Protein/Arabinoxylans Gels: Effect of Mass Ratio on the Rheological, Microstructural and Diffusional Characteristics
Abstract
:1. Introduction
2. Results and Discussion
2.1. WBAX and Protein/WBAX Gel
| Characteristic | t = 0 h | t = 6 h |
|---|---|---|
| FA a | 0.020 ± 0.001 | 0.006 ± 0.001 |
| di-FA a | 0.020 ± 0.001 | 0.008 ± 0.001 |
| tri-FA a | 0.0005 ± 0.001 | 0.0003 ± 0.0001 |
| G' b | 4 | 177 ± 3 |
| G'' b | 6 | 20 ± 1 |
| Mc c × 103 | - | 29 ± 1 |
| ρcd × 10−6 | - | 59 ± 2 |
| ξ e | - | 57 ± 8 |
| Characteristic | Insulin/WBAX Mass Ratio | ||||
|---|---|---|---|---|---|
| 0.06 | 0.12 | 0.25 | 0.50 | 1.0 | |
| FA a | 0.005 ± 0.001 | 0.006 ± 0.001 | 0.005 ± 0.001 | 0.006 ± 0.001 | 0.005 ± 0.001 |
| di-FA a | 0.007 ± 0.001 | 0.008 ± 0.001 | 0.007 ± 0.001 | 0.007 ± 0.001 | 0.007 ± 0.001 |
| tri-FA a | 0.0002 ± 0.0001 | 0.0003 ± 0.0001 | 0.0002 ± 0.0001 | 0.0002 ± 0.0001 | 0.0002 ± 0.0001 |
| G' b | 178 ± 4 | 176 ± 4 | 165 ± 3 | 155 ± 3 | 145 ± 4 |
| G'' b | 21 ± 2 | 20 ± 2 | 19 ± 2 | 18 ± 3 | 16 ± 2 |
| Ovalbumin/WBAX Mass Ratio | |||||
| 0.06 | 0.12 | 0.25 | 0.50 | 1.0 | |
| FA a | 0.005 ± 0.001 | 0.005 ± 0.001 | 0.005 ± 0.001 | 0.006 ± 0.001 | 0.006 ± 0.001 |
| di-FA a | 0.007 ± 0.001 | 0.006 ± 0.001 | 0.007 ± 0.001 | 0.007 ± 0.001 | 0.006 ± 0.001 |
| tri-FA a | 0.0003 ± 0.0001 | 0.0003 ± 0.0001 | 0.0002 ± 0.0001 | 0.0003 ± 0.0001 | 0.0002 ± 0.0001 |
| G' b | 176 ± 4 | 177 ± 3 | 160 ± 3 | 158 ± 3 | 140 ± 3 |
| G'' b | 20 ± 2 | 20 ± 3 | 19 ± 2 | 17 ± 3 | 17 ± 2 |
| BSA/WBAX Mass Ratio | |||||
| 0.06 | 0.12 | 0.25 | 0.50 | 1.0 | |
| FA a | 0.006 ± 0.001 | 0.006 ± 0.001 | 0.006 ± 0.001 | 0.005 ± 0.001 | 0.006 ± 0.001 |
| di-FA a | 0.007 ± 0.001 | 0.006 ± 0.001 | 0.008 ± 0.001 | 0.006 ± 0.001 | 0.007 ± 0.001 |
| tri-FA a | 0.0002 ± 0.0001 | 0.0002 ± 0.0001 | 0.0002 ± 0.0001 | 0.0002 ± 0.0001 | 0.0003 ± 0.0001 |
| G' b | 176 ± 5 | 174 ± 5 | 165± 3 | 155 ± 4 | 138 ± 5 |
| G'' b | 20 ± 2 | 21 ± 3 | 18 ± 2 | 16 ± 3 | 15 ± 2 |
2.2. Protein Distribution in the Gels
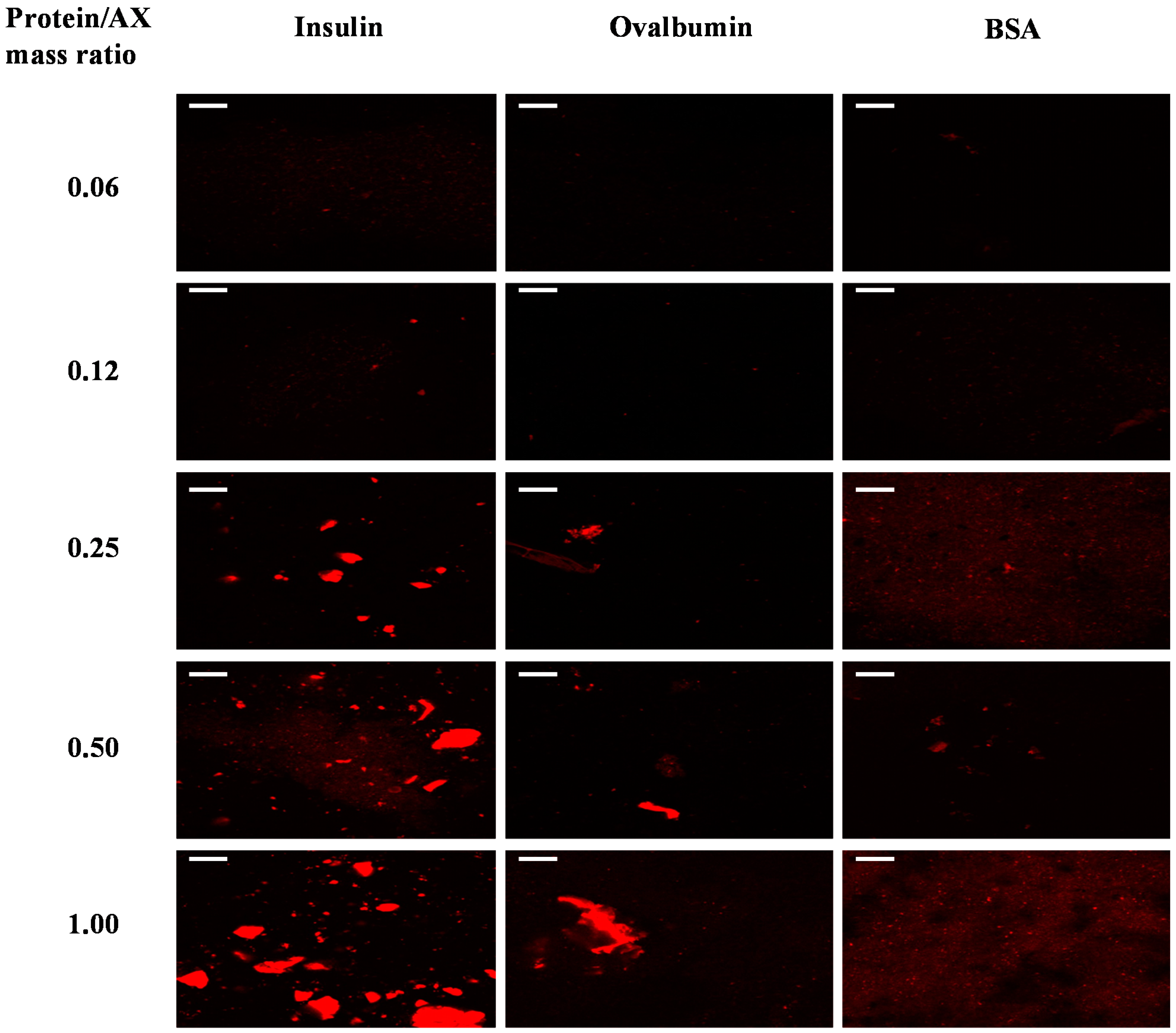
2.3. Protein Release
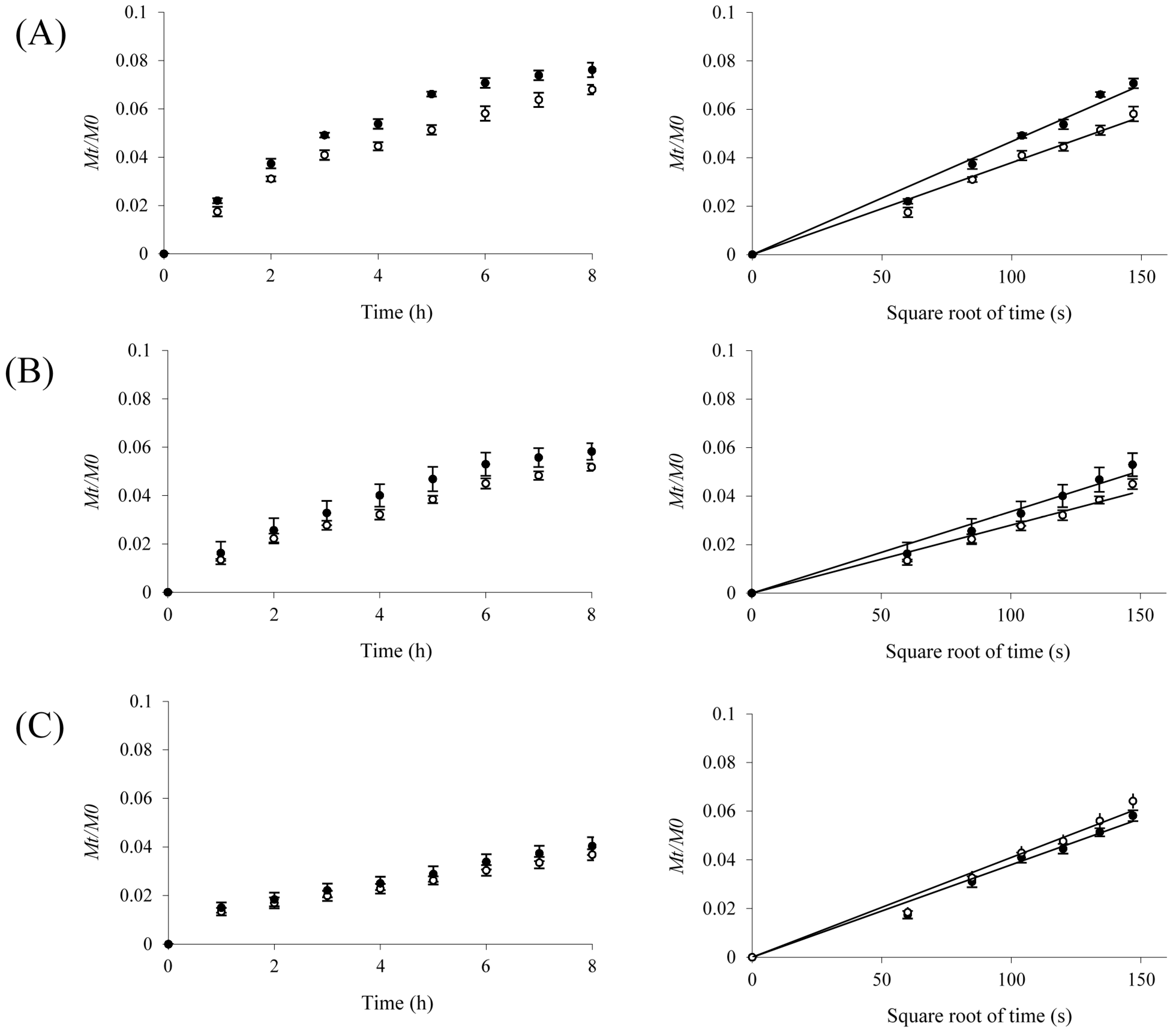
| Protein | MW a (kDa) | Do b × 10−7 (cm2/s) | Dm c × 10−7 (cm2/s) | Protein Released (%) | ||
|---|---|---|---|---|---|---|
| Protein/WBAX Mass Ratio | Protein/WBAX Mass Ratio | |||||
| 0.06 | 0.12 | 0.06 | 0.12 | |||
| Insulin | 5 | 15.9 | 2.64 ± 0.07 | 3.20 ± 0.06 | 6.2 ± 0.4 | 7.5 ± 0.5 |
| Ovalbumin | 43 | 8.40 | 1.47 ± 0.01 | 1.49 ± 0.04 | 5.1 ± 0.5 | 4.4 ± 0.3 |
| BSA d | 67 | 6.80 | 1.20 ± 0.01 | 1.25 ± 0.03 | 3.5 ± 0.2 | 3.2 ± 0.3 |
3. Experimental Section
3.1. Materials
3.2. Preparation of WBAX Gels
3.3. Phenolic Acids Content
3.4. Rheological Tests
3.5. WBAX Gel Swelling
3.6. WBAX Gel Structure
3.7. Preparation of Protein/WBAX Gels
3.8. CLSM
3.9. Protein Release
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Peppas, N.A.; Khare, A.R. Preparation, structure and diffusional behavior of hydrogels in controlled release. Adv. Drug Deliv. Rev. 1993, 11, 1–35. [Google Scholar]
- Peppas, N.A.; Bures, P.; Leobandung, W.; Ichikawa, H. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar]
- Rodríguez, R.; Alvarez-Lorenzo, C.; Concheiro, A. Cationic cellulose hydrogels: Kinetics of the cross-linking process and characterization as pH-/ion-sensitive drug delivery systems. J. Control. Release 2003, 86, 253–265. [Google Scholar]
- González-Rodríguez, M.L.; Holgado, M.A.; Sánchez-Lafuente, C.; Rabasco, A.M.; Fini, A. Alginate/chitosan particulate systems for sodium diclofenac release. Int. J. Pharm. 2002, 232, 225–234. [Google Scholar]
- Hennink, W.E.; van Nostrum, C.F. Novel crosslinking methods to design hydrogels. Adv. Drug Deliv. Rev. 2002, 54, 13–36. [Google Scholar]
- Mulhbacher, J.; Ispas-Szabo, P.; Lenaerts, V.; Mateescu, M.A. Cross-linked high amylose starch derivatives as matrices for controlled release of high drug loadings. J. Control. Release 2001, 76, 51–58. [Google Scholar]
- Brondsted, H.; Hovgaard, L.; Simonsen, L. Dextran hydrogels for colon-specific drug delivery. 2. Synthesis and characterization. Eur. J. Pharm. Biopharm. 1996, 42, 85–89. [Google Scholar]
- Van Laere, K.M.J.; Hartemink, R.; Bosveld, M.; Schols, H.A.; Voragen, A.G.J. Fermentation of plant cell wall derived polysaccharides and their corresponding oligosaccharides by intestinal bacteria. J. Agric. Food Chem. 2000, 48, 1644–1652. [Google Scholar] [CrossRef]
- Hopkins, M.J.; Englyst, H.N.; Macfarlane, S.; Furrie, E.; Macfarlane, G.T.; McBain, A.J. Degradation of cross-linked and non-cross-linked arabinoxylans by the intestinal microbiota in children. Appl. Environ. Microbiol. 2003, 69, 6354–6360. [Google Scholar] [CrossRef]
- Rascón-Chu, A.; Martínez-López, A.L.; Berlanga-Reyes, C.; Carvajal-Millan, E.; Campa-Mada, A.; Gardea, A.; Orozco-Avitia, A. Arabinoxylans gels as lycopene carriers: In vitro degradation by colonic bacteria. MRS Online Proc. Libr. 2012, 1487. imrc12-s4b-p035. [Google Scholar] [CrossRef]
- Izydorczyk, M.S.; Biliaderis, C.G. Cereal arabinoxylans: Advances in structure and physicochemical properties. Carbohydr. Polym. 1995, 28, 33–48. [Google Scholar]
- Geissman, T.; Neukom, H. On the composition of the water-soluble wheat flour pentosans and their oxidative gelation. Lebensm.-Wiss. Technol. 1973, 6, 59–62. [Google Scholar]
- Hoseney, R.C.; Faubion, J.M. A mechanism for the oxidative gelation of wheat flour water-soluble pentosans. Cereal Chem. 1981, 58, 421–424. [Google Scholar]
- Izydorczyk, M.S.; Biliaderis, C.G.; Bushuk, W. Oxidative gelation studies of water-soluble pentosans from wheat. J. Cereal Sci. 1990, 11, 153–169. [Google Scholar]
- Figueroa-Espinoza, M.C.; Rouau, X. Oxidative cross-linking of pentosans by a fungal laccase and horseradish peroxidase: Mechanism of linkage between feruloylated arabinoxylans. Cereal Chem. 1998, 75, 259–265. [Google Scholar] [CrossRef]
- Figueroa-Espinoza, M.C.; Morel, M.H.; Rouau, X. Effect of lysine, tyrosine, cysteine, and glutathione on the oxidative cross-linking of feruloylated arabinoxylans by a fungal laccase. J. Agric. Food Chem. 1998, 46, 2583–2589. [Google Scholar] [CrossRef]
- Schooneveld-Bergmans, M.E.F.; Dignum, M.J.W.; Grabber, J.H.; Beldman, G.; Voragen, A.G.J. Studies on the oxidative cross-linking of feruloylated arabinoxylans from wheat flour and wheat bran. Carbohydr. Polym. 1999, 38, 309–317. [Google Scholar] [CrossRef]
- Vansteenkiste, E.; Babot, C.; Rouau, X.; Micard, V. Oxidative gelation of feruloylated arabinoxylan as affected by protein. Influence on protein enzymatic hydrolysis. Food Hydrocoll. 2004, 18, 557–564. [Google Scholar] [CrossRef]
- Carvajal-Millan, E.; Guigliarelli, B.; Belle, V.; Rouau, X.; Micard, V. Storage stability of laccase induced arabinoxylan gels. Carbohydr. Polym. 2005, 59, 181–188. [Google Scholar] [CrossRef]
- Carvajal-Millan, E.; Landillon, V.; Morel, M.H.; Rouau, X.; Doublier, J.L.; Micard, V. Arabinoxylan gels: Impact of the feruloylation degree on their structure and properties. Biomacromolecules 2005, 6, 309–317. [Google Scholar] [CrossRef]
- Berlanga-Reyes, C.M.; Carvajal-Millan, E.; Caire-Juvera, G.; Rascón-Chu, A.; Marquez-Escalante, J.A.; Martínez-López, A.L. Laccase induced maize bran arabinoxylan gels: Structural and rheological properties. J. Food Sci. Biotechnol. 2009, 18, 1027–1029. [Google Scholar]
- Martínez-López, A.L.; Carvajal-Millan, E.; Lizardi-Mendoza, J.; López-Franco, Y.L.; Rascón-Chu, A.; Salas-Muñoz, E.; Barron, C.; Micard, V. The peroxidase/H2O2 system as a free radical-generating agent for gelling maize bran arabinoxylans: Rheological and structural properties. Molecules 2011, 16, 8410–8418. [Google Scholar]
- Ross-Murphy, S.B. Rheological methods. In Biophysical Methods in Food Research (Critical Reports on Applied Chemistry); Chan, H.W.-S., Ed.; SCI Blackwell: Oxford, UK, 1984; Volume 5, pp. 138–199. [Google Scholar]
- Lapierre, C.; Pollet, B.; Ralet, M.C.; Saulnier, L. The phenolic fraction of maize bran: Evidence for lignin-heteroxylan association. Phytochemistry 2001, 57, 765–772. [Google Scholar]
- Carvajal-Millan, E.; Rascón-Chu, A.; Márquez-Escalante, J.; Ponce de León, N.; Micard, V.; Gardea, A. Maize bran gum: Extraction, characterization and functional properties. Carbohydr. Polym. 2007, 69, 280–285. [Google Scholar] [CrossRef]
- Berlanga-Reyes, C.M.; Carvajal-Millan, E.; Lizardi-Mendoza, J.; Islas-Rubio, A.R.; Rascón-Chu, A. Enzymatic cross-linking of alkali extracted arabinoxylans: Gel rheological and structural characteristics. Int. J. Mol. Sci. 2011, 12, 5853–5861. [Google Scholar] [CrossRef]
- Carvajal-Millan, E.; Guilbert, S.; Morel, M.H.; Micard, V. Impact of the structure of arabinoxylan gels on their rheological and protein transport properties. Carbohydr. Polym. 2005, 60, 431–438. [Google Scholar] [CrossRef]
- Berlanga-Reyes, C.M.; Carvajal-Millan, E.; Lizardi-Mendoza, J.; Rascón-Chu, A.; Marquez-Escalante, J.A.; Martínez-López, A.L. Maize arabinoxylan gels as protein delivery matrices. Molecules 2009, 14, 1475–1482. [Google Scholar] [CrossRef]
- Carvajal-Millan, E.; Guilbert, S.; Doublier, J.L.; Micard, V. Arabinoxylan/protein gels: Structural, rheological and controlled release properties. Food Hydrocoll. 2006, 20, 53–61. [Google Scholar] [CrossRef]
- Grinberg, V.Y.; Tolstoguzov, V.B. Thermodynamic incompatibility of proteins and polysaccharides in solutions. Food Hydrocoll. 1997, 11, 145–158. [Google Scholar]
- Amsden, B. Solute diffusion in hydrogels: An examination of the retardation effect. Polym. Gels Netw. 1998, 6, 13–43. [Google Scholar] [CrossRef]
- Cole, D.G.; Diener, D.R.; Himelblau, A.L.; Beech, P.L.; Fuster, J.C.; Rosenbaum, J.L. Chlamydomonas Kinesin-II-dependent intraflagellar transport (IFT): IFT particles contain proteins required for ciliary assembly in Caenorhabditis elegans sensory neurons. J. Cell Biol. 1998, 141, 993–1008. [Google Scholar] [CrossRef]
- Nauman, J.V.; Campbell, P.G.; Lanni, F.; Anderson, J.L. Diffusion of insulin-like growth factor-I and ribonuclease through fibrin gels. Biophys. J. 2007, 92, 4444–4450. [Google Scholar] [CrossRef]
- Gehrke, S.H.; Uhden, L.H.; McBride, J.F. Enhanced loading and activity retention of bioactive proteins in hydrogel delivery systems. J. Control. Release 1998, 55, 21–33. [Google Scholar] [CrossRef]
- Sinha, V.R.; Kumria, R. Polysaccharides in colon-specific drug delivery. Int. J. Pharm. 2001, 224, 19–38. [Google Scholar] [CrossRef]
- Saulnier, L.; Crépeau, M.J.; Lahaye, M.; Thibault, J.F.; Garcia-Conesa, M.T.; Kroon, P.A.; Williamson, G. Isolation and structural determination of two 5,5'-diferuloyl oligosaccharides indicate that maize heteroxylans are covalently cross-linked by oxidatively coupled ferulates. Carbohydr. Res. 1999, 320, 82–92. [Google Scholar] [CrossRef]
- Rouau, X.; Cheynier, V.; Surget, A.; Gloux, D.; Barron, C.; Meudec, E.; Montero, J.L.; Criton, M. A dehydrotrimer of ferulic acid from maize bran. Phytochemistry 2003, 63, 899–903. [Google Scholar] [CrossRef]
- Flory, P.J.; Rehner, J. Statistical mechanics of cross-linked polymer networks. II. Swelling. J. Chem. Phys. 1943, 11, 521–526. [Google Scholar] [CrossRef]
- Peppas, N.A.; Merrill, E.W. Poly(vinyl alcohol) hydrogels: Reinforcement of radiation-crosslinked networks by crystallization. J. Polym. Sci. 1976, 14, 441–457. [Google Scholar]
- Sheen, S.; Bao, G.; Cooke, P. Food surface texture measurement using reflective confocal laser scanning microscopy. J. Food Sci. 2008, 73, E227–E234. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Oxford University Press: London, UK, 1975; pp. 44–68. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berlanga-Reyes, C.M.; Carvajal-Millan, E.; Hicks, K.B.; Yadav, M.P.; Rascón-Chu, A.; Lizardi-Mendoza, J.; Toledo-Guillén, A.R.; Islas-Rubio, A.R. Protein/Arabinoxylans Gels: Effect of Mass Ratio on the Rheological, Microstructural and Diffusional Characteristics. Int. J. Mol. Sci. 2014, 15, 19106-19118. https://doi.org/10.3390/ijms151019106
Berlanga-Reyes CM, Carvajal-Millan E, Hicks KB, Yadav MP, Rascón-Chu A, Lizardi-Mendoza J, Toledo-Guillén AR, Islas-Rubio AR. Protein/Arabinoxylans Gels: Effect of Mass Ratio on the Rheological, Microstructural and Diffusional Characteristics. International Journal of Molecular Sciences. 2014; 15(10):19106-19118. https://doi.org/10.3390/ijms151019106
Chicago/Turabian StyleBerlanga-Reyes, Claudia M., Elizabeth Carvajal-Millan, Kevin B. Hicks, Madhav P. Yadav, Agustín Rascón-Chu, Jaime Lizardi-Mendoza, Alma R. Toledo-Guillén, and Alma R. Islas-Rubio. 2014. "Protein/Arabinoxylans Gels: Effect of Mass Ratio on the Rheological, Microstructural and Diffusional Characteristics" International Journal of Molecular Sciences 15, no. 10: 19106-19118. https://doi.org/10.3390/ijms151019106
APA StyleBerlanga-Reyes, C. M., Carvajal-Millan, E., Hicks, K. B., Yadav, M. P., Rascón-Chu, A., Lizardi-Mendoza, J., Toledo-Guillén, A. R., & Islas-Rubio, A. R. (2014). Protein/Arabinoxylans Gels: Effect of Mass Ratio on the Rheological, Microstructural and Diffusional Characteristics. International Journal of Molecular Sciences, 15(10), 19106-19118. https://doi.org/10.3390/ijms151019106







