The Role of Food Peptides in Lipid Metabolism during Dyslipidemia and Associated Health Conditions
Abstract
:1. Dyslipidemia and Metabolic Syndrome
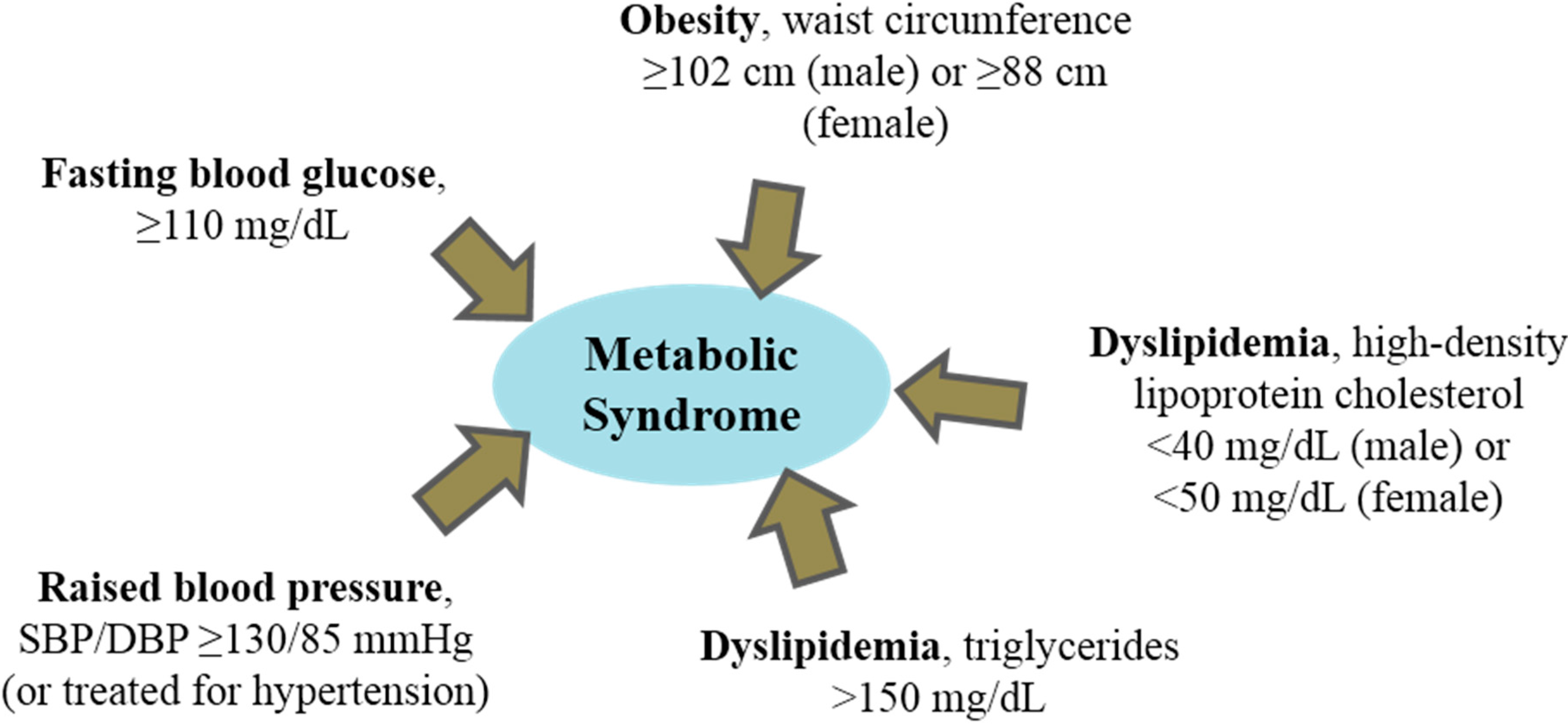
2. Dietary Peptides and Hyperlipidemia
| Peptide | Source | Activity/Mechanism | Reference |
|---|---|---|---|
| KNPQLR | Soybean β-conglycinin | Binding the active site and inhibition of FAS activity in vitro by interacting with FAS thioesterase domain/activity | [24] |
| EITPEKNPQLR | |||
| RKQEEDEDEEQQRE | |||
| LPYPR | Soybean proteins (glycinin) | HMGCoAR inhibition in vitro (LPYPR); disruption of cholesterol micellar solubility in vitro; upregulated lipogenic genes CYP51, LDLR, LPL and CYP7A1 resulting in reduction in plasma VLDL-C, TG, but increased plasma TC with low fecal sterol excretion in diet-induced hyperlipidemic mice | [23] |
| WGAPSL | |||
| WE | Synthetic | Direct binding and transactivation of PPARα; increased expression of PPARα-responsive genes of fatty acid metabolism, FATP4, ACS, CPT1 and ACOX, and reduced intracellular cholesterol and TG levels in hepatic cell culture | [25] |
| KRES | Synthetic | Increased plasma HDL-C and reduced atherosclerosis (in addition to its antioxidative activities) in apoE null mice; no known mechanism | [15] |
| KDW | Synthetic | Increased plasma HDL-C and decreased plasma LDL-C, TC, TG and atherogenic index in diet-induced hyperlipidemic rats; no known mechanism | [22] |
| YPFVV (soymorphin-5) | Soybean protein (β-conglycinin) | Decreased plasma and liver TG, and liver weight; increased plasma adiponectin, hepatic adiponectin receptor and PPARα expression leading to upregulation of genes involved in fatty acid β-oxidation in diabetic KKAy mice | [26] |
| HIRL (β-lactotensin) | Milk protein (β-lactoglobulin) | Decreased serum LDL-C and TC in diet-induced hyperlipidemic mice mediated by neurotensin (NT2) and dopamine (D2) receptors, and stimulated bile acid secretion | [27] |
3. Intestinal Functions of Hypolipidemic Peptides
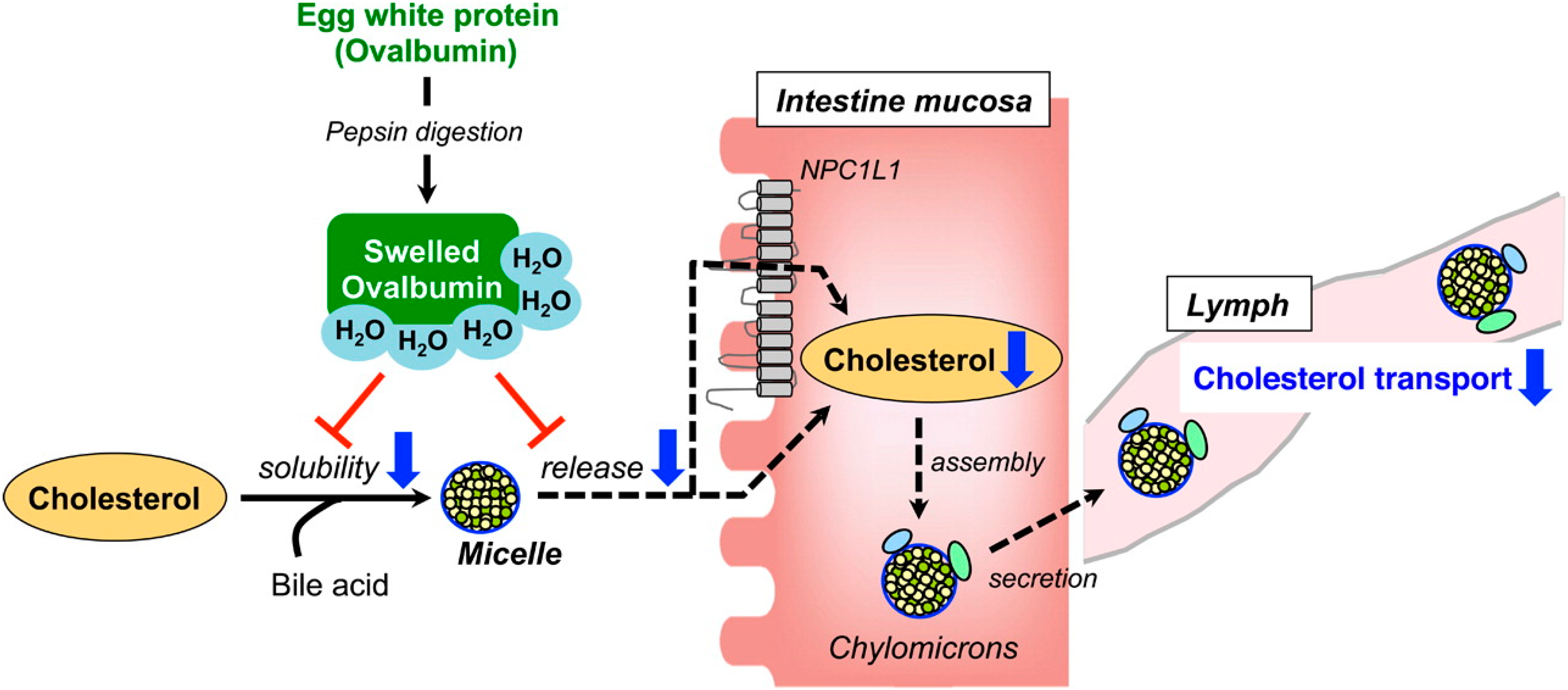
4. Adipocytic Functions of Hypolipidemic Peptides
5. Hepatic Functions of Hypolipidemic Peptides
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Malik, V.S.; Willett, W.C.; Hu, F.B. Global obesity: Trends, risk factors and policy implications. Nat. Rev. Endocrinol. 2013, 9, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Cornier, M.A.; Dabelea, D.; Hernandez, T.L.; Lindstrom, R.C.; Steig, A.J.; Stob, N.R.; van Pelt, R.E.; Wang, H.; Eckel, R.H. The metabolic syndrome. Endocr. Rev. 2008, 29, 777–822. [Google Scholar] [CrossRef] [PubMed]
- Misra, A.; Khurana, L. Obesity and the metabolic syndrome in developing countries. J. Clin. Endocrinol. Metab. 2008, 93, S9–S30. [Google Scholar] [CrossRef] [PubMed]
- WHO (World Health Organization). World Health Statistics; Geneva, Switzerland, 2012. [Google Scholar]
- Roberts, C.K.; Sindhu, K.K. Oxidative stress and metabolic syndrome. Life Sci. 2009, 84, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Abete, I.; Goyenechea, E.; Zulet, M.A.; Martíneza, J.A. Obesity and metabolic syndrome: Potential benefit from specific nutritional components. Nutr. Metab. Cardiovasc. Dis. 2011, 21, B1–B15. [Google Scholar] [CrossRef] [PubMed]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). J. Am. Med. Assoc. 2001, 285, 2486–2497. [Google Scholar]
- Sirtori, C.R.; Galli, C.; Anderson, J.W.; Arnoldi, A. Nutritional and nutraceutical approaches to dyslipidemia and atherosclerosis prevention: Focus on dietary proteins. Atherosclerosis 2009, 203, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Wanezaki, S.; Tachibana, N.; Nagata, M.; Saito, S.; Nagao, K.; Yanagita, T.; Kohno, M. Soy β-conglycinin improves obesity-induced metabolic abnormalities in a rat model of nonalcoholic fatty liver disease. Obes. Res. Clin. Pract. 2015, 9, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Nagata, Y.; Noguchi, Y.; Tamaru, S.; Kuwahara, K.; Okamoto, A.; Suruga, K.; Koba, K.; Tanaka, K. Hypolipidemic potential of squid homogenate irrespective of a relatively high content of cholesterol. Lipids Health Dis. 2014, 13, 165. [Google Scholar] [CrossRef]
- Vik, R.; Bjørndal, B.; Bohov, P.; Brattelid, T.; Svardal, A.; Nygård, O.K.; Nordrehaug, J.E.; Skorve, J.; Berge, R.K. Hypolipidemic effect of dietary water-soluble protein extract from chicken: Impact on genes regulating hepatic lipid and bile acid metabolism. Eur. J. Nutr. 2015, 54, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wood, C.; Gagnon, C.; Cober, E.R.; Frégeau-Reid, J.A.; Gleddie, S.; Xiao, C.W. The α' subunit of β-conglycinin and the A1–5 subunits of glycinin are not essential for many hypolipidemic actions of dietary soy proteins in rats. Eur. J. Nutr. 2014, 53, 1195–1207. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Figueroa, J.C.; González-Córdova, A.F.; Astiazaran-García, H.; Hernández-Mendoza, A.; Vallejo-Cordoba, B. Antihypertensive and hypolipidemic effect of milk fermented by specific Lactococcus lactis strains. J. Dairy Sci. 2013, 96, 4094–4099. [Google Scholar] [CrossRef] [PubMed]
- Bähr, M.; Fechner, A.; Kiehntopf, M.; Jahreis, G. Consuming a mixed diet enriched with lupin protein beneficially affects plasma lipids in hypercholesterolemic subjects: A randomized controlled trial. Clin. Nutr. 2015, 34, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Navab, M.; Anantharamaiah, G.M.; Reddy, S.T.; Hama, S.; Hough, G.; Frank, J.S.; Grijalva, V.R.; Ganesh, V.K.; Mishra, V.K.; Palgunachari, M.N.; et al. Oral small peptides render HDL antiinflammatory in mice and monkeys and reduce atherosclerosis in ApoE null mice. Circ. Res. 2005, 97, 524–532. [Google Scholar]
- Lillefosse, H.H.; Tastesen, H.S.; Du, Z.Y.; Ditlev, D.B.; Thorsen, F.A.; Madsen, L.; Kristiansen, K.; Liaset, B. Hydrolyzed casein reduces diet-induced obesity in male C57BL/6J mice. J. Nutr. 2013, 143, 1367–1375. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Kitagawa, S.; Tada, T.; Iefuji, H.; Inoue, Y.; Izawa, S. Modification of yeast characteristics by soy peptides: Cultivation with soy peptides represses the formation of lipid bodies. Appl. Microbiol. Biotechnol. 2011, 89, 1971–1977. [Google Scholar] [CrossRef] [PubMed]
- Howard, A.; Udenigwe, C.C. Mechanisms and prospects of food protein hydrolysates and peptide-induced hypolipidaemia. Food Funct. 2013, 4, 40–51. [Google Scholar] [CrossRef] [PubMed]
- González-Ortega, O.; López-Limón, A.R.; Morales-Domínguez, J.F.; Soria-Guerra, R.E. Production and purification of recombinant hypocholesterolemic peptides. Biotechnol. Lett. 2015, 37, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Ktari, N.; Mnafgui, K.; Nasri, R.; Hamden, K.; Bkhairia, I.; Hadj, A.B.; Boudaouara, T.; Elfeki, A.; Nasri, M. Hypoglycemic and hypolipidemic effects of protein hydrolysates from zebra blenny (Salaria basilisca) in alloxan-induced diabetic rats. Food Funct. 2013, 4, 1691–1699. [Google Scholar] [CrossRef] [PubMed]
- Kapravelou, G.; Martínez, R.; Andrade, A.M.; Sánchez, C.; Chaves, C.L.; López-Jurado, M.; Aranda, P.; Cantarero, S.; Arrebola, F.; Fernández-Segura, E.; et al. Health promoting effects of Lupin (Lupinus albus var. multolupa) protein hydrolyzate and insoluble fiber in a diet-induced animal experimental model of hypercholesterolemia. Food Res. Int. 2013, 54, 1471–1481. [Google Scholar]
- Malinin, V.V.; Savateeva-Lyubimova, T.N.; Sivak, K.V. Hypolipidemic and hypoglycemic effect of peptide Lys-Glu-Trp-NH2 in rats with associated metabolic disorders. Bull. Exp. Biol. Med. 2014, 156, 338–341. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Bartley, G.E.; Zhang, H.; Jing, W.; Fagerquist, C.K.; Zhong, F.; Yokoyama, W. Peptides identified in soybean protein increase plasma cholesterol in mice on hypercholesterolemic diets. J. Agric. Food Chem. 2013, 61, 8389–8395. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Villaluenga, C.; Rupasinghe, S.G.; Schuler, M.A.; Gonzalez de Mejia, E. Peptides from purified soybean β-conglycinin inhibit fatty acid synthase by interaction with the thioesterase catalytic domain. FEBS J. 2010, 277, 1481–1493. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Kim, J.H.; Nam, B.; Kim, J.; Lee, J.H.; Hwang, K.Y.; Lee, S.J. The dipeptide H-Trp-Glu-OH (WE) shows agonistic activity to peroxisome proliferator-activated protein-α and reduces hepatic lipid accumulation in lipid-loaded H4IIE cells. Bioorg. Med. Chem. Lett. 2014, 24, 2957–2962. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Muraki, A.; Oie, M.; Kanegawa, N.; Oda, A.; Sawashi, Y.; Kaneko, K.; Yoshikawa, M.; Goto, T.; Takahashi, N.; et al. Soymorphin-5, a soy-derived μ-opioid peptide, decreases glucose and triglyceride levels through activating adiponectin and PPARα systems in diabetic KKAy mice. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E433–E440. [Google Scholar]
- Yamauchi, R.; Ohinata, K.; Yoshikawa, M. β-Lactotensin and neurotensin rapidly reduce serum cholesterol via NT 2 receptor. Peptides 2003, 24, 1955–1961. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Lee, M.H.; Patel, S.B. Dietary cholesterol absorption; more than just bile. Trends Endocrinol. Metab. 2001, 12, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Marques, M.R.; Freitas, R.A.M.S.; Carlos, A.C.C.; Siguemoto, É.S.; Fontanari, G.G.; Arêas, J.A.G. Peptides from cowpea present antioxidant activity, inhibit cholesterol synthesis and its solubilisation into micelles. Food Chem. 2015, 168, 288–293. [Google Scholar]
- Matsuoka, R.; Shirouchi, B.; Kawamura, S.; Baba, S.; Shiratake, S.; Nagata, K.; Imaizumi, K.; Sato, M. Dietary egg white protein inhibits lymphatic lipid transport in thoracic lymph duct-cannulated rats. J. Agric. Food Chem. 2014, 62, 10694–10700. [Google Scholar] [CrossRef] [PubMed]
- Udenigwe, C.C. Bioinformatics approaches, prospects and challenges of food bioactive peptide research. Trends Food Sci. Technol. 2014, 36, 137–143. [Google Scholar] [CrossRef]
- De Mejia, E.G.; Martinez-Villaluenga, C.; Roman, M.; Bringe, N.A. Fatty acid synthase and in vitro adipogenic response of human adipocytes inhibited by α and α' subunits of soybean β-conglycinin hydrolysates. Food Chem. 2010, 119, 1571–1577. [Google Scholar] [CrossRef]
- Goto, T.; Mori, A.; Nagaoka, S. Soluble soy protein peptic hydrolysate stimulates adipocyte differentiation in 3T3-L1 cells. Mol. Nutr. Food Res. 2013, 57, 1435–1445. [Google Scholar] [CrossRef] [PubMed]
- Liaset, B.; Hao, Q.; Jørgensen, H.; Hallenborg, P.; Du, Z.Y.; Ma, T.; Marschall, H.U.; Kruhøffer, M.; Li, R.; Li, Q.; et al. Nutritional regulation of bile acid metabolism is associated with improved pathological characteristics of the metabolic syndrome. J. Biol. Chem. 2011, 286, 28382–28395. [Google Scholar]
- Lammi, C.; Zanoni, C.; Scigliuolo, G.M.; D’Amato, A.; Arnoldi, A. Lupin peptides lower low-density lipoprotein (LDL) cholesterol through an up-regulation of the LDL receptor/sterol regulatory element binding protein 2 (SREBP2) pathway at HepG2 cell line. J. Agric. Food Chem. 2014, 62, 7151–7159. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, T.; Kishimoto, K.; Miura, S.; Ezaki, O. Dietary β-conglycinin prevents fatty liver induced by a high-fat diet by a decrease in peroxisome proliferator-activated receptor γ2 protein. J. Nutr. Biochem. 2012, 23, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Zhang, Z.S.; Luo, H.B.; Shen, J.H.; Chen, K.X.; Shen, X.; Jiang, H.L. The dipeptide H-Trp-Glu-OH shows highly antagonistic activity against PPARγ: Bioassay with molecular modeling simulation. ChemBioChem 2006, 7, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Inoue, N.; Nagao, K.; Sakata, K.; Yamano, N.; Gunawardena, P.E.R.; Han, S.Y.; Matsui, T.; Nakamori, T.; Furuta, H.; Takamatsu, K.; et al. Screening of soy protein-derived hypotriglyceridemic di-peptides in vitro and in vivo. Lipids Health Dis. 2011, 10, 85. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Udenigwe, C.C.; Rouvinen-Watt, K. The Role of Food Peptides in Lipid Metabolism during Dyslipidemia and Associated Health Conditions. Int. J. Mol. Sci. 2015, 16, 9303-9313. https://doi.org/10.3390/ijms16059303
Udenigwe CC, Rouvinen-Watt K. The Role of Food Peptides in Lipid Metabolism during Dyslipidemia and Associated Health Conditions. International Journal of Molecular Sciences. 2015; 16(5):9303-9313. https://doi.org/10.3390/ijms16059303
Chicago/Turabian StyleUdenigwe, Chibuike C., and Kirsti Rouvinen-Watt. 2015. "The Role of Food Peptides in Lipid Metabolism during Dyslipidemia and Associated Health Conditions" International Journal of Molecular Sciences 16, no. 5: 9303-9313. https://doi.org/10.3390/ijms16059303
APA StyleUdenigwe, C. C., & Rouvinen-Watt, K. (2015). The Role of Food Peptides in Lipid Metabolism during Dyslipidemia and Associated Health Conditions. International Journal of Molecular Sciences, 16(5), 9303-9313. https://doi.org/10.3390/ijms16059303




