Organ-Specific Diversity of Secoiridoids in Ligustrum japonicum Thunb.
Abstract
1. Introduction
2. Results and Discussions
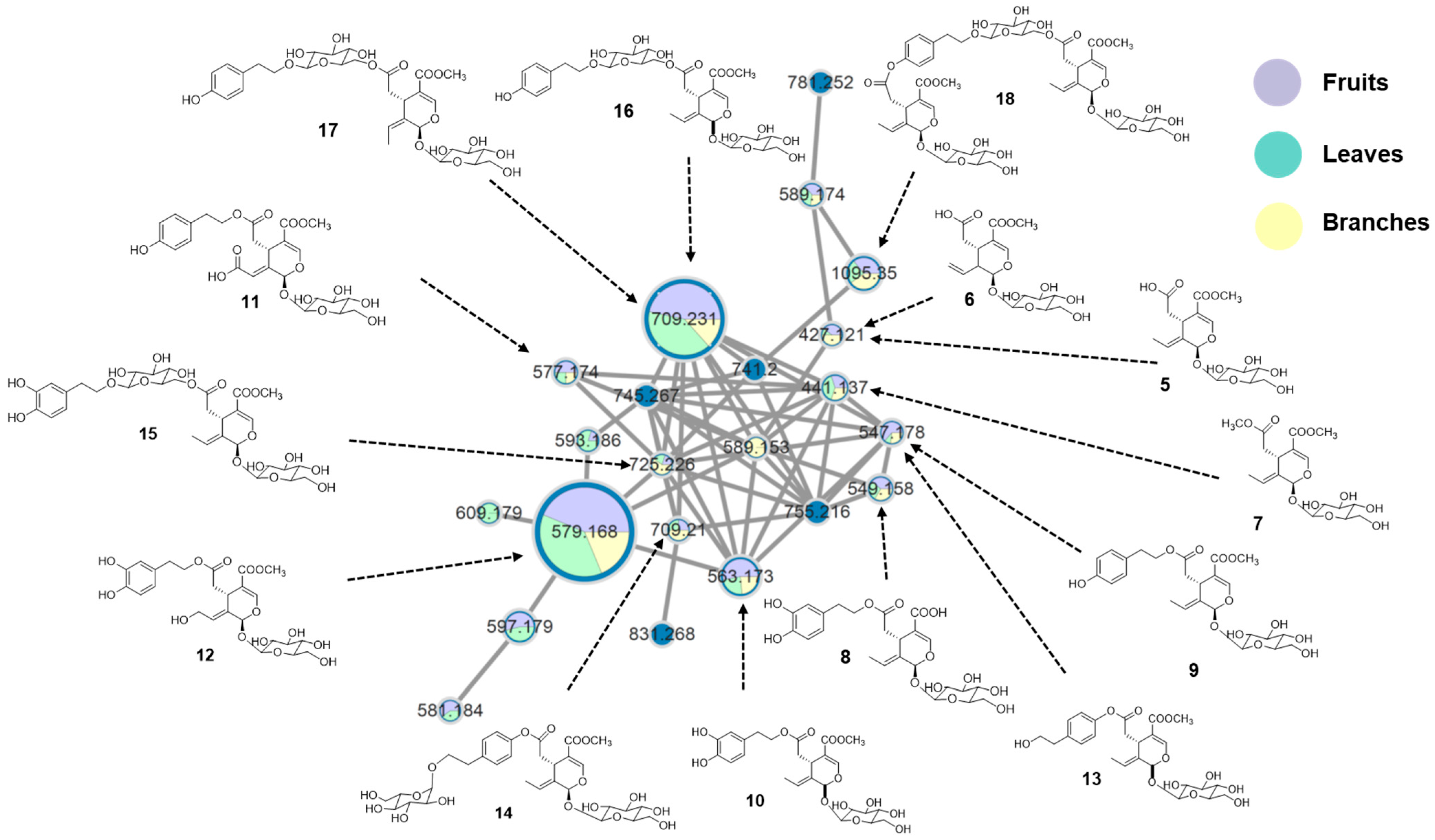
2.1. Molecular Networking Analysis of L. japonicum Extracts
2.2. Isolation of New Secoiridoids from L. japonicum
2.3. Proliferative Effects of Major Secoiridoids on Dermal Papilla Cells
2.4. Underutilized L. japonicum Parts as Potential Sources of Secoiridoids
3. Materials and Methods
3.1. Plant Material
3.2. General Experimental Procedure
3.3. Extraction of the Fruits, Leaves, and Branches of L. japonicum
3.4. UPLC-MS/MS and Molecular Networking
3.5. Isolation of Compounds
3.6. Identification of Structures
3.7. Evaluation of Biological Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HPLC–MS/MS | High-performance liquid chromatography–tandem mass spectrometry |
| GNPS | Directory of open access journals Global Natural Products Social |
| DP | Dermal papilla |
References
- Lee, Y.N. Flora of Korea; Kyohak Publishing Co.: Seoul, Republic of Korea, 2006; pp. 850–852. [Google Scholar]
- Gao, L.; Li, C.; Wang, Z.; Liu, X.; You, Y.; Wei, H.; Guo, T. Ligustri lucidi fructus as a traditional Chinese medicine: A review of its phytochemistry and pharmacology. Nat. Prod. Res. 2015, 29, 493–510. [Google Scholar] [CrossRef]
- Kim, Y.J.; Park, S.Y.; Koh, Y.J.; Lee, J.H. Anti-neuroinflammatory effects and mechanism of action of Fructus Ligustri lucidi extract in BV2 microglia. Plants 2021, 10, 688. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kong, C.S.; Seo, Y. Salidroside, 8(E)-nuezhenide, and ligustroside from Ligustrum japonicum Fructus inhibit expressions of MMP-2 and -9 in HT 1080 fibrosarcoma. Int. J. Mol. Sci. 2022, 23, 2660. [Google Scholar] [CrossRef] [PubMed]
- Katsube, T.; Tabata, H.; Ohta, Y.; Yamasaki, Y.; Anuurad, E.; Shiwaku, K.; Yamane, Y. Screening for antioxidant activity in edible plant products: Comparison of low-density lipoprotein oxidation assay, DPPH radical scavenging assay, and Folin-Ciocalteu assay. J. Agric. Food Chem. 2004, 52, 2391–2396. [Google Scholar] [CrossRef]
- Jang, J.Y.; Shin, H.; Lim, J.W.; Ahn, J.H.; Jo, Y.H.; Lee, K.Y.; Hwang, B.Y.; Jung, S.J.; Kang, S.Y.; Lee, M.K. Comparison of antibacterial activity and phenolic constituents of bark, lignum, leaves and fruit of Rhus verniciflua. PLoS ONE 2018, 13, e0200257. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.H.; Lee, S.; Yeon, S.W.; Turk, A.; Lee, J.H.; Hong, S.M.; Han, Y.K.; Lee, K.Y.; Hwang, B.Y.; Kim, S.Y.; et al. Anti-diabetic potential of Masclura tricuspidata leaves: Prenylated isoflavonoids with alpha-glucosidase inhibitory and anti-glycation activity. Bioorg. Chem. 2021, 114, 105098. [Google Scholar] [CrossRef]
- Anyamele, T.; Onwuegbuchu, P.N.; Ugbogu, E.A.; Ibe, C. Phytochemical composition, bioactive properties, and toxicological profile of Tetrapleura tetraptera. Bioorg. Chem. 2023, 131, 106288. [Google Scholar] [CrossRef]
- Ngo, Q.T.; Lee, H.S.; Nguyen, V.T.; Kim, J.A.; Woo, M.H.; Min, B.S. Chemical constituents from the fruits of Ligustrum japonicum and their inhibitory effects on T cell activation. Phytochemistry 2017, 141, 147–155. [Google Scholar] [CrossRef]
- Fukuyama, Y.; Koshino, K.; Hasegawa, T.; Yamada, T.; Nakagawa, K. New secoiridoid glucosides from Ligustrum japonicum. Planta Med. 1987, 53, 427–431. [Google Scholar] [CrossRef]
- Sung, S.H.; Kim, E.S.; Lee, K.Y.; Lee, M.K.; Kim, Y.C. A new neuroprotective compound of Ligustrum japonicum leaves. Planta Med. 2006, 72, 62–64. [Google Scholar] [CrossRef]
- Park, S.Y.; Han, Y.K.; Vinh, L.B.; Lee, K.Y. Analysis of α-glucosidase inhibitory constituents from Acer tegmentosum using LC-QTOF MS/MS and molecular networking. Nat. Prod. Sci. 2023, 29, 242–250. [Google Scholar] [CrossRef]
- Kim, J.G.; Le, T.P.L.; Han, J.S.; Cho, Y.B.; Lee, D.; Lee, M.K.; Hwang, B.Y. Molecular networking-assisted isolation of chlorophenolic glycosides from the rhizomes of Curculigo orchioides and their inhibitory effect on α-glucosidase. Phytochemistry 2023, 214, 113820. [Google Scholar] [CrossRef] [PubMed]
- Machida, K.; Kaneko, A.; Hosogai, T.; Kakuda, R.; Yaoita, Y.; Kikuchi, M. Studies on the constituents of Syringa species. X. Five new iridoid glycosides from the leaves of Syringa reticulata (Blume) Hara. Chem. Pharm. Bull. 2002, 50, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Calis, I.; Sticher, O. Secoiridoid glucosides from Lonicera periclymenum. Phytochemistry 1984, 23, 2539–2540. [Google Scholar] [CrossRef]
- Kwak, J.H.; Kang, M.W.; Roh, J.H.; Choi, S.U.; Zee, O.P. Cytotoxic Phenolic compounds from Chionanthus retusus. Arch. Pharm. Res. 2009, 32, 1681–1687. [Google Scholar] [CrossRef] [PubMed]
- He, Z.D.; Dong, H.; Xu, H.X.; Ye, W.C.; Sun, H.D.; But, P.P. Secoiridoid constituents from the fruits of Ligustrum lucidum. Phytochemistry 2001, 56, 327–330. [Google Scholar] [CrossRef]
- Huang, X.J.; Wang, Y.; Yin, Z.Q.; Ye, W.C. Two new dimeric secoiridoid glycosides from the fruits of Ligustrum lucidum. J. Asian Nat. Prod. Res. 2010, 12, 685–690. [Google Scholar] [CrossRef]
- Shen, Y.C.; Lin, C.Y.; Chen, C.H. Secoiridoid glycosides from Jasminum multiflorum. Phytochemistry 1990, 29, 2905–2912. [Google Scholar] [CrossRef]
- Rubio-Senent, F.; Martos, S.; García, A.; Fernández-Bolaños, J.G.; Rodríguez-Gutiérrez, G.; Fernández-Bolaños, J. Isolation and characterization of a secoiridoid derivative from two-phase olive waste (Alperujo). J. Agric. Food Chem. 2015, 63, 1151–1159. [Google Scholar] [CrossRef]
- Enshell-Seijffers, D.; Lindon, C.; Wu, E.; Taketo, M.M.; Morgan, B.A. β-Catenin activity in the dermal papilla of the hair follicle regulates pigment-type switching. Proc. Natl. Acad. Sci. USA 2010, 107, 21564–21569. [Google Scholar] [CrossRef]
- Hughes, K.; Ho, R.; Greff, S.; Filaire, E.; Ranouille, E.; Chazaud, C.; Herbette, G.; Butaud, J.F.; Berthon, J.Y.; Raharivelomanana, P. Hair growth activity of three plants of the polynesian cosmetopoeia and their regulatory effect on dermal papilla cells. Molecules 2020, 25, 4360. [Google Scholar] [CrossRef] [PubMed]
- Pérez, J.A.; Hernández, J.M.; Trujillo, J.M.; López, H. Iridoids and secoiridoids from Oleaceae. Stud. Nat. Prod. Chem. 2005, 32, 303–363. [Google Scholar]
- Hashmi, M.A.; Khan, A.; Hanif, M.; Farooq, U.; Perveen, S. Traditional uses, phytochemistry, and pharmacology of Olea europaea (Olive). Evid.-Based Complement. Alternat. Med. 2015, 2015, 541591. [Google Scholar]



| No | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| 1 | 6.09 (s) | 5.44 (d, 7.0) | 3.82 (m) | 3.83 (m) |
| 3 | 7.58 (s) | 7.51 (s) | 7.54 (s) | 7.55 (s) |
| 5 | 4.96 * | 3.40 * | 3.74 (t, 4.7) | 3.77 (t, 4.8) |
| 6 | 2.76 (t, 3.5) | 2.52 (2H, m) | 2.74 (dd, 14.4, 4.8) | 2.68 (dd, 15.2, 5.6) |
| 2.61 (dd, 14.4, 5.2) | 2.55 (dd, 15.2, 4.8) | |||
| 8 | 6.39 (brs) | 2.50 (m) | 6.55 (s) | 6.58 (s) |
| 9 | 2.35 (m), 2.48 (m) | |||
| 11 | 1.31 (3H, d, 6.4) | 1.30 (3H, d, 6.4) | ||
| 1′ | 4.82 (d, 7.7) | 4.69 (d, 7.7) | 4.31 (d, 7.8) | 4.31 (d, 8.0) |
| 2′ | 3.42, m | 3.25 (m) | 3.21 (m) | 3.22 * |
| 3′ | 3.36 * | 3.40 * | 3.43 (m) | 3.40 * |
| 4′ | 3.36 * | 3.40 * | 3.40 * | 3.40 * |
| 5′ | 3.43 (m) | 3.40 * | 3.41 (m) | 3.44 (m) |
| 6′ | 3.99 (m) | 3.82 (m) | 4.36 (m) | 4.38 (dd, 11.6, 2.0) |
| 3.62 (m) | 3.71 (m) | 4.16 (dd, 11.6, 5.2) | 4.15 (dd, 11.6, 6.0) | |
| 1″ | 4.10 (m), 4.04 (m) | |||
| 2″ | 1.63 (2H, m) | 7.08 (d, 8.4) | 7.08 (d, 8.4) | 7.07 (d, 8.8) |
| 3″ | 1.41 (2H, m) | 6.73 (d, 8.4) | 6.71 (d, 8.8) | 6.71 (d, 8.8) |
| 4″ | 0.97 (3H, t, 7.2) | |||
| 5″ | 6.73 (d, 8.4) | 6.71 (d, 8.4) | 6.71 (d, 8.8) | |
| 6″ | 7.08 (d, 8.4) | 7.08 (d, 8.4) | 7.07 (d, 8.8) | |
| 7″ | 2.85 (2H, m) | 2.85 (2H, m) | 2.85 (2H, m) | |
| 8″ | 4.21 (2H, m) | 3.99 (m), 3.71 (m) | 3.95 (m), 3.71 (m) | |
| 8-OCH3 | 3.22 (3H, s) | |||
| 10-OCH3 | 3.72 (3H, s) | 3.72 (3H, s) | ||
| 11-OCH3 | 3.74 (3H, s) | 3.67 (3H, s) | 3 | 4 |
| No | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| 1 | 93.1 | 96.3 | 76.6 | 76.7 |
| 3 | 153.0 | 152.7 | 151.7 | 151.6 |
| 4 | 107.6 | 108.8 | 108.5 | 108.8 |
| 5 | 31.2 | 29.3 | 27.1 | 26.4 |
| 6 | 39.8 | 34.9 | 39.6 | 39.9 |
| 7 | 171.9 | 172.8 | 171.8 | 171.6 |
| 8 | 119.9 | 32.2 | 137.6 | 139.1 |
| 9 | 141.8 | 36.7 | 120.6 | 116.9 |
| 10 | 157.7 | 167.2 | 167.1 | |
| 11 | 166.9 | 167.4 | 19.4 | 17.0 |
| 1′ | 99.6 | 99.1 | 103.1 | 103.0 |
| 2′ | 73.3 | 73.2 | 73.6 | 73.6 |
| 3′ | 77.0 | 77.0 | 73.8 | 76.5 |
| 4′ | 70.0 | 70.1 | 70.1 | 70.2 |
| 5′ | 76.4 | 76.4 | 76.5 | 73.9 |
| 6′ | 61.3 | 61.3 | 63.3 | 63.5 |
| 1″ | 64.3 | 128.6 | 129.3 | 129.3 |
| 2″ | 30.2 | 129.5 | 129.5 | 129.5 |
| 3″ | 18.7 | 114.9 | 114.8 | 114.8 |
| 4″ | 12.6 | 155.7 | 155.4 | 155.4 |
| 5″ | 114.9 | 114.8 | 114.8 | |
| 6″ | 129.5 | 129.5 | 129.5 | |
| 7″ | 33.7 | 35.0 | 35.0 | |
| 8″ | 65.5 | 70.9 | 70.8 | |
| 8-OCH3 | 54.7 | |||
| 10-OCH3 | 50.7 | 50.7 | ||
| 11-OCH3 | 50.4 | 50.4 | 3 | 4 |
| Compounds | Proliferation (%) | ||
|---|---|---|---|
| 60 μM | 40 μM | 20 μM | |
| Control | 100.0 ± 6.3 | ||
| Oleuropein (10) | 125.2 ± 7.3 | 109.4 ± 5.6 | 107.8 ± 7.1 |
| 10-Hydroxyoleuropein (12) | 98.9 ± 9.1 | 100.1 ± 8.0 | 99.0 ± 8.4 |
| Neonüezhenide (15) | 99.8 ± 5.2 | 98.3 ± 4.5 | 99.5 ± 1.4 |
| 8Z-Nüezhenide (17) | 118.9 ± 9.8 | 115.4 ± 7.6 | 116.3 ± 8.9 |
| GL-3 (18) CHIR99021 (2.5 μM) (a) | 115.7 ± 7.9 | 114.1 ± 7.3 158.8 ± 3.7 | 101.8 ± 3.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Yeon, S.W.; Liu, Q.; Lee, H.H.; Kim, S.J.; Lee, S.H.; Kim, M.-O.; Hwang, B.Y.; Lee, M.K. Organ-Specific Diversity of Secoiridoids in Ligustrum japonicum Thunb. Molecules 2026, 31, 174. https://doi.org/10.3390/molecules31010174
Yeon SW, Liu Q, Lee HH, Kim SJ, Lee SH, Kim M-O, Hwang BY, Lee MK. Organ-Specific Diversity of Secoiridoids in Ligustrum japonicum Thunb. Molecules. 2026; 31(1):174. https://doi.org/10.3390/molecules31010174
Chicago/Turabian StyleYeon, Sang Won, Qing Liu, Hak Hyun Lee, Se Jeong Kim, Su Hyeon Lee, Mun-Ock Kim, Bang Yeon Hwang, and Mi Kyeong Lee. 2026. "Organ-Specific Diversity of Secoiridoids in Ligustrum japonicum Thunb." Molecules 31, no. 1: 174. https://doi.org/10.3390/molecules31010174
APA StyleYeon, S. W., Liu, Q., Lee, H. H., Kim, S. J., Lee, S. H., Kim, M.-O., Hwang, B. Y., & Lee, M. K. (2026). Organ-Specific Diversity of Secoiridoids in Ligustrum japonicum Thunb. Molecules, 31(1), 174. https://doi.org/10.3390/molecules31010174







