Molecular Mechanisms of Nickel Allergy
Abstract
1. Introduction
2. Metal Allergy
3. Animal Models and Molecular Mechanism of Metal Allergy
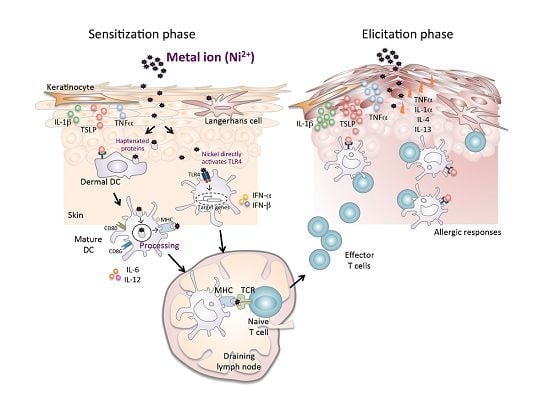
3.1. Keratinocytes and APCs in Ni Allergy Models
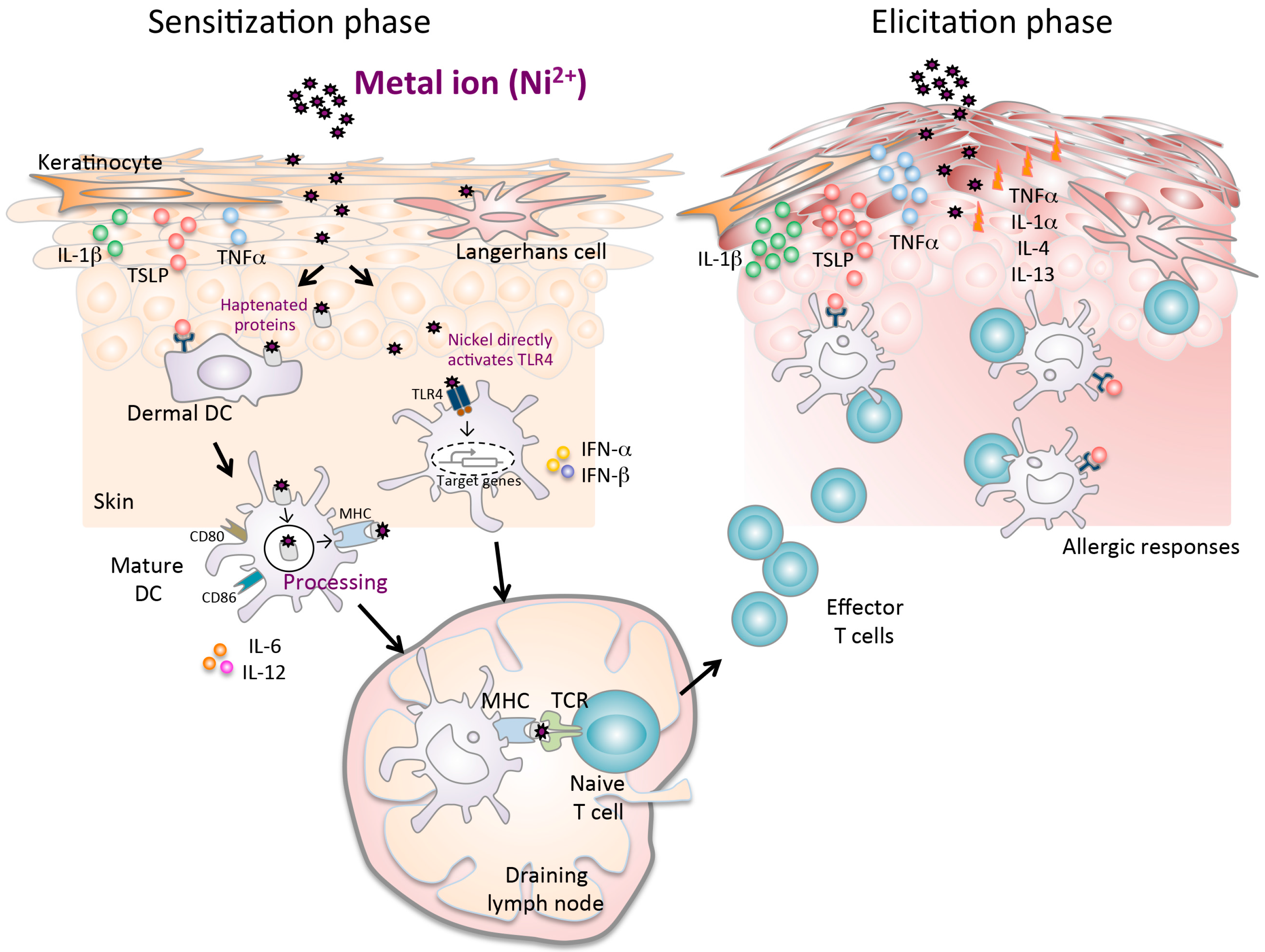
3.2. Critical Role of Toll-Like Receptor 4 in Ni Allergy
3.3. Thymic Stromal Lymphopoietin and Its Receptor in Ni Allergy
4. Adsorption and Excretion of Metals
5. Conclusions and Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Loh, J.; Fraser, J. Metal-derivatized major histocompatibility complex: Zeroing in on contact hypersensitivity. J. Exp. Med. 2003, 197, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Budinger, L.; Hertl, M. Immunologic mechanisms in hypersensitivity reactions to metal ions: An overview. Allergy 2000, 55, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Peltonen, L. Nickel sensitivity in the general population. Contact Dermat. 1979, 5, 27–32. [Google Scholar] [CrossRef]
- Nielsen, N.H.; Menne, T. Allergic contact sensitization in an unselected Danish population. Acta Derm. Venereol. 1992, 72, 456–460. [Google Scholar] [PubMed]
- Pigatto, P.D.; Guzzi, G. Systemic allergic dermatitis syndrome caused by mercury. Contact Dermat. 2008, 59, 66. [Google Scholar]
- Yoshihisa, Y.; Shimizu, T. Metal allergy and systemic contact dermatitis: An overview. Dermatol. Res. Pract. 2012, 2012, 749561. [Google Scholar] [CrossRef] [PubMed]
- Yokozeki, H.; Katayama, I.; Nishioka, K.; Kinoshita, M.; Nishiyama, S. The role of metal allergy and local hyperhidrosis in the pathogenesis of pompholyx. J. Dermatol. 1992, 19, 964–967. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Yin, W.; Ma, Q. Allergic palmoplantar pustulosis caused by cobalt in cast dental crowns: A case report. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2011, 111, e8–e10. [Google Scholar] [CrossRef] [PubMed]
- Stejskal, V.; Reynolds, T.; Bjorklund, G. Increased frequency of delayed type hypersensitivity to metals in patients with connective tissue disease. J. Trace Elem. Med. Biol. 2015, 31, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Mahler, V.; Geier, J.; Schnuch, A. Current trends in patch testing—new data from the German Contact Dermatitis Research Group (DKG) and the Information Network of Departments of Dermatology (IVDK). JDDG 2014, 12, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Garner, L.A. Contact dermatitis to metals. Dermatol. Ther. 2004, 17, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Peiser, M.; Tralau, T.; Heidler, J.; Api, A.M.; Arts, J.H.; Basketter, D.A.; English, J.; Diepgen, T.L.; Fuhlbrigge, R.C.; Gaspari, A.A.; et al. Allergic contact dermatitis: Epidemiology, molecular mechanisms, in vitro methods and regulatory aspects. Current knowledge assembled at an international workshop at BfR, Germany. Cell. Mol. Life Sci. 2012, 69, 763–781. [Google Scholar] [CrossRef] [PubMed]
- Schram, S.E.; Warshaw, E.M.; Laumann, A. Nickel hypersensitivity: A clinical review and call to action. Int. J. Dermatol. 2010, 49, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Wataha, J.C.; Drury, J.L.; Chung, W.O. Nickel alloys in the oral environment. Expert Rev. Med. Devices 2013, 10, 519–539. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaka, K.; Mabuchi, R.; Nagasaka, H.; Yoshinari, M.; Inoue, T. Improvement of eczematous symptoms after removal of amalgam-like metal in alveolar bone. Bull. Tokyo Dent. Coll. 2006, 47, 13–17. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Laeijendecker, R.; Dekker, S.K.; Burger, P.M.; Mulder, P.G.; Van Joost, T.; Neumann, M.H. Oral lichen planus and allergy to dental amalgam restorations. Arch. Dermatol. 2004, 140, 1434–1438. [Google Scholar] [CrossRef] [PubMed]
- Yaqob, A.; Danersund, A.; Stejskal, V.D.; Lindvall, A.; Hudecek, R.; Lindh, U. Metal-specific lymphocyte reactivity is downregulated after dental metal replacement. Neuro Endocrinol. Lett. 2006, 27, 189–197. [Google Scholar] [PubMed]
- Kapsenberg, M.L.; Wierenga, E.A.; Stiekema, F.E.; Tiggelman, A.M.; Bos, J.D. Th1 lymphokine production profiles of nickel-specific CD4+T-lymphocyte clones from nickel contact allergic and non-allergic individuals. J. Investig. Dermatol. 1992, 98, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Mortz, C.G.; Lauritsen, J.M.; Bindslev-Jensen, C.; Andersen, K.E. Prevalence of atopic dermatitis, asthma, allergic rhinitis, and hand and contact dermatitis in adolescents. The Odense Adolescence Cohort Study on Atopic Diseases and Dermatitis. Br. J. Dermatol. 2001, 144, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Thierse, H.J.; Gamerdinger, K.; Junkes, C.; Guerreiro, N.; Weltzien, H.U. T cell receptor (TCR) interaction with haptens: Metal ions as non-classical haptens. Toxicology 2005, 209, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Curtis, A.; Morton, J.; Balafa, C.; MacNeil, S.; Gawkrodger, D.J.; Warren, N.D.; Evans, G.S. The effects of nickel and chromium on human keratinocytes: Differences in viability, cell associated metal and IL-1alpha release. Toxicol. In Vitro 2007, 21, 809–819. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.M.; Bonefeld, C.M.; Poulsen, S.S.; Geisler, C.; Skov, L. IL-23 and T(H)17-mediated inflammation in human allergic contact dermatitis. J. Allergy Clin. Immunol. 2009, 123, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Sebastiani, S.; Albanesi, C.; Nasorri, F.; Girolomoni, G.; Cavani, A. Nickel-specific CD4(+) and CD8(+) T cells display distinct migratory responses to chemokines produced during allergic contact dermatitis. J. Investig. Dermatol. 2002, 118, 1052–1058. [Google Scholar] [CrossRef] [PubMed]
- Steinman, R.M.; Pack, M.; Inaba, K. Dendritic cells in the T-cell areas of lymphoid organs. Immunol. Rev. 1997, 156, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Ishimaru, N.; Ashrin, M.N.; Arakaki, R.; Yamada, A.; Ichikawa, T.; Hayashi, Y. A novel DC therapy with manipulation of MKK6 gene on nickel allergy in mice. PLoS ONE 2011, 6, e19017. [Google Scholar] [CrossRef] [PubMed]
- Roake, J.A.; Rao, A.S.; Morris, P.J.; Larsen, C.P.; Hankins, D.F.; Austyn, J.M. Dendritic cell loss from nonlymphoid tissues after systemic administration of lipopolysaccharide, tumor necrosis factor, and interleukin 1. J. Exp. Med. 1995, 181, 2237–2247. [Google Scholar] [CrossRef] [PubMed]
- Lore, K.; Sonnerborg, A.; Spetz, A.L.; Andersson, U.; Andersson, J. Immunocytochemical detection of cytokines and chemokines in Langerhans cells and in vitro derived dendritic cells. J. Immunol. Methods 1998, 214, 97–111. [Google Scholar] [CrossRef]
- Riedl, E.; Stockl, J.; Majdic, O.; Scheinecker, C.; Rappersberger, K.; Knapp, W.; Strobl, H. Functional involvement of E-cadherin in TGF-beta 1-induced cell cluster formation of in vitro developing human Langerhans-type dendritic cells. J. Immunol. 2000, 165, 1381–1386. [Google Scholar] [CrossRef] [PubMed]
- Geissmann, F.; Dieu-Nosjean, M.C.; Dezutter, C.; Valladeau, J.; Kayal, S.; Leborgne, M.; Brousse, N.; Saeland, S.; Davoust, J. Accumulation of immature Langerhans cells in human lymph nodes draining chronically inflamed skin. J. Exp. Med. 2002, 196, 417–430. [Google Scholar] [CrossRef] [PubMed]
- Villadangos, J.A.; Cardoso, M.; Steptoe, R.J.; van Berkel, D.; Pooley, J.; Carbone, F.R.; Shortman, K. MHC class II expression is regulated in dendritic cells independently of invariant chain degradation. Immunity 2001, 14, 739–749. [Google Scholar] [CrossRef]
- Verhasselt, V.; Buelens, C.; Willems, F.; De Groote, D.; Haeffner-Cavaillon, N.; Goldman, M. Bacterial lipopolysaccharide stimulates the production of cytokines and the expression of costimulatory molecules by human peripheral blood dendritic cells: Evidence for a soluble CD14-dependent pathway. J. Immunol. 1997, 158, 2919–2925. [Google Scholar] [PubMed]
- Kyriakis, J.M. Life-or-death decisions. Nature 2001, 414, 265–266. [Google Scholar] [CrossRef] [PubMed]
- Arrighi, J.F.; Rebsamen, M.; Rousset, F.; Kindler, V.; Hauser, C. A critical role for p38 mitogen-activated protein kinase in the maturation of human blood-derived dendritic cells induced by lipopolysaccharide, TNF-alpha, and contact sensitizers. J. Immunol. 2001, 166, 3837–3845. [Google Scholar] [CrossRef] [PubMed]
- Jorgl, A.; Platzer, B.; Taschner, S.; Heinz, L.X.; Hocher, B.; Reisner, P.M.; Gobel, F.; Strobl, H. Human Langerhans-cell activation triggered in vitro by conditionally expressed MKK6 is counterregulated by the downstream effector RelB. Blood 2007, 109, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Raghavan, B.; Muller, V.; Vogl, T.; Fejer, G.; Tchaptchet, S.; Keck, S.; Kalis, C.; Nielsen, P.J.; Galanos, C.; et al. Crucial role for human Toll-like receptor 4 in the development of contact allergy to nickel. Nat. Immunol. 2010, 11, 814–819. [Google Scholar] [CrossRef] [PubMed]
- Rachmawati, D.; Bontkes, H.J.; Verstege, M.I.; Muris, J.; von Blomberg, B.M.; Scheper, R.J.; van Hoogstraten, I.M. Transition metal sensing by Toll-like receptor-4: Next to nickel, cobalt and palladium are potent human dendritic cell stimulators. Contact Dermat. 2013, 68, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Sato, N.; Kinbara, M.; Kuroishi, T.; Kimura, K.; Iwakura, Y.; Ohtsu, H.; Sugawara, S.; Endo, Y. Lipopolysaccharide promotes and augments metal allergies in mice, dependent on innate immunity and histidine decarboxylase. Clin. Exp. Allergy 2007, 37, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Suga, H.; Sugaya, M.; Fujita, H.; Asano, Y.; Tada, Y.; Kadono, T.; Sato, S. TLR4, rather than TLR2, regulates wound healing through TGF-beta and CCL5 expression. J. Dermatol. Sci. 2014, 73, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Reche, P.A.; Soumelis, V.; Gorman, D.M.; Clifford, T.; Liu, M.; Travis, M.; Zurawski, S.M.; Johnston, J.; Liu, Y.J.; Spits, H.; et al. Human thymic stromal lymphopoietin preferentially stimulates myeloid cells. J. Immunol. 2001, 167, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Rachmawati, D.; Buskermolen, J.K.; Scheper, R.J.; Gibbs, S.; von Blomberg, B.M.; van Hoogstraten, I.M. Dental metal-induced innate reactivity in keratinocytes. Toxicol. In Vitro 2015, 30, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Ashrin, M.N.; Arakaki, R.; Yamada, A.; Kondo, T.; Kurosawa, M.; Kudo, Y.; Watanabe, M.; Ichikawa, T.; Hayashi, Y.; Ishimaru, N. A critical role for thymic stromal lymphopoietin in nickel-induced allergy in mice. J. Immunol. 2014, 192, 4025–4031. [Google Scholar] [CrossRef] [PubMed]
- Ying, S.; O'Connor, B.; Ratoff, J.; Meng, Q.; Mallett, K.; Cousins, D.; Robinson, D.; Zhang, G.; Zhao, J.; Lee, T.H.; et al. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J. Immunol. 2005, 174, 8183–8190. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.; Omori, M.; Gyarmati, D.; Zhou, B.; Aye, T.; Brewer, A.; Comeau, M.R.; Campbell, D.J.; Ziegler, S.F. Spontaneous atopic dermatitis in mice expressing an inducible thymic stromal lymphopoietin transgene specifically in the skin. J. Exp. Med. 2005, 202, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Cavani, A. Breaking tolerance to nickel. Toxicology 2005, 209, 119–121. [Google Scholar] [CrossRef] [PubMed]
- Cavani, A.; Nasorri, F.; Ottaviani, C.; Sebastiani, S.; De Pita, O.; Girolomoni, G. Human CD25+ regulatory T cells maintain immune tolerance to nickel in healthy, nonallergic individuals. J. Immunol. 2003, 171, 5760–5768. [Google Scholar] [CrossRef] [PubMed]
- Christensen, J.M. Human exposure to toxic metals: Factors influencing interpretation of biomonitoring results. Sci. Total Environ. 1995, 166, 89–135. [Google Scholar] [CrossRef]
- Tossavainen, A.; Nurminen, M.; Mutanen, P.; Tola, S. Application of mathematical modelling for assessing the biological half-times of chromium and nickel in field studies. Br. J. Ind. Med. 1980, 37, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Albohn, H. Comparative epicutaneous tests with contact allergens and croton oil in 5 different body rions. Z. Haut Gechlechtskr. 1966, 40, 118–124. (In Gemany) [Google Scholar]
- Fullerton, A.; Menne, T.; Hoelgaard, A. Patch testing with nickel chloride in a hydrogel. Contact Dermat. 1989, 20, 17–20. [Google Scholar] [CrossRef]
- Fullerton, A.; Andersen, J.R.; Hoelgaard, A.; Menne, T. Permeation of nickel salts through human skin in vitro. Contact Dermat. 1986, 15, 173–177. [Google Scholar] [CrossRef]
- Sunderman, F.W., Jr.; Hopfer, S.M.; Sweeney, K.R.; Marcus, A.H.; Most, B.M.; Creason, J. Nickel absorption and kinetics in human volunteers. Proc. Soc. Exp. Biol. Med. 1989, 191, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Leggett, R.W. The biokinetics of inorganic cobalt in the human body. Sci. Total Environ. 2008, 389, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Lauwerys, R.; Lison, D. Health risks associated with cobalt exposure—An overview. Sci. Total Environ. 1994, 150, 1–6. [Google Scholar] [CrossRef]
- Mosconi, G.; Bacis, M.; Vitali, M.T.; Leghissa, P.; Sabbioni, E. Cobalt excretion in urine: Results of a study on workers producing diamond grinding tools and on a control group. Sci. Total Environ. 1994, 150, 133–139. [Google Scholar] [CrossRef]
- Simonsen, L.O.; Harbak, H.; Bennekou, P. Cobalt metabolism and toxicology—A brief update. Sci. Total Environ. 2012, 432, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Ng, E.; Lind, P.M.; Lindgren, C.; Ingelsson, E.; Mahajan, A.; Morris, A.; Lind, L. Genome-wide association study of toxic metals and trace elements reveals novel associations. Hum. Mol. Genet. 2015, 24, 4739–4745. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saito, M.; Arakaki, R.; Yamada, A.; Tsunematsu, T.; Kudo, Y.; Ishimaru, N. Molecular Mechanisms of Nickel Allergy. Int. J. Mol. Sci. 2016, 17, 202. https://doi.org/10.3390/ijms17020202
Saito M, Arakaki R, Yamada A, Tsunematsu T, Kudo Y, Ishimaru N. Molecular Mechanisms of Nickel Allergy. International Journal of Molecular Sciences. 2016; 17(2):202. https://doi.org/10.3390/ijms17020202
Chicago/Turabian StyleSaito, Masako, Rieko Arakaki, Akiko Yamada, Takaaki Tsunematsu, Yasusei Kudo, and Naozumi Ishimaru. 2016. "Molecular Mechanisms of Nickel Allergy" International Journal of Molecular Sciences 17, no. 2: 202. https://doi.org/10.3390/ijms17020202
APA StyleSaito, M., Arakaki, R., Yamada, A., Tsunematsu, T., Kudo, Y., & Ishimaru, N. (2016). Molecular Mechanisms of Nickel Allergy. International Journal of Molecular Sciences, 17(2), 202. https://doi.org/10.3390/ijms17020202