Classification of 27 Tumor-Associated Antigens by Histochemical Analysis of 36 Freshly Resected Lung Cancer Tissues
Abstract
:1. Introduction
2. Results
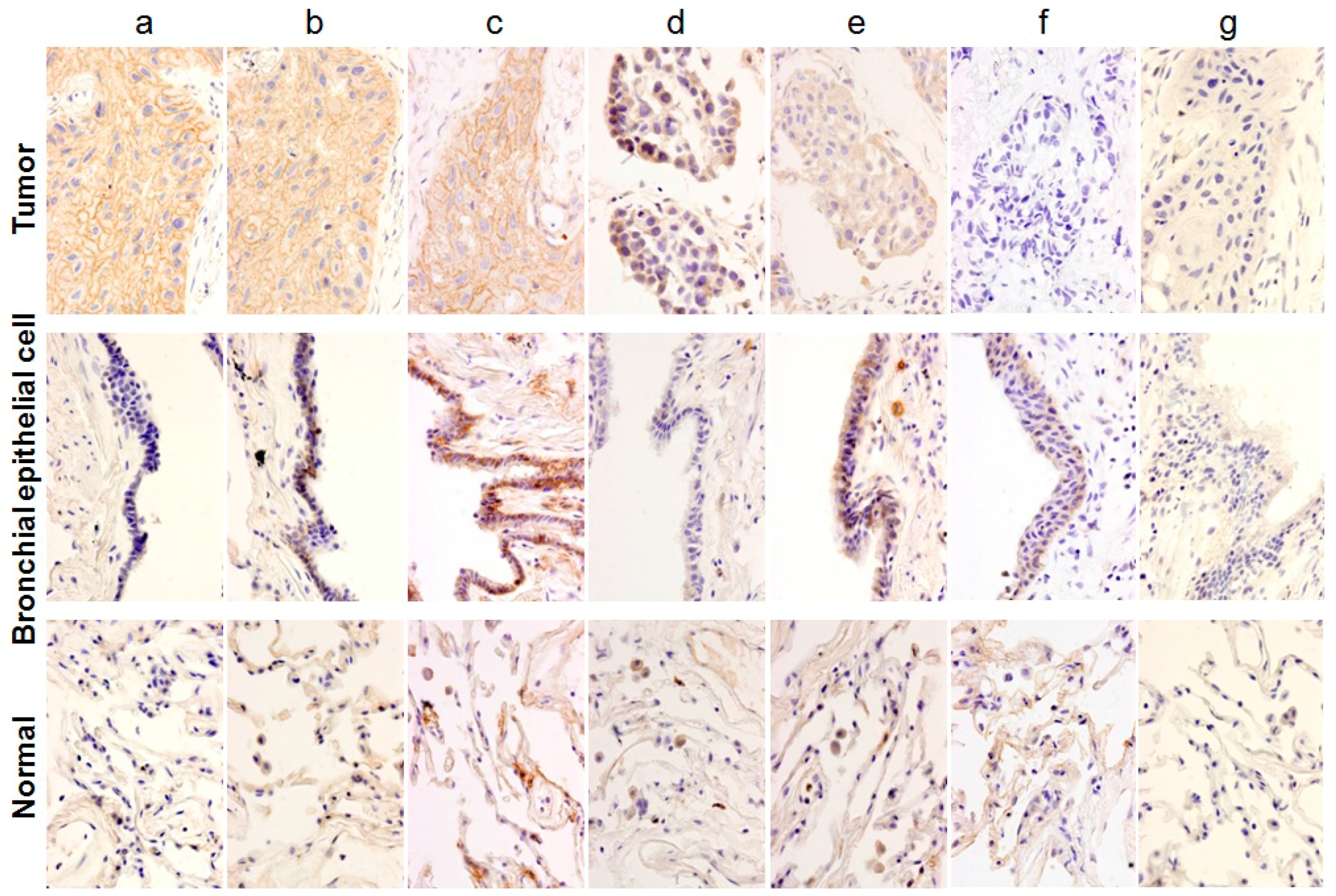
2.1. Classification of Staining Patterns
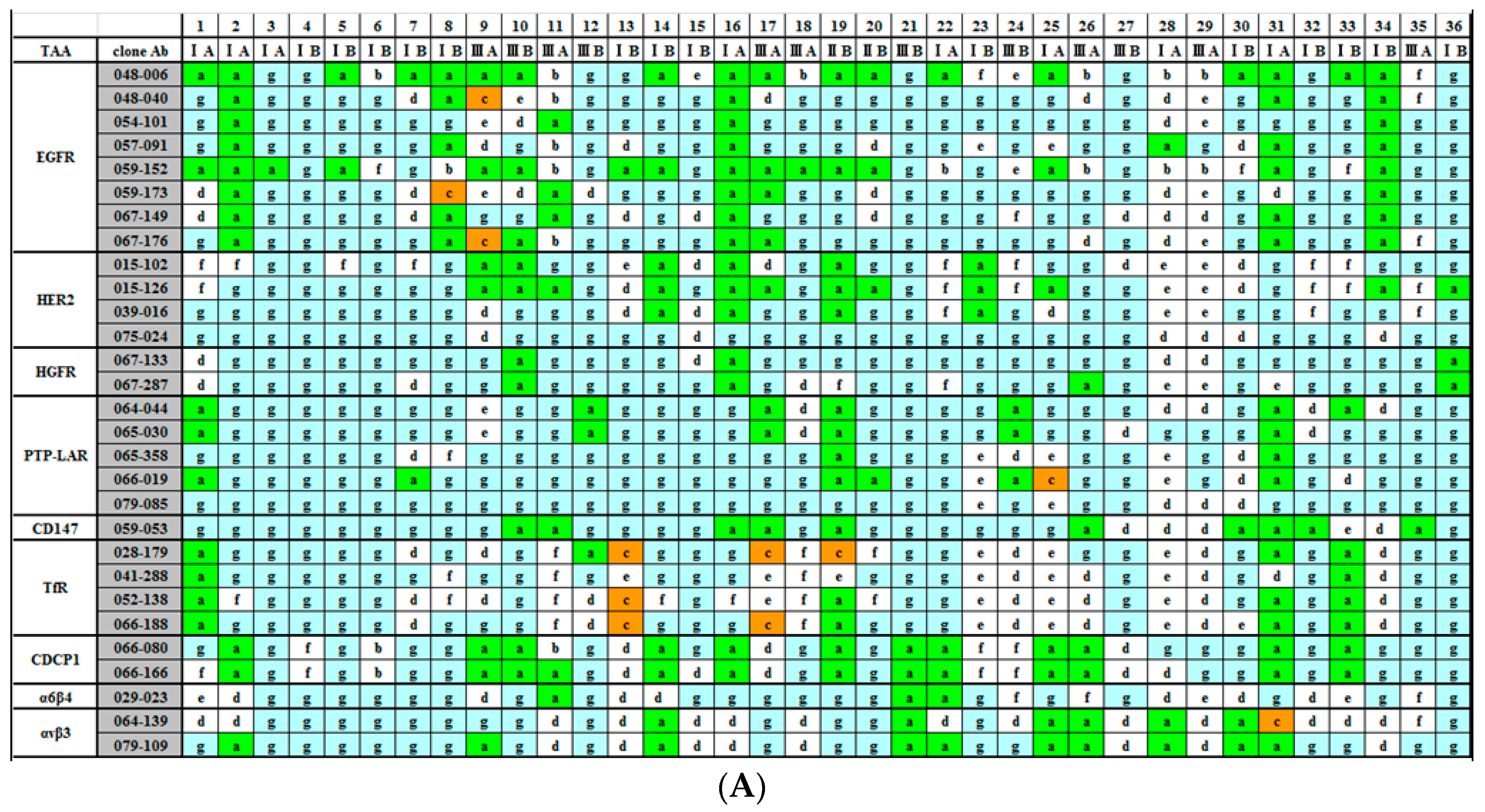
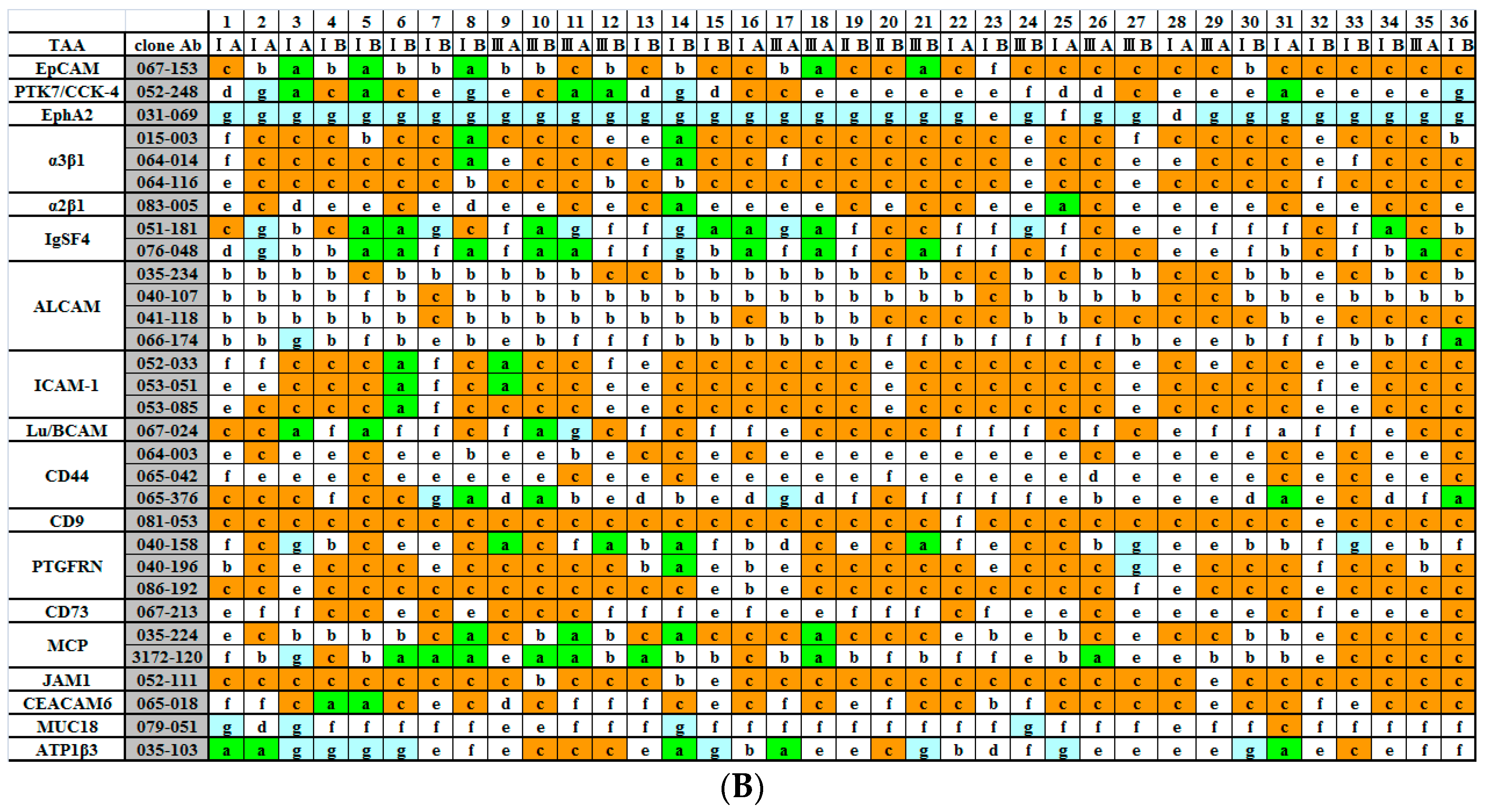
2.2. Immunohistochemical (IHC) Analyses of 36 Fresh Lung Cancer Specimens with 60 Monoclonal Antibodies (mAbs) against 27 Tumor-Associated Antigens (TAAs)
3. Discussion
4. Materials and Methods
4.1. mAbs
4.2. Immunostaining of Lung Cancer Tissues
4.3. Ethics Statement
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Carter, P.; Presta, L.; Gorman, C.M.; Ridgway, J.B.; Henner, D.; Wong, W.L.; Rowland, A.M.; Kotts, C.M.; Carver, M.E.; Shepard, H.M. Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc. Natl. Acad. Sci. USA 1992, 89, 4285–4289. [Google Scholar] [CrossRef] [PubMed]
- Reichert, J.M.; Valge-Archer, V.E. Development trends for monoclonal antibody cancer therapeutics. Nat. Rev. Drug Discov. 2007, 6, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Sliwkowski, M.X.; Mellman, I. Antibody therapeutics in Cancer. Science 2013, 341, 1192–1198. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.H.; Paulos, C.M. Novel immunotherapies for hematologic malignancies. Immnol. Rev. 2015, 263, 90–105. [Google Scholar] [CrossRef] [PubMed]
- Clynes, R.A.; Towers, T.L.; Presta, L.G.; Ravetch, J.V. Inhibitory Fc receptors modulate In Vivo cytotoxicity against tumor targets. Nat. Med. 2000, 6, 443–446. [Google Scholar] [PubMed]
- Di Gaetano, N.; Cittera, E.; Nota, R.; Vecchi, A.; Grieco, V.; Scanziani, E.; Botto, M.; Introna, M.; Golay, J. Complement activation determines the therapeutic activity of rituximab In Vivo. J. Immunol. 2003, 171, 1581–1587. [Google Scholar] [CrossRef] [PubMed]
- Wurz, G.T.; Kao, C.J.; DeGregorio, M.W. Novel cancer antigens for personalized immunotherapies: Latest evidence and clinical potential. Ther. Adv. Med. Oncol. 2016, 8, 4–31. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Faghfuri, E.; Faramarzi, M.A.; Nikfar, S.; Abdollahi, M. Nivolumab and pembrolizumab as immune-modulating monoclonal antibodies targeting the PD-1 receptor to treat melanoma. Expert Rev. Anticancer Ther. 2015, 15, 981–993. [Google Scholar] [CrossRef] [PubMed]
- Kurosawa, G.; Akahori, Y.; Morita, M.; Sumitomo, M.; Sato, N.; Muramatsu, C.; Eguchi, K.; Matsuda, K.; Takasaki, A.; Tanaka, M.; et al. Comprehensive screening for antigens overexpressed on carcinomas via isolation of human mAbs that may be therapeutic. Proc. Natl. Acad. Sci. USA 2008, 105, 7287–7292. [Google Scholar] [CrossRef] [PubMed]
- Kurosawa, G.; Sumitomo, M.; Ukai, Y.; Subere, J.; Muramatsu, C.; Eguchi, K.; Tanaka-Hashiba, M.; Sugiura, M.; Ando, M.; Sato, N.; et al. Selection and analysis of anti-cancer antibodies for cancer therapy obtained from antibody phage library. Cancer Sci. 2011, 102, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Borlak, J.; Laenger, F.; Spanel, R.; Schöndorfer, G.; Dittrich, C. Immune-mediated liver injury of the cancer therapeutic antibody catumaxomab targeting EpCAM, AD3 and Fcγ receptors. Oncotarget 2016, 7, 28059–28074. [Google Scholar] [PubMed]
- Tykvart, J.; Navratil, V.; Sedlak, F.; Corey, E.; Colombatti, M.; Fracasso, G.; Koukolik, F.; Bařinka, C.; Šácha, P.; Konvalinka, J. Comparative analysis of monoclonal antibodies against prostate-specific membrane antigen (PSMA). Prostate 2014, 74, 1674–1690. [Google Scholar] [CrossRef] [PubMed]
- Sattler, M.; Hasina, R.; Reddy, M.M.; Gangadhar, T.; Salgia, R. The role of the c-Met pathway in lung cancer and the potential for targeted therapy. Ther. Adv. Med. Oncol. 2011, 3, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Chagnon, M.J.; Uetani, N.; Tremblay, M.L. Functional significance of the LAR receptor protein tyrosine phosphatase family in development and diseases. Biochem. Cell Biol. 2004, 82, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Levea, C.M.; Mcgary, C.T.; Symons, J.R.; Mooney, R.A. PTP LAR expression compared to prognostic indices in metastatic and non-metastatic breast cancer. Breast Cancer Res. Treat. 2000, 64, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Nagai, K.; Nakahata, S.; Shimosaki, S.; Tamura, T.; Kondo, Y.; Baba, T.; Taki, T.; Taniwaki, M.; Kurosawa, G.; Sudo, Y.; et al. Development of a complete human anti-human transferrin receptor C antibody as a novel marker of oral dysplasia and oral cancer. Cancer Med. 2014, 3, 1085–1099. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, N.; Kaneda, K.; Saito, Y.; Ichihara, E.; Morishita, K. The increased expression of integrin α6 (ITGA6) enhances drug resistance in EVI1 high leukemia. PLoS ONE 2012, 7, e30706. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.; Auernheimer, J.; Modilinger, A.; Kessler, H. Targeting RGD recognizing integrins: Drug development, biomaterial research, tumor imaging and targeting. Curr. Pharm. Des. 2006, 12, 2723–2743. [Google Scholar] [CrossRef] [PubMed]
- Uekita, T.; Jia, L.; Narisawa-Saito, M.; Yokota, J.; Kiyono, T.; Sakai, R. CUB domain-containing protein 1 is a novel regulator of anoikis resistance in lung adenocarcinoma. Mol. Cell. Biol. 2007, 27, 7649–7660. [Google Scholar] [CrossRef] [PubMed]
- Siva, A.C.; Wild, M.A.; Kirkland, R.E.; Nolan, M.J.; Lin, B.; Maruyama, T.; Yantiri-Wernimont, F.; Frederickson, S.; Bowdish, K.S.; Xin, H. Targeting CUB domain-containing protein 1 with a monoclonal antibody inhibits metastasis in a prostate cancer model. Cancer Res. 2008, 68, 3759–3766. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, Y.; Sun, Q.; Feng, F.; Huhe, M.; Mi, L.; Chen, Z. Preclinical pharmacokinetics, tolerability, and pharmacodynamics of Metuzumab, a novel CD147 human-mouse chimeric and glycoengineered antibody. Mol. Cancer Ther. 2015, 14, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Kurosawa, G.; Kondo, M.; Kurosawa, Y. A variety of human monoclonal antibodies against epidermal growrth factor receptor isolated froma phage antibody libraray. Biochem. Biophys. Res. Commun. 2016, 480, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, Y.; Kurosawa, G.; Tanaka, M.; Sumitomo, M.; Muramatsu, C.; Eguchi, K.; Akahori, Y.; Iba, Y.; Tsuda, H.; Sugiura, M.; et al. Frequent overexpression of CADM1/IGSF4 in lung adenocarcinoma. Biochem. Biophys. Res. Commun. 2009, 383, 480–484. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurosawa, G.; Sugiura, M.; Hattori, Y.; Tsuda, H.; Kurosawa, Y. Classification of 27 Tumor-Associated Antigens by Histochemical Analysis of 36 Freshly Resected Lung Cancer Tissues. Int. J. Mol. Sci. 2016, 17, 1862. https://doi.org/10.3390/ijms17111862
Kurosawa G, Sugiura M, Hattori Y, Tsuda H, Kurosawa Y. Classification of 27 Tumor-Associated Antigens by Histochemical Analysis of 36 Freshly Resected Lung Cancer Tissues. International Journal of Molecular Sciences. 2016; 17(11):1862. https://doi.org/10.3390/ijms17111862
Chicago/Turabian StyleKurosawa, Gene, Mototaka Sugiura, Yoshinobu Hattori, Hiroyuki Tsuda, and Yoshikazu Kurosawa. 2016. "Classification of 27 Tumor-Associated Antigens by Histochemical Analysis of 36 Freshly Resected Lung Cancer Tissues" International Journal of Molecular Sciences 17, no. 11: 1862. https://doi.org/10.3390/ijms17111862
APA StyleKurosawa, G., Sugiura, M., Hattori, Y., Tsuda, H., & Kurosawa, Y. (2016). Classification of 27 Tumor-Associated Antigens by Histochemical Analysis of 36 Freshly Resected Lung Cancer Tissues. International Journal of Molecular Sciences, 17(11), 1862. https://doi.org/10.3390/ijms17111862





