Conformational Dynamics of Ago-Mediated Silencing Processes
Abstract
:1. Introduction
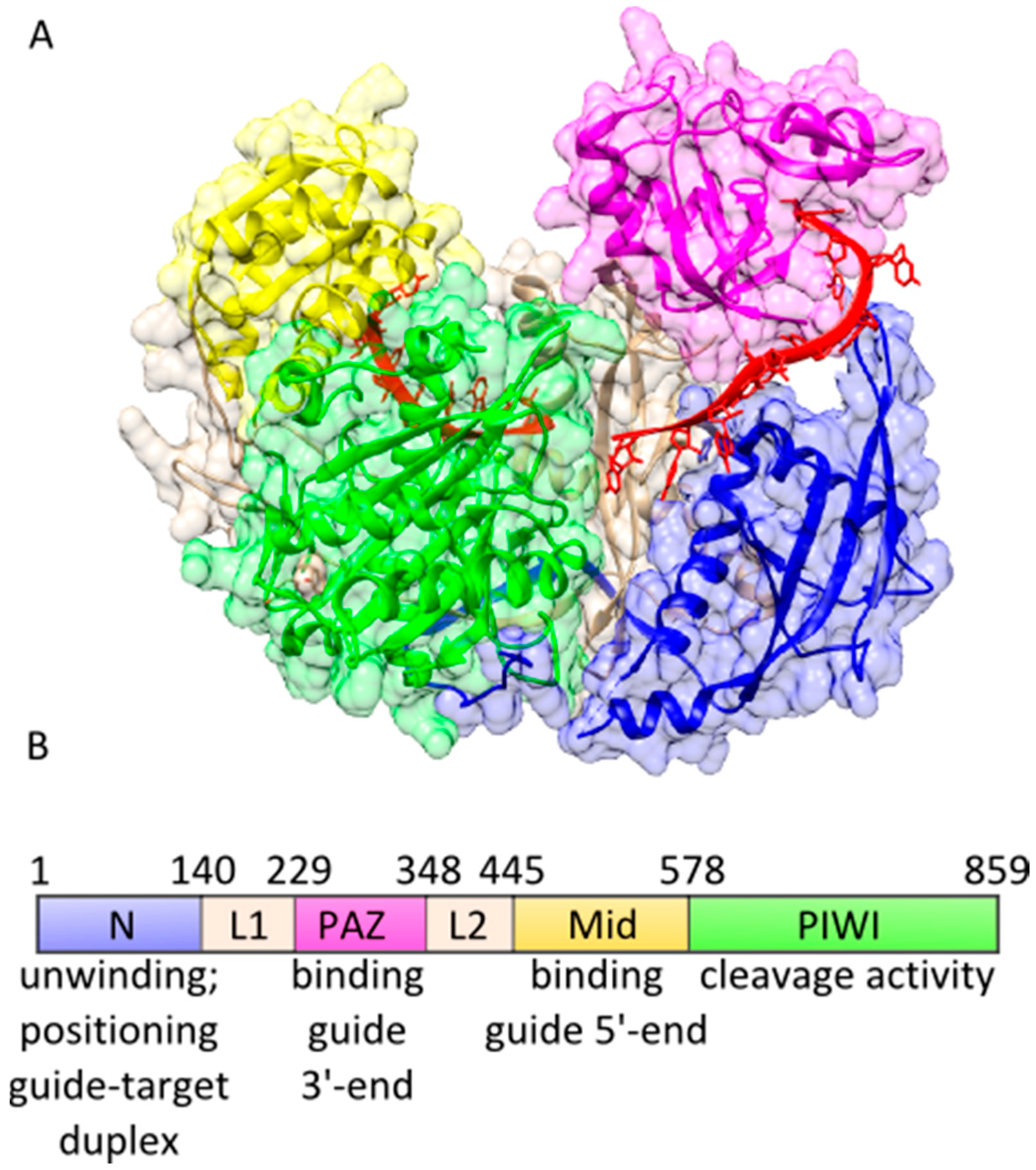
2. Functional Loading of Ago Proteins with Guide Strands
2.1. The Guide 5′-End Mainly Determines the Affinity of Binary Complexes
| Collision Complex | 5′-End Binding | 3′-End Binding | ||||||
|---|---|---|---|---|---|---|---|---|
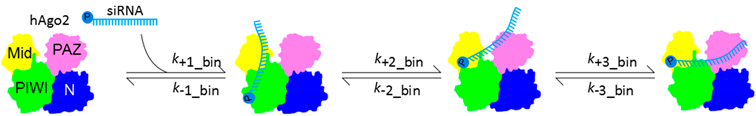 | ||||||||
| Ago Protein | k1_bin (M−1·s−1) | k-1_bin (s−1) | k2_bin (s−1) | k-2_bin (s−1) | k3_bin (s−1) | k-3_bin (s−1) | KD_bin (nM) | Reference |
| wt hAgo2 | 0.6 × 108 | 6.2 | 0.26 | 0.17 | 0.012 | 0.007 | 37 | [46] |
| wt hAgo2 | 1.2 × 105 | n.d. | n.d. | n.d. | n.d. | 0.007 | 57 | [47] |
| AaAgo | n.d. | n.d. | n.d. | n.d. | n.d. | 0.004 | 10 | [4,48] |
| hAgo2-PAZ9 | 0.2 × 108 | 7.8 | 0.18 | 0.024 | – | – | 49.5 | [46] |
| Guide Substrate | KD (nM) | Reference |
|---|---|---|
| hAgo2 | ||
| 19-Mer guide RNA | 83 | [47] |
| 21-Mer guide RNA | 7 | [46] |
| OH-19-Mer guide RNA | 395 | [47] |
| OH-21-Mer guide RNA | 106 | [46] |
| 19-Mer guide RNA Pos 1 abasic | 225 | [47] |
| Blunt end 19-Mer dsRNA | 6297 | [47] |
| 21-Mer siRNA | 48 | [46] |
| 19-Mer DNA | 565 | [47] |
| Methoxyethyl-Substituted Guides | ||
| 19-Mer pos 1–3 | 1100 | [47] |
| 19-Mer pos 12–14 | 234 | [47] |
| hAgo2 Mid Domain | ||
| UMP | 1.2 × 105 | [42] |
| AMP | 2.6 × 105 | [42] |
| CMP | 3.6 × 106 | [42] |
| GMP | 3.3 × 106 | [42] |
| DmAgo1 | ||
| 23-Mer guide RNA | 2.9 | [56] |
| DmAgo1-PAZ6 | ||
| 23-Mer guide RNA | 2.3 | [56] |
| DmAgo1-∆N-PAZ | ||
| 23-Mer guide RNA | 2.7 | [56] |
| DmAgo2 | ||
| 23-Mer guide RNA | 10 | [56] |
| DmAgo2-∆N-PAZ | ||
| 23-Mer guide RNA | 9.5 | [56] |
| AaAgo | ||
| ssDNA | 10 | [4] |
| ssDNA | 2.9–270 | [48] |
| dsDNA | 1000 | [4] |
| ssRNA | 970 | [4] |
| dsRNA | >10,000 | [4] |
| DNA/RNA | 640 | [4] |
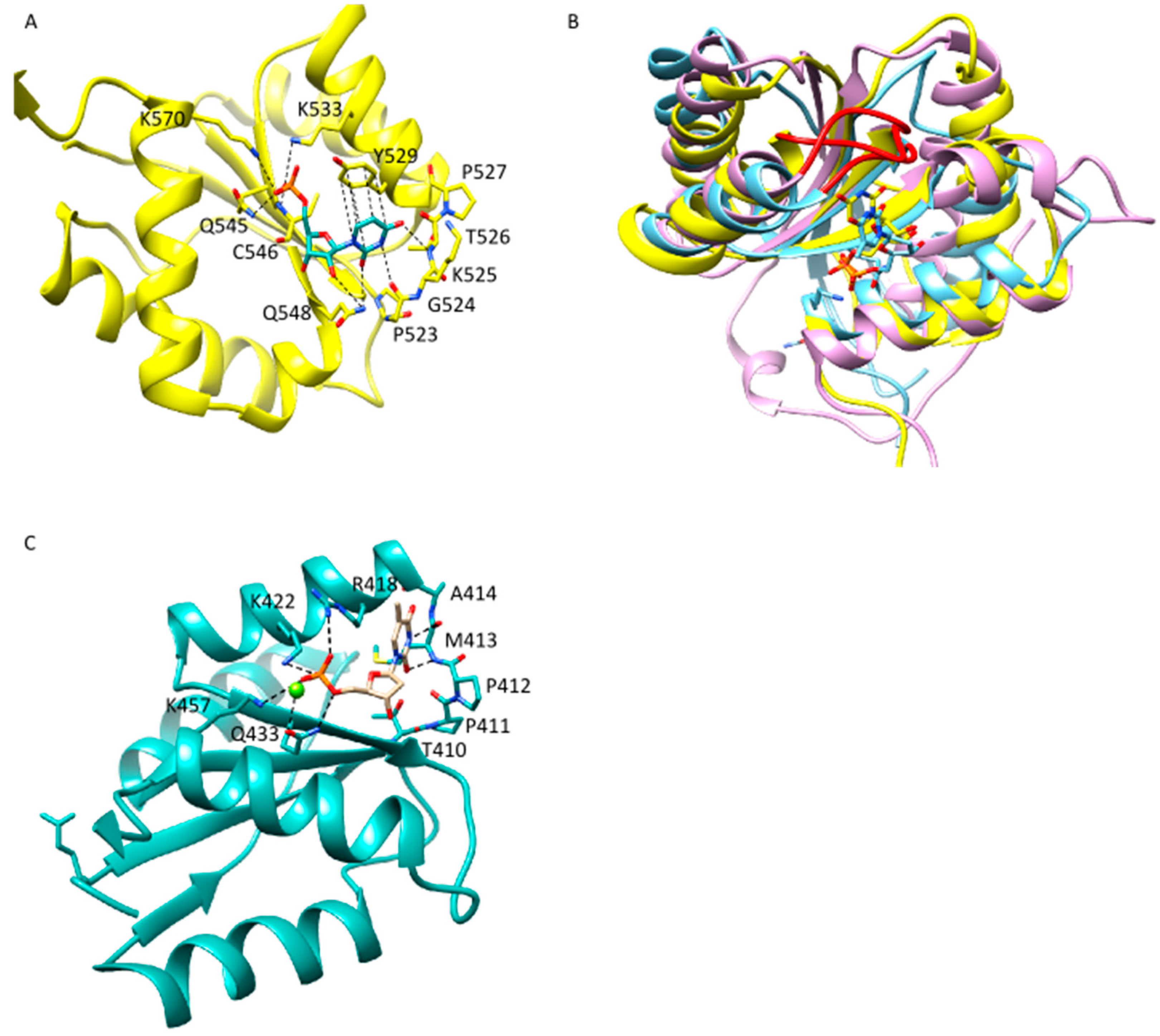
2.2. Anchoring of the Guide 3′-End Is Decisive for the Formation of Functional Binary Ago-Guide Complexes
3. Binding of Target Strands to Binary Ago-Guide Complexes
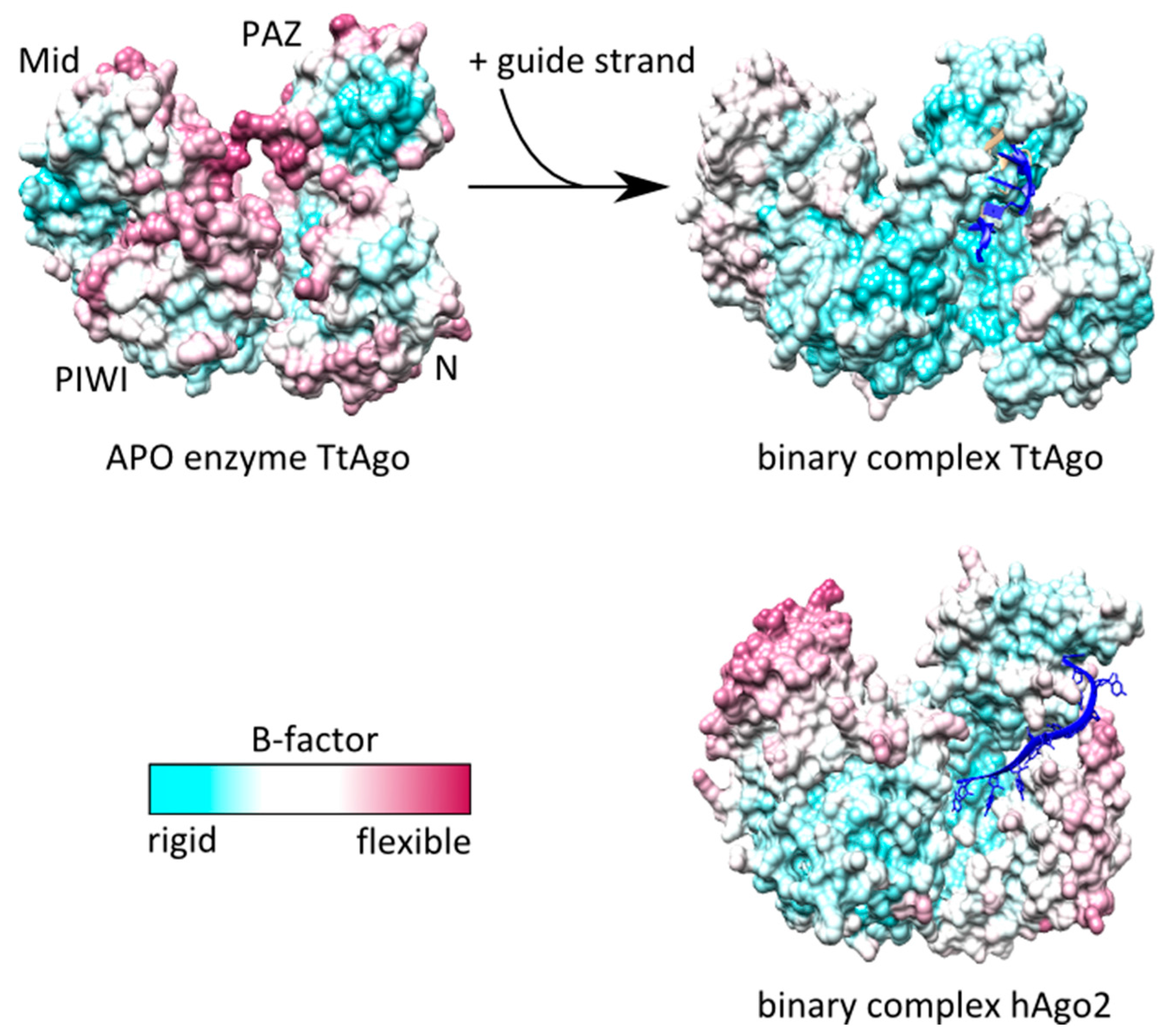
3.1. Dynamic Behavior of Ago Is the Basis of Target Turnover
| Collision Complex | Seed Pairing | PAZ Release | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
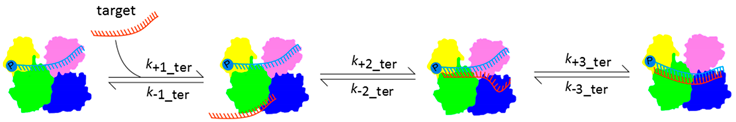 | ||||||||||
| Ago Protein and Guide-Target Complementarity | k1_ter (M−1·s−1) | k-1_ter (s−1) | k2_ter (s−1) | k-2_ter (s−1) | k3_ter (s−1) | k-3_ter (s−1) | KD_ter (nM) | Reference | ||
| hAgo2 | 3.2 × 108 | 2.0 | 0.01 | 0.002 | 0.003 | 0.0002 | 0.2 | [46] | ||
| hAgo2-PAZ9 | 2.9 × 108 | 9.4 | 0.01 | 0.02 | – | – | 47.2 | [46] | ||
| Fly Ago2 | 0.2 × 108 | n.d. | n.d. | n.d. | n.d. | 0.00009 | 0.004 | [69] | ||
| Fly Ago2-seed | 2.1 × 108 | n.d. | n.d. | 0.0045 | – | – | 210 | [69] | ||
| Fly Ago2-seed + 3′-sup | 3.1 × 108 | n.d. | n.d. | 0.005 | – | – | 120 | [69] | ||
| Mouse Ago2 | 0.4 × 108 | n.d. | n.d. | n.d. | n.d. | 0.0008 | 0.02 | [69] | ||
| Mouse Ago2-seed | 0.2 × 108 | n.d. | n.d. | 0.0005 | – | – | 0.03 | [69] | ||
| Mouse Ago2-seed + 3′-sup | 0.2 × 108 | n.d. | n.d. | 0.0005 | – | – | 0.01 | [69] | ||
3.2. Ago Modulates the Affinity of Binary Complexes for Target Strands
| Substrate | KD (nM) | Reference |
|---|---|---|
| hAgo2 | ||
| 21-Mer RNA | 0.2 | [46] |
| 19-Mer RNA | 204 | [47] |
| 20-Mer RNA | 104 | [47] |
| 29-Mer RNA | 43 | [47] |
| Sod1-RNA (complementarity for guide nt 2–7) | 20 | [39] |
| Sod1-RNA (complementarity for guide nt 2–8) | 1.9 | [39] |
| Sod1-RNA (complementarity for guide nt 2–9) | 4.0 | [39] |
| Sod1-RNA (complementarity for guide nt 2–10) | 2.4 | [39] |
| Fly Ago2 | ||
| 21-Mer RNA | 0.004 | [69] |
| Mouse Ago2 | ||
| 21-Mer RNA | 0.02 | [69] |
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Swarts, D.C.; Makarova, K.; Wang, Y.; Nakanishi, K.; Ketting, R.F.; Koonin, E.V.; Patel, D.J.; van der Oost, J. The evolutionary journey of Argonaute proteins. Nat. Struct. Mol. Biol. 2014, 21, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Bohmert, K.; Camus, I.; Bellini, C.; Bouchez, D.; Caboche, M.; Benning, C. Ago1 defines a novel locus of Arabidopsis controlling leaf development. EMBO J. 1998, 17, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Smith, S.K.; Hannon, G.J.; Joshua-Tor, L. Crystal structure of Argonaute and its implications for RISC slicer activity. Science 2004, 305, 1434–1437. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.R.; Pei, Y.; Ma, J.B.; Kuryavyi, V.; Zhadina, M.; Meister, G.; Chen, H.Y.; Dauter, Z.; Tuschl, T.; Patel, D.J. Crystal structure of A. aeolicus argonaute, a site-specific DNA-guided endoribonuclease, provides insights into RISC-mediated mRNA cleavage. Mol. Cell 2005, 19, 405–419. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Juranek, S.; Li, H.; Sheng, G.; Tuschl, T.; Patel, D.J. Structure of an argonaute silencing complex with a seed-containing guide DNA and target RNA duplex. Nature 2008, 456, 921–926. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Juranek, S.; Li, H.; Sheng, G.; Wardle, G.S.; Tuschl, T.; Patel, D.J. Nucleation, propagation and cleavage of target RNAs in Ago silencing complexes. Nature 2009, 461, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sheng, G.; Juranek, S.; Tuschl, T.; Patel, D.J. Structure of the guide-strand-containing argonaute silencing complex. Nature 2008, 456, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Fire, A.; Xu, S.Q.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Hutvagner, G.; Simard, M.J. Argonaute proteins: Key players in RNA silencing. Nat. Rev. Mol. Cell Biol. 2008, 9, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Lewis, B.P.; Shih, I.H.; Jones-Rhoades, M.W.; Bartel, D.P.; Burge, C.B. Prediction of mammalian microRNA targets. Cell 2003, 115, 787–798. [Google Scholar] [CrossRef]
- Brennecke, J.; Stark, A.; Russell, R.B.; Cohen, S.M. Principles of microRNA-target recognition. PLoS Biol. 2005, 3, e85. [Google Scholar] [CrossRef] [PubMed]
- Elbashir, S.M.; Martinez, J.; Patkaniowska, A.; Lendeckel, W.; Tuschl, T. Functional anatomy of siRNAs for mediating efficient RNAi in Drosophila melanogaster embryo lysate. EMBO J. 2001, 20, 6877–6888. [Google Scholar] [CrossRef] [PubMed]
- Sheng, G.; Zhao, H.; Wang, J.; Rao, Y.; Tian, W.; Swarts, D.C.; van der Oost, J.; Patel, D.J.; Wang, Y. Structure-based cleavage mechanism of Thermus thermophilus Argonaute DNA guide strand-mediated DNA target cleavage. Proc. Natl. Acad. Sci. USA 2014, 111, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Meister, G.; Landthaler, M.; Patkaniowska, A.; Dorsett, Y.; Teng, G.; Tuschl, T. Human Argonaute2 mediates RNA cleavage targeted by miRNAs and siRNAs. Mol. Cell 2004, 15, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Carmell, M.A.; Rivas, F.V.; Marsden, C.G.; Thomson, J.M.; Song, J.; Hammond, S.M.; Joshua-Tor, L.; Hannon, G.J. Argonaute2 is the catalytic engine of mammalian RNAi. Science 2004, 305, 1437–1441. [Google Scholar] [CrossRef] [PubMed]
- Elbashir, S.M.; Lendeckel, W.; Tuschl, T. RNA interference is mediated by 21- and 22-nucleotide RNAs. Genes Dev. 2001, 15, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, E.; Caudy, A.A.; Hammond, S.M.; Hannon, G.J. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature 2001, 409, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Noland, C.; Siridechadilok, B.; Taylor, D.W.; Ma, E.; Felderer, K.; Doudna, J.A.; Nogales, E. Structural insights into RNA processing by the human RISC-loading complex. Nat. Struct. Mol. Biol. 2009, 16, 1148–1153. [Google Scholar] [CrossRef] [PubMed]
- Tomari, Y.; Matranga, C.; Haley, B.; Martinez, N.; Zamore, P.D. A protein sensor for siRNA asymmetry. Science 2004, 306, 1377–1380. [Google Scholar] [CrossRef] [PubMed]
- MacRae, I.J.; Ma, E.; Zhou, M.; Robinson, C.V.; Doudna, J.A. In vitro reconstitution of the human RISC-loading complex. Proc. Natl. Acad. Sci. USA 2008, 105, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Hur, I.; Park, S.; Kim, Y.; Suh, M.; Kim, V.N. The role of PACT in the RNA silencing pathway. EMBO J. 2006, 25, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Chendrimada, T.P.; Gregory, R.I.; Kumaraswamy, E.; Norman, J.; Cooch, N.; Nishikura, K.; Shiekhattar, R. TRBP recruits the Dicer complex to Ago2 for microRNA processing and gene silencing. Nature 2005, 436, 740–744. [Google Scholar] [CrossRef] [PubMed]
- Matranga, C.; Tomari, Y.; Shin, C.; Bartel, D.P.; Zamore, P.D. Passenger-strand cleavage facilitates assembly of siRNA into Ago2-containing RNAi enzyme complexes. Cell 2005, 123, 607–620. [Google Scholar] [CrossRef] [PubMed]
- Leuschner, P.J.; Ameres, S.L.; Kueng, S.; Martinez, J. Cleavage of the siRNA passenger strand during RISC assembly in human cells. EMBO Rep. 2006, 7, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Rand, T.A.; Petersen, S.; Du, F.; Wang, X. Argonaute2 cleaves the anti-guide strand of siRNA during RISC activation. Cell 2005, 123, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Hammond, S.M.; Bernstein, E.; Beach, D.; Hannon, G.J. An RNA-directed nuclease mediates post-transcriptional gene silencing in Drosophila cells. Nature 2000, 404, 293–296. [Google Scholar] [PubMed]
- Haley, B.; Zamore, P.D. Kinetic analysis of the RNAi enzyme complex. Nat. Struct. Mol. Biol. 2004, 11, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Filipowicz, W.; Bhattacharyya, S.N.; Sonenberg, N. Mechanisms of post-transcriptional regulation by microRNAs: Are the answers in sight? Nat. Rev. Genet. 2008, 9, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Pillai, R.S.; Bhattacharyya, S.N.; Artus, C.G.; Zoller, T.; Cougot, N.; Basyuk, E.; Bertrand, E.; Filipowicz, W. Inhibition of translational initiation by Let-7 microRNA in human cells. Science 2005, 309, 1573–1576. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, D.T.; Westman, B.J.; Martin, D.I.K.; Preiss, T. MicroRNAs control translation initiation by inhibiting eukaryotic initiation factor 4E/cap and poly(A) tail function. Proc. Natl. Acad. Sci. USA 2005, 102, 16961–16966. [Google Scholar] [CrossRef] [PubMed]
- Fukaya, T.; Iwakawa, H.O.; Tomari, Y. MicroRNAs block assembly of eIF4F translation initiation complex in Drosophila. Mol. Cell 2014, 56, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Zander, A.; Holzmeister, P.; Klose, D.; Tinnefeld, P.; Grohmann, D. Single-molecule FRET supports the two-state model of Argonaute action. RNA Biol. 2014, 11, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Yuan, Y.; Meister, G.; Pei, Y.; Tuschl, T.; Patel, D.J. Structural basis for 5′-end-specific recognition of guide RNA by the A. fulgidus Piwi protein. Nature 2005, 434, 666–670. [Google Scholar] [CrossRef] [PubMed]
- Swarts, D.C.; Jore, M.M.; Westra, E.R.; Zhu, Y.; Janssen, J.H.; Snijders, A.P.; Wang, Y.; Patel, D.J.; Berenguer, J.; Brouns, S.J.J.; et al. DNA-guided DNA interference by a prokaryotic Argonaute. Nature 2014, 507, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Olovnikov, I.; Chan, K.; Sachidanandam, R.; Newman, D.K.; Aravin, A. Bacterial argonaute samples the transcriptome to identify foreign DNA. Mol. Cell 2013, 51, 594–605. [Google Scholar] [CrossRef] [PubMed]
- Willkomm, S.; Zander, A.; Gust, A.; Grohmann, D. A prokaryotic twist on argonaute function. Life 2015, 5, 538–553. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, K.; Weinberg, D.E.; Bartel, D.P.; Patel, D.J. Structure of yeast Argonaute with guide RNA. Nature 2012, 486, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Schirle, N.T.; MacRae, I.J. The crystal structure of human Argonaute2. Science 2012, 336, 1037–1040. [Google Scholar] [CrossRef] [PubMed]
- Schirle, N.T.; Sheu-Gruttadauria, J.; MacRae, I.J. Structural basis for microRNA targeting. Science 2014, 346, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Elkayam, E.; Kuhn, C.-D.; Tocilj, A.; Haase, A.D.; Greene, E.M.; Hannon, G.J.; Joshua-Tor, L. The structure of human Argonaute-2 in complex with miR-20a. Cell 2012, 150, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, K.; Ascano, M.; Gogakos, T.; Ishibe-Murakami, S.; Serganov, A.A.; Briskin, D.; Morozov, P.; Tuschl, T.; Patel, D.J. Eukaryote-specific insertion elements control human Argonaute slicer activity. Cell Rep. 2013, 3, 1893–1900. [Google Scholar] [CrossRef] [PubMed]
- Frank, F.; Sonenberg, N.; Nagar, B. Structural basis for 5′-nucleotide base-specific recognition of guide RNA by human Ago2. Nature 2010, 465, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Frank, F.; Hauver, J.; Sonenberg, N.; Nagar, B. Arabidopsis Argonaute MID domains use their nucleotide specificity loop to sort small RNAs. EMBO J. 2012, 31, 3588–3595. [Google Scholar] [CrossRef] [PubMed]
- Boland, A.; Huntzinger, E.; Schmidt, S.; Izaurralde, E.; Weichenrieder, O. Crystal structure of the MID-PIWI lobe of a eukaryotic Argonaute protein. Proc. Natl. Acad. Sci. USA 2011, 108, 10466–10471. [Google Scholar] [CrossRef] [PubMed]
- Rivas, F.V.; Tolia, N.H.; Song, J.; Aragon, J.P.; Liu, J.; Hannon, G.J.; Joshua-Tor, L. Purified Argonaute2 and an siRNA form recombinant human RISC. Nat. Struct. Mol. Biol. 2005, 12, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Deerberg, A.; Willkomm, S.; Restle, T. Minimal mechanistic model of siRNA-dependent target RNA slicing by recombinant human Argonaute 2 protein. Proc. Natl. Acad. Sci. USA 2013, 110, 17850–17855. [Google Scholar] [CrossRef] [PubMed]
- Lima, W.F.; Wu, H.; Nichols, J.G.; Sun, H.; Murray, H.M.; Crooke, S.T. Binding and cleavage specificities of human Argonaute2. J. Biol. Chem. 2009, 284, 26017–26028. [Google Scholar] [CrossRef] [PubMed]
- Rashid, U.J.; Paterok, D.; Koglin, A.; Gohlke, H.; Piehler, J.; Chen, J. Structure of Aquifex aeolicus argonaute highlights conformational flexibility of the PAZ domain as a potential regulator of RNA-induced silencing complex function. J. Biol. Chem. 2007, 282, 13824–13832. [Google Scholar] [CrossRef] [PubMed]
- Lau, N.C.; Lim, L.P.; Weinstein, E.G.; Bartel, D.P. An abundant class of tiny RNAs with probable regulatory roles in Caenorhabditis elegans. Science 2001, 294, 858–862. [Google Scholar] [CrossRef] [PubMed]
- Ghildiyal, M.; Seitz, H.; Horwich, M.D.; Li, C.; Du, T.; Lee, S.; Xu, J.; Kittler, E.L.; Zapp, M.L.; Weng, Z.; et al. Endogenous siRNAs derived from transposons and mRNAs in Drosophila somatic cells. Science 2008, 320, 1077–1081. [Google Scholar] [CrossRef] [PubMed]
- Ghildiyal, M.; Xu, J.; Seitz, H.; Weng, Z.; Zamore, P.D. Sorting of Drosophila small silencing RNAs partitions microRNA* strands into the RNA interference pathway. RNA 2010, 16, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.Y.; Yan, Z.; Xu, Y.; Hu, H.; Menzel, C.; Zhou, Y.H.; Chen, W.; Khaitovich, P. Sequence features associated with microRNA strand selection in humans and flies. BMC Genomics 2009, 10, 413. [Google Scholar] [CrossRef] [PubMed]
- Felice, K.M.; Salzman, D.W.; Shubert-Coleman, J.; Jensen, K.P.; Furneaux, H.M. The 5′ terminal uracil of let-7a is critical for the recruitment of mRNA to Argonaute2. Biochem. J. 2009, 422, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, D.S.; Hutvágner, G.; Du, T.; Xu, Z.; Aronin, N.; Zamore, P.D. Asymmetry in the assembly of the RNAi enzyme complex. Cell 2003, 115, 199–208. [Google Scholar] [CrossRef]
- Khvorova, A.; Reynolds, A.; Jayasena, S.D. Functional siRNAs and miRNAs exhibit strand bias. Cell 2003, 115, 209–216. [Google Scholar] [CrossRef]
- Hur, J.K.; Zinchenko, M.K.; Djuranovic, S.; Green, R. Regulation of Argonaute slicer activity by guide RNA 3′ end interactions with the N-terminal lobe. J. Biol. Chem. 2013, 288, 7829–7840. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Kim, E.; Hwang, W.; Shin, S.; Song, J.; Hohng, S. Dynamic anchoring of the 3′-end of the guide strand controls the target dissociation of Argonaute-guide complex. J. Am. Chem. Soc. 2013, 135, 16865–16871. [Google Scholar] [CrossRef] [PubMed]
- Ming, D.; Wall, M.E.; Sanbonmatsu, K.Y. Domain motions of Argonaute, the catalytic engine of RNA interference. BMC Bioinform. 2007, 8, 470. [Google Scholar] [CrossRef] [PubMed]
- Winter, J.; Diederichs, S. Argonaute proteins regulate microRNA stability: Increased microRNA abundance by Argonaute proteins is due to microRNA stabilization. RNA Biol. 2011, 8, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Jin, L.; Huang, Y.; Zhang, F.; Kay, M.A. Slicing-independent RISC activation requires the argonaute PAZ domain. Curr. Biol. 2012, 22, 1536–1542. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Rivas, F.V.; Wohlschlegel, J.; Yates, J.R.; Parker, R.; Hannon, G.J. A role for the P-body component GW182 in microRNA function. Nat. Cell Biol. 2005, 7, 1261–1266. [Google Scholar] [CrossRef] [PubMed]
- Ameres, S.L.; Martinez, J.; Schroeder, R. Molecular basis for target RNA recognition and cleavage by human RISC. Cell 2007, 130, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Lambert, N.J.; Gu, S.G.; Zahler, A.M. The conformation of microRNA seed regions in native microRNPs is prearranged for presentation to mRNA targets. Nucleic Acids Res. 2011, 39, 4827–4835. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.S.; Parizotto, E.A.; Wang, M.; Roe, S.M.; Barford, D. Enhancement of the seed-target recognition step in RNA silencing by a PIWI/MID domain protein. Mol. Cell 2009, 33, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Schürmann, N.; Trabuco, L.G.; Bender, C.; Russell, R.B.; Grimm, D. Molecular dissection of human Argonaute proteins by DNA shuffling. Nat. Struct. Mol. Biol. 2013, 20, 818–826. [Google Scholar] [CrossRef] [PubMed]
- Kwak, P.B.; Tomari, Y. The N domain of Argonaute drives duplex unwinding during RISC assembly. Nat. Struct. Mol. Biol. 2012, 19, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Hauptmann, J.; Dueck, A.; Harlander, S.; Pfaff, J.; Merkl, R.; Meister, G. Turning catalytically inactive human Argonaute proteins into active slicer enzymes. Nat. Struct. Mol. Biol. 2013, 20, 814–817. [Google Scholar] [CrossRef] [PubMed]
- Faehnle, C.R.; Elkayam, E.; Haase, A.D.; Hannon, G.J.; Joshua-Tor, L. The making of a slicer: Activation of human Argonaute1. Cell Rep. 2013, 3, 1901–1909. [Google Scholar] [CrossRef] [PubMed]
- Wee, L.M.; Flores-Jasso, C.F.; Salomon, W.E.; Zamore, P.D. Argonaute divides its RNA guide into domains with distinct functions and RNA-binding properties. Cell 2012, 151, 1055–1067. [Google Scholar] [CrossRef] [PubMed]
- Bramsen, J.B.; Laursen, M.B.; Nielsen, A.F.; Hansen, T.B.; Bus, C.; Langkjaer, N.; Babu, B.R.; Højland, T.; Abramov, M.; van Aerschot, A.; et al. A large-scale chemical modification screen identifies design rules to generate siRNAs with high activity, high stability and low toxicity. Nucleic Acids Res. 2009, 37, 2867–2881. [Google Scholar] [CrossRef] [PubMed]
- Chounga, S.; Kimb, Y.J.; Kima, S.; Parka, H.; Choi, Y. Chemical modification of siRNAs to improve serum stability without loss of efficacy. Biochem. Biophys. Res. Commun. 2006, 342, 919–927. [Google Scholar] [CrossRef]
- Allerson, C.R.; Sioufi, N.; Jarres, R.; Prakash, T.P.; Naik, N.; Berdeja, A.; Wanders, L.; Griffey, R.H.; Swayze, E.E.; Bhat, B. Fully 2′-modified oligonucleotide duplexes with improved in vitro potency and stability compared to unmodified small interfering RNA. J. Med. Chem. 2005, 48, 901–904. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Willkomm, S.; Restle, T. Conformational Dynamics of Ago-Mediated Silencing Processes. Int. J. Mol. Sci. 2015, 16, 14769-14785. https://doi.org/10.3390/ijms160714769
Willkomm S, Restle T. Conformational Dynamics of Ago-Mediated Silencing Processes. International Journal of Molecular Sciences. 2015; 16(7):14769-14785. https://doi.org/10.3390/ijms160714769
Chicago/Turabian StyleWillkomm, Sarah, and Tobias Restle. 2015. "Conformational Dynamics of Ago-Mediated Silencing Processes" International Journal of Molecular Sciences 16, no. 7: 14769-14785. https://doi.org/10.3390/ijms160714769
APA StyleWillkomm, S., & Restle, T. (2015). Conformational Dynamics of Ago-Mediated Silencing Processes. International Journal of Molecular Sciences, 16(7), 14769-14785. https://doi.org/10.3390/ijms160714769





