Biochemical Properties, Antioxidant Activity, and In Vitro Ruminal Fermentation of Four Medicinal Plant Species Grown in Northwestern Tunisia
Abstract
1. Introduction
2. Results
2.1. Chemical Composition of the Studied Plant Species
2.2. Extraction Yield, Phenolic Compound Content, and DPPH Activity
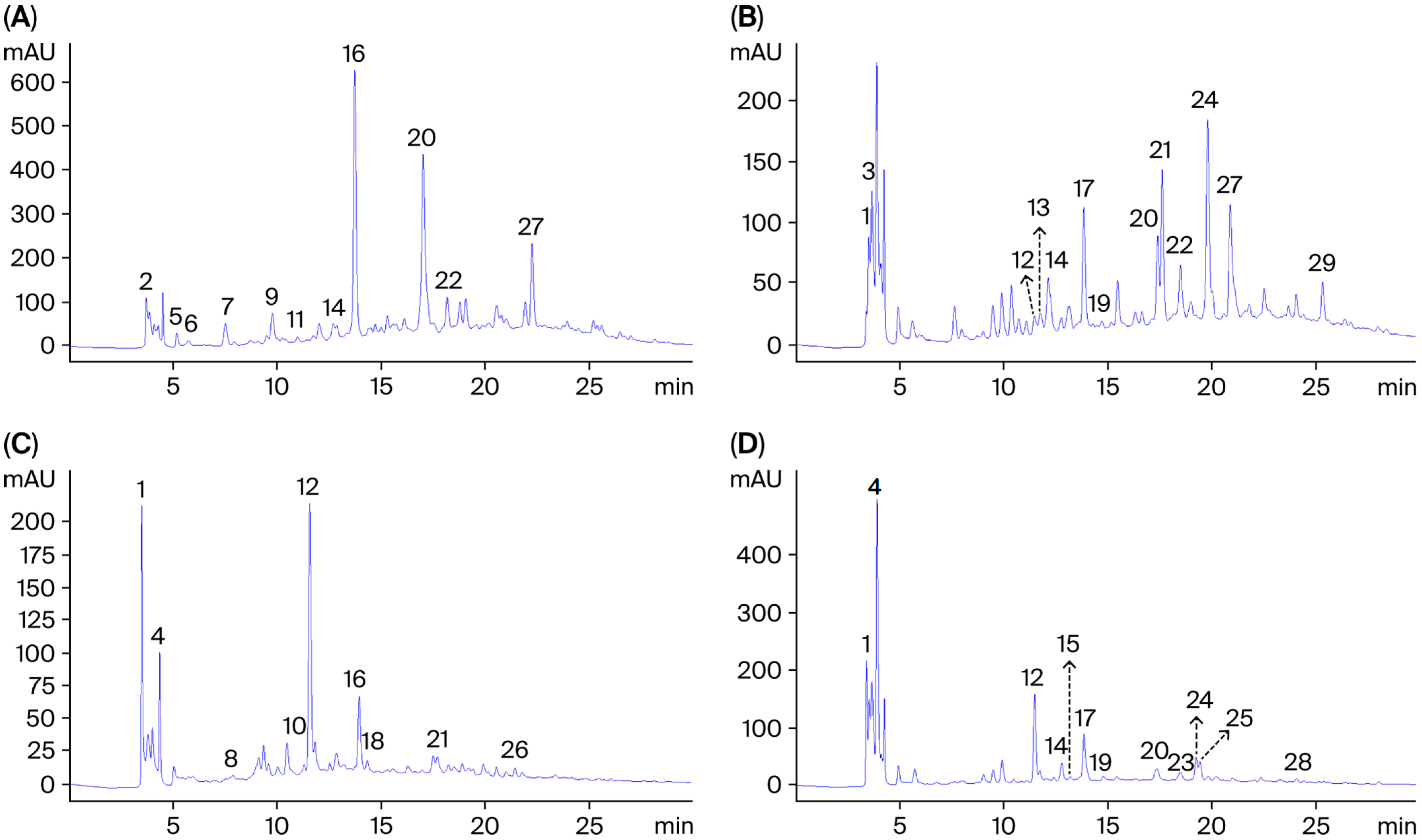
2.3. Compounds Profile Identified in the Aqueous Extract of P. vulgare, C.nobile, O. forsskaolii and L. stoechas
2.4. Effects of Plant Extracts on the In Vitro Kinetics of Gas Production
2.5. In Vitro Impact of Plant Extracts on Ruminal Fermentation Parameters
2.6. GC–MS Profile of Volatile Fatty Acids (VFAs)
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Animal Material
4.3. Phytochemical Analysis
4.3.1. Dry Matter, Ash, Total Nitrogen and Dietary Fiber Determination
4.3.2. Preparation and Yield of Plant Extracts
4.3.3. Total Polyphenol Determination
4.3.4. Total Flavonoid Determination
4.3.5. Antioxidant Activity
4.3.6. HPLC Analysis of Plant Aqueous Extracts
4.4. In Vitro Evaluation of Gas Production
4.5. In Vitro Fermentation Parameters
4.6. GC–MS Analysis of Volatile Fatty Acids (VFAs)
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Estimations des Émissions de gaz à Effet de Serre en Agriculture: Un Manuel pour Répondre aux Exigences de Données des pays en Développement; Organisation des Nations Unies pour l’alimentation et l’agriculture: Rome, Italy, 2015; Available online: https://openknowledge.fao.org/items/49ce77d7-499f-49f0-a254-e2210235eaa8 (accessed on 1 October 2025).
- Agriculture Victoria. Livestock Methane and Nitrogen Emissions. 2024. Available online: https://agriculture.vic.gov.au (accessed on 1 October 2025).
- Climate and Clean Air Coalition (CCAC). Enteric Fermentation and Methane Reduction. 2024. Available online: https://www.ccacoalition.org (accessed on 1 October 2025).
- Patra, A.; Park, T.; Kim, M.; Yu, Z. Rumen methanogens and mitigation of methane emission by anti-methanogenic compounds and substances. J. Anim. Sci. Biotechnol. 2017, 8, 13. [Google Scholar] [CrossRef]
- Yang, H.; Tan, S.; Chen, S.; Wu, Y.; Yang, Y.; Li, H.; Yu, H. Effects of fermented Yupingfeng on intramuscular fatty acids and ruminal microbiota in Qingyuan black goats. Anim. Sci. J. 2021, 92, 13554. [Google Scholar] [CrossRef]
- Iommelli, P.; Spina, A.A.; Vastolo, A.; Infascelli, L.; Lotito, D.; Musco, N.; Tudisco, R. Functional and economic role of some Mediterranean medicinal plants in dairy ruminants’ feeding: A review of the effects of garlic, oregano, and rosemary. Animals 2025, 15, 657. [Google Scholar] [CrossRef]
- Aziz, S.; Ölmez, M.; Nazir, R.; Mobashar, M.; Iqbal, Z.; Naeem, M.I.; Muzaffar, H.A.; Saif, D.; Faizan, M.; Kamran, M. Herbal feed additives for ruminant nutrition. In Complementary and Alternative Medicine: Feed Additives; Abbas, R.Z., Akhtar, T., Asrar, R., Khan, A.M.A., Saeed, Z., Eds.; Unique Scientific Publishers: Faisalabad, Pakistan, 2024; pp. 15–21. [Google Scholar] [CrossRef]
- Bouzazi, M.; Tajini, F.; Selmi, H.; Ouerghui, A.; Sebai, H. Ethno-Veterinary Survey on the Traditional Use of Medicinal Plants with Therapeutic Properties in the Northwestern Regions of Tunisia. IOSR J. Agric. Vet. Sci. 2025, 18, 1–9. [Google Scholar] [CrossRef]
- Farràs, A.; Mitjans, M.; Maggi, F.; Caprioli, G.; Vinardell, M.P.; López, V. Polypodium vulgare L. (Polypodiaceae) as a source of bioactive compounds: Polyphenolic profile, cytotoxicity, and cytoprotective properties in different cell lines. Front. Pharmacol. 2021, 12, 727528. [Google Scholar] [CrossRef]
- Sah, A.; Naseef, P.P.; Kuruniyan, M.S.; Jain, G.K.; Zakir, F.; Aggarwal, G. A Comprehensive Study of Therapeutic Applications of Chamomile. Pharmaceuticals 2022, 15, 1284. [Google Scholar] [CrossRef]
- Alharbi, A.A. In vitro and in vivo anticancer, anti-inflammatory, and antioxidant activity of Dhimran (Ocimum forsskaolii Benth) extract and essential oil on carbon tetrachloride-induced hepatotoxicity in mice. Kafkas Univ. Vet. Fak. Derg. 2024, 30, 729–740. [Google Scholar] [CrossRef]
- Domingues, J.; Delgado, F.; Gonçalves, J.C.; Zuzarte, M.; Duarte, A.P. Mediterranean Lavenders from Section Stoechas: An Undervalued Source of Secondary Metabolites with Pharmacological Potential. Metabolites 2023, 13, 337. [Google Scholar] [CrossRef]
- Dupont, J.; Martin, P. Study of insoluble fibers in Mediterranean plants: Comparison between Asteraceae. J. Medit. Bot. 2017, 24, 145–157. [Google Scholar]
- George, C.F.; Lawrence, N.; Brian, L.; David, R.M. Critical Factors in Determining Fiber Content of Feeds and Foods and Their Ingredients. J. AOAC Int. 2019, 102, 52–62. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, G.; Li, Y.; Zhang, Y. Effects of high forage/concentrate diet on volatile fatty acid production and the microorganisms involved in VFA production in cow rumen. Animals 2020, 10, 223. [Google Scholar] [CrossRef]
- Sebastian, D.M.; Kim, L.J.; Walter, G.; de Sonja, V. Insoluble fibers affect digesta transit behavior in the upper gastrointestinal tract of growing pigs, regardless of particle size. J. Anim. Sci. 2023, 101, skad299. [Google Scholar] [CrossRef] [PubMed]
- Chuang, W.-Y.; Lin, L.-J.; Shih, H.-D.; Shy, Y.-M.; Chang, S.-C.; Lee, T.-T. The potential utilization of high-fiber agricultural by-products as monogastric animal feed and feed additives: A review. Animals 2021, 11, 2098. [Google Scholar] [CrossRef] [PubMed]
- Brinsi, C.; Jedidi, S.; Dhiffalah, A.; Selmi, H.; Sammari, H.; Sebai, H. Nutritional Value, Phytochemical Properties of Different Parts of Dill (Anethum graveolens L.) and Their Effects on in vitro Digestibility in Ruminants. Nat. Prod. Commun. 2024, 19, 1934578X241288854. [Google Scholar] [CrossRef]
- Ez zoubi, Y.; Bousta, D.; Farah, A. A phytopharmacological review of a Mediterranean plant: Lavandula stoechas L. Clin. Phytosci. 2020, 6, 9. [Google Scholar] [CrossRef]
- Polcaro, L.M.; Cerulli, A.; Montella, F.; Ciaglia, E.; Masullo, M.; Piacente, S. Chemical Profile and Antioxidant and Tyrosinase Inhibitory Activity of Chamaemelum nobile L. Green Extracts. Cosmetics 2024, 11, 94. [Google Scholar] [CrossRef]
- Patel, F.; Nainesh, R.M. Estimation of total phenolic content in selected varieties of Ocimum species grown in different environmental conditions. J. Pharmacogn. Phytochem. 2018, 7, 144–148. [Google Scholar]
- Zongo, E.; Busuioc, A.; Meda, R.N.T.; Botezatu, A.V.; Mihaila, M.D.; Mocanu, A.M.; Avramescu, S.M.; Koama, B.K.; Kam, S.E.; Belem, H.; et al. Exploration of the antioxidant and anti-inflammatory potential of Cassia sieberiana DC and Piliostigmathonningii (Schumach.) Milne-Redh, traditionally used in the treatment of hepatitis in the Hauts-Bassins region of Burkina Faso. Pharmaceuticals 2023, 16, 133. [Google Scholar] [CrossRef]
- Tajini, F.; Jelassi, A.; Hamdani, A.; Salem, A.; Abdelhedi, O.; Ouerghui, A.; Sebai, H. Antioxidant Activities and Laxative Effect of Bioactive Compounds from Cynara cardunculus var. sylvestris. Sains Malays. 2024, 53, 1617–1630. [Google Scholar] [CrossRef]
- Dai, Y.-L.; Li, Y.; Wang, Q.; Niu, F.-J.; Li, K.-W.; Wang, Y.-Y.; Wang, J.; Zhou, C.-Z.; Gao, L.-N. Chamomile: A Review of Its Traditional Uses, Chemical Constituents, Pharmacological Activities and Quality Control Studies. Molecules 2023, 28, 133. [Google Scholar] [CrossRef]
- Ndhlala, A.R.; Işık, M.; Kavaz Yüksel, A.; Dikici, E. Phenolic Content Analysis of Two Species Belonging to the Lamiaceae Family: Antioxidant, Anticholinergic, and Antibacterial Activities. Molecules 2024, 29, 480. [Google Scholar] [CrossRef]
- Calabrò, S.; Cutrignelli, M.I.; Bovera, F.; Piccolo, G.; Infascelli, F. In vitro fermentation kinetics of carbohydrate fractions of fresh forage, silage and hay of Avena sativa. J. Sci. Food Agric. 2005, 85, 1528–1536. [Google Scholar] [CrossRef]
- Freitas, R.S.X.d.; da Silva, J.S.; Furtado, A.J.; Perna Junior, F.; de Oliveira, A.L.; da Silva Bueno, I.C. The production of Marandu grass (Urochloabrizantha) extracts as a natural modifier of rumen fermentation kinetics using an in vitro technique. Fermentation 2024, 10, 447. [Google Scholar] [CrossRef]
- Gonzalez Ronquillo, M.; Ghavipanje, N.; Sainz-Ramírez, A.; Danaee Celis-Alvarez, M.; Andrea Plata-Reyes, D.; Robles Jimenez, L.E.; Vargas-Bello Perez, E. Effects of plant extracts on in vitro gas production kinetics and ruminal fermentation of four fibrous feeds: Towards sustainable animal diets. Chil. J. Agric. Anim. Sci. 2023, 39, 319–326. [Google Scholar] [CrossRef]
- Ferchichi, A.; Ben Salem, H.; Makkar, H.P.S.; Fodelianakis, S. Influence of phenolic-rich plants on in vitro ruminal fermentation and methane production. Anim. Feed Sci. Technol. 2019, 248, 138–146. [Google Scholar] [CrossRef]
- Huang, Q.; Chen, Y.; Wang, X.; Wei, Y.; Pan, M.; Zhao, G. Effects of Phlorotannins from Sargassum on In Vitro Rumen Fermentation, Microbiota and Fatty Acid Profile. Animals 2023, 13, 2854. [Google Scholar] [CrossRef]
- Messaoudi, Z.; El Gharbi, S.; Khammassi, S. Antioxidant and antimicrobial properties of Lavandula stoechas extracts and their effect on ruminant digestion. Environ. Toxicol. Pharmacol. 2018, 63, 21–28. [Google Scholar] [CrossRef]
- Elghandour, M.M.M.Y.; Acosta-Lozano, N.; Díaz Alvarado, T.; Castillo-Lopez, E.; Cipriano-Salazar, M.; Barros-Rodríguez, M.; Inyang, U.A.; Purba, R.A.P.; Salem, A.Z.M. Influence of Azadirachta indica and Cnidoscolus angustidens aqueous extract on cattle ruminal gas production and degradability in vitro. Front. Vet. Sci. 2023, 10, 1090729. [Google Scholar] [CrossRef]
- Gao, R.; Du, J.; Gang, G.; Jin, X.; Xing, Y.; Xu, Y.; Hong, L.; Yan, S.; Shi, B. Effects of Artemisia argyi aqueous extract on rumen fermentation parameters and microbiota in lambs. Fermentation 2025, 11, 53. [Google Scholar] [CrossRef]
- Chen, J.; Harstad, O.M.; McAllister, T.; Dörsch, P.; Holo, H. Propionic acid bacteria enhance ruminal feed degradation and reduce methane production in vitro. Acta Agric. Scand. Sect. A Anim. Sci. 2020, 69, 169–175. [Google Scholar] [CrossRef]
- Bedford, A.; Beckett, L.; Hardin, K.; Dias, N.W.; Davis, T.; Mercadante, V.R.G.; Ealy, A.D.; White, R.R. Propionate affects insulin signaling and progesterone profiles in dairy heifers. Sci. Rep. 2018, 8, 17629. [Google Scholar] [CrossRef] [PubMed]
- Patra, A.K.; Amasheh, S.; Aschenbach, J.R. Modulation of gastrointestinal barrier and nutrient transport function in farm animals by natural plant bioactive compounds—A comprehensive review. Crit. Rev. Food Sci. Nutr. 2019, 59, 3237–3266. [Google Scholar] [CrossRef] [PubMed]
- El Tawab, A.M.A.; Khattab, M.S.A.; Hadhoud, F.I.; Shaaban, M.M. Effects of a mixture of herbal plants on ruminal fermentation, degradability and gas production. Acta Scientiarum. Anim. Sci. 2021, 43, e48549. [Google Scholar] [CrossRef]
- Guo, G.; Shen, C.; Liu, Q.; Zhang, S.L.; Shao, T.; Wang, C.; Wang, Y.-X.; Xu, Q.-F.; Huo, W.-J. The effect of lactic acid bacteria inoculums on in vitro rumen fermentation, methane production, ruminal cellulolytic bacteria populations and cellulase activities of corn stover silage. J. Integr. Agric. 2020, 19, 838–847. [Google Scholar] [CrossRef]
- Xiao, M.; Du, L.; Wei, M.; Wang, Y.; Dong, C.; Ju, J.; Zhang, R.; Peng, W.; Wang, Y.; Zheng, Y.; et al. Effects of quercetin on in vitro rumen fermentation parameters, gas production and microflora of beef cattle. Front. Microbiol. 2025, 16, 1527405. [Google Scholar] [CrossRef]
- Sinz, S.; Kunz, C.; Liesegang, A.; Braun, U.; Marquardt, S.; Soliva, C.R.; Kreuzer, M. In vitro bioactivity of various pure flavonoids in ruminal fermentation, with special reference to methane formation. Czech J. Anim. Sci. 2018, 63, 293–304. [Google Scholar] [CrossRef]
- Tánori-Lozano, A.; López-Baca, M.Á.; Muhlia-Almazán, A.; Montalvo-Corral, M.; Pinelli-Saavedra, A.; Islava-Lagarda, T.Y.; Dávila-Ramírez, J.L.; Valenzuela-Melendres, M.; González-Ríos, H. Ferulic Acid and Clinoptilolite Affect In Vitro Rumen Fermentation Characteristics and Bacterial Abundance. Fermentation 2024, 10, 549. [Google Scholar] [CrossRef]
- Manoni, M.; Gschwend, F.; Amelchanka, S.; Terranova, M.; Pinotti, L.; Widmer, F.; Silacci, P.; Tretola, M. Gallic and ellagic acids differentially affect microbial community structures and methane emission when using a rumen simulation technique. J. Agric. Food Chem. 2024, 72, 27163–27176. [Google Scholar] [CrossRef]
- Ramin, M.; Chagas, J.C.; Smidt, H.; Exposito, R.G.; Krizsan, S.J. Enteric and Fecal Methane Emissions from Dairy Cows Fed Grass or Corn Silage Diets Supplemented with Rapeseed Oil. Animals 2021, 11, 1322. [Google Scholar] [CrossRef]
- Malik, P.K.; Kolte, A.P.; Baruah, L.; Saravanan, M.; Bakshi, B.; Bhatta, R. Enteric methane mitigation in sheep through leaves of selected tanniniferous tropical tree species. Livest. Sci. 2017, 200, 29–34. [Google Scholar] [CrossRef]
- Journal Officiel de la République Tunisienne, Loi n° 2005-95 du 18 Octobre 2005 Relative à L’élevage et aux Produits Animaux, JORT n° 83 du 18 Octobre 2005, p. 2688. Available online: https://faolex.fao.org/docs/pdf/tun60846.pdf (accessed on 1 October 2025).
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Method for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Olsen, S.R.; Sommer, L.E. Phosphorus. Chem. Microbiol. Prop. 1982, 9, 403. [Google Scholar]
- Tajini, F.; Boualy, Y.; Ouerghui, A. Study of the Nutritional Quality of Phoenix dactylifera L. fruit: Measurement of Biochemical Parameters. Rev. Nat. Technol. 2020, 12, 39–49. [Google Scholar]
- Sarr, S.O.; Fall, A.D.; Gueye, R.; Diop, A.; Sene, B.; Diatta, K.; Ndiaye, B.; Diop, Y.M. Evaluation de l’activitéantioxydante des extraits des feuilles de Aphania senegalensis (Sapindaceae) et de Saba senegalensis (Apocynaceae). Int. J. Biol. Chem. Sci. 2015, 9, 2676. [Google Scholar] [CrossRef]
- Jedidi, S.; Selmi, H.; Aloui, F.; Rtibi, K.; Jridi, M.; Chaâbane, A.; Sebai, H. Comparative studies of phytochemical screening, HPLC-PDA-ESI-MS/MS-LC-HRESIMS analysis, antioxidant capacity, and in vitro fermentation of officinal sage (Salvia officinalis L.) cultivated in different biotopes of Northwestern Tunisia. Chem. Biodivers. 2019, 10, 1002. [Google Scholar] [CrossRef]
- McDougall, E.I. Studies on ruminant saliva. The composition and output of sheep’s saliva. Biochem. J. 1948, 43, 99–109. [Google Scholar] [CrossRef]
- Orskov, E.R.; Macdonald, I. The estimation of protein degradability in the rumen from incubation measurements weighted according to rate of passage. J. Agric. Sci. Camb. 1979, 92, 499–502. [Google Scholar] [CrossRef]
- Menke, K.H.; Steingass, H. Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim. Res. Dev. 1988, 28, 7–55. [Google Scholar]
- Makkar, H.P.S. Development and field evaluation of animal feed supplementation packages. In Proceedings of the Final Review Meeting of an IAEA Technical Cooperation Regional AFRA, Joint FAO/IAEA Division of Nuclear Techniques in Food and Agriculture, Cairo, Egypt, 25–29 November 2000; pp. 1–66. [Google Scholar]
- SAS. Statistical Analysis System, User’s Guide, Version 8.2; SAS Institute Inc.: Carry, NC, USA, 2000. [Google Scholar]

| Parameters | Content (%DM) | |||
|---|---|---|---|---|
| P. vulgare | C. nobile | O. forsskaolii | L. stoechas | |
| DM | 24.25 ± 0.04 b | 21.46 ± 0.03 c | 19.87 ± 0.17 c | 27.49 ± 0.12 a |
| Ash | 4.55 ± 0.01 ab | 3.99 ± 0.01 b | 3.67 ± 0.03 b | 6.11 ± 0.02 a |
| TN | 1.97 ± 0.08 b | 3.39 ± 0.42 a | 3.20 ± 0.69 a | 1.78 ± 0.14 b |
| CP | 12.30 ± 0.49 b | 21.16 ± 2.62 a | 20.02 ± 4.31 a | 11.15 ± 0.86 b |
| NDF | 48.23 ± 0.70 b | 41.04 ± 0.99 d | 43.48 ± 0.41 cd | 62.39 ± 0.71 a |
| ADF | 27.13 ± 0.11 b | 23.94 ± 0.24 c | 26.46 ± 1.01 b | 40.22 ± 1.12 a |
| ADL | 14.58 ± 0.06 a | 6.72 ± 0.05 bc | 7.92 ± 0.50 b | 5.65 ± 0.15 c |
| HC | 21.10 ± 0.59 a | 17.10 ± 0.75 b | 17.02 ± 0.60 b | 22.17 ± 1.84 a |
| CC | 12.55 ± 0.18 c | 17.22 ± 0.19 b | 18.54 ± 0.50 b | 34.50 ± 0.97 a |
| Assay | P. vulgare | C. nobile | O. forsskaolii | L. stoechas |
|---|---|---|---|---|
| Yield (%) | 12.42 ± 0.37 b | 16.22 ± 0.41 a | 17.04 ± 0.85 a | 16.44 ± 0.62 a |
| Polyphenols (mg GAE/g DW) | 30.45 ± 0.26 c | 73.88 ± 0.79 a | 60.70 ± 0.62 b | 72.69 ± 0.05 a |
| Flavonoids (mg QE/g DW) | 9.03 ± 0.24 c | 27.85 ± 0.54 a | 19.25 ± 0.20 b | 20.11 ± 0.12 b |
| DPPH IC50 (mg/mL) | 1.79 ± 0.08 a | 0.38 ± 0.002 b | 0.36 ± 0.006 b | 0.40 ± 0.003 b |
| Peaks Numbers | Compounds a | RT b | P. vulgare | C. nobile | O. forsskaolii | L. stoechas |
|---|---|---|---|---|---|---|
| 1 | Ascorbic acid | 3.69 | - | 5.81 | 14.22 | 9.44 |
| 2 | Shikimic acid | 3.71 | 2.52 | - | - | - |
| 3 | Apigenine-7-O-glucoside | 3.93 | - | 9.39 | - | - |
| 4 | Apigenin | 4.29 | - | - | 6.89 | 21.40 |
| 5 | Gallic acid | 5.18 | 0.88 | - | - | - |
| 6 | 5-O-caffeoylquinic acid | 5.74 | 0.50 | - | - | - |
| 7 | 3-O-caffeoylquinic acid | 7.51 | 2.29 | - | - | - |
| 8 | Rosmarinic acid | 7.83 | - | - | 1.06 | - |
| 9 | Hyperoside | 9.77 | 2.75 | - | - | - |
| 10 | Kaempferol | 10.44 | - | - | 3.76 | - |
| 11 | 3,5-Dicaffeoylquinic acid | 10.98 | 0.41 | - | - | - |
| 12 | Chlorogenic acid | 11.50 | - | 0.99 | 23.53 | 10.30 |
| 13 | Résorcinol | 11.84 | - | 1.31 | - | - |
| 14 | Catechin | 12.70 | 1.37 | 3.92 | - | 2.56 |
| 15 | Vanillic acid | 13.29 | - | - | - | 0.66 |
| 16 | Catechol | 13.73 | 22.12 | - | 7.63 | - |
| 17 | Epicatechin | 13.94 | - | 5.80 | - | 7.63 |
| 18 | Syringic acid | 14.32 | - | - | 1.17 | - |
| 19 | Caffeic acid | 14.88 | - | 0.34 | - | 0.73 |
| 20 | Ellagic acid | 17.02 | 20.59 | 3.92 | - | 2.65 |
| 21 | Luteolin | 17.74 | - | 6.83 | 1.97 | - |
| 22 | Sinapic acid | 18.17 | 3.68 | 3.13 | - | - |
| 23 | p-Coumaric acid | 18.61 | - | - | - | 1.94 |
| 24 | Ferulic acid | 19.36 | - | 10.42 | - | 3.02 |
| 25 | m-Coumaric acid | 19.55 | - | - | - | 2.45 |
| 26 | Quercetin | 21.80 | - | - | 0.51 | - |
| 27 | Myricetin | 22.26 | 6.89 | 7.56 | - | - |
| 28 | Cinnamic acid | 24.22 | - | - | - | 0.41 |
| 29 | Ferulic-1-O-glucoside acid | 25.49 | - | 1.79 | - | - |
| Animals | Plants | Doses (µL) | a (mL) | b (mL) | c (mL/h) | Gp at 24 h(mL) | Gp at 48 h(mL) | CH4 (mL) |
|---|---|---|---|---|---|---|---|---|
| Sheep | P. vulgare | D0 | −1.36 ± 0.01 a | 61.82 ± 0.02 b | 0.012 b | 14.67 ± 0.29 b | 25.33 ± 0.29 b | 10.50 ± 0.50 b |
| D10 | −1.19 ± 0.01 a | 76.10 ± 0.01 a | 0.015 b | 20.50 ± 0.50 a | 36.00 ± 0.00 a | 11.50 ± 1.32 ab | ||
| D20 | −2.07 ± 0.01 b | 56.49 ± 0.04 b | 0.023 a | 21.17 ± 0.58 a | 33.50 ± 0.00 a | 13.83 ± 1.04 a | ||
| C. nobile | D0 | −1.36 ± 0.01 a | 61.82 ± 0.02 c | 0.012 b | 14.67 ± 0.29 c | 25.33 ± 0.29 c | 10.50 ± 0.50 b | |
| D10 | −3.45 ± 0.01 b | 82.87 ± 0.01 b | 0.023 a | 35.17 ± 0.28 b | 50.33 ± 0.29 b | 8.17 ± 0.76 b | ||
| D20 | −3.68 ± 0.01 b | 112.70 ± 0.04 a | 0.020 a | 40.50 ± 0.87 a | 62.00 ± 0.00 a | 22.00 ± 0.00 a | ||
| O. forsskaolii | D0 | −1.36 ± 0.01 a | 61.82 ± 0.02 c | 0.012 b | 14.67 ± 0.29 c | 25.33 ± 0.29 c | 10.50 ± 0.50 b | |
| D10 | −3.18 ± 0.01 b | 69.00 ± 0.20 b | 0.022 a | 25.17 ± 0.29 b | 38.50 ± 0.00 b | 12.16 ± 1.26 b | ||
| D20 | −5.38 ± 0.01 c | 82.81 ± 0.01 a | 0.023 a | 30.33 ± 0.28 a | 46.00 ± 0.00 a | 24.83 ± 0.29 a | ||
| L. stoechas | D0 | −1.36 ± 0.01 a | 61.82 ± 0.02 c | 0.012 b | 14.67 ± 0.29 b | 25.33 ± 0.29 c | 10.50 ± 0.50 b | |
| D10 | −3.39 ± 0.01 b | 82.13 ± 0.31 b | 0.016 a | 22.33 ± 0.29 a | 37.00 ± 0.00 b | 16.00 ± 1.00 a | ||
| D20 | −4.66 ± 0.05 c | 106.10 ± 0.01 a | 0.014 ab | 26.50 ± 0.50 a | 42.00 ± 0.00 a | 17.00 ± 0.01 a | ||
| Goats | P. vulgare | D0 | −1.42 ± 0.01 b | 50.38 ± 0.01 c | 0.016 a | 14.50 ± 0.50 a | 24.17 ± 0.29 a | 10.50 ± 0.50 a |
| D10 | 1.47 ± 0.01 a | 395.30 ± 4.01 a | 0.001 c | 13.17 ± 0.76 a | 26.00 ± 0.01 a | 9.33 ± 1.04 a | ||
| D20 | −1.84 ± 0.01 b | 67.67 ± 0.01 b | 0.013 b | 16.17 ± 0.76 a | 27.00 ± 0.01 a | 10.0 ± 0.50 a | ||
| C. nobile | D0 | −1.42 ± 0.01 b | 50.38 ± 0.01 b | 0.016 b | 14.50 ± 0.50 c | 24.17 ± 0.29 c | 10.50 ± 0.50 b | |
| D10 | −0.69 ± 0.01 a | 87.81 ± 0.03 a | 0.015 b | 25.50 ± 1.00 b | 42.66 ± 0.58 b | 12.33 ± 1.04 b | ||
| D20 | −1. 90 ± 0.01 b | 93.12 ± 0.01 a | 0.020 a | 34.00 ± 0.50 a | 52.00 ± −0.00 a | 24.00 ± 2.00 a | ||
| O. forsskaolii | D0 | −1.42 ± 0.01 a | 50.38 ± 0.01 b | 0.016 c | 14.50 ± 0.50 b | 24.17 ± 0.29 b | 10.50 ± 0.50 b | |
| D10 | −2.27 ± 0.01 b | 64.21 ± 0.01 a | 0.025 b | 28.33 ± 0.76 a | 40.50 ± 0.00 a | 13.33 ± 1.53 a | ||
| D20 | −1.37 ± 0.01 a | 60.89 ± 0.41 a | 0.029 a | 29.83 ± 0.29 a | 42.00 ± 0.00 a | 14.33 ± 0.58 a | ||
| L. stoechas | D0 | −1.42 ± 0.01 a | 50.38 ± 0.01 c | 0.016 b | 14.50 ± 0.50 c | 24.17 ± 0.29 c | 10.50 ± 0.50 b | |
| D10 | −2.52 ± 0.01 b | 177.9 ± 0.06 a | 0.007 c | 22.83 ± 0.29 a | 41.50 ± 0.00 a | 9.33 ± 0.50 b | ||
| D20 | −3.38 ± 0.01 c | 64.45 ± 0.01 b | 0.025 a | 26.67 ± 0.58 a | 39.00 ± 0.00 a | 19.00 ± 1.50 a | ||
| Plant effect | *** | * | *** | *** | *** | *** | ||
| Animal effect | *** | ns | ns | ** | ** | ns | ||
| Dose effect | *** | ** | *** | *** | *** | *** | ||
| Plants | Doses | ME (MJ/kg DM) | OMd (%) | VFA (mmol) | |||
|---|---|---|---|---|---|---|---|
| Sheep | Goats | Sheep | Goats | Sheep | Goats | ||
| P. vulgare | D0 | 4.33 ± 0.04 c | 4.31 ± 0.07 a | 29.53 ± 0.26 b | 29.38 ± 0.44 b | 0.29 ± 0.01 b | 0.28 ± 0.01 b |
| D10 | 5.13 ± 0.07 a | 4.13 ± 0.10 a | 34.71 ± 0.44 a | 28.19 ± 0.68 b | 0.43 ± 0.01 a | 0.25 ± 0.02 b | |
| D20 | 5.22 ± 0.08 a | 4.54 ± 0.10 a | 35.30 ± 0.51 a | 30.86 ± 0.68 a | 0.45 ± 0.01 a | 0.33 ± 0.02 a | |
| C. nobile | D0 | 4.33 ± 0.04 c | 4.31 ± 0.07 c | 29.53 ± 0.26 c | 29.38 ± 0.44 c | 0.29 ± 0.01 c | 0.28 ± 0.01 c |
| D10 | 7.12 ± 0.04 b | 5.81 ± 0.14 b | 47.75 ± 0.26 b | 39.16 ± 0.89 b | 0.78 ± 0.01 b | 0.55 ± 0.02 b | |
| D20 | 7.85 ± 0.12 a | 6.96 ± 0.07 a | 52.49 ± 0.77 a | 46.71 ± 0.44 a | 0.90 ± 0.02 a | 0.75 ± 0.01 a | |
| O. forsskaolii | D0 | 4.33 ± 0.04 c | 4.31 ± 0.07 b | 29.53 ± 0.26 c | 29.38 ± 0.44 b | 0.29 ± 0.01 c | 0.28 ± 0.01 b |
| D10 | 5.76 ± 0.04 b | 6.19 ± 0.10 a | 38.86 ± 0.25 b | 41.68 ± 0.68 a | 0.54 ± 0.01 b | 0.62 ± 0.02 a | |
| D20 | 6.49 ± 0.01 a | 6.40 ± 0.03 a | 43.60 ± 0.02 a | 43.05 ± 0.22 a | 0.66 ± 0.01 a | 0.65 ± 0.01 a | |
| L. stoechas | D0 | 4.33 ± 0.04 c | 4.31 ± 0.07 b | 29.53 ± 0.26 c | 29.38 ± 0.44 c | 0.29 ± 0.01 c | 0.28 ± 0.01 c |
| D10 | 5.38 ± 0.04 b | 5.44 ± 0.04 b | 36.34 ± 0.26 b | 36.79 ± 0.26 b | 0.47 ± 0.01 b | 0.48 ± 0.01 b | |
| D20 | 5.94 ± 0.07 a | 5.97 ± 0.08 a | 40.05 ± 0.44 a | 40.19 ± 0.51 a | 0.57 ± 0.01 a | 0.57 ± 0.01 a | |
| Plant effect | *** | *** | *** | ||||
| Animal effect | * | * | * | ||||
| Dose effect | *** | *** | *** | ||||
| Animals | Plants | Doses (µL) | Compounds (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetic Acid | Propanoic Acid | n-Butyric Acid | Isobutyric Acid | 3-Methylbut- Anoic Acid | 2-Methylbut- Anoic Acid | n-Pent- Anoic Acid | Isopropyl- Acetate | n-Propyl- Acetate | 4-Methyl-2- Pentanone | A/P Ratio | |||
| Sheep | Control | D0 | 23.96 | 5.23 | 29.11 | 4.02 | 6.88 | 6.52 | 5.52 | 0.76 | 4.12 | 11.89 | 4.58 |
| P. vulgare | D10 | 8.85 | 17.71 | 32.44 | 3.78 | 6.24 | 6.21 | 4.94 | 1.00 | 4.93 | 2.97 | 0.50 | |
| D20 | 21.61 | 13.59 | 39.95 | 3.48 | 7.28 | 5.96 | — | 1.29 | 1.75 | — | 1.59 | ||
| C. nobile | D10 | 6.01 | 19.00 | 32.55 | — | 6.42 | 6.00 | 4.92 | 1.02 | 4.85 | — | 0.32 | |
| D20 | 22.67 | 16.96 | 35.03 | 3.51 | 6.7 | 5.52 | 3.83 | 1.44 | 1.22 | — | 1.34 | ||
| O. forsskaolii | D10 | 14.7 | 22.95 | 25.98 | 2.43 | 3.75 | 3.15 | 2.69 | 0.49 | — | — | 0.64 | |
| D20 | 33.66 | 15.95 | 37.24 | 2.41 | 3.21 | 1.35 | — | 1.69 | 1.64 | 0.55 | 2.11 | ||
| L. stoechas | D10 | 26.2 | — | 31.59 | 3.36 | 6.02 | 5.62 | 4.92 | 0.84 | 3.77 | 10.88 | — | |
| D20 | 40.41 | — | — | — | — | — | — | — | — | — | — | ||
| Goats | Control | D0 | 17.07 | 13.67 | 29.15 | 8.40 | 4.45 | 4.74 | 4.45 | 2.85 | 14.32 | 0.90 | 1.25 |
| P. vulgare | D10 | 10.73 | — | 29.21 | — | 4.42 | 5.75 | — | 6.88 | 27.25 | 2.02 | — | |
| D20 | 32.72 | — | — | — | — | — | — | — | — | — | — | ||
| C. nobile | D10 | 4.38 | 10.79 | 32.61 | — | 4.76 | 5.29 | 4.24 | 4.65 | 20.66 | 1.43 | 0.40 | |
| D20 | 9.69 | 11.29 | 31.29 | 11.03 | 6.07 | 7.48 | — | 4.15 | 17.75 | 1.25 | 0.86 | ||
| O. forsskaolii | D10 | 18.63 | 5.07 | 28.16 | 5.35 | 3.98 | 4.13 | — | 3.67 | 19.92 | 1.34 | 3.67 | |
| D20 | 35.00 | 12.49 | 22.47 | 6.44 | 4.03 | 4.12 | 3.50 | 2.59 | 11.58 | 0.84 | 2.48 | ||
| L. stoechas | D10 | 14.68 | 33.05 | 29.87 | — | 4.10 | 5.48 | — | 4.72 | — | 1.70 | 0.44 | |
| D20 | 38.53 | — | — | — | — | — | — | — | — | — | — | ||
| Components | Oat Hay | Concentrate |
|---|---|---|
| Dry matter (%) | 85.00 | 92.00 |
| Mineral matter (%) | 7.28 | 8.09 |
| Organic matter (%) | 92.72 | 91.91 |
| Crude protein (%) | 2.53 | 23.41 |
| NDF (%) | 68.77 | 42.60 |
| ADF (%) | 36.43 | 8.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouzazi, M.; Selmi, H.; Tajini, F.; Jridi, M.; Jallouli, S.; Ouerghui, A.; Sebai, H. Biochemical Properties, Antioxidant Activity, and In Vitro Ruminal Fermentation of Four Medicinal Plant Species Grown in Northwestern Tunisia. Molecules 2025, 30, 4451. https://doi.org/10.3390/molecules30224451
Bouzazi M, Selmi H, Tajini F, Jridi M, Jallouli S, Ouerghui A, Sebai H. Biochemical Properties, Antioxidant Activity, and In Vitro Ruminal Fermentation of Four Medicinal Plant Species Grown in Northwestern Tunisia. Molecules. 2025; 30(22):4451. https://doi.org/10.3390/molecules30224451
Chicago/Turabian StyleBouzazi, Monia, Houcine Selmi, Fatma Tajini, Mourad Jridi, Selim Jallouli, Abid Ouerghui, and Hichem Sebai. 2025. "Biochemical Properties, Antioxidant Activity, and In Vitro Ruminal Fermentation of Four Medicinal Plant Species Grown in Northwestern Tunisia" Molecules 30, no. 22: 4451. https://doi.org/10.3390/molecules30224451
APA StyleBouzazi, M., Selmi, H., Tajini, F., Jridi, M., Jallouli, S., Ouerghui, A., & Sebai, H. (2025). Biochemical Properties, Antioxidant Activity, and In Vitro Ruminal Fermentation of Four Medicinal Plant Species Grown in Northwestern Tunisia. Molecules, 30(22), 4451. https://doi.org/10.3390/molecules30224451





