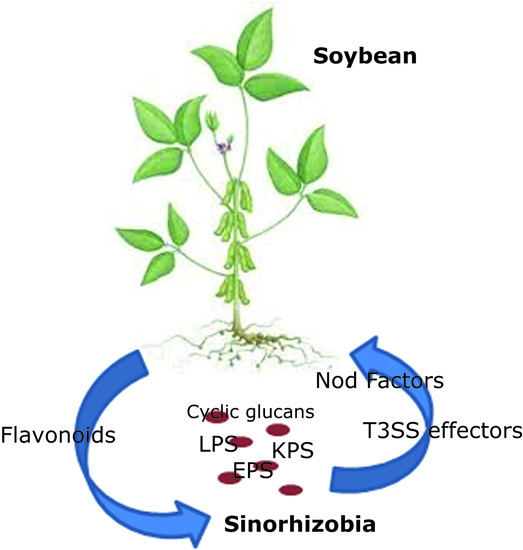
Bacterial Molecular Signals in the Sinorhizobium fredii-Soybean Symbiosis
Abstract
:1. Introduction
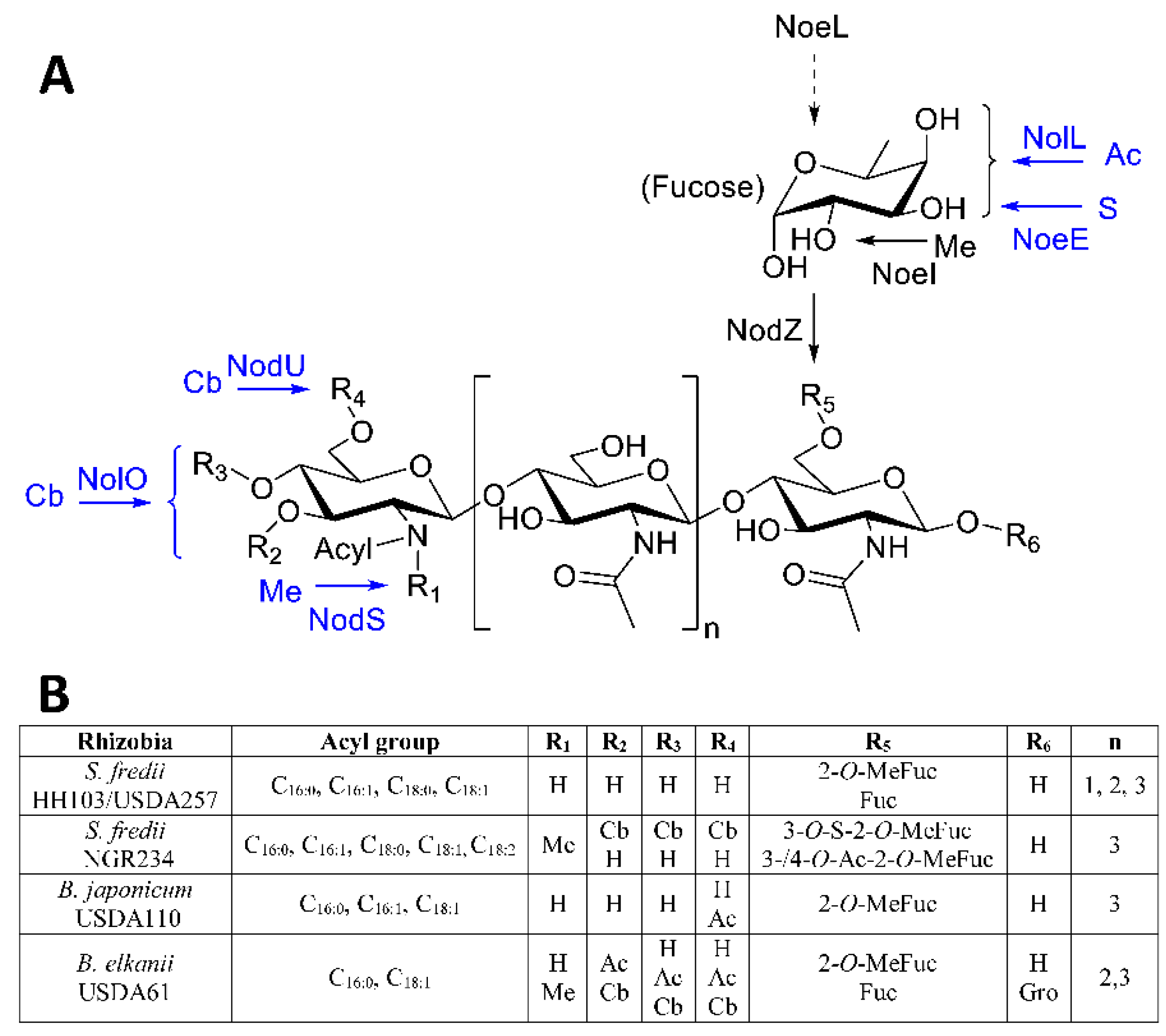
2. Nod Factors and Nodulation Genes
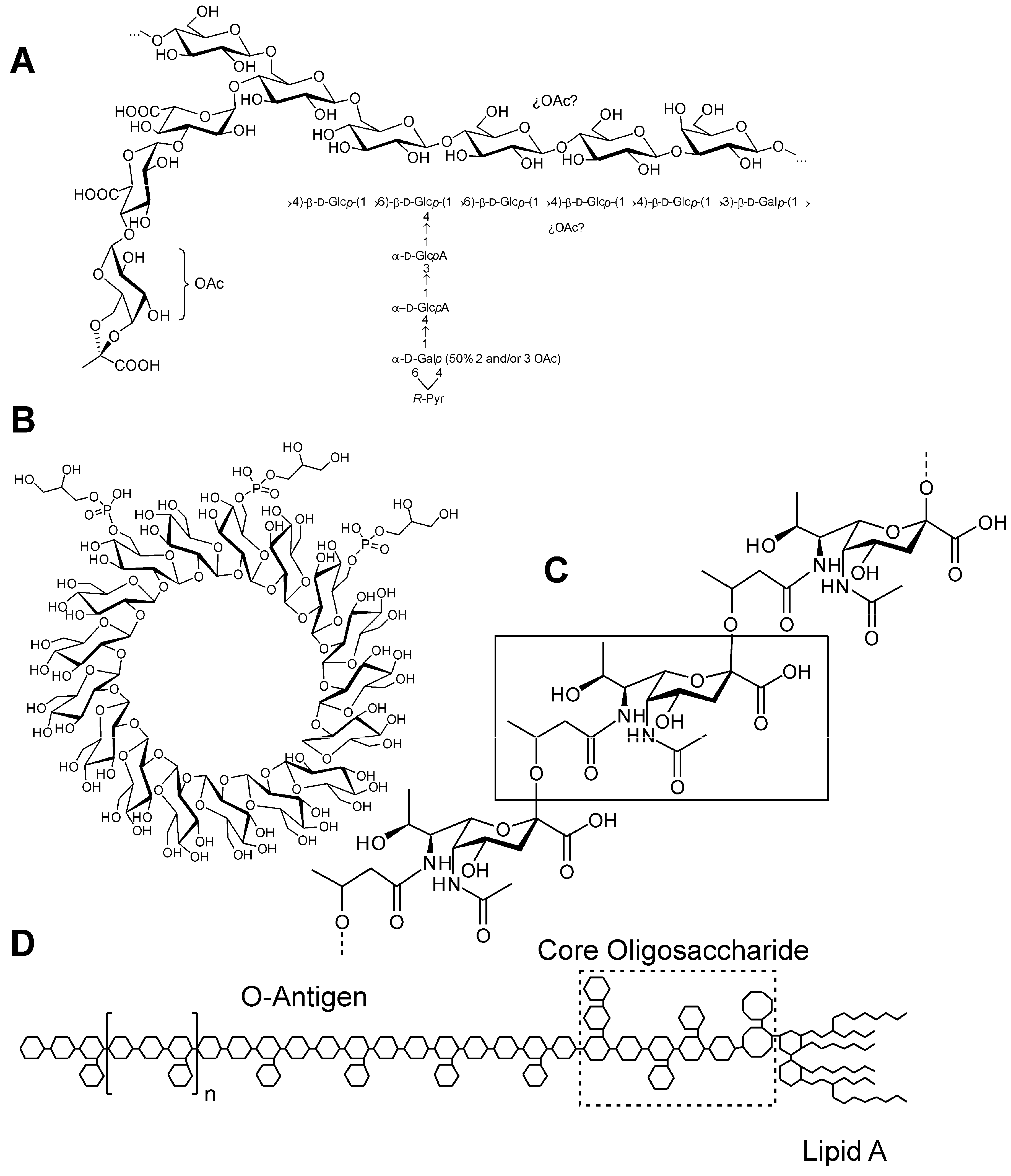
3. Surface Polysaccharides
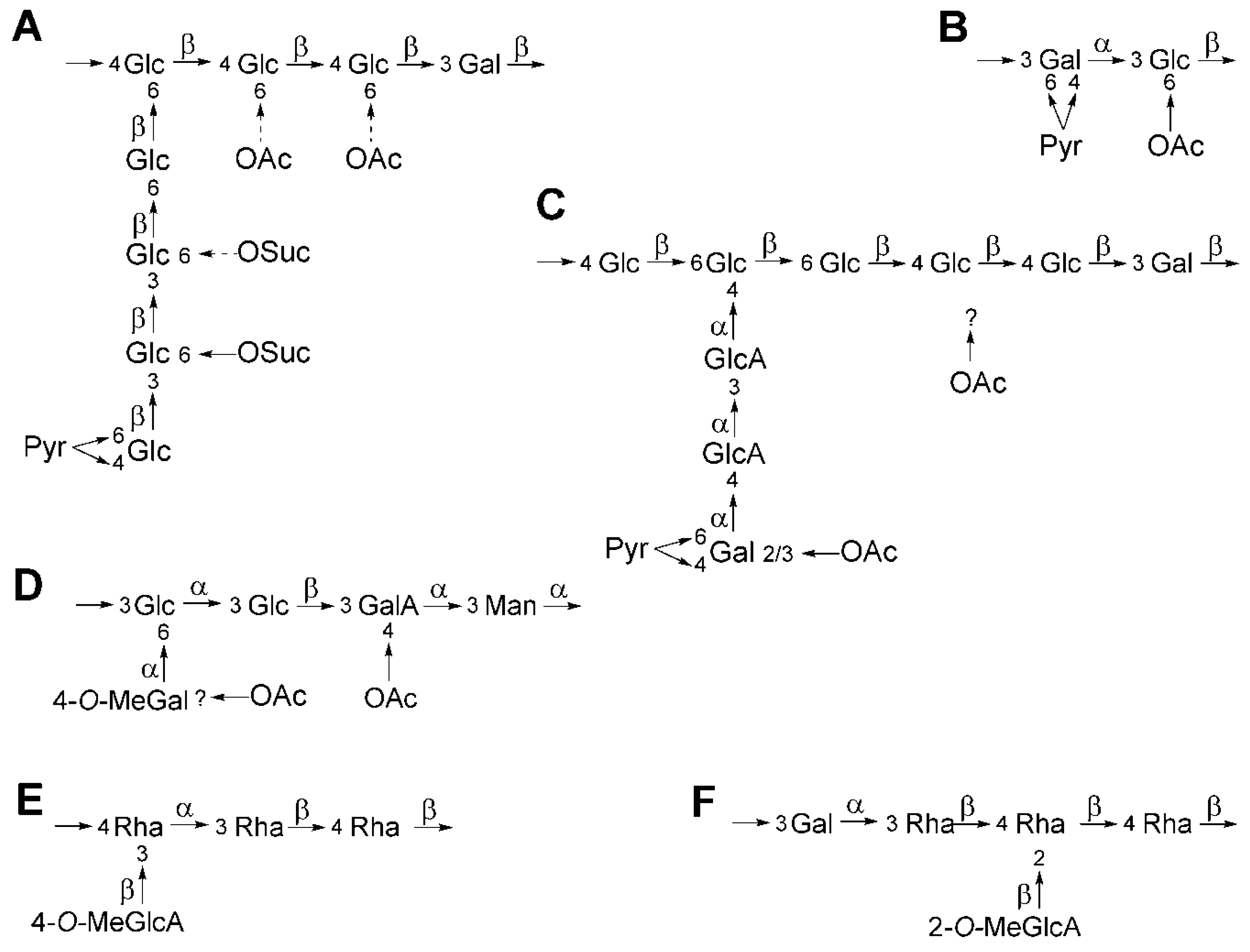
4. K-Antigen Polysaccharides
5. Exopolysaccharides
6. Cyclic Glucans
7. Lipopolysaccharides (LPS)
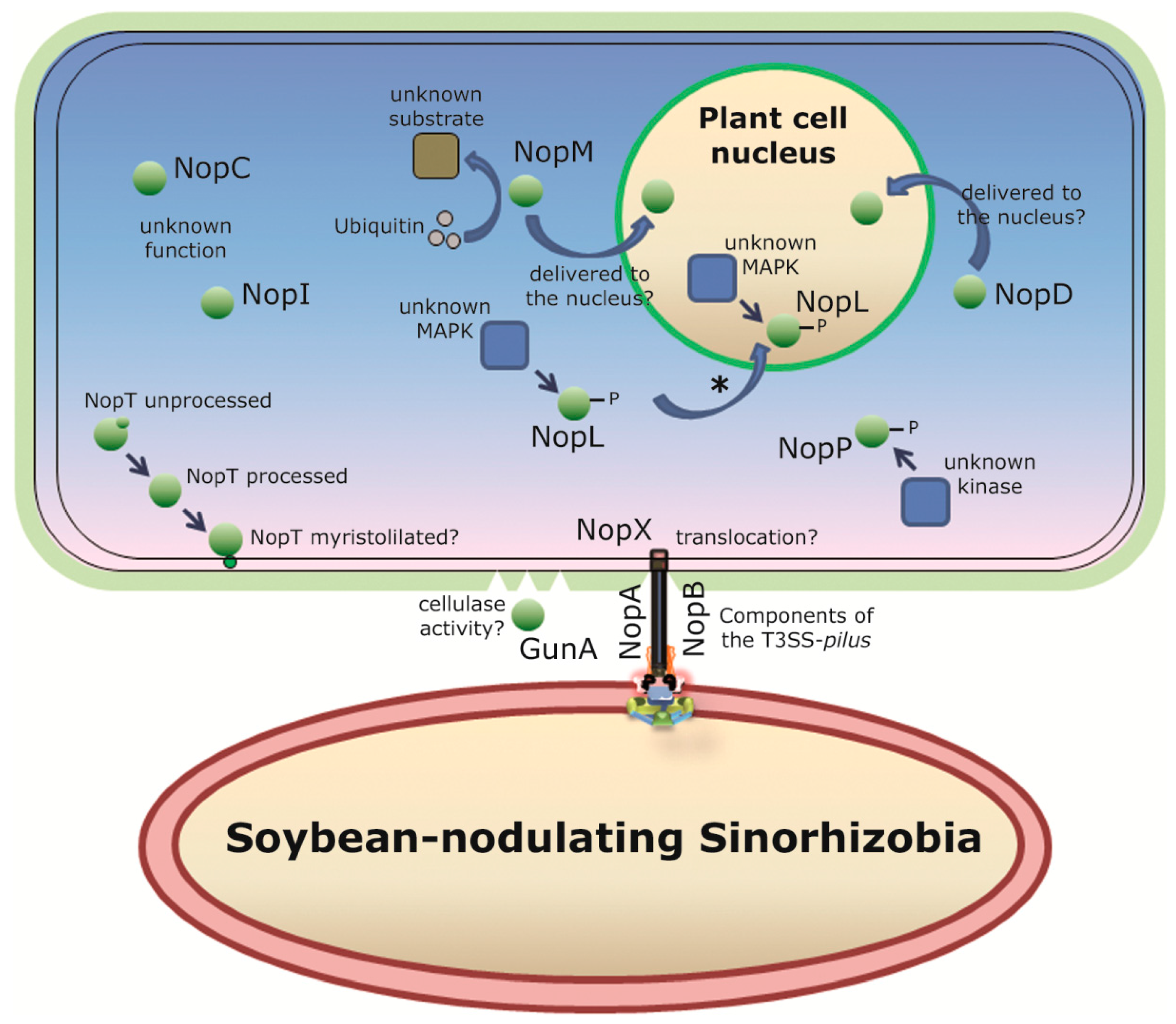
8. The Type 3 Secretion System
9. Conclusions and Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Downie, J.A. The roles of extracellular proteins, polysaccharides and signals in the interactions of rhizobia with legume roots. FEMS Microbiol. Rev. 2010, 34, 150–170. [Google Scholar] [CrossRef] [PubMed]
- Saldaña, G.; Martinez-Alcántara, V.; Vinardell, J.M.; Bellogín, R.A.; Ruiz-Sainz, J.E.; Balatti, P.A. Genetic diversity of fast-growing rhizobia that nodulate soybean (Glycine max L. Merr). Arch. Microbiol. 2003, 180, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Hungría, M.; Chueire, L.M.O.; Megías, M.; Lamrabet, Y.; Probanza, A.; Gutiérrez-Mañero, F.J.; Campo, R.J. Genetic diversity of indigenous tropical fast-growing rhizobia isolated from soybean nodules. Plant Soil 2006, 288, 343–356. [Google Scholar] [CrossRef]
- Ruiz-Sainz, J.E.; Zhou, J.C.; Rodriguez-Navarro, D.N.; Vinardell, J.M.; Thomas-Oates, J.E. Soybean cultivation and BNF in China. In Nitrogen Fixation in Agriculture, Forestry, Ecology, and the Environment; Werner, D., Newton, W.E., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 67–87. [Google Scholar]
- Pueppke, S.G.; Broughton, W.J. Rhizobium sp. strain NGR234 and R. fredii USDA257 share exceptionally broad, nested host ranges. Mol. Plant Microbe Interact. 1999, 12, 293–318. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.; Mathesius, U. The role of flavonoids in root-rhizosphere signaling: Opportunities and challenges for improving plant-microbe interactions. J. Exp. Bot. 2012, 63, 3429–3444. [Google Scholar] [CrossRef] [PubMed]
- D’Arcy-Lameta, A. Study of soybean and lentil root exudates. II. Identification of some polyphenolic compounds, relation with plantlet physiology. Plant Soil 1986, 92, 113–123. [Google Scholar]
- Kosslak, R.M.; Brookland, R.; Barkei, J.; Paaren, H.E.; Appelbaum, E.R. Induction of Bradyrhizobium japonicum common nod genes by isoflavones isolated from Glycine max. Proc. Natl. Acad. Sci. USA 1987, 84, 7428–7432. [Google Scholar] [CrossRef] [PubMed]
- Schlaman, H.R.M.; Phillips, D.A.; Kondorosi, E. Genetic organization and transcriptional regulation of rhizobial nodulation genes. In Molecular Biology of Model Plant-Associated Bacteria; Spaink, H.P., Kondorosi, A., Hooykaas, P.J.J., Eds.; Kluwer Academic Publisher: Dordrecht, The Netherlands, 1998; pp. 361–386. [Google Scholar]
- Oldroyd, G.E. Speak, friend, and enter: Signaling systems that promote beneficial symbiotic associations in plants. Nat. Rev. Microbiol. 2013, 11, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Perret, X.; Staehelin, C.; Broughton, W.J. Molecular basis of symbiotic promiscuity. Microbiol. Mol. Biol. Rev. 2000, 64, 180–201. [Google Scholar] [CrossRef] [PubMed]
- Price, N.P.; Relić, B.; Talmont, F.; Lewin, A.; Promé, D.; Pueppke, S.G.; Maillet, F.; Dénarié, J.; Promé, J.C.; Broughton, W.J. Broad-host-range Rhizobium species strain NGR234 secretes a family of carbamoylated, and fucosylated, nodulation signals that are O-acetylated or sulphated. Mol. Microbiol. 1992, 6, 3575–3584. [Google Scholar] [CrossRef] [PubMed]
- Bec-Ferté, M.P.; Krishnan, H.B.; Savagnac, A.; Pueppke, S.G.; Promé, J.C. Structures of nodulation factors from the nitrogen-fixing soybean symbiont Rhizobium fredii USDA257. Biochemistry 1994, 33, 11782–11788. [Google Scholar] [CrossRef] [PubMed]
- Gil-Serrano, A.M.; Franco-Rodríguez, G.; Tejero-Mateo, P.; Thomas-Oates, J.; Spaink, H.P.; Ruiz-Sainz, J.E.; Megías, M.; Lamrabet, Y. Structural determination of the lipo-chitin oligosaccharide nodulation signals produced by Rhizobium fredii HH103. Carbohydr. Res. 1997, 303, 435–443. [Google Scholar] [CrossRef]
- Lamrabet, Y.; Bellogín, R.A.; Cubo, T.; Espuny, R.; Gil, A.; Krishnan, H.B.; Megías, M.; Ollero, F.J.; Pueppke, S.G.; Ruiz-Sainz, J.E.; et al. Mutation in GDP-fucose synthesis genes of Sinorhizobium fredii alters Nod factors and significantly decreases competitiveness to nodulate soybeans. Mol. Plant Microbe Interact. 1999, 12, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Madinabeitia, N.; Bellogín, R.A.; Buendía-Clavería, A.M.; Camacho, M.; Cubo, T.; Espuny, M.R.; Gil-Serrano, A.; de Lyra, M.C.C.P.; Moussaid, A.; Ollero, F.J.; et al. Sinorhizobium fredii HH103 has a truncated nolO gene due to a-1 frameshift mutation that is conserved among other geographically distant S. fredii strains. Mol. Plant Microbe Interact. 2002, 15, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Jabbouri, S.; Fellay, R.; Talmont, F.; Kamalaprija, P.; Burger, U.; Relić, B.; Promé, J.C.; Broughton, W.J. Involvement of nodS in N-methylation and nodU in 6-O-carbamoylation of Rhizobium sp. NGR234 Nod factors. J. Biol. Chem. 1995, 270, 22968–22973. [Google Scholar] [CrossRef] [PubMed]
- Jabbouri, S.; Relić, B.; Hanin, M.; Kamalaprija, P.; Burger, U.; Promé, D.; Promé, J.C.; Broughton, W.J. nolO and noeI (HsnIII) of Rhizobium sp. NGR234 are involved in 3-O-carbamoylation and 2-O-methylation of Nod factors. J. Biol. Chem. 1998, 273, 12047–12055. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, H.B.; Lewin, A.; Fellay, R.; Broughton, W.J.; Pueppke, S.G. Differential expression of nodS accounts for the varied abilities of Rhizobium fredii USDA257 and Rhizobium sp. strain NGR234 to nodulate Leucaena spp. Mol. Microbiol. 1992, 6, 3321–3330. [Google Scholar] [CrossRef] [PubMed]
- Hanin, M.; Jabbouri, S.; Quesada-Vincens, D.; Freiberg, C.; Perret, X.; Promé, J.C.; Broughton, W.J.; Fellay, R. Sulphation of Rhizobium sp. NGR234 Nod factors is dependent on noeE, a new host-specificity gene. Mol. Microbiol. 1997, 24, 1119–1129. [Google Scholar] [CrossRef] [PubMed]
- Vinardell, J.M.; Acosta-Jurado, S.; Zehner, S.; Göttfert, M.; Becker, A.; Baena, I.; Blom, J.; Crespo-Rivas, J.C.; Goesmann, A.; Jaenicke, S.; et al. The Sinorhizobium fredii HH103 genome: A comparative analysis with S. fredii strains differing in their symbiotic behavior with soybean. Mol. Plant Microbe Interact. 2015, 28, 811–824. [Google Scholar] [CrossRef] [PubMed]
- Thomas-Oates, J.; Bereszczak, J.; Edwards, E.; Gill, A.; Noreen, S.; Zhou, J.C.; Chen, M.Z.; Miao, L.H.; Xie, F.L.; Yang, J.K.; et al. A catalogue of molecular, physiological and symbiotic properties of soybean-nodulating rhizobial strains from different soybean cropping areas of China. Syst. Appl. Microbiol. 2003, 26, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Machado, D.; Pueppke, S.G.; Vinardell, J.M.; Ruiz-Sainz, J.E.; Krishnan, H.B. Expression of nodD1 and nodD2 in Sinorhizobium fredii, a nitrogen-fixing symbiont of soybean and other legumes. Mol. Plant Microbe Interact. 1998, 11, 375–382. [Google Scholar] [CrossRef]
- Vinardell, J.M.; López-Baena, F.J.; Hidalgo, A.; Ollero, F.J.; Bellogín, R.; Espuny, M.R.; Temprano, F.; Romero, F.; Krishnan, H.B.; Pueppke, S.G.; et al. The effect of FITA mutations on the symbiotic properties of Sinorhizobium fredii varies in a chromosomal-background-dependent manner. Arch. Microbiol. 2004, 181, 144–154. [Google Scholar] [CrossRef] [PubMed]
- López-Baena, F.J.; Vinardell, J.M.; Pérez-Montaño, F.; Crespo-Rivas, J.C.; Bellogín, R.A.; Espuny, M.R.; Ollero, F.J. Regulation and symbiotic significance of nodulation outer proteins secretion in Sinorhizobium fredii HH103. Microbiology 2008, 154, 1825–1836. [Google Scholar] [CrossRef] [PubMed]
- Vinardell, J.M.; Ollero, F.J.; Hidalgo, A.; López-Baena, F.J.; Medina, C.; Ivanov-Vangelov, K.; Parada, M.; Madinabeitia, N.; Espuny, M.R.; Bellogín, R.A.; et al. NolR regulates diverse symbiotic signals of Sinorhizobium fredii HH103. Mol. Plant Microbe Interact. 2004, 17, 676–685. [Google Scholar] [CrossRef] [PubMed]
- Lang, K.; Lindemann, A.; Hauser, F.; Göttfert, M. The genistein stimulon of Bradyrhizobium japonicum. Mol. Genet. Genom. 2008, 279, 203–211. [Google Scholar] [CrossRef] [PubMed]
- D’Haeze, W.; Holsters, M. Nod factor structures, responses, and perception during initiation of nodule development. Glycobiology 2002, 12, 79–105. [Google Scholar] [CrossRef]
- Margaret, I.; Becker, A.; Blom, J.; Bonilla, I.; Goesmann, A.; Göttfert, M.; Lloret, J.; Mittard-Runte, V.; Rückert, C.; Ruiz-Sainz, J.E.; et al. Symbiotic properties and first analyses of the genomic sequence of the fast growing model strain Sinorhizobium fredii HH103 nodulating soybean. J. Biotechnol. 2011, 155, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Giraud, E.; Moulin, L.; Vallenet, D.; Barbe, V.; Cytryn, E.; Avarre, J.C.; Jaubert, M.; Simon, D.; Cartieaux, F.; Prin, Y.; et al. Legumes symbioses: Absence of nod genes in photosynthetic bradyrhizobia. Science 2007, 316, 1307–1312. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, S.; Kaneko, T.; Sato, S.; Saeki, K. Hijacking of leguminous nodulation signaling by the rhizobial type III secretion system. Proc. Natl. Acad. Sci. USA 2013, 110, 17131–17136. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Guerrero, I.; Pérez-Montaño, F.; Monreal, J.A.; Preston, G.M.; Fones, H.; Vioque, B.; Ollero, F.J.; López-Baena, F.J. The Sinorhizobium (Ensifer) fredii HH103 type 3 secretion system suppresses early defense responses to effectively nodulate soybean. Mol. Plant Microbe Interact. 2015, 28, 790–799. [Google Scholar] [CrossRef] [PubMed]
- Fraysse, N.; Couderc, F.; Poinsot, V. Surface polysaccharide involvement in establishing the rhizobium-legume symbiosis. Eur. J. Biochem. 2003, 270, 1365–1380. [Google Scholar] [CrossRef] [PubMed]
- D’Haeze, W.; Holsters, M. Surface polysaccharides enable bacteria to evade plant immunity. Trends Microbiol. 2004, 12, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.; Fraysse, N.; Sharypova, L. Recent advances in studies on structure and symbiosis-related function of rhizobial K-antigens and lipopolysaccharides. Mol. Plant Microbe Interact. 2005, 18, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, C. Biosynthesis and assembly of capsular polysaccharides in Escherichia coli. Annu. Rev. Biochem. 2006, 75, 39–68. [Google Scholar] [CrossRef] [PubMed]
- Reuhs, B.L.; Carlson, R.W.; Kim, J.S. Rhizobium fredii and Rhizobium meliloti produce 3-deoxy-d-manno-2-octulosonic acid containing polysaccharides that are structurally analogous to group II K antigens (capsular polysaccharides) found in Escherichia coli. J. Bacteriol. 1993, 175, 3570–3580. [Google Scholar] [PubMed]
- Reuhs, B.L.; Geller, D.P.; Kim, J.S.; Fox, J.E.; Kolli, V.S.; Pueppke, S.G. Sinorhizobium fredii and Sinorhizobium meliloti produce structurally conserved lipopolysaccharides and strain-specific K antigens. Appl. Environ. Microbiol. 1998, 64, 4930–4938. [Google Scholar] [PubMed]
- Townsend, G.E., 2nd; Forsberg, L.S.; Keating, D.H. Mesorhizobium loti produces nodPQ-dependent sulfated cell surface polysaccharides. J. Bacteriol. 2006, 188, 8560–8572. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.R.; Rodrigues, E.P.; Marcelino-Guimarães, F.C.; Oliveira, A.L.; Hungria, M. Fast induction of biosynthetic polysaccharide genes lpxA, lpxE, and rkpI of Rhizobium sp. strain PRF 81 by common bean seed exudates is indicative of a key role in symbiosis. Funct. Integr. Genom. 2013, 13, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Kannenberg, E.L.; Reuhs, B.L.; Fosberg, L.S.; Carlson, R.W. Lipopolysaccharides and K-antigens: Their structures, biosynthesis, and functions. In The Rhizobiaceae. Molecular Biology of Model Plant-Associated Bacteria; Spaink, H.P., Kondorosi, A., Hooykaas, P.J.J., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1998; pp. 119–154. [Google Scholar]
- Fraysse, N.; Lindner, B.; Kaczynski, Z.; Sharypova, L.; Holst, O.; Niehaus, K.; Poinsot, V. Sinorhizobium meliloti strain 1021 produces a low-molecular mass capsular polysaccharide that is a homopolymer of 3-deoxy-d-manno-oct-2-ulosonic acid harbouring a phospholipidic anchor. Glycobiology 2005, 15, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Chataigné, G.; Couderc, F.; Poinsot, V. Polysaccharides analysis of sinorhizobial capside by on-line anion exchange chromatography with pulsed amperometric detection and mass spectrometry coupling. J. Chromatogr. A 2008, 1185, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Le Quéré, A.J.L.; Deakin, W.K.; Schmeisser, C.; Carlson, R.W.; Streit, W.R.; Broughton, W.J.; Scott Forsberg, L. Structural characterization of a K-antigen capsular polysaccharide essential for normal symbiotic infection in Rhizobium sp. NGR234. J. Biol. Chem. 2006, 281, 28981–28992. [Google Scholar] [CrossRef] [PubMed]
- Gil-Serrano, A.M.; Rodríguez-Carvajal, M.A.; Tejero Mateo, P.; Espartero, J.L.; Menéndez, M.; Corzo, J.; Ruiz-Sainz, J.E.; Buendía-Clavería, A.M. Structural determination of a 5-acetamido-3,5,7,9-tetradeoxy-7-(3-hydroxybutyramido)-l-glycero-l-manno-nonulosonic acid-containing homopolysaccharide isolated from Sinorhizobium fredii HH103. Biochem. J. 1999, 342, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Carvajal, M.A.; Tejero-Mateo, P.; Espartero, J.L.; Ruiz-Sainz, J.E.; Buendía-Clavería, A.M.; Ollero, F.J.; Yang, S.S.; Gil-Serrano, A.M. Determination of the chemical structure of the capsular polysaccharide of strain B33, a fast-growing soya bean nodulating bacterium isolated from an arid region of China. Biochem. J. 2001, 357, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Carvajal, M.A.; Rodrigues, J.A.; Soria-Díaz, M.E.; Tejero-Mateo, P.; Buendía-Clavería, A.M.; Gutiérrez, R.; Ruiz-Sainz, J.E.; Thomas-Oates, J.; Gil-Serrano, A.M. Structural analysis of the capsular polysaccharide from Sinorhizobium fredii HWG35. Biomacromolecules 2005, 6, 1448–1456. [Google Scholar] [CrossRef] [PubMed]
- Forsberg, L.S.; Reuhs, B. Structural characterization of the K antigens from Rhizobium fredii USDA257: Evidence for a common structural motif, with strain-specific variation, in the capsular polysaccharides of Rhizobium spp. J. Bacteriol. 1997, 179, 5366–5371. [Google Scholar] [PubMed]
- Meinhardt, L.W.; Krishnan, H.B.; Balatti, P.A.; Pueppke, S.G. Molecular cloning and characterization of a sym plasmid locus that regulates cultivar-specific nodulation of soybean by Rhizobium fredii USDA257. Mol. Microbiol. 1993, 9, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Kiss, E.; Kereszt, A.; Barta, F.; Stephens, S.; Reuhs, B.L.; Kondorosi, A.; Putnoky, P. The rkp-3 gene region of Sinorhizobium meliloti Rm41 contains strain-specific genes that determine K antigen structure. Mol. Plant Microbe Interact. 2001, 14, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- Kiss, E.; Reuhs, B.L.; Kim, J.S.; Kereszt, A.; Petrovics, G.; Putnoky, P.; Dusha, I.; Carlson, R.W.; Kondorosi, A. The rkpGHI and -J genes are involved in capsular polysaccharide production by Rhizobium meliloti. J. Bacteriol. 1997, 179, 2132–2140. [Google Scholar] [PubMed]
- Müller, M.G.; Forsberg, L.S.; Keating, D.H. The rkp-1 cluster is required for secretion of Kdo homopolymeric capsular polysaccharide in Sinorhizobium meliloti strain Rm1021. J. Bacteriol. 2009, 191, 6988–7000. [Google Scholar] [CrossRef] [PubMed]
- Kereszt, A.; Kiss, E.; Rehus, B.L.; Carlson, R.W.; Kondorosi, A.; Putnoky, P. Novel rkp gene clusters of Sinorhizobium meliloti involved in capsular polysaccharide production and invasion of the symbiotic nodule: The rkpK gene codes a UDP-glucose dehydrogenase. J. Bacteriol. 1998, 180, 5426–5431. [Google Scholar] [PubMed]
- Pálvölgyi, A.; Deák, V.; Poinsot, V.; Nagy, T.; Nagy, E.; Kerepesi, I.; Putnoky, P. Genetic analysis of the rkp-3 gene region in Sinorhizobium meliloti 41: rkpY directs capsular polysaccharide synthesis to KR5 antigen production. Mol. Plant Microbe Interact. 2009, 22, 1422–1430. [Google Scholar] [CrossRef] [PubMed]
- Parada, M.; Vinardell, J.M.; Ollero, F.J.; Hidalgo, A.; Gutiérrez, R.; Buendía-Clavería, A.M.; Lei, W.; Margaret, I.; López-Baena, F.J.; Gil-Serrano, A.M.; et al. Sinorhizobium fredii HH103 mutants affected in capsular polysaccharide (KPS) are impaired for nodulation with soybean and Cajanus cajan. Mol. Plant Microbe Interact. 2006, 19, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, A.; Margaret, I.; Crespo-Rivas, J.C.; Parada, M.; Murdoch, P.S.; López, A.; Buendía-Clavería, A.M.; Moreno, J.; Albareda, M.; Gil-Serrano, A.M.; et al. The rkpU gene of Sinorhizobium fredii HH103 is required for bacterial K-antigen polysaccharide production and for efficient nodulation with soybean but not with cowpea. Microbiology 2010, 156, 3398–3411. [Google Scholar] [CrossRef] [PubMed]
- Margaret, I.; Crespo-Rivas, J.C.; Acosta-Jurado, S.; Buendía-Clavería, A.M.; Cubo, M.T.; Gil-Serrano, A.; Moreno, J.; Murdoch, P.S.; Rodríguez-Carvajal, M.A.; Rodríguez-Navarro, D.N.; et al. Sinorhizobium fredii HH103 rkp-3 genes are required for K-antigen polysaccharide biosynthesis, affect lipopolysaccharide structure and are essential for infection of legumes forming determinate nodules. Mol. Plant Microbe Interact. 2012, 25, 825–838. [Google Scholar] [CrossRef] [PubMed]
- Margaret-Oliver, I.; Lei, W.; Parada, M.; Rodríguez-Carvajal, M.A.; Crespo-Rivas, J.C.; Hidalgo, A.; Gil-Serrano, A.; Moreno, J.; Rodríguez-Navarro, D.N.; Buendía-Clavería, A.; et al. Sinorhizobium fredii HH103 does not strictly require KPS and/or EPS to nodulate Glycyrrhiza uralensis, an indeterminate nodule-forming legume. Arch. Microbiol. 2012, 194, 87–102. [Google Scholar] [CrossRef] [PubMed]
- Pellock, B.J.; Cheng, H.P.; Walker, G.C. Alfalfa root nodule invasion efficiency is dependent on Sinorhizobium meliloti polysaccharides. J. Bacteriol. 2000, 182, 4310–4318. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.; Pühler, A. Production of exopolysaccharides. In The Rhizobiaceae, Molecular Biology of Model Plant-Associated Bacteria; Spaink, H.P., Kondorosi, A., Hooykaas, P.J.J., Eds.; Kluwer Academic Publisher: Dordrecht, The Netherlands, 1998; pp. 97–108. [Google Scholar]
- Janczarek, M. Environmental signals and regulatory pathways that influence exopolysaccharide production in rhizobia. Int. J. Mol. Sci. 2011, 12, 7898–7933. [Google Scholar] [CrossRef] [PubMed]
- Zevenhuizen, L.P.T.M. Succinoglycan and galactoglucan. Carbohydr. Polym. 1997, 33, 139–144. [Google Scholar] [CrossRef]
- Rodríguez-Navarro, D.N.; Rodríguez-Carvajal, M.A.; Acosta-Jurado, S.; Soto, M.J.; Margaret, I.; Crespo-Rivas, J.C.; Sanjuan, J.; Temprano, F.; Gil-Serrano, A.; Ruiz-Sainz, J.E.; et al. Structure and biological roles of Sinorhizobium fredii HH103 exopolysaccharide. PLoS ONE 2014, 9, e115391. [Google Scholar]
- Schmeisser, C.; Liesegang, H.; Krysciak, D.; Bakkou, N.; Le Quéré, A.; Wollherr, A.; Heinemeyer, I.; Morgenstern, B.; Pommerening-Röser, A.; Flores, M.; et al. Rhizobium sp. strain NGR234 possesses a remarkable number of secretion systems. Appl. Environ. Microbiol. 2009, 75, 4035–4045. [Google Scholar] [CrossRef] [PubMed]
- Mort, A.J.; Bauer, W.D. Application of two new methods for cleavage of polysaccharides into specific oligosaccharide fragments. J. Biol. Chem. 1982, 257, 1870–1876. [Google Scholar] [PubMed]
- Poveda, A.; Santamaría, M.; Bernabé, M.; Prieto, A.; Bruix, M.; Corzo, J.; Jiménez-Barbero, J. Studies on the structure and the solution conformation of an acidic extracellular polysaccharide isolated from Bradyrhizobium. Carbohydr. Res. 1997, 304, 209–217. [Google Scholar] [CrossRef]
- An, J.; Carlson, R.W.; Glushka, J.; Streeter, J.G. The structure of a novel polysaccharide produced by Bradyrhizobium species within soybean nodules. Carbohydr. Res. 1995, 269, 303–317. [Google Scholar] [PubMed]
- Rolfe, B.G.; Carlson, R.W.; Ridge, R.W.; Dazzo, R.W.; Mateos, F.B.; Pankhurst, C.E. Defective infection and nodulation of clovers by exopolysaccharide mutants of Rhizobium leguminosarum bv. trifolii. Aust. J. Plant Physiol. 1996, 23, 285–303. [Google Scholar] [CrossRef]
- Cheng, H.-P.; Walker, G.C. Succinoglycan is required for initiation and elongation of infection threads during nodulation of alfalfa by Rhizobium meliloti. J. Bacteriol. 1998, 180, 5183–5191. [Google Scholar] [PubMed]
- Kelly, S.J.; Muszyński, A.; Kawaharada, Y.; Hubber, A.M.; Sullivan, J.T.; Sandal, N.; Carlson, R.W.; Stougaard, J.; Ronson, C.W. Conditional requirement for exopolysaccharide in the Mesorhizobium-Lotus symbiosis. Mol. Plant Microbe Interact. 2013, 26, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Quelas, J.I.; Mongiardini, E.J.; Casabuono, A.; López-García, S.L.; Althabegoiti, M.J.; Covelli, J.M.; Pérez-Giménez, J.; Couto, A.; Lodeiro, A.R. Lack of galactose or galacturonic acid in Bradyrhizobium japonicum USDA 110 exopolysaccharide leads to different symbiotic responses in soybean. Mol. Plant Microbe Interact. 2010, 23, 1592–1604. [Google Scholar] [CrossRef] [PubMed]
- Kawaharada, Y.; Kelly, S.; Nielsen, M.W.; Hjuler, C.T.; Gysel, K.; Muszyński, A.; Carlson, R.W.; Thygesen, M.B.; Sandal, N.; Asmussen, M.H.; et al. Receptor-mediated exopolysaccharide perception controls bacterial infection. Nature 2015, 523, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Djordjevic, S.P.; Chen, H.; Batley, M.; Redmond, J.W.; Rolfe, B.G. Nitrogen fixation ability of exopolysaccharide synthesis mutants of Rhizobium sp. strain NGR234 and Rhizobium trifolii is restored by addition of homologous exopolysaccharides. J. Bacteriol. 1987, 169, 53–60. [Google Scholar] [PubMed]
- Gonzalez, J.E.; Semino, C.E.; Wang, L.X.; Castellano-Torres, L. Biosynthetic control of molecular weight in the polymerization of the octasaccharide subunits of succinoglycan, a symbiotically important exopolysaccharide of Rhizobium meliloti. Proc. Natl. Acad. Sci. USA 1998, 95, 13477–13482. [Google Scholar] [CrossRef] [PubMed]
- Staehelin, C.; Forsberg, L.S.; D’Haeze, W.; Gao, M.Y.; Carlson, R.W.; Xie, Z.P.; Pellock, B.J.; Jones, K.M.; Walker, G.C.; Streit, W.R.; et al. Exo-oligosaccharides of Rhizobium sp. strain NGR234 are required for symbiosis with various legumes. J. Bacteriol. 2006, 188, 6168–6178. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Rivas, J.C.; Margaret, I.; Hidalgo, A.; Buendía-Clavería, A.M.; Ollero, F.J.; López-Baena, F.J.; del Socorro Murdoch, P.; Rodríguez-Carvajal, M.A.; Soria-Díaz, M.E.; Reguera, M.; et al. Sinorhizobium fredii HH103 cgs mutants are unable to nodulate determinate- and indeterminate nodule-forming legumes and overproduce an altered EPS. Mol. Plant Microbe Interact. 2009, 22, 575–588. [Google Scholar] [CrossRef] [PubMed]
- Breedveld, M.W.; Miller, K.J. Cyclic β-glucans of members of the family Rhizobiaceae. Microbiol. Rev. 1994, 58, 145–161. [Google Scholar] [PubMed]
- Bhagwat, A.A.; Keister, D.L. Site-directed mutagenesis of the cyclic β-(1→3)(1→6)-glucan synthesis locus of Bradyrhizobium japonicum. Mol. Plant Microbe Interact. 1995, 8, 366–370. [Google Scholar] [CrossRef]
- D’Antuono, A.L.; Casabuono, A.; Couto, A.; Ugalde, R.A.; Lepek, V.C. Nodule development induced by Mesorhizobium loti mutant strains affected in polysaccharide synthesis. Mol. Plant Microbe Interact. 2005, 18, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Rolin, D.B.; Pfeffer, P.E.; Osman, S.F.; Szwergold, S.; Fappler, F.; Benesi, A.J. Structural studies of a phosphocholine substituted β-(1,3);(1,6) macrocyclic glucan from Bradyrhizobium japonicum USDA 110. Biochim. Biophys. Acta 1992, 1116, 215–225. [Google Scholar] [CrossRef]
- Janczarek, M.; Rachwal, K.; Marzec, A.; Grzadziel, J. Signal molecules and cell-surface components involved in early stages of the legume-rhizobium interactions. Appl. Soil Ecol. 2015, 85, 94–113. [Google Scholar] [CrossRef]
- Breedveld, M.W.; Miller, K.J. Cell-surface β-glucans. In The Rhizobiaceae. Molecular Biology of Model Plant-Associated Bacteria; Spaink, H.P., Kondorosi, A., Hooykaas, P.J.J., Eds.; Kluwer Academic Publisher: Dordrecht, The Netherlands, 1998; pp. 81–96. [Google Scholar]
- Schuldes, J.; Rodríguez Orbegoso, M.; Schmeisser, C.; Krishnan, H.B.; Daniel, R.; Streit, W.R. Complete genome sequence of the broad-host-range strain Sinorhizobium fredii USDA257. J. Bacteriol. 2012, 194, 4483. [Google Scholar] [CrossRef] [PubMed]
- Ciocchini, A.E.; Guidolin, L.S.; Casabuono, A.C.; Couto, A.S.; de Iannino, N.I.; Ugalde, R.A. A glycosyltransferase with a length-controlling activity as a mechanism to regulate the size of polysaccharides. Proc. Natl. Acad. Sci. USA 2007, 104, 16492–16497. [Google Scholar] [CrossRef] [PubMed]
- Gay-Fraret, J.; Ardissone, S.; Kambara, K.; Broughton, W.J.; Deakin, W.J.; Le Quéré, A. Cyclic-β-glucans of Rhizobium (Sinorhizobium) sp. strain NGR234 are required for hypo-osmotic adaptation, motility, and efficient symbiosis with host plants. FEMS Microbiol. Lett. 2012, 333, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Bhagwat, A.A.; Mithöfer, A.; Pfeffer, P.E.; Kraus, C.; Spickers, N.; Hotchkiss, A.; Ebel, J.; Keister, D.L. Further studies of the role of cyclic β-glucans in symbiosis. An ndvC mutant of Bradyrhizobium japonicum synthesizes Cyclodecakis-(1→3)-β-Glucosyl. Plant Physiol. 1999, 119, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Ebel, J.; Mithöfer, A. Early events in the elicitation of plant defense. Planta 1998, 206, 335–348. [Google Scholar] [CrossRef]
- Dylan, T.; Nagpal, P.; Helinski, D.R.; Ditta, G.S. Symbiotic pseudorevertants of Rhizobium ndv mutants. J. Bacteriol. 1990, 172, 1409–1417. [Google Scholar] [PubMed]
- Carlson, R.W.; Forsberg, L.S.; Kannenberg, E.L. Lipopolysaccharides in Rhizobium-legume symbioses. In Endotoxins: Structure, Function and Recognition; Wang, W., Quinn, P.J., Eds.; Springer Science + Business Media: Dordrecht, The Netherlands, 2010; pp. 339–386. [Google Scholar]
- De Castro, C.; Molinaro, A.; Lanzetta, R.; Silipo, A.; Parrilli, M. Lipopolysaccharide structures from Agrobacterium and Rhizobium species. Carbohydr. Res. 2008, 343, 1924–1933. [Google Scholar] [CrossRef] [PubMed]
- Forsberg, L.S.; Carlson, R.W. Structural characterization of the primary O-antigenic polysaccharide of the Rhizobium leguminosarum 3841 lipopolysaccharide and identification of a new 3-acetimidoylamino-3-deoxyhexuronic acid glycosyl component: A unique O-methylated glycans of uniform size, containing 6-deoxy-3-O-methyl-d-talose, N-acetylquinovosamine, and rhizoaminuronic acid (3-acetimidoylamino-3-deoxy-d-gluco-hexuronic acid). J. Biol. Chem. 2008, 283, 16037–16050. [Google Scholar] [PubMed]
- Reuhs, B.L.; Kim, J.S.; Badgett, A.; Carlson, R.W. Production of cell-associated polysaccharides of Rhizobium fredii USDA257 is modulated by apigenin and host root extract. Mol. Plant Microbe Interact. 1994, 7, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Campbell, G.R.O.; Sharypova, L.A.; Scheidle, H.; Jones, K.M.; Niehaus, K.N.; Becker, A.; Walker, G.C. Striking complexity of lipopolysaccharides defects in a collection of Sinorhizobium meliloti mutants. J. Bacteriol. 2003, 185, 3853–3862. [Google Scholar] [CrossRef] [PubMed]
- Carlson, R.W.; Kalembasa, S.; Turowski, D.; Pachori, P.; Noel, K.D. Characterization of the lipopolysaccharide from a Rhizobium phaseoli mutant that is defective in infection thread development. J. Bacteriol. 1987, 169, 4923–4928. [Google Scholar] [PubMed]
- Forsberg, L.S.; Bhat, U.R.; Carlson, R.W. Structural characterization of the O-antigenic polysaccharide of the lipopolysaccharide from Rhizobium etli strain CE3. J. Biol. Chem. 2000, 275, 18851–18863. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, L.K.; Whitfield, C. Synthesis of lipopolysaccharide O-antigens by ABC transporter-dependent pathways. Carbohydr. Res. 2012, 356, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Reuhs, B.L.; Williams, M.N.V.; Kim, J.S.; Carlson, R.W.; Côté, F. Suppression of the FixZ phenotype of Rhizobium meliloti exoB mutants by lpsZ is correlated to a modified expression of the K polysaccharide. J. Bacteriol. 1995, 177, 4249–4296. [Google Scholar]
- Stacey, G.; So, J.S.; Roth, L.E.; Bhagya Lakshmi, S.K.; Carlson, R.W. A lipopolysaccharide mutant from Bradyrhizobium japonicum that uncouples plant from bacterial differentiation. Mol. Plant Microbe Interact. 1991, 4, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Campbell, G.R.; Reuhs, B.L.; Walker, G.C. Chronic intracellular infection of alfalfa nodules by Sinorhizobium meliloti requires correct lipopolysaccharide core. Proc. Natl. Acad. Sci. USA 2002, 99, 3938–3943. [Google Scholar] [CrossRef] [PubMed]
- Margaret, I.; Lucas, M.; Acosta-Jurado, S.; Buendía-Clavería, A.M.; Fedorova, E.; Hidalgo, A.; Rodríguez-Carvajal, M.A.; Rodriguez-Navarro, D.N.; Ruiz-Sainz, J.E.; Vinardell, J.M. The Sinorhizobiumfredii HH103 lipopolysaccharide is not only relevant at early soybean nodulation stages but also for symbiosome stability in mature nodules. PLoS ONE 2013, 8, e74717. [Google Scholar] [CrossRef] [PubMed]
- Niehaus, K.; Lagares, A.; Pühler, A. A Sinorhizobium meliloti lipopolysaccharide mutant induces effective nodules on the host plant Medicago sativa (Alfalfa) but fails to establish a symbiosis with Medicago truncatula. Mol. Plant Microbe Interact. 1998, 11, 906–914. [Google Scholar] [CrossRef]
- Pallen, M.J.; Chaudhuri, R.R.; Henderson, I.R. Genomic analysis of secretion systems. Curr. Opin. Microbiol. 2003, 6, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.D.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, K.; Katagiri, F. Comparing signaling mechanisms engaged in pattern-triggered and effector-triggered immunity. Curr. Opin. Plant Biol. 2010, 13, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Viprey, V.; del Greco, A.; Golinowski, W.; Broughton, W.J.; Perret, X. Symbiotic implications of type III protein secretion machinery in Rhizobium. Mol. Microbiol. 1998, 28, 1381–1389. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, H.B.; Pueppke, S.G. Flavonoid inducers of nodulation genes stimulate Rhizobium fredii USDA257 to export proteins into the environment. Mol. Plant Microbe Interact. 1993, 6, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, H.B.; Kuo, C-I.; Pueppke, S.G. Elaboration of flavonoid-induced proteins by nitrogen-fixing soybean symbiont Rhizobium fredii is regulated by both nodD1 and nodD2, and is dependent on the cultivar-specificity locus, nolXWBTUV. Microbiology 1995, 141, 2245–2251. [Google Scholar] [CrossRef]
- Marie, C.; Deakin, W.J.; Viprey, V.; Kopciñska, J.; Golinowski, W.; Krishnan, H.B.; Perret, X.; Broughton, W.J. Characterization of Nops, nodulation outer proteins, secreted via the type III secretion system of NGR234. Mol. Plant Microbe Interact. 2003, 16, 743–751. [Google Scholar] [CrossRef] [PubMed]
- De Lyra, M.C.C.P.; López-Baena, F.J.; Madinabeitia, N.; Vinardell, J.M.; Espuny, M.R.; Cubo, M.T.; Bellogín, R.A.; Ruiz-Sainz, J.E.; Ollero, F.J. Inactivation of the Sinorhizobiumfredii HH103 rhcJ gene abolishes nodulation outer proteins (Nops) secretion and decreases the symbiotic capacity with soybean. Int. Microbiol. 2006, 9, 125–133. [Google Scholar]
- Krishnan, H.B.; Lorio, J.; Kim, W.S.; Jiang, G.; Kim, K.Y.; de Boer, M.; Pueppke, S.G. Extracellular proteins involved in soybean cultivar-specific nodulation are associated with pilus-like surface appendages and exported by a type III protein secretion system in Sinorhizobium fredii USDA257. Mol. Plant Microbe Interact. 2003, 16, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Krishnan, H.B. Sinorhizobium fredii USDA257, a cultivar-specific soybean symbiont, carries two copies of y4yA and y4yB, two open reading frames that are located in a region that encodes the type III protein secretion system. Mol. Plant Microbe Interact. 2000, 13, 1010–1014. [Google Scholar] [CrossRef] [PubMed]
- Lorio, J.C.; Chronis, D.; Krishnan, H.B. Y4xP, an open reading frame located in a type III protein secretion system locus of Sinorhizobium fredii USDA257 and USDA191, encodes cysteine synthase. Mol. Plant Microbe Interact. 2006, 19, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Guerrero, I.; Pérez-Montaño, F.; Medina, C.; Ollero, F.J.; López-Baena, F.J. NopC is a Rhizobium-specific type 3 secretion system effector secreted by Sinorhizobium (Ensifer) fredii HH103. PLoS ONE 2015, 10, e0142866. [Google Scholar]
- Kim, W.S.; Krishnan, H.B. A nopA deletion mutant of Sinorhizobium fredii USDA257, a soybean symbiont, is impaired in nodulation. Curr. Microbiol. 2013, 68, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Lorio, J.C.; Kim, W.S.; Krishnan, H.B. NopB, a soybean cultivar-specificity protein from Sinorhizobium fredii USDA257, is a type III secreted protein. Mol. Plant Microbe Interact. 2004, 17, 1259–1268. [Google Scholar] [CrossRef] [PubMed]
- Bellato, C.; Krishnan, H.B.; Cubo, T.; Temprano, F.; Pueppke, S.G. The soybean cultivar specificity gene nolX is present, expressed in a nodD-dependent manner, and of symbiotic significance in cultivar-non specific strains of Rhizobium (Sinorhizobium) fredii. Microbiology 1997, 143, 1381–1388. [Google Scholar] [CrossRef] [PubMed]
- López-Baena, F.J.; Monreal, J.A.; Pérez-Montaño, F.; Guasch-Vidal, B.; Bellogín, R.A.; Vinardell, J.M.; Ollero, F.J. The absence of Nops secretion in Sinorhizobium fredii HH103 increases GmPR1 expression in Williams soybean. Mol. Plant Microbe Interact. 2009, 22, 1445–1454. [Google Scholar]
- Deakin, W.J.; Broughton, W.J. Symbiotic use of pathogenic strategies: Rhizobial protein secretion systems. Nat. Rev. Microbiol. 2009, 7, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Deakin, W.J.; Marie, C.; Saad, M.M.; Krishnan, H.B.; Broughton, W.J. NopA is associated with cell surface appendages produced by the type III secretion system of Rhizobium sp. strain NGR234. Mol. Plant Microbe Interact. 2005, 18, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Saad, M.M.; Kobayashi, H.; Marie, C.; Brown, I.R.; Mansfield, J.W.; Broughton, W.J.; Deakin, W.J. NopB, a type III secreted protein of Rhizobium sp. strain NGR234, is associated with pilus-like surface appendages. J. Bacteriol. 2005, 187, 1173–1181. [Google Scholar] [CrossRef] [PubMed]
- Saad, M.M.; Staehelin, C.; Broughton, W.J.; Deakin, W.J. Protein-protein interactions within type III secretion system-dependent pili of Rhizobium sp. strain NGR234. J. Bacteriol. 2008, 190, 750–754. [Google Scholar] [CrossRef] [PubMed]
- Tampakaki, A.P. Commonalities and differences of T3SSs in rhizobia and plant pathogenic bacteria. Front. Plant Sci. 2014, 5, 114. [Google Scholar] [CrossRef] [PubMed]
- Benabdillah, R.; Mota, L.J.; Lützelschwab, S.; Demoinet, E.; Cornelis, G.R. Identification of a nuclear targeting signal in YopM from Yersinia spp. Microb. Pathog. 2004, 36, 247–261. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.A.; López-Baena, F.J.; Ollero, F.J.; Vinardell, J.M.; Espuny, M.R.; Bellogín, R.A.; Ruiz-Sainz, J.E.; Thomas, J.R.; Sumpton, D.; Ault, J.; et al. Thomas-Oates, J. NopM and NopD are rhizobial nodulation outer proteins: Identification using LC-MALDI and LC-ESI with a monolithic capillary column. J. Proteome Res. 2007, 6, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Xin, D.W.; Liao, S.; Xie, Z.P.; Hann, D.R.; Steinle, L.; Boller, T.; Staehelin, C. Functional analysis of NopM, a novel E3 ubiquitin ligase (NEL) domain effector of Rhizobium sp. strain NGR234. PLoS Pathog. 2012, 8, e1002707. [Google Scholar] [CrossRef] [PubMed]
- Kambara, K.; Ardissone, S.; Kobayashi, H.; Saad, M.M.; Schumpp, O.; Broughton, W.J.; Deakin, W.J. Rhizobia utilize pathogen-like effector proteins during symbiosis. Mol. Microbiol. 2009, 71, 92–106. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhao, J.; Morgan, R.L.; Ma, W.; Jiang, T. Computational prediction of type III secreted proteins from gram-negative bacteria. BMC Bioinform. 2010, 11 (Suppl. 1), S47. [Google Scholar] [CrossRef] [PubMed]
- Hotson, A.; Chosed, R.; Shu, H.; Orth, K.; Mudgett, M.B. Xanthomonas type III effector XopD targets SUMO-conjugated proteins in planta. Mol. Microbiol. 2003, 50, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Sakata, T.; Kanesaki, Y.; Yoshikawa, H.; Tsurumaru, H.; Yamakawa, T. Draft genome sequence of Bradyrhizobium japonicum Is-1, which is incompatible with Rj2 genotype soybeans. Genome Announc. 2015, 3, e01219-15. [Google Scholar] [CrossRef] [PubMed]
- Faruque, O.M.; Miwa, H.; Yasuda, M.; Fujii, Y.; Kaneko, T.; Sato, S.; Okazaki, S. Identification of Bradyrhizobium elkanii genes involved in incompatibility with soybean plants carrying the Rj4 allele. Appl. Environ. Microbiol. 2015, 81, 6710–6717. [Google Scholar] [CrossRef] [PubMed]
- Shao, F.; Merrit, P.M.; Bao, Z.; Innes, R.W.; Dixon, J.E. A Yersinia effector and a Pseudomonas avirulence protein define a family of cysteine proteases functioning in bacterial pathogenesis. Cell 2002, 109, 575–588. [Google Scholar] [CrossRef]
- Dai, W.J.; Zeng, Y.; Xie, Z.P.; Staehelin, C. Symbiosis-promoting and deleterious effects of NopT, a novel type 3 effector of Rhizobium sp. strain NGR234. J. Bacteriol. 2008, 190, 5101–5110. [Google Scholar] [CrossRef] [PubMed]
- Fotiadis, C.T.; Dimou, M.; Georgakopoulos, D.G.; Katinakis, P.; Tampakaki, A.P. Functional characterization of NopT1 and NopT2, two type III effectors of Bradyrhizobium japonicum. FEMS Microbiol. Lett. 2012, 327, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Bartsev, A.V.; Boukli, N.M.; Deakin, W.J.; Staehelin, C.; Broughton, W.J. Purification and phosphorylation of the effector protein NopL from Rhizobium sp. NGR234. FEBS Lett. 2003, 554, 271–274. [Google Scholar] [CrossRef]
- Skorpil, P.; Saad, M.M.; Boukli, N.M.; Kobayashi, H.; Ares-Orpel, F.; Broughton, W.J.; Deakin, W.J. NopP, a phosphorylated effector of Rhizobium sp. strain NGR234, is a major determinant of nodulation of the tropical legumes Flemingia congesta and Tephrosia vogelii. Mol. Microbiol. 2005, 57, 1304–1317. [Google Scholar] [CrossRef] [PubMed]
- Schechter, L.M.; Guenther, J.; Olcay, E.A.; Jang, S.; Krishnan, H.B. Translocation of NopP by Sinorhizobium fredii USDA257 into Vigna unguiculata root nodules. Appl. Environ. Microbiol. 2010, 76, 3758–3761. [Google Scholar] [CrossRef] [PubMed]
- Bartsev, A.V.; Deakin, W.J.; Boukli, N.M.; McAlvin, C.B.; Stacey, G.; Malnoë, P.; Broughton, W.J.; Staehelin, C. NopL, an effector protein of Rhizobium sp. NGR234, thwarts activation of plant defense reactions. Plant Physiol. 2004, 134, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, X.J.; Lu, H.B.; Xie, Z.P.; Staehelin, C. Functional analysis of the type 3 effector nodulation outer protein L (NopL) from Rhizobium sp. NGR234: Symbiotic effects, phosphorylation, and interference with mitogen-activated protein kinase signaling. J. Biol. Chem. 2011, 286, 32178–32187. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.Y.; Xiang, Q.W.; Wagner, C.; Zhang, D.; Xie, Z.P.; Staehelin, C. The type 3 effector NopL of Sinorhizobium sp. strain NGR234 is a mitogen-activated protein kinase substrate. J. Exp. Bot. 2016, 67, 2483–2494. [Google Scholar] [CrossRef] [PubMed]
- Berrabah, F.; Ratet, P.; Gourion, B. Multiple steps control immunity during the intracellular accommodation of rhizobia. J. Exp. Bot. 2015, 66, 1977–1985. [Google Scholar] [CrossRef] [PubMed]
- Györgypal, Z.; Kondorosi, E.; Kondorosi, A. Diverse signal sensitivity of NodD protein homologs from narrow and broad host range rhizobia. Mol. Plant Microbe Interact. 1991, 4, 356–364. [Google Scholar] [CrossRef]
- Laranjo, M.; Alexandre, A.; Oliveira, S. Legume growth-promoting rhizobia: An overview on the Mesorhizobium genus. Microbiol. Res. 2014, 169, 2–17. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Saeki, Y.; Haga, M.; Harada, K.; Kouchi, H.; Umehara, Y. Rj (rj) genes involved in nitrogen-fixing root nodule formation in soybean. Breed. Sci. 2012, 61, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Videira, L.B.; Pastorino, G.N.; Balatti, P.A. Incompatibility may not be the rule in the Sinorhizobium fredii-soybean interaction. Soil Biol. Biochem. 2001, 33, 833–840. [Google Scholar] [CrossRef]
- Yang, S.; Tang, F.; Gao, M.; Krishnan, H.B.; Zhu, H. R gene-controlled host specificity in the legume-rhizobia symbiosis. Proc. Natl. Acad. Sci. USA 2010, 107, 18735–18740. [Google Scholar] [CrossRef] [PubMed]
| Species | Strain | KPS Structure [Reference] | Symbiotic Phenotype with Asiatic/American Soybeans |
|---|---|---|---|
| Sinorhizobium meliloti | Rm41 | [-β-GlcA→Pse5N(3-OH-But)7NAc-]n [38] | NA |
| Sm1021 | [→7)-β-Kdop-(2→]n [42] | NA | |
| Sinorhizobium fredii | USDA201 | [-α-Gal→β-Kdo→2-O-Me-α-Hex→β-Kdo-]n [38] | Fix+/Nod− |
| USDA205 | [→3)-α-d-Galp-(1→5)-β-Kdop-(2→]n | Fix+/Nod− | |
| [-2-O-MeManp→β-Kdo-]n [37] | |||
| USDA208 | [-α-Gal→β-Kdo-]n [38] | Fix+/Nod− | |
| USDA257 | [→3)-β-d-Manp-(1→5)–β-Kdop-(2→]n | Fix+/Nod− | |
| [→3)-β-d-2-O-MeManp-(1→5)–β-d-Kdop-(2→]n [48] | |||
| NGR234 | [-β-Glc→Pse5NAc7NAc-]n [38] | Nod−/Nod− | |
| HH103 | [→3′)-α-Pse5NAc7(3-OH-Bu)-(2→]n [45] | Fix+/Fix+ | |
| HH303 | [Rha, GalA]n [38] | Fix+/Fix+ | |
| B33 | [→6)-4-O-Me-α-d-Glcp-(1→4)-3-O-Me-β-d-GlcpA-(1→]n [46] | Fix+/Fix+ | |
| HGW35 | [→6)-2,4-di-O-Me-α-d-Galp-(1→4)-β-d-GlcpA-(1→]n [47] | Fix+/Fix+ |
| tts Gene Mutated | Function | Symbiotic Phenotype in American or Asiatic Soybean Cultivars | |
|---|---|---|---|
| HH103 | USDA257 | ||
| rhcJ | T3SS machinery | American and Asiatic: reduced nodule number, mass of nodules and plant-top dry mass [109] | American: Fix− to Fix+ [49] |
| rhcC1 | T3SS machinery | – | American: Fix− to Fix+ [49] |
| nolU | T3SS machinery | – | American: Fix− to Fix+ [49] |
| rhcL | T3SS machinery | – | American: Fix− to Fix+ [49] |
| rhcN | T3SS machinery | – | American: Fix− to Fix+ [48,110] |
| Asiatic: negative effect [110] | |||
| y4yA | Unknown | – | American and Asiatic: no effect [111] |
| y4yB | Unknown | – | American and Asiatic: no effect [112] |
| y4xP | Cysteine synthase | – | American and Asiatic: no effect [112] |
| nopA | T3SS pilus | American: reduced nodulation [113] | American: Fix− to Fix+ |
| Asiatic: reduced nodulation [114] | |||
| nopB | T3SS pilus | – | American: Fix− to Fix+ [49] |
| Asiatic: negative effect [115] | |||
| nopX | T3SS pilus/effector translocation? | American: reduced nodulation [116] | American: Fix− to Fix+ [49] |
| Asiatic: delayed nodulation [116] | |||
| nopC | Effector | American and Asiatic: reduced nodulation [113] | – |
| nopP | Effector | American and Asiatic: increased nodule number and plant-top dry mass [117] | - |
| Nop | Size (kDa) | Function | Detected in Induced Culture Supernatant * | Gene Present in the Sequenced Genome | ||||
|---|---|---|---|---|---|---|---|---|
| HH103 | USDA257 | NGR234 | HH103 | USDA257 | NGR234 | |||
| NopA | ~6 | T3SS pilus | Yes | Yes | Yes | Yes | Yes | Yes |
| NopC | ~11 | Effector | Yes | Yes | Yes | Yes | Yes | Yes |
| NopB | ~21 | T3SS pilus | Yes | Yes | Yes | Yes | Yes | Yes |
| NopI | ~27 | Putative effector | NC | NC | - | Yes | Yes | No |
| NopT | ~28 | Putative effector | NC | NC | Yes | Yes | Yes | Yes |
| NopJ | ~29 | Putative effector | - | - | ND | No | No | Yes |
| NopP | ~32 | Effector | Yes | Yes | Yes | Yes | Yes | Yes |
| NopL | ~37 | Putative effector | Yes | NC | Yes | Yes | Yes | Yes |
| NopX | ~60 | Translocation | Yes | Yes | Yes | Yes | Yes | Yes |
| NopM | ~60 | Putative effector | Yes | NC | Yes | Yes (x2) | Yes | Yes |
| NopD | ~150 | Putative effector | Yes | NC | – | Yes | Yes | No |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Baena, F.J.; Ruiz-Sainz, J.E.; Rodríguez-Carvajal, M.A.; Vinardell, J.M. Bacterial Molecular Signals in the Sinorhizobium fredii-Soybean Symbiosis. Int. J. Mol. Sci. 2016, 17, 755. https://doi.org/10.3390/ijms17050755
López-Baena FJ, Ruiz-Sainz JE, Rodríguez-Carvajal MA, Vinardell JM. Bacterial Molecular Signals in the Sinorhizobium fredii-Soybean Symbiosis. International Journal of Molecular Sciences. 2016; 17(5):755. https://doi.org/10.3390/ijms17050755
Chicago/Turabian StyleLópez-Baena, Francisco J., José E. Ruiz-Sainz, Miguel A. Rodríguez-Carvajal, and José M. Vinardell. 2016. "Bacterial Molecular Signals in the Sinorhizobium fredii-Soybean Symbiosis" International Journal of Molecular Sciences 17, no. 5: 755. https://doi.org/10.3390/ijms17050755
APA StyleLópez-Baena, F. J., Ruiz-Sainz, J. E., Rodríguez-Carvajal, M. A., & Vinardell, J. M. (2016). Bacterial Molecular Signals in the Sinorhizobium fredii-Soybean Symbiosis. International Journal of Molecular Sciences, 17(5), 755. https://doi.org/10.3390/ijms17050755