Marine Macroalgal Polysaccharides in Nanomedicine: Blue Biotechnology Contributions in Advanced Therapeutics
Abstract
1. Introduction
2. Algal Biotechnology and Algal Polysaccharides
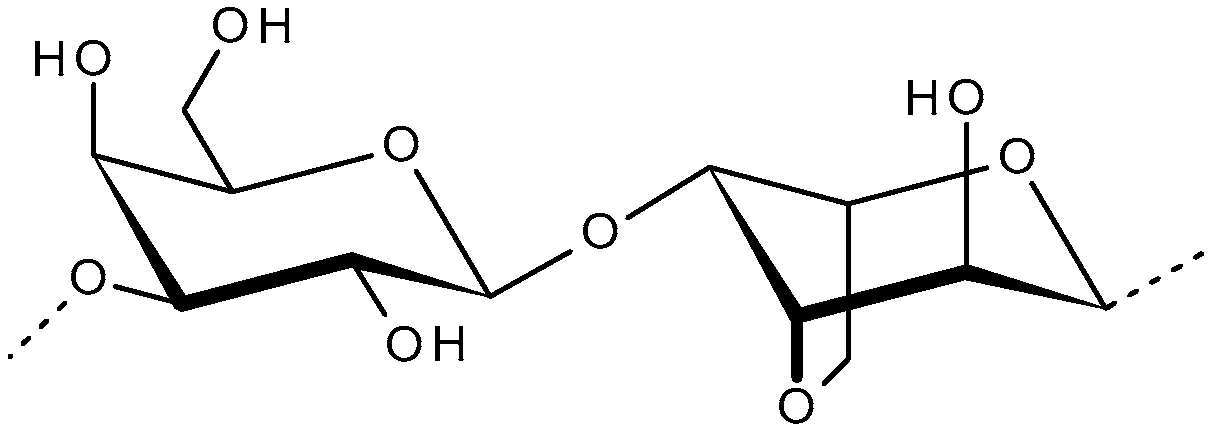
2.1. Agarans (Agar, Agarose, Funoran and Porphyran)
2.1.1. Agar and Agarose
2.1.2. Funoran
2.1.3. Phorphyran
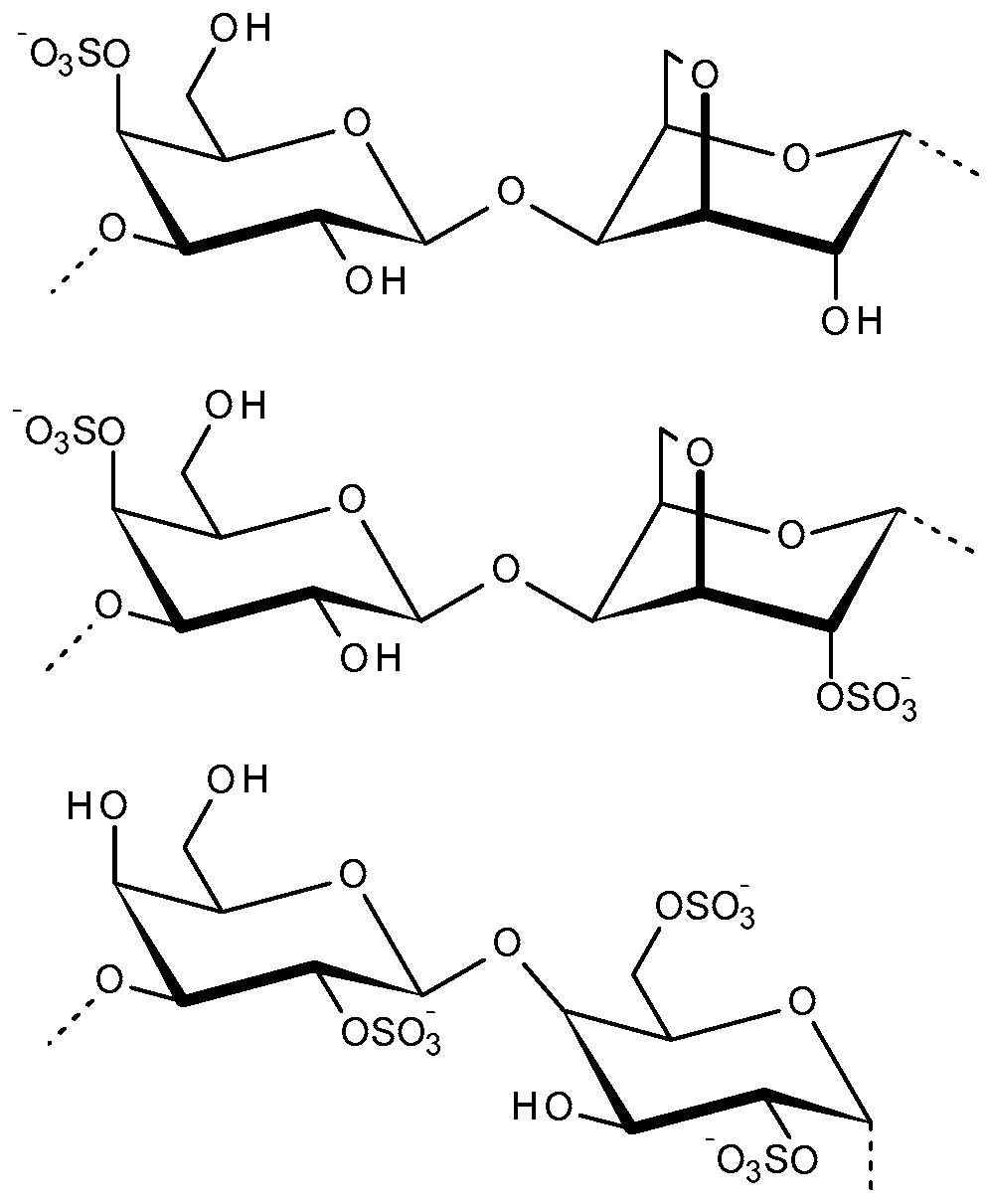
2.2. Carrageenans
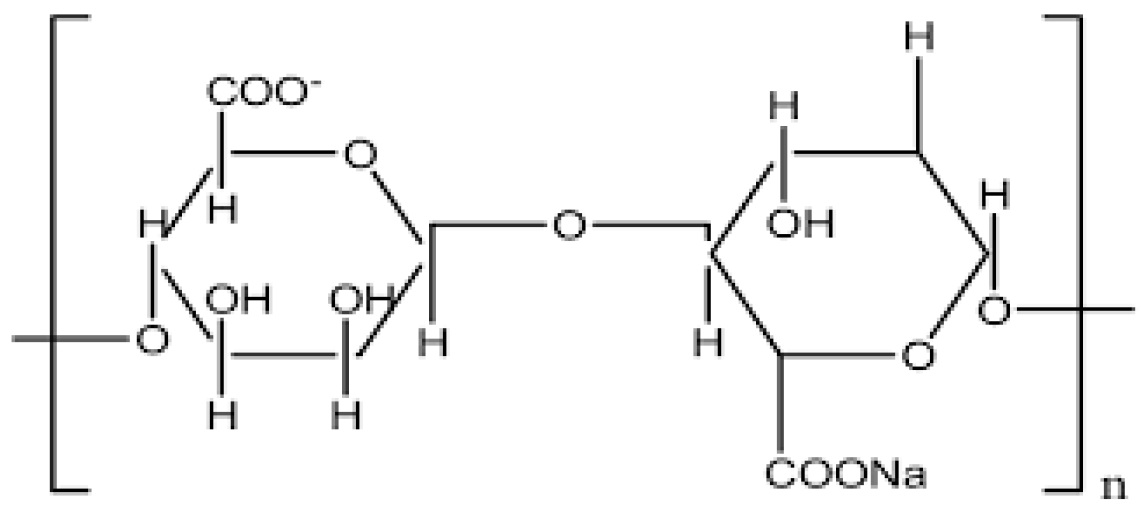
2.3. Alginate
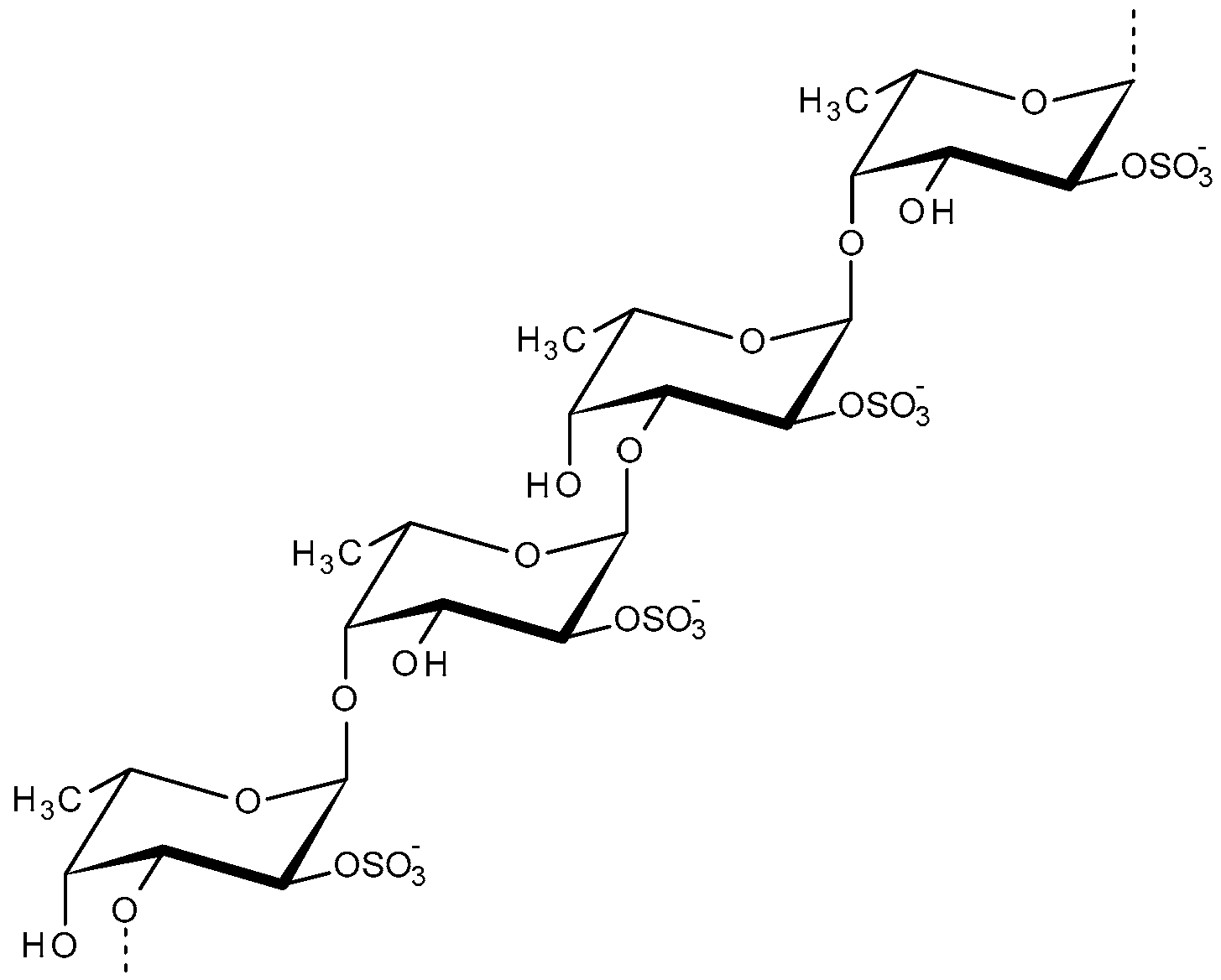
2.4. Fucoidan
3. Nanotechnology and Algae: Biosynthesis of Nanomaterials
4. Nanomedical Applications of Seaweed Polysaccharides
5. Recent Advances and Future Directions in Marine Polysaccharide Nanomedicine
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rioux, L.-E.; Beaulieu, L.; Turgeon, S.L. Seaweeds: A traditional ingredients for new gastronomic sensation. Food Hydrocoll. 2017, 68, 255–265. [Google Scholar] [CrossRef]
- Cardoso, S.M.; Pereira, O.R.; Seca, A.M.L.; Pinto, D.C.G.A.; Silva, A.M.S. Seaweeds as preventive agents for cardiovascular diseases: From nutrients to functional foods. Mar. Drugs 2015, 13, 6838–6865. [Google Scholar] [CrossRef] [PubMed]
- MacArtain, P.; Gill, C.I.; Brooks, M.; Campbell, R.; Rowland, I.R. Nutritional value of edible seaweeds. Nutr. Rev. 2007, 65, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Chapman, V. Seaweeds and Their Uses; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Ruperez, P. Mineral content of edible marine seaweeds. Food Chem. 2002, 79, 23–26. [Google Scholar] [CrossRef]
- Demarco, M.; de Moraes, J.O.; Matos, Â.P.; Derner, R.B.; Neves, F.d.F.; Tribuzi, G. Digestibility, bioaccessibility and bioactivity of compounds from algae. Trends Food Sci. Technol. 2022, 121, 114–128. [Google Scholar] [CrossRef]
- Burtin, P. Nutritional value of seaweeds. Electron. J. Environ. Agric. Food Chem. 2003, 2, 498–503. [Google Scholar]
- Yu, Y.; Shen, M.; Song, Q.; Xie, J. Biological activities and pharmaceutical applications of polysaccharide from natural resources: A review. Carbohydr. Polym. 2018, 183, 91–101. [Google Scholar] [CrossRef]
- Pereira, L.; Valado, A. Beyond Nutrition: The Therapeutic Promise of Seaweed-Derived Polysaccharides Against Bacterial and Viral Threats. Mar. Drugs 2025, 23, 407. [Google Scholar] [CrossRef]
- Krylova, N.V.; Kravchenko, A.O.; Likhatskaya, G.N.; Iunikhina, O.V.; Glazunov, V.P.; Zaporozhets, T.S.; Shchelkanov, M.Y.; Yermak, I.M. Carrageenans and the Carrageenan-Echinochrome Complex as Anti-SARS-CoV-2 Agents. Int. J. Mol. Sci. 2025, 26, 6175. [Google Scholar] [CrossRef]
- Bhuyan, P.P.; Nayak, R.; Patra, S.; Abdulabbas, H.S.; Jena, M.; Pradhan, B. Seaweed-derived sulfated polysaccharides; the new age chemopreventives: A comprehensive review. Cancers 2023, 15, 715. [Google Scholar] [CrossRef]
- Ouyang, Y.; Qiu, Y.; Liu, Y.; Zhu, R.; Chen, Y.; El-Seedi, H.R.; Chen, X.; Zhao, C. Cancer-fighting potentials of algal polysaccharides as nutraceuticals. Food Res. Int. 2021, 147, 110522. [Google Scholar] [CrossRef]
- Quach, T.T.M.; Nguyen, N.T.; Yuguchi, Y.; Do, X.T.T.; Nguyen, T.Q.; Thanh, T.T.T. Structural characteristics and cytotoxic activity of sulfated polysaccharide from green seaweed Codium geppiorum. Polym. Bull. 2023, 81, 6921–6934. [Google Scholar] [CrossRef]
- Sellimi, S.; Maalej, H.; Rekik, D.M.; Benslima, A.; Ksouda, G.; Hamdi, M.; Sahnoun, Z.; Li, S.; Nasri, M.; Hajji, M. Antioxidant, antibacterial and in vivo wound healing properties of laminaran purified from Cystoseira barbata seaweed. Int. J. Biol. Macromol. 2018, 119, 633–644. [Google Scholar] [CrossRef]
- Bai, R.G.; Tuvikene, R. Potential antiviral properties of industrially important marine algal polysaccharides and their significance in fighting a future viral pandemic. Viruses 2021, 13, 1817. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-C.; Hou, M.-F.; Huang, H.-W.; Chang, F.-R.; Yeh, C.-C.; Tang, J.-Y.; Chang, H.-W. Marine algal natural products with anti-oxidative, anti-inflammatory, and anti-cancer properties. Cancer Cell Int. 2013, 13, 55–57. [Google Scholar] [CrossRef]
- Kuznetsova, T.A.; Andryukov, B.G.; Besednova, N.N.; Zaporozhets, T.S.; Kalinin, A.V. Marine algae polysaccharides as basis for wound dressings, drug delivery, and tissue engineering: A review. J. Mar. Sci. Eng. 2020, 8, 481. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, Q.; Xu, W.; Yang, M.; Guo, W.; He, S.; Liu, W. Alginate-based hydrogels mediated biomedical applications: A review. Int. J. Biol. Macromol. 2024, 279, 135019. [Google Scholar] [CrossRef]
- Tian, J.; Peng, J.; Hu, C.; Lei, S.; Wu, D. A general strategy for prepared multifunction double-ions agarose hydrogel dressing promotes wound healing. Mater. Des. 2024, 240, 112854. [Google Scholar] [CrossRef]
- Mazurek, Ł.; Kuś, M.; Jurak, J.; Rybka, M.; Kuczeriszka, M.; Stradczuk-Mazurek, M.; Konop, M. Biomedical potential of alginate wound dressings—From preclinical studies to clinical applications: A review. Int. J. Biol. Macromol. 2025, 309, 142908. [Google Scholar] [CrossRef]
- Soleymani, H.; Ghorbani, M.; Sedghi, M.; Allahverdi, A.; Naderi-Manesh, H. Microfluidics single-cell encapsulation reveals that poly-l-lysine-mediated stem cell adhesion to alginate microgels is crucial for cell-cell crosstalk and its self-renewal. Int. J. Biol. Macromol. 2024, 274, 133418. [Google Scholar] [CrossRef] [PubMed]
- Borowitzka, M.A. Algal biotechnology. In The Algae World; Sahoo, D., Seckbach, J., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 319–338. [Google Scholar]
- Cannell, R.J.P. Algal biotechnology. Appl. Biochem. Biotechnol. 1990, 26, 85–105. [Google Scholar] [CrossRef]
- Stephen, C.; Joshi, L. Algal biotechnology: An emerging resource with diverse application and potential. In Transgenic crop plants; Springer: Berlin/Heidelberg, Germany, 2010; pp. 343–357. [Google Scholar]
- Dang, B.-T.; Bui, X.-T.; Tran, D.P.; Ngo, H.H.; Nghiem, L.D.; Hoang, T.-K.; Nguyen, P.-T.; Nguyen, H.H.; Vo, T.-K.; Lin, C.; et al. Current application of algae derivatives for bioplastic production: A review. Bioresour. Technol. 2022, 347, 126698. [Google Scholar] [CrossRef]
- Feng, Y.; Wassie, T.; Gan, R.; Wu, X. Structural characteristics and immunomodulatory effects of sulfated polysaccharides derived from marine algae. Crit. Rev. Food Sci. Nutr. 2022, 63, 7180–7196. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Yadav, D.; Lee, P.C.; Jin, J. Immunomodulatory effects of polysaccharides from marine algae for treating cancer, infectious disease, and inflammation. Phytother. Res. 2021, 36, 761–777. [Google Scholar] [CrossRef] [PubMed]
- Gomes-Dias, J.S.; Teixeira, J.A.; Rocha, C.M. Recent Advances in the Valorization of Algae Polysaccharides for Food and Nutraceutical Applications: A Review on the Role of Green Processing Technologies. Food Bioprocess Technol. 2022, 15, 1948–1976. [Google Scholar] [CrossRef]
- Nigam, S.; Singh, R.; Bhardwaj, S.K.; Sami, R.; Nikolova, M.P.; Chavali, M.; Sinha, S. Perspective on the therapeutic applications of algal polysaccharides. J. Polym. Environ. 2021, 30, 785–809. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.K.; Lim, Y.Y.; Leow, A.T.C.; Namasivayam, P.; Ong Abdullah, J.; Ho, C.L. Biosynthesis of agar in red seaweeds: A review. Carbohydr. Polym. 2017, 164, 23–30. [Google Scholar] [CrossRef]
- Oliveira, E.C.; Alveal, K.; Anderson, R.J. Mariculture of the agar-producing Gracilarioid red algae. Rev. Fish. Sci. 2000, 8, 345–377. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, X.; Duan, D.; Xu, J.; Gao, X. Preparation and characterization of agar, agarose, and agaropectin from the red alga Ahnfeltia plicata. J. Oceanol. Limnol. 2019, 37, 815–824. [Google Scholar] [CrossRef]
- Souza, J.M.C.; Yokoya, N.S. Effects of cytokinins on physiological and biochemical responses of the agar-producing red alga Gracilaria caudata (Gracilariales, Rhodophyta). J. Appl. Phycol. 2016, 28, 3491–3499. [Google Scholar] [CrossRef]
- Yang, E.C.; Kim, K.M.; Kim, S.Y.; Yoon, H.S. Complete mitochondrial genome of agar-producing red alga Gracilariopsis chorda (Gracilariales). Mitochondrial DNA 2014, 25, 339–341. [Google Scholar] [CrossRef]
- Schroeder, D.C.; Jaffer, M.A.; Coyne, V.E. Investigation of the role of a β(1–4) agarase produced by Pseudoalteromonas gracilis B9 in eliciting disease symptoms in the red alga Gracilaria gracilis. Microbiology 2003, 149, 2919–2929. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.C.; Kim, K.M.; Boo, G.H.; Lee, J.-H.; Boo, S.M.; Yoon, H.S. Complete mitochondrial genome of the agarophyte red alga Gelidium vagum(Gelidiales). Mitochondrial DNA 2014, 25, 267–268. [Google Scholar] [CrossRef] [PubMed]
- Falcão, V.D.R.; Tonon, A.P.; Oliveira, M.C.; Colepicolo, P. RNA Isolation method for polysaccharide rich algae: Agar producing Gracilaria tenuistipitata (Rhodophyta). J. Appl. Phycol. 2007, 20, 9–12. [Google Scholar] [CrossRef]
- Matsuhashi, T. Agar. In Food gels; Springer: Dordrecht, The Netherlands, 1990; pp. 1–51. [Google Scholar]
- Armisen, R.; Galatas, F. Production, properties and uses of agar. Production and utilization of products from commercial seaweeds. FAO Fish. Tech. Pap. 1987, 288, 1–57. [Google Scholar]
- Lee, W.-K.; Lim, Y.-Y.; Leow, A.T.-C.; Namasivayam, P.; Abdullah, J.O.; Ho, C.-L. Factors affecting yield and gelling properties of agar. J. Appl. Phycol. 2016, 29, 1527–1540. [Google Scholar] [CrossRef]
- Harrold, J.; Wyszomirska-Noga, Z. Funori: The use of a traditional Japanese adhesive in the preservation and conservation treatment of Western objects. In Proceedings of the International Conference of the Icon Book & Paper Group, London, UK, 8–10 April 2015. [Google Scholar]
- Takigami, S.; Yamaguch, H.; Takahashi, R.; Nagasawa, N. 2-14 Preparation and Characterization of the Low Molecular Weight Funoran; JAEA Takasaki Annual Report 2010; JAEA: Ibaraki, Japan, 2012. [Google Scholar]
- Takano, R.; Hayashi, K.; Hara, S.; Hirase, S. Funoran from the red seaweed, Gloiopeltis complanata: Polysaccharides with sulphated agarose structure and their precursor structure. Carbohydr. Polym. 1995, 27, 305–311. [Google Scholar] [CrossRef]
- Tuvikene, R.; Robal, M.; Fujita, D.; Saluri, K.; Truus, K.; Tashiro, Y.; Ogawa, H.; Matsukawa, S. Funorans from Gloiopeltis species. Part I. Extraction and structural characteristics. Food Hydrocoll. 2015, 43, 481–492. [Google Scholar] [CrossRef]
- Tuvikene, R.; Robal, M.; Mändar, H.; Fujita, D.; Saluri, K.; Truus, K.; Brenner, T.; Tashiro, Y.; Ogawa, H.; Matsukawa, S. Funorans from Gloiopeltis species. Part II. Rheology and thermal properties. Food Hydrocoll. 2015, 43, 649–657. [Google Scholar] [CrossRef]
- Ren, D.L.; Wang, J.Z.; Noda, H.; Amano, H.; Ogawa, S. The effects of an algal polysaccharide from Gloiopeltis tenaxon transplantable tumors and immune activities in mice. Planta Medica 1995, 61, 120–125. [Google Scholar] [CrossRef]
- Bhatia, S. Novel algal polysaccharides from marine source: Porphyran. Pharmacogn. Rev. 2008, 2, 271. [Google Scholar]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Rees, D.; Conway, E. The structure and biosynthesis of porphyran: A comparison of some samples. Biochem. J. 1962, 84, 411–416. [Google Scholar] [CrossRef]
- Venkatraman, K.L.; Mehta, A. Health benefits and pharmacological effects of porphyra species. Plant. Food Hum. Nutr. 2019, 74, 10–17. [Google Scholar] [CrossRef]
- Qiu, Y.; Jiang, H.; Fu, L.; Ci, F.; Mao, X. Porphyran and oligo-porphyran originating from red algae Porphyra: Preparation, biological activities, and potential applications. Food Chem. 2021, 349, 129209. [Google Scholar] [CrossRef]
- He, D.; Wu, S.; Yan, L.; Zuo, J.; Cheng, Y.; Wang, H.; Liu, J.; Zhang, X.; Wu, M.; Choi, J.; et al. Antitumor bioactivity of porphyran extracted from Pyropia yezoensis Chonsoo2 on human cancer cell lines. J. Sci. Food Agric. 2019, 99, 6722–6730. [Google Scholar] [CrossRef]
- Qiu, H.-M.; Veeraperumal, S.; Lv, J.-H.; Wu, T.-C.; Zhang, Z.-P.; Zeng, Q.-K.; Liu, Y.; Chen, X.-Q.; Aweya, J.J.; Cheong, K.-L. Physicochemical properties and potential beneficial effects of porphyran from Porphyra haitanensis on intestinal epithelial cells. Carbohydr. Polym. 2020, 246, 116626. [Google Scholar] [CrossRef]
- Bhatia, S.; Rathee, P.; Sharma, K.; Chaugule, B.; Kar, N.; Bera, T. Immuno-modulation effect of sulphated polysaccharide (porphyran) from Porphyra vietnamensis. Int. J. Biol. Macromol. 2013, 57, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.-Y.; Zhang, H.; Liu, J.; Ouyang, J.-M. Repair activity and crystal adhesion inhibition of polysaccharides with different molecular weights from red algae Porphyra yezoensis against oxalate-induced oxidative damage in renal epithelial cells. Food Funct. 2019, 10, 3851–3867. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Sun, X.-Y.; Chen, X.-W.; Ouyang, J.-M. Degraded Porphyra yezoensis polysaccharide protects HK-2 cells and reduces nano-COM crystal toxicity, adhesion and endocytosis. J. Mater. Chem. B 2020, 8, 7233–7252. [Google Scholar] [CrossRef] [PubMed]
- Tuvikene, R.; Truus, K.; Vaher, M.; Kailas, T.; Martin, G.; Kersen, P. Extraction and quantification of hybrid carrageenans from the biomass of the red algae Furcellaria lumbricalis and Coccotylus truncatus. Proc. Est. Acad. Sci. Chem. 2006, 55, 40–53. [Google Scholar] [CrossRef]
- Suganya, A.M.; Sanjivkumar, M.; Chandran, M.N.; Palavesam, A.; Immanuel, G. Pharmacological importance of sulphated polysaccharide carrageenan from red seaweed Kappaphycus alvarezii in comparison with commercial carrageenan. Biomed. Pharmacother. 2016, 84, 1300–1312. [Google Scholar] [CrossRef] [PubMed]
- Cicinskas, E.; Begun, M.A.; Tiasto, V.A.; Belousov, A.S.; Vikhareva, V.V.; Mikhailova, V.A.; Kalitnik, A.A. In vitro antitumor and immunotropic activity of carrageenans from red algae Chondrus armatus and their low-molecular weight degradation products. J. Biomed. Mater. Res. Part A 2020, 108, 254–266. [Google Scholar] [CrossRef]
- Ghannam, A.; Abbas, A.; Alek, H.; Al-Waari, Z.; Al-Ktaifani, M. Enhancement of local plant immunity against tobacco mosaic virus infection after treatment with sulphated-carrageenan from red alga (Hypnea musciformis). Physiol. Mol. Plant Pathol. 2013, 84, 19–27. [Google Scholar] [CrossRef]
- Reunov, A.; Nagorskaya, V.; Lapshina, L.; Yermak, I.; Barabanova, A. Effect of κ/ß-Carrageenan from red alga Tichocarpus crinitus (Tichocarpaceae) on infection of detached tobacco leaves with tobacco mosaic virus. J. Plant Dis. Prot. 2004, 111, 165–172. [Google Scholar] [CrossRef]
- Kim, S.-K. Marine Carbohydrates: Fundamentals and Applications; Academic Press: New York, NY, USA, 2014. [Google Scholar]
- Qin, Y. Seaweed hydrocolloids as thickening, gelling, and emulsifying agents in functional food products. In Bioactive Seaweeds for Food Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 135–152. [Google Scholar]
- BeMiller, J.N. Carbohydrate Chemistry for Food Scientists, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Therkelsen, G.H. Carrageenan, in Industrial Gums; Elsevier: Amsterdam, The Netherlands, 1993; pp. 145–180. [Google Scholar]
- Blakemore, W.R. Polysaccharide ingredients: Carrageenan; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Déléris, P.; Nazih, H.; Bard, J.-M. Seaweeds in human health. In Seaweed in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2016; pp. 319–367. [Google Scholar]
- Draget, K.I. Alginates. In Handbook of Hydrocolloids; Elsevier: Amsterdam, The Netherlands, 2009; pp. 807–828. [Google Scholar]
- Sutherland, I.W. Alginates. In Biomaterials; Springer: London, UK, 1991; pp. 307–331. [Google Scholar]
- Alba, K.; Kontogiorgos, V. Seaweed polysaccharides (agar, alginate carrageenan). In Encyclopedia of Food Chemistry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 240–250. [Google Scholar] [CrossRef]
- Murata, K.; Inose, T.; Hisano, T.; Abe, S.; Yonemoto, Y.; Yamashita, T.; Takagi, M.; Sakaguchi, K.; Kimura, A.; Imanaka, T. Bacterial alginate lyase: Enzymology, genetics and application. J. Ferment. Bioeng. 1993, 76, 427–437. [Google Scholar] [CrossRef]
- Varelis, P.; Melton, L.; Shahidi, F. Encyclopedia of Food Chemistry; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Ertesvåg, H.; Valla, S. Biosynthesis and applications of alginates. Polym. Degrad. Stab. 1998, 59, 85–91. [Google Scholar] [CrossRef]
- May, T.B.; Chakrabarty, A. Pseudomonas aeruginosa: Genes and enzymes of alginate synthesis. Trends Microbiol. 1994, 2, 151–157. [Google Scholar] [CrossRef]
- Pindar, D.F.; Bucke, C. The biosynthesis of alginic acid by Azotobacter vinelandii. Biochem. J. 1975, 152, 617–622. [Google Scholar] [CrossRef]
- Nesic, A.R.; Seslija, S.I. The influence of nanofillers on physical–chemical properties of polysaccharide-based film intended for food packaging. In Food Packaging; Elsevier: Amsterdam, The Netherlands, 2017; pp. 637–697. [Google Scholar]
- Toldra, F.; Kim, S.-K. Marine Enzymes Biotechnology: Production and Industrial Applications, Part III—Application of Marine Enzymes; Academic Press: New York, NY, USA, 2017. [Google Scholar]
- Clare, K. Algin. In Industrial Gums; Elsevier: Amsterdam, The Netherlands, 1993; pp. 105–143. [Google Scholar]
- Titlyanov, A.E.; Titlyanova, V.T.; Li, X.; Huang, H. Coral Reef Marine Plants of Hainan Island; Academic Press: New York, NY, USA, 2016. [Google Scholar]
- Vo, T.-S.; Kim, S.-K. Fucoidans as a natural bioactive ingredient for functional foods. J. Funct. Foods 2013, 5, 16–27. [Google Scholar] [CrossRef]
- Senthilkumar, K.; Ramajayam, G.; Venkatesan, J.; Kim, S.K.; Ahn, B.C. Biomedical applications of fucoidan, seaweed polysaccharides. In Seaweed Polysaccharides; Elsevier: Amsterdam, The Netherlands, 2017; pp. 269–281. [Google Scholar]
- Wijesinghe, W.A.J.P.; Jeon, Y.-J. Biological activities and potential industrial applications of fucose rich sulfated polysaccharides and fucoidans isolated from brown seaweeds: A review. Carbohydr. Polym. 2012, 88, 13–20. [Google Scholar] [CrossRef]
- Fitton, J.H.; Stringer, D.N.; Karpiniec, S.S. Therapies from fucoidan: An update. Mar. Drugs 2015, 13, 5920–5946. [Google Scholar] [CrossRef]
- Kusaykin, M.; Bakunina, I.; Sova, V.; Ermakova, S.; Kuznetsova, T.; Besednova, N.; Zaporozhets, T.; Zvyagintseva, T. Structure, biological activity, and enzymatic transformation of fucoidans from the brown seaweeds. Biotechnol. J. 2008, 3, 904–915. [Google Scholar] [CrossRef] [PubMed]
- Etman, S.M.; Elnaggar, Y.S.R.; Abdallah, O.Y. Fucoidan, a natural biopolymer in cancer combating: From edible algae to nanocarrier tailoring. Int. J. Biol. Macromol. 2019, 147, 799–808. [Google Scholar] [CrossRef]
- Senthilkumar, K.; Manivasagan, P.; Venkatesan, J.; Kim, S.-K. Brown seaweed fucoidan: Biological activity and apoptosis, growth signaling mechanism in cancer. Int. J. Biol. Macromol. 2013, 60, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Okimura, T.; Yokose, T.; Yamasaki, Y.; Yamaguchi, K.; Oda, T. Effects of sulfated fucan, ascophyllan, from the brown Alga Ascophyllum nodosum on various cell lines: A comparative study on ascophyllan and fucoidan. J. Biosci. Bioeng. 2010, 110, 113–117. [Google Scholar] [CrossRef]
- Malyarenko, O.S.; Ermakova, S.P. Fucoidans: Anticancer activity and molecular mechanisms of action. In Seaweed Polysaccharides; Elsevier: Amsterdam, The Netherlands, 2017; pp. 175–203. [Google Scholar]
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef] [PubMed]
- Cooney, O.C.; Morrin, S.T.; Buck, R.H.; Owens, R.A.; Hickey, R.M. Seaweed-derived polysaccharides as antibacterial and antiviral ingredients. Int. J. Biol. Macromol. 2025, 321, 145823. [Google Scholar] [CrossRef]
- Alfinaikh, R.S.; Alamry, K.A.; Hussein, M.A. Sustainable and biocompatible hybrid materials-based sulfated polysaccharides for biomedical applications: A review. RSC Adv. 2025, 15, 4708–4767. [Google Scholar] [CrossRef]
- John, R.M.; Raj, A.L.T.; Dinakarkumar, Y.; Selvaraj, A.; Radhakrishnan, M. Review on seaweed derived sulfated and non-sulfated marine polysaccharides as multifunctional food ingredients: Structure–function relationships, bioactivities and applications in functional foods. Int. J. Biol. Macromol. 2025, 330, 148071. [Google Scholar] [CrossRef]
- Bai, R.G.; Sabouni, R.; Husseini, G. Green nanotechnology—A road map to safer nanomaterials. In Applications of Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2018; pp. 133–159. [Google Scholar]
- Bai, R.G.; Ninan, N.; Muthoosamy, K.; Manickam, S. Graphene: A versatile platform for nanotheranostics and tissue engineering. Prog. Mater. Sci. 2018, 91, 24–69. [Google Scholar] [CrossRef]
- Bai, R.G.; Muthoosamy, K.; Manickam, S.; Hilal-Alnaqbi, A. Graphene-based 3D scaffolds in tissue engineering: Fabrication, applications, and future scope in liver tissue engineering. Int. J. Nanomed. 2019, 14, 5753–5783. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, Q.; Hou, Y.; Zhang, J.; Liang, X.-J. Nanomaterials in medicine and pharmaceuticals: Nanoscale materials developed with less toxicity and more efficacy. Eur. J. Nanomed. 2013, 5, 61–79. [Google Scholar] [CrossRef]
- Barabadi, H.; Ovais, M.; Shinwari, Z.K.; Saravanan, M. Anti-cancer green bionanomaterials: Present status and future prospects. Green Chem. Lett. Rev. 2017, 10, 285–314. [Google Scholar] [CrossRef]
- Dahoumane, S.A.; Jeffryes, C.; Mechouet, M.; Agathos, S.N. Biosynthesis of Inorganic Nanoparticles: A Fresh Look at the Control of Shape, Size and Composition. Bioengineering 2017, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.G.; Muthoosamy, K.; Zhou, M.; Ashokkumar, M.; Huang, N.M.; Manickam, S. Sonochemical and sustainable synthesis of graphene-gold (G-Au) nanocomposites for enzymeless and selective electrochemical detection of nitric oxide. Biosens. Bioelectron. 2017, 87, 622–629. [Google Scholar] [CrossRef]
- Bai, R.G.; Muthoosamy, K.; Shipton, F.N.; Pandikumar, A.; Rameshkumar, P.; Huang, N.M.; Manickam, S. The biogenic synthesis of a reduced graphene oxide–silver (RGO–Ag) nanocomposite and its dual applications as an antibacterial agent and cancer biomarker sensor. RSC Adv. 2016, 6, 36576–36587. [Google Scholar] [CrossRef]
- Manickam, S.; Muthoosamy, K.; Bai, R.G.; Abubakar, I.B.; Sudheer, S.M.; Hongngee, L.; Hwei-San, L.; Nayming, H.; Ch, C. Exceedingly biocompatible and thin-layered reduced graphene oxide nanosheets using an eco-friendly mushroom extract strategy. Int. J. Nanomed. 2015, 10, 1505–1519. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Sharma, S.; Sharma, K.; Chetri, S.P.K.; Vashishtha, A.; Singh, P.; Kumar, R.; Rathi, B.; Agrawal, V. Algae as crucial organisms in advancing nanotechnology: A systematic review. J. Appl. Phycol. 2015, 28, 1759–1774. [Google Scholar] [CrossRef]
- Khanna, P.; Kaur, A.; Goyal, D. Algae-based metallic nanoparticles: Synthesis, characterization and applications. J. Microbiol. Methods 2019, 163, 105656. [Google Scholar] [CrossRef]
- Naveena, B.E.; Prakash, S. Biological synthesis of gold nanoparticles using marine algae Gracilaria corticata and its application as a potent antimicrobial and antioxidant agent. Asian J. Pharm. Clin. Res. 2013, 6, 179–182. [Google Scholar]
- González-Ballesteros, N.; Prado-López, S.; Rodríguez-González, J.; Lastra, M.; Rodríguez-Argüelles, M. Green synthesis of gold nanoparticles using brown algae Cystoseira baccata: Its activity in colon cancer cells. Colloids Surf. B Biointerfaces 2017, 153, 190–198. [Google Scholar] [CrossRef]
- Ramakrishna, M.; Babu, D.R.; Gengan, R.M.; Chandra, S.; Rao, G.N. Green synthesis of gold nanoparticles using marine algae and evaluation of their catalytic activity. J. Nanostruct. Chem. 2015, 6, 1–13. [Google Scholar] [CrossRef]
- Khan, A.U.; Khan, M.; Malik, N.; Cho, M.H.; Khan, M.M. Recent progress of algae and blue–green algae-assisted synthesis of gold nanoparticles for various applications. Bioprocess Biosyst. Eng. 2018, 42, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Prasad, T.N.V.K.V.; Kambala, V.S.R.; Naidu, R. Phyconanotechnology: Synthesis of silver nanoparticles using brown marine algae Cystophora moniliformis and their characterisation. J. Appl. Phycol. 2012, 25, 177–182. [Google Scholar] [CrossRef]
- Kathiraven, T.; Sundaramanickam, A.; Shanmugam, N.; Balasubramanian, T. Green synthesis of silver nanoparticles using marine algae Caulerpa racemosa and their antibacterial activity against some human pathogens. Appl. Nanosci. 2014, 5, 499–504. [Google Scholar] [CrossRef]
- Venkatesan, J.; Kim, S.-K.; Shim, M.S. Antimicrobial, Antioxidant, and Anticancer Activities of Biosynthesized Silver Nanoparticles Using Marine Algae Ecklonia cava. Nanomaterials 2016, 6, 235. [Google Scholar] [CrossRef]
- Li, X.; Schirmer, K.; Bernard, L.; Sigg, L.; Pillai, S.; Behra, R. Silver nanoparticle toxicity and association with the alga Euglena gracilis. Environ. Sci. Nano 2015, 2, 594–602. [Google Scholar] [CrossRef]
- Ibraheem, I.B.M.; Abd-Elaziz, B.E.E.; Saad, W.F.; Fathy, W.A. Green biosynthesis of silver nanoparticles using marine Red Algae Acanthophora specifera and its antimicrobial activity. J. Nanomed. Nanotechnol. 2016, 7, 1–4. [Google Scholar]
- Sajidha Parveen, K.; Lakshmi, D. Biosynthesis of silver nanoparticles using red algae, Amphiroa fragilissima and its antibacterial potential against gram positive and gram negative bacteria. Int. J. Curr. Sci. 2016, 19, 93–100. [Google Scholar]
- Bhattacharya, P.; Swarnakar, S.; Ghosh, S.; Majumdar, S.; Banerjee, S. Disinfection of drinking water via algae mediated green synthesized copper oxide nanoparticles and its toxicity evaluation. J. Environ. Chem. Eng. 2019, 7, 102867. [Google Scholar] [CrossRef]
- Edison, T.N.J.I.; Atchudan, R.; Kamal, C.; Lee, Y.R. Caulerpa racemosa: A marine green alga for eco-friendly synthesis of silver nanoparticles and its catalytic degradation of methylene blue. Bioprocess Biosyst. Eng. 2016, 39, 1401–1408. [Google Scholar] [CrossRef] [PubMed]
- Aboelfetoh, E.F.; El-Shenody, R.A.; Ghobara, M.M. Eco-friendly synthesis of silver nanoparticles using green algae (Caulerpa serrulata): Reaction optimization, catalytic and antibacterial activities. Environ. Monit. Assess. 2017, 189, 349. [Google Scholar] [CrossRef]
- Rao, M.D.; Pennathur, G. Green synthesis and characterization of cadmium sulphide nanoparticles from Chlamydomonas reinhardtii and their application as photocatalysts. Mater. Res. Bull. 2017, 85, 64–73. [Google Scholar] [CrossRef]
- Mahajan, A.; Arya, A.; Chundawat, T.S. Green synthesis of silver nanoparticles using green alga (Chlorella vulgaris) and its application for synthesis of quinolines derivatives. Synth. Commun. 2019, 49, 1926–1937. [Google Scholar] [CrossRef]
- Mishra, V.; Arya, A.; Chundawat, T.S. High Catalytic Activity of Pd Nanoparticles Synthesized from Green Alga Chlorella vulgaris in Buchwald-hartwig Synthesis of N-Aryl Piperazines. Curr. Organocatalysis 2019, 7, 23–33. [Google Scholar] [CrossRef]
- Calangian, M.T.F.; Ildefonzo, A.B.; Manzano, V.K.S.; Agcaoili, G.J.T.; Ganado, R.J.J.; Yago, A.C.C.; Magdaluyo, E.R., Jr.; Vasquez, R.D.; Franco, C.F., Jr. Facile Synthesis of Biologically derived Fluorescent Carbon Nanoparticles (FCNPs) from an Abundant Marine Alga and its Biological Activities. Orient. J. Chem. 2018, 34, 791–799. [Google Scholar] [CrossRef]
- Graily-Moradi, F.; Mallak, A.M.; Ghorbanpour, M. Biogenic Synthesis of Gold Nanoparticles and Their Potential Application in Agriculture. In Biogenic Nano-Particles and their Use in Agro-Ecosystems; Springer: Singapore, 2020; pp. 187–204. [Google Scholar]
- Abdel-Raouf, N.; Al-Enazi, N.M.; Ibraheem, I.B. Green biosynthesis of gold nanoparticles using Galaxaura elongata and characterization of their antibacterial activity. Arab. J. Chem. 2017, 10, S3029–S3039. [Google Scholar] [CrossRef]
- Pugazhendhi, A.; Prabakar, D.; Jacob, J.M.; Karuppusamy, I.; Saratale, R.G. Synthesis and characterization of silver nanoparticles using Gelidium amansii and its antimicrobial property against various pathogenic bacteria. Microb. Pathog. 2018, 114, 41–45. [Google Scholar] [CrossRef]
- Öztürk, B.Y.; Gürsu, B.Y.; Dağ, İ. Antibiofilm and antimicrobial activities of green synthesized silver nanoparticles using marine red algae Gelidium corneum. Process Biochem. 2020, 89, 208–219. [Google Scholar] [CrossRef]
- Senthilkumar, P.; Surendran, L.; Sudhagar, B.; Kumar, D.S.R.S. Facile green synthesis of gold nanoparticles from marine algae Gelidiella acerosa and evaluation of its biological Potential. SN Appl. Sci. 2019, 1, 284. [Google Scholar] [CrossRef]
- de Aragao, A.P.; de Oliveira, T.M.; Quelemes, P.V.; Perfeito, M.L.G.; Araujo, M.C.; Santiago, J.D.A.S.; Cardoso, V.S.; Quaresma, P.; de Almeida, J.R.D.S.; da Silva, D.A. Green synthesis of silver nanoparticles using the seaweed Gracilaria birdiae and their antibacterial activity. Arab. J. Chem. 2019, 12, 4182–4188. [Google Scholar] [CrossRef]
- Lavakumar, V.; Masilamani, K.; Ravichandiran, V.; Venkateshan, N.; Saigopal, D.V.R.; Kumar, C.K.A.; Sowmya, C. Promising upshot of silver nanoparticles primed from Gracilaria crassa against bacterial pathogens. BMC Chem. 2015, 9, 42. [Google Scholar] [CrossRef]
- Chellapandian, C.; Ramkumar, B.; Puja, P.; Shanmuganathan, R.; Pugazhendhi, A.; Kumar, P. Gold nanoparticles using red seaweed Gracilaria verrucosa: Green synthesis, characterization and biocompatibility studies. Process Biochem. 2019, 80, 58–63. [Google Scholar] [CrossRef]
- Vinosha, M.; Palanisamy, S.; Muthukrishnan, R.; Selvam, S.; Kannapiran, E.; You, S.; Prabhu, N.M. Biogenic synthesis of gold nanoparticles from Halymenia dilatata for pharmaceutical applications: Antioxidant, anti-cancer and antibacterial activities. Process Biochem. 2019, 85, 219–229. [Google Scholar] [CrossRef]
- Abdel-Raouf, N.; Alharbi, R.M.; Al-Enazi, N.M.; Alkhulaifi, M.M.; Ibraheem, I.B.M. Rapid biosynthesis of silver nanoparticles using the marine red alga Laurencia catarinensis and their characterization. Beni-Suef Univ. J. Basic Appl. Sci. 2018, 7, 150–157. [Google Scholar] [CrossRef]
- Bao, Z.; Cao, J.; Kang, G.; Lan, C.Q. Effects of reaction conditions on light-dependent silver nanoparticle biosynthesis mediated by cell extract of green alga Neochloris oleoabundans. Environ. Sci. Pollut. Res. 2018, 26, 2873–2881. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Raouf, N.; Al-Enazi, N.M.; Ibraheem, I.B.M.; Alharbi, R.M.; Alkhulaifi, M.M. Biosynthesis of silver nanoparticles by using of the marine brown alga Padina pavonia and their characterization. Saudi J. Biol. Sci. 2019, 26, 1207–1215. [Google Scholar] [CrossRef]
- Moshfegh, A.; Jalali, A.; Salehzadeh, A.; Jozani, A.S. Biological synthesis of silver nanoparticles by cell-free extract of Polysiphonia algae and their anticancer activity against breast cancer MCF-7 cell lines. Micro Nano Lett. 2019, 14, 581–584. [Google Scholar] [CrossRef]
- Fatima, R.; Priya, M.; Indurthi, L.; Radhakrishnan, V.; Sudhakaran, R. Biosynthesis of silver nanoparticles using red algae Portieria hornemannii and its antibacterial activity against fish pathogens. Microb. Pathog. 2020, 138, 103780. [Google Scholar] [CrossRef]
- Duygu, D.Y.; Erkaya, I.A.; Erdem, B.; Yalcin, B.M. Characterization of silver nanoparticle produced by Pseudopediastrum boryanum (Turpin) E. Hegewald and its antimicrobial effects on some pathogens. Int. J. Environ. Sci. Technol. 2019, 16, 7093–7102. [Google Scholar] [CrossRef]
- Murugesan, S.; Bhuvaneswari, S.; Sivamurugan, V. Green synthesis, characterization of silver nanoparticles of a marine red alga Spyridia Fusiformis and their antibacterial activity. Int. J. Pharm. Pharm. Sci. 2017, 9, 192–197. [Google Scholar] [CrossRef]
- El-Khateeb, A.Y.; Hamed, E.; Ibrahim, F.Y.; Hamed, S.E. Eco-Friendly Synthesis of Selenium and Zinc Nanoparticles with Biocompatible Sargassum latifolium Algae Extract in Preservation of Edible Oils. J. Food Dairy Sci. 2019, 10, 141–146. [Google Scholar] [CrossRef]
- Sanaeimehr, Z.; Javadi, I.; Namvar, F. Antiangiogenic and antiapoptotic effects of green-synthesized zinc oxide nanoparticles using Sargassum muticum algae extraction. Cancer Nanotechnol. 2018, 9, 3. [Google Scholar] [CrossRef]
- Ramaswamy, S.V.P.; Narendhran, S.; Sivaraj, R. Potentiating effect of ecofriendly synthesis of copper oxide nanoparticles using brown alga: Antimicrobial and anticancer activities. Bull. Mater. Sci. 2016, 39, 361–364. [Google Scholar] [CrossRef]
- Subbiah, M.; Pandithurai, M.; Vajiravelu, S. Spatoglossum asperum J Agardh mediated synthesis of silver nanoparticles, characterization and evaluation antifungal activities. J. Pharmacogn. Phytochem. 2019, 8, 1991–1995. [Google Scholar]
- Sayadi, M.H.; Salmani, N.; Heidari, A.; Rezaei, M.R. Bio-synthesis of palladium nanoparticle using Spirulina platensis alga extract and its application as adsorbent. Surf. Interfaces 2018, 10, 136–143. [Google Scholar] [CrossRef]
- Valarmathi, N.; Ameen, F.; Almansob, A.; Kumar, P.; Arunprakash, S.; Govarthanan, M. Utilization of marine seaweed Spyridia filamentosa for silver nanoparticles synthesis and its clinical applications. Mater. Lett. 2020, 263, 127244. [Google Scholar] [CrossRef]
- Sathishkumar, R.; Sundaramanickam, A.; Srinath, R.; Ramesh, T.; Saranya, K.; Meena, M.; Surya, P. Green synthesis of silver nanoparticles by bloom forming marine microalgae Trichodesmium erythraeum and its applications in antioxidant, drug-resistant bacteria, and cytotoxicity activity. J. Saudi Chem. Soc. 2019, 23, 1180–1191. [Google Scholar] [CrossRef]
- Massironi, A.; Morelli, A.; Grassi, L.; Puppi, D.; Braccini, S.; Maisetta, G.; Esin, S.; Batoni, G.; Della Pina, C.; Chiellini, F. Ulvan as novel reducing and stabilizing agent from renewable algal biomass: Application to green synthesis of silver nanoparticles. Carbohydr. Polym. 2019, 203, 310–321. [Google Scholar] [CrossRef]
- Mukhoro, O.C.; Roos, W.D.; Jaffer, M.; Bolton, J.J.; Stillman, M.J.; Beukes, D.R.; Antunes, E. Very Green Photosynthesis of Gold Nanoparticles by a Living Aquatic Plant: Photoreduction of AuIII by the Seaweed Ulva armoricana. Chem.—A Eur. J. 2017, 24, 1657–1666. [Google Scholar] [CrossRef] [PubMed]
- Mashjoor, S.; Yousefzadi, M.; Zolgharnain, H.; Kamrani, E.; Alishahi, M. Organic and inorganic nano-Fe3O4: Alga Ulva flexuosa-based synthesis, antimicrobial effects and acute toxicity to briny water rotifer Brachionus rotundiformis. Environ. Pollut. 2018, 237, 50–64. [Google Scholar] [CrossRef] [PubMed]
- González-Ballesteros, N.; Diego-González, L.; Lastra-Valdor, M.; Rodríguez-Argüelles, M.C.; Grimaldi, M.; Cavazza, A.; Bigi, F.; Simón-Vázquez, R. Immunostimulant and biocompatible gold and silver nanoparticles synthesized using the Ulva intestinalis L. aqueous extract. J. Mater. Chem. B 2019, 7, 4677–4691. [Google Scholar] [CrossRef]
- González-Ballesteros, N.; Rodríguez-Argüelles, M.C.; Prado-López, S.; Lastra, M.; Grimaldi, M.; Cavazza, A.; Nasi, L.; Salviati, G.; Bigi, F. Macroalgae to nanoparticles: Study of Ulva lactuca L. role in biosynthesis of gold and silver nanoparticles and of their cytotoxicity on colon cancer cell lines. Mater. Sci. Eng. C 2019, 97, 498–509. [Google Scholar] [CrossRef]
- Varshosaz, J.; Minaiyan, M.; Zaki, M.R.; Fathi, M.; Jaleh, H. In vitro/in vivo evaluation of agar nano spheres for pulmonary delivery of bupropion HCl. Drug Deliv. 2016, 23, 1948–1954. [Google Scholar]
- Díaz-Bleis, D.; Alvarado-Gil, J.J.; I Martínez, A.; Gómez-Y-Gómez, Y.; Freile-Pelegrín, Y. On the preparation and characterization of superparamagnetic nanoparticles with Gelidium robustum agar coating for biomedical applications. Bull. Mater. Sci. 2018, 41, 39. [Google Scholar] [CrossRef]
- Ziabari, S.A.M.; Babamoradi, M.; Hajizadeh, Z.; Maleki, A. The effect of magnetic field on the magnetic property of Agar/Fe3O4 nanocomposite. Eurasian Chem. Commun. 2020, 2, 456–464. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W.; Jaiswal, L. Bioactive agar-based functional composite film incorporated with copper sulfide nanoparticles. Food Hydrocoll. 2019, 93, 156–166. [Google Scholar] [CrossRef]
- Thakur, S.S.; Shenoy, S.K.; Suk, J.S.; Hanes, J.S.; Rupenthal, I.D. Validation of hyaluronic acid-agar-based hydrogels as vitreous humor mimetics for in vitro drug and particle migration evaluations. Eur. J. Pharm. Biopharm. 2020, 148, 118–125. [Google Scholar] [CrossRef]
- de Lima, G.G.; de Lima, D.W.; de Oliveira, M.J.; Lugão, A.B.; Alcântara, M.T.; Devine, D.M.; de Sá, M.J. Synthesis and in vivo behavior of PVP/CMC/agar hydrogel membranes impregnated with silver nanoparticles for wound healing applications. ACS Appl. Bio Mater. 2018, 1, 1842–1852. [Google Scholar] [CrossRef]
- Venkatpurwar, V.; Shiras, A.; Pokharkar, V. Porphyran capped gold nanoparticles as a novel carrier for delivery of anticancer drug: In vitro cytotoxicity study. Int. J. Pharm. 2011, 409, 314–320. [Google Scholar] [CrossRef]
- Xu, Y.-Y.; Huo, Y.-F.; Xu, L.; Zhu, Y.-Z.; Wu, Y.-T.; Wei, X.-Y.; Zhou, T. Resveratrol-loaded ovalbumin/Porphyra haitanensis polysaccharide composite nanoparticles: Fabrication, characterization and antitumor activity. J. Drug Deliv. Sci. Technol. 2021, 66, 102811. [Google Scholar] [CrossRef]
- Xu, X.; Cao, J.; Deng, W.; Cao, X.; Chen, J.; Wang, Y.; Wang, S.; Yu, J.; Gao, X.; Yu, Q.; et al. Efficient gene delivery to human umbilical cord mesenchymal stem cells by cationized Porphyra yezoensis polysaccharide nanoparticles. Int. J. Nanomed. 2015, 10, 7097–7107. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Q.; Zhou, J.; Deng, W.; Yu, Q.; Cao, X.; Wang, J.; Shao, F.; Li, Y.; Ma, P.; et al. Porphyra polysaccharide-derived carbon dots for non-viral co-delivery of different gene combinations and neuronal differentiation of ectodermal mesenchymal stem cells. Nanoscale 2017, 9, 10820–10831. [Google Scholar] [CrossRef]
- Sarkar, S.D.; Farrugia, B.L.; Dargaville, T.R.; Dhara, S. Chitosan–collagen scaffolds with nano/microfibrous architecture for skin tissue engineering. J. Biomed. Mater. Res. Part A 2013, 101, 3482–3492. [Google Scholar] [CrossRef] [PubMed]
- Ghanbarzadeh, M.; Golmoradizadeh, A.; Homaei, A. Carrageenans and carrageenases: Versatile polysaccharides and promising marine enzymes. Phytochem. Rev. 2018, 17, 535–571. [Google Scholar] [CrossRef]
- González Ocampo, J.I.; Bassous, N.; Ossa Orozco, C.P.; Webster, T.J. Evaluation of cytotoxicity and antimicrobial activity of an injectable bone substitute of carrageenan and nano hydroxyapatite. J. Biomed. Mater. Res. Part A 2018, 106, 2984–2993. [Google Scholar] [CrossRef]
- Baradaran, S.; Hajizadeh Moghaddam, A.; Khanjani Jelodar, S. P73: Nano-phytosome of curcumin improve behavioral impairment on carrageenan-induced acute inflammation model in mice. Neurosci. J. Shefaye Khatam 2018, 6, 104. [Google Scholar]
- Maldonado, L.; Chough, S.; Bonilla, J.; Kim, K.; Kokini, J. Mechanism of fabrication and nano-mechanical properties of α-lactalbumin/chitosan and BSA/κ-carrageenan nanotubes through layer-by-layer assembly for curcumin encapsulation and determination of in vitro cytotoxicity. Food Hydrocoll. 2019, 93, 293–307. [Google Scholar] [CrossRef]
- Zepon, K.M.; Marques, M.S.; Paula, M.M.d.S.; Morisso, F.D.P.; Kanis, L.A. Facile, green and scalable method to produce carrageenan-based hydrogel containing in situ synthesized AgNPs for application as wound dressing. Int. J. Biol. Macromol. 2018, 113, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, L.; Shankar, S.; Rhim, J.-W. Carrageenan-based functional hydrogel film reinforced with sulfur nanoparticles and grapefruit seed extract for wound healing application. Carbohydr. Polym. 2019, 224, 115191. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Huang, X.; Wu, F.; Feng, S.; Cheng, R.; Xu, J.; Cui, T.; Li, J. 3D-Printed Hydrogel Scaffolds Loaded with Flavanone@ZIF-8 Nanoparticles for Promoting Bacteria-Infected Wound Healing. Gels 2024, 10, 835. [Google Scholar] [CrossRef]
- Azizi, S.; Mohamad, R.; Rahim, R.A.; Mohammadinejad, R.; Bin Ariff, A. Hydrogel beads bio-nanocomposite based on Kappa-Carrageenan and green synthesized silver nanoparticles for biomedical applications. Int. J. Biol. Macromol. 2017, 104, 423–431. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Doroudian, M.; Ahadpour, A.; Azari, S. Injectable chitosan/κ-carrageenan hydrogel designed with au nanoparticles: A conductive scaffold for tissue engineering demands. Int. J. Biol. Macromol. 2019, 126, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Sequeira, R.; Ardao, I.; Starbird, R.; García-González, C.A. Conductive nanostructured materials based on poly-(3,4-ethylenedioxythiophene) (PEDOT) and starch/κ-carrageenan for biomedical applications. Carbohydr. Polym. 2018, 189, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.; Sen, K.K.; Gandhi, A. Alginate based nanocarriers for drug delivery applications. Curr. Pharm. Des. 2016, 22, 3399–3410. [Google Scholar] [CrossRef]
- Reddy, B.H.N.; Rauta, P.R.; Lakshmi, V.V.; Sreenivasa, S. Development, formulation and evaluation of sodium alginate-g-poly (Acrylamide-Co-Acrylic Acid/Cloiste-30B) AGNPs Hydrogel composites and their applications in paclitaxel drug delivery and anticancer activity. Int. J. Appl. Pharm. 2018, 10, 141–150. [Google Scholar] [CrossRef]
- Wang, X.; Guo, C.; Hao, W.; Ullah, N.; Chen, L.; Li, Z.; Feng, X. Development and characterization of agar-based edible films reinforced with nano-bacterial cellulose. Int. J. Biol. Macromol. 2018, 118, 722–730. [Google Scholar] [CrossRef]
- Amiri, M.; Salavati-Niasari, M.; Pardakhty, A.; Ahmadi, M.; Akbari, A. Caffeine: A novel green precursor for synthesis of magnetic CoFe2O4 nanoparticles and pH-sensitive magnetic alginate beads for drug delivery. Mater. Sci. Eng. C 2017, 76, 1085–1093. [Google Scholar] [CrossRef]
- Joshy, K.; Susan, M.A.; Snigdha, S.; Nandakumar, K.; Laly, A.P.; Sabu, T. Encapsulation of zidovudine in PF-68 coated alginate conjugate nanoparticles for anti-HIV drug delivery. Int. J. Biol. Macromol. 2018, 107, 929–937. [Google Scholar] [CrossRef]
- Lakkakula, J.R.; Matshaya, T.; Krause, R.W.M. Cationic cyclodextrin/alginate chitosan as 5-fluorouracil drug delivery system. Mater. Sci. Eng. C 2017, 70, 169–177. [Google Scholar] [CrossRef]
- Prabha, G.; Raj, V. Sodium alginate–polyvinyl alcohol–bovin serum albumin coated Fe3O4 nanoparticles as anticancer drug delivery vehicle: Doxorubicin loading and in vitro release study and cytotoxicity to HepG2 and L02 cells. Mater. Sci. Eng. C 2017, 79, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, S.M.; Sharipov, M.; Huy, B.T.; Gerelkhuu, Z.; Biechele-Speziale, D.; Lee, Y.-I. Naturally modified nonionic alginate functionalized upconversion nanoparticles for the highly efficient targeted pH-responsive drug delivery and enhancement of NIR-imaging. J. Ind. Eng. Chem. 2018, 57, 424–435. [Google Scholar] [CrossRef]
- Wang, F.; Yang, S.; Yuan, J.; Gao, Q.; Huang, C. Effective method of chitosan-coated alginate nanoparticles for target drug delivery applications. J. Biomater. Appl. 2016, 31, 3–12. [Google Scholar] [CrossRef]
- Joshy, K.; George, A.; Jose, J.; Kalarikkal, N.; Pothen, L.A.; Thomas, S. Novel dendritic structure of alginate hybrid nanoparticles for effective anti-viral drug delivery. Int. J. Biol. Macromol. 2017, 103, 1265–1275. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.H.; Duan, W.; Tran, T.T. Fucoidan-based nanostructures: A focus on its combination with chitosan and the surface functionalization of metallic nanoparticles for drug delivery. Int. J. Pharm. 2019, 575, 118956. [Google Scholar] [CrossRef]
- Aquib, M.; Farooq, M.A.; Filli, M.S.; Boakye-Yiadom, K.O.; Kesse, S.; Maviah, M.B.J.; Mavlyanova, R.; Wang, B. A review on the chemotherapeutic role of fucoidan in cancer as nanomedicine. Res. J. Life Sci. Bioinform. Pharm. Chem. Sci. 2019, 5, 512–539. [Google Scholar]
- Lu, H.-T.; Lu, T.-W.; Chen, C.-H.; Mi, F.-L. Development of genipin-crosslinked and fucoidan-adsorbed nano-hydroxyapatite/hydroxypropyl chitosan composite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2019, 128, 973–984. [Google Scholar] [CrossRef]
- Lowe, B.; Venkatesan, J.; Anil, S.; Shim, M.S.; Kim, S.-K. Preparation and characterization of chitosan-natural nano hydroxyapatite-fucoidan nanocomposites for bone tissue engineering. Int. J. Biol. Macromol. 2016, 93, 1479–1487. [Google Scholar] [CrossRef]
- Young, A.T.; Kang, J.H.; Kang, D.J.; Venkatesan, J.; Chang, H.K.; Bhatnagar, I.; Chang, K.-Y.; Hwang, J.-H.; Salameh, Z.; Kim, S.-K.; et al. Interaction of stem cells with nano hydroxyapatite-fucoidan bionanocomposites for bone tissue regeneration. Int. J. Biol. Macromol. 2016, 93, 1488–1491. [Google Scholar] [CrossRef]
- Devi GV, Y.; Nagendra, A.H.; Shenoy P, S.; Chatterjee, K.; Venkatesan, J. Fucoidan-incorporated composite scaffold stimulates osteogenic differentiation of mesenchymal stem cells for bone tissue engineering. Mar. Drugs 2022, 20, 589. [Google Scholar] [CrossRef]
- Abdollah, M.R.A.; Carter, T.J.; Jones, C.; Kalber, T.L.; Rajkumar, V.; Tolner, B.; Gruettner, C.; Zaw-Thin, M.; Torres, J.B.; Ellis, M.; et al. Fucoidan prolongs the circulation time of dextran-coated iron oxide nanoparticles. ACS Nano 2018, 12, 1156–1169. [Google Scholar] [CrossRef] [PubMed]
- Moorthy, M.S.; Subramanian, B.; Panchanathan, M.; Mondal, S.; Kim, H.; Lee, K.D.; Oh, J. Fucoidan-coated core–shell magnetic mesoporous silica nanoparticles for chemotherapy and magnetic hyperthermia-based thermal therapy applications. New J. Chem. 2017, 41, 15334–15346. [Google Scholar] [CrossRef]
- Lu, K.-Y.; Li, R.; Hsu, C.-H.; Lin, C.-W.; Chou, S.-C.; Tsai, M.-L.; Mi, F.-L. Development of a new type of multifunctional fucoidan-based nanoparticles for anticancer drug delivery. Carbohydr. Polym. 2017, 165, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Nguyen, V.P.; Manivasagan, P.; Jung, M.J.; Kim, S.W.; Oh, J.; Kang, H.W. Doxorubicin-fucoidan-gold nanoparticles composite for dual-chemo-photothermal treatment on eye tumors. Oncotarget 2017, 8, 113719–113733. [Google Scholar] [CrossRef]
- Manivasagan, P.; Bharathiraja, S.; Bui, N.Q.; Jang, B.; Oh, Y.-O.; Lim, I.G.; Oh, J. Doxorubicin-loaded fucoidan capped gold nanoparticles for drug delivery and photoacoustic imaging. Int. J. Biol. Macromol. 2016, 91, 578–588. [Google Scholar] [CrossRef]
- Fan, J.; Liu, Y.; Wang, S.; Liu, Y.; Li, S.; Long, R.; Zhang, R.; Kankala, R.K. Synthesis and characterization of innovative poly(lactide-co-glycolide)-(poly-l-ornithine/fucoidan) core–shell nanocarriers by layer-by-layer self-assembly. RSC Adv. 2017, 7, 32786–32794. [Google Scholar] [CrossRef]
- Barbosa, A.I.; Lima, S.A.C.; Reis, S. Development of methotrexate loaded fucoidan/chitosan nanoparticles with anti-inflammatory potential and enhanced skin permeation. Int. J. Biol. Macromol. 2019, 124, 1115–1122. [Google Scholar] [CrossRef]
- Chiang, C.-S.; Lin, Y.-J.; Lee, R.; Lai, Y.-H.; Cheng, H.-W.; Hsieh, C.-H.; Shyu, W.-C.; Chen, S.-Y. Combination of fucoidan-based magnetic nanoparticles and immunomodulators enhances tumour-localized immunotherapy. Nat. Nanotechnol. 2018, 13, 746–754. [Google Scholar] [CrossRef]
- Choi, D.G.; Venkatesan, J.; Shim, M.S. Selective anticancer therapy using pro-oxidant drug-loaded chitosan–fucoidan nanoparticles. Int. J. Mol. Sci. 2019, 20, 3220. [Google Scholar] [CrossRef]
- Jang, H.; Kang, K.; El-Sayed, M.A. Real-time tracking of the autophagy process in living cells using plasmonically enhanced Raman spectroscopy of fucoidan-coated gold nanoparticles. J. Mater. Chem. B 2018, 6, 5460–5465. [Google Scholar] [CrossRef]
- Jayakumar, R.; Ramachandran, R.; Divyarani, V.; Chennazhi, K.; Tamura, H.; Nair, S. Fabrication of chitin–chitosan/nano TiO2-composite scaffolds for tissue engineering applications. Int. J. Biol. Macromol. 2011, 48, 336–344. [Google Scholar] [CrossRef]
- Hasan, A.; Waibhaw, G.; Saxena, V.; Pandey, L.M. Nano-biocomposite scaffolds of chitosan, carboxymethyl cellulose and silver nanoparticle modified cellulose nanowhiskers for bone tissue engineering applications. Int. J. Biol. Macromol. 2018, 111, 923–934. [Google Scholar] [CrossRef]
- Li, X.; Chen, S.; Zhang, B.; Li, M.; Diao, K.; Zhang, Z.; Li, J.; Xu, Y.; Wang, X.; Chen, H. In situ injectable nano-composite hydrogel composed of curcumin, N,O-carboxymethyl chitosan and oxidized alginate for wound healing application. Int. J. Pharm. 2012, 437, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Archana, D.; Dutta, J.; Dutta, P. Evaluation of chitosan nano dressing for wound healing: Characterization, in vitro and in vivo studies. Int. J. Biol. Macromol. 2013, 57, 193–203. [Google Scholar] [CrossRef]
- Monti, S.; Jose, J.; Sahajan, A.; Kalarikkal, N.; Thomas, S. Structure and dynamics of gold nanoparticles decorated with chitosan–gentamicin conjugates: ReaxFF molecular dynamics simulations to disclose drug delivery. Phys. Chem. Chem. Phys. 2019, 21, 13099–13108. [Google Scholar] [CrossRef]
- Silva, M.M.; Calado, R.; Marto, J.; Bettencourt, A.; Almeida, A.J.; Gonçalves, L.M.D. Chitosan nanoparticles as a mucoadhesive drug delivery system for ocular administration. Mar. Drugs 2017, 15, 370. [Google Scholar] [CrossRef]
- Mansur, H.S.; Mansur, A.A.P.; Curti, E.; De Almeida, M.V. Functionalized-chitosan/quantum dot nano-hybrids for nanomedicine applications: Towards biolabeling and biosorbing phosphate metabolites. J. Mater. Chem. B 2013, 1, 1696–1711. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhang, C.; Wang, Y.; Chen, G. A review of the current and emerging detection methods of marine harmful microalgae. Sci. Total. Environ. 2022, 815, 152913. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Li, Y.; Wu, Q.; Gong, C. Nanotechnology-based CRISPR/Cas9 delivery system for genome editing in cancer treatment. MedComm–Biomater. Appl. 2024, 3, e70. [Google Scholar] [CrossRef]
- Hwang, B.; Korsnick, L.; Shen, M.; Jin, L.; Singh, Y.; Abdalla, M.; Bauser-Heaton, H.; Serpooshan, V. FSTL-1 loaded 3D bioprinted vascular patch regenerates the ischemic heart tissue. iScience 2024, 27, 110770. [Google Scholar] [CrossRef]
- Binsuwaidan, R.; El-Masry, T.A.; El-Sheekh, M.; Seadawy, M.G.; Makhlof, M.E.M.; Aboukhatwa, S.M.; El-Shitany, N.A.; Elmorshedy, K.E.; El-Nagar, M.M.F.; El-Bouseary, M.M. Prospective Antiviral Effect of Ulva lactuca Aqueous Extract against COVID-19 Infection. Mar. Drugs 2023, 22, 30. [Google Scholar] [CrossRef]
- Shefer, S.; Robin, A.; Chemodanov, A.; Lebendiker, M.; Bostwick, R.; Rasmussen, L.; Lishner, M.; Gozin, M.; Golberg, A. Fighting SARS-CoV-2 with green seaweed Ulva sp. extract: Extraction protocol predetermines crude ulvan extract anti-SARS-CoV-2 inhibition properties in in vitro Vero-E6 cells assay. PeerJ 2021, 9, e12398. [Google Scholar] [CrossRef] [PubMed]
- Huhulea, E.N.; Huang, L.; Eng, S.; Sumawi, B.; Huang, A.; Aifuwa, E.; Hirani, R.; Tiwari, R.K.; Etienne, M. Artificial Intelligence Advancements in Oncology: A Review of Current Trends and Future Directions. Biomedicines 2025, 13, 951. [Google Scholar] [CrossRef] [PubMed]
- Duong, T.K.N.; Truong, T.T.; Phan, T.N.L.; Nguyen, T.X.; Doan, V.H.M.; Vo, T.T.; Choi, J.; Pal, U.; Dhar, P.; Lee, B.; et al. Hydrogel-Based Smart Materials for Wound Healing and Sensing. Aggregate 2025, 6, e70047. [Google Scholar] [CrossRef]
- Golzary, A.; Zarei, Z. Advances in technologies for biotransformation of algal biomass into high-value products. In Algal Biorefinery; Elsevier: Amsterdam, The Netherlands, 2025; pp. 289–305. [Google Scholar]
- Narayanan, M. Promising biorefinery products from marine macro and microalgal biomass: A review. Renew. Sustain. Energy Rev. 2023, 190, 114081. [Google Scholar] [CrossRef]
- Patel, A.K.; Vadrale, A.P.; Singhania, R.R.; Michaud, P.; Pandey, A.; Chen, S.-J.; Chen, C.-W.; Dong, C.-D. Algal polysaccharides: Current status and future prospects. Phytochem. Rev. 2023, 22, 1167–1196. [Google Scholar] [CrossRef]
- Pörtner, R. Biopharmaceutical Manufacturing: Progress, Trends and Challenges; Springer: Cham, Switzerland, 2024. [Google Scholar]
- Guo, B.; Lu, X.; Jiang, X.; Shen, X.-L.; Wei, Z.; Zhang, Y. Artificial Intelligence in Advancing Algal Bioactive Ingredients: Production, Characterization, and Application. Foods 2025, 14, 1783. [Google Scholar] [CrossRef]
- Abdullah, M.; Malik, H.A.; Ali, A.; Boopathy, R.; Vo, P.H.N.; Danaee, S.; Ralph, P.; Malik, S. AI-Driven Algae Biorefineries: A New Era for Sustainable Bioeconomy. Curr. Pollut. Rep. 2025, 11, 21. [Google Scholar] [CrossRef]
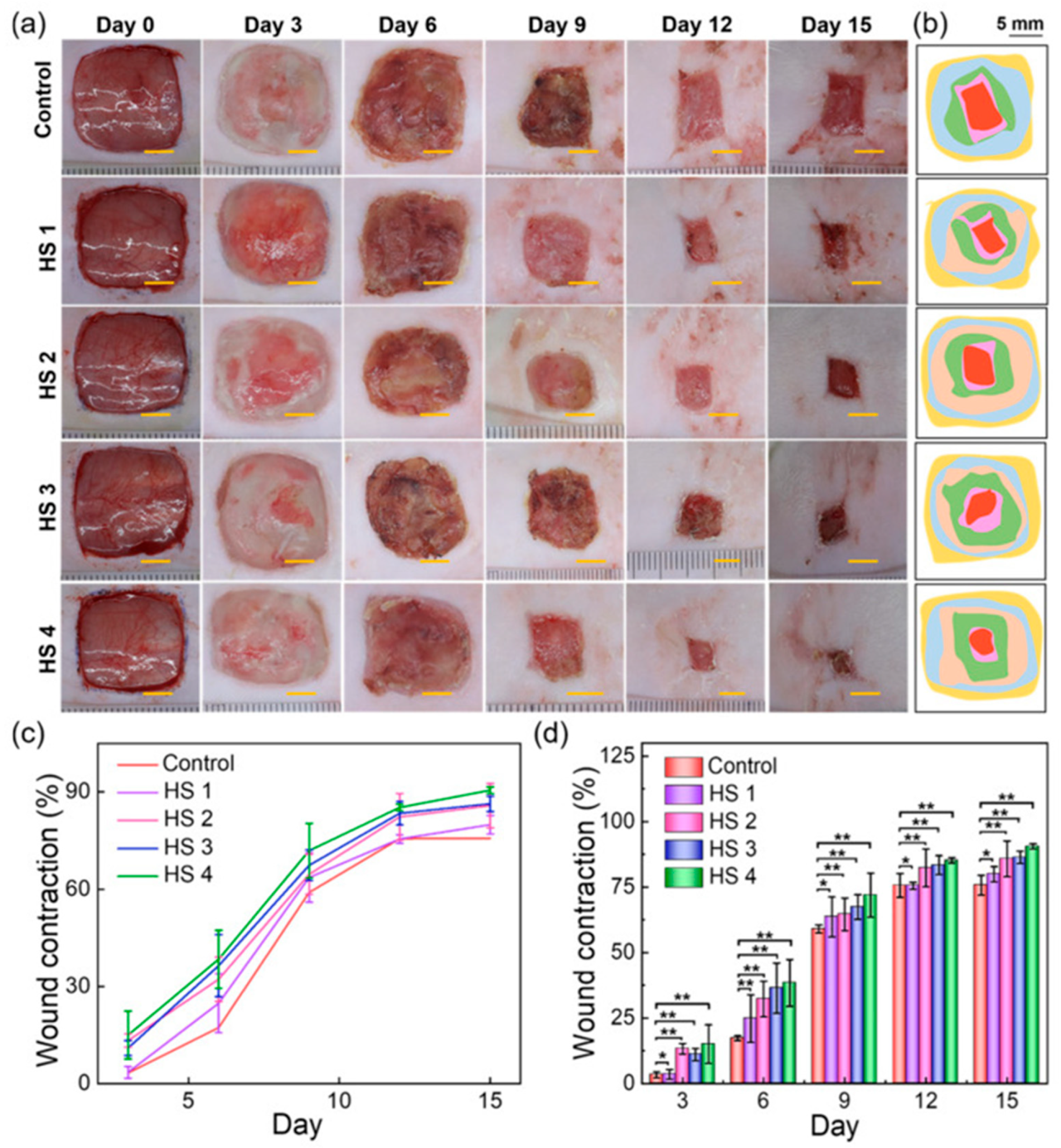
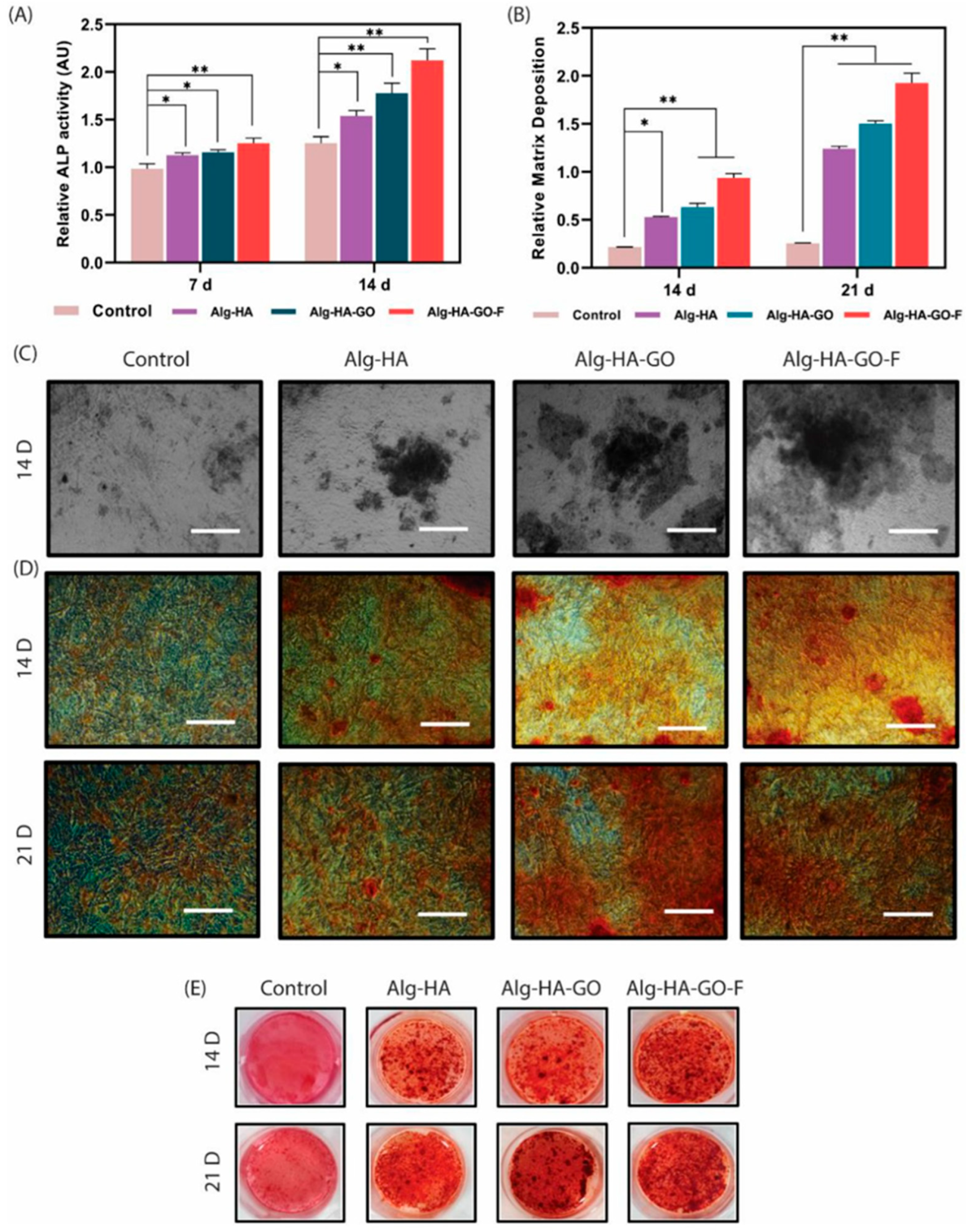
| Algae | Nanomaterial | Size | Application. Target Pathogen/Catalytic Action | Reference |
|---|---|---|---|---|
| Acanthophora specifera | silver | 33–81 nm | antimicrobial activity. Staphylococcus aureus, Bacillus subtillis, Salmonella spp., Escherichia coli | [112] |
| Amphiroa fragilissima | silver | - | antibacterial activity, Escherichia coli, Bacillus subtilis, Klebsiella pneumonia, Staphylococcus aureus, Pseudomonas aeruginosa | [113] |
| Anabaena cylindrica | copper oxide | 3.6 nm | drinking water disinfection. Escherichia coli. | [114] |
| Caulerpa racemosa | silver | 25 nm | catalytic activity towards methylene blue. | [115] |
| Caulerpa serrulata | silver | 10 ± 2 nm | catalytic and antibacterial activity. Staphylococcus aureus, Pseudomonas aeruginosa, Shigella sp., Salmonella typhi, Escherichia coli | [116] |
| Chlamydomonas reinhardtii | cadmium sulphide | 5 nm | photocatalysis towards organic dye degradation. | [117] |
| Chlorella vulgaris | silver | 40–90 nm | synthesis of substituted quinolines. | [118] |
| Chlorella vulgaris | palladium | 70 nm | catalytic activity for the synthesis of N-aryl piperazines. | [119] |
| Cladophora vagabunda | fluorescent carbon | 42.78 nm | fluorescent material for biological activities. Staphylococcus aureus, Escherichia coli | [120] |
| Galaxaura elongate | gold | - | agriculture application- protection against plant pathogens. | [121] |
| Galaxaura elongata | gold | 3.85–77.13 nm | antimicrobial applications. Escherichia coli, Klebsiella pneumoniae, MRSA, Staphylococcus aureus, Pseudomonas aeruginosa. | [122] |
| Gelidium amansii | silver | 20–95 nm | antibacterial agents. Staphylococcus aureus, Bacillus pumilus, Escherichia coli, Pseudomonas aeruginosa, Vibrio parahaemolyticus, Aeromonas hydrophila | [123] |
| Gelidium corneum | silver | 20–50 nm | antibiofilm, antimicrobial activity. Candida albicans, Escherichia coli | [124] |
| Gelidiella acerosa | gold | 5.81–117.59 nm | antioxidant activity and antibacterial activity. Escherichia coli, Serratia marcescens, Klebsiella pneumonia, Bacillus subtilis | [125] |
| Gracilaria birdiae | silver | 20.2–94.9 nm | antibacterial activity. Escherichia coli, Staphylococcus aureus | [126] |
| Gracilaria crassa | silver | 122.7 nm | antibacterial activity. Escherichia coli, Proteus mirabilis, Bacillus subtilis, Pseudomonas aeruginosa. | [127] |
| Gracilaria verrucosa | gold | 20–80 nm | anticancer activity. human embryonic kidney HEK-293 cell lines. | [128] |
| Halymenia dilatata | gold | 16 nm | antioxidant, anticancer and antibacterial activity. Aeromonas hydrophila, human colorectal adenocarcinoma HT-29 cell line | [129] |
| Laurencia catarinensis | silver | 39.41–77.71 nm | green chemistry. | [130] |
| Neochloris oleoabundans | silver | 16.63 nm | antibacterial activity Escherichia coli | [131] |
| Padina pavonia | silver | 49.58–86.37 nm | green synthesis. | [132] |
| Polysiphonia sp. | silver | 5–25 nm | anticancer activity breast cancer MCF-7 cell line | [133] |
| Portieria hornemannii | silver | 70–75 nm | anti fish pathogen activity Vibrio harveyii, Vibrio parahaemolyticus, Vibrio alginolyticus, Vibrio anguillarum | [134] |
| Pseudopediastrum boryanum | silver | - | antimicrobial activity Proteus vulgaris, parapsilosis, Pseudomonas aeruginosa, Candida parapsilosis, Aeromonas hydrophila, Staphylococcus epidermidis, Candida parapsilosis, Candida albicans | [135] |
| Spyridia fusiformis | silver | 5–50 nm | antibacterial activity. Escherichia coli, Klebsiella pneumonia, Staphylococcus aureus, Pseudomonas aeruginosa | [136] |
| Sargassum latifolium | selenium zinc | 22.31–95.16 nm | edible oils preservation via bio-reduction reaction to prevent oils oxidation and rancidity. | [137] |
| Sargassum muticum | zinc oxide | 30–57 nm. | liver cancer therapy human liver cancer HepG2 cell line | [138] |
| Sargassum polycystum | copper oxide | - | antimicrobial and anticancer activities. Pseudomonas aeruginosa, Aspergillus niger. MCF-7 cells | [139] |
| Spatoglossum asperum | silver | 28.8 nm | antifungal activity. Aspergillus flavus, Candida albicans, Candida tropicalis, Trichophyton mentagrophytes | [140] |
| Spirulina platensis | palladium | 10–20 nm | adsorption activity in lead removal. | [141] |
| Spyridia filamentosa | silver | 20–30 nm | antibacterial and anticancer activity. Staphylococcus sp. and Klebsiella sp. MCF-7 cells. | [142] |
| Trichodesmium erythraeum | silver | 26.5 nm | antioxidant, drug-resistant antibacterial activity, and cytotoxicity activity. Staphylococcus aureus, Proteus mirabilis, E. coli (AmikacinR), S. aureus (TetracyclineR), S. pneumoniae (PenicillinR). HeLa, MCF-7 cell lines | [143] |
| Ulva armoricana | silver | 215 nm | anticancer, antimicrobial activity. Balb/3T3 mouse embryo fibroblasts. Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, Staphylococcus epidermidis | [144] |
| Ulva armoricana | gold | ~200 nm | catalytic activity towards the reduction of 4-nitrophenol. | [145] |
| Ulva flexuosa | iron oxide | 12.3 ± 1.7 nm | antimicrobial activity. Brachionus rotundiformis. | [146] |
| Ulva intestinalis | gold silver | 17.8± 2.7 nm 14.2± 2 nm | anticancer activity, therapeutic vaccines. Raw 264.7 cells, HL-60 cells, HL-60 cells | [147] |
| Ulva lactuca | gold silver | 7.9 ± 1.7 nm 31 ± 8 nm | colorectal cancer therapy. colon cancer cell lines HT-29 and Caco-2 | [148] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Geetha Bai, R.; Sudheer, S.; Premarathna, A.D.; Tuvikene, R. Marine Macroalgal Polysaccharides in Nanomedicine: Blue Biotechnology Contributions in Advanced Therapeutics. Molecules 2026, 31, 175. https://doi.org/10.3390/molecules31010175
Geetha Bai R, Sudheer S, Premarathna AD, Tuvikene R. Marine Macroalgal Polysaccharides in Nanomedicine: Blue Biotechnology Contributions in Advanced Therapeutics. Molecules. 2026; 31(1):175. https://doi.org/10.3390/molecules31010175
Chicago/Turabian StyleGeetha Bai, Renu, Surya Sudheer, Amal D. Premarathna, and Rando Tuvikene. 2026. "Marine Macroalgal Polysaccharides in Nanomedicine: Blue Biotechnology Contributions in Advanced Therapeutics" Molecules 31, no. 1: 175. https://doi.org/10.3390/molecules31010175
APA StyleGeetha Bai, R., Sudheer, S., Premarathna, A. D., & Tuvikene, R. (2026). Marine Macroalgal Polysaccharides in Nanomedicine: Blue Biotechnology Contributions in Advanced Therapeutics. Molecules, 31(1), 175. https://doi.org/10.3390/molecules31010175







