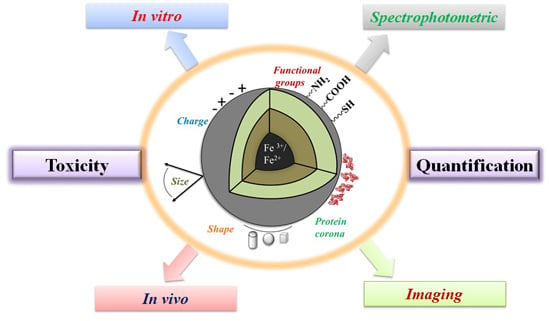
In Vitro/In Vivo Toxicity Evaluation and Quantification of Iron Oxide Nanoparticles
Abstract
:1. Introduction
2. Mechanism of Toxicity of Iron Oxide Nanoparticles (IONPs)
2.1. Shape, Size and Surface Chemistry of IONPs
2.2. Chemical Nature of IONPs
2.3. Morphological Changes Induced by IONPs Exposure
2.4. Genotoxic Effects of IONPs
2.5. Role of Protein Corona on ROS Formation
3. In Vitro and in Vivo Techniques to Evaluate Toxicity of IONPs
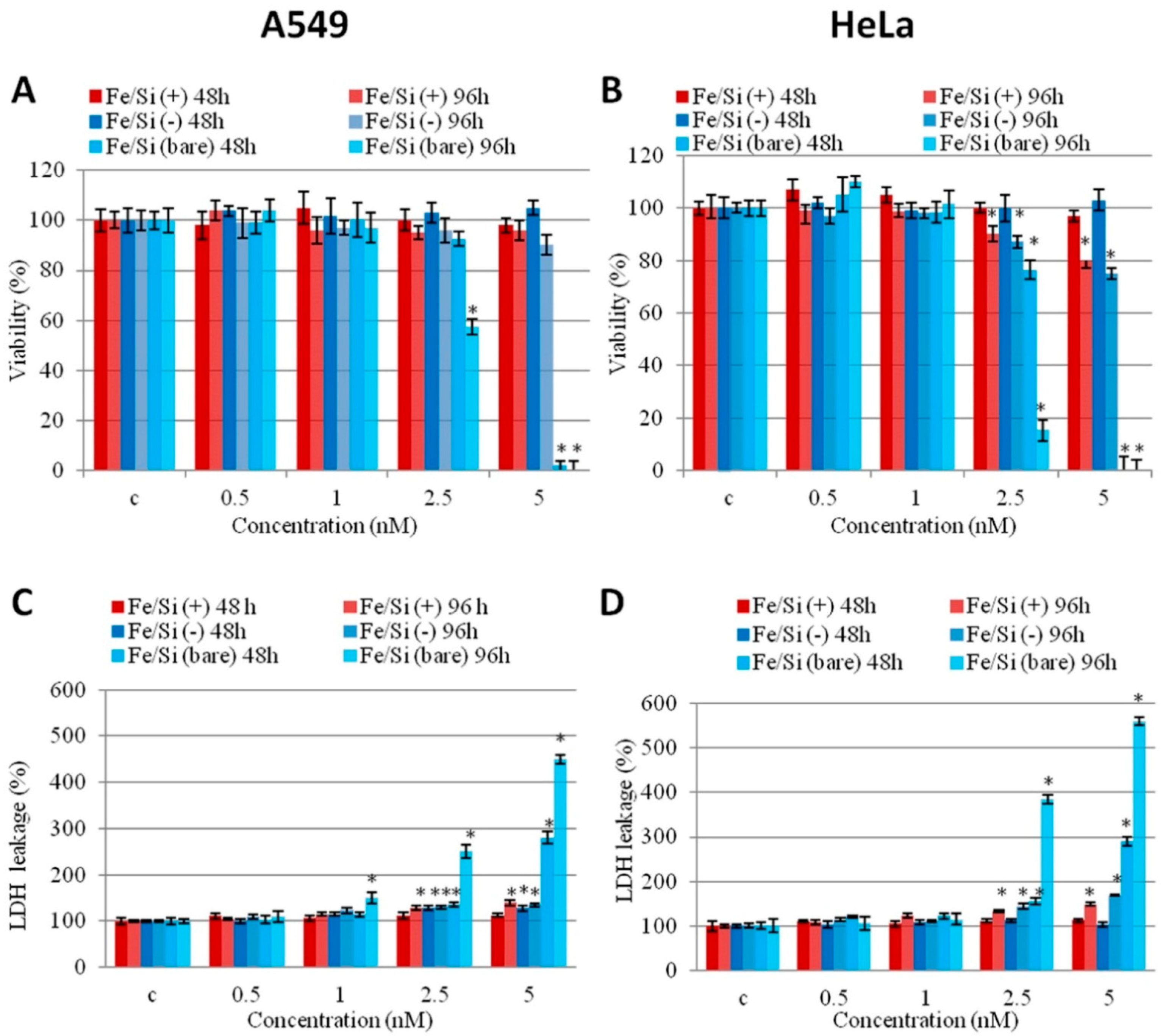
3.1. In Vitro Toxicity Evaluation of IONPs
| Coating Agent | Types of IONPs | Diameter (nm) | Type of Cells | Dose | Incubation Time | Types of Assay | Brief Results | Ref. |
|---|---|---|---|---|---|---|---|---|
| Silica | Bare IONPs | 10 ± 3 | Human dermal fibroblasts (HDFs) and human fibrosarcoma (HT-1080) in DMEM media | 200–1000 μg/mL | 24 h | CCK-8 and LDH | APTMS-TEOS-Fe3O4 showed more cytotoxicity in terms of metabolic activity compared to other MNPs in HDFs. All MNPs induced LDH leakage in HDFs and HT-1080 cells. | [62] |
| TEOS-IONPs | 100–150 | |||||||
| APTMS-TEOS-IONPs | 100–150 | |||||||
| Bare IONPs | 10–50 | Peripheral blood lymphocytes in RPMI media | 1–100 μg/mL | 2 and 24 h | Annexin V-FITC apoptosis detection | No significant difference between treated and untreated lymphocytes for 2 and 24 h. | [104] | |
| VTES-TEOS-IONPs | 10–50 | |||||||
| APTES/VTES-TEOS-IONPs | 10–50 | |||||||
| Bare IONPs | 150–200 | L929 fibroblasts in DMEM media | 15–1000 mg/L | 24–72 h | MTT | Silica coating reduced cell toxicity. Sulfhydryl modification improved cell-compatibility and haemocompatibility. | [105] | |
| TEOS-IONPs | ||||||||
| DMSA-TEOS-IONPs | ||||||||
| TEOS-IONPs | 15–20 | MCF-7 and HeLa cells in DMEM media | 0–200 μg/mL | 24 h | MTT | MCF-7 and HeLa cells showed good biocompatibility at various concentrations. | [106] | |
| PEG | PEG-IONPs | ~30 | Hela cells and C6 cells in DMEM media | 0.01–1 mg/mL | 12 h | MTT | Cell viability was not affected at the concentration of 1 mg/mL. | [107] |
| PEG-IONPs | 10–15 | NIH/3T3 in DMEM | 1.5 to 192 μM | 24 and 48 h | MTT | PEG-IONPs showed good compatibility, 86% (24 h) and 67% (48 h) at 192 μM. | [108] | |
| Bare IONPs | 10–13 | Macrophages (mice) in RPMI media | 100 μg/mL | 1 h | MTT | No significant changes in viability after 1 h by all IONPs. Bare IONPs produced highest ROS compared to PEG and COOH-PEG-IONPs. | [109] | |
| PEG- IONPs | 100 | |||||||
| COOH-PEG-IONPs | 100 | |||||||
| PEG-550-IONPs | 8–11 | Bovine vascular smooth muscle cells (VSMCs) in DMEM media | 100–1000 ppm | 5–24 h | LIVE/DEAD viability/Cytotoxicity Kit | Dose dependent cytotoxic response was found. PEG-2K showed higher cell viability compared to PEG-10K at 100 ppm. | [110] | |
| PEG-2K-IONPs | ||||||||
| PEG-5K-IONPs | ||||||||
| PEG-10K-IONPs | ||||||||
| PEPABC: IONPs | 36 ± 5 | Mouse brain endothelial cell line (bEnd.3) in DMEM media | 0–10 mg/mL | 30 h | Resazurin dye assay | No cell death reported after 30 h exposure at 10 mg/mL. | [111] | |
| Dextran | Dextran-IONPs | 200–250 | Head and neck squamous cell carcinoma: tonsilla (UT-SCC-60A) and the metastasis (UT-SCC-60B) in DMEM media | 0.2–1.8 mM | 0–120 h | MTT, Annexin-V-apoptosis detection assay | MTT: Decreased cell toxicity of dextran-IONPs compared to Resovist® Annexin-V-apoptosis: no changes in cell viability when cells were treated at the concentration of 1.8 mM. | [112] |
| Dextran-IONPs | 100 | Mouse melanoma cells (B16) and Chinese hamster lung; fibroblast cells (V79) in DMEM media | 0–400 μg/mL | 24 h | MTT | Slight changes in the cell viability were noticed as compared to control. | [113] | |
| Dextran-IONPs | 9.12 ± 1.46 | L929 fibroblast cells | 50–1000 μg/mL | 24 h | MTT | Significant reduction in cell viability at 1 mg/mL. Cells were 90% viable at 0.75 mg/mL. | [114] | |
| DEAE-dextran-IONPs | 27–50 | Murine mesenchymal stem/stromal cell (MSC) in DMEM media | 50 μg/mL | 3 h | CCK-8 | No significant changes I the cell viability were noticed. | [115] | |
| Bare Fe2O3 | 7 | Human bone marrow mesenchymal stromal cells (hBMSCs) hBMSCs-1: age 12 years; hBMSCs-2: age 54 years in α-modified eagle media (α MEM) | 15.4 g of iron/mL | 72 h | WST-1 | The study compared physicochemical properties of bare Fe2O3 and nanoparticles coated with different coating agents. hBMSCs-1: significant reduction in cell viability by PLL-Fe2O3and mannose-Fe2O3 NPs; hBMSCs-2: reduction in cell viability by all IONPs, mostly by uncoated-Fe2O3 and PLL-Fe2O3 NPs. | [116] | |
| Endorem® (Fe3O4 coated with dextran) | 5.5 | |||||||
| PLL | PLL-Fe2O3 | 5.5 | ||||||
| PLL-dextran | PLL-Endorem® | 5.6 | ||||||
| PDMAAm | PDMAAm-Fe2O3 | 7.5 | ||||||
| Mannose | Mannose-Fe2O3 | 7 | ||||||
| Mono-meric citrate layer | IONPs-R1 | 6.5–7.5 | Murine primary brain cells (primary microglia, primary hippocampal neurons, and neuron–glia co-cultures) in DMEM media | 0.5, 1.5 or 3.0 mM | 6–24 h | PI staining | Extended incubation and dose dependent cell death was observed by all IONPs except Ferumoxytol. Ferumoxytol surprisingly increased the number of viable cells. IONPs-R1, R2 and Ferucarbotran were quickly ingested by microglial cells compared to Ferumoxytol. | [117] |
| IONPs-R2 | 7.5–8.7 | |||||||
| Carboxy-dextran | Ferucarbotran (Resovist®) | 60 | ||||||
| Carboxymethyl-dextran | Ferumoxytol (Feraheme®) | 30 | ||||||
| Chitosan | Bare IONPs | 50-100 | Human L-O2 hepatocytes in RPMI media | 1.25–20 μg/mL | 24 h | MTT | Bare IONPs showed more cytotoxicity compared to FAPLCS-IONPs in L-O2 hepatocytes. | [118] |
| FAPLCS-IONPs | 136.60 ± 3.90 | |||||||
| Bare IONPs | 18 | Primary human osteoblast cells (SV40) in DMEM media | 20–300 μg/mL | 48 h | CCK-8 | Decreased viability found when cells were treated with bare IONPs at 100 and 300 μg/mL. | [119] | |
| CS-IONPs | 35 | |||||||
| CS-IONPs | 2–8 | Cervical carcinoma cell lines (HeLa and SiHa) | 0–1000 μg/mL | 24 h | XTT | Bare and CS-IONPs showed reduction in cell viability by 5% and 2% respectively. SiHa cells showed 8% reduction in cell viability at 1000 μg/mL. | [120] | |
| Carbon | Fe@C/C | 5–140 | Human (HTB140), murine (B16-F10) melanoma cells and human dermal fibroblasts (HDF) in DMEM | 0.0001–100 μg/mL | 24 h | MTT | Decreased cell viability in melanoma cells. Murine melanoma cells were more sensitive to bare IONPs than human cells. Fe@C-COOH and Fe@C-CH2CH2-COOH showed weaker response to cells, and 80%–100% cells remained viable. | [121] |
3.2. In Vivo Toxicity Evaluation of IONPs
4. Quantification of IONPs
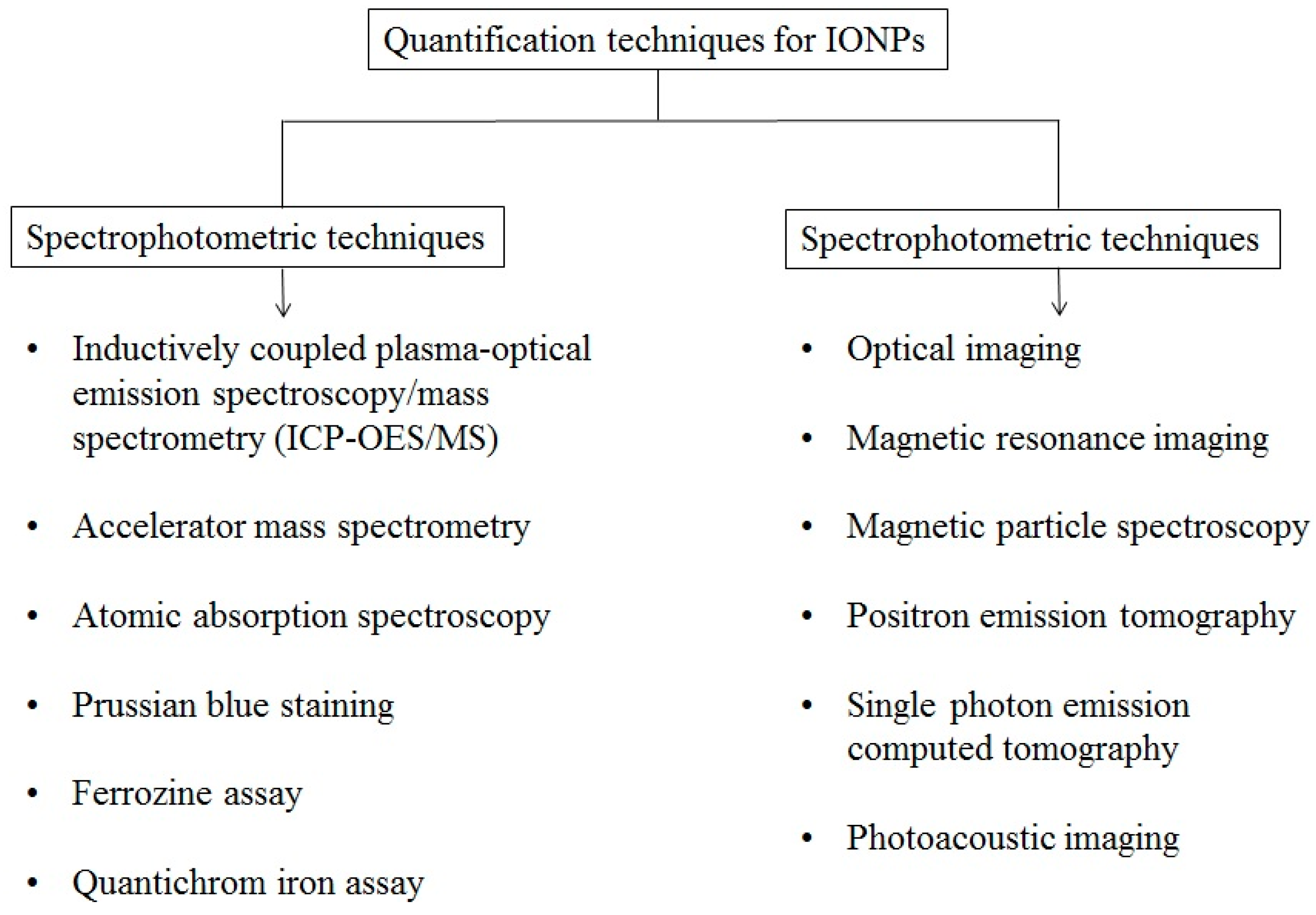
4.1. Spectrophotometric Quantification of IONPs
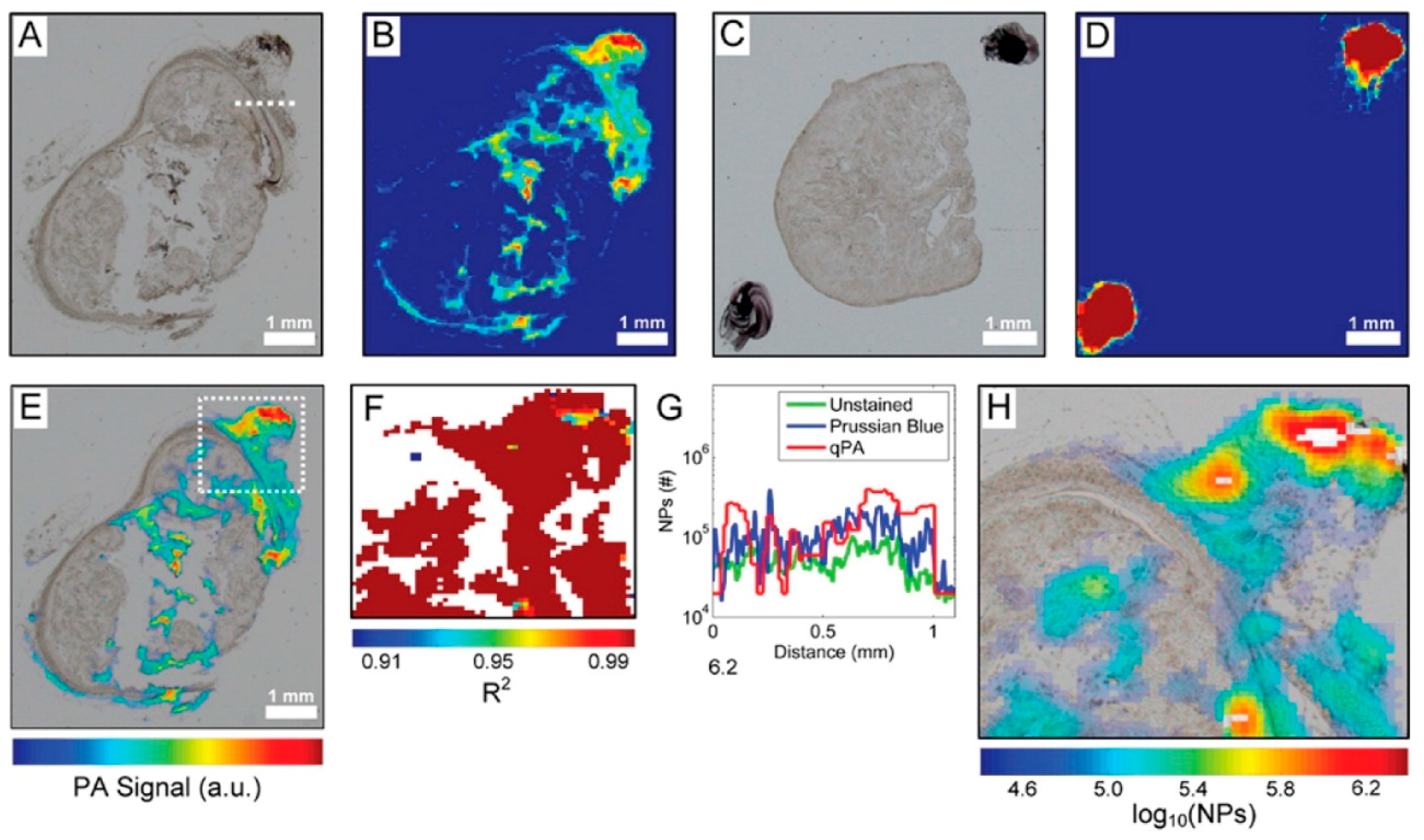
4.2. Imaging Based Quantification of IONPs
5. Conclusions and Perspective
Acknowledgments
Conflicts of Interest
References
- Gu, L.Z.; Hong, Q.; Xiang, C.J. The application of nanotechnology for mechanical manufacturing. Key Eng. Mater. 2010, 447–448, 86–90. [Google Scholar] [CrossRef]
- Cavalcanti, A.; Freitas, R.A., Jr. Nanorobotics control design: A collective behavior approach for medicine. IEEE Trans. Nanobiosci. 2005, 4, 133–140. [Google Scholar] [CrossRef]
- Hobson, D.W. Commercialization of nanotechnology. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2009, 1, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Venkatraman, S. Has nanomedicine lived up to its promise? Nanotechnology 2014, 25, 372501. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Sun, S. New forms of superparamagnetic nanoparticles for biomedical applications. Adv. Drug Deliv. Rev. 2013, 65, 732–743. [Google Scholar] [CrossRef] [PubMed]
- Estelrich, J.; Sanchez-Martin, M.J.; Busquets, M.A. Nanoparticles in magnetic resonance imaging: From simple to dual contrast agents. Int. J. Nanomed. 2015, 10, 1727–1741. [Google Scholar]
- Estelrich, J.; Escribano, E.; Queralt, J.; Busquets, M.A. Iron oxide nanoparticles for magnetically-guided and magnetically-responsive drug delivery. Int. J. Mol. Sci. 2015, 16, 8070–8101. [Google Scholar] [CrossRef] [PubMed]
- Sawdon, A.; Weydemeyer, E.; Peng, C.A. Antitumor therapy using nanomaterial-mediated thermolysis. J. Biomed. Nanotechnol. 2014, 10, 1894–1917. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, A.; Canete, M.; Roca, A.G.; Calero, M.; Veintemillas-Verdaguer, S.; Serna, C.J.; del Puerto Morales, M.; Miranda, R. The influence of surface functionalization on the enhanced internalization of magnetic nanoparticles in cancer cells. Nanotechnology 2009, 20, 115103. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, M.; Prakash, S. Targeted drug delivery across blood-brain-barrier using cell penetrating peptides tagged nanoparticles. Curr. Nanosci. 2011, 7, 81–93. [Google Scholar] [CrossRef]
- Ansciaux, E.; Burtea, C.; Laurent, S.; Crombez, D.; Nonclercq, D.; Vander Elst, L.; Muller, R.N. In vitro and in vivo characterization of several functionalized ultrasmall particles of iron oxide, vectorized against amyloid plaques and potentially able to cross the blood–brain barrier: Toward earlier diagnosis of Alzheimer’s disease by molecular imaging. Contrast Media Mol. Imaging 2015, 10, 211–224. [Google Scholar] [PubMed]
- Koh, I.; Josephson, L. Magnetic nanoparticle sensors. Sensors 2009, 9, 8130–8145. [Google Scholar] [CrossRef] [PubMed]
- Patil, U.S.; Qu, H.; Caruntu, D.; O’Connor, C.J.; Sharma, A.; Cai, Y.; Tarr, M.A. Labeling primary amine groups in peptides and proteins with N-hydroxysuccinimidyl ester modified Fe3O4@SiO2 nanoparticles containing cleavable disulfide-bond linkers. Bioconjug. Chem. 2013, 24, 1562–1569. [Google Scholar] [CrossRef] [PubMed]
- Patil, U.S.; Osorno, L.; Ellender, A.; Grimm, C.; Tarr, M.A. Cleavable ester-linked magnetic nanoparticles for labeling of solvent-exposed primary amine groups of peptides/proteins. Anal. Biochem. 2015, 484, 18–20. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Chatterjee, S.; Bandyopadhyay, A.; Sarkar, K. Potential application of superparamagnetic nanoparticles for extraction of bacterial genomic DNA from contaminated food and environmental samples. J. Sci. Food Agric. 2013, 93, 788–793. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.; Xia, T.; Madler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Nalwa, H.S. Nanotechnology and health safety—Toxicity and risk assessments of nanostructured materials on human health. J. Nanosci. Nanotechnol. 2007, 7, 3048–3070. [Google Scholar] [CrossRef] [PubMed]
- Nanotechnology Characterization Laboratory. Available online: http://ncl.cancer.gov/ (accesses on 5 July 2015).
- Juillerat-Jeanneret, L.; Dusinska, M.; Fjellsbo, L.M.; Collins, A.R.; Handy, R.D.; Riediker, M. Biological impact assessment of nanomaterial used in nanomedicine. introduction to the NanoTEST project. Nanotoxicology 2015, 9 (Suppl. 1), 5–12. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, J.S.; Steinauer, K.K.; Hornung, B.; Irish, J.M.; Lecane, P.; Birrell, G.W.; Peehl, D.M.; Knox, S.J. Role of glutathione depletion and reactive oxygen species generation in apoptotic signaling in a human B lymphoma cell line. Cell Death Differ. 2002, 9, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Ayala, A.; Muoz, M.F.; Arguelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Med. Cell. Longev. 2014, 2014, 31. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, A.; Lehmler, H.J.; Robertson, L.W.; Ludewig, G. Production of DNA strand breaks in vitro and reactive oxygen species in vitro and in HL-60 cells by PCB metabolites. Toxicol. Sci. 2001, 60, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Ziech, D.; Franco, R.; Pappa, A.; Panayiotidis, M.I. Reactive oxygen species (ROS)—Induced genetic and epigenetic alterations in human carcinogenesis. Mutat. Res. 2011, 711, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 26. [Google Scholar] [CrossRef]
- Sugamura, K.; Keaney, J.F., Jr. Reactive oxygen species in cardiovascular disease. Free Radic. Biol. Med. 2011, 51, 978–992. [Google Scholar] [CrossRef] [PubMed]
- Liou, G.Y.; Storz, P. Reactive oxygen species in cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Motherwell, M.S. The impact of reactive oxygen species and genetic mitochondrial mutations in Parkinson’s disease. Gene 2013, 532, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Gelderman, K.A.; Hultqvist, M.; Olsson, L.M.; Bauer, K.; Pizzolla, A.; Olofsson, P.; Holmdahl, R. Rheumatoid arthritis: The role of reactive oxygen species in disease development and therapeutic strategies. Antioxid. Redox Signal. 2007, 9, 1541–1567. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Yin, J.J.; Wamer, W.G.; Zeng, M.; Lo, Y.M. Reactive oxygen species-related activities of nano-iron metal and nano-iron oxides. J. Food Drug Anal. 2014, 22, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, S.; Meyerstein, D.; Czapski, G. The Fenton reagents. Free Radic. Biol. Med. 1993, 15, 435–445. [Google Scholar] [CrossRef]
- Voinov, M.A.; Pagán, J.O.S.; Morrison, E.; Smirnova, T.I.; Smirnov, A.I. Surface-mediated production of hydroxyl radicals as a mechanism of iron oxide nanoparticle biotoxicity. J. Am. Chem. Soc. 2011, 133, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Valdiglesias, V.; Kilic, G.; Costa, C.; Fernandez-Bertolez, N.; Pasaro, E.; Teixeira, J.P.; Laffon, B. Effects of iron oxide nanoparticles: Cytotoxicity, genotoxicity, developmental toxicity, and neurotoxicity. Environ. Mol. Mutagen. 2015, 56, 125–148. [Google Scholar] [CrossRef] [PubMed]
- Ying, E.; Hwang, H.-M. In vitro evaluation of the cytotoxicity of iron oxide nanoparticles with different coatings and different sizes in A3 human T lymphocytes. Sci. Total Environ. 2010, 408, 4475–4481. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Kuang, H.; Zhang, W.; Aguilar, Z.P.; Xiong, Y.; Lai, W.; Xu, H.; Wei, H. Size dependent biodistribution and toxicokinetics of iron oxide magnetic nanoparticles in mice. Nanoscale 2015, 7, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, H.L.; Gustafsson, J.; Cronholm, P.; Möller, L. Size-dependent toxicity of metal oxide particles—A comparison between nano- and micrometer size. Toxicol. Lett. 2009, 188, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Ju, J.E.; Kim, B.I.; Pak, P.J.; Choi, E.K.; Lee, H.S.; Chung, N. Rod-shaped iron oxide nanoparticles are more toxic than sphere-shaped nanoparticles to murine macrophage cells. Environ. Toxicol. Chem. 2014, 33, 2759–2766. [Google Scholar] [CrossRef] [PubMed]
- Magdolenova, Z.; Drlickova, M.; Henjum, K.; Runden-Pran, E.; Tulinska, J.; Bilanicova, D.; Pojana, G.; Kazimirova, A.; Barancokova, M.; Kuricova, M.; et al. Coating-dependent induction of cytotoxicity and genotoxicity of iron oxide nanoparticles. Nanotoxicology 2015, 9 (Suppl. 1), 44–56. [Google Scholar] [CrossRef] [PubMed]
- Malvindi, M.A.; de Matteis, V.; Galeone, A.; Brunetti, V.; Anyfantis, G.C.; Athanassiou, A.; Cingolani, R.; Pompa, P.P. Toxicity assessment of silica coated iron oxide nanoparticles and biocompatibility improvement by surface engineering. PLoS ONE 2014, 9, e85835. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.; Lagarde, F.; Maraloiu, V.A.; Blanchin, M.G.; Gendron, F.; Wilhelm, C.; Gazeau, F. Degradability of superparamagnetic nanoparticles in a model of intracellular environment: Follow-up of magnetic, structural and chemical properties. Nanotechnology 2010, 21, 395103. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Hanna, K.; Deng, N. Fenton-like oxidation of Rhodamine B in the presence of two types of iron (II, III) oxide. J. Hazard. Mater. 2009, 166, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Gorski, C.A.; Nurmi, J.T.; Tratnyek, P.G.; Hofstetter, T.B.; Scherer, M.M. Redox Behavior of Magnetite: Implications for contaminant reduction. Environ. Sci. Technol. 2010, 44, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yin, J.-J.; Zhou, Y.-T.; Zhang, Y.; Song, L.; Song, M.; Hu, S.; Gu, N. Dual enzyme-like activities of iron oxide nanoparticles and their implication for diminishing cytotoxicity. ACS Nano 2012, 6, 4001–4012. [Google Scholar] [CrossRef] [PubMed]
- Hermanek, M.; Zboril, R.; Medrik, I.; Pechousek, J.; Gregor, C. Catalytic efficiency of iron(III) oxides in decomposition of hydrogen peroxide: Competition between the surface area and crystallinity of nanoparticles. J. Am. Chem. Soc. 2007, 129, 10929–10936. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Umh, H.N.; Choi, D.H.; Cho, M.H.; Choi, W.; Kim, S.W.; Kim, Y.; Kim, J.H. Magnetite- and maghemite-induced different toxicity in murine alveolar macrophage cells. Arch. Toxicol. 2014, 88, 1607–1618. [Google Scholar] [PubMed]
- Cromer Berman, S.M.; Kshitiz; Wang, C.J.; Orukari, I.; Levchenko, A.; Bulte, J.W.M.; Walczak, P. Cell motility of neural stem cells is reduced after SPIO-labeling, which is mitigated after exocytosis. Magn. Reson. Med. 2013, 69, 255–262. [Google Scholar] [PubMed]
- Wu, X.; Tan, Y.; Mao, H.; Zhang, M. Toxic effects of iron oxide nanoparticles on human umbilical vein endothelial cells. Int. J. Nanomed. 2010, 5, 385–399. [Google Scholar] [CrossRef]
- Buyukhatipoglu, K.; Clyne, A.M. Superparamagnetic iron oxide nanoparticles change endothelial cell morphology and mechanics via reactive oxygen species formation. J. Biomed. Mater. Res. Part A 2011, 96, 186–195. [Google Scholar]
- Zhang, W.; Kalive, M.; Capco, D.G.; Chen, Y. Adsorption of hematite nanoparticles onto Caco-2 cells and the cellular impairments: Effect of particle size. Nanotechnology 2010, 21, 355103. [Google Scholar] [CrossRef] [PubMed]
- Kalive, M.; Zhang, W.; Chen, Y.; Capco, D. Human intestinal epithelial cells exhibit a cellular response indicating a potential toxicity upon exposure to hematite nanoparticles. Cell Biol. Toxicol. 2012, 28, 343–368. [Google Scholar] [CrossRef]
- Astanina, K.; Simon, Y.; Cavelius, C.; Petry, S.; Kraegeloh, A.; Kiemer, A.K. Superparamagnetic iron oxide nanoparticles impair endothelial integrity and inhibit nitric oxide production. Acta Biomater. 2014, 10, 4896–4911. [Google Scholar] [CrossRef] [PubMed]
- Mesarosova, M.; Kozics, K.; Babelova, A.; Regendova, E.; Pastorek, M.; Vnukova, D.; Buliakova, B.; Razga, F.; Gabelova, A. The role of reactive oxygen species in the genotoxicity of surface-modified magnetite nanoparticles. Toxicol. Lett. 2014, 226, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Kedziorek, D.A.; Muja, N.; Walczak, P.; Ruiz-Cabello, J.; Gilad, A.A.; Jie, C.C.; Bulte, J.W. Gene expression profiling reveals early cellular responses to intracellular magnetic labeling with superparamagnetic iron oxide nanoparticles. Magn. Reson. Med. 2010, 63, 1031–1043. [Google Scholar] [PubMed]
- Alarifi, S.; Ali, D.; Alkahtani, S.; Alhader, M.S. Iron oxide nanoparticles induce oxidative stress, DNA damage, and caspase activation in the human breast cancer cell line. Biol. Trace Elem. Res. 2014, 159, 416–424. [Google Scholar] [PubMed]
- Ahamed, M.; Alhadlaq, H.A.; Alam, J.; Khan, M.A.; Ali, D.; Alarafi, S. Iron oxide nanoparticle-induced oxidative stress and genotoxicity in human skin epithelial and lung epithelial cell lines. Curr. Pharma. Des. 2013, 19, 6681–6690. [Google Scholar]
- Couto, D.; Freitas, M.; Porto, G.; Lopez-Quintela, M.A.; Rivas, J.; Freitas, P.; Carvalho, F.; Fernandes, E. Polyacrylic acid-coated and non-coated iron oxide nanoparticles induce cytokine activation in human blood cells through TAK1, p38 MAPK and JNK pro-inflammatory pathways. Arch. Toxicol. 2015, 89, 1759–1769. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, J. Effects of DMSA-coated Fe3O4 nanoparticles on the transcription of genes related to iron and osmosis homeostasis. Toxicol. Sci. 2013, 131, 521–536. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Rahman, M.F.; Murty, U.S.; Mahboob, M.; Grover, P. Comparative study of genotoxicity and tissue distribution of nano and micron sized iron oxide in rats after acute oral treatment. Toxicol. Appl. Pharmacol. 2013, 266, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Faust, J.J.; Zhang, W.; Chen, Y.; Capco, D.G. α-Fe2O3 elicits diameter-dependent effects during exposure to an in vitro model of the human placenta. Cell Biol. Toxicol. 2014, 30, 31–53. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xia, Q.; Liu, Y.; Zhang, S.; Cheng, F.; Zhong, Z.; Wang, L.; Li, H.; Xiao, K. Genotoxicity assessment of magnetic iron oxide nanoparticles with different particle sizes and surface coatings. Nanotechnology 2014, 25, 425101. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Lee, J.; Hong, S.; Lee, J.; Lee, J.; Han, D.-W. Difference between toxicities of iron oxide magnetic nanoparticles with various surface-functional groups against human normal fibroblasts and fibrosarcoma cells. Materials 2013, 6, 4689–4706. [Google Scholar] [CrossRef]
- Singh, N.; Jenkins, G.J.; Nelson, B.C.; Marquis, B.J.; Maffeis, T.G.; Brown, A.P.; Williams, P.M.; Wright, C.J.; Doak, S.H. The role of iron redox state in the genotoxicity of ultrafine superparamagnetic iron oxide nanoparticles. Biomaterials 2012, 33, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Sheibani, S.; Milani, A.S.; Rezaee, F.; Gauberti, M.; Dinarvand, R.; Vali, H. Crucial role of the protein corona for the specific targeting of nanoparticles. Nanomedicine 2015, 10, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Burtea, C.; Thirifays, C.; Rezaee, F.; Mahmoudi, M. Significance of cell “observer” and protein source in nanobiosciences. J. Colloid Interface Sci. 2013, 392, 431–445. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Abdelmonem, A.M.; Behzadi, S.; Clement, J.H.; Dutz, S.; Ejtehadi, M.R.; Hartmann, R.; Kantner, K.; Linne, U.; Maffre, P.; et al. Temperature: The “ignored” factor at the NanoBio interface. ACS Nano 2013, 7, 6555–6562. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Burtea, C.; Thirifays, C.; Hafeli, U.O.; Mahmoudi, M. Crucial ignored parameters on nanotoxicology: The importance of toxicity assay modifications and “cell vision”. PLoS ONE 2012, 7, e29997. [Google Scholar] [CrossRef] [PubMed]
- Mbeh, D.A.; Mireles, L.K.; Stanicki, D.; Tabet, L.; Maghni, K.; Laurent, S.; Sacher, E.; Yahia, L. Human alveolar epithelial cell responses to core-shell superparamagnetic iron oxide nanoparticles (SPIONs). Langmuir 2015, 31, 3829–3839. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Shokrgozar, M.A.; Behzadi, S. Slight temperature changes affect protein affinity and cellular uptake/toxicity of nanoparticles. Nanoscale 2013, 5, 3240–3244. [Google Scholar] [CrossRef] [PubMed]
- Weissleder, R.; Stark, D.D.; Engelstad, B.L.; Bacon, B.R.; Compton, C.C.; White, D.L.; Jacobs, P.; Lewis, J. Superparamagnetic iron oxide: Pharmacokinetics and toxicity. Am. J. Roentgenol. 1989, 152, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Josephson, L.; Groman, E.V.; Menz, E.; Lewis, J.M.; Bengele, H. A functionalized superparamagnetic iron oxide colloid as a receptor directed MR contrast agent. Magn. Reson. Imaging 1990, 8, 637–646. [Google Scholar] [CrossRef]
- Malvindi, M.A.; Greco, A.; Conversano, F.; Figuerola, A.; Corti, M.; Bonora, M.; Lascialfari, A.; Doumari, H.A.; Moscardini, M.; Cingolani, R.; et al. Magnetic/silica nanocomposites as dual-mode contrast agents for combined magnetic resonance imaging and ultrasonography. Adv. Funct. Mater. 2011, 21, 2548–2555. [Google Scholar] [CrossRef]
- Wang, Y.-X.J. Superparamagnetic iron oxide based MRI contrast agents: Current status of clinical application. Quant. Imaging Med. Surg. 2011, 1, 35–40. [Google Scholar] [PubMed]
- Castaneda, R.T.; Khurana, A.; Khan, R.; Daldrup-Link, H.E. Labeling stem cells with ferumoxytol, an FDA-approved iron oxide nanoparticle. J. Vis. Exp. 2011. [Google Scholar] [CrossRef] [PubMed]
- Kolhatkar, A.G.; Jamison, A.C.; Litvinov, D.; Willson, R.C.; Lee, T.R. Tuning the magnetic properties of nanoparticles. Int. J. Mol. Sci. 2013, 14, 15977–16009. [Google Scholar] [CrossRef] [PubMed]
- Chiriaco, F.; Soloperto, G.; Greco, A.; Conversano, F.; Ragusa, A.; Menichetti, L.; Casciaro, S. Magnetically-coated silica nanospheres for dual-mode imaging at low ultrasound frequency. World J. Radiol. 2013, 5, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Urbanova, V.; Magro, M.; Gedanken, A.; Baratella, D.; Vianello, F.; Zboril, R. Nanocrystalline iron oxides, composites, and related materials as a platform for electrochemical, magnetic, and chemical biosensors. Chem. Mater. 2014, 26, 6653–6673. [Google Scholar] [CrossRef]
- Doak, S.H.; Griffiths, S.M.; Manshian, B.; Singh, N.; Williams, P.M.; Brown, A.P.; Jenkins, G.J. Confounding experimental considerations in nanogenotoxicology. Mutagenesis 2009, 24, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Simchi, A.; Imani, M.; Shokrgozar, M.A.; Milani, A.S.; Hafeli, U.O.; Stroeve, P. A new approach for the in vitro identification of the cytotoxicity of superparamagnetic iron oxide nanoparticles. Coll. Surf. B Biointerfaces 2010, 75, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, M.; Mitsumori, L.M.; Kushleika, J.V.; Rosenfeld, M.E.; Krishnan, K.M. Cytotoxicity of iron oxide nanoparticles made from the thermal decomposition of organometallics and aqueous phase transfer with Pluronic F127. Contrast Media Mol. Imaging 2010, 5, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, T.; Colognato, R.; Nelissen, I.; Favilli, F.; Casals, E.; Ooms, D.; Leppens, H.; Ponti, J.; Stritzinger, R.; Puntes, V.; et al. The suitability of different cellular in vitro immunotoxicity and genotoxicity methods for the analysis of nanoparticle-induced events. Nanotoxicology 2010, 4, 52–72. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; An, X.; Cui, J.; Li, J.; Wen, S.; Li, K.; Shen, M.; Zheng, L.; Zhang, G.; Shi, X. Facile hydrothermal synthesis and surface functionalization of polyethyleneimine-coated iron oxide nanoparticles for biomedical applications. ACS Appl. Mater. Interfaces 2013, 5, 1722–1731. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Huang, S.; Yu, K.J.; Clyne, A.M. Dextran and polymer polyethylene glycol (PEG) coating reduce both 5 and 30 nm iron oxide nanoparticle cytotoxicity in 2D and 3D cell culture. Int. J. Mol. Sci. 2012, 13, 5554–5570. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Jadaun, A.; Arora, V.; Sinha, R.K.; Biyani, N.; Jain, V.K. In vitro toxicity assessment of chitosan oligosaccharide coated iron oxide nanoparticles. Toxicol. Rep. 2015, 2, 27–39. [Google Scholar] [CrossRef]
- Ebrahiminezhad, A.; Rasoul-Amini, S.; Kouhpayeh, A.; Davaran, S.; Barar, J.; Ghasemi, Y. Impacts of amine functionalized iron oxide nanoparticles on HepG2 cell line. Curr. Nanosci. 2015, 11, 113–119. [Google Scholar] [CrossRef]
- Chang, Y.K.; Liu, Y.P.; Ho, J.H.; Hsu, S.C.; Lee, O.K. Amine-surface-modified superparamagnetic iron oxide nanoparticles interfere with differentiation of human mesenchymal stem cells. J. Orthop. Res. 2012, 30, 1499–1506. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Cai, H.; Wang, X.; Cao, X.; Li, K.; Wang, S.H.; Guo, R.; Zheng, L.; Zhang, G.; Shi, X. Facile one-pot preparation, surface functionalization, and toxicity assay of APTS-coated iron oxide nanoparticles. Nanotechnology 2012, 23, 105601. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Kodali, V.; Gaffrey, M.; Wang, W.; Minard, K.R.; Karin, N.J.; Teeguarden, J.G.; Thrall, B.D. Iron oxide nanoparticle agglomeration influences dose rates and modulates oxidative stress-mediated dose-response profiles in vitro. Nanotoxicology 2014, 8, 663–675. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.C.; Lee, J.H.; Lee, J.; Kim, H.Y.; Park, J.Y.; Cho, J.; Lee, J.; Han, D.W. Subtle cytotoxicity and genotoxicity differences in superparamagnetic iron oxide nanoparticles coated with various functional groups. Int. J. Nanomed. 2011, 6, 3219–3231. [Google Scholar]
- Sun, Z.; Yathindranath, V.; Worden, M.; Thliveris, J.A.; Chu, S.; Parkinson, F.E.; Hegmann, T.; Miller, D.W. Characterization of cellular uptake and toxicity of aminosilane-coated iron oxide nanoparticles with different charges in central nervous system-relevant cell culture models. Int. J. Nanomed. 2013, 8, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Hofmann, H.; Rothen-Rutishauser, B.; Petri-Fink, A. Assessing the in vitro and in vivo toxicity of superparamagnetic iron oxide nanoparticles. Chem. Rev. 2012, 112, 2323–2338. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, S.; Samim, M.; Abdin, M.; Ahmed, F.J.; Maitra, A.; Prashant, C.; Dinda, A.K. Concentration-dependent toxicity of iron oxide nanoparticles mediated by increased oxidative stress. Int. J. Nanomed. 2010, 5, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.G.; Koch, B.; Bachmatiuk, A.; El-Gendy, A.A.; Krupskaya, Y.; Springer, A.; Klingeler, R.; Schmidt, O.; Büchner, B.; Sanchez, S.; et al. Synthesis and toxicity characterization of carbon coated iron oxide nanoparticles with highly defined size distributions. Biochim. Biophys. Acta 2014, 1840, 160–169. [Google Scholar] [CrossRef]
- Dwivedi, S.; Siddiqui, M.A.; Farshori, N.N.; Ahamed, M.; Musarrat, J.; Al-Khedhairy, A.A. Synthesis, characterization and toxicological evaluation of iron oxide nanoparticles in human lung alveolar epithelial cells. Coll. Surf. B Biointerfaces 2014, 122, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Taupitz, M.; Wagner, S.; Schnorr, J.; Kravec, I.; Pilgrimm, H.; Bergmann-Fritsch, H.; Hamm, B. Phase I clinical evaluation of citrate-coated monocrystalline very small superparamagnetic iron oxide particles as a new contrast medium for magnetic resonance imaging. Investig. Radiol. 2004, 39, 394–405. [Google Scholar] [CrossRef]
- Hoskins, C.; Cuschieri, A.; Wang, L. The cytotoxicity of polycationic iron oxide nanoparticles: Common endpoint assays and alternative approaches for improved understanding of cellular response mechanism. J. Nanobiotechnol. 2012, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, M.; Ozkan, M.; Ozkan, C.S. Magnetic force microscopy of iron oxide nanoparticles and their cellular uptake. Biotechnol. Prog. 2009, 25, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Love, S.A.; Maurer-Jones, M.A.; Thompson, J.W.; Lin, Y.S.; Haynes, C.L. Assessing nanoparticle toxicity. Annu. Rev. Anal. Chem. 2012, 5, 181–205. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.M.; Chen, C.S. Deconstructing the third dimension: How 3D culture microenvironments alter cellular cues. J. Cell Sci. 2012, 125, 3015–3024. [Google Scholar] [CrossRef] [PubMed]
- Da Rocha, E.L.; Porto, L.M.; Rambo, C.R. Nanotechnology meets 3D in vitro models: Tissue engineered tumors and cancer therapies. Mater. Sci. Eng. C 2014, 34, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lilly, G.D.; Doty, R.C.; Podsiadlo, P.; Kotov, N.A. In vitro toxicity testing of nanoparticles in 3D cell culture. Small 2009, 5, 1213–1221. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Wang, C.; Hossain, M.; Qiao, Y.; Ma, L.; An, J.; Su, M. Three-dimensional microtissue assay for high-throughput cytotoxicity of nanoparticles. Anal. Chem. 2012, 84, 6731–6738. [Google Scholar] [CrossRef] [PubMed]
- Nancy, A.; Monteiro-Riviere, C.L.T. Nanotoxicology: Progress toward Nanomedicine, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Lankoff, A.; Arabski, M.; Wegierek-Ciuk, A.; Kruszewski, M.; Lisowska, H.; Banasik-Nowak, A.; Rozga-Wijas, K.; Wojewodzka, M.; Slomkowski, S. Effect of surface modification of silica nanoparticles on toxicity and cellular uptake by human peripheral blood lymphocytes in vitro. Nanotoxicology 2013, 7, 235–250. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Mao, F.; Wang, W.; Yang, Y.; Bai, Z. Sulfhydryl-modified Fe3O4@SiO2 core/shell nanocomposite: Synthesis and toxicity assessment in vitro. ACS Appl. Mater. Interfaces 2015, 7, 14983–14991. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.Z.; Ma, X.; Chen, T.; Zhang, L.E.; Ren, W.; Xiang, L.; Wu, A. Silica-coated super-paramagnetic iron oxide nanoparticles (SPIONPs): A new type contrast agent of T1 magnetic resonance imaging (MRI). J. Mater. Chem. B 2015, 3, 5172–5181. [Google Scholar] [CrossRef]
- Yuan, G.; Yuan, Y.; Xu, K.; Luo, Q. Biocompatible PEGylated Fe3O4 nanoparticles as photothermal agents for near-infrared light modulated cancer therapy. Int. J. Mol. Sci. 2014, 15, 18776–18788. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Liu, Y.; Wang, Z.; Guo, F.; Shi, D.; Zhang, B. One-pot facile synthesis of PEGylated superparamagnetic iron oxide nanoparticles for MRI contrast enhancement. Mater. Sci. Eng. C 2014, 41, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Al Faraj, A. Preferential magnetic nanoparticle uptake by bone marrow derived macrophages sub-populations: Effect of surface coating on polarization, toxicity, and in vivo MRI detection. J. Nanopart. Res. 2013, 15, 1–13. [Google Scholar] [CrossRef]
- Park, Y.C.; Smith, J.B.; Pham, T.; Whitaker, R.D.; Sucato, C.A.; Hamilton, J.A.; Bartolak-Suki, E.; Wong, J.Y. Effect of PEG molecular weight on stability, T2 contrast, cytotoxicity, and cellular uptake of superparamagnetic iron oxide nanoparticles (SPIONs). Coll. Surf. B Biointerfaces 2014, 119, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Dan, M.; Scott, D.F.; Hardy, P.A.; Wydra, R.J.; Hilt, J.Z.; Yokel, R.A.; Bae, Y. Block copolymer cross-linked nanoassemblies improve particle stability and biocompatibility of superparamagnetic iron oxide nanoparticles. Pharm. Res. 2013, 30, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Lindemann, A.; Ludtke-Buzug, K.; Fraderich, B.M.; Grafe, K.; Pries, R.; Wollenberg, B. Biological impact of superparamagnetic iron oxide nanoparticles for magnetic particle imaging of head and neck cancer cells. Int. J. Nanomed. 2014, 9, 5025–5040. [Google Scholar] [CrossRef] [PubMed]
- Zavisova, V.; Koneracka, M.; Kovac, J.; Kubovcikova, M.; Antal, I.; Kopcansky, P.; Bednarikova, M.; Muckova, M. The cytotoxicity of iron oxide nanoparticles with different modifications evaluated in vitro. J. Magn. Magn. Mater. 2015, 380, 85–89. [Google Scholar] [CrossRef]
- Easo, S.L.; Mohanan, P.V. Dextran stabilized iron oxide nanoparticles: Synthesis, characterization and in vitro studies. Carbohydr. Polym. 2013, 92, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Barrow, M.; Taylor, A.; Nieves, D.J.; Bogart, L.K.; Mandal, P.; Collins, C.M.; Moore, L.R.; Chalmers, J.J.; Levy, R.; Williams, S.R.; et al. Tailoring the surface charge of dextran-based polymer coated SPIONs for modulated stem cell uptake and MRI contrast. Biomater. Sci. 2015, 3, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Novotna, B.; Jendelova, P.; Kapcalova, M.; Rossner, P., Jr.; Turnovcova, K.; Bagryantseva, Y.; Babic, M.; Horak, D.; Sykova, E. Oxidative damage to biological macromolecules in human bone marrow mesenchymal stromal cells labeled with various types of iron oxide nanoparticles. Toxicol. Lett. 2012, 210, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Neubert, J.; Wagner, S.; Kiwit, J.; Brauer, A.U.; Glumm, J. New findings about iron oxide nanoparticles and their different effects on murine primary brain cells. Int. J. Nanomed. 2015, 10, 2033–2049. [Google Scholar]
- Xiao, Y.; Lin, Z.T.; Chen, Y.; Wang, H.; Deng, Y.L.; Le, D.E.; Bin, J.; Li, M.; Liao, Y.; Liu, Y.; et al. High molecular weight chitosan derivative polymeric micelles encapsulating superparamagnetic iron oxide for tumor-targeted magnetic resonance imaging. Int. J. Nanomed. 2015, 10, 1155–1172. [Google Scholar]
- Shi, S.F.; Jia, J.F.; Guo, X.K.; Zhao, Y.P.; Chen, D.S.; Guo, Y.Y.; Cheng, T.; Zhang, X.L. Biocompatibility of chitosan-coated iron oxide nanoparticles with osteoblast cells. Int. J. Nanomed. 2012, 7, 5593–5602. [Google Scholar]
- Unsoy, G.; Yalcin, S.; Khodadust, R.; Gunduz, G.; Gunduz, U. Synthesis optimization and characterization of chitosan-coated iron oxide nanoparticles produced for biomedical applications. J. Nanopart. Res. 2012, 14, 1–13. [Google Scholar] [CrossRef]
- Grudzinski, I.P.; Bystrzejewski, M.; Cywinska, M.A.; Kosmider, A.; Poplawska, M.; Cieszanowski, A.; Ostrowska, A. Cytotoxicity evaluation of carbon-encapsulated iron nanoparticles in melanoma cells and dermal fibroblasts. J. Nanopart. Res. 2013, 15, 1835. [Google Scholar] [CrossRef] [PubMed]
- NDong, C.; Tate, J.A.; Kett, W.C.; Batra, J.; Demidenko, E.; Lewis, L.D.; Hoopes, P.J.; Gerngross, T.U.; Griswold, K.E. Tumor cell targeting by iron oxide nanoparticles is dominated by different factors in vitro versus in vivo. PLoS ONE 2015, 10, e0115636. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Fang, R.H.; Sailor, M.J.; Park, J.-H. In vivo clearance and toxicity of monodisperse iron oxide nanocrystals. ACS Nano 2012, 6, 4947–4954. [Google Scholar] [CrossRef] [PubMed]
- Jain, T.K.; Reddy, M.K.; Morales, M.A.; Leslie-Pelecky, D.L.; Labhasetwar, V. Biodistribution, clearance, and biocompatibility of iron oxide magnetic nanoparticles in rats. Mol. Pharm. 2008, 5, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, A.; Hernandez, Y.; Cabal, C.; Gonzalez, E.; Veintemillas-Verdaguer, S.; Martinez, E.; Morales, M.P. Biodistribution and pharmacokinetics of uniform magnetite nanoparticles chemically modified with polyethylene glycol. Nanoscale 2013, 5, 11400–11408. [Google Scholar] [CrossRef] [PubMed]
- Di Bona, K.R.; Xu, Y.; Ramirez, P.A.; DeLaine, J.; Parker, C.; Bao, Y.; Rasco, J.F. Surface charge and dosage dependent potential developmental toxicity and biodistribution of iron oxide nanoparticles in pregnant CD-1 mice. Reprod. Toxicol. 2014, 50, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Bellusci, M.; La Barbera, A.; Padella, F.; Mancuso, M.; Pasquo, A.; Grollino, M.G.; Leter, G.; Nardi, E.; Cremisini, C.; Giardullo, P.; et al. Biodistribution and acute toxicity of a nanofluid containing manganese iron oxide nanoparticles produced by a mechanochemical process. Int. J. Nanomed. 2014, 9, 1919–1929. [Google Scholar]
- Kim, J.S.; Yoon, T.J.; Yu, K.N.; Kim, B.G.; Park, S.J.; Kim, H.W.; Lee, K.H.; Park, S.B.; Lee, J.K.; Cho, M.H. Toxicity and tissue distribution of magnetic nanoparticles in mice. Toxicol. Sci. 2006, 89, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Ganong, W.F. Circumventricular organs: Definition and role in the regulation of endocrine and autonomic function. Clin. Exp. Pharmacol. Physiol. 2000, 27, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Li, D.; Sun, H.; Guo, X.; Chen, Y.; Zhou, S. Quantitative control of active targeting of nanocarriers to tumor cells through optimization of folate ligand density. Biomaterials 2014, 35, 8015–8027. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, Y.; Chen, B.; Ding, J.; Xia, G.; Gao, C.; Cheng, J.; Jin, N.; Zhou, Y.; Li, X.; et al. Pharmacokinetic parameters and tissue distribution of magnetic Fe3O4 nanoparticles in mice. Int. J. Nanomed. 2010, 5, 861–866. [Google Scholar]
- Boehm, I. Magnetic resonance cell-tracking studies: Spectrophotometry-based method for the quantification of cellular iron content after loading with superparamagnetic iron oxide nanoparticles. Mol. Imaging 2011, 10, 270–277. [Google Scholar]
- Hellborg, R.; Skog, G. Accelerator mass spectrometry. Mass Spectrom. Rev. 2008, 27, 398–427. [Google Scholar] [CrossRef]
- Malfatti, M.A.; Palko, H.A.; Kuhn, E.A.; Turteltaub, K.W. Determining the pharmacokinetics and long-term biodistribution of SiO2 nanoparticles in vivo using accelerator mass spectrometry. Nano Lett. 2012, 12, 5532–5538. [Google Scholar] [CrossRef] [PubMed]
- Carter, P. Spectrophotometric determination of serum iron at the submicrogram level with a new reagent (ferrozine). Anal. Biochem. 1971, 40, 450–458. [Google Scholar] [CrossRef]
- Viollier, E.; Inglett, P.W.; Hunter, K.; Roychoudhury, A.N.; van Cappellen, P. The ferrozine method revisited: Fe(II)/Fe(III) determination in natural waters. Appl. Geochem. 2000, 15, 785–790. [Google Scholar] [CrossRef]
- Im, J.; Lee, J.; Löffler, F.E. Interference of ferric ions with ferrous iron quantification using the ferrozine assay. J. Microbiol. Methods 2013, 95, 366–367. [Google Scholar] [CrossRef] [PubMed]
- Anastácio, A.S.; Harris, B.; Yoo, H.-I.; Fabris, J.D.; Stucki, J.W. Limitations of the ferrozine method for quantitative assay of mineral systems for ferrous and total iron. Geochim. Cosmochim. Acta 2008, 72, 5001–5008. [Google Scholar] [CrossRef]
- Wu, Y.J.; Muldoon, L.L.; Varallyay, C.; Markwardt, S.; Jones, R.E.; Neuwelt, E.A. In vivo leukocyte labeling with intravenous ferumoxides/protamine sulfate complex and in vitro characterization for cellular magnetic resonance imaging. Am. J. Physiol. Cell Physiol. 2007, 293, C1698–C1708. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cuschieri, A. Tumour cell labelling by magnetic nanoparticles with determination of intracellular iron content and spatial distribution of the intracellular iron. Int. J. Mol. Sci. 2013, 14, 9111–9125. [Google Scholar] [CrossRef] [PubMed]
- Gramoun, A.; Crowe, L.A.; Maurizi, L.; Wirth, W.; Tobalem, F.; Grosdemange, K.; Coullerez, G.; Eckstein, F.; Koenders, M.I.; van den Berg, W.B.; et al. Monitoring the effects of dexamethasone treatment by MRI using in vivo iron oxide nanoparticle-labeled macrophages. Arthritis Res. Ther. 2014, 16, R131. [Google Scholar] [CrossRef] [PubMed]
- Scharlach, C.; Kratz, H.; Wiekhorst, F.; Warmuth, C.; Schnorr, J.; Genter, G.; Ebert, M.; Mueller, S.; Schellenberger, E. Synthesis of acid-stabilized iron oxide nanoparticles and comparison for targeting atherosclerotic plaques: Evaluation by MRI, quantitative MPS, and TEM alternative to ambiguous Prussian blue iron staining. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1085–1095. [Google Scholar] [CrossRef] [PubMed]
- Langheinrich, A.C.; Michniewicz, A.; Sedding, D.G.; Lai, B.; Jorgensen, S.M.; Bohle, R.M.; Ritman, E.L. Quantitative X-ray imaging of intraplaque hemorrhage in aortas of apoE−/−/LDL−/− double knockout mice. Investig. Radiol. 2007, 42, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Di Corato, R.; Bigall, N.C.; Ragusa, A.; Dorfs, D.; Genovese, A.; Marotta, R.; Manna, L.; Pellegrino, T. Multifunctional nanobeads based on quantum dots and magnetic nanoparticles: Synthesis and cancer cell targeting and sorting. ACS Nano 2011, 5, 1109–1121. [Google Scholar] [CrossRef] [PubMed]
- Rosca, E.V.; Wright, M.; Gonitel, R.; Gedroyc, W.; Miller, A.D.; Thanou, M. Thermosensitive, near infrared-labelled nanoparticles for topotecan delivery to tumours. Mol. Pharm. 2015, 12, 1335–1346. [Google Scholar] [CrossRef] [PubMed]
- Galbiati, E.; Cassani, M.; Verderio, P.; Martegani, E.; Colombo, M.; Tortora, P.; Mazzucchelli, S.; Prosperi, D. Peptide-nanoparticle ligation mediated by cutinase fusion for the development of cancer cell-targeted nanoconjugates. Bioconjug. Chem. 2015, 26, 680–689. [Google Scholar] [CrossRef]
- Shim, W.; Paik, M.J.; Nguyen, D.-T.; Lee, J.-K.; Lee, Y.; Kim, J.-H.; Shin, E.-H.; Kang, J.S.; Jung, H.-S.; Choi, S.; et al. Analysis of changes in gene expression and metabolic profiles induced by silica-coated magnetic nanoparticles. ACS Nano 2012, 6, 7665–7680. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Kim, B.; Shin, J.-Y.; Ryu, S.; Noh, M.; Woo, J.; Park, J.-S.; Lee, Y.; Lee, N.; Hyeon, T.; et al. Iron oxide nanoparticle-mediated development of cellular gap junction crosstalk to improve mesenchymal stem cells’ therapeutic efficacy for myocardial infarction. ACS Nano 2015, 9, 2805–2819. [Google Scholar] [CrossRef] [PubMed]
- Vanhecke, D.; Rodriguez-Lorenzo, L.; Clift, M.J.; Blank, F.; Petri-Fink, A.; Rothen-Rutishauser, B. Quantification of nanoparticles at the single-cell level: An overview about state-of-the-art techniques and their limitations. Nanomedicine 2014, 9, 1885–1900. [Google Scholar] [CrossRef] [PubMed]
- Graves, E.E.; Ripoll, J.; Weissleder, R.; Ntziachristos, V. A submillimeter resolution fluorescence molecular imaging system for small animal imaging. Med. Phys. 2003, 30, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Jiang, H. Diffuse optical tomography guided quantitative fluorescence molecular tomography. Appl. Opt. 2008, 47, 2011–2016. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Cao, Z.; Sajja, H.K.; Lipowska, M.; Wang, Y.A.; Yang, L.; Jiang, H. DOT corrected fluorescence molecular tomography using targeted contrast agents for small animal tumor imaging. J. X-ray Sci. Technol. 2013, 21, 43–52. [Google Scholar]
- Liu, W.; Frank, J.A. Detection and quantification of magnetically labeled cells by cellular MRI. Eur. J. Radiol. 2009, 70, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, Y.; Zhang, C. A concise review of magnetic resonance molecular imaging of tumor angiogenesis by targeting integrin αvβ3 with magnetic probes. Int. J. Nanomed. 2013, 8, 1083–1093. [Google Scholar]
- Young, I.R.; Cox, I.J.; Bryant, D.J.; Bydder, G.M. The benefits of increasing spatial resolution as a means of reducing artifacts due to field inhomogeneities. Magn. Reson. Imaging 1988, 6, 585–590. [Google Scholar] [CrossRef]
- Frahm, J.; Merboldt, K.D.; Hanicke, W. Direct FLASH MR imaging of magnetic field inhomogeneities by gradient compensation. Magn. Reson. Med. 1988, 6, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Kuhlpeter, R.; Dahnke, H.; Matuszewski, L.; Persigehl, T.; von Wallbrunn, A.; Allkemper, T.; Heindel, W.L.; Schaeffter, T.; Bremer, C. R2 and R2* mapping for sensing cell-bound superparamagnetic nanoparticles: In vitro and murine in vivo testing. Radiology 2007, 245, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Walczak, P.; Kedziorek, D.A.; Gilad, A.A.; Barnett, B.P.; Bulte, J.W. Applicability and limitations of MR tracking of neural stem cells with asymmetric cell division and rapid turnover: The case of the shiverer dysmyelinated mouse brain. Magn. Reson. Med. 2007, 58, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, L.; Mejias, R.; Barber, D.F.; Veintemillas-Verdaguer, S.; Serna, C.J.; Lazaro, F.J.; Morales, M.P. Ac magnetic susceptibility study of in vivo nanoparticle biodistribution. J. Phys. D Appl. Phys. 2011, 44, 255002. [Google Scholar] [CrossRef]
- Mejías, R.; Gutiérrez, L.; Salas, G.; Pérez-Yagüe, S.; Zotes, T.M.; Lázaro, F.J.; Morales, M.P.; Barber, D.F. Long term biotransformation and toxicity of dimercaptosuccinic acid-coated magnetic nanoparticles support their use in biomedical applications. J. Control. Release 2013, 171, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Maurizi, L.; Sakulkhu, U.; Gramoun, A.; Vallee, J.-P.; Hofmann, H. A fast and reproducible method to quantify magnetic nanoparticle biodistribution. Analyst 2014, 139, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Zysler, R.D.; Lima, E., Jr.; Vasquez Mansilla, M.; Troiani, H.E.; Mojica Pisciotti, M.L.; Gurman, P.; Lamagna, A.; Colombo, L. A new quantitative method to determine the uptake of SPIONs in animal tissue and its application to determine the quantity of nanoparticles in the liver and lung of Balb-c mice exposed to the SPIONs. J. Biomed. Nanotechnol. 2013, 9, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Dobosz, B.; Krzyminiewski, R.; Schroeder, G.; Kurczewska, J. Electron paramagnetic resonance as an effective method for a characterization of functionalized iron oxide. J. Phys. Chem. Solids 2014, 75, 594–598. [Google Scholar] [CrossRef]
- Chertok, B.; Cole, A.J.; David, A.E.; Yang, V.C. Comparison of electron spin resonance spectroscopy and inductively-coupled plasma optical emission spectroscopy for biodistribution analysis of iron-oxide nanoparticles. Mol. Pharm. 2010, 7, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Hoopes, P.J.; Petryk, A.A.; Gimi, B.; Giustini, A.J.; Weaver, J.B.; Bischof, J.; Chamberlain, R.; Garwood, M. In vivo imaging and quantification of iron oxide nanoparticle uptake and biodistribution. Proc. SPIE 2012. [Google Scholar] [CrossRef]
- Wang, L.; Corum, C.A.; Idiyatullin, D.; Garwood, M.; Zhao, Q. T1 estimation for aqueous iron oxide nanoparticle suspensions using a variable flip angle SWIFT sequence. Magn. Reson. Med. 2013, 70, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Gleich, B.; Weizenecker, J. Tomographic imaging using the nonlinear response of magnetic particles. Nature 2005, 435, 1214–1217. [Google Scholar] [CrossRef] [PubMed]
- Loewa, N.; Wiekhorst, F.; Gemeinhardt, I.; Ebert, M.; Schnorr, J.; Wagner, S.; Taupitz, M.; Trahms, L. Cellular uptake of magnetic nanoparticles quantified by magnetic particle spectroscopy. Magn. IEEE Trans. 2013, 49, 275–278. [Google Scholar] [CrossRef]
- Wang, H.; Kumar, R.; Nagesha, D.; Duclos, R.I., Jr.; Sridhar, S.; Gatley, S.J. Integrity of 111In-radiolabeled superparamagnetic iron oxide nanoparticles in the mouse. Nucl. Med. Biol. 2015, 42, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Bargheer, D.; Giemsa, A.; Freund, B.; Heine, M.; Waurisch, C.; Stachowski, G.M.; Hickey, S.G.; Eychmuller, A.; Heeren, J.; Nielsen, P. The distribution and degradation of radiolabeled superparamagnetic iron oxide nanoparticles and quantum dots in mice. Beilstein J. Nanotechnol. 2015, 6, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.R.; Frey, W.; Emelianov, S. Quantitative photoacoustic imaging of nanoparticles in cells and tissues. ACS Nano 2013, 7, 1272–1280. [Google Scholar] [CrossRef] [PubMed]
- Freund, B.; Tromsdorf, U.I.; Bruns, O.T.; Heine, M.; Giemsa, A.; Bartelt, A.; Salmen, S.C.; Raabe, N.; Heeren, J.; Ittrich, H.; et al. A simple and widely applicable method to 59Fe-radiolabel monodisperse superparamagnetic iron oxide nanoparticles for in vivo quantification studies. ACS Nano 2012, 6, 7318–7325. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Hong, H.; Grailer, J.J.; Rowland, I.J.; Javadi, A.; Hurley, S.A.; Xiao, Y.; Yang, Y.; Zhang, Y.; Nickles, R.J.; et al. cRGD-functionalized, DOX-conjugated, and 64Cu-labeled superparamagnetic iron oxide nanoparticles for targeted anticancer drug delivery and PET/MR imaging. Biomaterials 2011, 32, 4151–4160. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, D.; Sun, M.; Yang, L.; McDonagh, P.R.; Corwin, F.; Sundaresan, G.; Wang, L.; Vijayaragavan, V.; Thadigiri, C.; Lamichhane, N.; et al. Intrinsically radiolabelled [59Fe]-SPIONs for dual MRI/radionuclide detection. Am. J. Nucl. Med. Mol. Imaging 2014, 4, 548–560. [Google Scholar]
- Chakravarty, R.; Valdovinos, H.F.; Chen, F.; Lewis, C.M.; Ellison, P.A.; Luo, H.; Meyerand, M.E.; Nickles, R.J.; Cai, W. Intrinsically germanium-69-labeled iron oxide nanoparticles: Synthesis and in vivo dual-modality PET/MR imaging. Adv. Mater. 2014, 26, 5119–5123. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.Q.; Nothdurft, R.E.; Erpelding, T.N.; Wang, L.V.; Culver, J.P. Quantitative photoacoustic imaging: Correcting for heterogeneous light fluence distributions using diffuse optical tomography. J. Biomed. Opt. 2011, 16, 096016. [Google Scholar] [CrossRef] [PubMed]
- Vetten, M.A.; Yah, C.S.; Singh, T.; Gulumian, M. Challenges facing sterilization and depyrogenation of nanoparticles: Effects on structural stability and biomedical applications. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1391–1399. [Google Scholar] [CrossRef] [PubMed]
- Pirani, P.; Patil, U.; Apsunde, T.; Trudell, M.; Cai, Y.; Tarr, M. Protein surface labeling reactivity of N-hydroxysuccinimide esters conjugated to Fe3O4@SiO2 magnetic nanoparticles. J. Nanopart. Res. 2015, 17, 1–11. [Google Scholar] [CrossRef]
- Oberdorster, G.; Oberdorster, E.; Oberdorster, J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005, 113, 823–839. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Manshian, B.; Jenkins, G.J.S.; Griffiths, S.M.; Williams, P.M.; Maffeis, T.G.G.; Wright, C.J.; Doak, S.H. NanoGenotoxicology: The DNA damaging potential of engineered nanomaterials. Biomaterials 2009, 30, 3891–3914. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patil, U.S.; Adireddy, S.; Jaiswal, A.; Mandava, S.; Lee, B.R.; Chrisey, D.B. In Vitro/In Vivo Toxicity Evaluation and Quantification of Iron Oxide Nanoparticles. Int. J. Mol. Sci. 2015, 16, 24417-24450. https://doi.org/10.3390/ijms161024417
Patil US, Adireddy S, Jaiswal A, Mandava S, Lee BR, Chrisey DB. In Vitro/In Vivo Toxicity Evaluation and Quantification of Iron Oxide Nanoparticles. International Journal of Molecular Sciences. 2015; 16(10):24417-24450. https://doi.org/10.3390/ijms161024417
Chicago/Turabian StylePatil, Ujwal S., Shiva Adireddy, Ashvin Jaiswal, Sree Mandava, Benjamin R. Lee, and Douglas B. Chrisey. 2015. "In Vitro/In Vivo Toxicity Evaluation and Quantification of Iron Oxide Nanoparticles" International Journal of Molecular Sciences 16, no. 10: 24417-24450. https://doi.org/10.3390/ijms161024417
APA StylePatil, U. S., Adireddy, S., Jaiswal, A., Mandava, S., Lee, B. R., & Chrisey, D. B. (2015). In Vitro/In Vivo Toxicity Evaluation and Quantification of Iron Oxide Nanoparticles. International Journal of Molecular Sciences, 16(10), 24417-24450. https://doi.org/10.3390/ijms161024417