Extraction of Natural Antioxidants from the Thelephora ganbajun Mushroom by an Ultrasound-Assisted Extraction Technique and Evaluation of Antiproliferative Activity of the Extract against Human Cancer Cells
Abstract
:1. Introduction
2. Results and Discussion
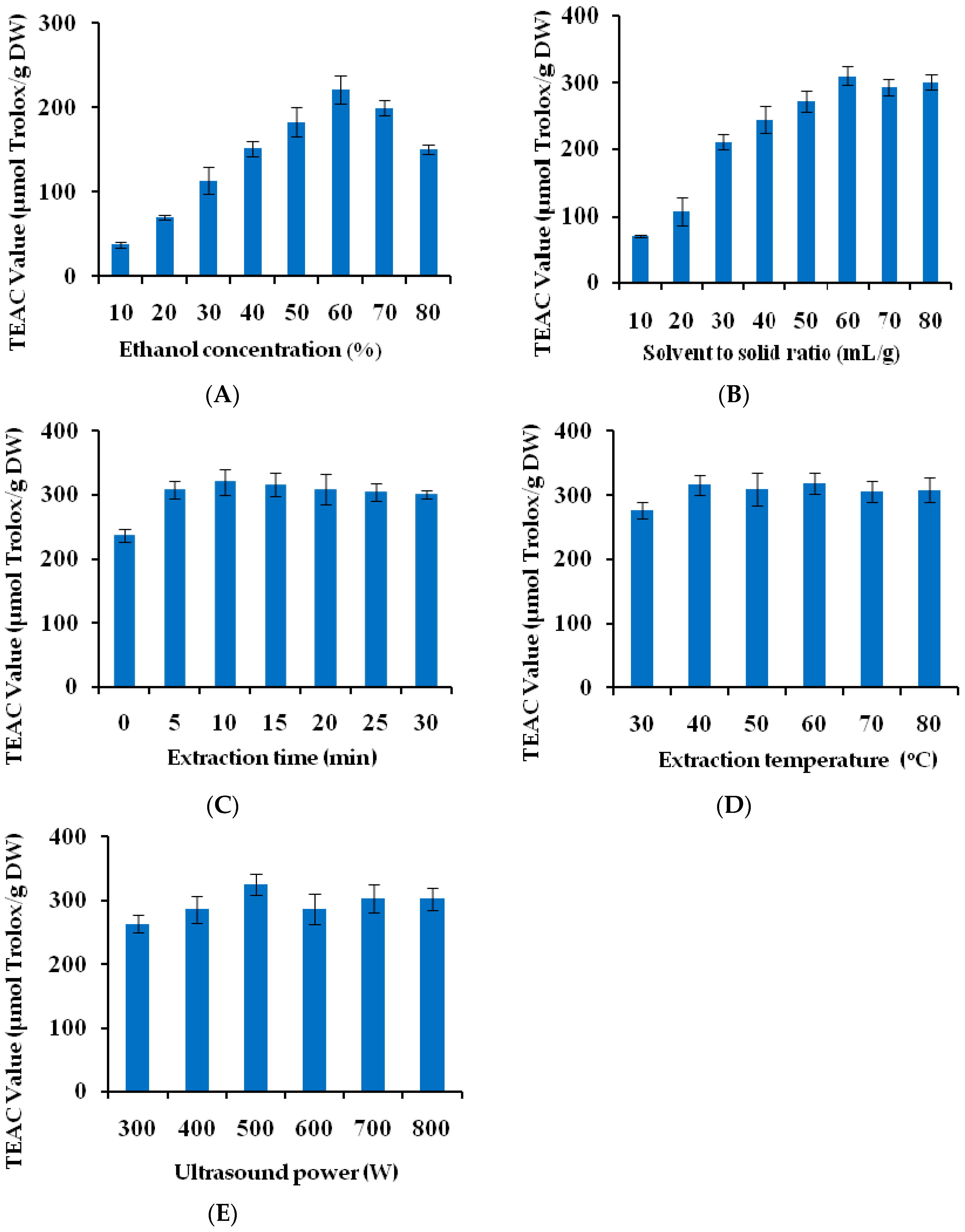
2.1. Single Factor Experimental Analysis
2.1.1. Influence of Ethanol Concentration
2.1.2. Influence of Solvent to Solid Ratio
2.1.3. Influence of Extraction Time
2.1.4. Influence of Temperature
2.1.5. Influence of Ultrasound Power
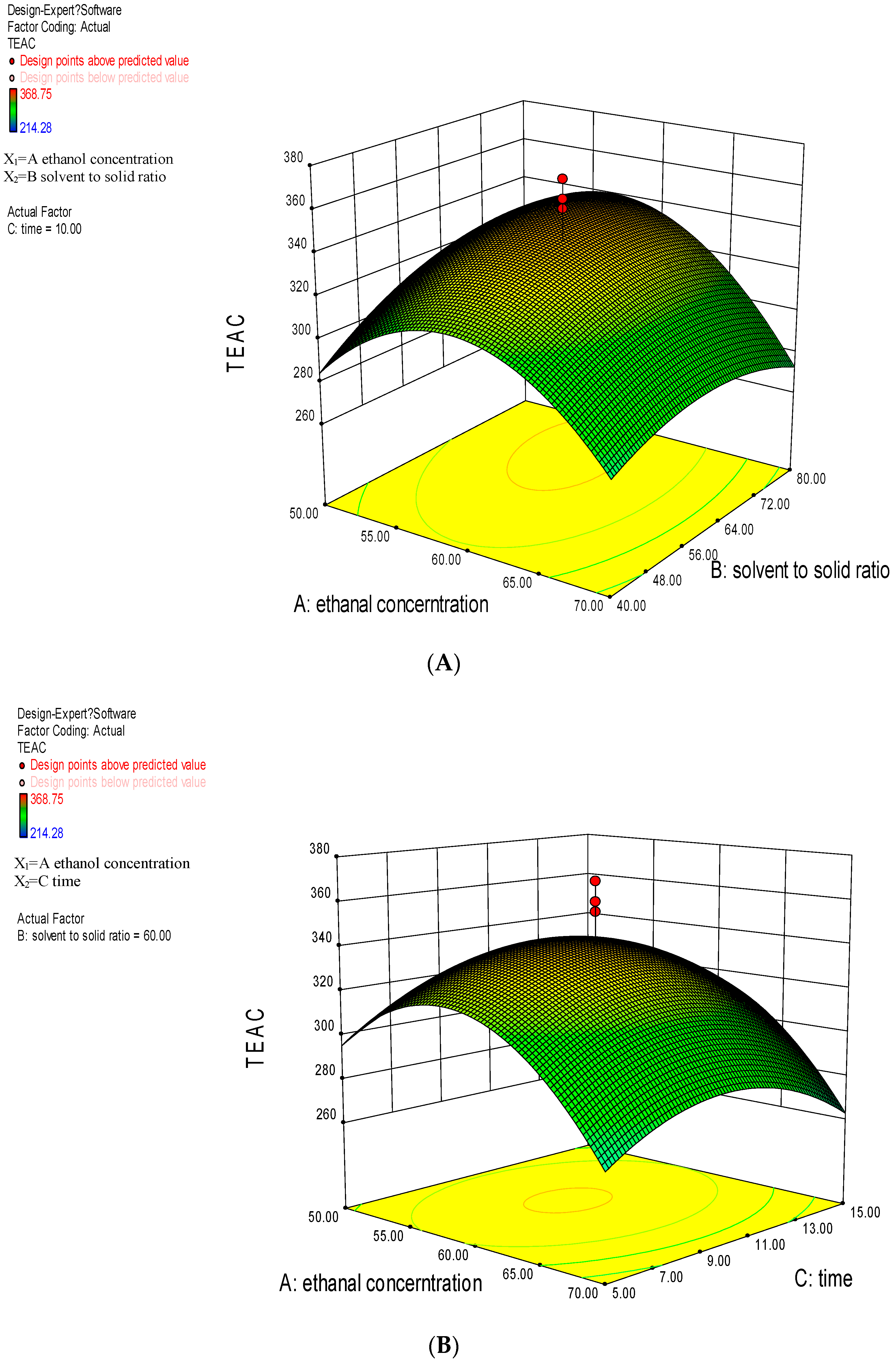
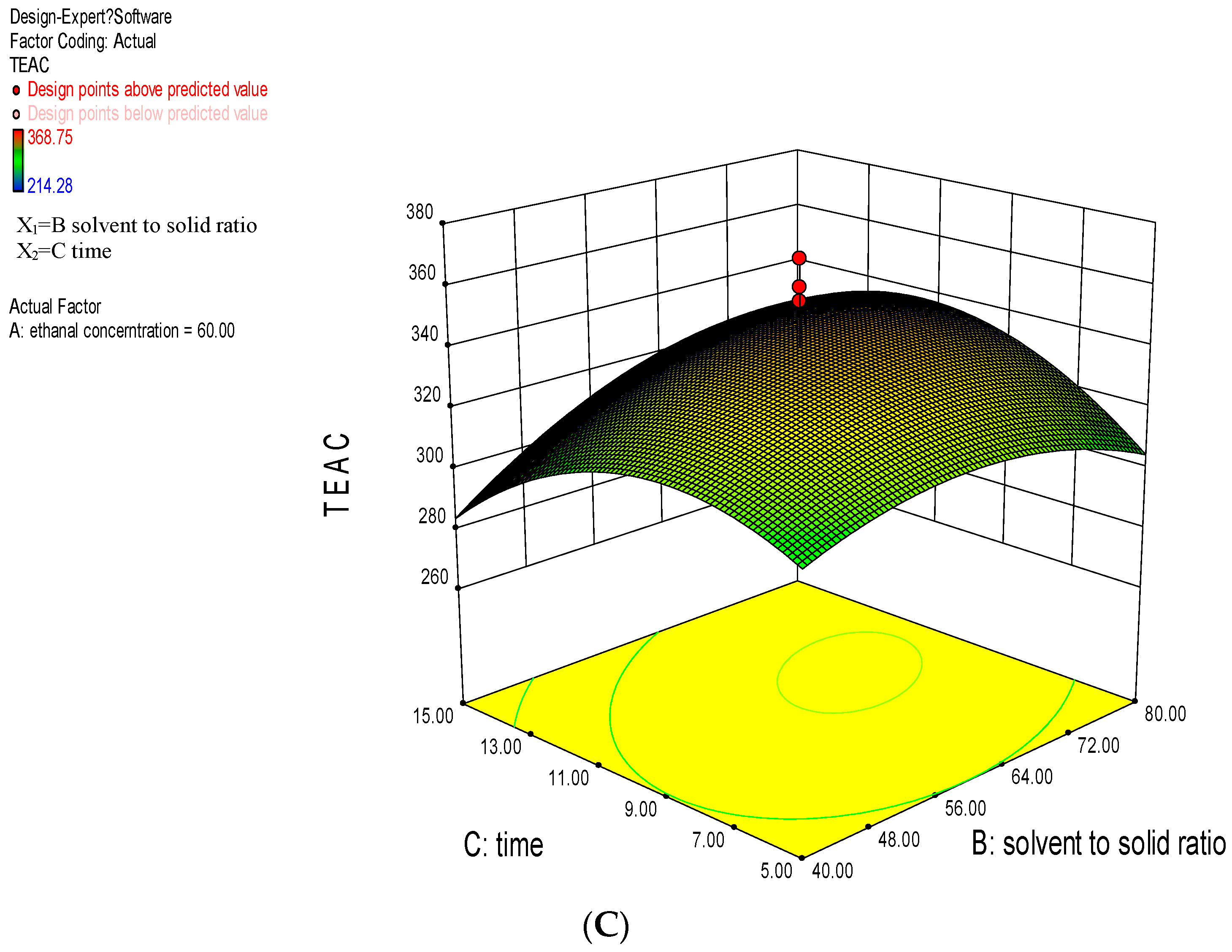
2.2. Response Surface Methodology Analysis
2.2.1. Model Fitting
2.2.2. Analysis of Response Surface Plots
2.2.3. Predicted Value Verification
2.2.4. Comparison of Ultrasound-Assisted Extraction and the Conventional Extraction Methods
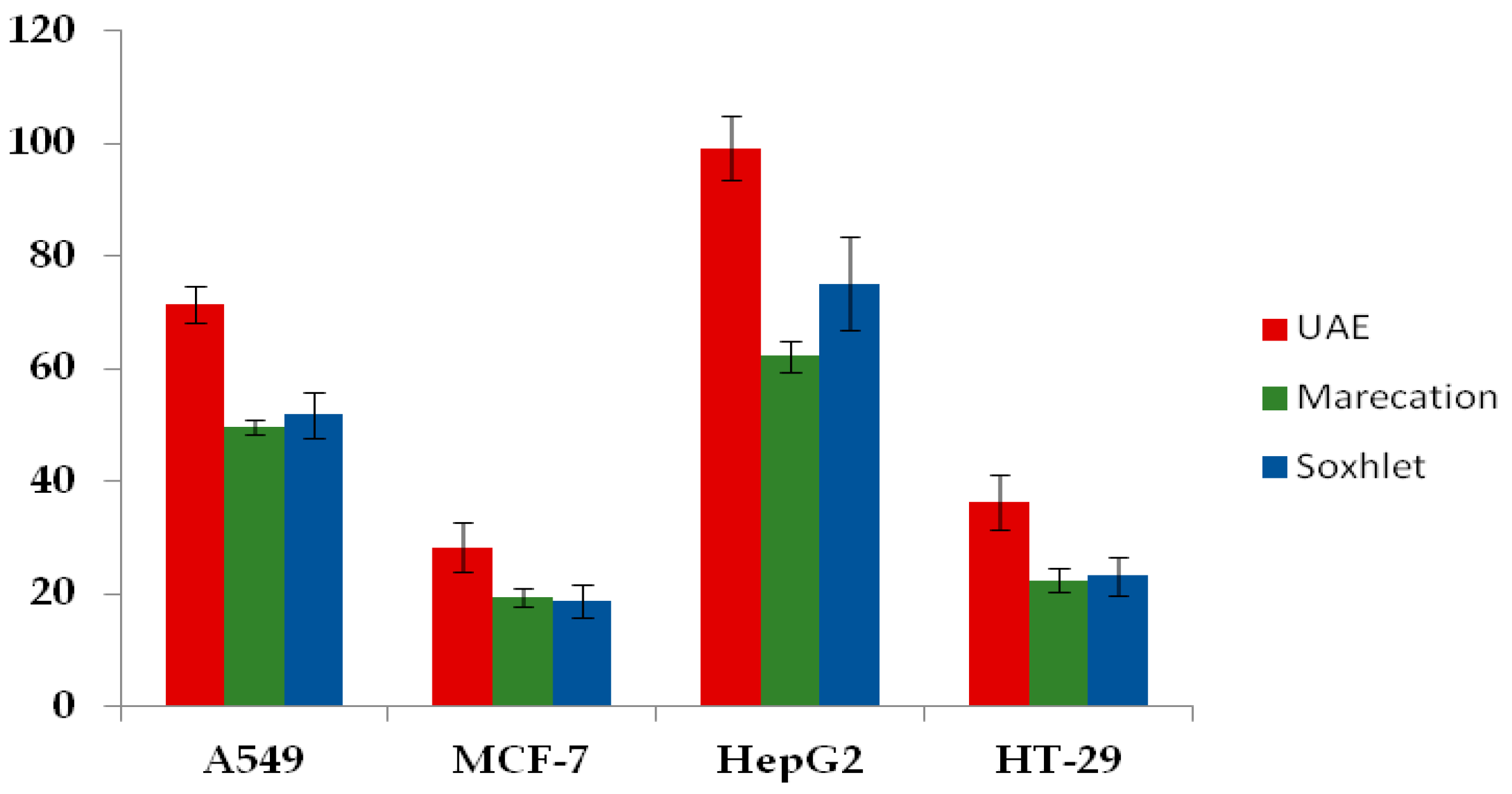
2.3. Antiproliferative Effects of Thelephora Ganbajun Extract
2.4. Polyphenolic Compound Profile in Thelephora Ganbajun Extract
3. Materials and Methods
3.1. Materials and Reagents
3.2. Extraction of Antioxidants
3.2.1. Ultrasound-Assisted Extraction
3.2.2. Maceration
3.2.3. Soxhlet Extraction
3.3. Experimental Design
3.3.1. Single-Factor Experiments
3.3.2. Response Surface Methodology
3.4. Measurement of Antioxidant Activity
3.5. Measurement of Total Phenolic Content
3.6. Measurement of Total Flavonoid Content
3.7. Evaluation of Antiproliferative Abilities of Extracts toward Four Human Cancer Cells
3.7.1. Sample Preparation
3.7.2. Cell Culture
3.7.3. Determination of Antiproliferative Effects
3.8. High-Performance Liquid Chromatography with Photodiode Array Detection (HPLC-PAD) Analysis
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Obrenovich, M.E.; Li, Y.; Parvathaneni, K.; Yendluri, B.B.; Palacios, H.H.; Leszek, J.; Aliev, G. Antioxidants in health, disease and aging. CNS Neurol. Disord. Drug Targets 2011, 10, 192–207. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, P.; Nandakumar, N.; Rengarajan, T.; Palaniswami, R.; Gnanadhas, E.N.; Lakshminarasaiah, U.; Gopas, J.; Nishigaki, I. Antioxidants and human diseases. Clin. Chim. Acta 2014, 436, 332–347. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.; Lee, S.H. Anticancer properties of capsaicin against human cancer. Anticancer Res. 2016, 36, 837–843. [Google Scholar] [PubMed]
- Wang, S.; Meckling, K.A.; Marcone, M.F.; Kakuda, Y.; Tsao, R. Can phytochemical antioxidant rich foods act as anti-cancer agents? Food Res. Int. 2011, 44, 2545–2554. [Google Scholar] [CrossRef]
- Subramanian, A.P.; John, A.A.; Vellayappan, M.V.; Balaji, A.; Jaganathan, S.K.; Supriyanto, E.; Yusof, M. Gallic acid: Prospects and molecular mechanisms of its anticancer activity. RSC Adv. 2015, 5, 35608–35621. [Google Scholar] [CrossRef]
- Yasueda, A.; Urushima, H.; Ito, T. Efficacy and interaction of antioxidant supplements as adjuvant therapy in cancer treatment: A systematic review. Integr. Cancer Ther. 2016, 15, 17–39. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhou, Z.M.; Yang, H.X.; Xu, J.C. Integrative management of commercialized wild mushroom: A case study of Thelephora ganbajun in Yunnan, southwest China. Environ. Manag. 2011, 48, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Luo, X.Y.; Wu, Y.; Chen, Y.M.; Xu, W.J.; Yi, X.X. Optimization of submerged fermentation of Thelephora ganbajun zang. J. Basic Microbiol. 2014, 54, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Sha, T.; Xu, J.P.; Palanichamy, M.G.; Zhang, H.B.; Li, T.; Zhao, Z.W.; Zhang, Y.P. Genetic diversity of the endemic gourmet mushroom Thelephora ganbajun from southwestern China. Microbiology 2008, 154, 3460–3468. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.M.; Liu, J.K.; Hu, L.; Dong, Z.J.; Wu, W.L.; Chen, Z.H. Antioxidant properties of natural p-terphenyl derivatives from the mushroom Thelephora ganbajun. Z. Naturforsch. C 2004, 59, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Gao, J.M.; Liu, J.K. Unusual poly(phenylacetyloxy)-substituted 1,1’: 4’,1”-terphenyl derivatives from fruiting bodies of the basidiomycete Thelephora ganbajun. Helv. Chim. Acta 2001, 84, 3342–3349. [Google Scholar] [CrossRef]
- Hu, L.; Liu, J.K. Two novel phenylacetoxylated p-terphenyls from Thelephora ganbajun Zang. Z. Naturforsch. C 2001, 56, 983–987. [Google Scholar]
- Guo, Y.J.; Deng, G.F.; Xu, X.R.; Wu, S.; Li, S.; Xia, E.Q.; Li, F.; Chen, F.; Ling, W.H.; Li, H.B. Antioxidant capacities, phenolic compounds and polysaccharide contents of 49 edible macro-fungi. Food Funct. 2012, 3, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.P.; Zhou, Y.; Zheng, J.; Li, S.; Li, A.N.; Li, H.B. Optimization of ultrasound-assisted extraction of natural antioxidants from the flower of Jatropha integerrima by response surface methodology. Molecules 2016, 21, 218. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Chen, H.; Zhou, X.; Deng, Q.; Zhao, Y.; Zhao, C.; Gong, X. Optimization of the microwave-assisted extraction and antioxidant activities of anthocyanins from blackberry using a response surface methodology. RSC Adv. 2015, 5, 19686–19695. [Google Scholar] [CrossRef]
- Mushtaq, M.; Sultana, B.; Bhatti, H.N.; Asghar, M. RSM based optimized enzyme-assisted extraction of antioxidant phenolics from underutilized watermelon (Citrullus lanatus Thunb.) rind. J. Food Sci. Technol. 2015, 52, 5048–5056. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Sanchez, J.; Castro-Puyana, M.; Mendiola, J.A.; Segura-Carretero, A.; Cifuentes, A.; Ibanez, E. Recovering bioactive compounds from olive oil filter cake by advanced extraction techniques. Int. J. Mol. Sci. 2014, 15, 16270–16283. [Google Scholar] [CrossRef] [PubMed]
- Heleno, S.A.; Diz, P.; Prieto, M.A.; Barros, L.; Rodrigues, A.; Barreiro, M.F.; Ferreira, I.C. Optimization of ultrasound-assisted extraction to obtain mycosterols from Agaricus bisporus L. by response surface methodology and comparison with conventional Soxhlet extraction. Food Chem. 2016, 197, 1054–1063. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Huang, J.; Wu, G.; Tong, J.H.; Xie, G.Y.; Duan, J.A.; Qin, M.J. Multiple responses optimization of ultrasonic-assisted extraction by response surface methodology (RSM) for rapid analysis of bioactive compounds in the flower head of Chrysanthemum morifolium Ramat. Ind. Crop. Prod. 2015, 74, 192–199. [Google Scholar] [CrossRef]
- Xu, J.L.; Wang, W.C.; Liang, H.; Zhang, Q.; Li, Q.Y. Optimization of ionic liquid based ultrasonic assisted extraction of antioxidant compounds from Curcuma Longa L. using response surface methodology. Ind. Crop. Prod. 2015, 76, 487–493. [Google Scholar] [CrossRef]
- Zou, T.B.; Wang, M.; Gan, R.Y.; Ling, W.H. Optimization of ultrasound-assisted extraction of anthocyanins from mulberry, using response surface methodology. Int. J. Mol. Sci. 2011, 12, 3006–3017. [Google Scholar] [CrossRef] [PubMed]
- Salarbashi, D.; Attaran Dowom, S.; Fazly Bazzaz, B.S.; Khanzadeh, F.; Soheili, V.; Mohammadpour, A. Evaluation, prediction and optimization the ultrasound-assisted extraction method using response surface methodology: Antioxidant and biological properties of Stachys parviflora L. Iran. J. Basic Med. Sci. 2016, 19, 829–841. [Google Scholar]
- Pradal, D.; Vauchel, P.; Decossin, S.; Dhulster, P.; Dimitrov, K. Kinetics of ultrasound-assisted extraction of antioxidant polyphenols from food by-products: Extraction and energy consumption optimization. Ultrason. Sonochem. 2016, 32, 137–146. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Zhang, L.L.; Yue, X.Y.; Liang, J.; Jiang, J.; Gao, X.L.; Yue, P.X. Optimization of ultrasound-assisted extraction of phenolic compounds and anthocyanins from blueberry (Vaccinium ashei) wine pomace. Food Chem. 2016, 204, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Vajic, U.J.; Grujic-Milanovic, J.; Zivkovic, J.; Savikin, K.; Godevac, D.; Miloradovic, Z.; Bugarski, B.; Mihailovic-Stanojevic, N. Optimization of extraction of stinging nettle leaf phenolic compounds using response surface methodology. Ind. Crop. Prod. 2015, 74, 912–917. [Google Scholar] [CrossRef]
- Akalin, M.K.; Tekin, K.; Akyuz, M.; Karagoz, S. Sage oil extraction and optimization by response surface methodology. Ind. Crop. Prod. 2015, 76, 829–835. [Google Scholar] [CrossRef]
- Dailey, A.; Vuong, Q.V. Optimization of aqueous extraction conditions for recovery of phenolic content and antioxidant properties from macadamia (Macadamia tetraphylla) skin waste. Antioxidants 2015, 4, 699–718. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.Y.; Bao, Y.H. Response Surface Optimization of extraction and antioxidant activity of total flavonoids from seed shell of Juglans mandshurica. Food Sci. Technol. Res. 2014, 20, 715–724. [Google Scholar] [CrossRef]
- Ramic, M.; Vidovic, S.; Zekovic, Z.; Viadic, J.; Cvejin, A.; Pavlic, B. Modeling and optimization of ultrasound-assisted extraction of polyphenolic compounds from Aronia melanocarpa by-products from filter-tea factory. Ultrason. Sonochem. 2015, 23, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wan, C.; Peng, X.; Chen, Y.; Chen, M.; Chen, J. Optimization of antifungal extracts from Ficus hirta fruits using response surface methodology and antifungal activity tests. Molecules 2015, 20, 19647–19659. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; You, L.J.; Abbasi, A.M.; Fu, X.; Liu, R.H. Optimization for ultrasound extraction of polysaccharides from mulberry fruits with antioxidant and hyperglycemic activity in vitro. Carbohydr. Polym. 2015, 130, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.; Ghavidel, R.A.; Sheikholeslami, Z.; Elahi, M.; Elahi, S. Optimization of ultrasonic assisted extraction of antioxidants and phenolic compounds from red basil using response surface methodology. Agro Food Ind. Hi Tech 2015, 26, 28–32. [Google Scholar]
- Szydlowska-Czerniak, A.; Tulodziecka, A. Optimization of ultrasound-assisted extraction procedure to determine antioxidant capacity of rapeseed cultivars. Food Anal. Meth. 2015, 8, 778–789. [Google Scholar] [CrossRef]
- Pan, G.; Yu, G.; Zhu, C.; Qiao, J. Optimization of ultrasound-assisted extraction (UAE) of flavonoids compounds (FC) from hawthorn seed (HS). Ultrason. Sonochem. 2012, 19, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Olsson, M.E.; Gustavsson, K.E.; Andersson, S.; Nilsson, A.; Duan, R.D. Inhibition of cancer cell proliferation in vitro by fruit and berry extracts and correlations with antioxidant levels. J. Agric. Food Chem. 2004, 52, 7264–7271. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Alonso, J.; Ros, G.; Periago, M.J. Antiproliferative and cytoprotective activities of a phenolic-rich juice in HepG2 cells. Food Res. Int. 2006, 39, 982–991. [Google Scholar] [CrossRef]
- Valentao, P.; Andrade, P.B.; Rangel, J.; Ribeiro, B.; Silva, B.M.; Baptista, P.; Seabra, R.M. Effect of the conservation procedure on the contents of phenolic compounds and organic acids in chanterelle (Cantharellus cibarius) mushroom. J. Agric. Food Chem. 2005, 53, 4925–4931. [Google Scholar] [CrossRef] [PubMed]
- Li, A.N.; Li, S.; Li, H.B.; Xu, D.P.; Xu, X.R.; Chen, F. Total phenolic contents and antioxidant capacities of 51 edible and wild flowers. J. Funct. Food. 2014, 6, 319–330. [Google Scholar] [CrossRef]
- Kalia, K.; Sharma, K.; Singh, H.P.; Singh, B. Effects of extraction methods on phenolic contents and antioxidant activity in aerial parts of Potentilla atrosanguinea Lodd. and quantification of its phenolic constituents by RP-HPLC. J. Agric. Food Chem. 2008, 56, 10129–10134. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Li, S.; Li, H.B.; Deng, G.F.; Ling, W.H.; Wu, S.; Xu, X.R.; Chen, F. Antiproliferative activity of peels, pulps and seeds of 61 fruits. J. Funct. Food 2013, 5, 1298–1309. [Google Scholar] [CrossRef]
- Cai, Y.Z.; Luo, Q.; Sun, M.; Corke, H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004, 74, 2157–2184. [Google Scholar] [CrossRef] [PubMed]
| Run | X1 (Concentration of Ethanol, %) | X2 (Solvent to Solid Ratio, mL/g) | X3 (Ultrasound Time, min) | Y (TEAC Value, µmol Trolox/g DW) |
|---|---|---|---|---|
| 1 | 70 | 40 | 5 | 263.08 |
| 2 | 50 | 40 | 5 | 259.96 |
| 3 | 70 | 40 | 15 | 214.3 |
| 4 | 50 | 80 | 5 | 312.87 |
| 5 | 60 | 60 | 10 | 314.54 |
| 6 | 60 | 26.36 | 10 | 307.88 |
| 7 | 43.18 | 60 | 10 | 247.7 |
| 8 | 60 | 60 | 1.59 | 279.67 |
| 9 | 60 | 60 | 10 | 336.34 |
| 10 | 50 | 40 | 15 | 255.81 |
| 11 | 70 | 80 | 5 | 236.27 |
| 12 | 76.82 | 60 | 10 | 214.28 |
| 13 | 60 | 60 | 10 | 355.12 |
| 14 | 70 | 80 | 15 | 273.64 |
| 15 | 60 | 60 | 10 | 368.75 |
| 16 | 60 | 60 | 10 | 307.28 |
| 17 | 50 | 80 | 15 | 312.87 |
| 18 | 60 | 60 | 18.41 | 281.12 |
| 19 | 60 | 93.64 | 10 | 300.01 |
| 20 | 60 | 60 | 10 | 359.59 |
| Source | Sum of Squares | df | Mean Square | F Value | p Value | Significant |
|---|---|---|---|---|---|---|
| Model | 33,516.29 | 9 | 3724.03 | 6.57 | 0.0035 | significant |
| X1 (ethanol concentration) | 3242.25 | 1 | 3242.25 | 5.72 | 0.0379 | |
| X2 (solvent to solid ratio) | 1223.51 | 1 | 1223.51 | 2.16 | 0.1727 | |
| X3 (extraction time) | 12.61 | 1 | 12.61 | 0.022 | 0.8845 | |
| X1X2 | 749.62 | 1 | 749.62 | 1.32 | 0.2771 | |
| X1X3 | 6.59 | 1 | 6.59 | 0.012 | 0.9163 | |
| X2X3 | 1019.26 | 1 | 1019.26 | 1.8 | 0.2097 | |
| X12 | 21,871.78 | 1 | 21,871.78 | 38.56 | 0.0001 | |
| X22 | 2497.34 | 1 | 2497.34 | 4.40 | 0.0623 | |
| X32 | 6655.52 | 1 | 6655.52 | 11.73 | 0.0065 | |
| Residual | 5672.24 | 10 | 567.22 | |||
| Lack of fit | 2501.52 | 5 | 500.30 | 0.79 | 0.5994 | not significant |
| Pure error | 3170.71 | 5 | 634.14 | |||
| Cor total | 39,188.53 | 19 | ||||
| R-squared | 0.8553 | |||||
| Adj R-squared | 0.7250 |
| Extracting Method | Ethanol Concerntration | Temperature | Time | TEAC Value (µmol Trolox/g DW) | TPC (mg GAE/g) | TFC (mg QE/g) |
|---|---|---|---|---|---|---|
| UAE | 57.38% | 40 °C | 10.58 min | 346.98 ± 12.19 | 91.51 ± 4.38 | 5.90 ± 0.27 |
| Maceration extraction | 57.38% | 25 °C | 24 h | 204.34 ± 7.86 | 70.06 ± 3.63 | 3.84 ± 0.11 |
| Soxhlet extraction | 57.38% | 95 °C | 4 h | 276.76 ± 16.39 | 72.68 ± 2.99 | 3.87 ± 0.12 |
| Component | Retention Time (min) | Wavelength (nm) | Content (mg/kg DW) |
|---|---|---|---|
| Epicatechin | 23.306 | 280 | 11.28 ± 1.56 |
| Rutin | 32.481 | 250 | 122.81 ± 5.23 |
| 2-Hydrocinnamic acid | 35.410 | 280 | 11.90 ± 1.02 |
| Unknown 1 | 15.855 | 260 | 9.14% * |
| Unknown 2 | 23.775 | 280 | 6.89% * |
| Unknown 3 | 38.166 | 320 | 9.72% * |
| Unknown 4 | 42.639 | 260 | 8.73% * |
| Variable | Units | Symbol | Code Levels | ||||
|---|---|---|---|---|---|---|---|
| −1.68 | −1 | 0 | 1 | 1.68 | |||
| Ethanol concentration | % (v/v) | X1 | 43.18 | 50 | 60 | 70 | 76.82 |
| Solvent to solid ratio | mL/g | X2 | 26.36 | 40 | 60 | 80 | 93.64 |
| Extraction time | min | X3 | 1.59 | 5 | 10 | 15 | 18.41 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, D.-P.; Zheng, J.; Zhou, Y.; Li, Y.; Li, S.; Li, H.-B. Extraction of Natural Antioxidants from the Thelephora ganbajun Mushroom by an Ultrasound-Assisted Extraction Technique and Evaluation of Antiproliferative Activity of the Extract against Human Cancer Cells. Int. J. Mol. Sci. 2016, 17, 1664. https://doi.org/10.3390/ijms17101664
Xu D-P, Zheng J, Zhou Y, Li Y, Li S, Li H-B. Extraction of Natural Antioxidants from the Thelephora ganbajun Mushroom by an Ultrasound-Assisted Extraction Technique and Evaluation of Antiproliferative Activity of the Extract against Human Cancer Cells. International Journal of Molecular Sciences. 2016; 17(10):1664. https://doi.org/10.3390/ijms17101664
Chicago/Turabian StyleXu, Dong-Ping, Jie Zheng, Yue Zhou, Ya Li, Sha Li, and Hua-Bin Li. 2016. "Extraction of Natural Antioxidants from the Thelephora ganbajun Mushroom by an Ultrasound-Assisted Extraction Technique and Evaluation of Antiproliferative Activity of the Extract against Human Cancer Cells" International Journal of Molecular Sciences 17, no. 10: 1664. https://doi.org/10.3390/ijms17101664
APA StyleXu, D.-P., Zheng, J., Zhou, Y., Li, Y., Li, S., & Li, H.-B. (2016). Extraction of Natural Antioxidants from the Thelephora ganbajun Mushroom by an Ultrasound-Assisted Extraction Technique and Evaluation of Antiproliferative Activity of the Extract against Human Cancer Cells. International Journal of Molecular Sciences, 17(10), 1664. https://doi.org/10.3390/ijms17101664