Mushroom Lectins: Specificity, Structure and Bioactivity Relevant to Human Disease
Abstract
:1. Introduction
2. Mushroom Lectins
| Source of Lectin (Current Species Names Given in Parentheses) | Specificity of Sugars/Glycoproteins * | Ref. |
|---|---|---|
| Agaricus arvensis | Inulin | [48] |
| Agaricus bisporus | GalNAc, Galβ1,3GalNAc (T antigen), sialyl-Galβ (ABL) ‡ | [49,50,51,52] |
| Agaricus bitorquis | Lac | [7] |
| Agaricus blazei (Agaricus subrufescens) | Methyl N-acetyl-α-o-galactosaminide, GalNAc, BSM, asialo-BSM, fetuin, asialofetuin | [53] |
| Agaricus campestris | GalNAc, Gal, Suc | [54,55] |
| Agaricus pilatianus | Lac, GlcNAc, Glc, Rham | [47] |
| Agrocybe aegerita (Cyclocybe aegerita) | Lac, BSM, Glycophorin A, k-Casein, β-galactosides, Gal (AAL galectin), terminal non-reducing GlcNAc (AAL2) | [56,57,58,59] |
| Agrocybe cylindracea (Cyclocybe cylindracea) | Trisaccharides containing Neu5Acα2,3Gal, Lac, sialic acid, inulin (ACG) | [60,61,62,63] |
| Aleuria aurantia | l-Fuc, fucosyl oligosaccharides (AAL) | [64,65] |
| Amanita muscaria | O-type glycans | [66] |
| Amanita ovoidea | Gal, GalNAc, Rham | [47] |
| Amantia pantherina | GIcNAcβ1, 4ManβpNP, Galβ1,4GlcNAcβ1,4GIcNAc, Galβ1,4GIcNAcβ1,4GlcNAc, BSM, asialo-BSM | [67] |
| Amanita phalloides | Ovomucin, human glycophorin A (A. phalloides lectin) | [66] |
| Amanita virosa | Blood group specific substance B, A and H, bovine thyreoglobulin, ovomucoid, asialo-ovomucoid transferrin, Ovine submaxillary mucin, 4-nitrophenyl-α-d-mannopyranoside, 4-nitrophenyl-β-d-glucopyranoside, 4-nitrophenyl-β-d-galactopyranoside | [68] |
| Amanita muscaria | O-type glycans | [66] |
| Armillaria luteovirens | Inulin (ALL) | [69] |
| Auricularia polytricha (Auricularia cornea) | Raf, Gal, ovomucoid and β-anomers of galactoside (Lac, p-nitrophenyl β-d-galactoside) | [70] |
| Boletopsis leucomelaena [as “leucomelas”] # | GlcNAcβ1,2Manα1,3(GlcNAcβ1,2Manα1,6)Manβ1,4GlcNAcβ1, 4GlcNAc, GlcNAc (BLL) | [71] |
| Boletus edulis | d(+)-Mel, d-Xyl (BEL) | [72] |
| Boletus satanas | d-Gal | [73] |
| Boletus subtomentosus (Xerocomus subtomentosus) | d-Lac | [74] |
| Boletus venenatus | Asialofetuin, Galβ1,4GlcNAcβ1,4Manβ1,4GlcNAcβ1,4GlcNAc residues in N-linked sugar chains | [75] |
| Chlorophyllum brunneum | Neu5Ac | [7] |
| Chlorophyllum molybdites | Neu5Gc, GalNAc, asialo-BSM, PSM | [76] |
| Ciborinia camelliae | GalNAc | [77] |
| Clavaria purpurea (Alloclavaria purpurea) | Asialo-BSM, α-Gal, Galα1,3Gal, Raf | [78] |
| Clitocybe geophyla ^ | GalNAc, Lac, Glc | [47] |
| Clitocybe nebularis | Asialo-fetuin, Lac, GalNAc, Gal, N,N-diacetyllactosediamine (GalNAcβ1,4GlcNAc, LacdiNAc) (CNL) | [79,80,81] |
| Coprinus atramentarius (Coprinopsis atramentaria) | d-Lac | [74] |
| Coprinus comatus | GlcNAc, Lac, Gal, Ara, Rib, Xyl | [7,47] |
| Coprinus cinereus (Coprinopsis cinerea) | β-Gal (CCL2), GlcNAcβ1,4(Fucα1,3)GlcNAc (CGL2), GalNAcβ1,4GlcNAc (CGL3) | [82,83,84] |
| Coprinus micaceus (Coprinellus micaceus) | Lac, Gal, GalNAc | [47] |
| Cordyceps militaris | Sialoglycoprotein, Neu5Ac (CML) | [85] |
| Cortinarius sp. TWM 1710 | Gal | [7] |
| Flammulina velutipes | β-d-Gal, fetuin, human transferrin, human glycophorin, lactoferrin (F. velutipes lectin) | [86,87] |
| Fomes fomentarius | GalNAc, α-d-Gal, Raf | [80] |
| Ganoderma capense | d-Gal, d(+)-Galactosamine (G. capense lectin) | [88] |
| Ganoderma lucidum | Asialo-triantennary N-glycan, N-and O-linked glycans. | [89,90] |
| Grifola frondosa | Terminal GalNAc residues, porcine stomach mucin, linear d-Rham, PSM (GFL) | [76,91,92] |
| Hericium erinaceus [as “erinaceum”] # | Neu5Gc, Neu5Ac, inulin (HEA) | [62,93,94] |
| Hygrophorus hypothejus | Lac, d-Gal, d-GalNAc, Galβ1,4GlcNAc, o-nitrophenylα-d-GalNAc, p-nitrophenyl-β-d-GalNAc, asialo-BSM | [95,96] |
| Hygrophorus russula | α1,6-mannobiose, Isomaltose (Glcα1,6Glc), isomaltotriose, isomaltotetraose, isomaltopentaose, isomaltohexaose, methyl α-mannoside, α1,3-mannobiose, methyl β-mannoside, α1,2-mannobiose, α1,4-mannobiose, methyl α-glucoside, Man, lacturose (HRL) | [97] |
| Inocybe fastigiata (Inocybe rimosa) | GalNAc | [98] |
| Inocybe umbrinella | Raf, d-Mel, α-Lac, d-Gal (I. umbrinella lectin) | [99] |
| Ischnoderma resinosum | Methyl-β-galactoside, Fuc, l-Ara | [53] |
| Kuehneromyces mutabilis | Asialo-PSM, asialofetuin, fetuin, α1-acid glycoprotein, Ovomucoid | [100] |
| Laccaria amethystina | l-Fuc (LAF), d-Lac and GalNAc, BSM, asialo-BSM,PSM, asialo-PSM, human glycophorin A (LAL) | [101,102] |
| Laccaria laccata | l-Fuc | [103] |
| Lactarius deliciosus | Galβ1,3GalNAc | [104] |
| Lactarius deterrimus | Galβ1,3GalNAc | [105] |
| Lactarius flavidulus | d-Mel, d-Fru, l(+)-Rham, Sor, d-Gal, d(+)-Man, Lac, d(+)-Xyl, l(+)-Ara, d-Glu, Raf, Inulin, p-nitrophenyl-α,d-glucopyranoside, p-nitrophenyl-β,d-glucopyranoside, inositol (LFL) | [106] |
| Lactarius lignyotus | Asialofetuin, asialo-PSM and other desialylated glycoproteins | [107] |
| Lactarius pergamenus | GalNAc, 4-nitrophenyl-β-d-galactopyranoside, α-phenyl N-acetyl-d-glucosaminopyranoside, Bovine thyroglobulin, human transferrin, Orosomucoid (α-glycoprotein), sheep submaxillary mucin, BSM, asialo-BSM, fetuin | [108] |
| Lactarius rufus | α-phenyl N-acetyl-d-glucosaminopyranoside, 4-nitrophenyl-β-d-glucosamine, asialo-BSM, human and bovine thyroglobulin, group specific substances from human erythrocytes | [109] |
| Lactarius salmonicolor | Galβ1,3GalNAc | [110] |
| Laetiporus sulphureus [as “sulfureus”] # | LacNAc, l-Rham, salicine, asialo-BSM,BSM, asialofetuin, lacto-N-neotetraose (Galβ1,4GlcNAcβ1,3Galβ1,4Glc) (LSL) | [111,112,113] |
| Lentinus edodes (Lentinula edodes) | GlcNAc, GalNAc, Man, d-Mel, Gal | [114,115,116,117] |
| Lentinus squarrosulus | Raf, d-Suc, Rib | [118] |
| Lepiota leucothites (Leucoagaricus leucothites) | Glc, GlcNAc, Man | [47] |
| Lepiota rhacodes (Macrolepiota rachodes) | Lac, Arab, MethGLc, GalNAc | [47] |
| Lepista nuda | Gal,Fuc, Suc, Arab | [47] |
| Lyophyllum decastes | Galabiose-Galα1,4Gal, non-reducing α-Gal | [119] |
| Macrolepiota procera | Terminal N-acetyl-lactosamine, β-galactosides (MPL) | [120] |
| Marasmius oreades | Galα1,3Galβ1,4GlcNAc, blood group Btrisaccharide (Galα1,3Gal2,1αFuc), Man, thyroglobulin, asialofetuin, complex type N-glycans (MOA) | [121,122,123,124,125] |
| Melanoleuca brevipes | Gal, Rham, Lac | [47] |
| Melastiza chateri | l-Fuc | [126] |
| Mycoleptodonoides aitchisonii | Asialo-BSM, BSM | [127] |
| Omphalotus nidiformis | Lac, Gal, Ara, Rib | [7] |
| Oudemansiella platyphylla (Megacollybia platyphylla) | β-GalNAc, terminal GlcNAc | [47,76,128] |
| Panus conchatus | d-Gal | [40,129] |
| Paxillus involutus | Asialo-PSM, asialofetuin, fetuin, α1-acid glycoprotein (P. involutus lectin) | [103] |
| Paecilomyes japonica ^ | Sialic acid and sialoglycoprotein (PJA) | [130] |
| Peziza silvestris [as “sylvestris”] # (Peziza arvernensis) | l-Ara (P. silvestris lectin) | [131] |
| Phaeolepiota aurea | GalNAc (PAL1 and PAL2) | [132] |
| Phallus impudicus | Fetuin | [76] |
| Phlebopus marginatus | Lac, Gal | [7] |
| Pholiota adiposa | Inulin (PAL) | [133] |
| Pholiota aurivella | Asialofetuin | [134] |
| Pholiota squarrosa | α1,6-fucosylated N-glycans | [135] |
| Pleurocybella porrigens | GalNAc, asialo-BSM, O-linked glycans | [136] |
| Pleurotus citrinopileatus | Mal, o-nitrophenyl-β-d-galactopyranoside, o/p-nitrophenyl-β-d-glucuronide, inulin (P. citrinopileatus lectin) | [137] |
| Pleurotus cornucopiae | Asialo-mucin | [138] |
| Pleurotus eous | Methyl-α-d-galactoside, galactosamine, mannosamine, asialofetuin (PEL) | [139] |
| Pleurotus ostreatus | Me-α-GalNAc and 2'-fucosyllactose (Fucα1,2Galβ1,4Glc), d-Mel, d-Gal, Raf, NeuNAc, Inulin, Lac, Galactosyl and N-Acetyl galactosaminyl groups, BSM, asialo-BSM (POL) | [32,140,141,142] |
| Pleurotus serotinus (Sarcomyxa serotina) | GalNAc | [129] |
| Pleurotus spodoleucus | Lac | [140] |
| Pleurotus tuber-regium | GlcNAc | [143] |
| Polyporus adustus [as “adusta”] # (Bjerkandera adusta) | d-Mel, d-Fru, d-Ara, d-Glu, d-Raf, p-nitro-α-d-glucopyranoside (P. adustus lectin) | [144] |
| Polyporus squamosus | Neu5Acα2,6Galβ1,4Glc/GlcNAc (6'-sialylated type II chain) of N-glycans (PSL) | [145,146,147,148] |
| Psathyrella asperospora | GlcNAc (PAL) | [7] |
| Psathyrella velutina | GlcNAc, Neu5Acα2,3Galβ1,4GlcNAc, Heparin and Pectin (PVL) | [62,149,150,151,152] |
| Psilocybe barrerae | d-Gal, Glycophorin, BSM, asialo-BSM, human serum and milk transferrin | [153] |
| Russula delica | Inulin, o-nitrophenyl-β-d-galactopyranoside (R. delica lectin) | [154] |
| Russula lepida | Inulin, o-nitrophenyl-β-d-galactopyranoside | [155] |
| Russula nigricans | Asialofetuin, asialo-PSM, fetuin, ovomucoid, α1-acid glycoprotein | [103] |
| Schizophyllum commune | GalNAc (S. commune lectin, species from Thailand), Lac (S. commune lectin, species from China) | [156,157] |
| Stereum hirsutum | l-Xyl | [158] |
| Trametes versicolor | Gal | [47] |
| Tricholoma fracticum [as “fractum”] # | Lac, Gal, GalNAc | [47] |
| Tricholoma mongolicum (Leucocalocybe mongolica) | Lac (TML1), GalNAc and Gal (TML2) | [45] |
| Volvariella volvacea | Thyroglobulin (VVL) | [31] |
| Xerocomus chrysenteron | Asialofetuin, asialo-PSM and other desialyzed glycoproteins Sychrova, GalNAc, Gal,TF antigen (XCL) | [107,159,160] |
| Xerocomus spadiceus (Xerocomus ferrugineus) | Inulin (X. spadiceus lectin) | [161] |
| Xylaria hypoxylon | Inulin, Xyl (X. hypoxylon lectin) | [162] |
| Lectin | Purification Strategy | Description of Lectin/Lectin Complex and Their PDB ID * | Resolution (Å) | Type of Fold #, PDB Structure and ID | Similarity to Other Structures and Their PDB ID | Ref. |
|---|---|---|---|---|---|---|
| Unique lectin-fold | ||||||
| Lyophyllum decastes lectin (LDL) | Melibiose-sepharose | LDL (Ligand free)/4NDS | 1.00 | Two β-sheets linked by disulfide bridges: 4NDS | Ginkbilobin-2/3A2E | [165] |
| LDL globotriose complex/4NDV | 1.00 | |||||
| LDL orthorhombic form/4NDT | 1.30 | |||||
| LDL α-methylgalactoside complex/4NDU | 1.03 | |||||
| β-propeller-fold | ||||||
| Aleuria aurantia lectin (AAL) | (NH4)2SO4 precipitation, fucose-starch AC ^ | AAL (Ligand free)/1OFZ | 1.50 | Six-bladed β-propeller fold 1OFZ | A. fumigatus Fuc-binding lectin (AFL)/4AGI | [76,166,167,168] |
| AAL complexed with Fuc/1IUC | 2.24 | |||||
| AAL (Hg-derivative from)/1IUB | 2.31 | |||||
| Psathyrella velutina lectin (PVL) | Chitin-sepharose AC, DEAE-cellulofine, CM-sepharose CL-6B | PVL (Ligand free)/2BWR | 1.50 | Integrin-like 7-blade β-propeller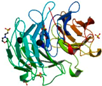 2BWR | Phanerochaete chrysosporium aldos-2-ulose dehydratase (AUDH)/4A7K | [76,149,150,152,169,170] |
| PVL GlcNAc complex/2C4D | 2.60 | |||||
| PVL Neu5Ac complex/2C25 | 1.80 | |||||
| PVL methyl 2-acetamido-1, 2-dideoxy-1-seleno-β-d-glucopyranoside complex/2BWM | 1.80 | |||||
| Galectin-like fold | ||||||
| Agrocybe aegerita lectin/galectin (AAL-galectin) | (NH4)2SO4 precipitation, DEAE-sepharose FF, Sephacryl S-200 HR, GF-250 HPLC | AAL-galectin (Ligand free)/2ZGK | 3.00 |  2ZGK | ACG complexed with blood type A antigen tetraose/3WG3 | [171,172] |
| Recombinant AAL-galectin (rAAL-galectin) (Ligand free)/2ZGL | 1.90 | |||||
| rAAL-galectin Lac complex/2ZGM | 1.90 | |||||
| rAAL-galectin Gal complex/2ZGN | 2.50 | |||||
| AAL-galectin mutant H59Q Lac complex/2ZGO | 2.00 | |||||
| AAL-galectin mutant I25G/2ZGP | 2.70 | |||||
| AAL-galectin mutant L33A/2ZGQ | 1.90 | |||||
| AAL-galectin mutant L33A/2ZGR | 1.90 | |||||
| AAL-galectin mutant L47A/2ZGS | 1.90 | |||||
| AAL-galectin mutant F93G/2ZGT | 2.80 | |||||
| AAL-galectin mutant I144G/2ZGU | 2.40 | |||||
| AAL-galectin TF antigen complex/3AFK | 1.95 | |||||
| AAL-galectin p-nitrophenyl TF disaccharide complex/3M3C | 2.00 | |||||
| AAL-galectin mutant E66A p-nitrophenyl TF disaccharide complex/3M3E | 2.10 | |||||
| AAL-galectin mutant R85A p-nitrophenyl TF disaccharide complex/3M3O | 2.10 | |||||
| AAL-galectin ganglosides complex GM1 pentasaccharide/3M3Q | 2.10 | |||||
| Agrocybe cylindracea galectin/lectin (ACG) | (NH4)2SO4 precipitation, DEAE-cellulofine A-200, DEAE-Toyopearl 650M, and Toyopearl HW | ACG (Ligand free)/1WW7 | 1.90 |  1WW7 | Recombinant (rAAL-galectin)/2ZGL | [60,173,174] |
| ACG Lac complex/1WW6 | 2.20 | |||||
| ACG 3'-sulfonyl Lac complex/1WW5 | 2.20 | |||||
| ACG α2,3-sialyllactose complex/1WW4 | 2.30 | |||||
| ACG mutant (N46A) blood type A antigen tetraose complex/3WG4 | 1.60 | |||||
| ACG blood type A antigen tetraose complex/3WG3 | 1.35 | |||||
| Coprinus cinereus lectin (CGL2) | Lactosyl-sepharose AC | CGL2 (Ligand free)/1UL9 | 2.22 |  1UL9 | CGL3 chitotetraose complex/2R0H | [82,175,176] |
| CGL2 Lac complex/1ULC | 2.60 | |||||
| CGL2 xeno linear trisaccharide complex/1ULE | 2.15 | |||||
| CGL2 blood group A tetrasaccharide complex/1ULF | 2.36 | |||||
| CGL2 TF antigen complex/1ULG | 2.20 | |||||
| CGL2 blood H Type II complex/1ULD | 2.20 | |||||
| CGL2 C. elegans N-glycan complex/2WKK | 2.10 | |||||
| Coprinopsis cinerea lectin (CGL3) | Lactosyl-sepharose AC | CGL3 (Ligand free)/2R0F | 2.00 | 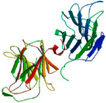 2R0F | CGL2 C. elegans N-glycan complex/2WKK | [82,84] |
| CGL3 chitotetraose complex/2R0H | 1.90 | |||||
| β-Trefoil fold | ||||||
| Boletus edulis lectin (BEL) | DEAE cellulose, Superdex G75, MonoQ, Lipidex 1000 | BEL (Ligand free) form 1/4I4O | 1.12 |  4I4P | LSL N-acetyllactoseamine complex/1W3F | [177] |
| BEL (Ligand free) form 2/4I4P | 1.28 | |||||
| BEL (Ligand free) form 3/4I4Q | 1.51 | |||||
| BEL (Ligand free) form 4/4I4R | 1.77 | |||||
| BEL lactose complex/4I4S | 1.40 | |||||
| BEL galactose complex/4I4U | 1.57 | |||||
| BEL N-acetylgalactosamine complex/4I4V | 1.50 | |||||
| BEL T-Antigen disaccharide complex/4I4X | 1.72 | |||||
| BEL T-Antigen complex/4I4Y | 1.90 | |||||
| Clitocybe nebularis lectin (CNL) | Lactosyl and glucosyl-sepharose AC, Chromsep HPLC | CNL Lac complex at pH 4.4/3NBC | 1.01 | 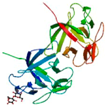 3NBC | Three Foil/3PG0 | [79,81] |
| CNL Lac complex at pH 7.1/3NBD | 1.15 | |||||
| CNL N,N'-diacetyllactosediamine complex/3NBE | 1.86 | |||||
| Coprinopsis cinerea lectin (CCL2) | Horseradish Peroxidase AC | CCL2 (Ligand free)/2LIE | NA |  2LIE | Mosquitocidal toxin/2VSE | [83] |
| CCL2 nematode glycan complex/2LIQ | ||||||
| Laetiporus sulphureus lectin (LSL) | Lactose-sepharose AC | LSL (Ligand free)/1W3A | 2.65 | 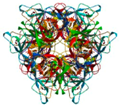 1W3A | Boletus edulis lectin (BEL)/4I4O | [76,111,112] |
| LSL N-acetyllactoseamine complex/1W3G | 2.68 | |||||
| LSL N-acetyllactoseamine complex in the Gamma motif/1W3F | 2.58 | |||||
| LSL (recombinant)/2Y9F | 1.47 | |||||
| LSL (recombinant) Lac complex/2Y9G | 1.67 | |||||
| Marasmius oreades lectin (MOA) | (NH4)2SO4 precipitation, melibiose-sepharose, Synsorb-type B trisaccharide, and Synsorb-type A trisaccharide AC | MOA Galβ1,3Galβ1,4-GlcNAc complex/2IHO | 2.41 | 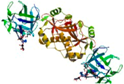 2IHO | Three Foil/3PG0 | [122,178,179] |
| MOA Galα1,3(Fucα1,2)Gal and calcium complex/3EF2 | 1.80 | |||||
| Macrolepiota procera (MPL) | Lactosyl-Sepharose AC | MPL (ligand free)/4ION | 1.60 | 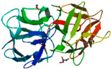 4ION | Rhizoctonia solani agglutinin/4G9M | [120] |
| MPL Gal complex/4IYB | 1.59 | |||||
| MPL Lac complex/4IZX | 1.1 | |||||
| MPL N-acetyllactoseamine complex/4J2S | 1.4 | |||||
| Polyporus squamosus lectin (PSL) | (NH4)2SO4 precipitation, β-d-galactosyl-Synsorb AC, DEAE-Sephacel | PSL bound to human-type influenza-binding epitope Neu5Ac α2-6Galβ1-4GlcNAc/3PHZ | 1.70 | 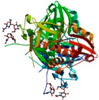 3PHZ | ThreeFoil/3PG0 | [145,180] |
| Actinoporin-like fold | ||||||
| Agaricus bisporus lectin (ABL) | Human erythrocytic stroma polyacrylamide gel AC, preparative isoelectric focusing to separate the 5 ABL isoforms | ABL (Ligand free)/1Y2T | 1.50 |  1Y2T | Sclerotium rolfsii lectin (SRL)/2OFC | [51] |
| ABL Lacto-N-biose complex/1Y2U | 1.85 | |||||
| ABL T-antigen complex/1Y2V | 1.90 | |||||
| Orthorhombic form of ABL T-antigen and GlcNAc complex/1Y2W | 1.74 | |||||
| Tetragonal form of ABL T-antigen and GlcNAc complex/1Y2X | 2.36 | |||||
| Boletus edulis lectin (BEL) | Chitin-sepharose AC, Superdex G-200 HR, Lipidex 1000; Human erythrocytic stroma polyacryl-amide gel AC, Superdex G-200 HR, Lipidex 1000 resin, MiniQ PE | BEL (Ligand free)/3QDS | 1.15 |  3QDS | Sclerotium rolfsii lectin (SRL)/2OFC | [177] |
| BEL T-antigen complex/3QDT | 1.30 | |||||
| BEL N,N-diacetyl chitobiose complex/3QDU | 2.00 | |||||
| Orthorhombic form of BEL GlcNAc and GalNAc complex/3QDV | 1.30 | |||||
| Hexagonal form of BEL GlcNAc and GalNAc complex/3QDW | 1.90 | |||||
| Orthorhombic form of BEL T-antigen disaccharide and N,N-diacetyl chitobiose complex/3QDX | 1.70 | |||||
| Hexagonal form of BEL T-antigen disaccharide and N,N-diacetyl chitobiose complex/3QDY | 2.00 | |||||
| Xerocomus chrysenteron lectin (XCL) | Fetuin-sepharose | Wild-type XCL (ligand free)/1XI0 | 2.00 | 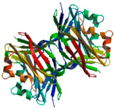 1XI0 | Sclerotium rolfsii lectin (SRL)/2OFC | [107,181] |
| XCL mutated at Q46M, V54M, L58M/1X99 | 1.40 | |||||
| Antiproliferative/Antitumor Activity | |||
|---|---|---|---|
| Source of Lectin | IC50 | Cell Type/Target | Ref. |
| Agaricus bisporus (ABL) | 50 µg/mL * | HT-29 | [50] |
| Agrocybe aegerita (AAL galectin) | NA | S-180, Hela, SW480,SGC 7901, MGC80-3, BGC-823, HL-60 | [56] |
| Amanita phalloides (A. phalloides lectin) | 1.7 µg/mL * | L1210 | [66] |
| Armillaria luteovirens (ALL) | 2.5 μM | MBL2 | [69] |
| Boletopsis leucomelaena (BLL) | 15 µg/mL | U937 | [182] |
| Clitocybe nebularis (CNL) | NA | Mo-T, Jurkat | [79] |
| Cordyceps militaris (CML) | 0.5–0.6 mg/mL * | HepG2 | [183] |
| Flammulina velutipes (F. velutipes lectin) | 13 μM | L1210 | [86] |
| Ganoderma capense (G. capense lectin) | 8 μM | L1210 | [88] |
| Grifola frondosa (GFL) | 25 μg/mL * | HeLa | [184] |
| Hericium erinaceus (HEA) | 56.1 μM | HepG2 | [94] |
| Inocybe umbrinella (I. umbrinella lectin) | 3.5 μM | HepG2 | [99] |
| 7.4 μM | MCF7 | ||
| Lactarius flavidulus (LFL) | 8.90 μM | HepG2 | [106] |
| Paecilomyces japonica (PJA) | NA | SNU-1, AsPc-1, MDAMB-231 | [130] |
| Paxillus involutus (P. involutus lectin) | NA | A-549, HCT-8 | [185] |
| Pholiota adiposa (PAL) | 2.1 μM | HepG2 | [133] |
| Pleurotus citrinopileatus (P. citrinopileatus lectin) | 5 mg/kg of body weight/day ¥ | S-180 in ICR mice | [137] |
| Pleurotus eous (PEL) | 2 μg/mL | MCF-7, K562, HEP-2 | [139] |
| Pleurotus ostreatus (POL) | 1.5 mg/kg bodyweight/day ¥ | S-180, H-22 | [32] |
| Polyporus adustus (P. adustus lectin) | NA | M1, Herto, S180 | [144] |
| Psathyrella asperospora (PAL) | 0.48 μM | HT29 | [186] |
| Russula delica | 0.88 μM | HepG2 | [154] |
| Schizophyllum commune (SCL) | 30 μg/mL | KB | [156] |
| Tricholoma mongolicum (TML1 & TML2) | NA | P815, PU5-1.8 | [187] |
| Volvariella volvacea (VVL) | 17.5 mg/kg body weight ¥ | S-180 | [188] |
| Xerocomus chrysenteron (XCL) | NA | Hela, NIH-3T3 | [189] |
| Xylaria hypoxylon (X. hypoxylon lectin) | 1.24 μM | M1 | [162] |
| Mitogenic Activity | |||
| Source of lectin | [Lectin] £ | Cell type/Target | Ref. |
| Agrocybe cylindracea (ACG) | 2 µM | Mouse splenocytes | [61] |
| Armillaria luteovirens (ALL) | 1 µM | Mouse splenocytes | [69] |
| Boletus edulis (BEL) | 1 µM | Mouse splenocytes | [72] |
| Cordyceps militaris (CML) | 26 µM | Mouse splenocytes | [85] |
| Flammulina velutipes (F. velutipes lectin) | 100 µM | Mouse spleen lymphocytes | [86,190] |
| Hericium erinaceus (HEA) | 20 µM | Mouse splenocytes | [94] |
| Ganoderma capense (G. capense lectin) | 1.5 µM | Mouse splenocytes | [88] |
| Hygrophorus russula (HRL) | 0.15 µM | Mouse splenocytes | [97] |
| Peziza silvestris (P. silvestris lectin) | 8 µM | Mouse splenocytes | [131] |
| Pleurotus citrinopileatus (P. citrinopileatus lectin) | 2 µM | Mouse splenocytes | [137] |
| Polyporus adustus (P. adustus lectin) | 62.5 µM | Mouse splenocytes | [144] |
| Schizophyllum commune (SCL) | 4 µM | Mouse splenocytes | [157] |
| Xerocomus spadiceus (X. spadiceus lectin) | 31.25 µM | Mouse splenocytes | [161] |
| Source of Lectin | IC50 | Ref. |
|---|---|---|
| Agaricus bisporus (ABL) | 8.0 µM | [191] |
| Boletus edulis (BEL) | 14.3 µM | [72] |
| Cordyceps militaris (CML) | 10.0 µM | [183] |
| Hericium erinaceus (HEA) | 31.7 µM | [94] |
| Inocybe umbrinella (I. umbrinella lectin) | 4.7 µM | [99] |
| Pleurotus citrinopileatus (P. citrinopileatus lectin) | 0.93 µM | [137] |
| Schizophyllum commune (SCL) | 1.2 µM | [157] |
3. Mushroom Lectin Structures
4. Biological Activity of Mushroom Lectins
4.1. Antiproliferative/Antitumor Activity
4.2. Mitogenic/Antimitogenic Activity
4.3. Immunomodulatory Activity
5. Conclusions and Future Perspectives
Author Contributions
Conflicts of Interest
References
- Sharon, N.; Lis, H. Lectins as cell recognition molecules. Science 1989, 246, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Kocourek, J.; Horejsi, V. A note on the recent discussion on definition of the term “Lectin”. Lectins Biol. Biochem. Clin. Biochem. 1983, 3, 3–6. [Google Scholar]
- Ashwell, G.; Harford, J. Carbohydrate-specific receptors of the liver. Annu. Rev. Biochem. 1982, 51, 531–554. [Google Scholar] [CrossRef] [PubMed]
- Springer, T.A.; Lasky, L.A. Sticky sugars for selectins. Nature 1991, 349, 196–197. [Google Scholar] [CrossRef] [PubMed]
- Cash, H.L.; Whitham, C.V.; Behrendt, C.L.; Hooper, L.V. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science 2006, 313, 1126–1130. [Google Scholar] [CrossRef] [PubMed]
- Imberty, A.; Gautier, C.; Lescar, J.; Pérez, S.; Wyns, L.; Loris, R. An unusual carbohydrate binding site revealed by the structures of two Maackia amurensis lectins complexed with sialic acid-containing oligosaccharides. J. Biol. Chem. 2000, 275, 17541–17548. [Google Scholar] [CrossRef] [PubMed]
- Rouf, R.; Tiralongo, E.; Krahl, A.; Maes, K.; Spaan, L.; Wolf, S.; May, T.W.; Tiralongo, J. Comparative study of hemagglutination and lectin activity in Australian medicinal mushrooms (higher Basidiomycetes). Int. J. Med. Mushrooms 2011, 13, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.S.; Tiwary, A.K.; Kennedy, J.F. Lectins: Sources, activities, and applications. Crit. Rev. Biotechnol. 1999, 19, 145–178. [Google Scholar] [CrossRef]
- Wright, L.M.; van Damme, E.J.; Barre, A.; Allen, A.K.; van Leuven, F.; Reynolds, C.D.; Rouge, P.; Peumans, W.J. Isolation, characterization, molecular cloning and molecular modelling of two lectins of different specificities from bluebell (Scilla campanulata) bulbs. Biochem. J. 1999, 340 Pt 1, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Wright, L.M.; Wood, S.D.; Reynolds, C.D.; Rizkallah, P.J.; Peumans, W.J.; van Damme, E.J.; Allen, A.K. Purification, crystallization and preliminary X-ray analysis of a mannose-binding lectin from bluebell (Scilla campanulata) bulbs. Acta Crystallogr. D 1996, 52, 1021–1023. [Google Scholar] [CrossRef] [PubMed]
- Van Damme, E.J.; Balzarini, J.; Smeets, K.; van Leuven, F.; Peumans, W.J. The monomeric and dimeric mannose-binding proteins from the Orchidaceae species Listera ovata and Epipactis helleborine: Sequence homologies and differences in biological activities. Glycoconj. J. 1994, 11, 321–332. [Google Scholar]
- Van Damme, J.M.; Smeets, K.; Torrekens, S.; van Leuven, F.; Peumans, W.J. Characterization and molecular cloning of mannose-binding lectins from the Orchidaceae species Listera ovata, Epipactis helleborine and Cymbidium hybrid. Eur. J. Biochem. 1994, 221, 769–777. [Google Scholar]
- Van Damme, E.J.; Barre, A.; Mazard, A.M.; Verhaert, P.; Horman, A.; Debray, H.; Rouge, P.; Peumans, W.J. Characterization and molecular cloning of the lectin from Helianthus tuberosus. Eur. J. Biochem. 1999, 259, 135–142. [Google Scholar]
- Lis, H.; Sharon, N. Lectins: Carbohydrate-Specific proteins that mediate cellular recognition. Chem. Rev. 1998, 98, 637–674. [Google Scholar] [CrossRef] [PubMed]
- Gatehouse, A.M.; Powell, K.S.; Peumans, W.J.; van Damme, E.J.; Gatehouse, J.A. Insecticidal properties of plant lectins: Their potential in plant protection. In Lectins Biomedical Perspectives; Taylor & Francis e-library: Bristol, PA, USA, 1995; pp. 35–57. [Google Scholar]
- Brock, T.; Madigan, M.; Martinko, J.; Parker, J. Biology of Microorganisms, 7th ed.; Prentice-Hall International, Inc.: Englewood Cliffs, NJ, USA, 1994. [Google Scholar]
- Varki, A.; Cummings, R.; Esko, J.; Freeze, H.; Hart, G.; Marth, J. Structures common to different types of glycans. In Essentials of Glycobiology; Cold Spring Harbor Laboraory Press: New York, NY, USA, 1999; pp. 211–252. [Google Scholar]
- Sharon, N. Lectin receptors as lymphocyte surface markers. Adv. Immunol. 1983, 34, 213–298. [Google Scholar] [PubMed]
- Wang, H.; Ng, T.B.; Ooi, V.E.C. Lectins from mushrooms. Mycol. Res. 1998, 102, 897–906. [Google Scholar] [CrossRef]
- Tiwary, A.; Singh, R. Lectins: Novel drug targeting molecules. Indian J. Pharm. Sci. 1999, 61, 259. [Google Scholar]
- Chang, S.T.; Buswell, J. Mushroom nutriceuticals. World J. Microbiol. Biotechnol. 1996, 12, 473–476. [Google Scholar] [CrossRef] [PubMed]
- Wasser, S.P.; Sokolov, D.; Reshetnikov, S.V.; Timor-Tismenetsky, M. Dietary supplements from medicinal mushrooms: Diversity of types and variety of regulations. Int. J. Med. Mushrooms 2000, 2, 1–19. [Google Scholar] [CrossRef]
- Breene, W.M. Nutritional and medicinal value of specialty mushrooms. J. Food Prot. 1990, 53, 883–894. [Google Scholar]
- Chang, S.T.; Miles, P.G. Mushrooms biology-a new discipline. Mycologist 1992, 6, 64–65. [Google Scholar] [CrossRef]
- Hibbett, D.S.; Binder, M.; Bischoff, J.F.; Blackwell, M.; Cannon, P.F.; Eriksson, O.E.; Huhndorf, S.; James, T.; Kirk, P.M.; Lücking, R. A higher-level phylogenetic classification of the Fungi. Mycol. Res. 2007, 111, 509–547. [Google Scholar] [CrossRef] [PubMed]
- Lindequist, U.; Niedermeyer, T.H.; Jülich, W.-D. The pharmacological potential of mushrooms. Evid.-Based Complement. Altern. Med. 2005, 2, 285–299. [Google Scholar] [CrossRef]
- Wasser, S.P. Current findings, future trends, and unsolved problems in studies of medicinal mushrooms. Appl. Microbiol. Biotechnol. 2011, 89, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Wasser, S.P. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl. Microbiol. Biotechnol. 2002, 60, 258–274. [Google Scholar] [CrossRef] [PubMed]
- Hawksworth, D.L. Mushrooms: The extent of the unexplored potential. Int. J. Med. Mushrooms 2001, 3, 333–340. [Google Scholar] [CrossRef]
- Erjavec, J.; Kos, J.; Ravnikar, M.; Dreo, T.; Sabotič, J. Proteins of higher fungi—From forest to application. Trends Biotechnol. 2012, 30, 259–273. [Google Scholar] [CrossRef] [PubMed]
- She, Q.B.; Ng, T.B.; Liu, W.K. A novel lectin with potent immunomodulatory activity isolated from both fruiting bodies and cultured mycelia of the edible mushroom Volvariella volvacea. Biochem. Biophys. Res. Commun. 1998, 247, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Gao, J.; Ng, T.B. A new lectin with highly potent antihepatoma and antisarcoma activities from the oyster mushroom Pleurotus ostreatus. Biochem. Biophys. Res. Commun. 2000, 275, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Weis, A.L.; Wasser, S.P. Therapeutic effects of substances occurring in higher basidiomycetes mushrooms: A modern perspective. Crit. Rev. Immunol. 1999, 19, 32. [Google Scholar] [CrossRef]
- Sliva, D. Ganoderma lucidum in cancer research. Leuk. Res. 2006, 30, 767–768. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.K.; Cho, H.Y.; Kim, J.S.; Kim, H.W.; Choi, E.C. Studies on constituents of higher Fungi of Korea (LXVIII). Studies 1993, 24, 203–212. [Google Scholar]
- Singh, R.S.; Bhari, R.; Kaur, H.P. Mushroom lectins: Current status and future perspectives. Crit. Rev. Biotechnol. 2010, 30, 99–126. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Khan, M.I. Fungal lectins: Current molecular and biochemical perspectives. Int. J. Biol. Chem. 2011, 5, 1–20. [Google Scholar] [CrossRef]
- Sharon, N.; Lis, H. History of lectins: From hemagglutinins to biological recognition molecules. Glycobiology 2004, 14, 53R–62R. [Google Scholar] [CrossRef] [PubMed]
- Końska, G. The lectins of higher fungi (macromycetes)—Their occurrence, physiological role and biological activity. Int. J. Med. Mushrooms 2006, 8, 19–30. [Google Scholar] [CrossRef]
- Guillot, J.; Konska, G. Lectins in higher fungi. Biochem. Syst. Ecol. 1997, 25, 203–230. [Google Scholar] [CrossRef]
- Singh, S.S.; Wang, H.; Chan, Y.S.; Pan, W.; Dan, X.; Yin, C.M.; Akkouh, O.; Ng, T.B. Lectins from Edible Mushrooms. Molecules 2014, 20, 446–469. [Google Scholar] [CrossRef] [PubMed]
- Peumans, W.J.; van Damme, E.J. Lectins as plant defense proteins. Plant Physiol. 1995, 109, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Swamy, B.M.; Bhat, A.G.; Hegde, G.V.; Naik, R.S.; Kulkarni, S.; Inamdar, S.R. Immunolocalization and functional role of Sclerotium rolfsii lectin in development of fungus by interaction with its endogenous receptor. Glycobiology 2004, 14, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Varrot, A.; Basheer, S.M.; Imberty, A. Fungal lectins: Structure, function and potential applications. Curr. Opin. Struct. Biol. 2013, 23, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.X.; Ng, T.B.; Liu, W.K.; Ooi, V.E.C.; Chang, S.T. Isolation and characterization of two distinct lectins with antiproliferative activity from the cultured mycelium of the edible mushroom Tricholoma mongolicum. Int. J. Pept. Protein Res. 1995, 46, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Kawagishi, H.; Mitsunaga, S.; Yamawaki, M.; Ido, M.; Shimada, A.; Kinoshita, T.; Murata, T.; Usui, T.; Kimura, A.; Chiba, S. A lectin from mycelia of the fungus Ganoderma lucidum. Phytochemistry 1997, 44, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Mikiashvili, N.; Elisashvili, V.; Wasser, S.P.; Nevo, E. Comparative study of lectin activity of higher Basidiomycetes. Int. J. Med. Mushrooms 2006, 8, 31–38. [Google Scholar] [CrossRef]
- Zhao, J.K.; Zhao, Y.C.; Li, S.H.; Wang, H.X.; Ng, T.B. Isolation and characterization of a novel thermostable lectin from the wild edible mushroom Agaricus arvensis. J. Basic Microbiol. 2011, 51, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Nakamura-Tsuruta, S.; Kominami, J.; Kuno, A.; Hirabayashi, J. Evidence that Agaricus bisporus agglutinin (ABA) has dual sugar-binding specificity. Biochem. Biophys. Res. Commun. 2006, 347, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Fernig, D.G.; Smith, J.A.; Milton, J.D.; Rhodes, J.M. Reversible inhibition of proliferation of epithelial cell lines by Agaricus bisporus (edible mushroom) lectin. Cancer Res. 1993, 53, 4627–4632. [Google Scholar] [PubMed]
- Carrizo, M.E.; Capaldi, S.; Perduca, M.; Irazoqui, F.J.; Nores, G.A.; Monaco, H.L. The antineoplastic lectin of the common edible mushroom (Agaricus bisporus) has two binding sites, each specific for a different configuration at a single epimeric hydroxyl. J. Biol. Chem. 2005, 280, 10614–10623. [Google Scholar] [CrossRef] [PubMed]
- Batterbury, M.; Tebbs, C.A.; Rhodes, J.M.; Grierson, I. Agaricus bisporus (edible mushroom lectin) inhibits ocular fibroblast proliferation and collagen lattice contraction. Exp. Eye Res. 2002, 74, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Kawagishi, H.; Aya, N.; Takayuki, Y.; Takashi, M.; Toshihiko, H.; Takuji, N. Isolation and properties of a lectin from the fruiting bodies of Agaricus blazei. Carbohydr. Res. 1988, 183, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Sage, H.J.; Vazquez, J.J. Studies on a hemagglutinin from the mushroom agaricus campestris. J. Biol. Chem. 1967, 242, 120–125. [Google Scholar] [PubMed]
- Sage, H.J.; Connett, S.L. Studies on a hemagglutinin from the meadow mushroom: II. purification, composition, and structure of agaricus campestris hemagglutinin. J. Biol. Chem. 1969, 244, 4713–4719. [Google Scholar] [PubMed]
- Zhao, C.; Sun, H.; Tong, X.; Qi, Y. An antitumour lectin from the edible mushroom Agrocybe aegerita. Biochem. J. 2003, 374, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Liang, Y.; Xiang, Y.; Zhang, Y.; Sun, H.; Wang, D.C. Crystallization and preliminary crystallographic studies of an antitumour lectin from the edible mushroom Agrocybe aegerita. Protein Pept. Lett. 2005, 12, 705–707. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhao, C.G.; Tong, X.; Qi, Y.P. A lectin with mycelia differentiation and antiphytovirus activities from the edible mushroom Agrocybe aegerita. J. Biochem. Mol. Biol. 2003, 36, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Chen, Y.; Wang, M.; Yin, Y.; Pan, Y.; Gu, B.; Yu, G.; Li, Y.; Wong, B.H.; Liang, Y.; et al. A novel lectin from Agrocybe aegerita shows high binding selectivity for terminal N-acetylglucosamine. Biochem. J. 2012, 443, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Yagi, F.; Miyamoto, M.; Abe, T.; Minami, Y.; Tadera, K.; Goldstein, I.J. Purification and carbohydrate-binding specificity of Agrocybe cylindracea lectin. Glycoconj. J. 1997, 14, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ng, T.B.; Liu, Q. Isolation of a new heterodimeric lectin with mitogenic activity from fruiting bodies of the mushroom Agrocybe cylindracea. Life Sci. 2002, 70, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, F.; Tiralongo, E.; Tiralongo, J. Sialic acid-specific lectins: Occurrence, specificity and function. Cell. Mol. Life Sci. 2006, 63, 1331–1354. [Google Scholar] [CrossRef] [PubMed]
- Yagi, F.; Hiroyama, H.; Kodama, S. Agrocybe cylindracea lectin is a member of the galectin family. Glycoconj. J. 2001, 18, 745–749. [Google Scholar] [CrossRef] [PubMed]
- Kochibe, N.; Furukawa, K. Purification and properties of a novel fucose-specific hemagglutinin of Aleuria aurantia. Biochemistry 1980, 19, 2841–2846. [Google Scholar] [CrossRef] [PubMed]
- Olausson, J.; Tibell, L.; Jonsson, B.H.; Pahlsson, P. Detection of a high affinity binding site in recombinant Aleuria aurantia lectin. Glycoconj. J. 2008, 25, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Lutsik-Kordovsky, M.D.; Stasyk, T.V.; Stoika, R.S. Analysis of cytotoxicity of lectin and non-lectin proteins from Amanita mushrooms. Eksp. Onkol. 2001, 23, 43–45. [Google Scholar]
- Zhuang, C.; Murata, T.; Usui, T.; Kawagishi, H.; Kobayashi, K. Purification and characterization of a lectin from the toxic mushroom Amanita pantherina. Biochim. Biophys. Acta Gen. Subj. 1996, 1291, 40–44. [Google Scholar] [CrossRef]
- Antonyuk, V.O.; Yu Klyuchivska, O.; Stoika, R.S. Cytotoxic proteins of Amanita virosa Secr. mushroom: Purification, characteristics and action towards mammalian cells. Toxicon 2010, 55, 1297–1305. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Liu, Q.H.; Ng, T.B.; Liu, H.Z.; Li, J.Q.; Chen, G.; Sheng, H.Y.; Xie, Z.L.; Wang, H.X. Isolation and characterization of a novel lectin from the mushroom Armillaria luteo-virens. Biochem. Biophys. Res. Commun. 2006, 345, 1573–1578. [Google Scholar] [CrossRef] [PubMed]
- Yagi, F.; Tadera, K. Purification and characterization of lectin from Auricularia polytricha. Agric. Biol. Chem. 1988, 52, 2077–2079. [Google Scholar] [CrossRef]
- Koyama, Y.; Suzuki, T.; Odani, S.; Nakamura, S.; Kominami, J.; Hirabayashi, J.; Isemura, M. Carbohydrate specificity of lectins from Boletopsis leucomelas and Aralia cordate. Biosci. Biotechnol. Biochem. 2006, 70, 542–545. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Li, C.; Ng, T.B.; Wang, H.X. A lectin with mitogenic activity from the edible wild mushroom Boletus edulis. Process Biochem. 2007, 42, 1620–1624. [Google Scholar] [CrossRef]
- Licastro, F.; Morini, M.C.; Kretz, O.; Dirheimer, G.; Creppy, E.E.; Stirpe, F. Mitogenic activity and immunological properties of bolesatine, a lectin isolated from the mushroom Boletus satanas Lenz. Int. J. Biochem. 1993, 25, 789–792. [Google Scholar] [CrossRef] [PubMed]
- Colceag, J.; Mogos, S.; Hulea, S. Studies on occurrence and characterization of phytolectins in some species of mushrooms. Rev. Roum. Biochim. 1984, 21, 263–266. [Google Scholar]
- Horibe, M.; Kobayashi, Y.; Dohra, H.; Morita, T.; Murata, T.; Usui, T.; Nakamura-Tsuruta, S.; Kamei, M.; Hirabayashi, J.; Matsuura, M.; et al. Toxic isolectins from the mushroom Boletus venenatus. Phytochemistry 2010, 71, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Ishizaki, T.; Kawagishi, H. Screening for lectins in wild and cultivated mushrooms from Japan and their sugar-binding specificities. Int. J. Med. Mushrooms 2004, 6, 117–129. [Google Scholar] [CrossRef]
- Otta, Y.; Amano, K.; Nishiyama, K.; Ando, A.; Ogawa, S.; Nagata, Y. Purification and properties of a lectin from ascomycete mushroom, Ciborinia camelliae. Phytochemistry 2002, 60, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Lyimo, B.; Funakuma, N.; Minami, Y.; Yagi, F. Characterization of a new alpha-Galactosyl-Binding Lectin from the Mushroom Clavaria purpurea. Biosci. Biotechnol. Biochem. 2012, 76, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Pohleven, J.; Obermajer, N.; Sabotic, J.; Anzlovar, S.; Sepcic, K.; Kos, J.; Kralj, B.; Strukelj, B.; Brzin, J. Purification, characterization and cloning of a ricin B-like lectin from mushroom Clitocybe nebularis with antiproliferative activity against human leukemic T cells. Biochim. Biophys. Acta 2009, 1790, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Horejsi, V.; Kocourek, J. Studies on lectins. XXXVI. Properties of some lectins prepared by affinity chromatography on O-glycosyl polyacrylamide gels. Biochim. Biophys. Acta 1978, 538, 299–315. [Google Scholar] [CrossRef] [PubMed]
- Pohleven, J.; Renko, M.; Magister, Š.; Smith, D.F.; Künzler, M.; Štrukelj, B.; Turk, D.; Kos, J.; Sabotič, J. Bivalent carbohydrate binding is required for biological activity of Clitocybe nebularis Lectin (CNL), the N,N'-Diacetyllactosediamine (GalNAcβ1–4GlcNAc, LacdiNAc)-specific Lectin from Basidiomycete C. nebularis. J. Biol. Chem. 2012, 287, 10602–10612. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.N.; Boulianne, R.P.; Charlton, S.; Farrell, E.M.; Sucher, A.; Lu, B.C. Fungal galectins, sequence and specificity of two isolectins from Coprinus cinereus. J. Biol. Chem. 1997, 272, 1514–1521. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.; Bleuler-Martinez, S.; Butschi, A.; Walti, M.A.; Egloff, P.; Stutz, K.; Yan, S.; Collot, M.; Mallet, J.M.; Wilson, I.B.; et al. Plasticity of the beta-trefoil protein fold in the recognition and control of invertebrate predators and parasites by a fungal defence system. PLoS Pathog. 2012, 8, e1002706. [Google Scholar] [CrossRef] [PubMed]
- Walti, M.A.; Walser, P.J.; Thore, S.; Grunler, A.; Bednar, M.; Kunzler, M.; Aebi, M. Structural basis for chitotetraose coordination by CGL3, a novel galectin-related protein from Coprinopsis cinerea. J. Mol. Biol. 2008, 379, 146–159. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.C.; Kim, K.D.; Bae, C.H.; Kim, J.C.; Kim, D.K.; Kim, H.H. A mushroom lectin from ascomycete Cordyceps militaris. Biochim. Biophys. Acta 2007, 1770, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.B.; Ngai, P.H.; Xia, L. An agglutinin with mitogenic and antiproliferative activities from the mushroom Flammulina velutipes. Mycologia 2006, 98, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Yatohgo, T.; Nakata, M.; Tsumuraya, Y.; Hashimoto, Y.; Yamamoto, S. Purification and properties of a lectin from the fruitbodies of Flammulina velutipes. Agric. Biol. Chem. 1988, 52, 1485–1493. [Google Scholar] [CrossRef]
- Ngai, P.H.K.; Ng, T.B. A mushroom (Ganoderma capense) lectin with spectacular thermostability, potent mitogenic activity on splenocytes, and antiproliferative activity toward tumor cells. Biochem. Biophys. Res. Commun. 2004, 314, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.; Rana, M.; Lakhanpal, T.N.; Ahmad, A.; Khan, M.I. Purification and characterization of lectin from fruiting body of Ganoderma lucidum. Biochim. Biophys. Acta Gen. Subj. 2007, 1770, 1404–1412. [Google Scholar] [CrossRef]
- Thakur, A.; Pal, L.; Ahmad, A.; Khan, M.I. Complex carbohydrate specificity of lectin from fruiting body of Ganoderma lucidum. A surface plasmon resonance study. IUBMB Life 2007, 59, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Nagata, Y.; Yamashita, M.; Honda, H.; Akabane, J.; Uehara, K.; Saito, A.; Sumisa, F.; Nishibori, K.; Oodaira, Y. Characterization, occurrence, and molecular cloning of a lectin from Grifola frondosa: Jacalin-related lectin of fungal origin. Biosci. Biotechnol. Biochem. 2005, 69, 2374–2380. [Google Scholar] [CrossRef] [PubMed]
- Stepanova, L.; Burygin, G.; Matora, L.; Bogatyrev, V.; Sokolova, M.; Nikitina, V. Localization and immunochemical characteristics of basidiomycete Grifola frondosa (Fr.) SF Gray lectin. Mikrobiologiia 2009, 78, 236. [Google Scholar] [PubMed]
- Kawagishi, H.; Mori, H.; Uno, A.; Kimura, A.; Chiba, S. A sialic acid-binding lectin from the mushroom Hericium erinaceum. FEBS Lett. 1994, 340, 56–58. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, G.; Ng, T.B.; Wang, H. A novel lectin with antiproliferative and HIV-1 reverse transcriptase inhibitory activities from dried fruiting bodies of the monkey head mushroom Hericium erinaceum. J. Biomed. Biotechnol. 2010, 2010, 716515. [Google Scholar] [PubMed]
- Veau, B.; Guillot, J.; Damez, M.; Dusser, M.; Konska, G.; Botton, B. Purification and characterization of an anti-(A+B) specific lectin from the mushroom Hygrophorus hypothejus. Biochim. Biophys. Acta 1999, 1428, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Guillot, J.; Coulet, M. Properties of the anti (A plus B) lectin of Hygrophorus hypothejus Fr. Fixation, elution, inhibition. Rev. Fr. Transfus. 1974, 17, 49. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Sugiyama, K.; Hirai, H.; Ito, H.; Morita, T.; Dohra, H.; Murata, T.; Usui, T.; Tateno, H.; Hirabayashi, J.; et al. Mannose-specific lectin from the mushroom Hygrophorus russula. Glycobiology 2012, 22, 616–629. [Google Scholar] [CrossRef] [PubMed]
- Coulet, M.; Guillot, J.; Betail, G. Action of certain mono- and polysaccharides on hemagglutinins of certain mushrooms. Acta Pol. Pharm. 1972, 29, 299–307. [Google Scholar] [PubMed]
- Zhao, J.K.; Wang, H.X.; Ng, T.B. Purification and characterization of a novel lectin from the toxic wild mushroom Inocybe umbrinella. Toxicon 2009, 53, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Musflek, M.; Ticha, M.; Volc, J.; Kocourek, J. Studies on lectins LXXI. Lectins in mycelial cultures of Kuehneromyces mutabilis, Pholiota squarrosa, and Flammulina velutipes. In Proceedings of the Tenth Lectin Meeting, Prague, Czech Republic, July 1988; Sigma Aldrich Corp: St. Louis, MI, USA, 1990; p. 53. [Google Scholar]
- Guillot, J.; Genaud, L.; Gueugnot, J.; Damez, M. Purification and properties of two hemagglutinins of the mushroom Laccaria amethystina. Biochemistry 1983, 22, 5365–5369. [Google Scholar] [CrossRef]
- Lyimo, B.; Yagi, F.; Minami, Y. Primary structure and specificity of a new member of galectin family from the Amethyst deceiver mushroom Laccaria amethystina. Biosci. Biotechnol. Biochem. 2011, 75, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Ticha, M.; Sychrova, H.; Kocourek, J. Saccharide binding properties of lectins of higher fungi. In Lectins; Bøg-Hansen, T.C., Freed, D.L.J., Eds.; Sigma chemical company: St. Louis, MI, USA, 1988; Volume 6, pp. 383–391. [Google Scholar]
- Guillot, J.; Giollant, M.; Damez, M.; Dusser, M. Isolation and characterization of a lectin from the mushroom, Lactarius deliciosus. J. Biochem. 1991, 109, 840–845. [Google Scholar] [PubMed]
- Giollant, M.; Guillot, J.; Damez, M.; Dusser, M.; Didier, P.; Didier, E. Characterization of a lectin from Lactarius deterrimus. Research on the possible involvement of the fungal lectin in recognition between mushroom and spruce during the early stages of mycorrhizae formation. Plant Physiol. 1993, 101, 513–522. [Google Scholar] [PubMed]
- Wu, Y.; Wang, H.; Ng, T.B. Purification and characterization of a lectin with antiproliferative activity toward cancer cells from the dried fruit bodies of Lactarius flavidulus. Carbohydr. Res. 2011, 346, 2576–2581. [Google Scholar] [CrossRef] [PubMed]
- Sychrova, H.; Ticha, M.; Kocourek, J. Studies on lectins. LIX. Isolation and properties of lectins from fruiting bodies of Xerocomus chrysenteron and Lactarius lignyotus. Can. J. Biochem. Cell Biol. 1985, 63, 700–704. [Google Scholar] [CrossRef] [PubMed]
- Panchak, L.V.; Antonyuk, V.O. Purification of a lectin from fruit bodies of Lactarius pergamenus (Fr.) Fr. and studies of its properties. Biochemistry 2011, 76, 438–449. [Google Scholar] [PubMed]
- Panchak, L.V.; Antoniuk, V.O. Purification of lectin from fruiting bodies of Lactarius rufus (Scop.: Fr.)Fr. and its carbohydrate specificity. Ukr. Biokhim. Zhurnal 2007, 79, 123–128. [Google Scholar]
- Giollant, M. Les Lectines des Lactaires du Groupe Dapestes: (L. deiciosus, L. deterrimus, L. salmonicolor). Purification, Étude Biochimique et Spécificité. Invervention des Lectines dans les Phénoménes de Reconnaissance Moléculaire au Cours des Événements Précoces de la Mycorrhization avec les Conifers Associés. Thése de Doctorat en Pharmacie, Univ. Clermont I, Clermont-Ferrand, France, 1991. [Google Scholar]
- Tateno, H.; Goldstein, I.J. Molecular cloning, expression, and characterization of novel hemolytic lectins from the mushroom Laetiporus sulphureus, which show homology to bacterial toxins. J. Biol. Chem. 2003, 278, 40455–40463. [Google Scholar] [CrossRef] [PubMed]
- Mancheno, J.M.; Tateno, H.; Goldstein, I.J.; Martinez-Ripoll, M.; Hermoso, J.A. Structural analysis of the Laetiporus sulphureus hemolytic pore-forming lectin in complex with sugars. J. Biol. Chem. 2005, 280, 17251–17259. [Google Scholar] [CrossRef] [PubMed]
- Konska, G.; Guillot, J.; Dusser, M.; Damez, M.; Botton, B. Isolation and characterization of an N-acetyllactosamine-binding lectin from the mushroom Laetiporus sulfureus. J. Biochem. 1994, 116, 519–523. [Google Scholar] [PubMed]
- Wang, H.X.; Ng, T.B.; Ooi, V.E.C. Studies on purification of a lectin from fruiting bodies of the edible shiitake mushroom Lentinus edodes. Int. J. Biochem. Cell Biol. 1999, 31, 595–599. [Google Scholar] [CrossRef]
- Vetchinkina, E.P.; Pozdnyakova, N.N.; Nikitina, V.E. Laccase and lectin activities of intracellular proteins produced in a submerged culture of the xylotrophic basidiomycete Lentinus edodes. Curr. Microbiol. 2008, 57, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Tsivileva, O.M.; Nikitina, V.E.; Garibova, L.V.; Zav’yalova, L.A.; Ignatov, V.V. Hemagglutinating activity of the fungus Lentinus edodes (Berk.) sing “Lentinus edodes (Berk.) Pegler”. Microbiology 2000, 69, 30–35. [Google Scholar] [CrossRef]
- Eghianruwa, Q.; Odekanyin, O.; Kuku, A. Physicochemical properties and acute toxicity studies of a lectin from the saline extract of the fruiting bodies of the shiitake mushroom, Lentinula edodes (Berk). Int. J. Biochem. Mol. Biol. 2011, 2, 309–317. [Google Scholar] [PubMed]
- Sharma, S.K.; Atri, N. Comparative study on lectin activity in mycelium of wild mushroom (Lentinus) species. Middle-East J. Sci. Res. 2012, 12, 499–503. [Google Scholar]
- Goldstein, I.J.; Winter, H.C.; Aurandt, J.; Confer, L.; Adamson, J.T.; Hakansson, K.; Remmer, H. A new alpha-galactosyl-binding protein from the mushroom Lyophyllum decastes. Arch. Biochem. Biophys. 2007, 467, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Zurga, S.; Pohleven, J.; Renko, M.; Bleuler-Martinez, S.; Sosnowski, P.; Turk, D.; Kunzler, M.; Kos, J.; Sabotic, J. A novel beta-trefoil lectin from the parasol mushroom (Macrolepiota procera) is nematotoxic. FEBS J. 2014, 281, 3489–3506. [Google Scholar] [CrossRef] [PubMed]
- Loganathan, D.; Winter, H.C.; Judd, W.J.; Petryniak, J.; Goldstein, I.J. Immobilized Marasmius oreades agglutinin: Use for binding and isolation of glycoproteins containing the xenotransplantation or human type B epitopes. Glycobiology 2003, 13, 955–960. [Google Scholar] [CrossRef] [PubMed]
- Winter, H.C.; Mostafapour, K.; Goldstein, I.J. The mushroom Marasmius oreades lectin is a blood group type B agglutinin that recognizes the Galalpha 1,3Gal and Galalpha 1,3Galbeta 1,4GlcNAc porcine xenotransplantation epitopes with high affinity. J. Biol. Chem. 2002, 277, 14996–15001. [Google Scholar] [CrossRef] [PubMed]
- Rempel, B.P.; Winter, H.C.; Goldstein, I.J.; Hindsgaul, O. Characterization of the recognition of blood group B trisaccharide derivatives by the lectin from Marasmius oreades using frontal affinity chromatography-mass spectrometry. Glycoconj. J. 2002, 19, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Tateno, H.; Goldstein, I.J. Partial identification of carbohydrate-binding sites of a Galalpha1,3Galbeta1,4GlcNAc-specific lectin from the mushroom Marasmius oreades by site-directed mutagenesis. Arch. Biochem. Biophys. 2004, 427, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Shimokawa, M.; Fukudome, A.; Yamashita, R.; Minami, Y.; Yagi, F.; Tateno, H.; Hirabayashi, J. Characterization and cloning of GNA-like lectin from the mushroom Marasmius oreades. Glycoconj. J. 2012, 29, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S.; Otta, Y.; Ando, A.; Nagata, Y. A lectin from an ascomycete mushroom, Melastiza chateri: No synthesis of the lectin in mycelial isolate. Biosci. Biotechnol. Biochem. 2001, 65, 686–689. [Google Scholar] [CrossRef] [PubMed]
- Kawagishi, H.; Takagi, J.; Taira, T.; Murata, T.; Usui, T. Purification and characterization of a lectin from the mushroom Mycoleptodonoides aitchisonii. Phytochemistry 2001, 56, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Natsume, A.; Ueda, H.; Saitoh, T.; Ogawa, H. Screening of a unique lectin from 16 cultivable mushrooms with hybrid glycoprotein and neoproteoglycan probes and purification of a novel N-acetylglucosamine-specific lectin from Oudemansiella platyphylla fruiting body. Biochim. Biophys. Acta 2001, 1526, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Gold, E.R.; Balding, P. Receptor-Specific Proteins: Plant and Animal Lectins; Excerpta Medica: Amsterdam, The Netherlands, 1975. [Google Scholar]
- Park, J.H.; Ryu, C.S.; Kim, H.N.; Na, Y.J.; Park, H.J.; Kim, H. A sialic acid-specific lectin from the mushroom Paecilomyces Japonica that exhibits hemagglutination activity and cytotoxicity. Protein Pept. Lett. 2004, 11, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ng, T.B. First report of an arabinose-specific fungal lectin. Biochem. Biophys. Res. Commun. 2005, 337, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Kawagishi, H.; Wasa, T.; Murata, T.; Usui, T.; Kimura, A.; Chiba, S. Two N-acetyl-d-galactosamine-specific lectins from Phaeolepiota aurea. Phytochemistry 1996, 41, 1013–1016. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.Q.; Sun, J.; Wang, H.X.; Ng, T.B. A novel lectin with antiproliferative activity from the medicinal mushroom Pholiota adiposa. Acta Biochim. Pol. 2009, 56, 415–421. [Google Scholar] [PubMed]
- Kawagishi, H.; Abe, Y.; Nagata, T.; Kimura, A.; Chiba, S. A lectin from the mushroom Pholiota aurivella. Agric. Biol. Chem. 1991, 55, 2485–2489. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Tateno, H.; Dohra, H.; Moriwaki, K.; Miyoshi, E.; Hirabayashi, J.; Kawagishi, H. A novel core fucose-specific lectin from the mushroom Pholiota squarrosa. J. Biol. Chem. 2012, 287, 33973–33982. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Amano, Y.; Fujita, M.; Kobayashi, Y.; Dohra, H.; Hirai, H.; Murata, T.; Usui, T.; Morita, T.; Kawagishi, H. Purification, characterization, and cDNA cloning of a lectin from the mushroom Pleurocybella porrigens. Biosci. Biotechnol. Biochem. 2009, 73, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.R.; Liu, Q.H.; Wang, H.X.; Ng, T.B. A novel lectin with potent antitumor, mitogenic and HIV-1 reverse transcriptase inhibitory activities from the edible mushroom Pleurotus citrinopileatus. Biochim. Biophys. Acta 2008, 1780, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Kato, S.; Oguri, S.; Nagata, Y. Purification and properties of lectins from a mushroom, Pleurotus cornucopiae. Biosci. Biotechnol. Biochem. 1994, 58, 498–501. [Google Scholar] [CrossRef]
- Mahajan, R.G.; Patil, S.I.; Mohan, D.R.; Shastry, P. Pleurotus Eous mushroom lectin (PEL) with mixed carbohydrate inhibition and antiproliferative activity on tumor cell lines. J. Biochem. Mol. Biol. Biophys. 2002, 6, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Kogure, T. On the specificity of mushroom Pleurotus ostreatus and Pleurotus spodoleucus extracts. Vox Sang. 1975, 29, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Nakamura, H.; Sekiguchi, T.; Takanami, R.; Murata, T.; Usui, T.; Kawagishi, H. Analysis of the carbohydrate binding specificity of the mushroom Pleurotus ostreatus lectin by surface plasmon resonance. Anal. Biochem. 2005, 336, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Kawagishi, H.; Suzuki, H.; Watanabe, H.; Nakamura, H.; Sekiguchi, T.; Murata, T.; Usui, T.; Sugiyama, K.; Suganuma, H.; Inakuma, T.; et al. A lectin from an edible mushroom Pleurotus ostreatus as a food intake-suppressing substance. Biochim. Biophys. Acta Gen. Subj. 2000, 1474, 299–308. [Google Scholar] [CrossRef]
- Wang, H.; Ng, T.B. Isolation of a novel N-acetylglucosamine-specific lectin from fresh sclerotia of the edible mushroom Pleurotus tuber-regium. Protein Expr. Purif. 2003, 29, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ng, T.B.; Liu, Q. A novel lectin from the wild mushroom Polyporus adusta. Biochem. Biophys. Res. Commun. 2003, 307, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Mo, H.; Winter, H.C.; Goldstein, I.J. Purification and characterization of a Neu5Acalpha2-6Galbeta1-4Glc/GlcNAc-specific lectin from the fruiting body of the polypore mushroom Polyporus squamosus. J. Biol. Chem. 2000, 275, 10623–10629. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Palcic, M.M.; Mo, H.; Goldstein, I.J.; Hindsgaul, O. Rapid determination of the binding affinity and specificity of the mushroom Polyporus squamosus lectin using frontal affinity chromatography coupled to electrospray mass spectrometry. Glycobiology 2001, 11, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Toma, V.; Zuber, C.; Winter, H.C.; Goldstein, I.J.; Roth, J. Application of a lectin from the mushroom Polysporus squamosus for the histochemical detection of the NeuAcalpha2,6Galbeta1,4Glc/GlcNAc sequence of N-linked oligosaccharides: A comparison with the Sambucus nigra lectin. Histochem. Cell Biol. 2001, 116, 183–193. [Google Scholar] [PubMed]
- Arigi, E.; Singh, S.; Kahlili, A.H.; Winter, H.C.; Goldstein, I.J.; Levery, S.B. Characterization of neutral and acidic glycosphingolipids from the lectin-producing mushroom, Polyporus squamosus. Glycobiology 2007, 17, 754–766. [Google Scholar] [CrossRef] [PubMed]
- Ueda, H.; Kojima, K.; Saitoh, T.; Ogawa, H. Interaction of a lectin from Psathyrella velutina mushroom with N-acetylneuraminic acid. FEBS Lett. 1999, 448, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Ueda, H.; Matsumoto, H.; Takahashi, N.; Ogawa, H. Psathyrella velutina mushroom lectin exhibits high affinity toward sialoglycoproteins possessing terminal N-acetylneuraminic acid alpha 2,3-linked to penultimate galactose residues of trisialyl N-glycans. comparison with other sialic acid-specific lectins. J. Biol. Chem. 2002, 277, 24916–24925. [Google Scholar] [CrossRef] [PubMed]
- Ueda, H.; Saitoh, T.; Kojima, K.; Ogawa, H. Multi-specificity of a Psathyrella velutina mushroom lectin: Heparin/pectin binding occurs at a site different from the N-acetylglucosamine/N-acetylneuraminic acid-specific site. J. Biochem. 1999, 126, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Kochibe, N.; Matta, K.L. Purification and properties of an N-acetylglucosamine-specific lectin from Psathyrella velutina mushroom. J. Biol. Chem. 1989, 264, 173–177. [Google Scholar] [PubMed]
- Hernandez, E.; Ortiz, R.; Lopez, F.; Masso, F.; Montano, L.F.; Martinage, A.; Zenteno, E. Purification and characterization of a galactose-specific lectin from Psilocybe barrerae. Phytochemistry 1993, 32, 1209–1211. [Google Scholar] [CrossRef]
- Zhao, S.; Zhao, Y.; Li, S.; Zhao, J.; Zhang, G.; Wang, H.; Ng, T.B. A novel lectin with highly potent antiproliferative and HIV-1 reverse transcriptase inhibitory activities from the edible wild mushroom Russula delica. Glycoconj. J. 2010, 27, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Sun, J.; Wang, H.; Ng, T.B. First isolation and characterization of a novel lectin with potent antitumor activity from a Russula mushroom. Phytomedicine 2010, 17, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Chumkhunthod, P.; Rodtong, S.; Lambert, S.J.; Fordham-Skelton, A.P.; Rizkallah, P.J.; Wilkinson, M.C.; Reynolds, C.D. Purification and characterization of an N-acetyl-d-galactosamine-specific lectin from the edible mushroom Schizophyllum commune. Biochim. Biophys. Acta 2006, 1760, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Han, C.H.; Liu, Q.H.; Ng, T.B.; Wang, H.X. A novel homodimeric lactose-binding lectin from the edible split gill medicinal mushroom Schizophyllum commune. Biochem. Biophys. Res. Commun. 2005, 336, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Ingram, G.A.; Murray-Rochard, S.; Dougherty, A.S. Agglutinins (lectins) and lysins in extracts of cultured and noncultured fungi. Lectins Biol. Biochem. Clin. Biochem. 1988, 6, 393–400. [Google Scholar]
- Damian, L.; Fournier, D.; Winterhalter, M.; Paquereau, L. Determination of thermodynamic parameters of Xerocomus chrysenteron lectin interactions with N-acetylgalactosamine and Thomsen-Friedenreich antigen by isothermal titration calorimetry. BMC Biochem. 2005, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Trigueros, V.; Lougarre, A.; Ali-Ahmed, D.; Rahbe, Y.; Guillot, J.; Chavant, L.; Fournier, D.; Paquereau, L. Xerocomus chrysenteron lectin: Identification of a new pesticidal protein. Biochim. Biophys. Acta 2003, 1621, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, H.; Ng, T.B. Isolation and characterization of a novel lectin from the wild mushroom Xerocomus spadiceus. Peptides 2004, 25, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, H.; Ng, T.B. First report of a xylose-specific lectin with potent hemagglutinating, antiproliferative and anti-mitogenic activities from a wild ascomycete mushroom. Biochim. Biophys. Acta Gen. Subj. 2006, 1760, 1914–1919. [Google Scholar] [CrossRef]
- Luangsa-ard, J.J.; Hywel-Jones, N.L.; Manoch, L.; Samson, R.A. On the relationships of Paecilomyces sect. Isarioidea species. Mycol. Res. 2005, 109, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Kobert, R. Lehrbuch der Intoxikationen; Fredinand Enke: Stuttgart, Germany, 1893. [Google Scholar]
- Van Eerde, A.; Grahn, E.M.; Winter, H.C.; Goldstein, I.J.; Krengel, U. Atomic-resolution structure of the alpha-galactosyl binding Lyophyllum decastes lectin reveals a new protein family found in both fungi and plants. Glycobiology 2014. [Google Scholar] [CrossRef]
- Wimmerova, M.; Mitchell, E.; Sanchez, J.F.; Gautier, C.; Imberty, A. Crystal structure of fungal lectin: Six-bladed beta-propeller fold and novel fucose recognition mode for Aleuria aurantia lectin. J. Biol. Chem. 2003, 278, 27059–27067. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S.; Nakajima, E.; Nagao, H.; Ohtoshi, M.; Ando, A.; Nagata, Y. Synthesis of a lectin in both mycelia and fruit bodies of the ascomycete mushroom Aleuria aurantia. Biosci. Biotechnol. Biochem. 1998, 62, 915–918. [Google Scholar] [CrossRef]
- Fujihashi, M.; Peapus, D.H.; Kamiya, N.; Nagata, Y.; Miki, K. Crystal Structure of Fucose-Specific Lectin from aleuria aurantia binding ligands at three of its five sugar recognition sites. Biochemistry 2003, 42, 11093–11099. [Google Scholar] [CrossRef] [PubMed]
- Cioci, G.; Mitchell, E.P.; Chazalet, V.; Debray, H.; Oscarson, S.; Lahmann, M.; Gautier, C.; Breton, C.; Perez, S.; Imberty, A. β-Propeller crystal structure of Psathyrella velutina lectin: An integrin-like fungal protein interacting with monosaccharides and calcium. J. Mol. Biol. 2006, 357, 1575–1591. [Google Scholar] [CrossRef] [PubMed]
- Kochibe, N. Manufacture of Novel Lectin with Psathyrella velutina. Japan Patent 88-151136, 1989. [Google Scholar]
- Feng, L.; Sun, H.; Zhang, Y.; Li, D.-F.; Wang, D.-C. Structural insights into the recognition mechanism between an antitumor galectin AAL and the Thomsen-Friedenreich antigen. FASEB J. 2010, 24, 3861–3868. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Li, D.-F.; Feng, L.; Xiang, Y.; Liu, W.; Sun, H.; Wang, D.-C. Structural basis for the tumor cell apoptosis-inducing activity of an antitumor lectin from the edible mushroom Agrocybe aegerita. J. Mol. Biol. 2009, 387, 694–705. [Google Scholar] [CrossRef] [PubMed]
- Ban, M.; Yoon, H.J.; Demirkan, E.; Utsumi, S.; Mikami, B.; Yagi, F. Structural basis of a fungal galectin from Agrocybe cylindracea for recognizing sialoconjugate. J. Mol. Biol. 2005, 351, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, N.; Hu, D.; Tateno, H.; Makyio, H.; Hirabayashi, J.; Kato, R. Conformational change of a unique sequence in a fungal galectin from Agrocybe cylindracea controls glycan ligand-binding specificity. FEBS Lett. 2013, 587, 3620–3625. [Google Scholar] [CrossRef] [PubMed]
- Walser, P.J.; Haebel, P.W.; Kunzler, M.; Sargent, D.; Kues, U.; Aebi, M.; Ban, N. Structure and functional analysis of the fungal galectin CGL2. Structure 2004, 12, 689–702. [Google Scholar] [CrossRef] [PubMed]
- Butschi, A.; Titz, A.; Walti, M.A.; Olieric, V.; Paschinger, K.; Nobauer, K.; Guo, X.; Seeberger, P.H.; Wilson, I.B.; Aebi, M.; et al. Caenorhabditis elegans N-glycan core beta-galactoside confers sensitivity towards nematotoxic fungal galectin CGL2. PLoS Pathog. 2010, 6, e1000717. [Google Scholar] [CrossRef] [PubMed]
- Bovi, M.; Carrizo, M.E.; Capaldi, S.; Perduca, M.; Chiarelli, L.R.; Galliano, M.; Monaco, H.L. Structure of a lectin with antitumoral properties in king bolete (Boletus edulis) mushrooms. Glycobiology 2011, 21, 1000–1009. [Google Scholar] [CrossRef] [PubMed]
- Grahn, E.; Askarieh, G.; Holmner, A.; Tateno, H.; Winter, H.C.; Goldstein, I.J.; Krengel, U. Crystal structure of the Marasmius oreades mushroom lectin in complex with a xenotransplantation epitope. J. Mol. Biol. 2007, 369, 710–721. [Google Scholar] [CrossRef] [PubMed]
- Grahn, E.M.; Winter, H.C.; Tateno, H.; Goldstein, I.J.; Krengel, U. Structural characterization of a lectin from the mushroom Marasmius oreades in complex with the blood group B trisaccharide and calcium. J. Mol. Biol. 2009, 390, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Kadirvelraj, R.; Grant, O.C.; Goldstein, I.J.; Winter, H.C.; Tateno, H.; Fadda, E.; Woods, R.J. Structure and binding analysis of Polyporus squamosus lectin in complex with the Neu5Ac{alpha}2-6Gal{beta}1-4GlcNAc human-type influenza receptor. Glycobiology 2011, 21, 973–984. [Google Scholar] [CrossRef] [PubMed]
- Birck, C.; Damian, L.; Marty-Detraves, C.; Lougarre, A.; Schulze-Briese, C.; Koehl, P.; Fournier, D.; Paquereau, L.; Samama, J.P. A new lectin family with structure similarity to actinoporins revealed by the crystal structure of Xerocomus chrysenteron lectin XCL. J. Mol. Biol. 2004, 344, 1409–1420. [Google Scholar] [CrossRef] [PubMed]
- Koyama, Y.; Katsuno, Y.; Miyoshi, N.; Hayakawa, S.; Mita, T.; Muto, H.; Isemura, S.; Aoyagi, Y.; Isemura, M. Apoptosis induction by lectin isolated from the mushroom boletopsis leucomelas in U937 cells. Biosci. Biotechnol. Biochem. 2002, 66, 784–789. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.H.; Wang, H.; Ng, T.B. A haemagglutinin from the medicinal fungus Cordyceps militaris. Biosci. Rep. 2009, 29, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Kawagishi, H.; Nomura, A.; Mizuno, T.; Kimura, A.; Chiba, S. Isolation and characterization of a lectin from Grifola frondosa fruiting bodies. Biochim. Biophys. Acta 1990, 1034, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.X.; Zhang, G.Q.; Zhao, S.; Xu, F.; Zhou, Y.; Geng, X.L.; Liu, Y.; Wang, H.X. Purification and characterization of a novel lectin with antiphytovirus activities from the wild mushroom Paxillus involutus. Protein Pept. Lett. 2012, 20, 767–774. [Google Scholar] [CrossRef]
- Rouf, R.; Stephens, A.S.; Spaan, L.; Arndt, N.X.; Day, C.J.; May, T.W.; Tiralongo, E.; Tiralongo, J. G(2)/M cell cycle arrest by an N-acetyl-d-glucosamine specific lectin from Psathyrella asperospora. Glycoconj. J. 2014, 31, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.X.; Liu, W.K.; Ng, T.B.; Ooi, V.E.C.; Chang, S.T. The immunomodulatory and antitumor activities of lectins from the mushroom Tricholoma mongolicum. Immunopharmacology 1996, 31, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.Y.; Chou, T.B. Isolation and characterization of a lectin from edible mushroom, Volvariella volvacea. J. Biochem. 1984, 96, 35–40. [Google Scholar] [PubMed]
- Marty-Detraves, C.; Francis, F.; Baricault, L.; Fournier, D.; Paquereau, L. Inhibitory action of a new lectin from Xerocomus chrysenteron on cell-substrate adhesion. Mol. Cell. Biochem. 2004, 258, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, M. Purification and characterization of a lectin from the mushroom, Flammulina veltipes. J. Biochem. 1979, 86, 1463–1468. [Google Scholar] [PubMed]
- Wang, H.X.; Ng, T.B. Examination of lectins, polysaccharopeptide, polysaccharide, alkaloid, coumarin and trypsin inhibitors for inhibitory activity against human immunodeficiency virus reverse transcriptase and glycohydrolases. Planta Med. 2001, 67, 669–672. [Google Scholar] [CrossRef] [PubMed]
- Entlicher, G.; Jesenska, K.; Jarsova-Dejlova, J.; Jarnik, M.; Kocourek, J. Studies on lectins LXIII. Isolation and characterization of a lectin from stinkhorn mushroom Phallus impudicus. In Lectins, Biology, Biochemistry, Clinical Biochemstry; Hansen, T.C.B., Breborowicz, J., Eds.; Walter de Gruyter: Berlin, Germany, 1985; Volume 4, pp. 491–503. [Google Scholar]
- Kobayashi, Y.; Kawagishi, H. Fungal lectins: A growing family. Methods Mol. Biol. 2014, 1200, 15–38. [Google Scholar] [PubMed]
- Houser, J.; Komarek, J.; Kostlanova, N.; Cioci, G.; Varrot, A.; Kerr, S.C.; Lahmann, M.; Balloy, V.; Fahy, J.V.; Chignard, M.; et al. A soluble fucose-specific lectin from Aspergillus fumigatus conidia—Structure, specificity and possible role in fungal pathogenicity. PLoS ONE 2013, 8, e83077. [Google Scholar] [CrossRef] [PubMed]
- Kostlanova, N.; Mitchell, E.P.; Lortat-Jacob, H.; Oscarson, S.; Lahmann, M.; Gilboa-Garber, N.; Chambat, G.; Wimmerova, M.; Imberty, A. The fucose-binding lectin from Ralstonia solanacearum. A new type of beta-propeller architecture formed by oligomerization and interacting with fucoside, fucosyllactose, and plant xyloglucan. J. Biol. Chem. 2005, 280, 27839–27849. [Google Scholar] [CrossRef] [PubMed]
- Rutenber, E.; Ready, M.; Robertus, J.D. Structure and evolution of ricin B chain. Nature 1987, 326, 624–626. [Google Scholar] [CrossRef] [PubMed]
- Hazes, B. The (QxW)3 domain: A flexible lectin scaffold. Protein Sci. 1996, 5, 1490–1501. [Google Scholar] [CrossRef] [PubMed]
- Wohlschlager, T.; Butschi, A.; Zurfluh, K.; Vonesch, S.C.; auf dem Keller, U.; Gehrig, P.; Bleuler-Martinez, S.; Hengartner, M.O.; Aebi, M.; Künzler, M. Nematotoxicity of Marasmius oreades Agglutinin (MOA) depends on glycolipid binding and cysteine protease activity. J. Biol. Chem. 2011, 286, 30337–30343. [Google Scholar] [CrossRef] [PubMed]
- Olsnes, S. The history of ricin, abrin and related toxins. Toxicon 2004, 44, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Eckhardt, A.E.; Goldstein, I.J. Occurrence of alpha-d-galactosyl-containing glycoproteins on Ehrlich tumor cell membranes. Biochemistry 1983, 22, 5280–5289. [Google Scholar] [CrossRef] [PubMed]
- Sharon, N.; Lis, H. Lectins: Cell-agglutinating and sugar-specific proteins. Science 1972, 177, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.E. T-cell activation through the antigen receptor. Part 1: Signaling components, signaling pathways, and signal integration at the T-cell antigen receptor synapse. J. Allergy Clin. Immunol. 2002, 109, 758–770. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.C.K.; Sze, S.C.W.; Shen, W.Z.; Liu, W.K. Mitogenic activity of edible mushroom lectins. Biochim. Biophys. Acta Gen. Subj. 2004, 1671, 9–17. [Google Scholar] [CrossRef]
- Greene, W.; Fleisher, T.; Waldmann, T. Suppression of human T and B lymphocyte activation by Agaricus bisporus lectin. I. Suggestive evidence for a surface “suppressor” receptor in human lymphocytes. J. Immunol. 1981, 126, 580–586. [Google Scholar] [PubMed]
- Chang, H.-H.; Chien, P.-J.; Tong, M.-H.; Sheu, F. Mushroom immunomodulatory proteins possess potential thermal/freezing resistance, acid/alkali tolerance and dehydration stability. Food Chem. 2007, 105, 597–605. [Google Scholar] [CrossRef]
- Ngai, P.H.; Wang, H.X.; Ng, T.B. Purification and characterization of a ubiquitin-like peptide with macrophage stimulating, antiproliferative and ribonuclease activities from the mushroom Agrocybe cylindracea. Peptides 2003, 24, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Sze, S.C.; Ho, J.C.; Liu, W.K. Volvariella volvacea lectin activates mouse T lymphocytes by a calcium dependent pathway. J. Cell. Biochem. 2004, 92, 1193–1202. [Google Scholar] [CrossRef] [PubMed]
- Bottcher, M.; Grosse, F. HIV-1 protease inhibits its homologous reverse transcriptase by protein-protein interaction. Nucleic Acids Res. 1997, 25, 1709–1714. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.H.; Ng, T.B. Vulgarinin, a broad-spectrum antifungal peptide from haricot beans (Phaseolus vulgaris). Int. J. Biochem. Cell Biol. 2005, 37, 1626–1632. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ng, T.B. Luffangulin, a novel ribosome inactivating peptide from ridge gourd (Luffa acutangula) seeds. Life Sci. 2002, 70, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, L.G.; Gronenborn, A.M. The highly specific carbohydrate-binding protein cyanovirin-N: Structure, anti-HIV/Ebola activity and possibilities for therapy. Mini Rev. Med. Chem. 2005, 5, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, P.; Simet, I.; Basu, S. Inhibition of human neuroblastoma DNA polymerase activities by plant lectins and toxins. Proc. Natl. Acad. Sci. USA 1979, 76, 2218–2221. [Google Scholar] [CrossRef] [PubMed]
- Koharudin, L.M.; Viscomi, A.R.; Jee, J.G.; Ottonello, S.; Gronenborn, A.M. The evolutionarily conserved family of cyanovirin-N homologs: Structures and carbohydrate specificity. Structure 2008, 16, 570–584. [Google Scholar] [CrossRef] [PubMed]
- Akkouh, O.; Ng, T.B.; Singh, S.S.; Yin, C.; Dan, X.; Chan, Y.S.; Pan, W.; Cheung, R.C. Lectins with Anti-HIV activity: A review. Molecules 2015, 20, 648–668. [Google Scholar] [CrossRef] [PubMed]
- Boyd, M.R.; Gustafson, K.R.; McMahon, J.B.; Shoemaker, R.H.; O’Keefe, B.R.; Mori, T.; Gulakowski, R.J.; Wu, L.; Rivera, M.I.; Laurencot, C.M.; et al. Discovery of cyanovirin-N, a novel human immunodeficiency virus-inactivating protein that binds viral surface envelope glycoprotein gp120: Potential applications to microbicide development. Antimicrob. Agents Chemother. 1997, 41, 1521–1530. [Google Scholar] [PubMed]
- Zappe, H.; Snell, M.E.; Bossard, M.J. PEGylation of cyanovirin-N, an entry inhibitor of HIV. Adv. Drug Deliv. Rev. 2008, 60, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Gueugnot, J.; Guillot, J.; Damez, M.; Coulet, M. Identification and taxonomy of human and animal leishmanias by lectin-mediated agglutination. Acta Trop. 1984, 41, 135–143. [Google Scholar] [PubMed]
- Aksoy, Ü.; Ahmet, Ü. Lectins and their application to parasitology. Turk. J. Infect. 2003, 17, 513–516. [Google Scholar]
- Gao, W.; Sun, Y.; Chen, S.; Zhang, J.; Kang, J.; Wang, Y.; Wang, H.; Xia, G.; Liu, Q.; Kang, Y. Mushroom lectin enhanced immunogenicity of HBV DNA vaccine in C57BL/6 and HBsAg-transgenic mice. Vaccine 2013, 31, 2273–2280. [Google Scholar] [CrossRef] [PubMed]
- Yazawa, S.; Furukawa, K.; Kochibe, N. Isolation of fucosyl glycoproteins from human erythrocyte membranes by affinity chromatography using Aleuria aurantia lectin. J. Biochem. 1984, 96, 1737–1742. [Google Scholar] [PubMed]
- Ohlson, C.; Karlsson, J.O. Glycoproteins of axonal transport: Polypeptides interacting with the lectin from Aleuria aurantia. Brain Res. 1983, 264, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, K.; Kochibe, N.; Ohkura, T.; Ueda, I.; Kobata, A. Fractionation of l-fucose-containing oligosaccharides on immobilized Aleuria aurantia lectin. J. Biol. Chem. 1985, 260, 4688–4693. [Google Scholar] [PubMed]
- Yazawa, S.; Kochibe, N.; Asao, T. A simple procedure for isolation of tumor-associated antigens by affinity chromatography using fucose-specific Aleuria aurantia lectin. Immunol. Investig. 1990, 19, 319–327. [Google Scholar] [CrossRef]
- Harada, H.; Kamei, M.; Tokumoto, Y.; Yui, S.; Koyama, F.; Kochibe, N.; Endo, T.; Kobata, A. Systematic fractionation of oligosaccharides of human immunoglobulin G by serial affinity chromatography on immobilized lectin columns. Anal. Biochem. 1987, 164, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.-C.; Li, Y.; Zhou, J.; Qian, J.; Schnaar, R.L.; Zhang, Y.; Goldstein, I.J.; Zhu, H.; Schneck, J.P. Lectin microarrays identify cell-specific and functionally significant cell surface glycan markers. Glycobiology 2008, 18, 761–769. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, M.A.A.; Rouf, R.; Tiralongo, E.; May, T.W.; Tiralongo, J. Mushroom Lectins: Specificity, Structure and Bioactivity Relevant to Human Disease. Int. J. Mol. Sci. 2015, 16, 7802-7838. https://doi.org/10.3390/ijms16047802
Hassan MAA, Rouf R, Tiralongo E, May TW, Tiralongo J. Mushroom Lectins: Specificity, Structure and Bioactivity Relevant to Human Disease. International Journal of Molecular Sciences. 2015; 16(4):7802-7838. https://doi.org/10.3390/ijms16047802
Chicago/Turabian StyleHassan, Mohamed Ali Abol, Razina Rouf, Evelin Tiralongo, Tom W. May, and Joe Tiralongo. 2015. "Mushroom Lectins: Specificity, Structure and Bioactivity Relevant to Human Disease" International Journal of Molecular Sciences 16, no. 4: 7802-7838. https://doi.org/10.3390/ijms16047802
APA StyleHassan, M. A. A., Rouf, R., Tiralongo, E., May, T. W., & Tiralongo, J. (2015). Mushroom Lectins: Specificity, Structure and Bioactivity Relevant to Human Disease. International Journal of Molecular Sciences, 16(4), 7802-7838. https://doi.org/10.3390/ijms16047802





