Cadmium Protection Strategies—A Hidden Trade-Off?
Abstract
:1. Introduction
2. Results and Discussion
2.1. Protection via Antioxidants
| Substance | Source | [Cd]/Duration/Experimental Animal | References |
|---|---|---|---|
| Curcumin a,b | Turmeric (Curcuma longa L.) | 24 h Cd exposure, in vivo, rodents In vitro, human airway epithelial cells | [20,21,22,23] |
| Ginger | Ginger (Zingiber officinale) | 200 mg/kg b.w., 12 weeks, in vivo, rabbits | [24] |
| Resveratrol b | Polyphenol from skin of grapes (Vitis vinifera) | 7 mg/kg b.w., 24 h exposure, in vivo, mice | [21] |
| Physalis extract | Physalis peruviana L. | 6.5 mg/kg b.w., 5 days, in vivo, rats | [25] |
| Grapefruit juice a | Grapefruit | 1.5 mg/kg b.w., from day 7 of gestation until day 17 of pregnancy, in vivo, mice | [26] |
| Garlic extract or Allicin b | Garlic | 5 or 10 ppm, 45 days, in vivo, Freshwater catfish (Clarias batrachus) | [27] |
| Royal jelly a | from Honey bees | 2 mg/kg b.w., 6–7 weeks, in vivo, mice | [28] |
| Spirulina a | Micro-algae spirulina (Arthrospira maxima) | 1.5 mg/kg b.w., 1 time Cd challenge, in vivo, pregnant mice; 3.5 mg/kg b.w., 1 time Cd intraperitoneal dose, in vivo, rats | [29,30] |
| Farnesol a | Isoprenoid from aromatic plants | 5 mg/kg b.w., 1 time Cd, in vivo, mice | [31] |
| Theaflavin | Polyphenol from black tea (Camellia sinensis) | 0.4 mg/kg b.w., once a day, for 5 weeks, in vivo, rats | [32] |
| Taxifolin | Bioflavonoid from conifers | 100 μM Cd, in vivo, Zebrafish (Danio rerio) | [33] |
| Quercetin | Bioflavonoid from apples and onions | 4 mg/kg b.w. for 2 weeks, in vivo, mice; 1.2 mg Cd/kg/day, 5 times/week during nine weeks, in vivo, rats 5 µM, in vitro, in cultured granulosa cells from chicken ovarian follicles | [34,35,36] |
| Naringenin | Bioflavonoid from grapefruit | 5 mg/kg, orally for 4 weeks, in vivo, rats | [37] |
| Rosemary extract b | Rosmarinus officinalis L. | 30 mg/kg b.w., 5 consecutive days/week for 8 weeks, in vivo, rats | [38] |
| Catechin a,b | Polyphenol from Green tea (Camellia sinensis) | 50 ppm ad libitum, 20 weeks, in vivo, rats | [39] |
| Sulforaphane a,b | Isothiocyanate from cruciferous vegetables | In vitro in human hepatocytes and in vivo in mice; 0.2 mg/kg, 15 days, in vivo, rats | [40,41] |
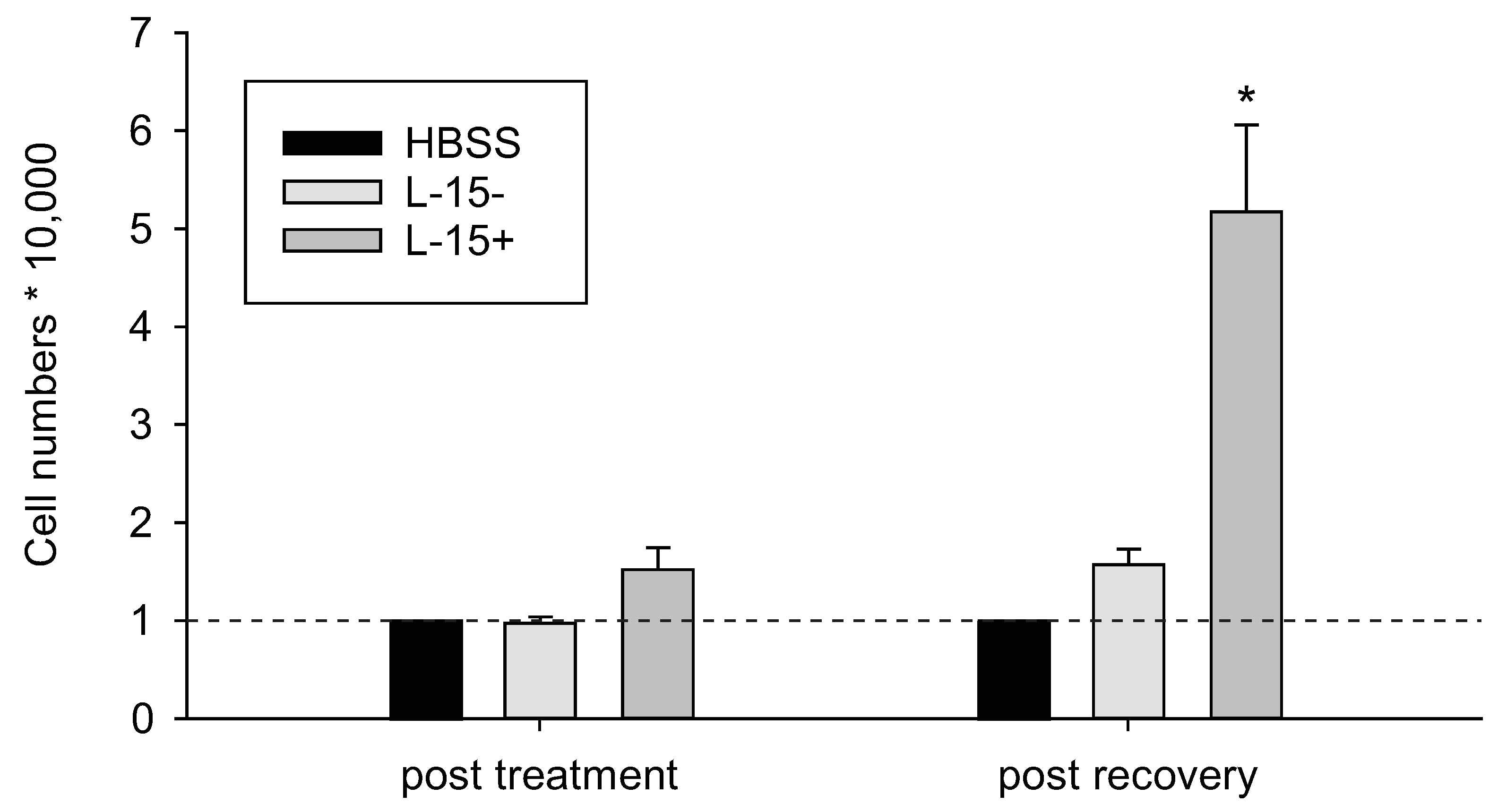
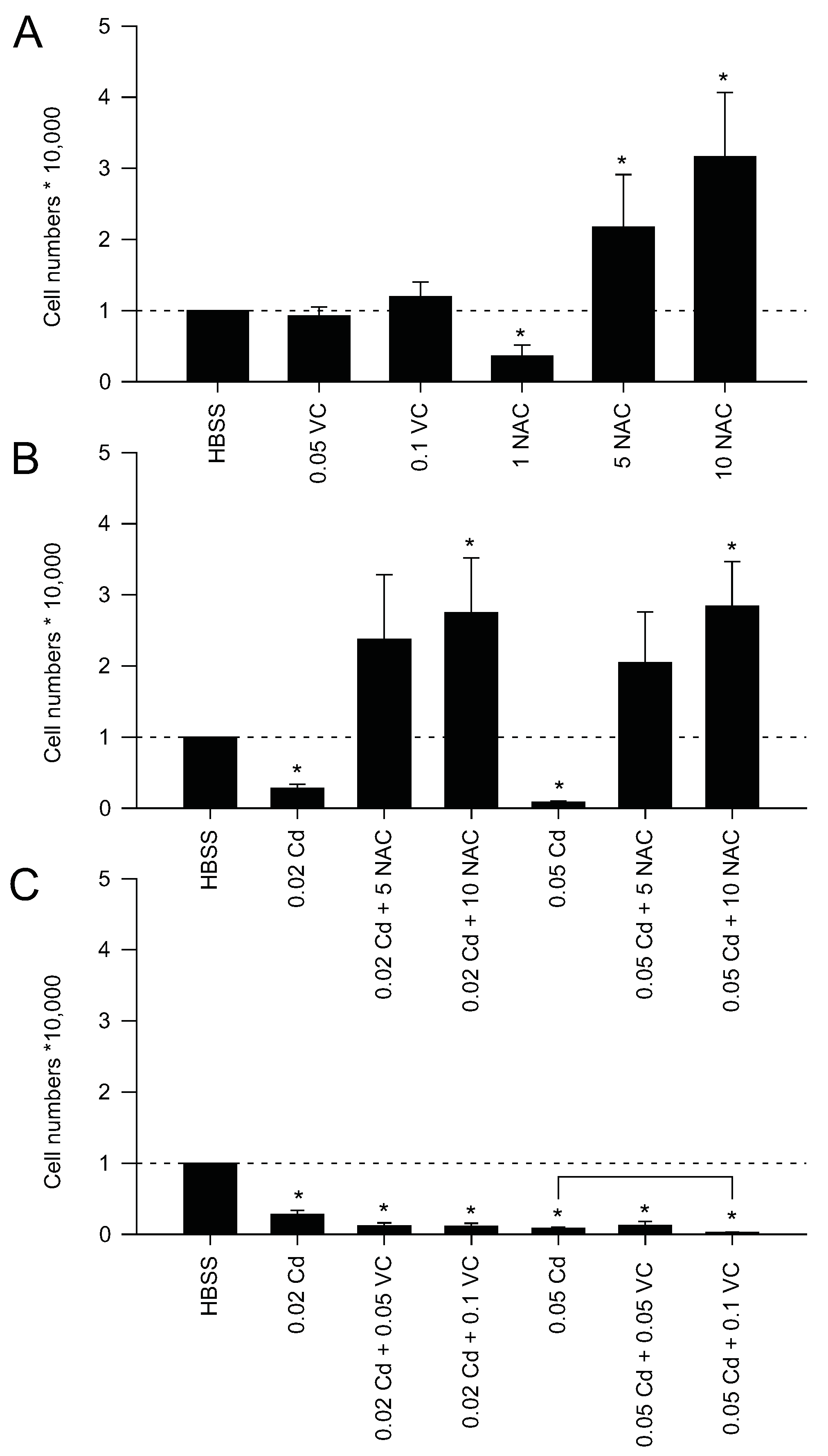
2.2. Mitochondrial Protection Counteracts Cd Insult
2.3. Protection by Metal Chelation
2.4. Protection against Macromolecular Damage
2.5. Cd Resistance and Cytoskeletal Rearrangements
2.6. Protection against Cd by Cd—Hormetic Responses
2.7. Protective Effect by Co-Exposure to Other Metals or Trace Elements
2.8. Protection by Reduced Uptake of Cd
2.9. Protection through Removal of Cd
2.10. Toxicity of Cd by Altered Environmental Factors
3. Experimental Section
3.1. Cell Culture
3.2. Treatments
3.3. Cell Density Assay
3.4. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Järup, L.; Akesson, A. Current status of Cd as an environmental health problem. Toxicol. Appl. Pharmacol. 2009, 238, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Choong, G.; Liu, Y.; Templeton, D.M. Interplay of Calcium and cadmium in mediating cadmium toxicity. Chem. Biol. Interact. 2014, 211, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Gardarin, A.; Chédin, S.; Lagniel, G.; Aude, J.-C.; Godat, E.; Catty, P.; Labarre, J. Endoplasmic reticulum is a major target of cadmium toxicity in yeast. Mol. Microbiol. 2010, 76, 1034–1048. [Google Scholar] [CrossRef] [PubMed]
- Stohs, S.J.; Bagchi, D. Oxidative mechanisms in the toxicity of metal ions. Free Radic. Biol. Med. 1995, 18, 321–336. [Google Scholar] [CrossRef]
- Valko, M.; Morris, H.; Cronin, M.T.D. Metals, toxicity and oxidative stress. Curr. Med. Chem. 2005, 12, 1161–1208. [Google Scholar] [CrossRef] [PubMed]
- Stohs, S.J.; Bagchi, D.; Hassoun, E.; Bagchi, M. Oxidative mechanisms in the toxicity of chromium and cadmium ions. J. Environ. Pathol. Toxicol. Oncol. 2000, 19, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Singhal, R.K.; Anderson, M.E.; Meister, A. Glutathione, a first line of defense against cadmium toxicity. FASEB J. 1987, 1, 220–223. [Google Scholar] [PubMed]
- Rana, S.V.S.; Singh, R. Influence of antioxidants on metallothionein-mediated protection in cadmium-fed rats. Biol. Trace Elem. Res. 2002, 88, 71–78. [Google Scholar] [CrossRef]
- Chubatsu, L.S.; Gennari, M.; Meneghini, R. Glutathione is the antioxidant responsible for resistance to oxidative stress in V79 Chinese hamster fibroblasts rendered resistant to cadmium. Chem. Biol. Interact. 1992, 82, 99–110. [Google Scholar] [CrossRef]
- Nair, A.R.; Lee, W.-K.; Smeets, K.; Swennen, Q.; Sanchez, A.; Thévenod, F.; Cuypers, A. Glutathione and mitochondria determine acute defense responses and adaptive processes in cadmium-induced oxidative stress and toxicity of the kidney. Arch. Toxicol. 2015, 89, 2273–2289. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, A.; Nishimoto, M.; Himeno, S.; Imura, N.; Tsujimoto, M.; Kunimoto, M.; Hara, S. Transcriptional regulation of thioredoxin reductase 1 expression by cadmium in vascular endothelial cells: Role of NF-E2-related factor-2. J. Cell. Physiol. 2005, 203, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Shaikh, Z.A. Activation of Nrf2 by cadmium and its role in protection against cadmium-induced apoptosis in rat kidney cells. Toxicol. Appl. Pharmacol. 2009, 241, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Arini, A.; Gourves, P.Y.; Gonzalez, P.; Baudrimont, M. Metal detoxification and gene expression regulation after a Cd and Zn contamination: An experimental study on Danio rerio. Chemosphere 2015, 128, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, W.; Su, Z.-Y.; Kong, A.-N.T. The complexity of the Nrf2 pathway: Beyond the antioxidant response. J. Nutr. Biochem. 2015, 26, 1401–1413. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q. Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Qu, W.; Kadiiska, M.B. Role of oxidative stress in cadmium toxicity and carcinogenesis. Toxicol. Appl. Pharmacol. 2009, 238, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Cuypers, A.; Plusquin, M.; Remans, T.; Jozefczak, M.; Keunen, E.; Gielen, H.; Opdenakker, K.; Nair, A.R.; Munters, E.; Artois, T.J.; et al. Cadmium stress: An oxidative challenge. BioMetals 2010, 23, 927–940. [Google Scholar] [CrossRef] [PubMed]
- Bertin, G.; Averbeck, D. Cadmium: Cellular effects, modifications of biomolecules, modulation of DNA repair and genotoxic consequences (a review). Biochimie 2006, 88, 1549–1559. [Google Scholar] [CrossRef] [PubMed]
- Bravard, A.; Campalans, A.; Vacher, M.; Gouget, B.; Levalois, C.; Chevillard, S.; Radicella, J.P. Inactivation by oxidation and recruitment into stress granules of hOGG1 but not APE1 in human cells exposed to sub-lethal concentrations of cadmium. Mutat. Res. 2010, 685, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Eybl, V.; Kotyzová, D.; Bludovská, M. The effect of curcumin on cadmium-induced oxidative damage and trace elements level in the liver of rats and mice. Toxicol. Lett. 2004, 151, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Eybl, V.; Kotyzova, D.; Koutensky, J. Comparative study of natural antioxidants—Curcumin, resveratrol and melatonin—In cadmium-induced oxidative damage in mice. Toxicology 2006, 225, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Daniel, S.; Limson, J.L.; Dairam, A.; Watkins, G.M.; Daya, S. Through metal binding, curcumin protects against lead- and cadmium-induced lipid peroxidation in rat brain homogenates and against lead-induced tissue damage in rat brain. J. Inorg. Biochem. 2004, 98, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Rennolds, J.; Malireddy, S.; Hassan, F.; Tridandapani, S.; Parinandi, N.; Boyaka, P.N.; Cormet-Boyaka, E. Curcumin regulates airway epithelial cell cytokine responses to the pollutant cadmium. Biochem. Biophys. Res. Commun. 2012, 417, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Baiomy, A.A.; Mansour, A.A. Genetic and histopathological responses to cadmium toxicity in rabbit’s kidney and liver: Protection by Ginger (Zingiber officinale). Biol. Trace Elem. Res. 2015, in press. [Google Scholar]
- Abdel Moneim, A.E.; Bauomy, A.A.; Diab, M.M.S.; Shata, M.T.M.; Al-Olayan, E.M.; El-Khadragy, M.F. The protective effect of Physalis peruviana L. against cadmium-induced neurotoxicity in rats. Biol. Trace Elem. Res. 2014, 160, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Argüelles, N.; Alvarez-González, I.; Chamorro, G.; Madrigal-Bujaidar, E. Protective effect of grapefruit juice on the teratogenic and genotoxic damage induced by cadmium in mice. J. Med. Food 2012, 15, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Prasad, Y.; Patra, A.K.; Ranjan, R.; Swarup, D.; Patra, R.C.; Pal, S. Ascorbic acid, garlic extract and taurine alleviate cadmium-induced oxidative stress in freshwater catfish (Clarias batrachus). Sci. Total Environ. 2009, 407, 5024–5030. [Google Scholar] [CrossRef] [PubMed]
- Cavuşoğlu, K.; Yapar, K.; Yalçin, E. Royal jelly (honey bee) is a potential antioxidant against cadmium-induced genotoxicity and oxidative stress in albino mice. J. Med. Food 2009, 12, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Argüelles-Velázquez, N.; Alvarez-González, I.; Madrigal-Bujaidar, E.; Chamorro-Cevallos, G. Amelioration of cadmium-produced teratogenicity and genotoxicity in mice given Arthrospira maxima (Spirulina) Treatment. Evid. Based Complement. Altern. Med. 2013, 2013, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Paniagua-Castro, N.; Escalona-Cardoso, G.; Hernández-Navarro, D.; Pérez-Pastén, R.; Chamorro-Cevallos, G. Spirulina (Arthrospira) protects against cadmium-induced teratogenic damage in mice. J. Med. Food 2011, 14, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Jahangir, T.; Khan, T.H.; Prasad, L.; Sultana, S. Alleviation of free radical mediated oxidative and genotoxic effects of cadmium by farnesol in Swiss albino mice. Redox Rep. 2005, 10, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Sun, Y.; Liu, J.; Wang, J.; Li, Y.; Li, H.; Zhang, W.; Liao, H. Protective effect of theaflavins on cadmium-induced testicular toxicity in male rats. Food Chem. Toxicol. 2012, 50, 3243–3250. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, M.; Jayaraj, R.L.; Jagatheesh, K.; Elangovan, N. Taxifolin mitigates oxidative DNA damage in vitro and protects zebrafish (Danio rerio) embryos against cadmium toxicity. Environ. Toxicol. Pharmacol. 2015, 39, 1252–1261. [Google Scholar]
- Jia, Y.; Lin, J.; Mi, Y.; Zhang, C. Quercetin attenuates cadmium-induced oxidative damage and apoptosis in granulosa cells from chicken ovarian follicles. Reprod. Toxicol. 2011, 31, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Sánchez, C.; Egido, J.; Sánchez-González, P.D.; Pérez-Barriocanal, F.; López-Novoa, J.M.; Morales, A.I. Effect of the flavonoid quercetin on cadmium-induced hepatotoxicity. Food Chem. Toxicol. 2008, 46, 2279–2287. [Google Scholar] [CrossRef] [PubMed]
- Bu, T.; Mi, Y.; Zeng, W.; Zhang, C. Protective effect of quercetin on cadmium-induced oxidative toxicity on germ cells in male mice. Anat. Rec. 2011, 294, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Renugadevi, J.; Prabu, S.M. Cadmium-induced hepatotoxicity in rats and the protective effect of naringenin. Exp. Toxicol. Pathol. 2010, 62, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Sakr, S.A.; Bayomy, M.F.; El-Morsy, A.M. Rosemary extract ameliorates cadmium-induced histological changes and oxidative damage in the liver of albino rat. J. Basic Appl. Zool. 2015, 71, 1–9. [Google Scholar] [CrossRef]
- Choi, J.-H.; Rhee, I.-K.; Park, K.-Y.; Park, K.-Y.; Kim, J.-K.; Rhee, S.-J. Action of green tea catechin on bone metabolic disorder in chronic cadmium-poisoned rats. Life Sci. 2003, 73, 1479–1489. [Google Scholar] [CrossRef]
- Jahan, S.; Khan, M.; Ahmed, S.; Ullah, H. Comparative analysis of antioxidants against cadmium induced reproductive toxicity in adult male rats. Syst. Biol. Reprod. Med. 2014, 60, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; He, Y.; Yu, G.; Li, B.; Sexton, D.W.; Wileman, T.; Roberts, A.A.; Hamilton, C.J.; Liu, R.; Chao, Y.; et al. Sulforaphane protects the liver against CdSe quantum dot-induced cytotoxicity. PLoS ONE 2015, 10, e0138771. [Google Scholar]
- Su, Z.-Y.; Shu, L.; Khor, T.O.; Lee, J.H.; Fuentes, F.; Kong, A.-N.T. A perspective on dietary phytochemicals and cancer chemoprevention: Oxidative stress, Nrf2, and epigenomics. Top. Curr. Chem. 2013, 329, 133–162. [Google Scholar] [PubMed]
- El-Sokkary, G.H.; Nafady, A.A.; Shabash, E.H. Melatonin administration ameliorates cadmium-induced oxidative stress and morphological changes in the liver of rat. Ecotoxicol. Environ. Saf. 2010, 73, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.Y.; Lee, M.J.; Lee, S.M.; Lee, W.C.; Kim, J.S. Effect of melatonin on cadmium-induced hepatotoxicity in male Sprague–Dawley rats. Tohoku J. Exp. Med. 1998, 186, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Pi, H.; Xu, S.; Reiter, R.J.; Guo, P.; Zhang, L.; Li, Y.; Li, M.; Cao, Z.; Tian, L.; Xie, J.; et al. SIRT3-SOD2-mROS-dependent autophagy in cadmium-induced hepatotoxicity and salvage by melatonin. Autophagy 2015, 11, 1037–1051. [Google Scholar] [CrossRef] [PubMed]
- García, M.T.A.; González, E.L.M. Natural antioxidants protect against cadmium-induced damage during pregnancy and lactation in rats’ pups. J. Food Sci. 2010, 75, T18–T23. [Google Scholar] [CrossRef] [PubMed]
- Ognjanović, B.I.; Pavlović, S.Z.; Maletić, S.D.; Zikić, R.V.; Stajn, A.S.; Radojicić, R.M.; Saicić, Z.S.; Petrović, V.M. Protective influence of vitamin E on antioxidant defense system in the blood of rats treated with cadmium. Physiol. Res. 2003, 52, 563–570. [Google Scholar] [PubMed]
- Novelli, J.; Novelli, E.L.B.; Manzano, M.A.; Lopes, A.M.; Cataneo, A.C.; Barbosa, L.L.; Ribas, B.O. Effect of α-tocopherol on superoxide radical and toxicity of cadmium exposure. Int. J. Environ. Health Res. 2000, 10, 125–134. [Google Scholar] [CrossRef]
- El-Sokkary, G.H.; Awadalla, E.A. The protective role of Vitamin C against cerebral and pulmonary damage induced by cadmium chloride in male adult albino rat. Open Neuroendocrinol. J. 2011, 4, 1–8. [Google Scholar] [CrossRef]
- Liu, T.; He, W.; Yan, C.; Qi, Y.; Zhang, Y. Roles of reactive oxygen species and mitochondria in cadmium-induced injury of liver cells. Toxicol. Ind. Health 2011, 27, 249–256. [Google Scholar] [PubMed]
- Abe, T.; Yamamura, K.; Gotoh, S.; Kashimura, M.; Higashi, K. Concentration-dependent differential effects of N-acetyl-l-cysteine on the expression of HSP70 and metallothionein genes induced by cadmium in human amniotic cells. Biochim. Biophys. Acta 1998, 1380, 123–132. [Google Scholar] [CrossRef]
- Odewumi, C.O.; Badisa, V.L.D.; Le, U.T.; Latinwo, L.M.; Ikediobi, C.O.; Badisa, R.B.; Darling-Reed, S.F. Protective effects of N-acetylcysteine against cadmium-induced damage in cultured rat normal liver cells. Int. J. Mol. Med. 2010, 27, 1193–1205. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhu, H.; Liu, X.; Liu, Z. N-acetylcysteine protects against cadmium-induced oxidative stress in rat hepatocytes. J. Vet. Sci. 2014, 15, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Wispriyono, B.; Matsuoka, M.; Igisu, H.; Matsuno, K. Protection from cadmium cytotoxicity by N-acetylcysteine in LLC-PK1 cells. J. Pharmacol. Exp. Ther. 1998, 287, 344–351. [Google Scholar] [PubMed]
- Wu, C.-A.; Chao, Y.; Shiah, S.-G.; Lin, W.-W. Nutrient deprivation induces the Warburg effect through ROS/AMPK-dependent activation of pyruvate dehydrogenase kinase. Biochim. Biophys. Acta 2013, 1833, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Nzengue, Y.; Steiman, R.; Garrel, C.; Lefèbvre, E.; Guiraud, P. Oxidative stress and DNA damage induced by cadmium in the human keratinocyte HaCaT cell line: Role of glutathione in the resistance to cadmium. Toxicology 2008, 243, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Khanna, S.; Mitra, S.; Lakhera, P.C.; Khandelwal, S. N-acetylcysteine effectively mitigates cadmium-induced oxidative damage and cell death in Leydig cells in vitro. Drug Chem. Toxicol. 2015, 39, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.-H.; Lim, S.-C. A rapid and transient ROS generation by cadmium triggers apoptosis via caspase-dependent pathway in HepG2 cells and this is inhibited through N-acetylcysteine-mediated catalase upregulation. Toxicol. Appl. Pharmacol. 2006, 212, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Odewumi, C.O.; Latinwo, L.M.; Ruden, M.L.; Badisa, V.L.D.; Fils-Aime, S.; Badisa, R.B. Modulation of cytokines and chemokines expression by NAC in cadmium chloride treated human lung cells. Environ. Toxicol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.-H.; Li, W.-X.; Sun, M.-Y.; Zhang, S.-B.; Fan, C.-X.; Wu, Q.; Zhu, W.; Xu, X. Cadmium Induced Apoptosis in MG63 Cells by Increasing ROS, Activation of p38 MAPK and Inhibition of ERK 1/2 Pathways. Cell. Physiol. Biochem. 2015, 36, 642–654. [Google Scholar] [CrossRef] [PubMed]
- Hsu, T.; Huang, K.-M.; Tsai, H.-T.; Sung, S.-T.; Ho, T.-N. Cadmium (Cd)-induced oxidative stress down-regulates the gene expression of DNA mismatch recognition proteins MutS homolog 2 (MSH2) and MSH6 in zebrafish (Danio rerio) embryos. Aquat. Toxicol. 2013, 126, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Luchese, C.; Zeni, G.; Rocha, J.B.T.; Nogueira, C.W.; Santos, F.W. Cadmium inhibits δ-aminolevulinate dehydratase from rat lung in vitro: Interaction with chelating and antioxidant agents. Chem. Biol. Interact. 2007, 165, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.-L.; Wang, Z.; Wang, H.; Zhang, C.; Zhang, Y.; Zhao, M.; Chen, Y.-H.; Meng, X.-H.; Xu, D.-X. Ascorbic acid protects against cadmium-induced endoplasmic reticulum stress and germ cell apoptosis in testes. Reprod. Toxicol. 2012, 34, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.; Sinha, M.; Sil, P.C. Taurine plays a beneficial role against cadmium-induced oxidative renal dysfunction. Amino Acids 2009, 36, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.; Diniz, Y.; Marques, S.F.; Faine, L.; Ribas, B.; Burneiko, R.; Novelli, E.L. The use of the oxidative stress responses as biomarkers in Nile tilapia (Oreochromis niloticus) exposed to in vivo cadmium contamination. Environ. Int. 2002, 27, 673–679. [Google Scholar] [CrossRef]
- Geret, F.; Serafim, A.; Bebianno, M.J. Antioxidant enzyme activities, metallothioneins and lipid peroxidation as biomarkers in Ruditapes decussatus? Ecotoxicology 2003, 12, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Cossu, C.; Doyotte, A.; Jacquin, M.C.; Babut, M.; Exinger, A.; Vasseur, P. Glutathione reductase, selenium-dependent glutathione peroxidase, glutathione levels, and lipid peroxidation in freshwater bivalves, Unio tumidus, as biomarkers of aquatic contamination in field studies. Ecotoxicol. Environ. Saf. 1997, 38, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Doyotte, A. Antioxidant enzymes, glutathione and lipid peroxidation as relevant biomarkers of experimental or field exposure in the gills and the digestive gland of the freshwater bivalve Unio tumidus. Aquat. Toxicol. 1997, 39, 93–110. [Google Scholar] [CrossRef]
- Thévenod, F.; Lee, W.-K. Cadmium and cellular signaling cascades: Interactions between cell death and survival pathways. Arch. Toxicol. 2013, 87, 1743–1786. [Google Scholar] [CrossRef] [PubMed]
- Gobe, G.; Crane, D. Mitochondria, reactive oxygen species and cadmium toxicity in the kidney. Toxicol. Lett. 2010, 198, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Cannino, G.; Ferruggia, E.; Luparello, C.; Rinaldi, A.M. Cadmium and mitochondria. Mitochondrion 2009, 9, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Poteet, E.; Winters, A.; Xie, L.; Ryou, M.-G.; Liu, R.; Yang, S.-H. In vitro protection by pyruvate against cadmium-induced cytotoxicity in hippocampal HT-22 cells. J. Appl. Toxicol. 2014, 34, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Qi, Y.; Zhang, X.; Qiu, Q.; Gu, X.; Tao, C.; Huang, D.; Zhang, Y. Cadmium induces mitophagy through ROS-mediated PINK1/Parkin pathway. Toxicol. Mech. Methods 2014, 24, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Pi, H.; Xu, S.; Zhang, L.; Guo, P.; Li, Y.; Xie, J.; Tian, L.; He, M.; Lu, Y.; Li, M.; et al. Dynamin 1-like-dependent mitochondrial fission initiates overactive mitophagy in the hepatotoxicity of cadmium. Autophagy 2013, 9, 1780–1800. [Google Scholar] [CrossRef] [PubMed]
- Belyaeva, E.A.; Sokolova, T.V.; Emelyanova, L.V.; Zakharova, I.O. Mitochondrial electron transport chain in heavy metal-induced neurotoxicity: Effects of cadmium, mercury, and copper. Sci. World J. 2012, 2012, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Dorta, D.J.; Leite, S.; deMarco, K.C.; Prado, I.M.R.; Rodrigues, T.; Mingatto, F.E.; Uyemura, S.A.; Santos, A.C.; Curti, C. A proposed sequence of events for cadmium-induced mitochondrial impairment. J. Inorg. Biochem. 2003, 97, 251–257. [Google Scholar] [CrossRef]
- Li, M.; Xia, T.; Jiang, C.S.; Li, L.J.; Fu, J.L.; Zhou, Z.C. Cadmium directly induced the opening of membrane permeability pore of mitochondria which possibly involved in cadmium-triggered apoptosis. Toxicology 2003, 194, 19–33. [Google Scholar] [CrossRef]
- Lee, W.-K.; Bork, U.; Gholamrezaei, F.; Thévenod, F. Cd2+-induced cytochrome c release in apoptotic proximal tubule cells: Role of mitochondrial permeability transition pore and Ca2+ uniporter. Am. J. Physiol. Ren. Physiol. 2005, 288, F27–F39. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Pi, H.; Chen, Y.; Zhang, N.; Guo, P.; Lu, Y.; He, M.; Xie, J.; Zhong, M.; Zhang, Y.; et al. Cadmium induced Drp1-dependent mitochondrial fragmentation by disturbing Calcium homeostasis in its hepatotoxicity. Cell Death Dis. 2013, 4, e540. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Pi, H.; Xu, S.; Zhang, L.; Li, Y.; Li, M.; Cao, Z.; Tian, L.; Xie, J.; Li, R.; et al. Melatonin Improves mitochondrial function by promoting MT1/SIRT1/PGC-1 α-dependent mitochondrial biogenesis in cadmium-induced hepatotoxicity in vitro. Toxicol. Sci. 2014, 142, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Kil, I.S.; Shin, S.W.; Yeo, H.S.; Lee, Y.S.; Park, J.-W. Mitochondrial NADP+-dependent isocitrate dehydrogenase protects cadmium-induced apoptosis. Mol. Pharmacol. 2006, 70, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Leung, K.M.Y.; Taylor, A.C.; Furness, R.W. Temperature-dependent physiological responses of the dogwhelk Nucella lapillus to cadmium exposure. J. Mar. Biol. Assoc. UK 2000, 80, 647–660. [Google Scholar] [CrossRef]
- Yang, J.; Liu, D.; He, Y.; Wang, L. Mitochondrial energy metabolism in the hepatopancreas of freshwater crabs (Sinopotamon henanense) after cadmium exposure. Environ. Sci. Process. Impacts 2015, 17, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Zhang, S.-L.; Liu, Z.-Y.; Tian, Y.; Sun, Q. Cadmium toxicity induces ER stress and apoptosis via impairing energy homeostasis in cardiomyocytes. Biosci. Rep. 2015, 35, e00214. [Google Scholar] [CrossRef] [PubMed]
- Andersen, O. Chelation of cadmium. Environ. Health Perspect. 1984, 54, 249–266. [Google Scholar] [CrossRef] [PubMed]
- Viarengo, A.; Burlando, B.; Ceratto, N.; Panfoli, I. Antioxidant role of metallothioneins: A comparative overview. Cell. Mol. Biol. 2000, 46, 407–417. [Google Scholar] [PubMed]
- Sato, M.; Kondoh, M. Recent studies on metallothionein: Protection against toxicity of heavy metals and oxygen free radicals. Tohoku J. Exp. Med. 2002, 196, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Cols, N.; Romero-Isart, N.; Bofill, R.; Capdevila, M.; Gonzàlez-Duarte, P.; Gonzàlez-Duarte, R.; Atrian, S. In vivo copper- and cadmium-binding ability of mammalian metallothionein beta domain. Protein Eng. 1999, 12, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Palacios, O.; Pagani, A.; Pérez-Rafael, S.; Egg, M.; Höckner, M.; Brandstätter, A.; Capdevila, M.; Atrian, S.; Dallinger, R. Shaping mechanisms of metal specificity in a family of metazoan metallothioneins: Evolutionary differentiation of mollusc metallothioneins. BMC Biol. 2011, 9. [Google Scholar] [CrossRef] [PubMed]
- Pinter, T.B.J.; Irvine, G.W.; Stillman, M.J. Domain Selection in Metallothionein 1A: Affinity-controlled mechanisms of zinc binding and cadmium exchange. Biochemistry 2015, 54, 5006–5016. [Google Scholar] [CrossRef] [PubMed]
- Jara-Biedma, R.; González-Dominguez, R.; García-Barrera, T.; Lopez-Barea, J.; Pueyo, C.; Gómez-Ariza, J.L. Evolution of metallotionein isoforms complexes in hepatic cells of Mus musculus along cadmium exposure. Biometals 2013, 26, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Palacios, O.; Pérez-Rafael, S.; Pagani, A.; Dallinger, R.; Atrian, S.; Capdevila, M. Cognate and noncognate metal ion coordination in metal-specific metallothioneins: The Helix pomatia system as a model. J. Biol. Inorg. Chem. 2014, 19, 923–935. [Google Scholar] [CrossRef] [PubMed]
- Höckner, M.; Stefanon, K.; de Vaufleury, A.; Monteiro, F.; Pérez-Rafael, S.; Palacios, O.; Capdevila, M.; Atrian, S.; Dallinger, R. Physiological relevance and contribution to metal balance of specific and non-specific Metallothionein isoforms in the garden snail, Cantareus aspersus. Biometals 2011, 24, 1079–1092. [Google Scholar] [CrossRef] [PubMed]
- Tomas, M.; Domènech, J.; Capdevila, M.; Bofill, R.; Atrian, S. The sea urchin metallothionein system: Comparative evaluation of the SpMTA and SpMTB metal-binding preferences. FEBS Open Biol. 2013, 3, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Egli, D.; Domènech, J.; Selvaraj, A.; Balamurugan, K.; Hua, H.; Capdevila, M.; Georgiev, O.; Schaffner, W.; Atrian, S. The four members of the Drosophila metallothionein family exhibit distinct yet overlapping roles in heavy metal homeostasis and detoxification. Genes Cells 2006, 11, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Höckner, M.; Dallinger, R.; Stürzenbaum, S.R. Nematode and snail metallothioneins. J. Biol. Inorg. Chem. 2011, 16, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Domènech, J.; Bofill, R.; Tinti, A.; Torreggiani, A.; Atrian, S.; Capdevila, M. Comparative insight into the Zn(II)-, Cd(II)- and Cu(I)-binding features of the protozoan Tetrahymena pyriformis MT1 metallothionein. Biochim. Biophys. Acta 2008, 1784, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xu, J.; Chai, B.; Liang, A.; Wang, W. Functional comparison of metallothioneins MTT1 and MTT2 from Tetrahymena thermophila. Arch. Biochem. Biophys. 2011, 509, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Eckschlager, T.; Adam, V.; Hrabeta, J.; Figova, K.; Kizek, R. Metallothioneins and cancer. Curr. Protein Pept. Sci. 2009, 10, 360–375. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, M.Ø.; Larsen, A.; Stoltenberg, M.; Penkowa, M. The role of metallothionein in oncogenesis and cancer prognosis. Prog. Histochem. Cytochem. 2009, 44, 29–64. [Google Scholar] [CrossRef] [PubMed]
- Günther, V.; Lindert, U.; Schaffner, W. The taste of heavy metals: Gene regulation by MTF-1. Biochim. Biophys. Acta 2012, 1823, 1416–1425. [Google Scholar] [CrossRef] [PubMed]
- Höckner, M.; Stefanon, K.; Schuler, D.; Fantur, R.; de Vaufleury, A.; Dallinger, R. Coping with cadmium exposure in various ways: The two helicid snails Helix pomatia and Cantareus aspersus share the metal transcription factor-2, but differ in promoter organization and transcription of their Cd-metallothionein genes. J. Exp. Zool. A Ecol. Genet. Physiol. 2009, 311, 776–787. [Google Scholar] [CrossRef] [PubMed]
- Höckner, M.; Dallinger, R.; Stürzenbaum, S.R. Metallothionein gene activation in the earthworm (Lumbricus rubellus). Biochem. Biophys. Res. Commun. 2015, 460, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Freisinger, E.; Vašák, M. Cadmium in metallothioneins. Met. Ions Life Sci. 2013, 11, 339–371. [Google Scholar] [PubMed]
- Langelueddecke, C.; Lee, W.-K.; Thévenod, F. Differential transcytosis and toxicity of the hNGAL receptor ligands cadmium-metallothionein and cadmium-phytochelatin in colon-like Caco-2 cells: Implications for in vivo cadmium toxicity. Toxicol. Lett. 2014, 226, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Wolff, N.A.; Lee, W.-K.; Thévenod, F. Role of Arf1 in endosomal trafficking of protein-metal complexes and cadmium-metallothionein-1 toxicity in kidney proximal tubule cells. Toxicol. Lett. 2011, 203, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Dong, F.; Xu, D.; Du, L.; Yan, S.; Hu, H.; Lobe, C.G.; Yi, F.; Kapron, C.M.; Liu, J. Short-term, low-dose cadmium exposure induces hyperpermeability in human renal glomerular endothelial cells. J. Appl. Toxicol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Onodera, A.; Tani, M.; Michigami, T.; Yamagata, M.; Min, K.-S.; Tanaka, K.; Nakanishi, T.; Kimura, T.; Itoh, N. Role of megalin and the soluble form of its ligand RAP in Cd-metallothionein endocytosis and Cd-metallothionein-induced nephrotoxicity in vivo. Toxicol. Lett. 2012, 212, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Delalande, O.; Desvaux, H.; Godat, E.; Valleix, A.; Junot, C.; Labarre, J.; Boulard, Y. Cadmium-glutathione solution structures provide new insights into heavy metal detoxification. FEBS J. 2010, 277, 5086–5096. [Google Scholar] [CrossRef] [PubMed]
- Liebeke, M.; Garcia-Perez, I.; Anderson, C.J.; Lawlor, A.J.; Bennett, M.H.; Morris, C.A.; Kille, P.; Svendsen, C.; Spurgeon, D.J.; Bundy, J.G. Earthworms produce phytochelatins in response to arsenic. PLoS ONE 2013, 8, e81271. [Google Scholar]
- Hall, J.; Haas, K.L.; Freedman, J.H. Role of MTL-1, MTL-2, and CDR-1 in mediating cadmium sensitivity in Caenorhabditis elegans. Toxicol. Sci. 2012, 128, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T.; Ueda, H.; Kawahara, A.; Fujimoto, S. Cadmium toxicity on cultured neonatal rat hepatocytes: Biochemical and ultrastructural analyses. Histol. Histopathol. 1991, 6, 127–133. [Google Scholar] [PubMed]
- Biagioli, M.; Pifferi, S.; Ragghianti, M.; Bucci, S.; Rizzuto, R.; Pinton, P. Endoplasmic reticulum stress and alteration in Calcium homeostasis are involved in cadmium-induced apoptosis. Cell Calcium 2008, 43, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Gorman, A.M.; Healy, S.J.M.; Jäger, R.; Samali, A. Stress management at the ER: Regulators of ER stress-induced apoptosis. Pharmacol. Ther. 2012, 134, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.; Lee, A.S. The critical roles of endoplasmic reticulum chaperones and unfolded protein response in tumorigenesis and anti-cancer therapies. Oncogene 2013, 32, 805–818. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Inageda, K.; Nishitai, G.; Matsuoka, M. Cadmium induces the expression of Grp78, an endoplasmic reticulum molecular chaperone, in LLC-PK1 renal epithelial cells. Environ. Health Perspect. 2006, 114, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wu, K.C.; Lu, Y.-F.; Ekuase, E.; Klaassen, C.D. Nrf2 protection against liver injury produced by various hepatotoxicants. Oxid. Med. Cell. Longev. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Du, K.; Takahashi, T.; Kuge, S.; Naganuma, A.; Hwang, G.-W. FBXO6 attenuates cadmium toxicity in HEK293 cells by inhibiting ER stress and JNK activation. J. Toxicol. Sci. 2014, 39, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.-C.; Hahm, K.-S.; Lee, S.-H.; Oh, S.-H. Autophagy involvement in cadmium resistance through induction of multidrug resistance-associated protein and counterbalance of endoplasmic reticulum stress WI38 lung epithelial fibroblast cells. Toxicology 2010, 276, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Gaubin, Y.; Vaissade, F.; Croute, F.; Beau, B.; Soleilhavoup, J.-P.; Murat, J.-C. Implication of free radicals and glutathione in the mechanism of cadmium-induced expression of stress proteins in the A549 human lung cell-line. Biochim. Biophys. Acta 2000, 1495, 4–13. [Google Scholar] [CrossRef]
- Liu, H.; He, J.; Chi, C.; Shao, J. Differential HSP70 expression in Mytilus coruscus under various stressors. Gene 2014, 543, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Luan, W.; Li, F.; Zhang, J.; Wen, R.; Li, Y.; Xiang, J. Identification of a novel inducible cytosolic Hsp70 gene in Chinese shrimp Fenneropenaeus chinensis and comparison of its expression with the cognate Hsc70 under different stresses. Cell Stress Chaperones 2010, 15, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Boutet, I.; Tanguy, A.; Rousseau, S.; Auffret, M.; Moraga, D. Molecular identification and expression of heat shock cognate 70 (HSC70) and heat shock protein 70 (HSP70) genes in the Pacific oyster Crassostrea gigas. Cell Stress Chaperones 2003, 8, 76–85. [Google Scholar] [CrossRef]
- Vilaboa, N.E.; Calle, C.; Pérez, C.; de Blas, E.; García-Bermejo, L.; Aller, P. cAMP increasing agents prevent the stimulation of heat-shock protein 70 (HSP70) gene expression by cadmium chloride in human myeloid cell lines. J. Cell Sci. 1995, 108 Pt 8, 2877–2883. [Google Scholar] [PubMed]
- Jing, J.; Liu, H.; Chen, H.; Hu, S.; Xiao, K.; Ma, X. Acute effect of copper and cadmium exposure on the expression of heat shock protein 70 in the Cyprinidae fish Tanichthys albonubes. Chemosphere 2013, 91, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Templeton, D.M. Cellular factors mediate cadmium-dependent actin depolymerization. Toxicol. Appl. Pharmacol. 1996, 139, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Go, Y.-M.; Orr, M.; Jones, D.P. Actin cytoskeleton redox proteome oxidation by cadmium. Am. J. Physiol. Lung Cell. Mol. Physiol. 2013, 305, L831–L843. [Google Scholar] [CrossRef] [PubMed]
- Colon Rodriguez, I.; Negron Berrios, J. Effects of cadmium on epigenetics of cytoskeletal genes in CHO cells. FASEB J. 2015, 29, 884.47. [Google Scholar]
- Templeton, D.M.; Liu, Y. Effects of cadmium on the actin cytoskeleton in renal mesangial cells. Can. J. Physiol. Pharmacol. 2013, 91, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Kagan, H.M.; Chou, I.N. Alterations in cytoskeletal organization and homeostasis of cellular thiols in cadmium-resistant cells. Toxicol. Appl. Pharmacol. 1994, 126, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Lau, A.T.Y.; Chiu, J.-F. The possible role of cytokeratin 8 in cadmium-induced adaptation and carcinogenesis. Cancer Res. 2007, 67, 2107–2113. [Google Scholar] [CrossRef] [PubMed]
- Somji, S.; Garrett, S.H.; Toni, C.; Zhou, X.D.; Zheng, Y.; Ajjimaporn, A.; Sens, M.A.; Sens, D. A Differences in the epigenetic regulation of MT-3 gene expression between parental and Cd+2 or As+3 transformed human urothelial cells. Cancer Cell Int. 2011, 11. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J. Converging concepts: Adaptive response, preconditioning, and the Yerkes–Dodson Law are manifestations of hormesis. Ageing Res. Rev. 2008, 7, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, G.R. A perspective on the scientific, philosophical, and policy dimensions of hormesis. Dose Response 2009, 7, 1–51. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Blain, R.B. The hormesis database: The occurrence of hormetic dose responses in the toxicological literature. Regul. Toxicol. Pharmacol. 2011, 61, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J. Hormetic mechanisms. Crit. Rev. Toxicol. 2013, 43, 580–606. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Zhu, J.Y.; Chan, K.M. Effects of cadmium on cell proliferation, apoptosis, and proto-oncogene expression in zebrafish liver cells. Aquat. Toxicol. 2014, 157, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Damelin, L.H.; Vokes, S.; Whitcutt, J.M.; Damelin, S.B.; Alexander, J.J. Hormesis: A stress response in cells exposed to low levels of heavy metals. Hum. Exp. Toxicol. 2000, 19, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi-Bardbori, A.; Rannug, A. Arsenic, cadmium, mercury and nickel stimulate cell growth via NADPH oxidase activation. Chem. Biol. Interact. 2014, 224, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.J.; Pane, E.F.; Wood, C.M. Physiological effects of dietary cadmium acclimation and waterborne cadmium challenge in rainbow trout: Respiratory, ionoregulatory, and stress parameters. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2004, 139, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Wirth, D.; Christians, E.; Li, X.; Benjamin, I.J.; Gustin, P. Use of HSF1(−/−) mice reveals an essential role for HSF1 to protect lung against cadmium-induced injury. Toxicol. Appl. Pharmacol. 2003, 192, 12–20. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, G.; Yu, Y.; Zhu, H. The hormetic effect of cadmium on the activity of antioxidant enzymes in the earthworm Eisenia fetida. Environ. Pollut. 2009, 157, 3064–3068. [Google Scholar] [CrossRef] [PubMed]
- Lefcort, H.; Freedman, Z.; House, S.; Pendleton, M. Hormetic effects of heavy metals in aquatic snails: Is a little bit of pollution good? Ecohealth 2008, 5, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Banjerdkij, P.; Vattanaviboon, P.; Mongkolsuk, S. Cadmium-induced adaptive resistance and cross-resistance to zinc in Xanthomonas campestris. Curr. Microbiol. 2003, 47, 260–262. [Google Scholar] [CrossRef] [PubMed]
- Fujishiro, H.; Kubota, K.; Inoue, D.; Inoue, A.; Yanagiya, T.; Enomoto, S.; Himeno, S. Cross-resistance of cadmium-resistant cells to manganese is associated with reduced accumulation of both cadmium and manganese. Toxicology 2011, 280, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Bhattacharyya, N.P. Oxidative stress-induced cadmium resistance in Chinese hamster V79 cells. Biochem. Biophys. Res. Commun. 1996, 228, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Mcclure, C.D.; Zhong, W.; Hunt, V.L.; Chapman, F.M.; Hill, F.V.; Priest, N.K. Hormesis results in trade-offs with immunity. Evolution 2014, 68, 2225–2233. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.Y.P.; Pereira, S.; Cheng, S.H.; Adam-Guillermin, C.; Garnier-Laplace, J.; Yu, K.N. Combined effects of depleted uranium and ionising radiation on zebrafish embryos. Radiat. Prot. Dosim. 2015, 167, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Belaidi, E.; Beguin, P.C.; Levy, P.; Ribuot, C.; Godin-Ribuot, D. Prevention of HIF-1 activation and iNOS gene targeting by low-dose cadmium results in loss of myocardial hypoxic preconditioning in the rat. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H901–H908. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Liu, L.Z.; Jiang, Y.; Zhu, Y.; Guo, N.L.; Barnett, J.; Rojanasakul, Y.; Agani, F.; Jiang, B.H. Cadmium increases HIF-1 and VEGF expression through ROS, ERK, and AKT signaling pathways and induces malignant transformation of human bronchial epithelial cells. Toxicol. Sci. 2012, 125, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Katakura, M.; Sugawara, N. Improvement of acute cadmium toxicity by pretreatment with copper salt. Bull. Environ. Contam. Toxicol. 1995, 54, 878–883. [Google Scholar] [CrossRef] [PubMed]
- El-Sharaky, A.S.; Newairy, A.A.; Badreldeen, M.M.; Eweda, S.M.; Sheweita, S.A. Protective role of selenium against renal toxicity induced by cadmium in rats. Toxicology 2007, 235, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Ognjanović, B.I.; Marković, S.D.; Pavlović, S.Z.; Zikić, R.V.; Stajn, A.S.; Saicić, Z.S. Effect of chronic cadmium exposure on antioxidant defense system in some tissues of rats: Protective effect of selenium. Physiol. Res. 2008, 57, 403–411. [Google Scholar]
- Liu, L.; Yang, B.; Cheng, Y.; Lin, H. Ameliorative effects of selenium on cadmium-induced oxidative stress and endoplasmic reticulum stress in the chicken kidney. Biol. Trace Elem. Res. 2015, 167, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.-J.; Zhang, S.-P.; Liu, C.-W.; Cai, Y.-Q. The protection of selenium on ROS mediated-apoptosis by mitochondria dysfunction in cadmium-induced LLC-PK1 cells. Toxicol. Vitro 2009, 23, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, Y.; Luo, K.; Liu, Y.; Zhou, M.; Yan, S.; Shi, H.; Cai, Y. The protective effects of selenium on cadmium-induced oxidative stress and apoptosis via mitochondria pathway in mice kidney. Food Chem. Toxicol. 2013, 58, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xu, F.; Yang, Z.; Li, M.; Min, Y.; Li, S. Cadmium-induced injury and the ameliorative effects of selenium on chicken splenic lymphocytes: Mechanisms of oxidative stress and apoptosis. Biol. Trace Elem. Res. 2014, 160, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Brzóska, M.M.; Rogalska, J. Protective effect of zinc supplementation against cadmium-induced oxidative stress and the RANK/RANKL/OPG system imbalance in the bone tissue of rats. Toxicol. Appl. Pharmacol. 2013, 272, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Coogan, T.P.; Bare, R.M.; Waalkes, M.P. Cadmium-induced DNA strand damage in cultured liver cells: Reduction in cadmium genotoxicity following zinc pretreatment. Toxicol. Appl. Pharmacol. 1992, 113, 227–233. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, J.; Gao, J.; Shahzad, M.; Han, Z.; Wang, Z.; Li, J.; Sjölinder, H. Zinc supplementation protects against cadmium accumulation and cytotoxicity in Madin–Darby bovine kidney cells. PLoS ONE 2014, 9, e103427. [Google Scholar] [CrossRef] [PubMed]
- Eybl, V.; Kotyzová, D. Protective effect of manganese in cadmium-induced hepatic oxidative damage, changes in cadmium distribution and trace elements level in mice. Interdiscip. Toxicol. 2010, 3, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Himeno, S.; Yanagiya, T.; Fujishiro, H. The role of zinc transporters in cadmium and manganese transport in mammalian cells. Biochimie 2009, 91, 1218–1222. [Google Scholar] [CrossRef] [PubMed]
- Thévenod, F. Catch me if you can! Novel aspects of cadmium transport in mammalian cells. Biometals 2010, 23, 857–875. [Google Scholar] [CrossRef] [PubMed]
- Leslie, E.M.; Liu, J.; Klaassen, C.D.; Waalkes, M.P. Acquired cadmium resistance in metallothionein-I/II(−/−) knockout cells: Role of the T-type Calcium channel Cacnα1G in cadmium uptake. Mol. Pharmacol. 2006, 69, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Santoyo-Sánchez, M.P.; Pedraza-Chaverri, J.; Molina-Jijón, E.; Arreola-Mendoza, L.; Rodríguez-Muñoz, R.; Barbier, O.C. Impaired endocytosis in proximal tubule from subchronic exposure to cadmium involves angiotensin II type 1 and cubilin receptors. BMC Nephrol. 2013, 14. [Google Scholar] [CrossRef] [PubMed]
- Gena, P.; Calamita, G.; Guggino, W.B. Cadmium impairs albumin reabsorption by down-regulating megalin and ClC5 channels in renal proximal tubule cells. Environ. Health Perspect. 2010, 118, 1551–1556. [Google Scholar] [CrossRef] [PubMed]
- Wolff, N.A.; Abouhamed, M.; Verroust, P.J.; Thévenod, F. Megalin-dependent internalization of cadmium-metallothionein and cytotoxicity in cultured renal proximal tubule cells. J. Pharmacol. Exp. Ther. 2006, 318, 782–791. [Google Scholar] [CrossRef] [PubMed]
- Jenkitkasemwong, S.; Wang, C.-Y.; Mackenzie, B.; Knutson, M.D. Physiologic implications of metal-ion transport by ZIP14 and ZIP8. Biometals 2012, 25, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Thévenod, F.; Wolff, N.A. Iron transport in the kidney: Implications for physiology and cadmium nephrotoxicity. Metallomics 2016. [Google Scholar] [CrossRef] [PubMed]
- Thevenod, F.; Friedmann, J.M.; Katsen, A.D.; Hauser, I.A. Up-regulation of multidrug resistance P-glycoprotein via nuclear factor-κB activation protects kidney proximal tubule cells from cadmium- and reactive oxygen species-induced apoptosis. J. Biol. Chem. 2000, 275, 1887–1896. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Li, Q.; Wang, Y.; Cui, Z. MRP proteins as potential mediators of heavy metal resistance in zebrafish cells. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2011, 153, 310–317. [Google Scholar] [CrossRef] [PubMed]
- L’hoste, S.; Chargui, A.; Belfodil, R.; Duranton, C.; Rubera, I.; Mograbi, B.; Poujeol, C.; Tauc, M.; Poujeol, P. CFTR mediates cadmium-induced apoptosis through modulation of ROS level in mouse proximal tubule cells. Free Radic. Biol. Med. 2009, 46, 1017–1031. [Google Scholar] [CrossRef] [PubMed]
- Bard, S.M. Multixenobiotic resistance as a cellular defense mechanism in aquatic organisms. Aquat. Toxicol. 2000, 48, 357–389. [Google Scholar] [CrossRef]
- Ferreira, M.; Costa, J.; Reis-Henriques, M.A. ABC transporters in fish species: A review. Front. Physiol. 2014, 5, 266. [Google Scholar] [CrossRef] [PubMed]
- Ivanina, A.; Sokolova, I. Effect of cadmium exposure on P-glycoprotein expression and activity in eastern oysters, Crassostrea virginica. FASEB J. 2008, 22, 757.11. [Google Scholar] [CrossRef] [PubMed]
- Legeay, A.; Achard-Joris, M.; Baudrimont, M.; Massabuau, J.-C.; Bourdineaud, J.-P. Impact of cadmium contamination and oxygenation levels on biochemical responses in the Asiatic clam Corbicula fluminea. Aquat. Toxicol. 2005, 74, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Achard, M. Induction of a multixenobiotic resistance protein (MXR) in the Asiatic clam Corbicula fluminea after heavy metals exposure. Aquat. Toxicol. 2004, 67, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Eufemia, N.A.; Epel, D. Induction of the multixenobiotic defense mechanism (MXR), P-glycoprotein, in the mussel Mytilus californianus as a general cellular response to environmental stresses. Aquat. Toxicol. 2000, 49, 89–100. [Google Scholar] [CrossRef]
- Zucchi, S.; Corsi, I.; Luckenbach, T.; Bard, S.M.; Regoli, F.; Focardi, S. Identification of five partial ABC genes in the liver of the Antarctic fish Trematomus bernacchii and sensitivity of ABCB1 and ABCC2 to Cd exposure. Environ. Pollut. 2010, 158, 2746–2756. [Google Scholar] [CrossRef] [PubMed]
- Sanni, B.; Cherkasov, A.; Sokolova, I.M. Mitochondrial aconitase is sensitive to oxidative stress induced by cadmium and elevated temperatures but not protected by uncoupling proteins in eastern oysters Crassostrea virginica. FASEB J. 2007, 21, A819–d–820. [Google Scholar]
- Cherkasov, A.S.; Biswas, P.K.; Ridings, D.M.; Ringwood, A.H.; Sokolova, I.M. Effects of acclimation temperature and cadmium exposure on cellular energy budgets in the marine mollusk Crassostrea virginica: Linking cellular and mitochondrial responses. J. Exp. Biol. 2006, 209, 1274–1284. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, I.M. Cadmium effects on mitochondrial function are enhanced by elevated temperatures in a marine poikilotherm, Crassostrea virginica Gmelin (Bivalvia: Ostreidae). J. Exp. Biol. 2004, 207, 2639–2648. [Google Scholar] [CrossRef] [PubMed]
- Ivanina, A.V.; Taylor, C.; Sokolova, I.M. Effects of elevated temperature and cadmium exposure on stress protein response in eastern oysters Crassostrea virginica (Gmelin). Aquat. Toxicol. 2009, 91, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Vergauwen, L.; Knapen, D.; Hagenaars, A.; Blust, R. Hypothermal and hyperthermal acclimation differentially modulate cadmium accumulation and toxicity in the zebrafish. Chemosphere 2013, 91, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.; Boudou, A.; Massabuau, J.-C. How water oxygenation level influences cadmium accumulation pattern in the Asiatic clam Corbicula fluminea : A laboratory and field study. Environ. Toxicol. Chem. 2001, 20, 2073–2080. [Google Scholar] [CrossRef] [PubMed]
- Leung, K.M.Y.; Svavarsson, J.; Crane, M.; Morritt, D. Influence of static and fluctuating salinity on cadmium uptake and metallothionein expression by the dogwhelk Nucella lapillus (L.). J. Exp. Mar. Biol. Ecol. 2002, 274, 175–189. [Google Scholar] [CrossRef]
- Pascoe, D.; Evans, S.A.; Woodworth, J. Heavy metal toxicity to fish and the influence of water hardness. Arch. Environ. Contam. Toxicol. 1986, 15, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Niyogi, S.; Wood, C.M. Biotic ligand model, a flexible tool for developing site-specific water quality guidelines for metals. Environ. Sci. Technol. 2004, 38, 6177–6192. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, M.; Fortin, C.; Campbell, P.G.C. Influence of essential elements on cadmium uptake and toxicity in a unicellular green alga: The protective effect of trace zinc and cobalt concentrations. Environ. Toxicol. Chem. 2012, 31, 1445–1452. [Google Scholar] [CrossRef] [PubMed]
- Vidal, M.-L.; Bassères, A.; Narbonne, J.-F. Influence of temperature, pH, oxygenation, water-type and substrate on biomarker responses in the freshwater clam Corbicula fluminea (Müller). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2002, 132, 93–104. [Google Scholar] [CrossRef]
- Pando, M.P.; Pinchak, A.B.; Cermakian, N.; Sassone-Corsi, P. A cell-based system that recapitulates the dynamic light-dependent regulation of the vertebrate clock. Proc. Natl. Acad. Sci. USA 2001, 98, 10178–10183. [Google Scholar] [CrossRef] [PubMed]
- Sandbichler, A.M.; Aschberger, T.; Pelster, B. A method to evaluate the efficiency of transfection reagents in an adherent zebrafish cell line. BioRes. Open Access 2013, 2, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Brzóska, M.M.; Borowska, S.; Tomczyk, M. Antioxidants as a Potential Preventive and Therapeutic Strategy for Cadmium. Curr. Drug Targets 2015, 16. [Google Scholar] [CrossRef]
- Ivanova, J.; Gluhcheva, Y.; Arpadjan, S.; Mitewa, M. Effects of cadmium and monensin on renal and cardiac functions of mice subjected to subacute cadmium intoxication. Interdiscip. Toxicol. 2014, 7, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.W. The role of chelation in the treatment of other metal poisonings. J. Med. Toxicol. 2013, 9, 355–369. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandbichler, A.M.; Höckner, M. Cadmium Protection Strategies—A Hidden Trade-Off? Int. J. Mol. Sci. 2016, 17, 139. https://doi.org/10.3390/ijms17010139
Sandbichler AM, Höckner M. Cadmium Protection Strategies—A Hidden Trade-Off? International Journal of Molecular Sciences. 2016; 17(1):139. https://doi.org/10.3390/ijms17010139
Chicago/Turabian StyleSandbichler, Adolf Michael, and Martina Höckner. 2016. "Cadmium Protection Strategies—A Hidden Trade-Off?" International Journal of Molecular Sciences 17, no. 1: 139. https://doi.org/10.3390/ijms17010139
APA StyleSandbichler, A. M., & Höckner, M. (2016). Cadmium Protection Strategies—A Hidden Trade-Off? International Journal of Molecular Sciences, 17(1), 139. https://doi.org/10.3390/ijms17010139






