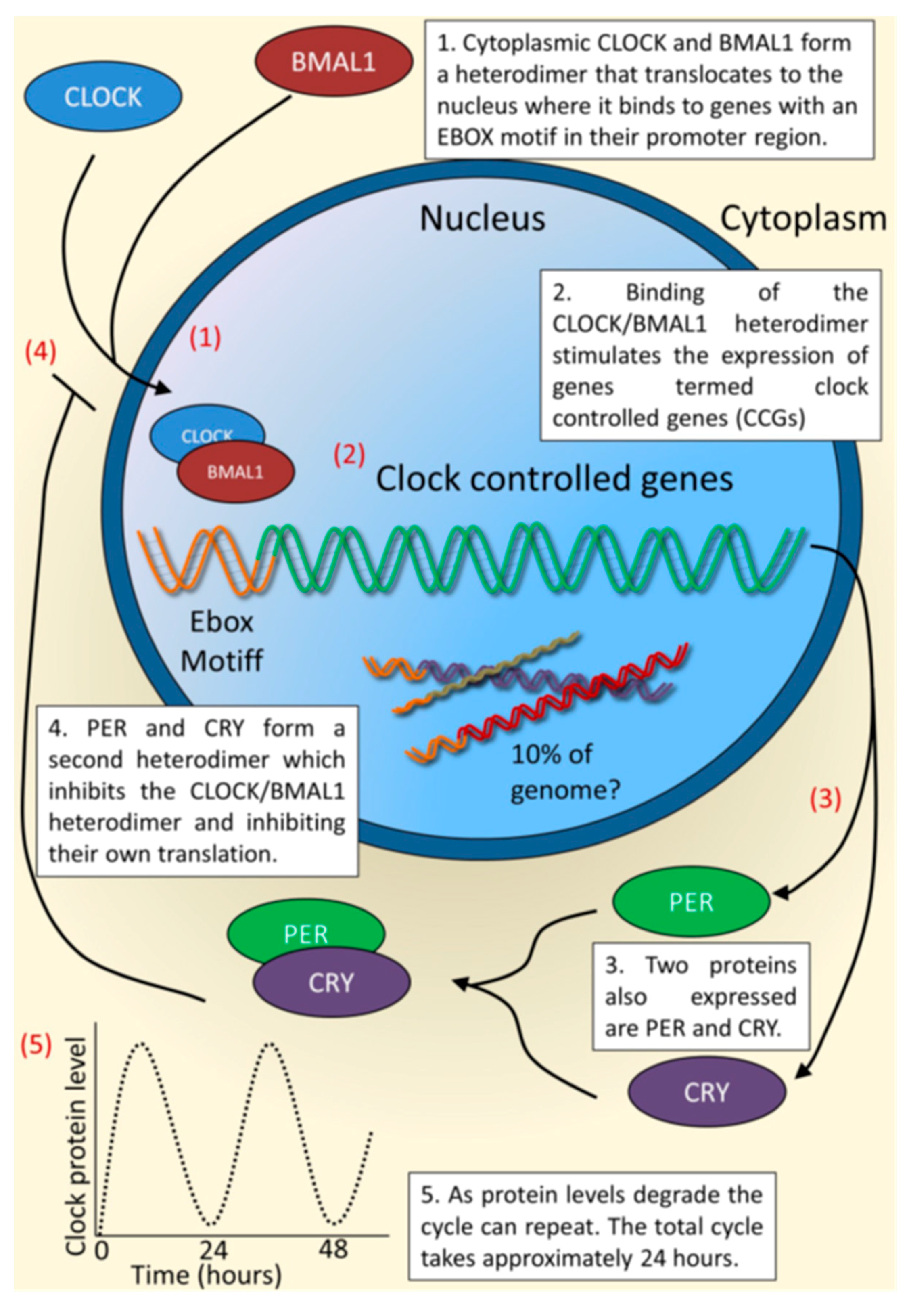
2. The cutaneous Circadian System
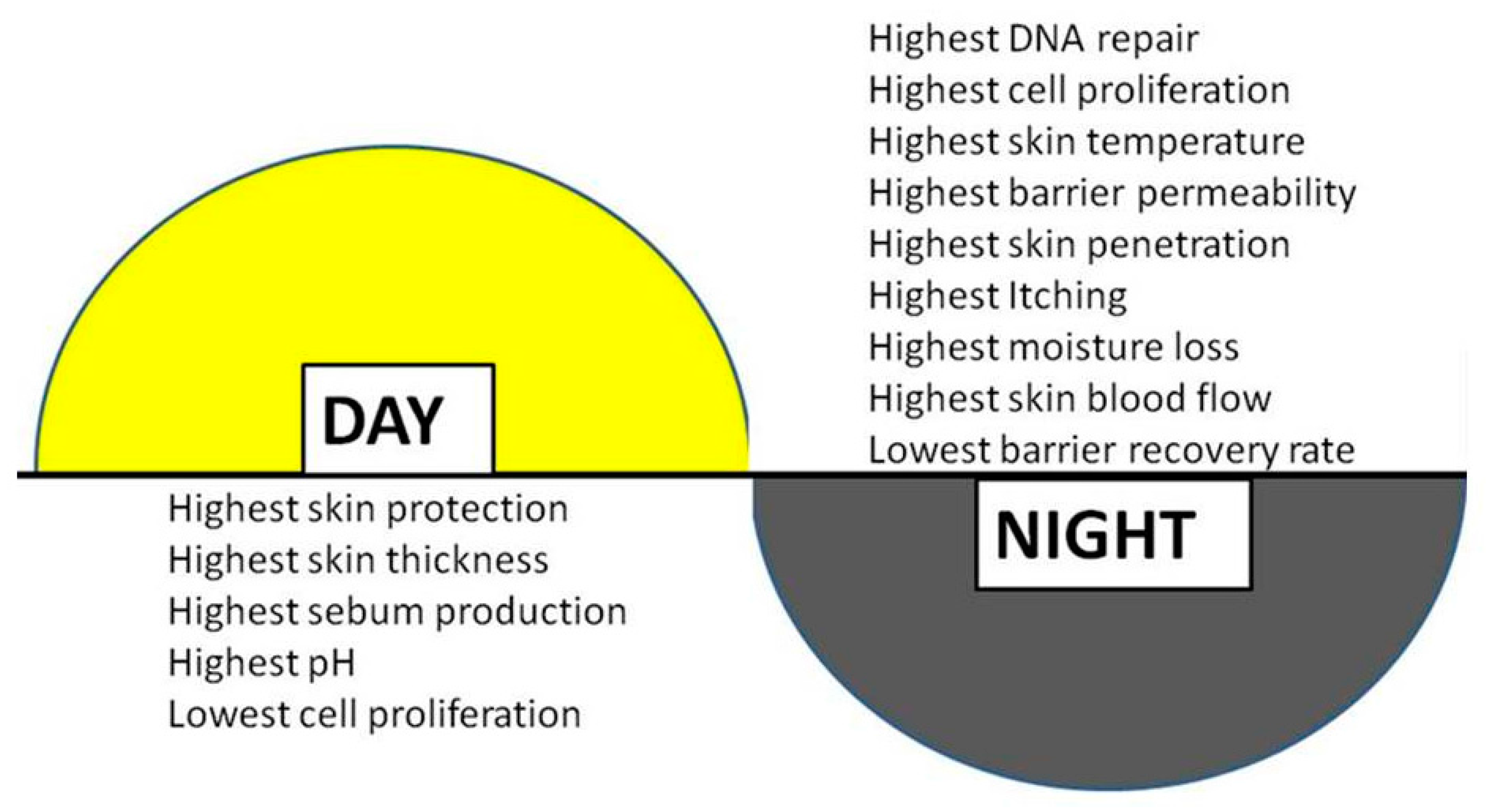
3. Clinical Phenomena of Cutaneous Rhythmicity
4. Regulatory Factors in Cutaneous Rhythms
5. Rhythms in Keratinocyte Differentiation and Proliferation
6. Circadian Influence on DNA Repair, Skin Cancer, and Skin Disease
7. Epidermal Nuclear Receptors and Transcription Factors
8. Aging and Cutaneous Rhythms: Circadian and Ultradian
9. Conclusions and Implications for Future Study
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| TEWL | Trans-epidermal water loss |
| Klf-9 | Krüppel like factor 9 |
| SCN | Suprachiasmatic nucleus |
| CCGs | Clock controlled genes |
| PER | Period |
| CRY | Cryptochrome |
| GREs | Glucocorticoid response elements |
| NRs | Nuclear receptors |
| ATP | Adenosine Triphosphate |
| ROS | Reactive oxygen species |
| UV | Ultraviolet |
| UVR | Ultraviolet radiation |
| UVB | Ultraviolet B |
References
- Ko, C.H.; Takahashi, J.S. Molecular components of the mammalian circadian clock. Hum. Mol. Genet. 2006, 15, R271–R277. [Google Scholar] [CrossRef] [PubMed]
- Zanello, S.B.; Jackson, D.M.; Holick, M.F. Expression of the circadian clock genes clock and period1 in human skin. J. Investig. Dermatol. 2000, 115, 757–760. [Google Scholar] [CrossRef] [PubMed]
- Desotelle, J.A.; Wilking, M.J.; Ahmad, N. The circadian control of skin and cutaneous photodamage. Photochem. Photobiol. 2012, 88, 1037–1047. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, L.; Brown, S.A. Measuring circadian clock function in human cells. Methods Enzymol. 2015, 552, 231–256. [Google Scholar] [PubMed]
- Sassone-Corsi, P. The time of your life. Cerebrum Dana Forum Brain Sci. 2014, PMC4445581. [Google Scholar]
- Su, Y.; Cailotto, C.; Foppen, E.; Jansen, R.; Zhang, Z.; Buijs, R.; Fliers, E.; Kalsbeek, A. The role of feeding rhythm, adrenal hormones and neuronal inputs in synchronizing daily clock gene rhythms in the liver. Mol. Cell. Endocrinol. 2016, 422, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Geyfman, M.; Andersen, B. How the skin can tell time. J. Investig. Dermatol. 2009, 129, 1063–1066. [Google Scholar] [CrossRef] [PubMed]
- Weigl, Y.; Harbour, V.L.; Robinson, B.; Dufresne, L.; Amir, S. Peripheral circadian clocks—A conserved phenotype? Chronobiol. Int. 2013, 30, 559–576. [Google Scholar] [CrossRef] [PubMed]
- Geyfman, M.; Kumar, V.; Liu, Q.; Ruiz, R.; Gordon, W.; Espitia, F.; Cam, E.; Millar, S.E.; Smyth, P.; Ihler, A.; et al. Brain and muscle Arnt-like protein-1 (BMAL1) controls circadian cell proliferation and susceptibility to UVB-induced DNA damage in the epidermis. Proc. Natl. Acad. Sci. USA 2012, 109, 11758–11763. [Google Scholar] [CrossRef] [PubMed]
- Al-Nuaimi, Y.; Hardman, J.A.; Biro, T.; Haslam, I.S.; Philpott, M.P.; Toth, B.I.; Farjo, N.; Farjo, B.; Baier, G.; Watson, R.E.; et al. A meeting of two chronobiological systems: Circadian proteins period1 and bmal1 modulate the human hair cycle clock. J. Investig. Dermatol. 2014, 134, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Plikus, M.V.; Vollmers, C.; de la Cruz, D.; Chaix, A.; Ramos, R.; Panda, S.; Chuong, C.M. Local circadian clock gates cell cycle progression of transient amplifying cells during regenerative hair cycling. Proc. Natl. Acad. Sci. USA 2013, 110, E2106–E2115. [Google Scholar] [CrossRef] [PubMed]
- Duffield, G.E. DNA microarray analyses of circadian timing: The genomic basis of biological time. J. Neuroendocrinol. 2003, 15, 991–1002. [Google Scholar] [CrossRef] [PubMed]
- Baron, K.G.; Reid, K.J. Circadian misalignment and health. Int. Rev. Psychiatry (Abingdon, Engl.) 2014, 26, 139–154. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Arnal, L.; Sassone-Corsi, P. Stem cells: The clock within. Nature 2011, 480, 185–187. [Google Scholar] [CrossRef] [PubMed]
- Orozco-Solis, R.; Ramadori, G.; Coppari, R.; Sassone-Corsi, P. Sirt1 relays nutritional inputs to the circadian clock through the sf1 neurons of the ventromedial hypothalamus. Endocrinology 2015, 156, 2174–2184. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.R.; Eckel-Mahan, K.; Sassone-Corsi, P.; Baldi, P. How pervasive are circadian oscillations? Trends Cell Biol. 2014, 24, 329–331. [Google Scholar] [CrossRef] [PubMed]
- Sahar, S.; Masubuchi, S.; Eckel-Mahan, K.; Vollmer, S.; Galla, L.; Ceglia, N.; Masri, S.; Barth, T.K.; Grimaldi, B.; Oluyemi, O.; et al. Circadian control of fatty acid elongation by sirt1 protein-mediated deacetylation of acetyl-coenzyme a synthetase 1. J. Biol. Chem. 2014, 289, 6091–6097. [Google Scholar] [CrossRef] [PubMed]
- Tamaru, T.; Hattori, M.; Ninomiya, Y.; Kawamura, G.; Vares, G.; Honda, K.; Mishra, D.P.; Wang, B.; Benjamin, I.; Sassone-Corsi, P.; et al. ROS stress resets circadian clocks to coordinate pro-survival signals. PLoS ONE 2013, 8, e82006. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.A. Circadian clock-mediated control of stem cell division and differentiation: Beyond night and day. Development (Camb., Engl.) 2014, 141, 3105–3111. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.A.; Pagani, L.; Cajochen, C.; Eckert, A. Systemic and cellular reflections on ageing and the circadian oscillator: A mini-review. Gerontology 2011, 57, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Dallmann, R.; Brown, S.A.; Gachon, F. Chronopharmacology: New insights and therapeutic implications. Annu. Rev. Pharmacol. Toxicol. 2014, 54, 339–361. [Google Scholar] [CrossRef] [PubMed]
- Dallmann, R.; Viola, A.U.; Tarokh, L.; Cajochen, C.; Brown, S.A. The human circadian metabolome. Proc. Natl. Acad. Sci. USA 2012, 109, 2625–2629. [Google Scholar] [CrossRef] [PubMed]
- Hardman, J.A.; Tobin, D.J.; Haslam, I.S.; Farjo, N.; Farjo, B.; Al-Nuaimi, Y.; Grimaldi, B.; Paus, R. The peripheral clock regulates human pigmentation. J. Investig. Dermatol. 2015, 135, 1053–1064. [Google Scholar] [CrossRef] [PubMed]
- Plikus, M.V.; van Spyk, E.N.; Pham, K.; Geyfman, M.; Kumar, V.; Takahashi, J.S.; Andersen, B. The circadian clock in skin: Implications for adult stem cells, tissue regeneration, cancer, aging, and immunity. J. Biol. Rhythms 2015, 30, 163–182. [Google Scholar] [CrossRef] [PubMed]
- Le Fur, I.; Reinberg, A.; Lopez, S.; Morizot, F.; Mechkouri, M.; Tschachler, E. Analysis of circadian and ultradian rhythms of skin surface properties of face and forearm of healthy women. J. Investig. Dermatol. 2001, 117, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Sporl, F.; Korge, S.; Jurchott, K.; Wunderskirchner, M.; Schellenberg, K.; Heins, S.; Specht, A.; Stoll, C.; Klemz, R.; Maier, B.; et al. Kruppel-like factor 9 is a circadian transcription factor in human epidermis that controls proliferation of keratinocytes. Proc. Natl. Acad. Sci. USA 2012, 109, 10903–10908. [Google Scholar] [CrossRef] [PubMed]
- Yosipovitch, G.; Xiong, G.L.; Haus, E.; Sackett-Lundeen, L.; Ashkenazi, I.; Maibach, H.I. Time-dependent variations of the skin barrier function in humans: Transepidermal water loss, stratum corneum hydration, skin surface pH, and skin temperature. J. Investig. Dermatol. 1998, 110, 20–23. [Google Scholar] [CrossRef] [PubMed]
- Luber, A.J.; Ensanyat, S.H.; Zeichner, J.A. Therapeutic implications of the circadian clock on skin function. J. Drugs Dermatol. 2014, 13, 130–134. [Google Scholar] [PubMed]
- Patel, T.; Ishiuji, Y.; Yosipovitch, G. Nocturnal itch: Why do we itch at night? Acta Derm. Venereol. 2007, 87, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Yosipovitch, G.; Sackett-Lundeen, L.; Goon, A.; Yiong Huak, C.; Leok Goh, C.; Haus, E. Circadian and ultradian (12 h) variations of skin blood flow and barrier function in non-irritated and irritated skin-effect of topical corticosteroids. J. Investig. Dermatol. 2004, 122, 824–829. [Google Scholar] [CrossRef] [PubMed]
- Van Someren, E.J. Mechanisms and functions of coupling between sleep and temperature rhythms. Prog. Brain Res. 2006, 153, 309–324. [Google Scholar] [PubMed]
- McDonnell, A.C.; Eiken, O.; Mekjavic, P.J.; Mekjavic, I.B. Circadian rhythm of peripheral perfusion during 10-day hypoxic confinement and bed rest. Eur. J. Appl. Physiol. 2014, 114, 2093–2104. [Google Scholar] [CrossRef] [PubMed]
- Pershing, L.K.; Corlett, J.L.; Lambert, L.D.; Poncelet, C.E. Circadian activity of topical 0.05% betamethasone dipropionate in human skin in vivo. J. Investig. Dermatol. 1994, 102, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Verschoore, M.; Poncet, M.; Krebs, B.; Ortonne, J.P. Circadian variations in the number of actively secreting sebaceous follicles and androgen circadian rhythms. Chronobiol. Int. 1993, 10, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Burton, J.L.; Cunliffe, W.J.; Shuster, S. Circadian rhythm in sebum excretion. Br. J. Dermatol. 1970, 82, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Reinberg, A.; Le Fur, I.; Tschachler, E. Problems related to circadian rhythms in human skin and their validation. J. Investig. Dermatol. 1998, 111, 708–709. [Google Scholar] [CrossRef] [PubMed]
- Reinberg, A.E.; Touitou, Y.; Soudant, E.; Bernard, D.; Bazin, R.; Mechkouri, M. Oral contraceptives alter circadian rhythm parameters of cortisol, melatonin, blood pressure, heart rate, skin blood flow, transepidermal water loss, and skin amino acids of healthy young women. Chronobiol. Int. 1996, 13, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Spruit, D. The diurnal variation of water vapour loss from the skin in relation to temperature. Br. J. Dermatol. 1971, 84, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Sporl, F.; Schellenberg, K.; Blatt, T.; Wenck, H.; Wittern, K.P.; Schrader, A.; Kramer, A. A circadian clock in hacat keratinocytes. J. Investig. Dermatol. 2011, 131, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Denda, M.; Tsuchiya, T. Barrier recovery rate varies time-dependently in human skin. Br. J. Dermatol. 2000, 142, 881–884. [Google Scholar] [CrossRef] [PubMed]
- Sandu, C.; Liu, T.; Malan, A.; Challet, E.; Pevet, P.; Felder-Schmittbuhl, M.P. Circadian clocks in rat skin and dermal fibroblasts: Differential effects of aging, temperature and melatonin. Cell. Mol. Life Sci. 2015, 72, 2237–2248. [Google Scholar] [CrossRef] [PubMed]
- Akashi, M.; Soma, H.; Yamamoto, T.; Tsugitomi, A.; Yamashita, S.; Yamamoto, T.; Nishida, E.; Yasuda, A.; Liao, J.K.; Node, K. Noninvasive method for assessing the human circadian clock using hair follicle cells. Proc. Natl. Acad. Sci. USA 2010, 107, 15643–15648. [Google Scholar] [CrossRef] [PubMed]
- Janich, P.; Toufighi, K.; Solanas, G.; Luis, N.M.; Minkwitz, S.; Serrano, L.; Lehner, B.; Benitah, S.A. Human epidermal stem cell function is regulated by circadian oscillations. Cell Stem Cell 2013, 13, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, E.; Ripperger, J.A.; Hoegger, D.C.; Bruegger, P.; Buch, T.; Birchler, T.; Mueller, A.; Albrecht, U.; Contaldo, C.; Brown, S.A. Nono couples the circadian clock to the cell cycle. Proc. Natl. Acad. Sci. USA 2013, 110, 1592–1599. [Google Scholar] [CrossRef] [PubMed]
- Scheving, L.E. Mitotic activity in the human epidermis. Anat. Rec. 1959, 135, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Livshits, G.; Kobielak, A.; Fuchs, E. Governing epidermal homeostasis by coupling cell-cell adhesion to integrin and growth factor signaling, proliferation, and apoptosis. Proc. Natl. Acad. Sci. USA 2012, 109, 4886–4891. [Google Scholar] [CrossRef] [PubMed]
- Pernodet, N.; Pelle, E. Chronobiology of the Skin, Skin Circadian Rhythm and Clock Genes: A New Approach to Slowing down the Aging Process, 9th ed.; Chemical Publishing: Los Angeles, CA, USA, 2015; Volume 2. [Google Scholar]
- Marchenay, C.; Cellarier, E.; Levi, F.; Rolhion, C.; Kwiatkowski, F.; Claustrat, B.; Madelmont, J.C.; Chollet, P. Circadian variation in O6-alkylguanine-DNA alkyltransferase activity in circulating blood mononuclear cells of healthy human subjects. Int. J. Cancer 2001, 91, 60–66. [Google Scholar] [CrossRef]
- Manzella, N.; Bracci, M.; Strafella, E.; Staffolani, S.; Ciarapica, V.; Copertaro, A.; Rapisarda, V.; Ledda, C.; Amati, M.; Valentino, M.; et al. Circadian modulation of 8-oxoguanine DNA damage repair. Sci. Rep. 2015, 5, 13752. [Google Scholar] [CrossRef] [PubMed]
- Gaddameedhi, S.; Selby, C.P.; Kemp, M.G.; Ye, R.; Sancar, A. The circadian clock controls sunburn apoptosis and erythema in mouse skin. J. Investig. Dermatol. 2015, 135, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Lengyel, Z.; Lovig, C.; Kommedal, S.; Keszthelyi, R.; Szekeres, G.; Battyani, Z.; Csernus, V.; Nagy, A.D. Altered expression patterns of clock gene mRNAs and clock proteins in human skin tumors. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2013, 34, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Kvaskoff, M.; Weinstein, P. Are some melanomas caused by artificial light? Med. Hypotheses 2010, 75, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Li, W.Q.; Qureshi, A.A.; Schernhammer, E.S.; Han, J. Rotating night-shift work and risk of psoriasis in US women. J. Investig. Dermatol. 2013, 133, 565–567. [Google Scholar] [CrossRef] [PubMed]
- Ando, N.; Nakamura, Y.; Aoki, R.; Ishimaru, K.; Ogawa, H.; Okumura, K.; Shibata, S.; Shimada, S.; Nakao, A. Circadian gene clock regulates psoriasis-like skin inflammation in mice. J. Investig. Dermatol. 2015, 135, 3001–3008. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Cho, H.; Yu, R.T.; Atkins, A.R.; Downes, M.; Evans, R.M. Nuclear receptors rock around the clock. EMBO Rep. 2014, 15, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Kino, T. Circadian rhythms of glucocorticoid hormone actions in target tissues: Potential clinical implications. Sci. Signal. 2012, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Kondratov, R.V.; Kondratova, A.A.; Gorbacheva, V.Y.; Vykhovanets, O.V.; Antoch, M.P. Early aging and age-related pathologies in mice deficient in bmal1, the core component of the circadian clock. Genes Dev. 2006, 20, 1868–1873. [Google Scholar] [CrossRef] [PubMed]
- Kawara, S.; Mydlarski, R.; Mamelak, A.J.; Freed, I.; Wang, B.; Watanabe, H.; Shivji, G.; Tavadia, S.K.; Suzuki, H.; Bjarnason, G.A.; et al. Low-dose ultraviolet b rays alter the mRNA expression of the circadian clock genes in cultured human keratinocytes. J. Investig. Dermatol. 2002, 119, 1220–1223. [Google Scholar] [CrossRef] [PubMed]
- Polefka, T.G.; Meyer, T.A.; Agin, P.P.; Bianchini, R.J. Cutaneous oxidative stress. J. Cosmet. Dermatol. 2012, 11, 55–64. [Google Scholar] [PubMed]
- Dong, K.; Pelle, E.; Yarosh, D.B.; Pernodet, N. Sirtuin 4 identification in normal human epidermal keratinocytes and its relation to sirtuin 3 and energy metabolism under normal conditions and UVB-induced stress. Exp. Dermatol. 2012, 21, 231–233. [Google Scholar] [CrossRef] [PubMed]
- Bednarova, A.; Kodrik, D.; Krishnan, N. Nature’s timepiece-molecular coordination of metabolism and its impact on aging. Int. J. Mol. Sci. 2013, 14, 3026–3049. [Google Scholar] [PubMed]
- Geyfman, M.; Andersen, B. Clock genes, hair growth and aging. Aging 2010, 2, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Andersen, B.; Takahashi, J.S. Epidermal stem cells ride the circadian wave. Genome Biol. 2013, 14, 140. [Google Scholar] [PubMed]
- Oyetakin-White, P.; Suggs, A.; Koo, B.; Matsui, M.S.; Yarosh, D.; Cooper, K.D.; Baron, E.D. Does poor sleep quality affect skin ageing? Clin. Exp. Dermatol. 2015, 40, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Pernodet, N.D.; Dong, K.; Pelle, E. Autophagy in human skin fibroblasts: Comparison between young and aged cells and evaluation of its cellular rhythm and response to ultraviolet a radiation. J. Cosmet. Sci. 2016, 67, 13–20. [Google Scholar]
- Ma, D.; Panda, S.; Lin, J.D. Temporal orchestration of circadian autophagy rhythm by C/EBPβ. EMBO J. 2011, 30, 4642–4651. [Google Scholar] [CrossRef] [PubMed]
- Stringari, C.; Wang, H.; Geyfman, M.; Crosignani, V.; Kumar, V.; Takahashi, J.S.; Andersen, B.; Gratton, E. In vivo single-cell detection of metabolic oscillations in stem cells. Cell Rep. 2015, 10, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.K.; Kleszczyński, K.; Janjetovic, Z.; Sweatman, T.; Lin, Z.; Li, W.; Reiter, R.J.; Fischer, T.W.; Slominski, A.T. Metabolism of melatonin and biological activity of intermediates of melatoninergic pathway in human skin cells. FASEB J. 2013, 27, 2742–2755. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Hardeland, R.; Reiter, R.J. When the circadian clock meets the melanin pigmentary system. J. Investig. Dermatol. 2015, 135, 943–945. [Google Scholar] [CrossRef] [PubMed]
- Erkekoglu, P.; Baydar, T. Chronopharmacokinetics of drugs in toxicological aspects: A short review for pharmacy practitioners. J. Res. Pharm. Pract. 2012, 1, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Metz, R.P.; Porter, W.W.; Cassone, V.M.; Earnest, D.J. Disruption of clock gene expression alters responses of the aryl hydrocarbon receptor signaling pathway in the mouse mammary gland. Mol. Pharmacol. 2007, 72, 1349–1358. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.X.; Wang, C.; Krager, S.L.; Bottum, K.M.; Tischkau, S.A. Aryl hydrocarbon receptor activation attenuates Per1 gene induction and influences circadian clock resetting. Toxicol. Sci. Off. J. Soc. Toxicol. 2013, 132, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Tanioka, M.; Yamada, H.; Doi, M.; Bando, H.; Yamaguchi, Y.; Nishigori, C.; Okamura, H. Molecular clocks in mouse skin. J. Investig. Dermatol. 2009, 129, 1225–1231. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsui, M.S.; Pelle, E.; Dong, K.; Pernodet, N. Biological Rhythms in the Skin. Int. J. Mol. Sci. 2016, 17, 801. https://doi.org/10.3390/ijms17060801
Matsui MS, Pelle E, Dong K, Pernodet N. Biological Rhythms in the Skin. International Journal of Molecular Sciences. 2016; 17(6):801. https://doi.org/10.3390/ijms17060801
Chicago/Turabian StyleMatsui, Mary S., Edward Pelle, Kelly Dong, and Nadine Pernodet. 2016. "Biological Rhythms in the Skin" International Journal of Molecular Sciences 17, no. 6: 801. https://doi.org/10.3390/ijms17060801
APA StyleMatsui, M. S., Pelle, E., Dong, K., & Pernodet, N. (2016). Biological Rhythms in the Skin. International Journal of Molecular Sciences, 17(6), 801. https://doi.org/10.3390/ijms17060801