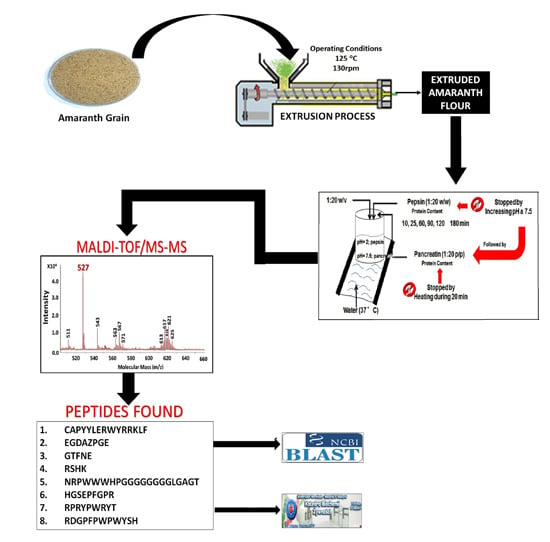
Characterization of Peptides Found in Unprocessed and Extruded Amaranth (Amaranthus hypochondriacus) Pepsin/Pancreatin Hydrolysates
Abstract
:1. Introduction
2. Results and Discussion
2.1. Pepsin and Pancreatin Hydrolysates
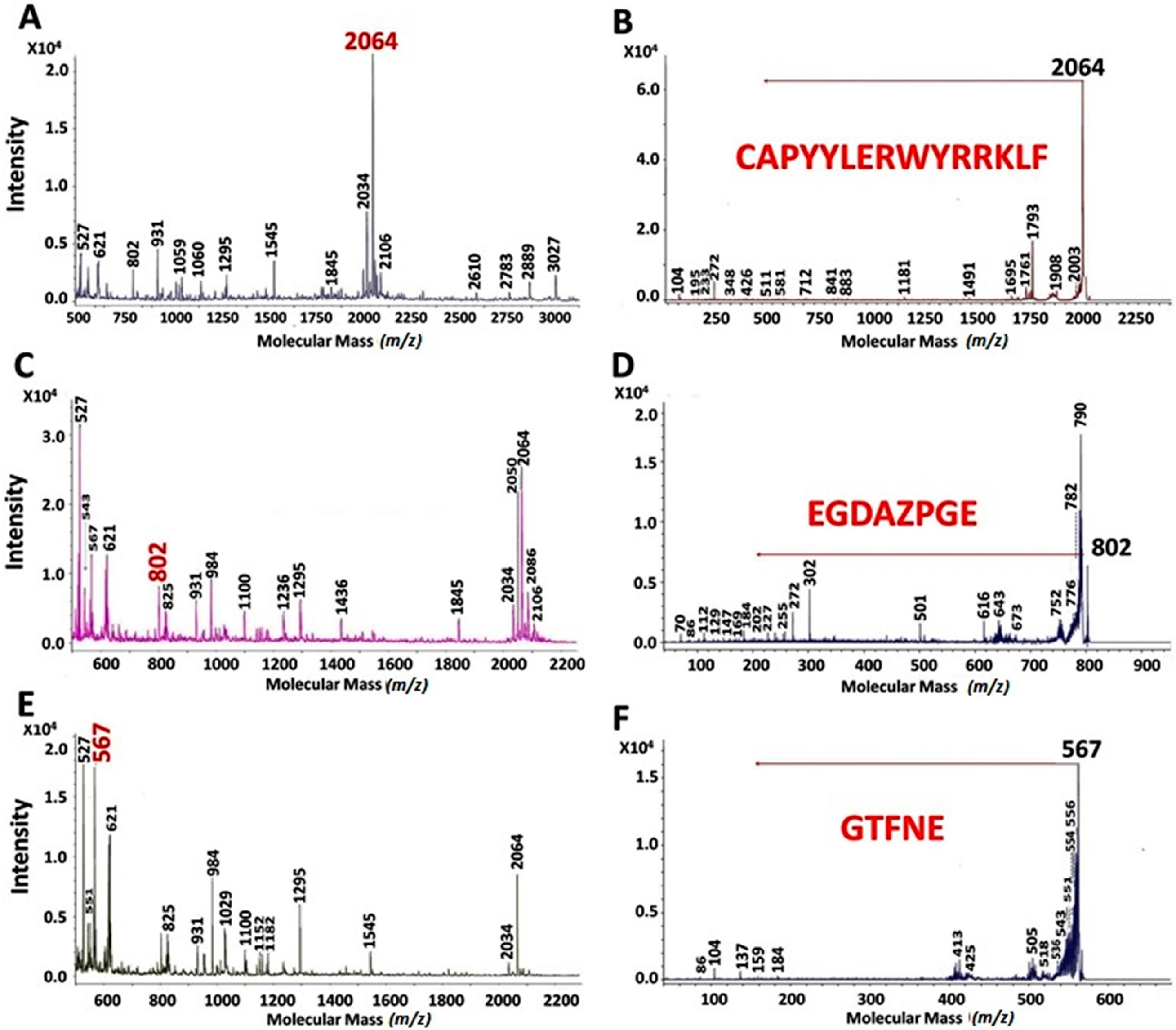
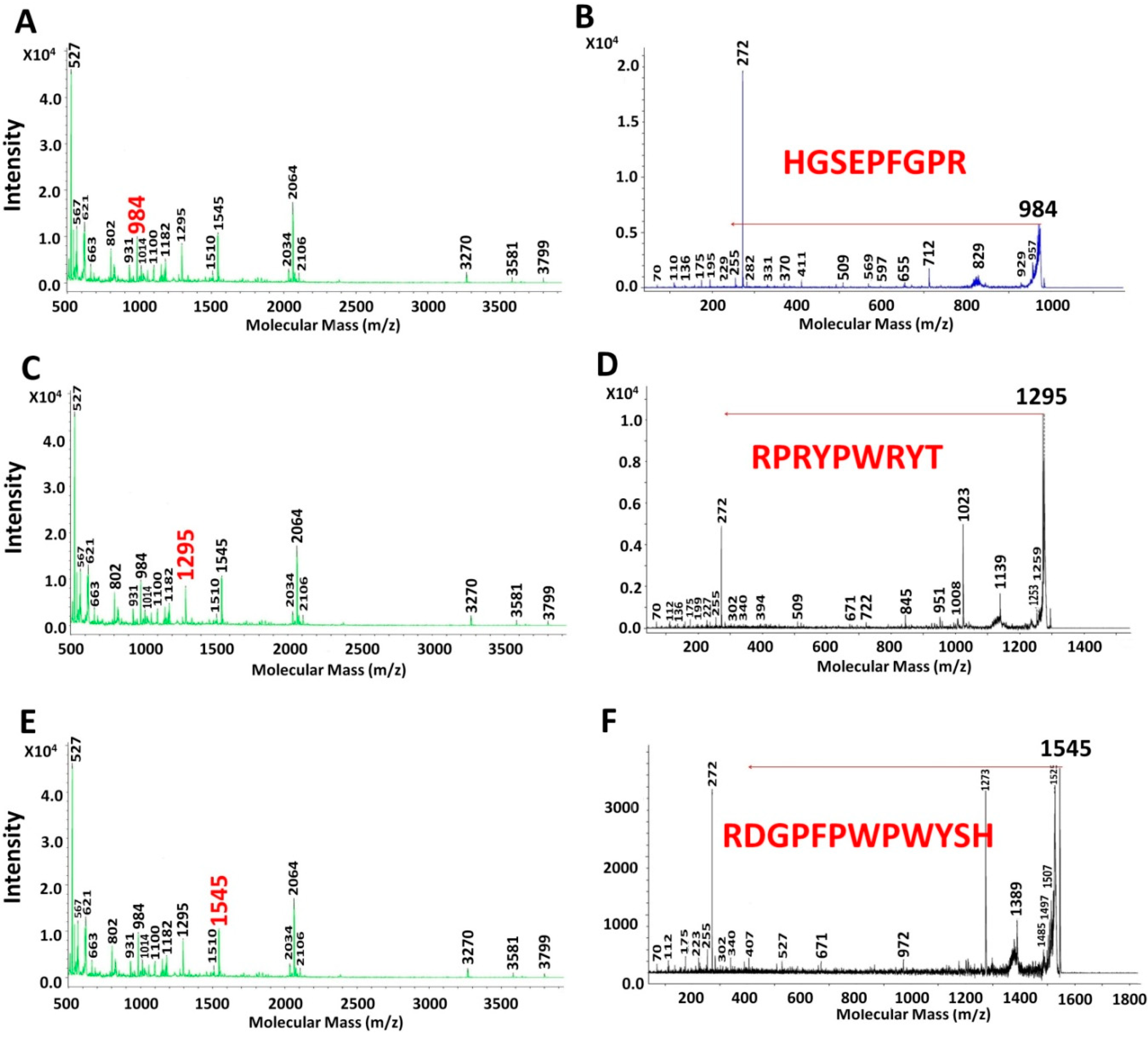
2.2. MALDI-TOF Hydrolysates Characterization
2.3. Characterization of UAH and EAH Amaranth Peptides by MS-MS
| MM (Da) | 10 RA | 25 RA | 60 RA | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Intensity | Area | % of Area | Intensity | Area | % of Area | Intensity | Area | % of Area | |
| 520 | - | - | - | 8533 | 2520 | 3.47 | - | - | - |
| 522 | 2796 | 698 | 0.97 | 16,061 | 5044 | 6.95 | - | - | - |
| 527 | 3847 | 938 | 1.31 | 5396 | 1374 | 1.89 | 23,053 | 4712 | 5.65 |
| 543 | - | - | - | - | - | - | 4617 | 1019 | 1.22 |
| 563 | - | - | - | 6290 | 1774 | 2.44 | 6816 | 1433 | 1.72 |
| 567 | 2805 | 826 | 1.15 | 11,598 | 3200 | 4.41 | 13,107 | 2797 | 3.35 |
| 571 | - | - | - | 3607 | 1051 | 1.45 | 3881 | 795 | 0.95 |
| 613 | - | - | - | 3298 | 1000 | 1.38 | 3515 | 774 | 0.93 |
| 616 | - | - | - | 4222 | 1357 | 1.87 | 4897 | 1117 | 1.34 |
| 617 | - | - | - | 10,310 | 3256 | 4.48 | 12,614 | 3045 | 3.65 |
| 619 | 2871 | 920 | 1.28 | 3505 | 1132 | 1.56 | 4704 | 1078 | 1.29 |
| 620 | - | - | - | 3905 | 1392 | 1.92 | 5409 | 1577 | 1.89 |
| 621 | 2911 | 911 | 1.27 | 10,788 | 3413 | 4.70 | 13,871 | 3477 | 4.17 |
| 623 | - | - | - | - | - | - | 3763 | 1152 | 1.38 |
| 625 | - | - | - | 4098 | 1435 | 1.98 | 5854 | 1470 | 1.76 |
| 802 | 2585 | 1005 | 1.40 | 3490 | 1541 | 2.12 | 3108 | 971 | 1.16 |
| 821 | - | - | - | - | - | - | 2560 | 771 | 0.92 |
| 825 | - | - | - | 3957 | 1729 | 2.38 | 5008 | 1591 | 1.91 |
| 829 | - | - | - | 3118 | 1561 | 2.15 | 4294 | 1408 | 1.69 |
| 931 | 4251 | 1907 | 2.66 | 4511 | 2259 | 3.11 | 3073 | 1065 | 1.28 |
| 984 | - | - | - | - | - | - | 2670 | 1094 | 1.31 |
| 1028 | 1892 | 754 | 1.05 | - | - | - | - | - | - |
| 1045 | 1405 | 720 | 1.00 | - | - | - | - | - | - |
| 1060 | 1952 | 1057 | 1.47 | 3020 | 1803 | 2.48 | - | - | - |
| 1160 | 1994 | 1101 | 1.53 | 1850 | 1217 | 1.68 | - | - | - |
| 1234 | - | - | - | - | - | - | 2487 | 1424 | 1.71 |
| 1295 | 2130 | 1393 | 1.94 | 3851 | 2850 | 3.93 | 3517 | 2107 | 2.52 |
| 1435 | - | - | - | - | - | - | 1668 | 1292 | 1.55 |
| 1545 | 3343 | 2703 | 3.77 | 2808 | 2565 | 3.53 | 1433 | 1134 | 1.36 |
| 1845 | 990 | 2515 | 3.51 | - | - | - | 1369 | 3123 | 3.74 |
| 1899 | - | - | - | 1124 | 1503 | 2.07 | - | - | - |
| 2014 | 2316 | 2825 | 3.94 | 912 | 1166 | 1.61 | 821 | 1040 | 1.25 |
| 2034 | 6795 | 8809 | 12.28 | 3669 | 4628 | 6.37 | 4647 | 5384 | 6.45 |
| 2050 | 1260 | 2559 | 3.57 | - | - | - | 1292 | 2289 | 2.74 |
| 2056 | - | - | - | - | - | - | 803 | 1358 | 1.63 |
| 2064 | 22,157 | 25,398 | 35.40 | 9809 | 13,092 | 18.03 | 16,883 | 20,859 | 24.99 |
| 2071 | - | - | - | - | - | - | 3278 | 3854 | 4.62 |
| 2076 | 3048 | 4023 | 5.61 | 2397 | 3203 | 4.41 | - | - | - |
| 2086 | 1684 | 2013 | 2.81 | - | - | - | 2818 | 3384 | 4.05 |
| 2106 | 1790 | 2404 | 3.35 | 1356 | 1873 | 2.58 | 1888 | 2063 | 2.47 |
| 2609 | 577 | 971 | 1.35 | - | - | - | - | - | - |
| 2783 | 491 | 732 | 1.02 | - | - | - | - | - | - |
| 2889 | 1142 | 1952 | 2.72 | 582 | 1299 | 1.79 | - | - | - |
| 3027 | 1380 | 2612 | 3.64 | 967 | 2367 | 3.26 | 331 | 656 | 0.79 |
| 3267 | - | - | - | - | - | - | 395 | 785 | 0.94 |
| 3581 | - | - | - | - | - | - | 583 | 1369 | 1.64 |
| MM (Da) | 90 RA | 120 RA | 180 RA | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Intensity | Area | % of Area | Intensity | Area | % of Area | Intensity | Area | % of Area | |
| 511 | - | - | - | 4456 | 1147 | 0.97 | - | - | - |
| 518 | 3942 | 911 | 1.86 | - | - | - | - | - | - |
| 520 | 10,311 | 2162 | 4.42 | 7918 | 1787 | 1.51 | - | - | - |
| 522 | 16,113 | 3407 | 6.97 | 12,115 | 2917 | 2.47 | - | - | - |
| 527 | 28,308 | 5819 | 11.90 | 30,901 | 6863 | 5.81 | 17,708 | 4186 | 7.01 |
| 543 | 6887 | 2151 | 4.40 | 6785 | 1717 | 1.45 | 3578 | 952 | 1.59 |
| 544 | 3402 | 765 | 1.57 | - | - | - | - | - | - |
| 551 | 3483 | 1701 | 3.48 | - | - | - | 3139 | 1601 | 2.68 |
| 563 | 8122 | 1639 | 3.35 | 6055 | 1348 | 1.14 | 6418 | 1641 | 2.75 |
| 567 | 15,285 | 3149 | 6.44 | 1290 | 2661 | 2.25 | 19,127 | 2849 | 4.77 |
| 571 | 4652 | 888 | 1.82 | 3815 | 762 | 0.65 | 3438 | 875 | 1.47 |
| 605 | 3702 | 855 | 1.75 | - | - | - | 2429 | 689 | 1.15 |
| 613 | 4086 | 923 | 1.89 | - | - | - | 3113 | 950 | 1.59 |
| 614 | 3559 | 942 | 1.93 | - | - | - | - | - | - |
| 616 | 5833 | 1374 | 2.81 | 3936 | 1192 | 1.01 | 4275 | 1254 | 2.10 |
| 617 | 14,666 | 3479 | 7.12 | 9341 | 2555 | 2.16 | 9820 | 2750 | 4.61 |
| 619 | 5729 | 1301 | 2.66 | 4213 | 1279 | 1.08 | 3311 | 1055 | 1.77 |
| 620 | 6434 | 1842 | 3.77 | 12,159 | 3405 | 2.88 | 3695 | 1151 | 1.93 |
| 621 | 16,898 | 4122 | 8.43 | - | - | - | 10,019 | 3052 | 5.11 |
| 623 | 4467 | 1394 | 2.85 | - | - | - | 2696 | 856 | 1.43 |
| 625 | 6945 | 1724 | 3.53 | 4569 | 1456 | 1.23 | 3693 | 1298 | 2.17 |
| 642 | 5163 | 1196 | 2.45 | - | - | - | - | - | - |
| 802 | - | - | - | 8474 | 3029 | 2.57 | 3646 | 1191 | 2.00 |
| 821 | 3265 | 957 | 1.96 | - | - | - | - | - | - |
| 825 | 6533 | 1986 | 4.06 | 4843 | 1616 | 1.37 | 3710 | 1403 | 2.35 |
| 829 | 5582 | 1748 | 3.58 | 4178 | 1447 | 1.23 | 2822 | 1227 | 2.06 |
| 931 | - | - | - | 5546 | 2509 | 2.12 | 2293 | 871 | 1.46 |
| 984 | - | - | - | 8651 | 4514 | 3.82 | 7974 | 3494 | 5.85 |
| 1028 | - | - | - | - | - | - | 3241 | 1271 | 2.13 |
| 1029 | - | - | - | - | - | - | 2988 | 1344 | 2.25 |
| 1100 | - | - | - | 3852 | 2177 | 1.84 | 1971 | 991 | 1.66 |
| 1103 | - | - | - | - | - | - | 1592 | 732 | 1.23 |
| 1152 | - | - | - | - | - | - | 1622 | 870 | 1.46 |
| 1161 | - | - | - | - | - | - | 1674 | 918 | 1.54 |
| 1182 | - | - | - | - | - | - | 1802 | 1041 | 1.74 |
| 1236 | 2061 | 1168 | 2.39 | 4094 | 2155 | 1.83 | - | - | - |
| 1295 | 2045 | 1276 | 2.61 | 5722 | 3977 | 3.37 | 5641 | 3503 | 5.87 |
| 1436 | - | - | - | 2914 | 2160 | 1.83 | - | - | - |
| 1545 | - | - | - | - | - | - | 1916 | 1568 | 2.63 |
| 1845 | - | - | - | 2880 | 6193 | 5.24 | - | - | - |
| 2034 | 1638 | 1764 | 3.61 | 4791 | 5546 | 4.70 | 849 | 1040 | 1.74 |
| 2046 | - | - | - | 1064 | 1693 | 1.43 | - | - | - |
| 2050 | - | - | - | 2476 | 4615 | 3.91 | - | - | - |
| 2056 | - | - | - | 1428 | 2673 | 2.26 | - | - | - |
| 2064 | 5467 | 6052 | 12.38 | 17,250 | 25,782 | 21.84 | 7614 | 9419 | 15.78 |
| 2076 | 1412 | 1592 | 3.26 | 2644 | 3473 | 2.94 | - | - | - |
| 2086 | 1374 | 1414 | 2.89 | 6138 | 7024 | 5.95 | - | - | - |
| 2102 | - | - | - | 1306 | 1510 | 1.28 | - | - | - |
| 2106 | 838 | 944 | 1.93 | 2060 | 2389 | 2.02 | - | - | - |
| 2118 | - | - | - | 1463 | 1556 | 1.32 | - | - | - |
| 3267 | 936 | 1670 | 3.42 | 877 | 1697 | 1.44 | 873 | 1812 | 3.04 |
| 3585 | - | - | - | - | - | - | 154 | 411 | 0.69 |
| 3910 | - | - | - | 278 | 598 | 0.51 | 143 | 377 | 0.63 |
| 3931 | - | - | - | 302 | 653 | 0.55 | - | - | - |
| MM (Da) | 10 EA | 25 EA | 60 EA | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Intensity | Area | % of Area | Intensity | Area | % of Area | Intensity | Area | % of area | |
| 511 | 4023 | 1200 | 1.18 | - | - | - | - | - | - |
| 527 | 44,864 | 8348 | 8.18 | 32,294 | 7546 | 5.70 | 32,765 | 7195 | 8.73 |
| 543 | 9619 | 2363 | 2.32 | 8061 | 1853 | 1.40 | 7333 | 1656 | 2.01 |
| 563 | 5331 | 1303 | 1.28 | 10,371 | 2528 | 1.91 | 6213 | 1356 | 1.64 |
| 567 | 8233 | 2054 | 2.01 | 10,797 | 4652 | 3.51 | 12,111 | 2745 | 3.33 |
| 571 | 2406 | 646 | 0.63 | 6062 | 1416 | 1.07 | 4350 | 949 | 1.15 |
| 613 | 2705 | 638 | 0.63 | 5618 | 1366 | 1.03 | 3196 | 841 | 1.02 |
| 616 | 3477 | 853 | 0.84 | 7149 | 1823 | 1.38 | 4765 | 1241 | 1.51 |
| 617 | 8638 | 2247 | 2.20 | 17,484 | 4759 | 3.60 | 10,565 | 2863 | 3.47 |
| 619 | 2880 | 908 | 0.89 | 6468 | 1833 | 1.38 | 3719 | 1172 | 1.42 |
| 620 | 4233 | 1065 | 1.04 | 7118 | 2387 | 1.80 | 4597 | 1078 | 1.31 |
| 621 | 9408 | 2591 | 2.54 | 19,361 | 5429 | 4.10 | 12,254 | 3405 | 4.13 |
| 623 | 2537 | 815 | 0.80 | 5323 | 1707 | 1.29 | 3638 | 1120 | 1.36 |
| 625 | 3861 | 1102 | 1.08 | 8395 | 2545 | 1.92 | 5284 | 1613 | 1.96 |
| 802 | - | - | - | - | - | - | 6918 | 3091 | 3.75 |
| 821 | - | - | - | 4037 | 1325 | 1.00 | - | - | - |
| 825 | 3562 | 1289 | 1.26 | 7130 | 2505 | 1.89 | 5265 | 1793 | 2.17 |
| 829 | 2853 | 1068 | 1.05 | 5784 | 2196 | 1.66 | 4384 | 1658 | 2.01 |
| 931 | - | - | - | - | - | - | 4247 | 2236 | 2.71 |
| 984 | - | - | - | - | - | - | 3431 | 1938 | 2.35 |
| 1029 | 1751 | 1067 | 1.05 | - | - | - | - | - | - |
| 1058 | - | - | - | 2853 | 2328 | 1.76 | - | - | - |
| 1152 | - | - | - | - | - | - | 2161 | 1564 | 1.90 |
| 1175 | - | - | - | - | - | - | 2287 | 1244 | 1.51 |
| 1295 | - | - | - | 2737 | 2253 | 1.70 | 3010 | 2317 | 2.81 |
| 1510 | - | - | - | - | - | - | - | 1719 | 2.08 |
| 1545 | - | - | - | 6383 | 6705 | 5.07 | 1520 | 3837 | 4.65 |
| 2014 | - | - | - | 1036 | 1568 | 1.18 | 3760 | 6488 | 7.87 |
| 2034 | 1618 | 2496 | 2.45 | 6796 | 9606 | 7.26 | 4402 | 14,649 | 17.77 |
| 2064 | 4010 | 6399 | 6.27 | 15,951 | 25,295 | 19.11 | 10,248 | 3654 | 4.43 |
| 2076 | 1055 | 1764 | 1.73 | 3864 | 5300 | 4.00 | 2681 | 2179 | 2.64 |
| 2106 | - | - | - | 2137 | 2953 | 2.23 | 1555 | 3359 | 4.07 |
| 3267 | - | - | - | 306 | 604 | 0.46 | 1184 | 1749 | 2.12 |
| 3581 | - | - | - | 561 | 1220 | 0.92 | 555 | 1749 | 2.12 |
| 4046 | 1119 | 13,322 | 13.06 | 490 | 6246 | 4.72 | - | - | - |
| 4059 | 813 | 11,555 | 11.33 | 292 | 3704 | 2.80 | - | - | - |
| 4080 | 1706 | 22,887 | 22.44 | 361 | 5643 | 4.26 | - | - | - |
| 4098 | - | - | - | 280 | 4109 | 3.10 | - | - | - |
| 4105 | 963 | 14033 | 13.76 | - | - | - | - | - | - |
| 4121 | - | - | - | 569 | 8944 | 6.76 | - | - | - |
| MM (Da) | 90 EA | 120 EA | 180 EA | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Intensity | Area | % of Area | Intensity | Area | % of Area | Intensity | Area | % of Area | |
| 511 | 3431 | 1035 | 1.28 | 4047 | 1672 | 1.44 | 2889 | 1225 | 2.14 |
| 527 | 33,333 | 6872 | 8.49 | 35,283 | 11664 | 10.07 | 27,734 | 6793 | 11.85 |
| 543 | 10,127 | 2156 | 2.67 | 10,824 | 2798 | 2.41 | 5629 | 1466 | 2.56 |
| 551 | 4242 | 1312 | 1.62 | - | - | - | - | - | - |
| 563 | 6457 | 1394 | 1.72 | 6494 | 1893 | 1.63 | 4824 | 1357 | 2.37 |
| 567 | 11,131 | 2447 | 3.02 | 11,454 | 3150 | 2.72 | 8491 | 2227 | 3.89 |
| 571 | 3204 | 634 | 0.78 | 3222 | 973 | 0.84 | - | - | - |
| 613 | 3580 | 854 | 1.06 | 3147 | 893 | 0.77 | 2492 | 731 | 1.28 |
| 616 | 4082 | 1250 | 1.55 | 4563 | 1156 | 1.00 | 2923 | 926 | 1.62 |
| 617 | 12,157 | 3044 | 3.76 | 9682 | 2752 | 2.37 | 8856 | 2501 | 4.36 |
| 619 | 4303 | 1077 | 1.33 | 3670 | 995 | 0.86 | 2839 | 920 | 1.61 |
| 620 | 5250 | 1438 | 1.78 | 4300 | 1373 | 1.18 | 3295 | 998 | 1.74 |
| 621 | 13,401 | 3485 | 4.31 | 10,859 | 3188 | 2.75 | 8726 | 2567 | 4.48 |
| 623 | 3494 | 1106 | 1.37 | 3165 | 1001 | 0.86 | 2480 | 784 | 1.37 |
| 625 | 5315 | 1439 | 1.78 | 4293 | 1402 | 1.21 | 3410 | 1121 | 1.96 |
| 663 | - | - | - | 3324 | 1116 | 0.96 | - | - | - |
| 802 | 7650 | 3318 | 4.10 | 6860 | 2890 | 2.49 | 7342 | 3409 | 5.95 |
| 825 | 4532 | 1523 | 1.88 | 4283 | 1651 | 1.42 | 2999 | 1248 | 2.18 |
| 829 | 4248 | 1491 | 1.84 | 3481 | 1514 | 1.31 | 2313 | 1077 | 1.88 |
| 931 | 5171 | 2313 | 2.86 | 3450 | 1701 | 1.47 | 3715 | 1977 | 3.45 |
| 984 | 6532 | 2978 | 3.68 | 9331 | 4683 | 4.04 | 9271 | 5183 | 9.04 |
| 1013 | - | - | - | 2806 | 1686 | 1.45 | - | - | - |
| 1030 | - | - | - | - | - | - | 1912 | 1081 | 1.89 |
| 1058 | - | - | - | 2526 | 1653 | 1.43 | - | - | - |
| 1100 | - | - | - | 3179 | 1841 | 1.59 | 3055 | 1820 | 3.18 |
| 1152 | 2864 | 1717 | 2.12 | 4156 | 2607 | 2.25 | 2741 | 1879 | 3.28 |
| 1175 | 2087 | 1039 | 1.28 | 2523 | 1638 | 1.41 | - | - | - |
| 1182 | 2422 | 1394 | 1.72 | 4423 | 2898 | 2.50 | 3769 | 2617 | 4.57 |
| 1295 | 3403 | 2304 | 2.85 | 7890 | 5901 | 5.09 | 3493 | 3048 | 5.32 |
| 1510 | 1718 | 1489 | 1.84 | 1582 | 1617 | 1.40 | - | - | - |
| 1545 | 3304 | 2987 | 3.69 | 10,228 | 10,310 | 8.90 | 2597 | 3021 | 5.27 |
| 1716 | 1209 | 1435 | 1.77 | - | - | - | - | - | - |
| 2034 | 3308 | 4371 | 5.40 | 2113 | 3570 | 22.03 | - | - | - |
| 2064 | 8506 | 12,656 | 15.64 | 14,575 | 25,523 | 2.33 | 3261 | 5052 | 8.81 |
| 2076 | 4310 | 6202 | 7.67 | 1898 | 2703 | 1.69 | - | - | - |
| 2106 | 2700 | 3706 | 4.58 | 1266 | 1964 | 1.88 | - | - | - |
| 3267 | 206 | 434 | 0.54 | 882 | 2181 | 0.68 | 859 | 2286 | 3.99 |
| 3581 | - | - | - | 284 | 785 | 0.46 | - | - | - |
| 3799 | - | - | - | 167 | 536 | 100 | - | - | - |
| Hydrolysis Time (min) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| - | UNPROCESSED (%AUC) | EXTRUDED (%AUC) | ||||||||||
| MM (Da) | 10 | 25 | 60 | 90 | 120 | 180 | 10 | 25 | 60 | 90 | 120 | 180 |
| 0–1000 | 10.04 | 48.26 | 37.55 | 72.75 | 35.74 | 57.63 | 27.93 | 34.66 | 46.02 | 50.89 | 41.82 | 63.70 |
| 1000–2000 | 14.28 | 13.69 | 10.88 | 3.86 | 14.11 | 20.50 | 1.05 | 8.53 | 12.95 | 15.28 | 26.02 | 23.50 |
| 2000–3000 | 72.04 | 34.79 | 48.20 | 18.43 | 47.65 | 17.52 | 10.45 | 33.79 | 36.78 | 33.29 | 29.13 | 8.81 |
| >3000 | 3.64 | 3.26 | 3.37 | 4.99 | 2.50 | 4.36 | 60.58 | 23.02 | 4.22 | 0.54 | 3.02 | 3.99 |

3. Experimental Section
3.1. Materials
3.2. Extrusion Process
3.3. Preparation of Amaranth Protein Hydrolysates
3.4. Peptide Characterization
4. Conclusions
Acknowledgments
Authors Contributions
Abbreviations
| ACE-Inhibitor | Angiotensin converting enzyme-inhibitor |
| DPP-IV-Inhibitor | Dipeptidyl peptidase IV inhibitor |
| EAH | Extruded amaranth hydrolysate |
| ET | Extrusion temperature |
| FAO | Food and agriculture organization |
| MALDI-TOF | Matrix-assisted laser desorption/ionization- time-of-flight mass spectrometer |
| MM | Molecular mass |
| MS-MS | Tandem mass spectrometry |
| RH | Relative humidity |
| rpm | Revolutions per minute |
| SS | Screw speed |
| UAH | Unprocessed amaranth hydrolysate |
| USP | Enzyme units (United States Pharmacopeia) |
| WHO | World health organization |
Conflicts of Interest
References
- Milán-Carrillo, J.; Montoya-Rodríguez, A.; Reyes-Moreno, C. High-antioxidant capacity beverages based on extruded and roasted amaranth (Amaranthus hypochondriacus) flour. In Hispanic Foods: Chemistry and Bioactive Compounds, 1st ed.; Tunick, M.H., González de Mejía, E., Eds.; American Chemical Society: Washington, DC, USA, 2012; Volume 1109, pp. 199–216. [Google Scholar]
- Pavlik, V. The revival of amaranth as a third-millennium food. Neuroendocrinol. Lett. 2012, 33, 3–7. [Google Scholar] [PubMed]
- Zapotoczny, P.; Markowski, M.; Majewska, K.; Ratajski, A.; Konopko, H. Effect of temperature on the physical, functional, and mechanical characteristics of hot-air-puffed amaranth seeds. J. Food Eng. 2006, 76, 469–476. [Google Scholar] [CrossRef]
- Gallegos-Tintoré, S.; Chel-Guerrero, L.; Corzo-Ríos, L.J.; Martínez-Ayala, A.L. Péptidos con actividadantioxidante de proteínas vegetales. In Bioactividad de Péptidos Derivados de Proteínas Alimentarias; Segura-Campos, M., Chel-Guerrero, L., Betancur-Ancona, D., Eds.; OmniaScience: Terrassa, Spain, 2013; Volume 4, pp. 111–122. [Google Scholar]
- Khandaker, L.; Masum-Akond, A.S.M.G.; Ali, M.B.; Oba, S. Biomass yield and accumulations of bioactive compounds in red amaranth (Amaranthus tricolor L.) grown under different colored shade polyethylene in spring season. Sci. Hortic. 2010, 123, 289–294. [Google Scholar]
- Avanza, M.V.; Puppo, M.C.; Añón, M.C. Structural characterization of amaranth protein gels. J. Food Sci. 2005, 70, 223–229. [Google Scholar] [CrossRef]
- Alvarez-Jubete, L.; Wijngaard, H.; Arendt, E.K.; Gallagher, E. Polyphenol composition and in vitro antioxidant activity of amaranth, quinoa buckwheat and wheat as affected by sprouting and baking. Food Chem. 2010, 119, 770–778. [Google Scholar] [CrossRef]
- Ferreira, T.A.; Gómez-Áreas, J.A. Calcium bioavailability of raw and extruded amaranth grains. Cienc. Tecnol. Aliment. 2010, 30, 532–538. [Google Scholar] [CrossRef]
- Repo-Carrasco-Valencia, R.; Hellstrom, J.K.; Pihlava, J.M.; Mattila, P.H. Flavonoids and other phenolic compounds in andean indigenous grains: Quinoa (Chenopodium quinoa), kaniwa (Chenopodium pallidicaule) and kiwicha (Amaranthus caudatus). Food Chem. 2010, 120, 128–133. [Google Scholar] [CrossRef]
- Aguilar, E.G.; Peiretti, E.G.; Uñates, M.A.; Marchevsky, E.J.; Escudero, N.L.; Camiña, J.M. Amaranth seed varieties: A chemometric approach. Food Measure 2013, 7, 199–206. [Google Scholar] [CrossRef]
- Silva-Sánchez, C.; Barba de la Rosa, A.P.; León-Galván, M.F.; De Lumen, B.O.; de León-Rodríguez, A.; González de Mejía, E. Bioactive peptides in amaranth (Amaranthus hypochondriacus) seed. J. Agric. Food Chem. 2008, 56, 1233–1240. [Google Scholar] [CrossRef] [PubMed]
- Grobelnik-Mlakar, S.; Turinek, M.; Jakop, M.; Bavec, M.; Bavec, F. Nutrition value and use of grain amaranth: potential future application in bread making. Agricultura 2009, 6, 43–53. [Google Scholar]
- Morales de León, J.; Camacho, M.E.; Bourges, H. Aminoacid composition of some Mexican foods. Arch. Latinoam. Nutr. 2005, 55, 172–186. [Google Scholar] [PubMed]
- Awasthi, C.P.; Kumar, A.; Singh, N.; Thakur, R. Biochemical composition of grain amaranth genotypes of himachal pradesh. Indian J. Agric. Biochem. 2011, 24, 141–144. [Google Scholar]
- Rastogi, A.; Shukla, S. Amaranth: A new millennium crop of nutraceutical values. Crit. Rev. Food Sci. 2013, 53, 109–125. [Google Scholar] [CrossRef]
- Shevkani, K.; Singh, N.; Chand-Rana, J.; Kaur, A. Relationship between physicochemical and functional properties of amaranth (Amaranthus hypochondriacus) protein isolates. Int. J. Food Sci. Technol. 2014, 49, 541–550. [Google Scholar] [CrossRef]
- Grobelnik-Mlakar, S.; Turinek, M.; Jakop, M.; Bavec, M.; Bavec, F. Grain amaranth as an alternative and perspective crop in temperate climate. J. Geogr. 2010, 5, 135–146. [Google Scholar]
- Gorinstein, S.; Pawelzik, E.; Delgado-Licon, E.; Haruenkit, R.; Weisz, M.; Trakhtenberg, S. Characterization of pseudocereal and cereal proteins by protein and amino acid analyses. J. Sci. Food Agric. 2002, 82, 886–891. [Google Scholar] [CrossRef]
- Alvarez-Jubete, L.; Arendt, E.K.; Gallagher, E. Nutritive value of pseudocereals and their increasing use as functional gluten free ingredients. Food Sci. Technol. 2010, 21, 106–113. [Google Scholar] [CrossRef]
- Pihlanto-Leppälä, A.L.; Koskinen, P.; Piilola, K.; Tupasela, T.; Korhonen, H. Angiotensin I-converting enzyme inhibitory properties of whey protein digests: Concentration and characterization of active peptides. J. Dairy Res. 2000, 67, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Milán-Carrillo, J.; Gutiérrez-Dorado, R.; Perales-Sánchez, J.X.K.; Cuevas-Rodríguez, E.O.; Ramírez-Wong, B.; Reyes-Moreno, C. The optimization of the extrusion process when using maize flour with a modified amino acid profile for making tortillas. Int. J. Food Sci. Technol. 2006, 41, 727–736. [Google Scholar] [CrossRef]
- Milán-Carrillo, J.; Montoya-Rodríguez, A.; Gutiérrez-Dorado, R.; Perales-Sánchez, X.; Reyes-Moreno, C. Optimization of extrusion process for producing high antioxidant instant amaranth (Amaranthus hypochondriacus L.) flour using response surface methodology. Appl. Math. 2012, 3, 1516–1525. [Google Scholar]
- Barba de la Rosa, A.P.; Fomsgaard, I.S.; Bente, L.; Mortensen, A.G.; Olvera-Martínez, L.; Silva-Sánchez, C.; Mendoza-Herrera, A.; González-Castañeda, J.; de León-Rodríguez, A. Amaranth (Amaranthus hypochondriacus) as an alternative crop for sustainable food production: Phenolic acids and flavonoids with potential impact on its nutraceutical quality. J. Cereal Sci. 2009, 49, 117–121. [Google Scholar] [CrossRef]
- Caselato-Sousa, V.M.; Amaya-Farfán, J. State of knowledge on amaranth grain: A comprehensive review. J. Food Sci. 2012, 77, 93–104. [Google Scholar] [CrossRef]
- Montoya-Rodríguez, A.; Milán-Carrillo, J.; Dia, V.P.; Reyes-Moreno, C.; González de Mejía, E. Pepsin-pancreatin protein hydrolysates from extruded amaranth inhibit markers of atherosclerosis in LPS-induced THP-1 macrophages-like human cells by reducing expression of proteins in LOX-1 signaling pathway. Proteome Sci. 2014, 12, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Montoya-Rodríguez, A.; González de Mejía, E.; Dia, V.P.; Reyes-Moreno, C.; Milán-Carrillo, J. Extrusion improved the anti-inflammatory effect of amaranth (Amaranthus hypochondriacus) hydrolysates in LPS-induced human THP-1 macrophage-like and mouse RAW 264.7 macrophages by preventing activation of NF-κB signaling. Mol. Nutr. Food Res. 2014, 58, 1028–1041. [Google Scholar]
- Benítez, R.; Ibarz, A.; Jordi-Pagan, J. Protein hydrolysates: Processes and applications. Acta Bioquim. Clin. Latinoam. 2008, 42, 227–236. [Google Scholar]
- Chun, C.; Haifeng, Z.; Mouming, Z.; Hua, C. Effects of extrusion treatment on enzymatic hydrolysis properties of wheat gluten. J. Food Process Eng. 2008, 34, 187–203. [Google Scholar]
- Sui, X.; Jiang, L.; Wang, C.; Li, Y. The comparison of amino acid content of the hydrolysate under extrusion pretreatment with non-extrusion pretreatment. Appl. Mech. Mater. 2011, 66–68, 702–708. [Google Scholar]
- Velarde-Salcedo, A.J.; Barrera-Pacheco, A.; Lara-González, S.; Montero-Morán, G.M.; Díaz-Gois, A.; González de Mejia, E.; Barba de la Rosa, A.P. In vitro inhibition of dipeptidyl peptidase IV by peptides derived from the hydrolysis of amaranth (Amaranthus hypochondriacus L.) proteins. Food Chem. 2013, 136, 758–764. [Google Scholar] [CrossRef]
- Dzubia, M.; Dzubia, B. In silico analysis of bioactive peptides. In Bioactive Proteins and Peptides as Functional Foods and Nutraceuticals; Yoshinori, M., Eunice, L.C., Bo, J., Eds.; Blackwell Publishing Ltd. and Institute of Food Technologists: Chicago, IL, USA, 2010; Volume 22, pp. 325–340. [Google Scholar]
- Tovar-Pérez, E.G.; Guerrero-Legarreta, I.; Farrés-González, A.; Soriano-Santos, J. Angiotensin I-converting enzyme inhibitory peptide fractions from albumin 1 and globulin as obtained of amaranth grain. Food Chem. 2009, 116, 437–444. [Google Scholar] [CrossRef]
- Vecchi, B.; Añón, M.C. ACE inhibitory tetrapeptides from Amaranthus hypochondriacus 11s globulin. Phytochemistry 2009, 70, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Cervantes, E.; Jeong, H.J.; León-Galván, F.; Barrera-Pacheco, A.; de León-Rodríguez, A.; González de Mejía, E.; de Lumen, B.O.; Barba de La Rosa, A.P. Amaranth lunasin-like peptide internalizes into the cell nucleus and inhibits chemical carcinogen-induced transformation of NIH-3T3 cells. Peptides 2010, 3, 11635–1642. [Google Scholar]
- González de Mejía, E.; Martínez-Villaluenga, C.; Fernández, D.; Urado, D.; Sato, K. Bioavailability and safety of food peptides. In Food proteins and Peptides: Chemistry, Functionality Interactions, and Commercialization; Hettiarachchy, N.S., Sato, K., Kannan, A., Eds.; Taylor & Francis: Boca Raton, FL, USA, 2012; pp. 297–330. [Google Scholar]
- Megías, C.; Yust, M.; Pedroche, J.; Lquari, H.; Girón-Calle, J.; Alaiz, M. Purification of an ACE inhibitory peptide after hydrolysis of sunflower (Helianthus annuus L.) protein isolates. J. Agric. Food Chem. 2009, 52, 1928–1932. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montoya-Rodríguez, A.; Milán-Carrillo, J.; Reyes-Moreno, C.; De Mejía, E.G. Characterization of Peptides Found in Unprocessed and Extruded Amaranth (Amaranthus hypochondriacus) Pepsin/Pancreatin Hydrolysates. Int. J. Mol. Sci. 2015, 16, 8536-8554. https://doi.org/10.3390/ijms16048536
Montoya-Rodríguez A, Milán-Carrillo J, Reyes-Moreno C, De Mejía EG. Characterization of Peptides Found in Unprocessed and Extruded Amaranth (Amaranthus hypochondriacus) Pepsin/Pancreatin Hydrolysates. International Journal of Molecular Sciences. 2015; 16(4):8536-8554. https://doi.org/10.3390/ijms16048536
Chicago/Turabian StyleMontoya-Rodríguez, Alvaro, Jorge Milán-Carrillo, Cuauhtémoc Reyes-Moreno, and Elvira González De Mejía. 2015. "Characterization of Peptides Found in Unprocessed and Extruded Amaranth (Amaranthus hypochondriacus) Pepsin/Pancreatin Hydrolysates" International Journal of Molecular Sciences 16, no. 4: 8536-8554. https://doi.org/10.3390/ijms16048536
APA StyleMontoya-Rodríguez, A., Milán-Carrillo, J., Reyes-Moreno, C., & De Mejía, E. G. (2015). Characterization of Peptides Found in Unprocessed and Extruded Amaranth (Amaranthus hypochondriacus) Pepsin/Pancreatin Hydrolysates. International Journal of Molecular Sciences, 16(4), 8536-8554. https://doi.org/10.3390/ijms16048536