Focal Adhesion Kinase-Dependent Role of the Soluble Form of Neurotensin Receptor-3/Sortilin in Colorectal Cancer Cell Dissociation
Abstract
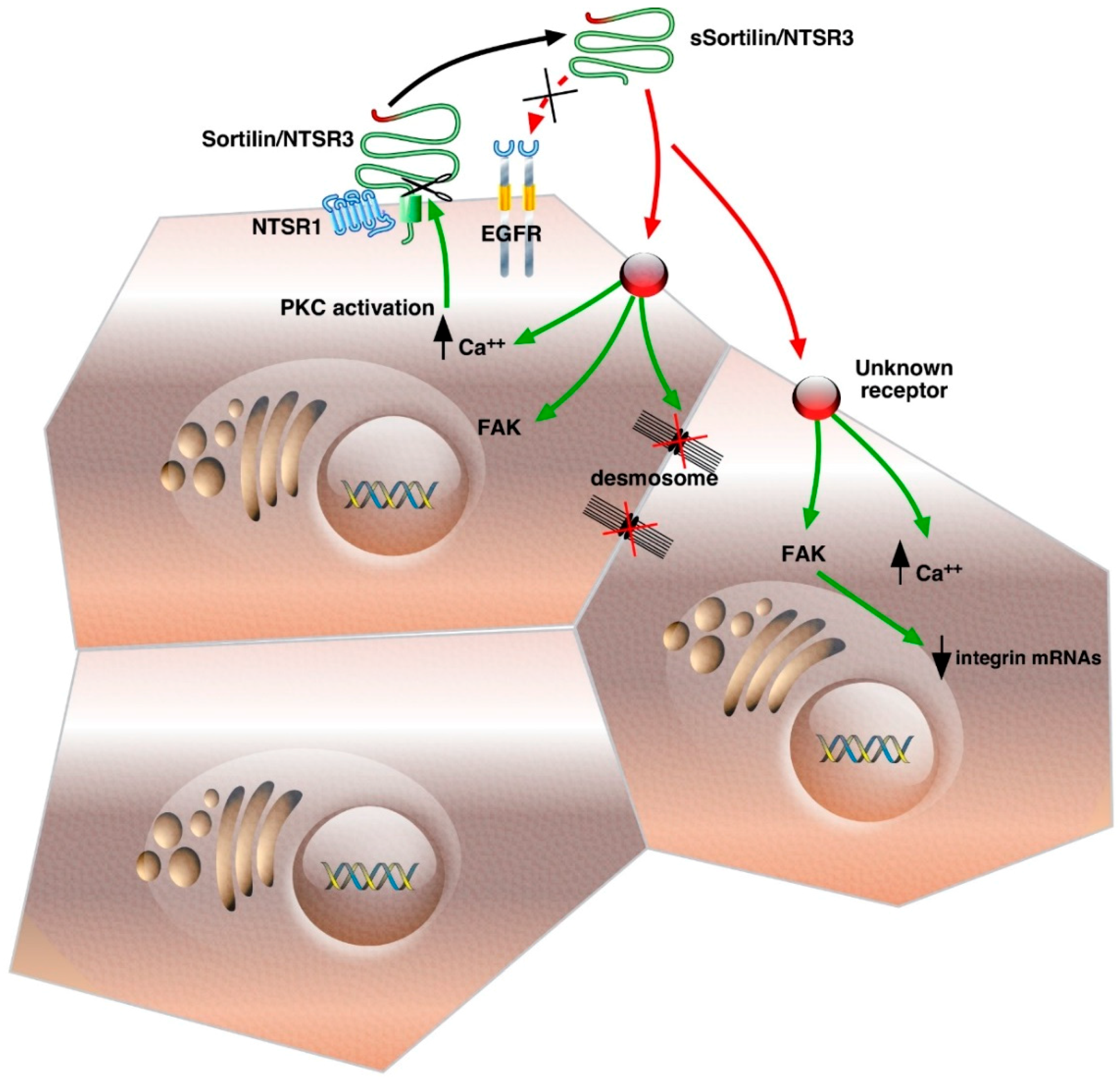
:1. Introduction
2. Release, Binding and Internalization Properties of sSortilin/NTSR3
2.1. Shedding
2.2. Binding of sSortilin/NTSR3 to a Specific Receptor Independent on the Epidermal Growth Factor Receptor (EGFR)
2.3. Internalization Properties of Sortilin/NTSR3
3. Signaling of sSortilin/NTSR3 in HT29 Cells
3.1. Calcium and Protein Kinase C (PKCα) Translocation
3.2. Focal Adhesion Kinase (FAK) Dependent Stimulation of the PI3 Kinase Pathway
4. Morphological Changes of HT29 Cells Induced by sSortilin/NTSR3
Cell Shape and Size, Cytoskeleton and Cell–Cell Junctions Modification
5. Cell–Cell and Cell–Matrix Junctions
5.1. Desmosomes Disruption
5.2. Modification of Cadherin and Integrins
5.3. Cancer Cell Detachment
6. Other Functions of sSortilin/NTSR3
Atherosclerosis and Depression
7. Conclusions
Acknowledgments
Conflicts of Interest
References
- Thiery, J.P. Epithelial-mesenchymal transitions in tumour progression. Nat. Rev. Cancer 2002, 2, 442–454. [Google Scholar] [CrossRef] [PubMed]
- Reubi, J.C. Peptide receptors as molecular targets for cancer diagnosis and therapy. Endocr. Rev. 2003, 24, 389–427. [Google Scholar] [CrossRef] [PubMed]
- Kasina, S.; Scherle, P.A.; Hall, C.L.; Macoska, J.A. ADAM-mediated amphiregulin shedding and EGFR transactivation. Cell Prolif. 2009, 42, 799–812. [Google Scholar] [CrossRef] [PubMed]
- Marcusson, E.G.; Horazdovsky, B.F.; Cereghino, J.L.; Gharakhanian, E.; Emr, S.D. The sorting receptor for yeast vacuolar carboxypeptidase Y is encoded by the VPS10 gene. Cell 1994, 77, 579–586. [Google Scholar] [CrossRef]
- Hermey, G. The Vps10p-domain receptor family. Cell. Mol. Life Sci. 2009, 66, 2677–2689. [Google Scholar] [CrossRef] [PubMed]
- Navarro, V.; Vincent, J.P.; Mazella, J. Shedding of the luminal domain of the neurotensin receptor-3/sortilin in the HT29 cell line. Biochem. Biophys. Res. Commun. 2002, 298, 760–764. [Google Scholar] [CrossRef]
- Hermey, G.; Sjogaard, S.S.; Petersen, C.M.; Nykjaer, A.; Gliemann, J. Tumour necrosis factor α-converting enzyme mediates ectodomain shedding of Vps10p-domain receptor family members. Biochem. J. 2006, 395, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Petersen, C.M.; Nielsen, M.S.; Nykjaer, A.; Jacobsen, L.; Tommerup, N.; Rasmussen, H.H.; Roigaard, H.; Gliemann, J.; Madsen, P.; Moestrup, S.K. Molecular identification of a novel candidate sorting receptor purified from human brain by receptor-associated protein affinity chromatography. J. Biol. Chem. 1997, 272, 3599–3605. [Google Scholar] [CrossRef] [PubMed]
- Mazella, J.; Zsurger, N.; Navarro, V.; Chabry, J.; Kaghad, M.; Caput, D.; Ferrara, P.; Vita, N.; Gully, D.; Maffrand, J.P.; et al. The 100-kDa neurotensin receptor is gp95/sortilin, a non-G-protein-coupled receptor. J. Biol. Chem. 1998, 273, 26273–26276. [Google Scholar] [CrossRef] [PubMed]
- Lefrancois, S.; Zeng, J.; Hassan, A.J.; Canuel, M.; Morales, C.R. The lysosomal trafficking of sphingolipid activator proteins (SAPs) is mediated by sortilin. EMBO J. 2003, 22, 6430–6437. [Google Scholar] [CrossRef] [PubMed]
- Willnow, T.E.; Petersen, C.M.; Nykjaer, A. VPS10P-domain receptors—Regulators of neuronal viability and function. Nat. Rev. Neurosci. 2008, 9, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.; Vincent, J.P.; Mazella, J. Involvement of the neurotensin receptor-3 in the neurotensin-induced migration of human microglia. J. Neurosci. 2003, 23, 1198–1205. [Google Scholar] [PubMed]
- Nielsen, M.S.; Jacobsen, C.; Olivecrona, G.; Gliemann, J.; Petersen, C.M. Sortilin/neurotensin receptor-3 binds and mediates degradation of lipoprotein lipase. J. Biol. Chem. 1999, 274, 8832–8836. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.; Navarro, V.; Vincent, J.P.; Mazella, J. Neurotensin receptor-1 and -3 complex modulates the cellular signaling of neurotensin in the HT29 cell line. Gastroenterology 2002, 123, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Beraud-Dufour, S.; Coppola, T.; Massa, F.; Mazella, J. Neurotensin receptor-2 and -3 are crucial for the anti-apoptotic effect of neurotensin on pancreatic β-TC3 cells. Int. J. Biochem. Cell Biol. 2009, 41, 2398–2402. [Google Scholar] [CrossRef] [PubMed]
- Nykjaer, A.; Lee, R.; Teng, K.K.; Jansen, P.; Madsen, P.; Nielsen, M.S.; Jacobsen, C.; Kliemannel, M.; Schwarz, E.; Willnow, T.E.; et al. Sortilin is essential for proNGF-induced neuronal cell death. Nature 2004, 427, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Teng, H.K.; Teng, K.K.; Lee, R.; Wright, S.; Tevar, S.; Almeida, R.D.; Kermani, P.; Torkin, R.; Chen, Z.Y.; Lee, F.S.; et al. ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin. J. Neurosci. 2005, 25, 5455–5463. [Google Scholar] [CrossRef] [PubMed]
- Carlo, A.S. Sortilin, a novel APOE receptor implicated in Alzheimer disease. Prion 2013, 7, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Dal Farra, C.; Sarret, P.; Navarro, V.; Botto, J.M.; Mazella, J.; Vincent, J.P. Involvement of the neurotensin receptor subtype NTR3 in the growth effect of neurotensin on cancer cell lines. Int. J. Cancer 2001, 92, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Fauchais, A.L.; Lalloue, F.; Lise, M.C.; Boumediene, A.; Preud'homme, J.L.; Vidal, E.; Jauberteau, M.O. Role of endogenous brain-derived neurotrophic factor and sortilin in B cell survival. J. Immunol. 2008, 181, 3027–3038. [Google Scholar] [CrossRef] [PubMed]
- Akil, H.; Perraud, A.; Melin, C.; Jauberteau, M.O.; Mathonnet, M. Fine-tuning roles of endogenous brain-derived neurotrophic factor, TrkB and sortilin in colorectal cancer cell survival. PLoS ONE 2011, 6, e25097. [Google Scholar] [CrossRef] [PubMed]
- Pinet, S.; Bessette, B.; Vedrenne, N.; Lacroix, A.; Richard, L.; Jauberteau, M.O.; Battu, S.; Lalloue, F. TrkB-containing exosomes promote the transfer of glioblastoma aggressiveness to YKL-40-inactivated glioblastoma cells. Oncotarget 2016. [Google Scholar] [CrossRef] [PubMed]
- Roselli, S.; Pundavela, J.; Demont, Y.; Faulkner, S.; Keene, S.; Attia, J.; Jiang, C.C.; Zhang, X.D.; Walker, M.M.; Hondermarck, H. Sortilin is associated with breast cancer aggressiveness and contributes to tumor cell adhesion and invasion. Oncotarget 2015, 6, 10473–10486. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.P.; Mazella, J.; Kitabgi, P. Neurotensin and neurotensin receptors. Trends Pharmacol. Sci. 1999, 20, 302–309. [Google Scholar] [CrossRef]
- Myers, R.M.; Shearman, J.W.; Kitching, M.O.; Ramos-Montoya, A.; Neal, D.E.; Ley, S.V. Cancer, chemistry, and the cell: Molecules that interact with the neurotensin receptors. ACS Chem. Biol. 2009, 4, 503–525. [Google Scholar] [CrossRef] [PubMed]
- Younes, M.; Wu, Z.; Dupouy, S.; Lupo, A.M.; Mourra, N.; Takahashi, T.; Flejou, J.F.; Tredaniel, J.; Regnard, J.F.; Damotte, D.; et al. Neurotensin (NTS) and its receptor (NTSR1) causes EGFR, HER2 and HER3 over-expression and their autocrine/paracrine activation in lung tumors, confirming responsiveness to erlotinib. Oncotarget 2014, 5, 8252–8269. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.M.; Naves, T.; Vincent, F.; Melloni, B.; Bonnaud, F.; Lalloue, F.; Jauberteau, M.O. Sortilin mediates the release and transfer of exosomes in concert with two tyrosine kinase receptors. J. Cell Sci. 2014, 127, 3983–3997. [Google Scholar] [CrossRef] [PubMed]
- Moody, T.W.; Moreno, P.; Jensen, R.T. Neuropeptides as lung cancer growth factors. Peptides 2015, 72, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Warhurst, G.; Fogg, K.E.; Higgs, N.B.; Tonge, A.; Grundy, J. Ca2+-mobilising agonists potentiate forskolin- and VIP-stimulated cAMP production in human colonic cell line, HT29-cl.19A: Role of [Ca2+]i and protein kinase C. Cell Calcium 1994, 15, 162–174. [Google Scholar] [CrossRef]
- Massa, F.; Devader, C.; Beraud-Dufour, S.; Brau, F.; Coppola, T.; Mazella, J. Focal adhesion kinase dependent activation of the PI3 kinase pathway by the functional soluble form of neurotensin receptor-3 in HT29 cells. Int. J. Biochem. Cell Biol. 2013, 45, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Hampe, W.; Riedel, I.B.; Lintzel, J.; Bader, C.O.; Franke, I.; Schaller, H.C. Ectodomain shedding, translocation and synthesis of SorLA are stimulated by its ligand head activator. J. Cell Sci. 2000, 113, 4475–4485. [Google Scholar] [PubMed]
- Nyborg, A.C.; Ladd, T.B.; Zwizinski, C.W.; Lah, J.J.; Golde, T.E. Sortilin, SorCS1b, and SorLA Vps10p sorting receptors, are novel gamma-secretase substrates. Mol. Neurodegener. 2006, 1, 3. [Google Scholar] [CrossRef] [PubMed]
- Willnow, T.E.; Andersen, O.M. Sorting receptor SORLA—A trafficking path to avoid Alzheimer disease. J. Cell Sci. 2013, 126, 2751–2760. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Zhan, Y.; Zeng, H.; Koon, H.W.; Moyer, M.P.; Pothoulakis, C. Neurotensin stimulates expression of early growth response gene-1 and EGF receptor through MAP kinase activation in human colonic epithelial cells. Int. J. Cancer 2007, 120, 1652–1656. [Google Scholar] [CrossRef] [PubMed]
- Moody, T.W.; Nuche-Berenguer, B.; Nakamura, T.; Jensen, R.T. EGFR Transactivation by Peptide G Protein-Coupled Receptors in Cancer. Curr. Drug Targets 2016, 17, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Dumaresq-Doiron, K.; Jules, F.; Lefrancois, S. Sortilin turnover is mediated by ubiquitination. Biochem. Biophys. Res. Commun. 2013, 433, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Evron, T.; Daigle, T.L.; Caron, M.G. GRK2: Multiple roles beyond G protein-coupled receptor desensitization. Trends Pharmacol. Sci. 2012, 33, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.M. Structural, biochemical and signaling properties of the low-density lipoprotein receptor gene family. Front. Biosci. 2001, 6, D417–D428. [Google Scholar] [CrossRef] [PubMed]
- Toker, A. Phosphoinositide 3-kinases—A historical perspective. Subcell. Biochem. 2012, 58, 95–110. [Google Scholar] [PubMed]
- Temraz, S.; Mukherji, D.; Shamseddine, A. Dual Inhibition of MEK and PI3K Pathway in KRAS and BRAF Mutated Colorectal Cancers. Int. J. Mol. Sci. 2015, 16, 22976–22988. [Google Scholar] [CrossRef] [PubMed]
- Buchheit, C.L.; Rayavarapu, R.R.; Schafer, Z.T. The regulation of cancer cell death and metabolism by extracellular matrix attachment. Semin. Cell Dev. Biol. 2012, 4, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Hall, J.E.; Schaller, M.D. Focal adhesion kinase-regulated signaling events in human cancer. Biomol. Concepts 2012, 3, 225–240. [Google Scholar] [CrossRef] [PubMed]
- Sackmann, E. How actin/myosin crosstalks guide the adhesion, locomotion and polarization of cells. Biochim. Biophys. Acta 2015, 1853, 3132–3142. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.T. Nuclear FAK: A new mode of gene regulation from cellular adhesions. Mol. Cells 2013, 36, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Parsons, J.T. Focal adhesion kinase: The first ten years. J. Cell Sci. 2003, 116, 1409–1416. [Google Scholar] [CrossRef] [PubMed]
- Massa, F.; Devader, C.; Lacas-Gervais, S.; Beraud-Dufour, S.; Coppola, T.; Mazella, J. Impairement of HT29 Cancer Cells Cohesion by the Soluble Form of Neurotensin Receptor-3. Genes Cancer 2014, 5, 240–249. [Google Scholar] [PubMed]
- Kalaji, R.; Wheeler, A.P.; Erasmus, J.C.; Lee, S.Y.; Endres, R.G.; Cramer, L.P.; Braga, V.M. ROCK1 and ROCK2 regulate epithelial polarisation and geometric cell shape. Biol. Cell 2012, 104, 435–451. [Google Scholar] [CrossRef] [PubMed]
- Farhadifar, R.; Roper, J.C.; Aigouy, B.; Eaton, S.; Julicher, F. The influence of cell mechanics, cell–cell interactions, and proliferation on epithelial packing. Curr. Biol. 2007, 17, 2095–2104. [Google Scholar] [CrossRef] [PubMed]
- Stutzmann, J.; Bellissent-Waydelich, A.; Fontao, L.; Launay, J.F.; Simon-Assmann, P. Adhesion complexes implicated in intestinal epithelial cell-matrix interactions. Microsc. Res. Tech. 2000, 51, 179–190. [Google Scholar] [CrossRef]
- Green, K.J.; Gaudry, C.A. Are desmosomes more than tethers for intermediate filaments? Nat. Rev. Mol. Cell Biol. 2000, 1, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Dusek, R.L.; Attardi, L.D. Desmosomes: New perpetrators in tumour suppression. Nat. Rev. Cancer 2011, 11, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Brooke, M.A.; Nitoiu, D.; Kelsell, D.P. Cell–cell connectivity: Desmosomes and disease. J. Pathol. 2012, 226, 158–171. [Google Scholar] [CrossRef] [PubMed]
- Takeichi, M. Dynamic contacts: Rearranging adherens junctions to drive epithelial remodelling. Nat. Rev. Mol. Cell Biol. 2014, 15, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Barczyk, M.; Carracedo, S.; Gullberg, D. Integrins. Cell Tissue Res. 2010, 339, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.K.; Schlaepfer, D.D. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr. Opin. Cell Biol. 2006, 18, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Adachi, M.; Taki, T.; Huang, C.; Higashiyama, M.; Doi, O.; Tsuji, T.; Miyake, M. Reduced integrin α3 expression as a factor of poor prognosis of patients with adenocarcinoma of the lung. J. Clin. Oncol. 1998, 16, 1060–1067. [Google Scholar] [PubMed]
- Takenaka, K.; Shibuya, M.; Takeda, Y.; Hibino, S.; Gemma, A.; Ono, Y.; Kudoh, S. Altered expression and function of β1 integrins in a highly metastatic human lung adenocarcinoma cell line. Int. J. Oncol. 2000, 17, 1187–1194. [Google Scholar] [PubMed]
- Koretz, K.; Schlag, P.; Boumsell, L.; Moller, P. Expression of VLA-α2, VLA-α6, and VLA-β1 chains in normal mucosa and adenomas of the colon, and in colon carcinomas and their liver metastases. Am. J. Pathol. 1991, 138, 741–750. [Google Scholar] [PubMed]
- Stallmach, A.; Riecken, E.O. Colorectal carcinoma—Current pathogenetic concepts. Significance of cell-matrix interaction for invasive growth and metastasis. Schweiz. Rundsch. Med. Prax. 1992, 81, 847–849. [Google Scholar] [PubMed]
- Ogawa, K.; Ueno, T.; Iwasaki, T.; Kujiraoka, T.; Ishihara, M.; Kunimoto, S.; Takayama, T.; Kanai, T.; Hirayama, A.; Hattori, H. Soluble sortilin is released by activated platelets and its circulating levels are associated with cardiovascular risk factors. Atherosclerosis 2016, 249, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Benjannet, S.; Rhainds, D.; Essalmani, R.; Mayne, J.; Wickham, L.; Jin, W.; Asselin, M.C.; Hamelin, J.; Varret, M.; Allard, D.; et al. NARC-1/PCSK9 and its natural mutants: Zymogen cleavage and effects on the low density lipoprotein (LDL) receptor and LDL cholesterol. J. Biol. Chem. 2004, 279, 48865–48875. [Google Scholar] [CrossRef] [PubMed]
- Nozue, T.; Hattori, H.; Ogawa, K.; Kujiraoka, T.; Iwasaki, T.; Michishita, I. Effects of Statin Therapy on Plasma Proprotein Convertase Subtilisin/kexin Type 9 and Sortilin Levels in Statin-Naive Patients with Coronary Artery Disease. J. Atheroscler. Thromb. 2016, 23, 848–856. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.Y.; Cayabyab, F.S.; Tang, C.K.; Zheng, X.L.; Peng, T.H.; Lv, Y.C. Sortilin: A novel regulator in lipid metabolism and atherogenesis. Clin. Chim. Acta 2016, 460, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Molgaard, S.; Demontis, D.; Nicholson, A.M.; Finch, N.A.; Petersen, R.C.; Petersen, C.M.; Rademakers, R.; Nykjaer, A.; Glerup, S. Soluble sortilin is present in excess and positively correlates with progranulin in CSF of aging individuals. Exp. Gerontol. 2016, 84, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Padukkavidana, T.; Vaegter, C.B.; Brady, O.A.; Zheng, Y.; Mackenzie, I.R.; Feldman, H.H.; Nykjaer, A.; Strittmatter, S.M. Sortilin-mediated endocytosis determines levels of the frontotemporal dementia protein, progranulin. Neuron 2010, 68, 654–667. [Google Scholar] [CrossRef] [PubMed]
- Buttenschon, H.N.; Demontis, D.; Kaas, M.; Elfving, B.; Molgaard, S.; Gustafsen, C.; Kaerlev, L.; Petersen, C.M.; Borglum, A.D.; Mors, O.; et al. Increased serum levels of sortilin are associated with depression and correlated with BDNF and VEGF. Transl. Psychiatry 2015, 5, e677. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Cao, H.; Shen, B.; Feng, J. Tumor-derived exosomes in cancer progression and treatment failure. Oncotarget 2015, 6, 37151–37168. [Google Scholar] [PubMed]
- Wilson, C.M.; Naves, T.; Al Akhrass, H.; Vincent, F.; Melloni, B.; Bonnaud, F.; Lalloue, F.; Jauberteau, M.O. A new role under sortilin’s belt in cancer. Commun. Integr. Biol. 2016, 9, e1130192. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Guan, J.L. Focal adhesion kinase and its signaling pathways in cell migration and angiogenesis. Adv. Drug Deliv. Rev. 2011, 63, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Cathcart, J.; Pulkoski-Gross, A.; Cao, J. Targeting matrix metalloproteinases in cancer: Bringing new life to old ideas. Genes Dis. 2015, 2, 26–34. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Béraud-Dufour, S.; Devader, C.; Massa, F.; Roulot, M.; Coppola, T.; Mazella, J. Focal Adhesion Kinase-Dependent Role of the Soluble Form of Neurotensin Receptor-3/Sortilin in Colorectal Cancer Cell Dissociation. Int. J. Mol. Sci. 2016, 17, 1860. https://doi.org/10.3390/ijms17111860
Béraud-Dufour S, Devader C, Massa F, Roulot M, Coppola T, Mazella J. Focal Adhesion Kinase-Dependent Role of the Soluble Form of Neurotensin Receptor-3/Sortilin in Colorectal Cancer Cell Dissociation. International Journal of Molecular Sciences. 2016; 17(11):1860. https://doi.org/10.3390/ijms17111860
Chicago/Turabian StyleBéraud-Dufour, Sophie, Christelle Devader, Fabienne Massa, Morgane Roulot, Thierry Coppola, and Jean Mazella. 2016. "Focal Adhesion Kinase-Dependent Role of the Soluble Form of Neurotensin Receptor-3/Sortilin in Colorectal Cancer Cell Dissociation" International Journal of Molecular Sciences 17, no. 11: 1860. https://doi.org/10.3390/ijms17111860
APA StyleBéraud-Dufour, S., Devader, C., Massa, F., Roulot, M., Coppola, T., & Mazella, J. (2016). Focal Adhesion Kinase-Dependent Role of the Soluble Form of Neurotensin Receptor-3/Sortilin in Colorectal Cancer Cell Dissociation. International Journal of Molecular Sciences, 17(11), 1860. https://doi.org/10.3390/ijms17111860





