A Precision Microbiome Approach Using Sucrose for Selective Augmentation of Staphylococcus epidermidis Fermentation against Propionibacterium acnes
Abstract
:1. Introduction
2. Results
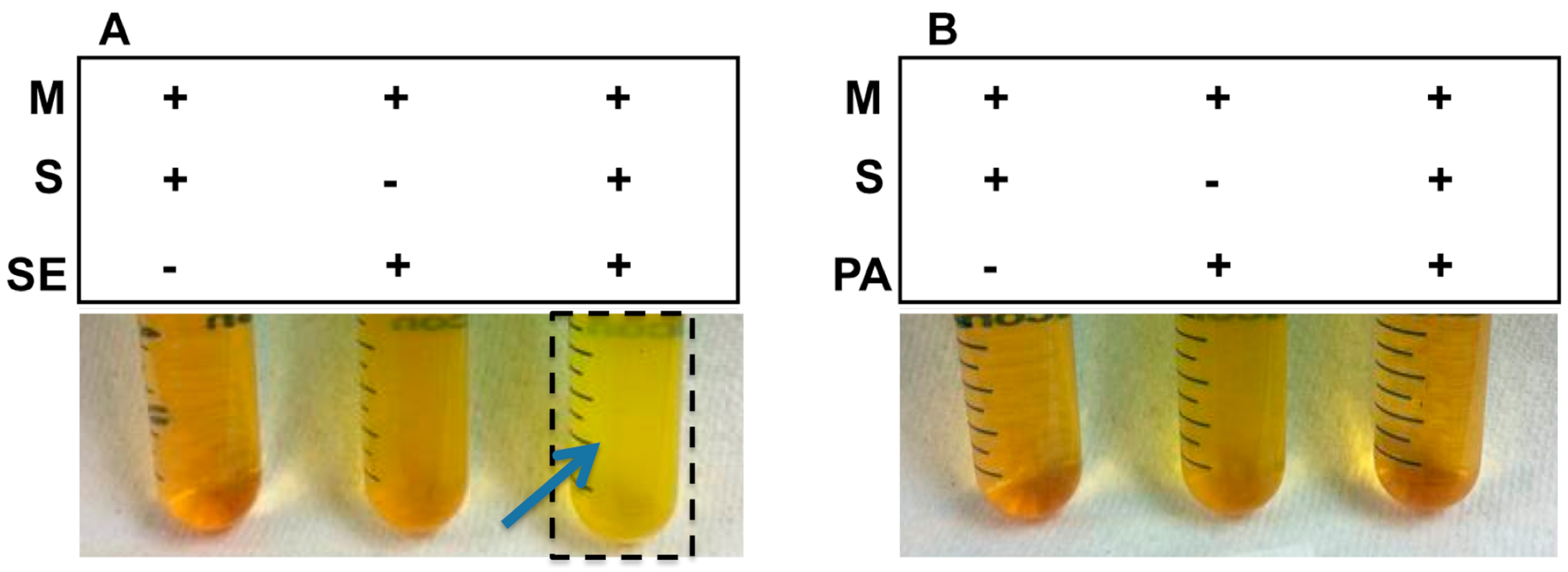
2.1. Sucrose Selectively Triggered S. epidermidis, but Not P. acnes, to Undergo Fermentation
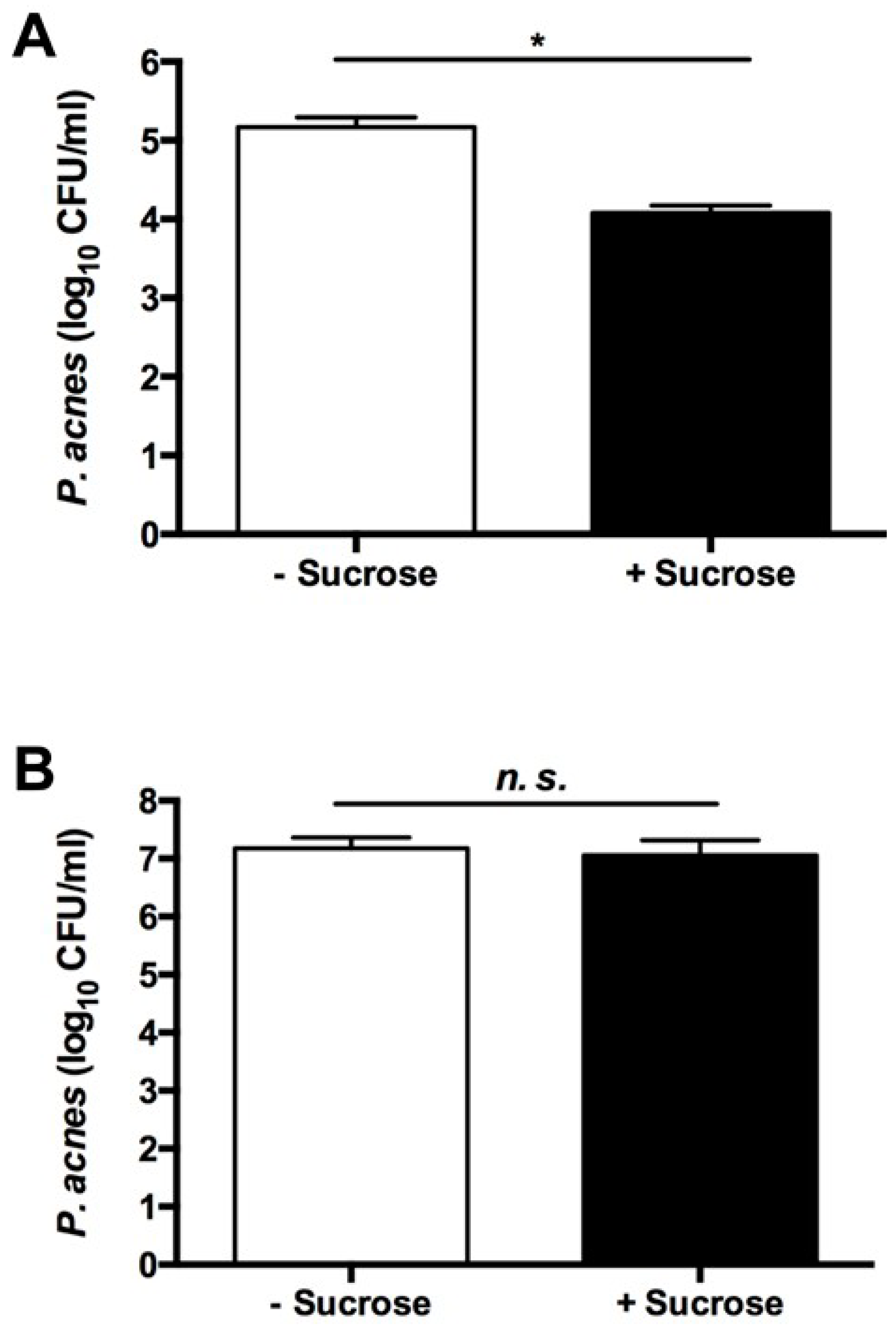
2.2. The Sucrose Fermentation of S. epidermidis Is Essential for Inhibition of P. acnes Growth
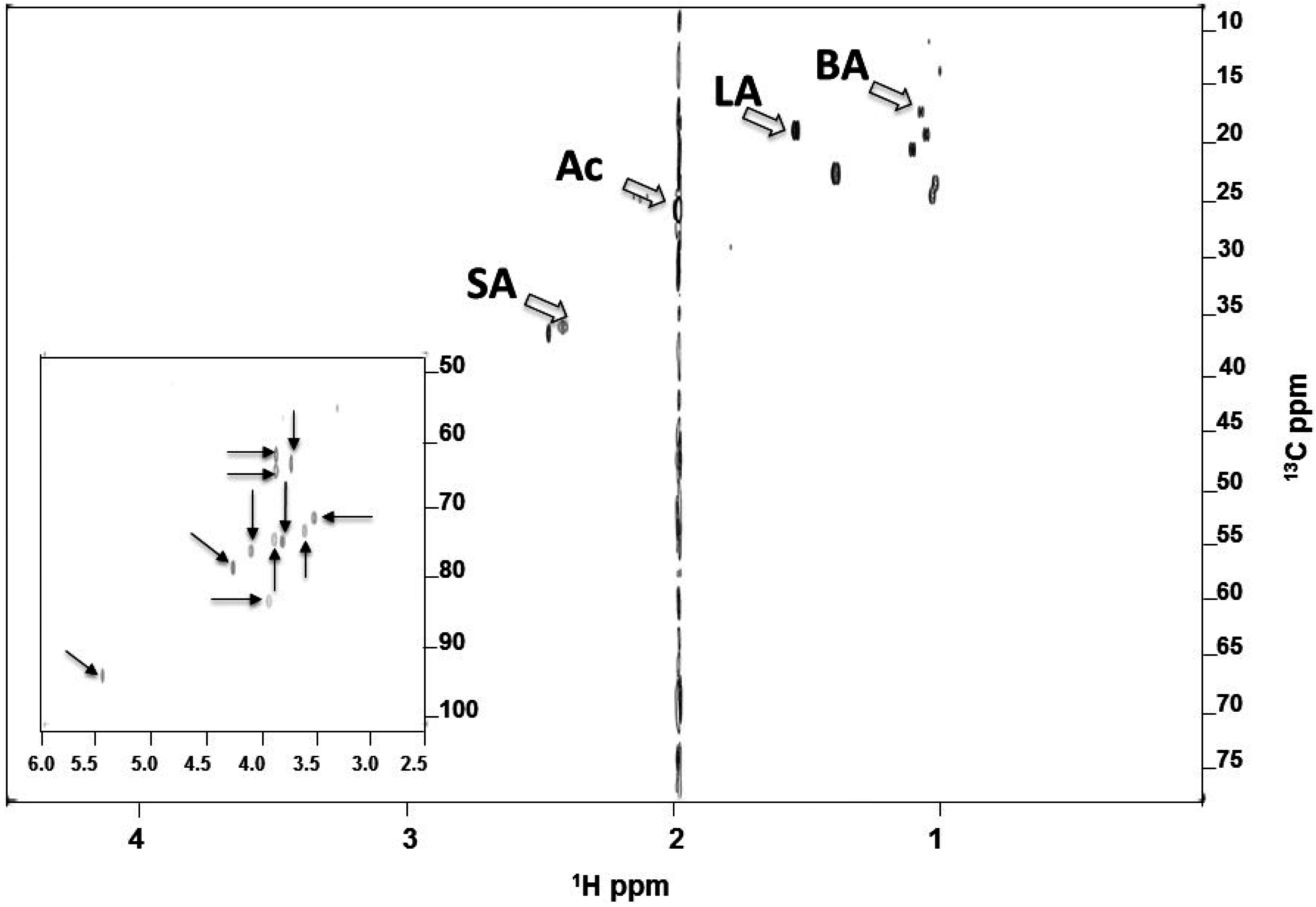
2.3. Identified SCFAs in Fermented Media of S. epidermidis
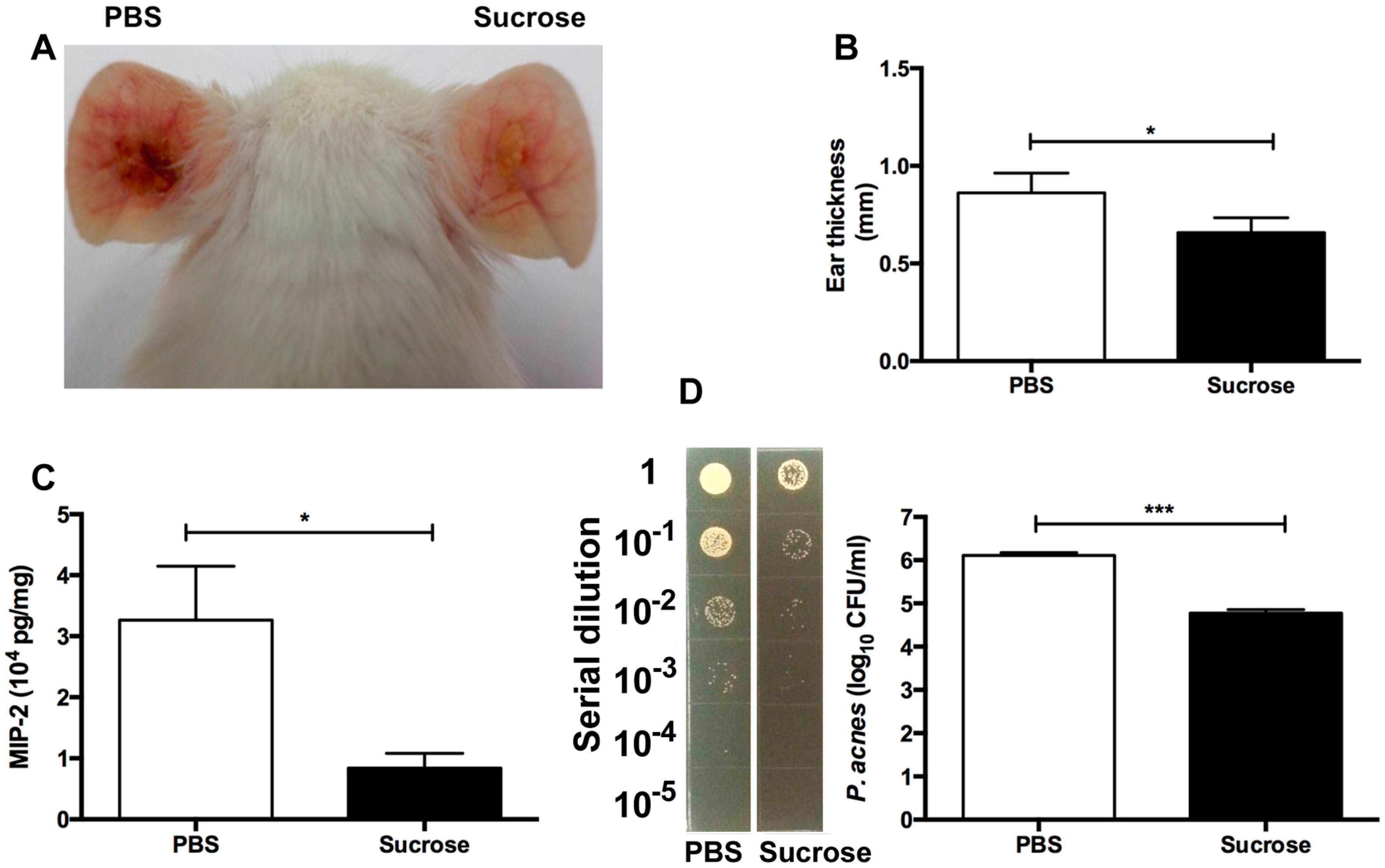
2.4. S. epidermidis Sucrose Fermentation Abrogated P. acnes-Induced Inflammation and Bacteria Colonization In Vivo
3. Discussion
4. Experimental Section
4.1. Ethics Statement
4.2. Bacterial Culture
4.3. Fermentation of Bacteria
4.4. Co-Culture Assays
4.5. NMR Analysis
4.6. The Sucrose Fermentation of S. epidermidis against P. acnes In Vivo
4.7. Bacterial Loads in Mouse Ears
4.8. ELISA
4.9. Statistics
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Ac | acetic acid |
| ATCC | American Type Culture Collection |
| BA | butyric acid |
| CFU | colony-forming unit |
| BSL-2 | biosafety level 2 |
| 1-D | one-dimensional |
| 2-D | two-dimensional |
| D2O | deuterium oxide |
| ELISA | enzyme-linked immunosorbent assay |
| FDA | Food and Drug Administration |
| Ffar1 | free fatty acid receptor 1 |
| Ffar2 | free fatty acid receptor 2 |
| GPR41 | G-protein coupled receptor 41 |
| GRAS | Generally Recognized As Safe |
| HDAC | histone deacetylase |
| HSQC | heteronuclear single quantum coherence |
| IACUC | Institutional Animal Care and Use Committee |
| IL | interleukin |
| MIP-2 | macrophage-inflammatory protein-2 |
| NCU | National Central University |
| NIH | National Institutes of Health |
| NF-S. epidermidis | non-fermentation S. epidermidis |
| NMR | nuclear magnetic resonance |
| NS | non-significant |
| P. acnes | Propionibacterium acnes |
| PBS | phosphate buffered saline |
| SCFA | short-chain fatty acids |
| SD | standard deviation |
| S. epidermidis | Staphylococcus epidermidis |
| SFI | selective fermentation initiator |
| 16S rRNA | 16S ribosomal RNA |
| TLR-2 | toll-like receptor 2 |
| TNFα | tumor necrosis factor α |
References
- Grice, E.A.; Segre, J.A. The skin microbiome. Nat. Rev. Microbiol. 2011, 9, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.H.; Segre, J.A. Skin microbiome: Looking back to move forward. J. Investig. Dermatol. 2012, 132, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Scanlan, P.D.; Buckling, A.; Kong, W.; Wild, Y.; Lynch, S.V.; Harrison, F. Gut dysbiosis in cystic fibrosis. J. Cyst. Fibros. 2012, 11, 454–455. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kuo, S.; Shu, M.; Yu, J.; Huang, S.; Dai, A.; Two, A.; Gallo, R.L.; Huang, C.M. Staphylococcus epidermidis in the human skin microbiome mediates fermentation to inhibit the growth of propionibacterium acnes: Implications of probiotics in acne vulgaris. Appl. Microbiol. Biotechnol. 2014, 98, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Moss, C.W.; Dowell, V.R., Jr.; Lewis, V.J.; Schekter, M.A. Cultural characteristics and fatty acid composition of corynebacterium acnes. J. Bacteriol. 1967, 94, 1300–1305. [Google Scholar] [PubMed]
- Robbins, G.B.; Lewis, K.H. Fermentation of sugar acids by bacteria. J. Bacteriol. 1940, 39, 399–404. [Google Scholar] [PubMed]
- Safonova, T.B.; Shcherbakova, N.A.; Afanas’eva, T.I.; Sobolev, V.R. Importance of carbohydrate tests for interspecies differentiation of staphylococci. Zh. Mikrobiol. Epidemiol. Immunobiol. 1978, 9, 98–101. [Google Scholar]
- Shu, M.; Wang, Y.; Yu, J.; Kuo, S.; Coda, A.; Jiang, Y.; Gallo, R.L.; Huang, C.M. Fermentation of propionibacterium acnes, a commensal bacterium in the human skin microbiome, as skin probiotics against methicillin-resistant staphylococcus aureus. PLoS ONE 2013, 8, e55380. [Google Scholar] [CrossRef] [PubMed]
- Eady, E.A.; Layton, A.M.; Cove, J.H. A honey trap for the treatment of acne: Manipulating the follicular microenvironment to control propionibacterium acnes. BioMed Res. Int. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.M.; Rasmussen, J.E. Intralesional corticosteroids in the treatment of nodulocystic acne. Arch. Dermatol. 1983, 119, 480–481. [Google Scholar] [CrossRef] [PubMed]
- Layton, A.M.; Dreno, B.; Gollnick, H.P.; Zouboulis, C.C. A review of the european directive for prescribing systemic isotretinoin for acne vulgaris. J. Eur. Acad. Dermatol. Venereol. 2006, 20, 773–776. [Google Scholar] [CrossRef] [PubMed]
- Lindh, J.M.; Terenius, O.; Faye, I. 16s rRNA gene-based identification of midgut bacteria from field-caught anopheles gambiae sensu lato and A. funestus mosquitoes reveals new species related to known insect symbionts. Appl. Environ. Microbiol. 2005, 71, 7217–7223. [Google Scholar] [CrossRef] [PubMed]
- Al-Naama, R.T. Evaluation of inhibitory effect of honey on some bacterial isolates. Iraqi J. Med. Sci. 2009, 7, 67–72. [Google Scholar]
- Ballesteros, S.A.; Chirife, J.; Bozzini, J.P. Specific solute effects on staphylococcus aureus cells subjected to reduced water activity. Int. J. Food Microbiol. 1993, 20, 51–66. [Google Scholar] [CrossRef]
- Wang, Y.; Dai, A.; Huang, S.; Kuo, S.; Shu, M.; Tapia, C.P.; Yu, J.; Two, A.; Zhang, H.; Gallo, R.L.; et al. Propionic acid and its esterified derivative suppress the growth of methicillin-resistant Staphylococcus aureus USA300. Benef. Microbes 2014, 5, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Pincus, N.B.; Reckhow, J.D.; Saleem, D.; Jammeh, M.L.; Datta, S.K.; Myles, I.A. Strain specific phage treatment for staphylococcus aureus infection is influenced by host immunity and site of infection. PLoS ONE 2015, 10, e0124280. [Google Scholar] [CrossRef] [PubMed]
- Haq, I.U.; Chaudhry, W.N.; Akhtar, M.N.; Andleeb, S.; Qadri, I. Bacteriophages and their implications on future biotechnology: A review. Virol. J. 2012, 9. [Google Scholar] [CrossRef] [PubMed]
- Drulis-Kawa, Z.; Majkowska-Skrobek, G.; Maciejewska, B.; Delattre, A.S.; Lavigne, R. Learning from bacteriophages—Advantages and limitations of phage and phage-encoded protein applications. Curr. Protein Pept. Sci. 2012, 13, 699–722. [Google Scholar] [CrossRef] [PubMed]
- Jahreis, K.; Pimentel-Schmitt, E.F.; Brückner, R.; Titgemeyer, F. Ins and outs of glucose transport systems in eubacteria. FEMS Microbiol. Rev. 2008, 32, 891–907. [Google Scholar] [CrossRef] [PubMed]
- Garland, S.H. Short chain fatty acids may elicit an innate immune response from preadipocytes: A potential link between bacterial infection and inflammatory diseases. Med. Hypotheses 2011, 76, 881–883. [Google Scholar] [CrossRef] [PubMed]
- Hobdy, E.; Murren, J. AN-9 (Titan). Curr. Opin. Investig. Drugs 2004, 5, 628–634. [Google Scholar] [PubMed]
- Vinolo, M.A.; Rodrigues, H.G.; Nachbar, R.T.; Curi, R. Regulation of inflammation by short chain fatty acids. Nutrients 2011, 3, 858–876. [Google Scholar] [CrossRef] [PubMed]
- Ulven, T. Short-chain free fatty acid receptors FFA2/GPR43 and FFA3/GPR41 as new potential therapeutic targets. Front. Endocrinol. 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Gottlicher, M. Valproic acid: An old drug newly discovered as inhibitor of histone deacetylases. Ann. Hematol. 2004, 83, S91–S92. [Google Scholar] [PubMed]
- Silva, F.; Serpa, J.; Domingues, G.; Silva, G.; Almeida, A.; Félix, A. Cell death induced by HDACS inhibitors in ovarian cancer cell lines (serous and clear cells carcinomas)—Role of NOTCH, TP53 and FN1. BMC Proc. 2010, 4. [Google Scholar] [CrossRef]
- Park, J.S.; Lee, E.J.; Lee, J.C.; Kim, W.K.; Kim, H.S. Anti-inflammatory effects of short chain fatty acids in IFN-γ-stimulated RAW 264.7 murine macrophage cells: Involvement of Nf-κB and ERK signaling pathways. Int. Immunopharmacol. 2007, 7, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Hirasawa, A.; Ichimura, A.; Kimura, I.; Tsujimoto, G. Free fatty acid receptors FFAR1 and GPR120 as novel therapeutic targets for metabolic disorders. J. Pharm. Sci. 2011, 100, 3594–3601. [Google Scholar] [CrossRef] [PubMed]
- Kim, J. Review of the innate immune response in acne vulgaris: Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. Dermatology 2005, 211, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Mastrofrancesco, A.; Kokot, A.; Eberle, A.; Gibbons, N.C.; Schallreuter, K.U.; Strozyk, E.; Picardo, M.; Zouboulis, C.C.; Luger, T.A.; Bohm, M. KdPT, a tripeptide derivative of α-melanocyte-stimulating hormone, suppresses IL-1β-mediated cytokine expression and signaling in human sebocytes. J. Immunol. 2010, 185, 1903–1911. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.F.; Nakatsuji, T.; Zhu, W.; Gallo, R.L.; Huang, C.M. Passive immunoprotection targeting a secreted CAMP factor of Propionibacterium acnes as a novel immunotherapeutic for acne vulgaris. Vaccine 2011, 29, 3230–3238. [Google Scholar] [CrossRef] [PubMed]
- Kligman, A.M. An overview of acne. J. Investig. Dermatol. 1974, 62, 268–287. [Google Scholar] [CrossRef] [PubMed]
- Valanne, S.; McDowell, A.; Ramage, G.; Tunney, M.M.; Einarsson, G.G.; O’Hagan, S.; Wisdom, G.B.; Fairley, D.; Bhatia, A.; Maisonneuve, J.F.; et al. CAMP factor homologues in Propionibacterium acnes: A new protein family differentially expressed by types I and II. Microbiology 2005, 151, 1369–1379. [Google Scholar] [CrossRef] [PubMed]
- Jahns, A.C.; Lundskog, B.; Ganceviciene, R.; Palmer, R.H.; Golovleva, I.; Zouboulis, C.C.; McDowell, A.; Patrick, S.; Alexeyev, O.A. An increased incidence of propionibacterium acnes biofilms in acne vulgaris: A case-control study. Br. J. Dermatol. 2012, 167, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Kelhälä, H.L.; Palatsi, R.; Fyhrquist, N.; Lehtimäki, S.; Väyrynen, J.P.; Kallioinen, M.; Kubin, M.E.; Greco, D.; Tasanen, K.; Alenius, H.; et al. IL-17/Th17 pathway is activated in acne lesions. PLoS ONE 2014, 9, e105238. [Google Scholar] [CrossRef] [PubMed]
- Del Rosso, J.Q.; Schmidt, N.F. A review of the anti-inflammatory properties of clindamycin in the treatment of acne vulgaris. Cutis 2010, 85, 15–24. [Google Scholar] [PubMed]
- Zavascki, A.P.; Bulitta, J.B.; Landersdorfer, C.B. Combination therapy for carbapenem-resistant Gram-negative bacteria. Expert Rev. Anti Infect. Ther. 2013, 11, 1333–1353. [Google Scholar] [CrossRef] [PubMed]
- Soares, D.N.; Antonio, A.G.; Iorio, N.L.; Pierro, V.S.; dos Santos, K.R.; Maia, L.C. Does the presence of sucrose in pediatric antibiotics influence the enamel mineral loss and the Streptococcus mutans counts in dental biofilm? Braz. Dent. J. 2015, 26, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Hurley, M.N.; Forrester, D.L.; Smyth, A.R. Antibiotic adjuvant therapy for pulmonary infection in cystic fibrosis. Cochrane Database Syst. Rev. 2013. [Google Scholar] [CrossRef]
- Marino, C.; Stoughton, R.B. Clinical use of a selective culture medium for wild and antibiotic-resistant propionibacterium acnes. J. Am. Acad. Dermatol. 1982, 6, 902–908. [Google Scholar] [CrossRef]
- Fried, R.G.; Wechsler, A. Psychological problems in the acne patient. Dermatol. Ther. 2006, 19, 237–240. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Kao, M.-S.; Yu, J.; Huang, S.; Marito, S.; Gallo, R.L.; Huang, C.-M. A Precision Microbiome Approach Using Sucrose for Selective Augmentation of Staphylococcus epidermidis Fermentation against Propionibacterium acnes. Int. J. Mol. Sci. 2016, 17, 1870. https://doi.org/10.3390/ijms17111870
Wang Y, Kao M-S, Yu J, Huang S, Marito S, Gallo RL, Huang C-M. A Precision Microbiome Approach Using Sucrose for Selective Augmentation of Staphylococcus epidermidis Fermentation against Propionibacterium acnes. International Journal of Molecular Sciences. 2016; 17(11):1870. https://doi.org/10.3390/ijms17111870
Chicago/Turabian StyleWang, Yanhan, Ming-Shan Kao, Jinghua Yu, Stephen Huang, Shinta Marito, Richard L. Gallo, and Chun-Ming Huang. 2016. "A Precision Microbiome Approach Using Sucrose for Selective Augmentation of Staphylococcus epidermidis Fermentation against Propionibacterium acnes" International Journal of Molecular Sciences 17, no. 11: 1870. https://doi.org/10.3390/ijms17111870
APA StyleWang, Y., Kao, M.-S., Yu, J., Huang, S., Marito, S., Gallo, R. L., & Huang, C.-M. (2016). A Precision Microbiome Approach Using Sucrose for Selective Augmentation of Staphylococcus epidermidis Fermentation against Propionibacterium acnes. International Journal of Molecular Sciences, 17(11), 1870. https://doi.org/10.3390/ijms17111870






