Angiotensin-I Converting Enzyme (ACE) Inhibitory and Anti-Oxidant Activities of Sea Cucumber (Actinopyga lecanora) Hydrolysates
Abstract
:1. Introduction
2. Results
2.1. Peptide Content
2.2. Amino Acid Composition
2.3. Effect of Enzymatic Hydrolysis on the Bioactivities of A. lecanora Hydrolysates
| Hydrolysis Time (h) | Peptide Content of Hydrolysates (mg Glutathione Equivalent/mL) | |||||
|---|---|---|---|---|---|---|
| Papain | Alcalase | Bromelain | Flavourzyme | Pepsin | Trypsin | |
| 0 | ND j | ND j | ND i | ND f | ND i | ND g |
| 1 | 1.93 ± 0.10 Ai | 1.55 ± 0.02 Bi | 1.78 ± 0.02 ABh | 1.10 ± 0.02 Ce | 1.10 ± 0.02 Ch | 1.01 ± 0.06 Ce |
| 2 | 2.20 ± 0.06 Ah | 1.95 ± 0.03 Ah | 1.90 ± 0.05 Agh | 1.14 ± 0.08 Be | 1.11 ± 0.02 Bh | 1.05 ± 0.04 Be |
| 3 | 2.43 ± 0.07 Ag | 2.15 ± 0.10 Bg | 2.06 ± 0.07 Bfg | 1.17 ± 0.02 Cde | 1.14 ± 0.03 Cgh | 1.03 ± 0.03 Ce |
| 4 | 2.80 ± 0.02 Af | 2.50 ± 0.05 Bf | 2.17 ± 0.08 Cef | 1.20 ± 0.06 Dde | 1.23 ± 0.04 Dfg | 1.08 ± 0.04 De |
| 5 | 2.79 ± 0.04 Af | 2.63 ± 0.09 Af | 2.25 ± 0.07 Bef | 1.27 ± 0.06 Cd | 1.30 ± 0.07 Cef | 1.00 ± 0.00 Dde |
| 6 | 3.01 ± 0.06 Ae | 2.98 ± 0.06 Ae | 2.36 ± 0.05 Be | 1.50 ± 0.03 Cc | 1.40 ± 0.04 Cde | 1.07 ± 0.03 Dde |
| 7 | 3.51 ± 0.03 Ad | 3.33 ± 0.04 Bd | 2.76 ± 0.06 Cd | 1.61 ± 0.04 Db | 1.50 ± 0.03 Dcd | 1.21 ± 0.02 Ecd |
| 8 | 3.91 ± 0.03 Ac | 3.70 ± 0.03 Bc | 3.09 ± 0.07 Cc | 1.70 ± 0.07 Db | 1.54 ± 0.04 Dbc | 1.30 ± 0.06 Ebc |
| 9 | 4.10 ± 0.05 Ab | 3.88 ± 0.05 Bb | 3.41 ± 0.08 Cb | 2.05 ± 0.07 Da | 1.62 ± 0.03 Eab | 1.35 ± 0.02 Fbc |
| 10 | 4.40 ± 0.06 Aa | 3.90 ± 0.04 Bb | 3.66 ± 0.09 Ca | 2.10 ± 0.07 Da | 1.67 ± 0.02 Ea | 1.41 ± 0.06 Fab |
| 24 | 4.47 ± 0.03 Aa | 4.40 ± 0.07 Aa | 3.80 ± 0.08 Ba | 2.13 ± 0.05 Ca | 1.69 ± 0.05 Da | 1.50 ± 0.03 Ea |
2.3.1. ACE Inhibitory Activity
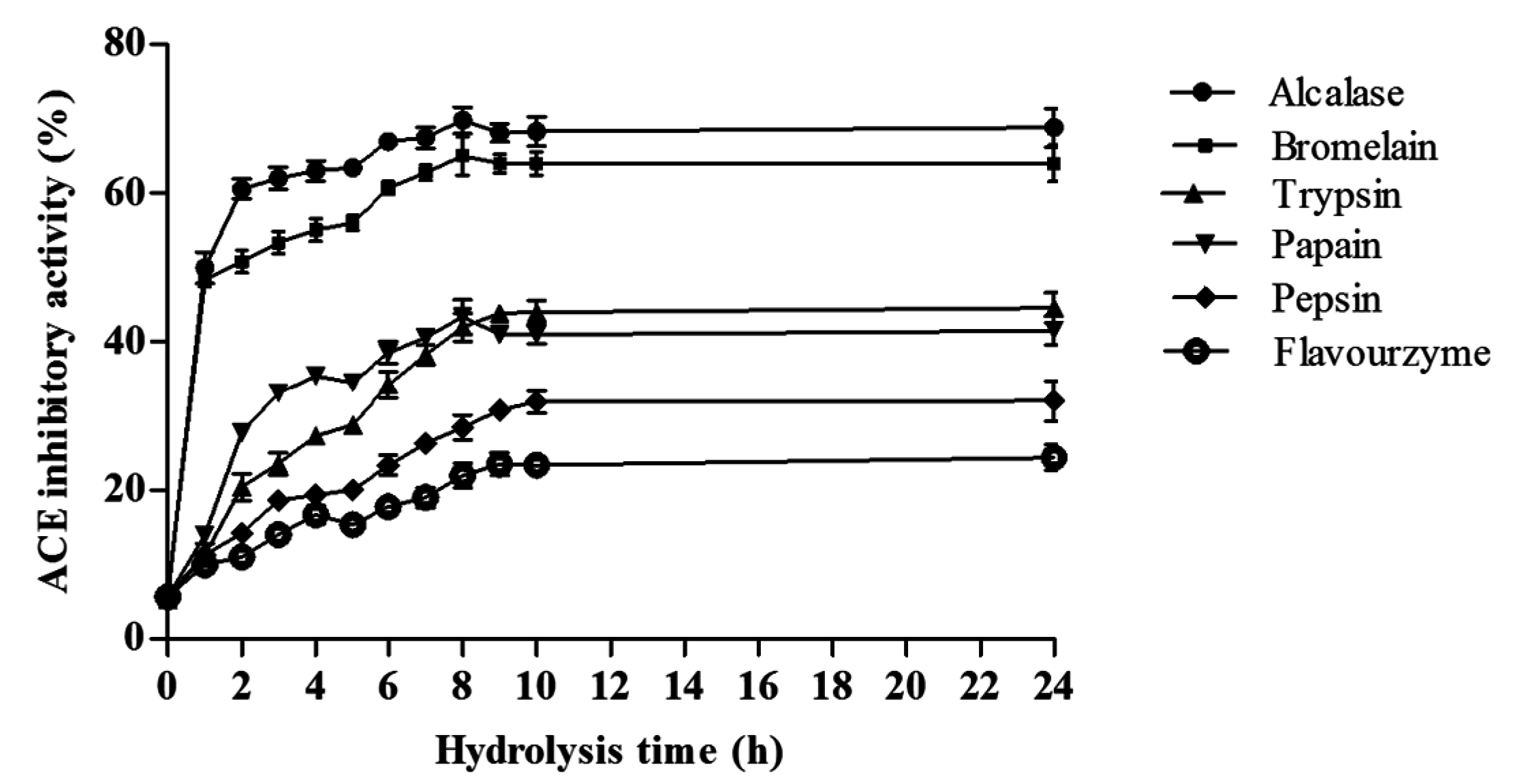

| Amino Acid | A. lecanora | Papain | Alcalase | Bromelain | Flavourzyme | Trypsin | Pepsin |
|---|---|---|---|---|---|---|---|
| Aspartic acid (D) | 78.83 ± 1.56 a | 50.33 ± 0.20 b | 55.96 ± 0.22 b | 53.16 ± 5.60 b | 36.07 ± 3.50 c | 17.27 ± 1.25 d | 49.21 ± 1.60 b |
| Glutamic acid (E) | 106.83 ± 3.90 a | 86.86 ± 1.10 b | 95.63 ± 1.25 b | 87.79 ± 6.74 b | 63.68 ± 4.68 c | 29.90 ± 2.12 d | 89.62 ± 1.21 b |
| Serine (S) | 34.11 ± 0.40 a | 23.03 ± 2.40 c | 27.80 ± 0.71 b | 28.35 ± 0.03 b | 18.85 ± 0.41 d | 7.99 ± 0.60 e | 20.47 ± 0.17 c,d |
| Histidine (H) | 10.93 ± 0.76 a | 4.30 ± 0.02 b,c | 5.88 ± 0.30 b | 4.83 ± 0.83 b,c | 4.55 ± 0.12 b,c | 1.60 ± 0.11 d | 3.58 ± 1.23 c |
| Arginine (R) | 65.98 ± 2.30 a | 45.62 ± 1.83 d | 56.62 ± 0.50 b,c | 58.64 ± 1.12 b | 32.73 ± 1.80 e | 16.55 ± 1.22 f | 54.40 ± 1.06 c |
| Thereonine (T) | 44.13 ± 1.43 a | 29.75 ± 2.60 c | 31.44 ± 0.80 b,c | 35.26 ± 1.18 b | 25.16 ± 1.58 d | 9.77 ± 0.30 e | 29.96 ± 0.72 c |
| Lysine (K) | 45.47 ± 2.10 a | 23.43 ± 4.10 b | 24.85 ± 1.00 b | 20.56 ± 1.26 b,c | 17.25 ± 2.83 c,d | 5.42 ± 0.35 e | 12.73 ± 0.23 d |
| Tyrosine (Y) | 44.14 ± 1.43 a | 29.65 ± 2.60 c | 31.44 ± 0.30 b,c | 35.26 ± 1.60 b | 25.16 ± 1.60 d | 9.77 ± 0.87 e | 29.96 ± 0.72 c |
| Valine (V) | 41.97 ± 1.41 a | 22.72 ± 0.34 d | 33.05 ± 0.60 b | 27.73 ± 0.54 c | 18.65 ± 1.03 e | 10.40 ± 0.17 f | 24.00 ± 0.32 d |
| Methionine (M) | 15.57 ± 0.60 a | 3.27 ± 0.10 c,d | 5.04 ± 0.045 b | 3.29 ± 0.90 c | 3.16 ± 0.30 c,d | 1.31 ± 0.01 e | 2.20 ± 0.18 d,e |
| Cystine (C) | 2.45 ± 0.10 a | 1.17 ± 0.01 c | 1.52 ± 0.25 b | 0.00 ± 0.00 e | 1.40 ± 0.13 b | 0.33 ± 0.06 d | 0.43 ± 0.01 d |
| Isoleucine (I) | 52.36 ± 3.42 a | 10.90 ± 0.20 b | 13.54 ± 0.30 b | 11.54 ± 1.10 b | 10.29 ± 0.12 b | 4.25 ± 0.41 c | 9.73 ± 0.15 b |
| Leucine (L) | 41.97 ± 2.20 b | 32.41 ± 0.31 d | 36.34 ± 2.10 c,d | 38.13 ± 0.81 b,c | 26.16 ± 1.72 e | 12.40 ± 2.01f | 48.22 ± 2.20 a |
| Phenylalanine (F) | 28.68 ± 1.37 a | 10.46 ± 0.30 d | 17.42 ± 0.041 b | 13.70 ± 1.45 c | 10.00 ± 1.28 d | 4.73 ± 0.05 e | 9.03 ± 0.40 d |
| Glycine (G) | 140.63 ± 1.33 a | 107.00 ± 2.02 c | 120.48 ± 4.10 b | 110.75 ± 1.50 c | 53.47 ± 2.40 d | 38.83 ± 3.53 e | 125.58 ± 3.52 b |
| Alanine (A) | 65.09 ± 1.50 a | 50.98 ± 0.87 c | 57.27 ± 1.71 b | 53.25 ± 0.80 c | 27.6 ± 1.52 d | 18.09 ± 0.70 e | 58.99 ± 1.60 b |
| Proline (P) | 59.77 ± 1.47 a | 48.86 ± 0.90 c | 54.64 ± 0.75 b | 50.36 ± 2.10 c | 27.92 ± 0.46 d | 16.91 ± 1.38 e | 57.60 ± 1.41 a,b |
| Total amino acid | 878.91 ± 30.90 a | 579.93 ± 12.40 c | 668.9 2± 10.45 b | 629.60 ± 12.66 b | 402.10 ± 23.24 d | 205.52 ± 15.92 e | 625.71 ± 6.51 b,c |
| Hydrophobic AA | 388.72 | 238.91 | 285.66 | 258.39 | 150.73 | 90.25 | 278.18 |
| Hydrophilic AA | 274.32 | 194.17 | 216.7 | 198.39 | 148.04 | 66.53 | 193.15 |
| Positively charged AA | 120.47 | 69.05 | 81.47 | 79.2 | 49.98 | 21.97 | 67.13 |
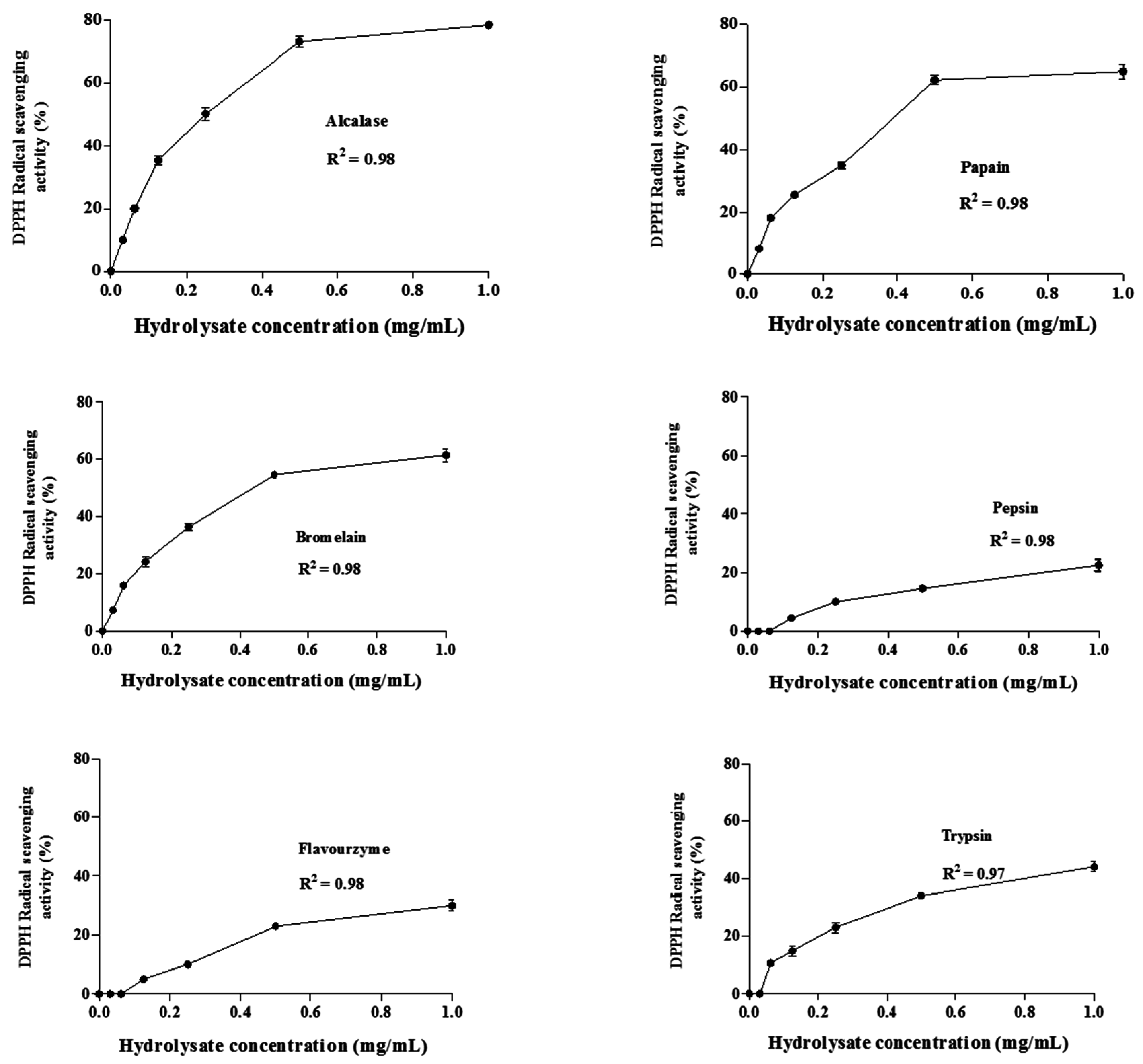
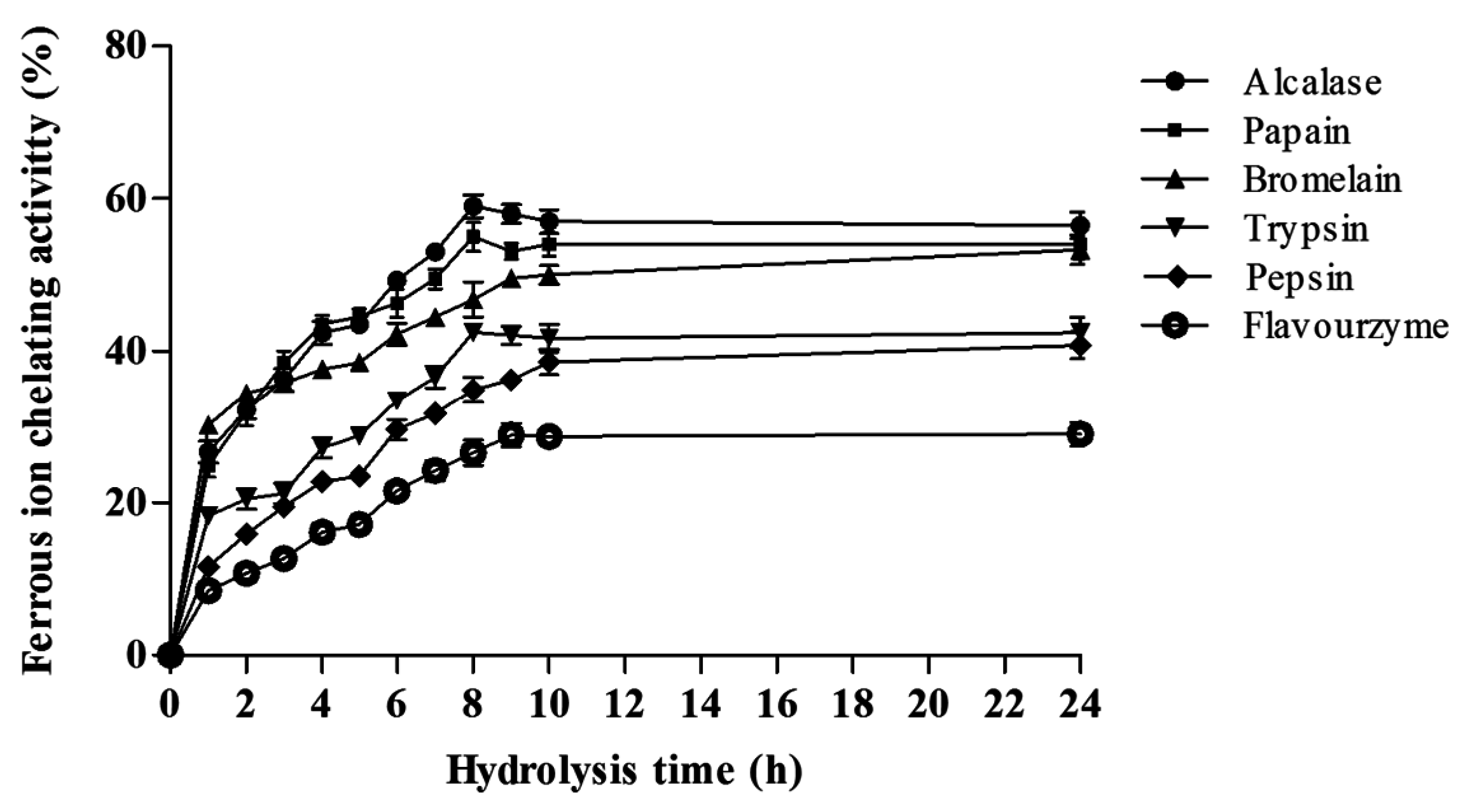
2.3.2. Anti-Oxidative Activities
DPPH Radical Scavenging Activity
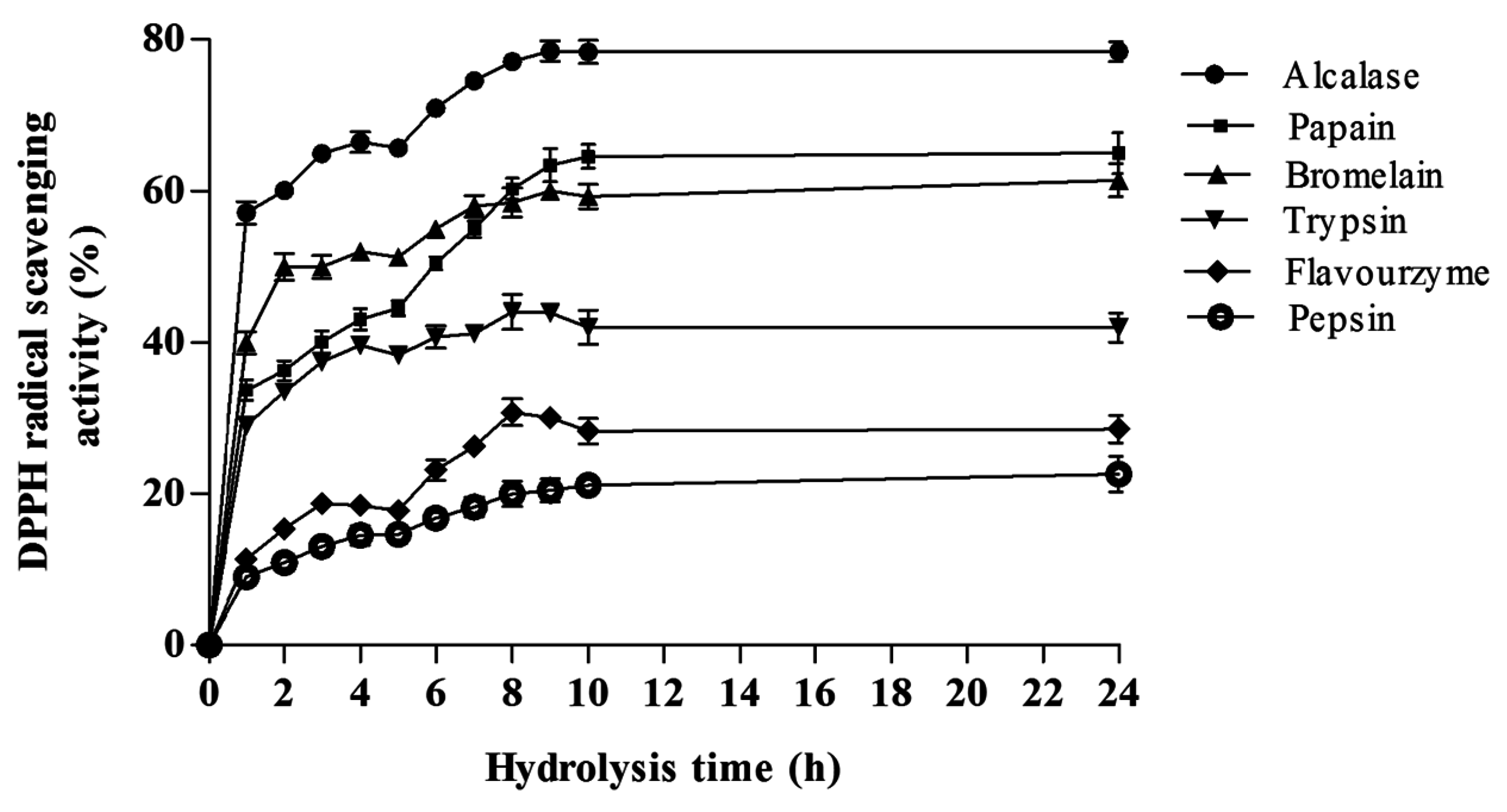
Ferrous Ion Chelating Activity (FIC)
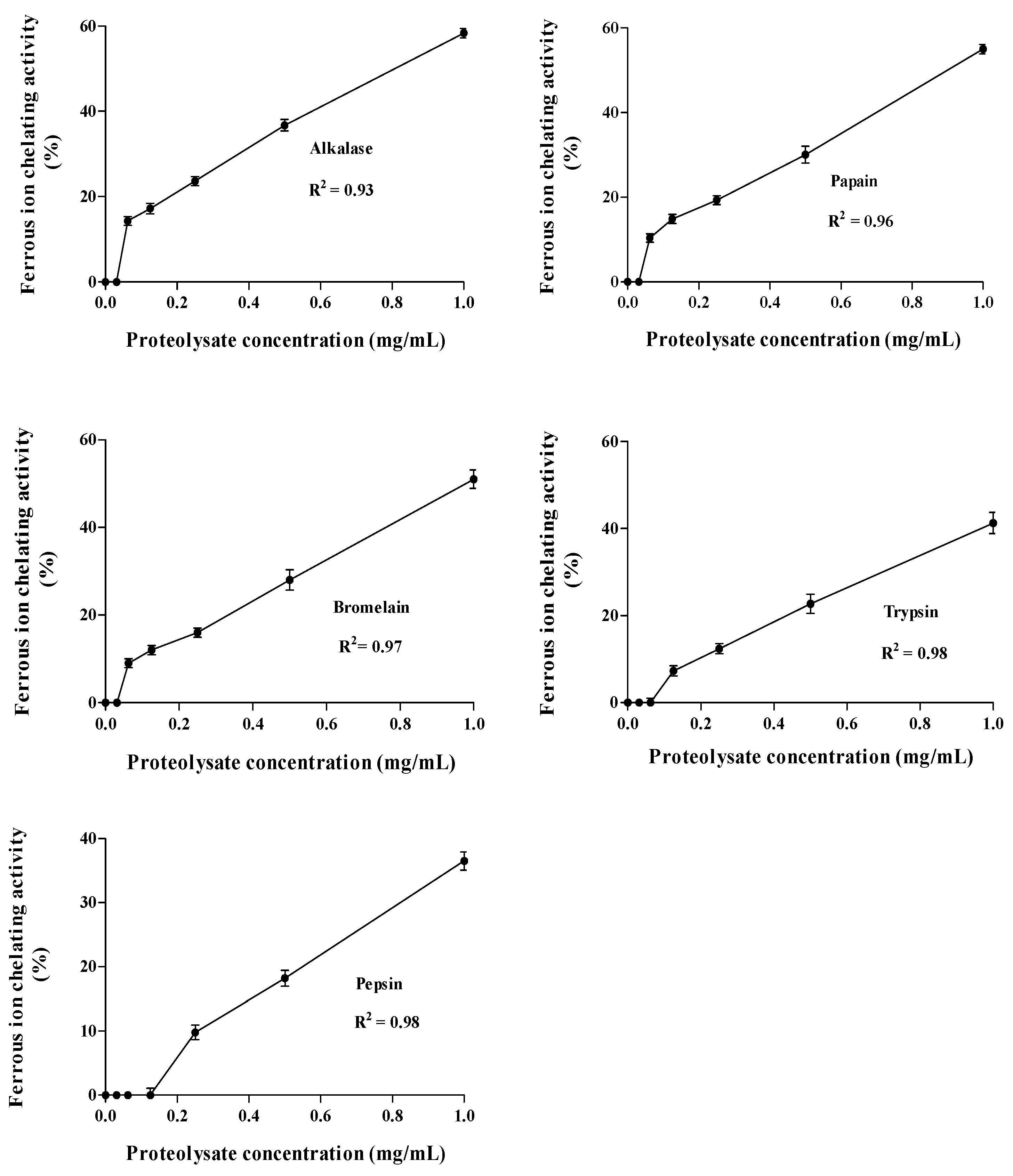
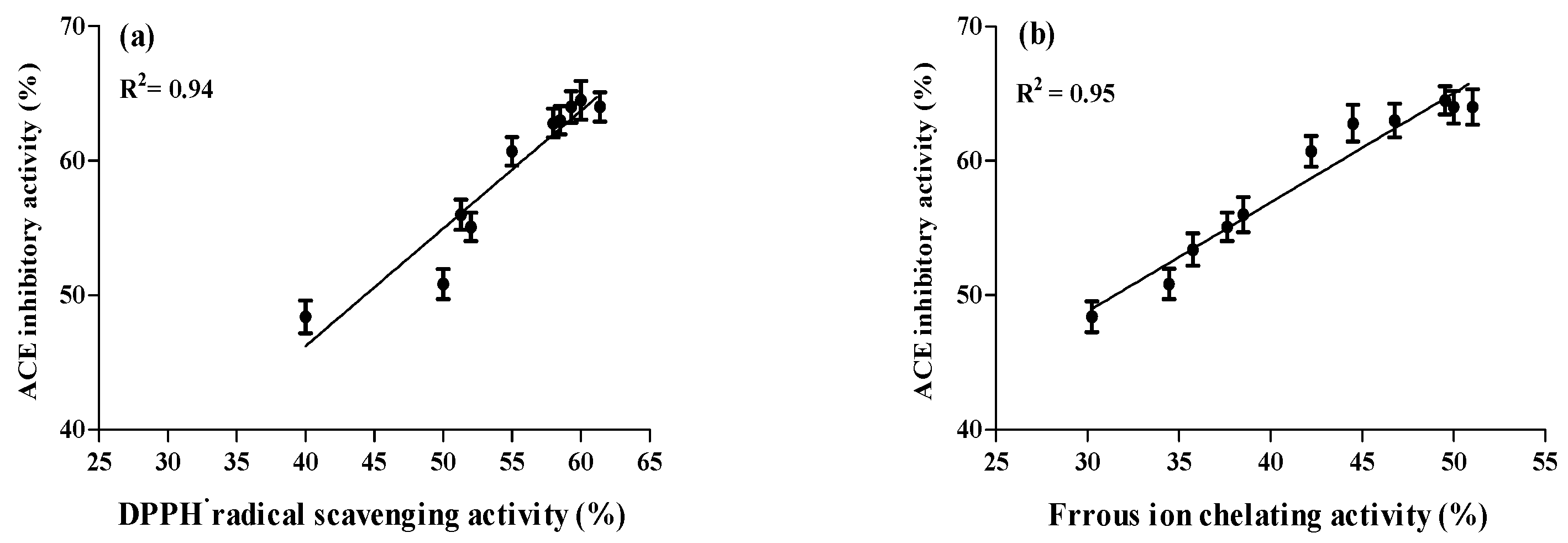
2.4. Correlation between ACE Inhibitory and Anti-Oxidative Activities of A. lecanora Alcalase-Generated Hydrolysates over 24 h of Hydrolysis
3. Experimental Section
3.1. Raw Material
3.2. Chemicals
3.3. Preparation of Enzymatic Hydrolysates from A. lecanora
3.4. Peptide Content Measurement
3.5. Amino Acid Composition
3.6. ACE Inhibitory Activity
3.7. DPPH Free Radical Scavenging Assay
3.8. Ferrous Ion-Chelating Activity
3.9. IC50 Determination of the Hydrolysates
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kuo, P.-L.; Pu, C. The contribution of depression to mortality among elderly with self-reported hypertension: Analysis using a national representative longitudinal survey. J. Hypertens. 2011, 29, 2084–2090. [Google Scholar] [CrossRef] [PubMed]
- Murray, B.A.; FitzGerald, R.J. Angiotensin converting enzyme inhibitory peptides derived from food proteins: Biochemistry, bioactivity and production. Curr. Pharm. Des. 2007, 13, 773–791. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-K.; Wijesekara, I. Development and biological activities of marine-derived bioactive peptides: A review. J. Funct. Foods 2010, 2, 1–9. [Google Scholar] [CrossRef]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008, 4, 89. [Google Scholar] [PubMed]
- Tain, Y.-L.; Baylis, C. Dissecting the causes of oxidative stress in an in vivo model of hypertension. Hypertension 2006, 48, 828–829. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.H.H.; Wu, K.L.H.; Chang, A.Y.W.; Tai, M.-H.; Chan, J.Y.H. Oxidative impairment of mitochondrial electron transport chain complexes in rostral ventrolateral medulla contributes to neurogenic hypertension. Hypertension 2009, 53, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Makinen, S.; Johannson, T.; VegarudGerd, E.; Pihlava, J.M.; Pihlanto, A. Angiotensin I-converting enzyme inhibitory and antioxidant properties of rapeseed hydrolysates. J. Funct. Foods 2012, 4, 575–583. [Google Scholar] [CrossRef]
- Schiffrin, E.L.; Touyz, R.M. From bedside to bench to bedside: role of renin-angiotensin-aldosterone system in remodeling of resistance arteries in hypertension. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Meisel, H. Biochemical properties of regulatory peptides derived from mil proteins. Pept. Sci. 1997, 43, 119–128. [Google Scholar] [CrossRef]
- Yea, C.S.; Ebrahimpour, A.; Hamid, A.A.; Bakar, J.; Muhammad, K.; Saari, N. Winged bean [Psophorcarpus tetragonolobus (L.) DC] seeds as an underutilised plant source of bifunctional proteolysate and biopeptides. Food Funct. 2014, 5, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Sheih, I.C.; Fang, T.J.; Wu, T.-K. Isolation and characterisation of a novel angiotensin I-converting enzyme (ACE) inhibitory peptide from the algae protein waste. Food Chem. 2009, 115, 279–284. [Google Scholar] [CrossRef]
- Pihlanto, A.; Akkanen, S.; Korhonen, H.J. ACE-inhibitory and antioxidant properties of potato (Solanum tuberosum). Food Chem. 2008, 109, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari, R.; Ebrahimpour, A.; Abdul-Hamid, A.; Ismail, A.; Saari, N. Actinopyga lecanora hydrolysates as natural antibacterial agents. Int. J. Mol. Sci. 2012, 13, 16796–16811. [Google Scholar] [CrossRef] [PubMed]
- Sowmya, R.; Rathinaraj, K.; Sachindra, N.M. An autolytic process for recovery of antioxidantactivity rich carotenoprotein from shrimp heads. Mar. Biotechnol. 2011, 13, 918–927. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Zhou, Y.; Lu, J.; Chen, A.; Li, Y.; Zheng, G. Enzymatic hydrolysis of Alaska pollack (Theragra chalcogramma) skin and antioxidant activity of the resulting hydrolysate. J. Sci. Food Agric. 2010, 90, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Bougatef, A.; Nedjar-Arroume, N.; Manni, L.; Ravallec, R.; Barkia, A.; Guillochon, D.; Nasri, M. Purification and identification of novel antioxidant peptides from enzymatic hydrolysates of sardinelle (Sardinella aurita) by-products proteins. Food Chem. 2010, 118, 559–565. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, C.; Jiang, A. Antioxidant peptides isolated from sea cucumber Stichopus Japonicus. Eur. Food Res. Technol. 2012, 234, 441–447. [Google Scholar] [CrossRef]
- Perez-Vega, J.A.; Olivera-Castillo, L.; Gomez-Ruiz, J.A.; Hernandez-Ledesma, B. Release of multifunctional peptides by gastrointestinal digestion of sea cucumber (Isostichopus badionotus). J. Funct. Foods 2013, 5, 869–877. [Google Scholar] [CrossRef]
- Duan, X.; Zhang, M.; Mujumdar, A.S.; Wang, S. Microwave freeze drying of sea cucumber (Stichopu sjaponicus). J. Food Eng. 2010, 96, 491–497. [Google Scholar] [CrossRef]
- Dong, S.; Zeng, M.; Wang, D.; Liu, Z.; Zhao, Y.; Yang, H. Antioxidant and biochemical properties of protein hydrolysates prepared from Silver carp (Hypophthalmichthys molitrix). Food Chem. 2008, 107, 1485–1493. [Google Scholar] [CrossRef]
- Je, J.-Y.; Lee, K.-H.; Lee, M.H.; Ahn, C.-B. Antioxidant and antihypertensive protein hydrolysates produced from tuna liver by enzymatic hydrolysis. Food Res. Int. 2009, 42, 1266–1272. [Google Scholar] [CrossRef]
- Nasri, R.; Chataigné, G.; Bougatef, A.; Chaabouni, M.K.; Dhulster, P.; Nasri, M.; Nedjar-Arroume, N. Novel angiotensin I-converting enzyme inhibitory peptides from enzymatic hydrolysates of goby (Zosterisessor ophiocephalus) muscle proteins. J. Proteom. 2013, 8, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Balti, R.; Bougatef, A.; El-Hadj Ali, N.; Zekri, D.; Barkia, A.; Nasri, M. Influence of degree of hydrolysis on functional properties and angiotensin I-converting enzyme-inhibitory activity of protein hydrolysates from cuttlefish (Sepia officinalis) by-products. J. Sci. Food Agric. 2010, 90, 2006–2014. [Google Scholar] [CrossRef] [PubMed]
- Amado, I.R.; Vazquez, J.A.; Gonzalez, P.; Esteban-Fernandez, D.; Carrera, M.; Pineiro, C. Identification of the major ACE-inhibitory peptides produced by enzymatic hydrolysis of a protein concentrate from cuttlefish wastewater. Mar. Drugs 2014, 12, 1390–1405. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Qian, Z.-J.; Kim, S.-K. A novel angiotensin I converting enzyme inhibitory peptide from tuna frame protein hydrolysate and its antihypertensive effect in spontaneously hypertensive rats. Food Chem. 2010, 118, 96–102. [Google Scholar] [CrossRef]
- He, H.-L.; Chen, X.-L.; Wu, H.; Sun, C.-Y.; Zhang, Y.-Z.; Zhou, B.-C. High throughput and rapid screening of marine protein hydrolysates enriched in peptides with angiotensin-I-converting enzyme inhibitory activity by capillary electrophoresis. Bioresour. Technol. 2007, 98, 3499–3505. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, B.; Dong, S.; Liu, Z.; Zhao, X.; Wang, J.; Zeng, M. A novel ACE inhibitory peptide isolated from Acaudina molpadioidea hydrolysate. Peptides 2009, 30, 1028–1033. [Google Scholar] [CrossRef] [PubMed]
- Matsufuji, H.; Matsui, T.; Seki, E.; Osajima, K.; Nakashima, M.; Osajima, Y. Angiotensin I-converting enzyme inhibitory peptides in an alkaline protease hydrolyzate derived from sardine muscle. Biosci. Biotechnol. Biochem. 1994, 58, 2244–2245. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-C.; Chen, H.-M.; Shiau, C.-Y. Free amino acids and peptides as related to antioxidant properties in protein hydrolysates of mackerel (Scomber austriasicus). Food Res. Int. 2003, 36, 949–957. [Google Scholar] [CrossRef]
- Wiriyaphan, C.; Chitsomboon, B.; Yongsawadigul, J. Antioxidant activity of protein hydrolysates derived from threadfin bream surimi byproducts. Food Chem. 2012, 132, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.-J.; Jung, W.-K.; Kim, S.-K. Free radical scavenging activity of a novel antioxidative peptide purified from hydrolysate of bullfrog skin, Ranacatesbeiana Shaw. Bioresour. Technol. 2008, 99, 1690–1698. [Google Scholar] [CrossRef] [PubMed]
- Adler-Nissen, J. Enzymic Hydrolysis of Food Proteins; Elsevier Applied Science Publishers: Barking, UK, 1986. [Google Scholar]
- Chen, H.-M.; Muramoto, K.; Yamauchi, F.; Fujimoto, K.; Nokihara, K. Antioxidative properties of histidine-containing peptides designed from peptide fragments found in the digests of a soybean protein. J. Agric. Food Chem. 1998, 46, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Rajapakse, N.; Mendis, E.; Byun, H.-G.; Kim, S.-K. Purification and in vitro antioxidative effects of giant squid muscle peptides on free radical-mediated oxidative systems. J. Nutr. Biochem. 2005, 16, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Aleman, A.; Prez-Santin, E.; Bordenave-Juchereau, S.; Arnaudin, I.; Gomez-Guillen, M.C.; Montero, P. Squid gelatin hydrolysates with antihypertensive, anticancer and antioxidant activity. Food Res. Int. 2011, 44, 1044–1051. [Google Scholar] [CrossRef]
- Byun, H.-G.; Lee, J.K.; Park, H.G.; Jeon, J.-K.; Kim, S.-K. Antioxidant peptides isolated from the marine rotifer, Brachionus rotundiformis. Process Biochem. 2009, 44, 842–846. [Google Scholar] [CrossRef]
- Elavarasan, K.; Naveen Kumar, V.; Shamasundar, B.A. Antioxidant and functional properties of fish protein hydrolysates from fresh water carp (Catla catla) as influenced by the nature of enzyme. J. Food Process. Preserv. 2013, 38, 1207–1214. [Google Scholar] [CrossRef]
- Bougatef, A.; Balti, R.; Haddar, A.; Jellouli, K.; Souissi, N.; Nasri, M. Protein hydrolysates from Bluefin Tuna (Thunnus thynnus) heads as influenced by the extent of enzymatic hydrolysis. Biotechnol. Bioprocess Eng. 2012, 17, 841–852. [Google Scholar] [CrossRef]
- Foh, M.B.K.; Qixing, J.; Amadou, I.; Xia, W.S. Influence of ultrafiltration on antioxidant activity of tilapia (Oreochromis niloticus) protein hydrolysate. Adv. J. Food Sci. Technol. 2010, 2, 227–235. [Google Scholar]
- Saiga, A.I.; Tanabe, S.; Nishimura, T. Antioxidant activity of peptides obtained from porcine myofibrillar proteins by protease treatment. J. Agric. Food Chem. 2003, 51, 3661–3667. [Google Scholar] [CrossRef] [PubMed]
- Cavanagh, E.M.V.; Inserra, F.; Ferder, L.; Fraga, C.G. Enalapril and captopril enhance glutathione-dependent antioxidant defenses in mouse tissues. Am. J. Phys. Regul. Integr. Comp. Phys. 2000, 278, 572–577. [Google Scholar]
- Church, F.C.; Swaisgood, H.E.; Porter, D.H.; Catignani, G.L. Spectrophotometric assay using o-Phthaldialdehyde for determination of proteolysis in milk and isolated milk proteins. J. Dairy Sci. 1983, 66, 1219–1227. [Google Scholar] [CrossRef]
- Zarei, M.; Ebrahimpour, A.; Abdul-Hamid, A.; Anwar, F.; Abu Bakar, F.; Philip, M.; Saari, N. Identification and characterization of papain-generated antioxidant peptides from palm kernel cake proteins. Food Res. Int. 2014, 62, 726–734. [Google Scholar] [CrossRef]
- Khan, J.K.; Kuo, Y.-H.; Kebede, N.; Lambein, F. Determination of non-protein amino acids and toxins in Lathyrus by high-performance liquid chromatography with precolumn phenyl isothiocyanate derivatization. J. Chromatogr. A 1994, 687, 113–119. [Google Scholar] [CrossRef]
- Jimsheena, V.K.; Gowda, L.R. Colorimetric, high-throughput assay for screening angiotensin I-converting enzyme inhibitors. Anal. Chem. 2009, 81, 9388–9394. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.-Y.; Shyu, Y.-S.; Wang, Y.-T.; Hsu, C.-K. Antioxidative properties of protein hydrolysate from defatted peanut kernels treated with esperase. LWT Food Sci. Technol. 2010, 43, 285–290. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Tang, Q.; Wang, Y.; Chang, Y.; Zhao, Q.; Xue, C. Antioxidation activities of low-molecular-weight gelatin hydrolysate isolated from the sea cucumber Stichopus Japonicus. J. Ocean Univ. China 2010, 9, 94–98. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghanbari, R.; Zarei, M.; Ebrahimpour, A.; Abdul-Hamid, A.; Ismail, A.; Saari, N. Angiotensin-I Converting Enzyme (ACE) Inhibitory and Anti-Oxidant Activities of Sea Cucumber (Actinopyga lecanora) Hydrolysates. Int. J. Mol. Sci. 2015, 16, 28870-28885. https://doi.org/10.3390/ijms161226140
Ghanbari R, Zarei M, Ebrahimpour A, Abdul-Hamid A, Ismail A, Saari N. Angiotensin-I Converting Enzyme (ACE) Inhibitory and Anti-Oxidant Activities of Sea Cucumber (Actinopyga lecanora) Hydrolysates. International Journal of Molecular Sciences. 2015; 16(12):28870-28885. https://doi.org/10.3390/ijms161226140
Chicago/Turabian StyleGhanbari, Raheleh, Mohammad Zarei, Afshin Ebrahimpour, Azizah Abdul-Hamid, Amin Ismail, and Nazamid Saari. 2015. "Angiotensin-I Converting Enzyme (ACE) Inhibitory and Anti-Oxidant Activities of Sea Cucumber (Actinopyga lecanora) Hydrolysates" International Journal of Molecular Sciences 16, no. 12: 28870-28885. https://doi.org/10.3390/ijms161226140
APA StyleGhanbari, R., Zarei, M., Ebrahimpour, A., Abdul-Hamid, A., Ismail, A., & Saari, N. (2015). Angiotensin-I Converting Enzyme (ACE) Inhibitory and Anti-Oxidant Activities of Sea Cucumber (Actinopyga lecanora) Hydrolysates. International Journal of Molecular Sciences, 16(12), 28870-28885. https://doi.org/10.3390/ijms161226140







