Asbestos Induces Oxidative Stress and Activation of Nrf2 Signaling in Murine Macrophages: Chemopreventive Role of the Synthetic Lignan Secoisolariciresinol Diglucoside (LGM2605)
Abstract
:1. Introduction
2. Results
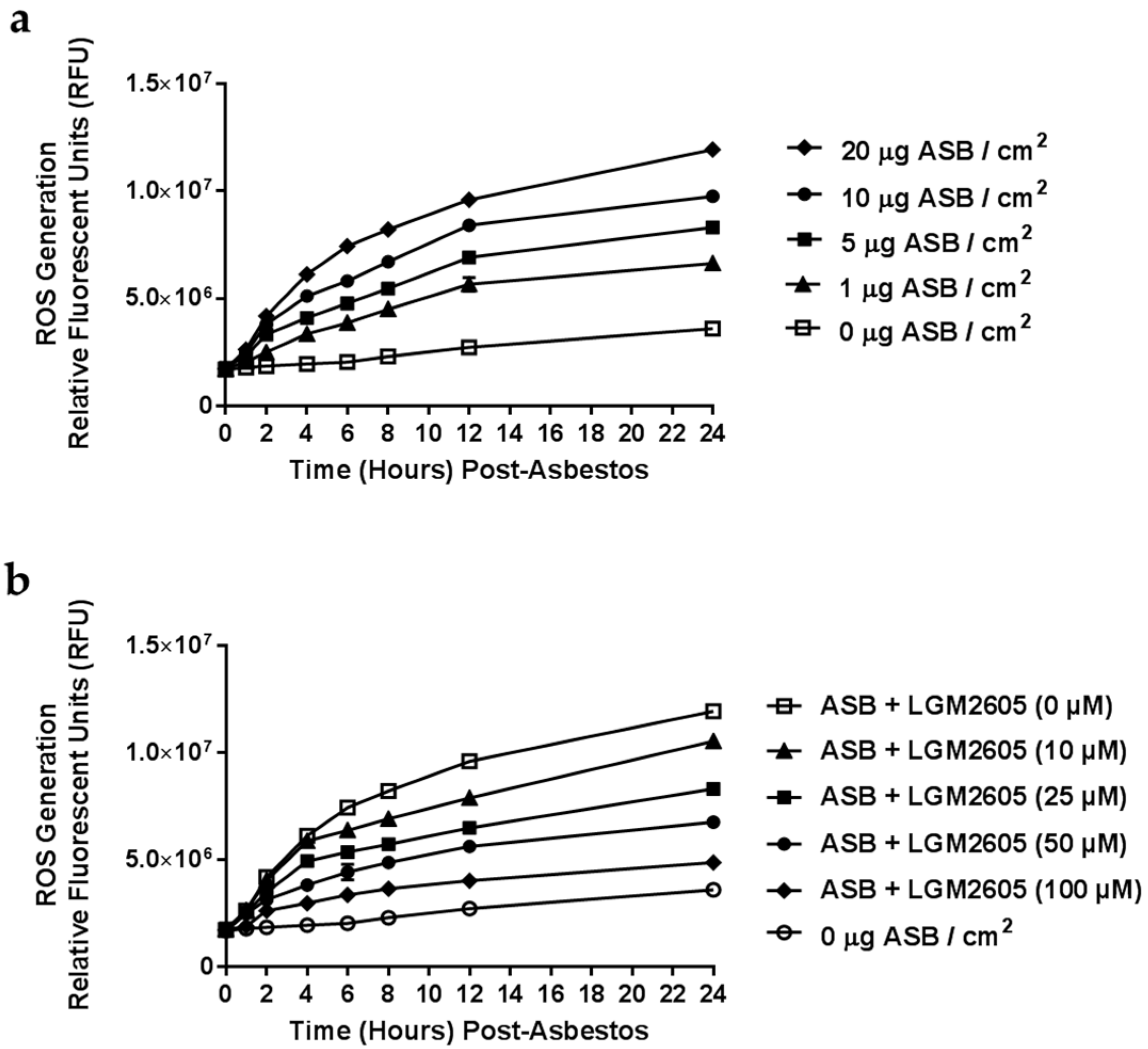
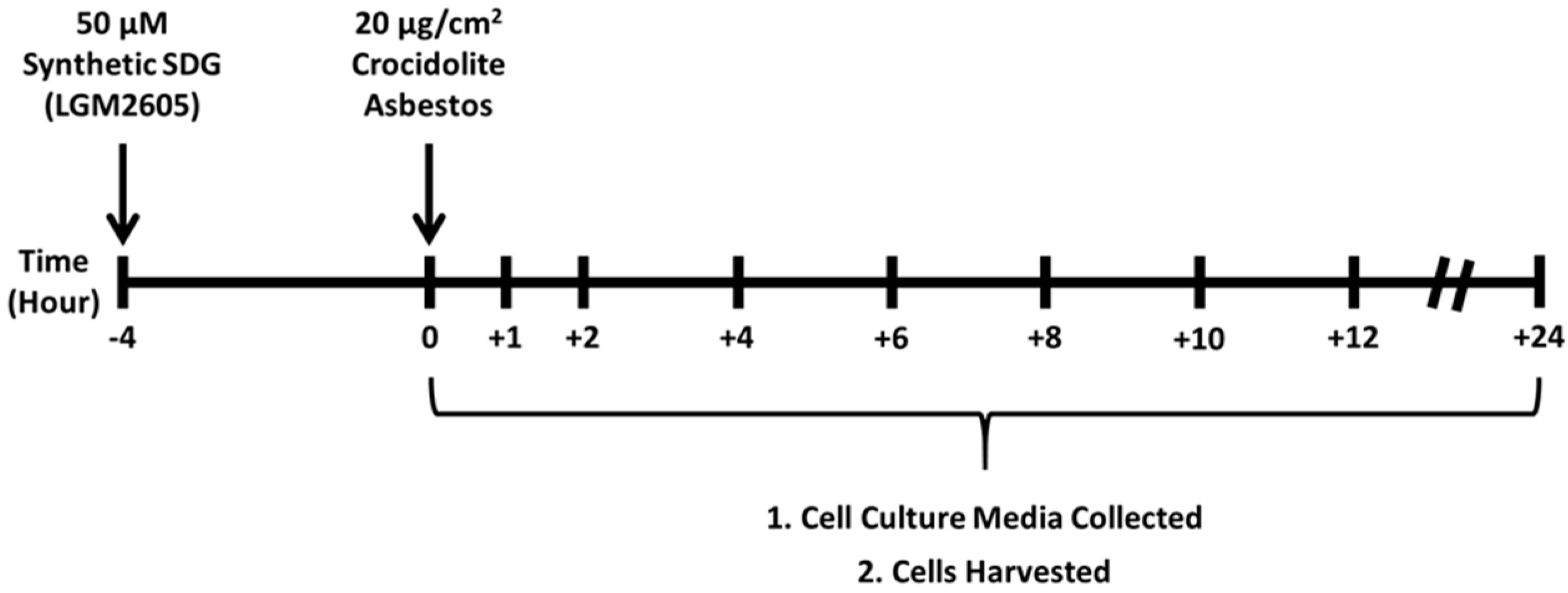
2.1. Determination of Dose-Response Following Asbestos Exposure and LGM2605
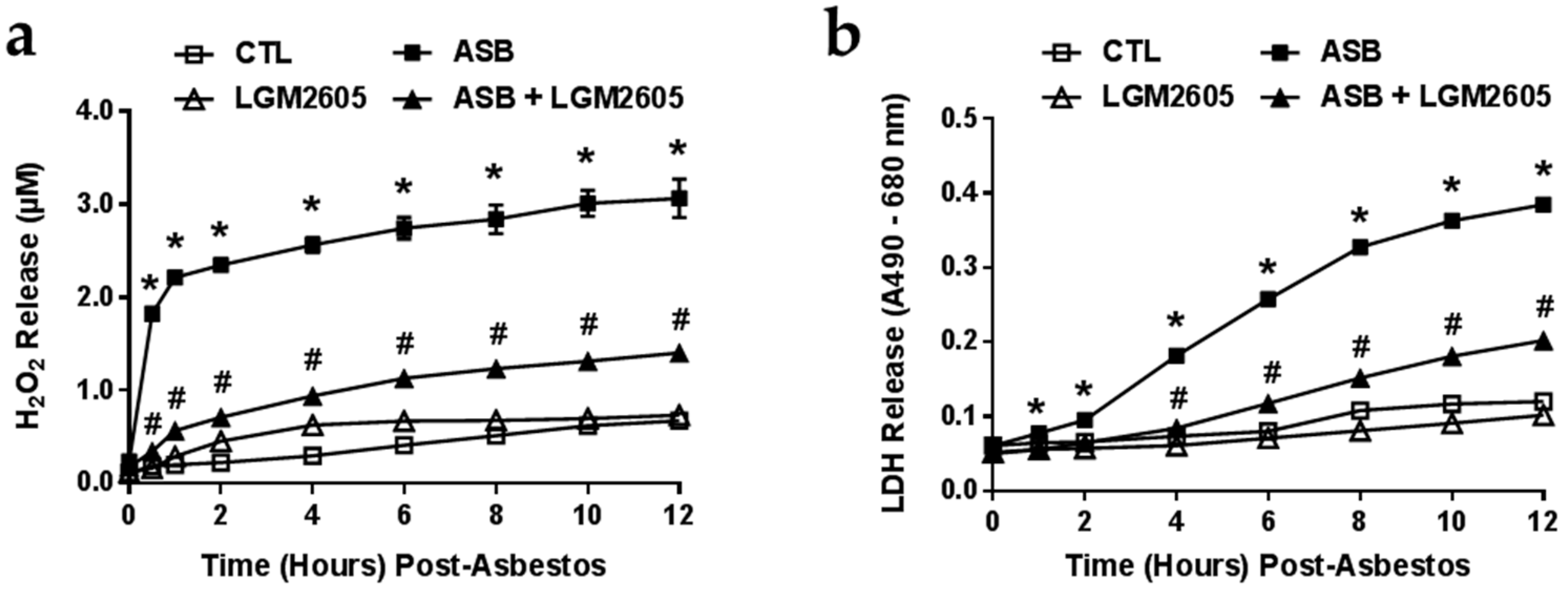
2.2. LGM2605 Reduces Asbestos-Induced ROS Generation and Cytotoxicity
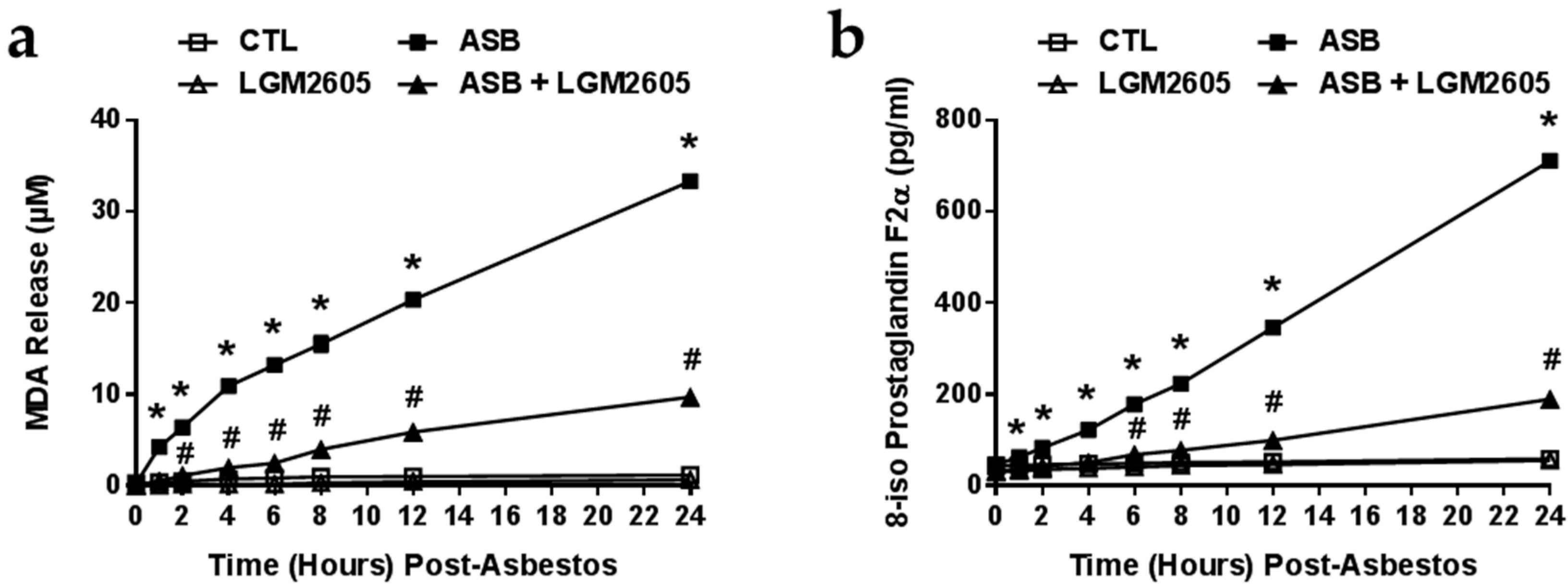
2.3. The Synthetic SDG (LGM2605) Reduces Asbestos-Induced Lipid Peroxidation and Oxidative Cell Damage
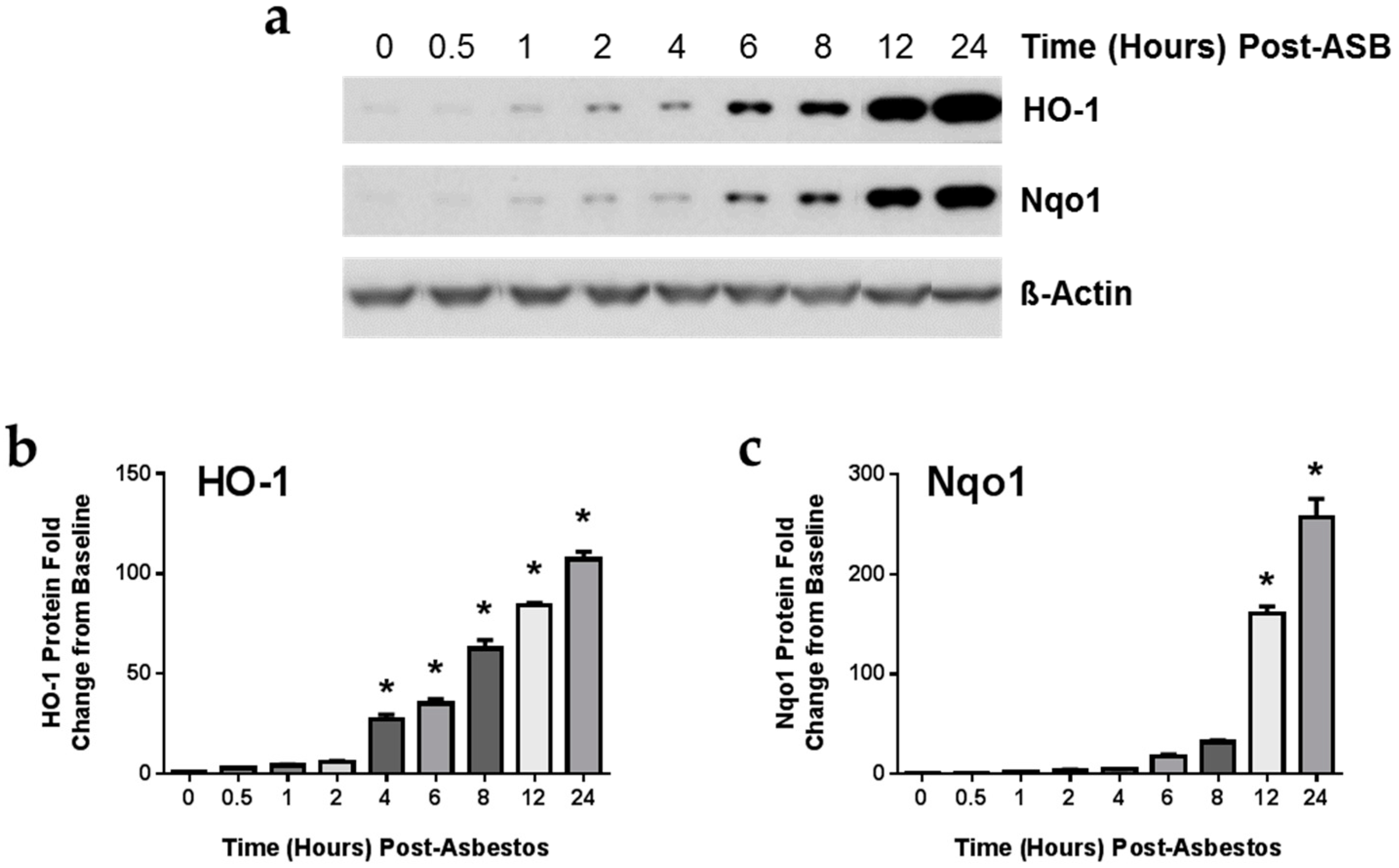
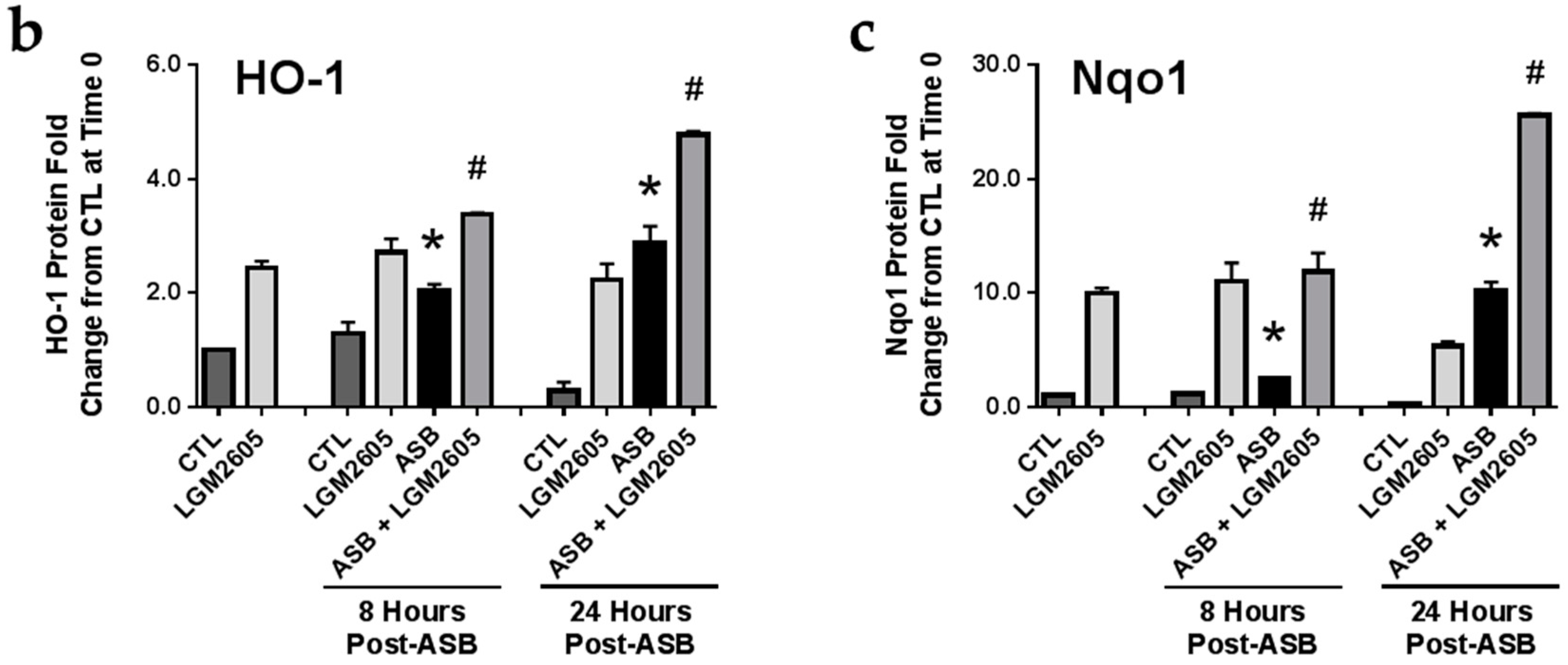
2.4. Exposure to Crocidolite Asbestos Fibers Induces Phase II Antioxidant Enzyme Expression in Murine Macrophages
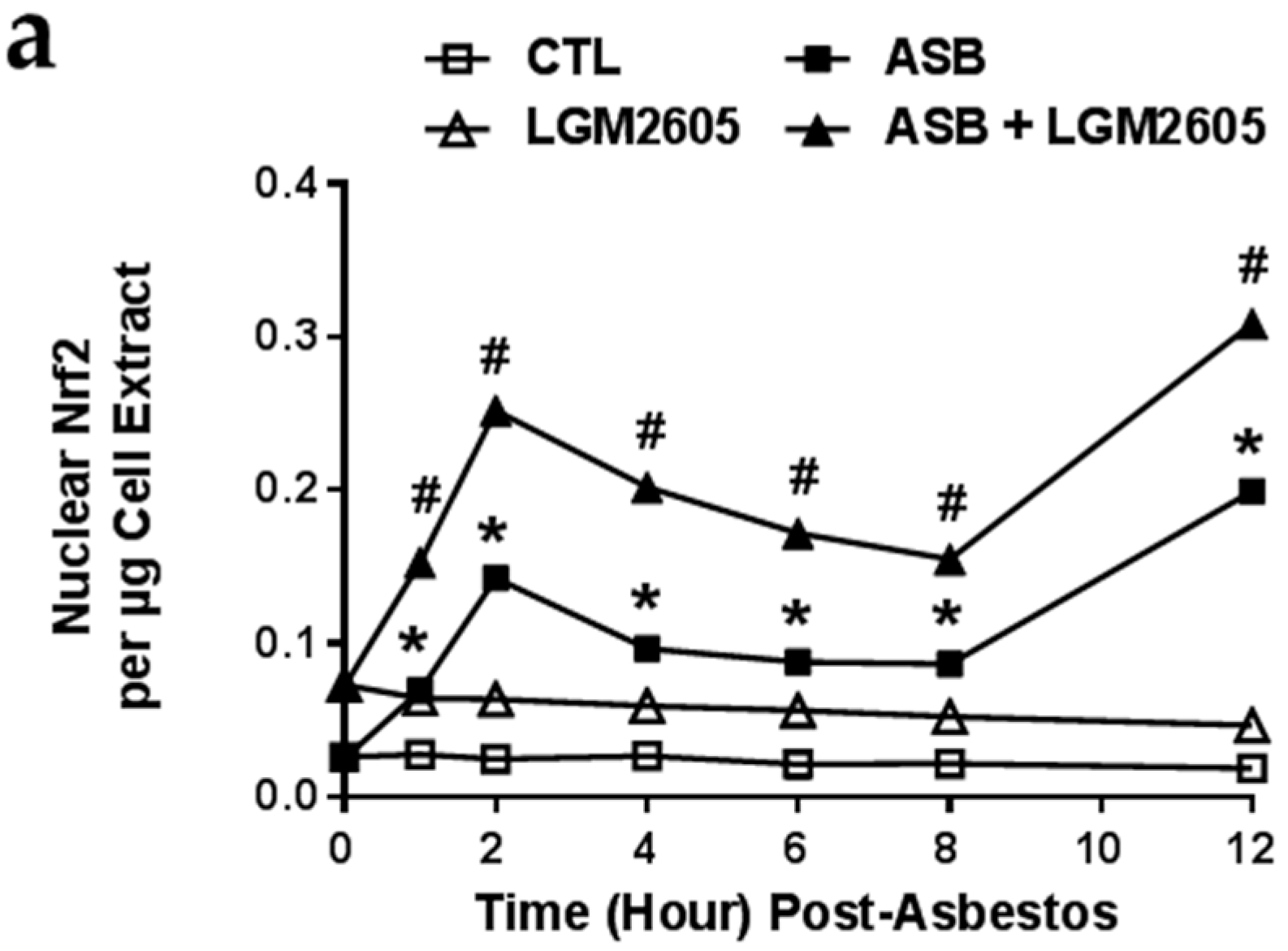
2.5. LGM2605 Enhances Asbestos-Induced Activatation Nrf2 Signaling and Gene Expression of Phase II Enzymes
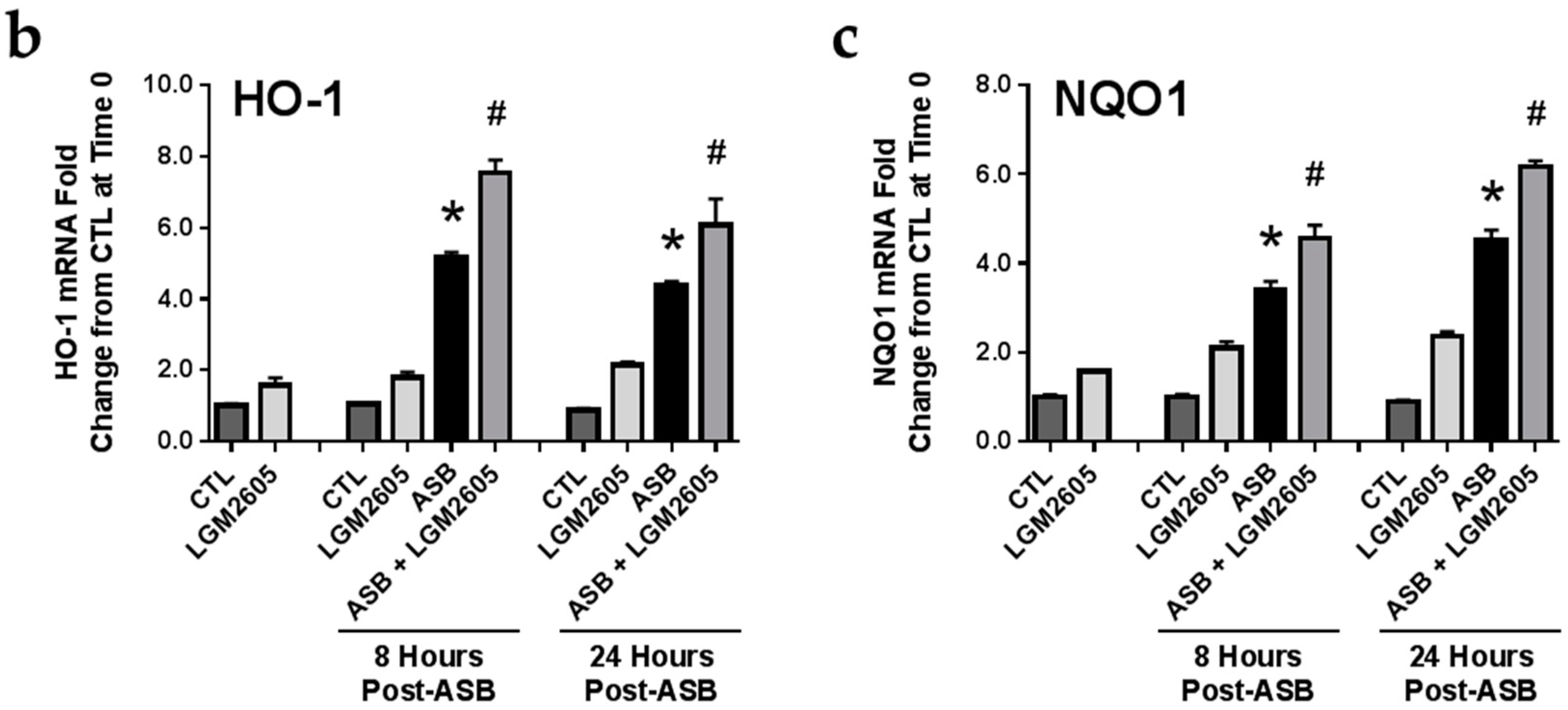
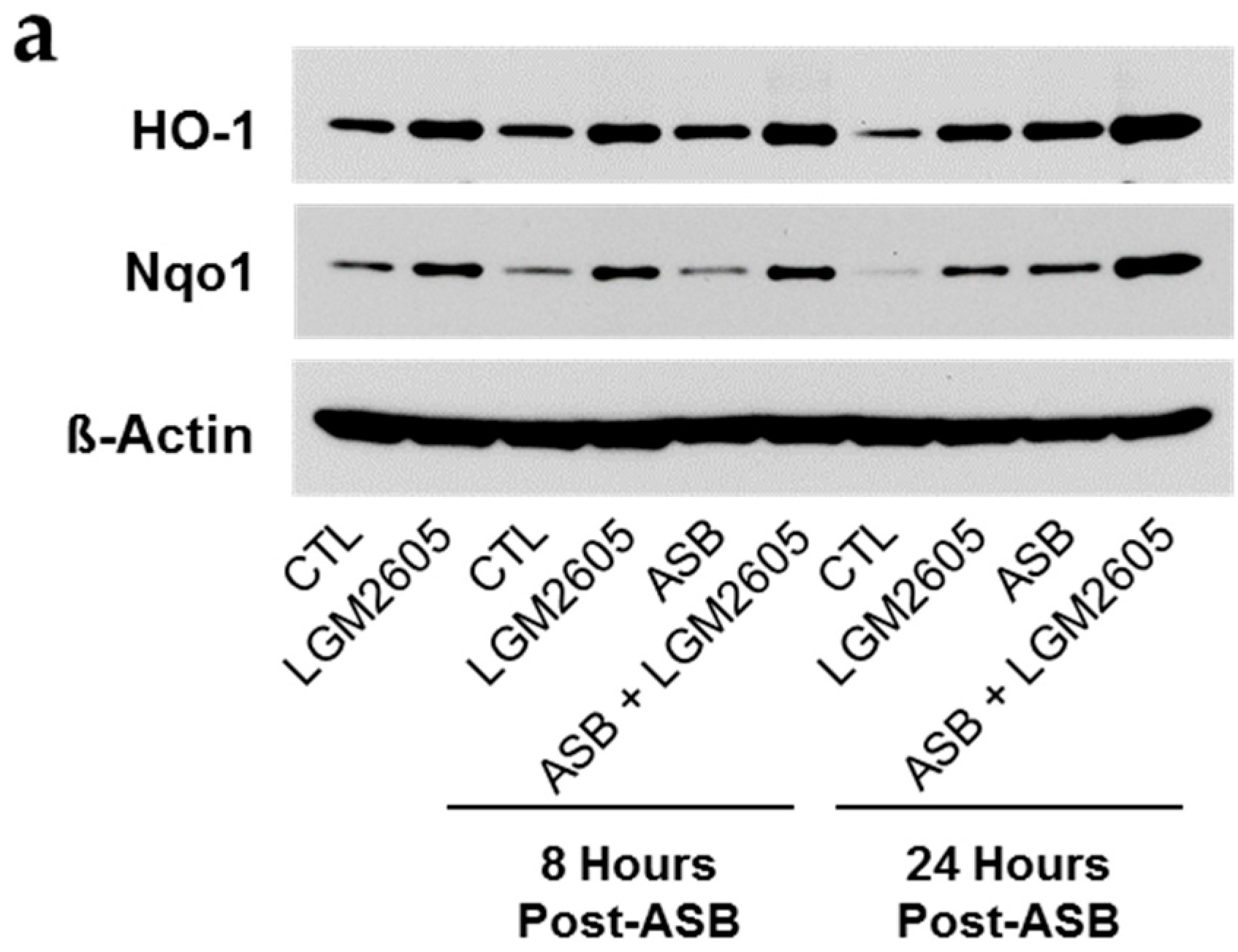
2.6. Induction of Cellular Antioxidant Enzymes by Asbestos and Further Activation by LGM2605
3. Discussion
4. Materials and Methods
4.1. Harvesting of Murine Peritoneal Macrophages
4.2. Crocidolite Asbestos Exposure
4.3. Synthetic SDG (LGM2605) Exposure
4.4. Determination of Intracellular Asbestos-Induced ROS Generation
4.5. Quantification of H2O2 Release from Peritoneal Macrophages Following Asbestos Exposure
4.6. Determination of Asbestos-Induced Cytotoxicity
4.7. Evaluation of Lipid Peroxidation
4.8. Analysis of 8-Iso Prostaglandin F2a Levels in the Cell Culture Medium
4.9. Nrf2 Transcription Factor Analysis
4.10. RNA Isolation and Gene Expression Analysis
4.11. Western Blot Analysis
4.12. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| 8-IsoP | 8-iso Prostaglandin F2a |
| ARE | antioxidant response element |
| CTL | control |
| ED | enterodiol |
| EL | enterolactone |
| ELISA | Enzyme-linked immunosorbent assays |
| FLC | Flaxseed Lignan Component |
| GSTM1 | glutathione S-transferase mu 1 |
| HO-1 | heme oxygenase-1 |
| IACUC | Institutional Animal Care and Use Committee |
| IP | intraperitoneal |
| KEAP1 | kelch-like ECH-associated protein 1 |
| LDH | lactate dehydrogenase |
| MF | macrophages |
| MDA | malondialdehyde |
| MDA-TBA | malondialdehyde-thiobarbituric acid |
| MM | Malignant Mesothelioma |
| Nrf2 | nuclear factor (erythroid-derived 2)-like 2 |
| Nqo1 | NADPH: quinone oxidoreductase-1 |
| PBS | phosphate-buffered saline |
| PL | peritoneal lavage |
| PLF | peritoneal lavage fluid |
| qPCR | quantitative polymerase chain reaction |
| RNS | reactive nitrogen species |
| ROS | reactive oxygen species |
| SDG | secoisolariciresinol diglucoside |
| UICC | Union Internationale Contre le Cancer |
| WBC | white blood cells |
References
- Sterman, D.H.; Recio, A.; Vachani, A.; Sun, J.; Cheung, L.; DeLong, P.; Amin, K.M.; Litzky, L.A.; Wilson, J.M.; Kaiser, L.R.; et al. Long-term follow-up of patients with malignant pleural mesothelioma receiving high-dose adenovirus herpes simplex thymidine kinase/ganciclovir suicide gene therapy. Clin. Cancer Res. 2005, 11, 7444–7453. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sterman, D.H.; Kaiser, L.R.; Albelda, S.M. Advances in the treatment of malignant pleural mesothelioma. Chest 1999, 116, 504–520. [Google Scholar] [CrossRef] [PubMed]
- Benard, F.; Sterman, D.; Smith, R.J.; Kaiser, L.R.; Albelda, S.M.; Alavi, A. Prognostic value of FDG pet imaging in malignant pleural mesothelioma. J. Nucl. Med. 1999, 40, 1241–1245. [Google Scholar] [PubMed]
- Carbone, M.; Yang, H. Molecular pathways: Targeting mechanisms of asbestos and erionite carcinogenesis in mesothelioma. Clin. Cancer Res. 2012, 18, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Neri, M.; Ugolini, D.; Boccia, S.; Canessa, P.A.; Cesario, A.; Leoncini, G.; Mutti, L.; Bonassi, S. Chemoprevention of asbestos-linked cancers: A systematic review. Anticancer Res. 2012, 32, 1005–1013. [Google Scholar] [PubMed]
- Sterman, D.H.; Albelda, S.M. Advances in the diagnosis, evaluation, and management of malignant pleural mesothelioma. Respirology 2005, 10, 266–283. [Google Scholar] [CrossRef] [PubMed]
- Bielefeldt-Ohmann, H.; Fitzpatrick, D.R.; Marzo, A.L.; Jarnicki, A.G.; Himbeck, R.P.; Davis, M.R.; Manning, L.S.; Robinson, B.W. Patho- and immunobiology of malignant mesothelioma: Characterisation of tumour infiltrating leucocytes and cytokine production in a murine model. Cancer Immunol. Immunother. 1994, 39, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Moalli, P.A.; MacDonald, J.L.; Goodglick, L.A.; Kane, A.B. Acute injury and regeneration of the mesothelium in response to asbestos fibers. Am. J. Pathol. 1987, 128, 426–445. [Google Scholar] [PubMed]
- Bielefeldt-Ohmann, H.; Jarnicki, A.G.; Fitzpatrick, D.R. Molecular pathobiology and immunology of malignant mesothelioma. J. Pathol. 1996, 178, 369–378. [Google Scholar] [CrossRef]
- Ramos-Nino, M.E.; Testa, J.R.; Altomare, D.A.; Pass, H.I.; Carbone, M.; Bocchetta, M.; Mossman, B.T. Cellular and molecular parameters of mesothelioma. J. Cell. Biochem. 2006, 98, 723–734. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.K.; Westbom, C.M.; MacPherson, M.B.; Mossman, B.T.; Heintz, N.H.; Spiess, P.; Shukla, A. Asbestos modulates thioredoxin-thioredoxin interacting protein interaction to regulate inflammasome activation. Part. Fibre Toxicol. 2014, 11. [Google Scholar] [CrossRef] [PubMed]
- Dostert, C.; Petrilli, V.; Van Bruggen, R.; Steele, C.; Mossman, B.T.; Tschopp, J. Innate immune activation through NALP3 inflammasome sensing of asbestos and silica. Science 2008, 320, 674–677. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.; Gulumian, M.; Hei, T.K.; Kamp, D.; Rahman, Q.; Mossman, B.T. Multiple roles of oxidants in the pathogenesis of asbestos-induced diseases. Free Radic. Biol. Med. 2003, 34, 1117–1129. [Google Scholar] [CrossRef]
- Moyer, V.D.; Cistulli, C.A.; Vaslet, C.A.; Kane, A.B. Oxygen radicals and asbestos carcinogenesis. Environ. Health Perspect. 1994, 102, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Greenwald, P. Cancer chemoprevention. BMJ 2002, 324, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Robinson, B.W.; Lake, R.A. Advances in malignant mesothelioma. N. Engl. J. Med. 2005, 353, 1591–1603. [Google Scholar] [CrossRef] [PubMed]
- Heintz, N.H.; Janssen-Heininger, Y.M.; Mossman, B.T. Asbestos, lung cancers, and mesotheliomas: From molecular approaches to targeting tumor survival pathways. Am. J. Respir. Cell Mol. Biol. 2010, 42, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Kinniry, P.; Amrani, Y.; Vachani, A.; Solomides, C.C.; Arguiri, E.; Workman, A.; Carter, J.; Christofidou-Solomidou, M. Dietary flaxseed supplementation ameliorates inflammation and oxidative tissue damage in experimental models of acute lung injury in mice. J. Nutr. 2006, 136, 1545–1551. [Google Scholar] [PubMed]
- Lee, J.C.; Bhora, F.; Sun, J.; Cheng, G.; Arguiri, E.; Solomides, C.C.; Chatterjee, S.; Christofidou-Solomidou, M. Dietary flaxseed enhances antioxidant defenses and is protective in a mouse model of lung ischemia-reperfusion injury. Am. J. Phys. Lung Cell. Mol. Phys. 2008, 294, L255–L265. [Google Scholar] [CrossRef] [PubMed]
- Razi, S.S.; Latif, M.J.; Li, X.; Afthinos, J.N.; Ippagunta, N.; Schwartz, G.; Sagalovich, D.; Belsley, S.J.; Connery, C.P.; Jour, G.; et al. Dietary flaxseed protects against lung ischemia reperfusion injury via inhibition of apoptosis and inflammation in a murine model. J. Surg. Res. 2011, 171, e113–e121. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Krochak, R.; Blouin, A.; Kanterakis, S.; Chatterjee, S.; Arguiri, E.; Vachani, A.; Solomides, C.C.; Cengel, K.A.; Christofidou-Solomidou, M. Dietary flaxseed prevents radiation-induced oxidative lung damage, inflammation and fibrosis in a mouse model of thoracic radiation injury. Cancer Biol. Ther. 2009, 8, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Mishra, O.P.; Simmons, N.; Tyagi, S.; Pietrofesa, R.; Shuvaev, V.V.; Valiulin, R.A.; Heretsch, P.; Nicolaou, K.C.; Christofidou-Solomidou, M. Synthesis and antioxidant evaluation of (S,S)- and (R,R)-secoisolariciresinol diglucosides (SDGs). Bioorg. Med. Chem. Lett. 2013, 23, 5325–5328. [Google Scholar] [CrossRef] [PubMed]
- Mishra, O.P.; Pietrofesa, R.; Christofidou-Solomidou, M. Novel synthetic (S,S) and (R,R)-secoisolariciresinol diglucosides (SDGs) protect naked plasmid and genomic DNA from γ radiation damage. Radiat. Res. 2014, 182, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Pietrofesa, R.A.; Velalopoulou, A.; Arguiri, E.; Menges, C.W.; Testa, J.R.; Hwang, W.T.; Albelda, S.M.; Christofidou-Solomidou, M. Flaxseed lignans enriched in secoisolariciresinol diglucoside prevent acute asbestos-induced peritoneal inflammation in mice. Carcinogenesis 2016, 37, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Velalopoulou, A.; Tyagi, S.; Pietrofesa, R.A.; Arguiri, E.; Christofidou-Solomidou, M. The flaxseed-derived lignan phenolic secoisolariciresinol diglucoside (SDG) protects non-malignant lung cells from radiation damage. Int. J. Mol. Sci. 2015, 17. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Kinniry, P.A.; Arguiri, E.; Serota, M.; Kanterakis, S.; Chatterjee, S.; Solomides, C.C.; Javvadi, P.; Koumenis, C.; Cengel, K.A.; et al. Dietary curcumin increases antioxidant defenses in lung, ameliorates radiation-induced pulmonary fibrosis, and improves survival in mice. Radiat. Res. 2010, 173, 590–601. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.L.; Spivack, S.D. Dietary chemoprevention strategies for induction of phase ii xenobiotic-metabolizing enzymes in lung carcinogenesis: A review. Lung Cancer 2009, 65, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Kwak, M.K.; Itoh, K.; Yamamoto, M.; Kensler, T.W. Enhanced expression of the transcription factor Nrf2 by cancer chemopreventive agents: Role of antioxidant response element-like sequences in the Nrf2 promoter. Mol. Cell. Biol. 2002, 22, 2883–2892. [Google Scholar] [CrossRef] [PubMed]
- Kwak, M.K.; Itoh, K.; Yamamoto, M.; Sutter, T.R.; Kensler, T.W. Role of transcription factor Nrf2 in the induction of hepatic phase 2 and antioxidative enzymes in vivo by the cancer chemoprotective agent, 3h-1, 2-dimethiole-3-thione. Mol. Med. 2001, 7, 135–145. [Google Scholar] [PubMed]
- Nakamura, H.; Nishikawa, A.; Furukawa, F.; Kasahara, K.; Miyauchi, M.; Okazaki, K.; Imazawa, T.; Uchida, K.; Hirose, M. Enhancing effects of oltipraz on the development of spontaneous hepatic lesions in LEC rats. Toxicol. Pathol. 2002, 30, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Zhu, H.; Trush, M.A.; Misra, H.P.; Li, Y. Generation of superoxide from reaction of 3h-1,2-dithiole-3-thione with thiols: Implications for dithiolethione chemoprotection. Mol. Cell. Biochem. 2008, 307, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Adare, A.; Afanasiev, S.; Aidala, C.; Ajitanand, N.N.; Akiba, Y.; Al-Bataineh, H.; Alexander, J.; Al-Jamel, A.; Aoki, K.; Aphecetche, L.; et al. Suppression pattern of neutral pions at high transverse momentum in Au + Au collisions at sqrt[SNN] = 200 GeV and constraints on medium transport coefficients. Phys. Rev. Lett. 2008, 101, 232301. [Google Scholar] [CrossRef] [PubMed]
- Kelley, M.J.; Glaser, E.M.; Herndon, J.E., 2nd; Becker, F.; Bhagat, R.; Zhang, Y.J.; Santella, R.M.; Carmella, S.G.; Hecht, S.S.; Gallot, L.; et al. Safety and efficacy of weekly oral oltipraz in chronic smokers. Cancer Epidemiol. Biomark. Prev. 2005, 14, 892–899. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Yuan, Y.V.; Kitts, D.D. Antioxidant activities of the flaxseed lignan secoisolariciresinol diglucoside, its aglycone secoisolariciresinol and the mammalian lignans enterodiol and enterolactone in vitro. Food Chem. Toxicol. 2007, 45, 2219–2227. [Google Scholar] [CrossRef] [PubMed]
- Kitts, D.D.; Yuan, Y.V.; Wijewickreme, A.N.; Thompson, L.U. Antioxidant activity of the flaxseed lignan secoisolariciresinol diglycoside and its mammalian lignan metabolites enterodiol and enterolactone. Mol. Cell. Biochem. 1999, 202, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Turowski, J.B.; Pietrofesa, R.A.; Lawson, J.A.; Christofidou-Solomidou, M.; Hadjiliadis, D. Flaxseed modulates inflammatory and oxidative stress biomarkers in cystic fibrosis: A pilot study. BMC Complement. Altern. Med. 2015, 15, 148. [Google Scholar] [CrossRef] [PubMed]
- Azrad, M.; Vollmer, R.T.; Madden, J.; Dewhirst, M.; Polascik, T.J.; Snyder, D.C.; Ruffin, M.T.; Moul, J.W.; Brenner, D.E.; Demark-Wahnefried, W. Flaxseed-derived enterolactone is inversely associated with tumor cell proliferation in men with localized prostate cancer. J. Med. Food 2013, 16, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Simbalista, R.L.; Sauerbronn, A.V.; Aldrighi, J.M.; Areas, J.A. Consumption of a flaxseed-rich food is not more effective than a placebo in alleviating the climacteric symptoms of postmenopausal women. J. Nutr. 2010, 140, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Pan, A.; Demark-Wahnefried, W.; Ye, X.; Yu, Z.; Li, H.; Qi, Q.; Sun, J.; Chen, Y.; Chen, X.; Liu, Y.; et al. Effects of a flaxseed-derived lignan supplement on C-reactive protein, IL-6 and retinol-binding protein 4 in type 2 diabetic patients. Br. J. Nutr. 2009, 101, 1145–1149. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K. Antioxidant activity of secoisolariciresinol diglucoside-derived metabolites, secoisolariciresinol, enterodiol, and enterolactone. Int. J. Angiol. 2000, 9, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Serraino, M.; Thompson, L.U. The effect of flaxseed supplementation on early risk markers for mammary carcinogenesis. Cancer Lett. 1991, 60, 135–142. [Google Scholar] [CrossRef]
- Serraino, M.; Thompson, L.U. The effect of flaxseed supplementation on the initiation and promotional stages of mammary tumorigenesis. Nutr. Cancer 1992, 17, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Jenab, M.; Thompson, L.U. The influence of flaxseed and lignans on colon carcinogenesis and β-glucuronidase activity. Carcinogenesis 1996, 17, 1343–1348. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Yee, J.A.; Li, D.; McGuire, M.H.; Thompson, L.U. Dietary flaxseed supplementation and experimental metastasis of melanoma cells in mice. Cancer Lett. 1998, 124, 181–186. [Google Scholar] [CrossRef]
- Bergman Jungestrom, M.; Thompson, L.U.; Dabrosin, C. Flaxseed and its lignans inhibit estradiol-induced growth, angiogenesis, and secretion of vascular endothelial growth factor in human breast cancer xenografts in vivo. Clin. Cancer Res. 2007, 13, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Saggar, J.K.; Corey, P.; Thompson, L.U. Flaxseed and pure secoisolariciresinol diglucoside, but not flaxseed hull, reduce human breast tumor growth (MCF-7) in athymic mice. J. Nutr. 2009, 139, 2061–2066. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Tan, K.P.; Ward, W.E.; Thompson, L.U. Exposure to flaxseed or its purified lignan during suckling inhibits chemically induced rat mammary tumorigenesis. Exp. Biol. Med. 2003, 228, 951–958. [Google Scholar]
- Chen, J.; Thompson, L.U. Lignans and tamoxifen, alone or in combination, reduce human breast cancer cell adhesion, invasion and migration in vitro. Breast Cancer Res. Treat. 2003, 80, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Christofidou-Solomidou, M.; Tyagi, S.; Pietrofesa, R.; Dukes, F.; Arguiri, E.; Turowski, J.; Grieshaber, P.A.; Solomides, C.C.; Cengel, K.A. Radioprotective role in lung of the flaxseed lignan complex enriched in the phenolic secoisolariciresinol diglucoside (SDG). Radiat. Res. 2012, 178, 568–580. [Google Scholar] [CrossRef] [PubMed]
- Blake, D.J.; Bolin, C.M.; Cox, D.P.; Cardozo-Pelaez, F.; Pfau, J.C. Internalization of libby amphibole asbestos and induction of oxidative stress in murine macrophages. Toxicol. Sci. 2007, 99, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.X.; Partridge, M.A.; Ghandhi, S.A.; Davidson, M.M.; Amundson, S.A.; Hei, T.K. Mitochondria-derived reactive intermediate species mediate asbestos-induced genotoxicity and oxidative stress-responsive signaling pathways. Environ. Health Perspect. 2012, 120, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Christofidou-Solomidou, M.; Tyagi, S.; Tan, K.S.; Hagan, S.; Pietrofesa, R.; Dukes, F.; Arguiri, E.; Heitjan, D.F.; Solomides, C.C.; Cengel, K.A. Dietary flaxseed administered post thoracic radiation treatment improves survival and mitigates radiation-induced pneumonopathy in mice. BMC Cancer 2011, 11, 269. [Google Scholar] [CrossRef] [PubMed]
- Robinson, C.; Walsh, A.; Larma, I.; O’Halloran, S.; Nowak, A.K.; Lake, R.A. Mextag mice exposed to asbestos develop cancer that faithfully replicates key features of the pathogenesis of human mesothelioma. Eur. J. Cancer 2011, 47, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Robinson, C.; Woo, S.; Walsh, A.; Nowak, A.K.; Lake, R.A. The antioxidants vitamins a and e and selenium do not reduce the incidence of asbestos-induced disease in a mouse model of mesothelioma. Nutr. Cancer 2012, 64, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Jube, S.; Rivera, Z.S.; Bianchi, M.E.; Powers, A.; Wang, E.; Pagano, I.; Pass, H.I.; Gaudino, G.; Carbone, M.; Yang, H. Cancer cell secretion of the damp protein HMGB1 supports progression in malignant mesothelioma. Cancer Res. 2012, 72, 3290–3301. [Google Scholar] [CrossRef] [PubMed]
- Grigoriu, B.; Chahine, B.; Zerimech, F.; Gregoire, M.; Balduyck, M.; Copin, M.C.; Devos, P.; Lassalle, P.; Scherpereel, A. Serum mesothelin has a higher diagnostic utility than hyaluronic acid in malignant mesothelioma. Clin. Biochem. 2009, 42, 1046–1050. [Google Scholar] [CrossRef] [PubMed]
- Pass, H.I.; Levin, S.M.; Harbut, M.R.; Melamed, J.; Chiriboga, L.; Donington, J.; Huflejt, M.; Carbone, M.; Chia, D.; Goodglick, L.; et al. Fibulin-3 as a blood and effusion biomarker for pleural mesothelioma. N. Engl. J. Med. 2012, 367, 1417–1427. [Google Scholar] [CrossRef] [PubMed]
- Pietrofesa, R.; Turowski, J.; Tyagi, S.; Dukes, F.; Arguiri, E.; Busch, T.M.; Gallagher-Colombo, S.M.; Solomides, C.C.; Cengel, K.A.; Christofidou-Solomidou, M. Radiation mitigating properties of the lignan component in flaxseed. BMC Cancer 2013, 13, 179. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pietrofesa, R.A.; Velalopoulou, A.; Albelda, S.M.; Christofidou-Solomidou, M. Asbestos Induces Oxidative Stress and Activation of Nrf2 Signaling in Murine Macrophages: Chemopreventive Role of the Synthetic Lignan Secoisolariciresinol Diglucoside (LGM2605). Int. J. Mol. Sci. 2016, 17, 322. https://doi.org/10.3390/ijms17030322
Pietrofesa RA, Velalopoulou A, Albelda SM, Christofidou-Solomidou M. Asbestos Induces Oxidative Stress and Activation of Nrf2 Signaling in Murine Macrophages: Chemopreventive Role of the Synthetic Lignan Secoisolariciresinol Diglucoside (LGM2605). International Journal of Molecular Sciences. 2016; 17(3):322. https://doi.org/10.3390/ijms17030322
Chicago/Turabian StylePietrofesa, Ralph A., Anastasia Velalopoulou, Steven M. Albelda, and Melpo Christofidou-Solomidou. 2016. "Asbestos Induces Oxidative Stress and Activation of Nrf2 Signaling in Murine Macrophages: Chemopreventive Role of the Synthetic Lignan Secoisolariciresinol Diglucoside (LGM2605)" International Journal of Molecular Sciences 17, no. 3: 322. https://doi.org/10.3390/ijms17030322
APA StylePietrofesa, R. A., Velalopoulou, A., Albelda, S. M., & Christofidou-Solomidou, M. (2016). Asbestos Induces Oxidative Stress and Activation of Nrf2 Signaling in Murine Macrophages: Chemopreventive Role of the Synthetic Lignan Secoisolariciresinol Diglucoside (LGM2605). International Journal of Molecular Sciences, 17(3), 322. https://doi.org/10.3390/ijms17030322






