Nitrogen-Doped Hollow Carbon Spheres-Decorated Co2SnO4/WS2 Heterostructures with Improved Visible-Light Photocatalytic Degradation of Organic Dye
Abstract
1. Introduction
2. Results and Discussion
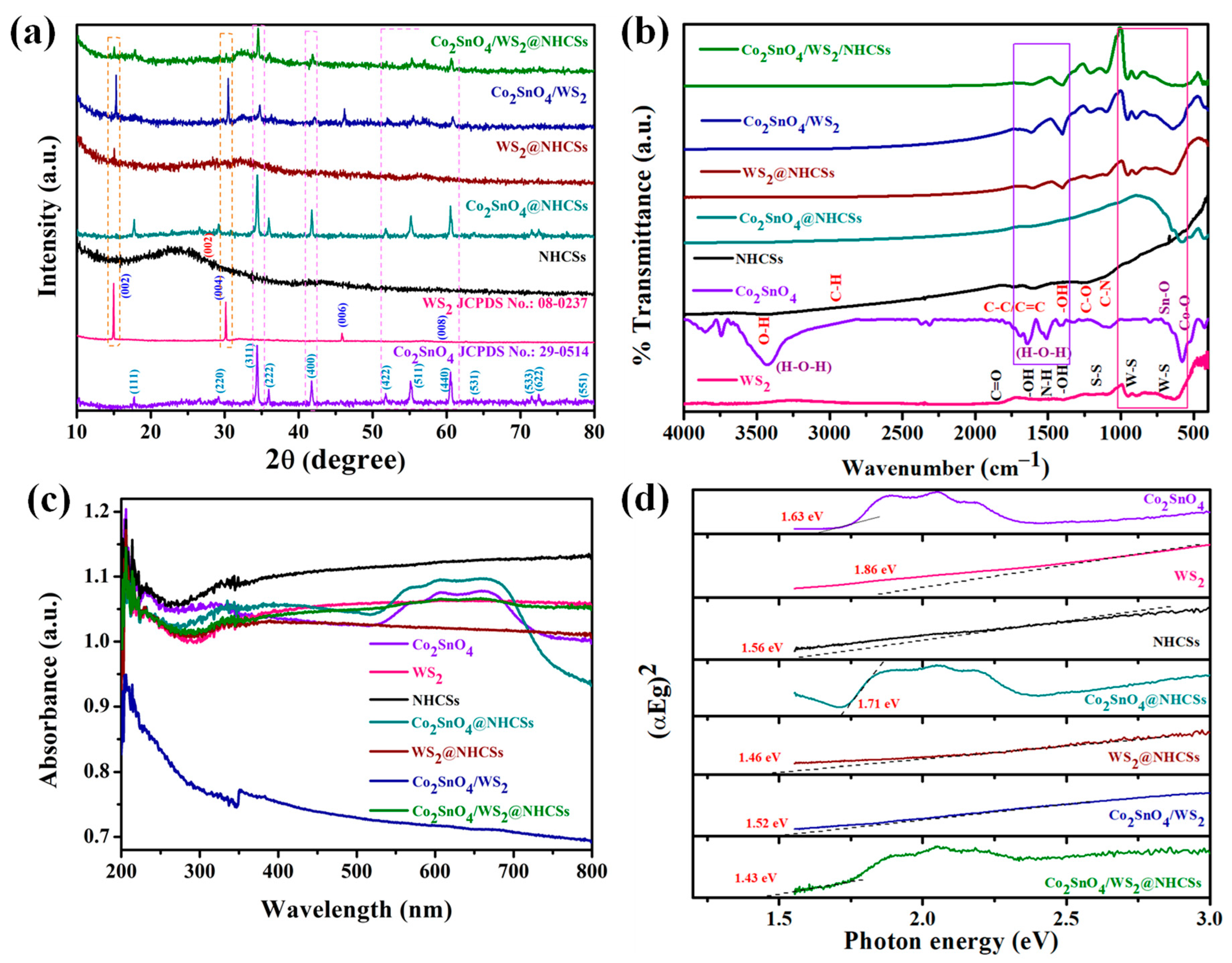
2.1. Crystalline Structure and Functional Group Analysis
2.2. Optical Properties Analysis
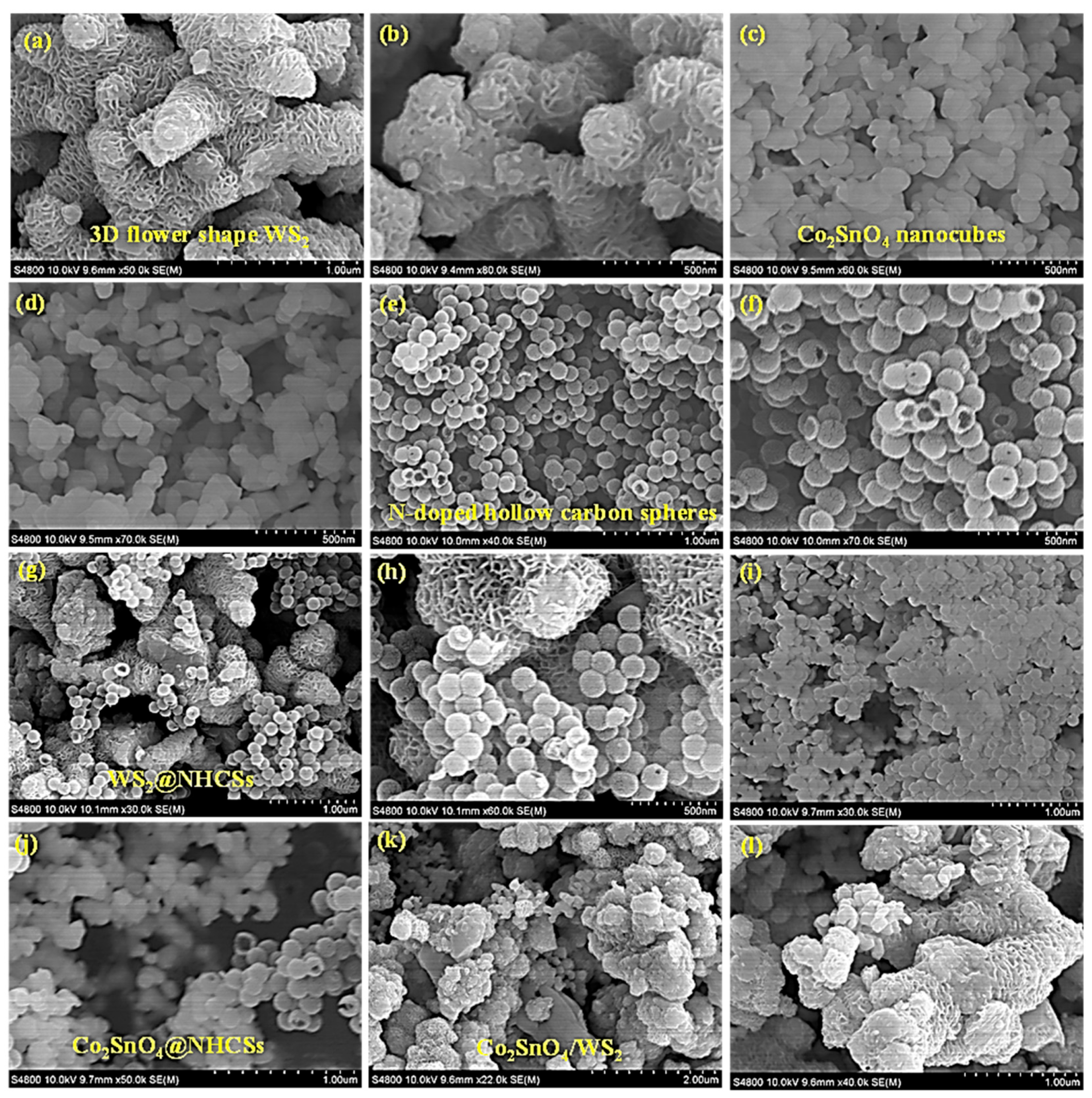
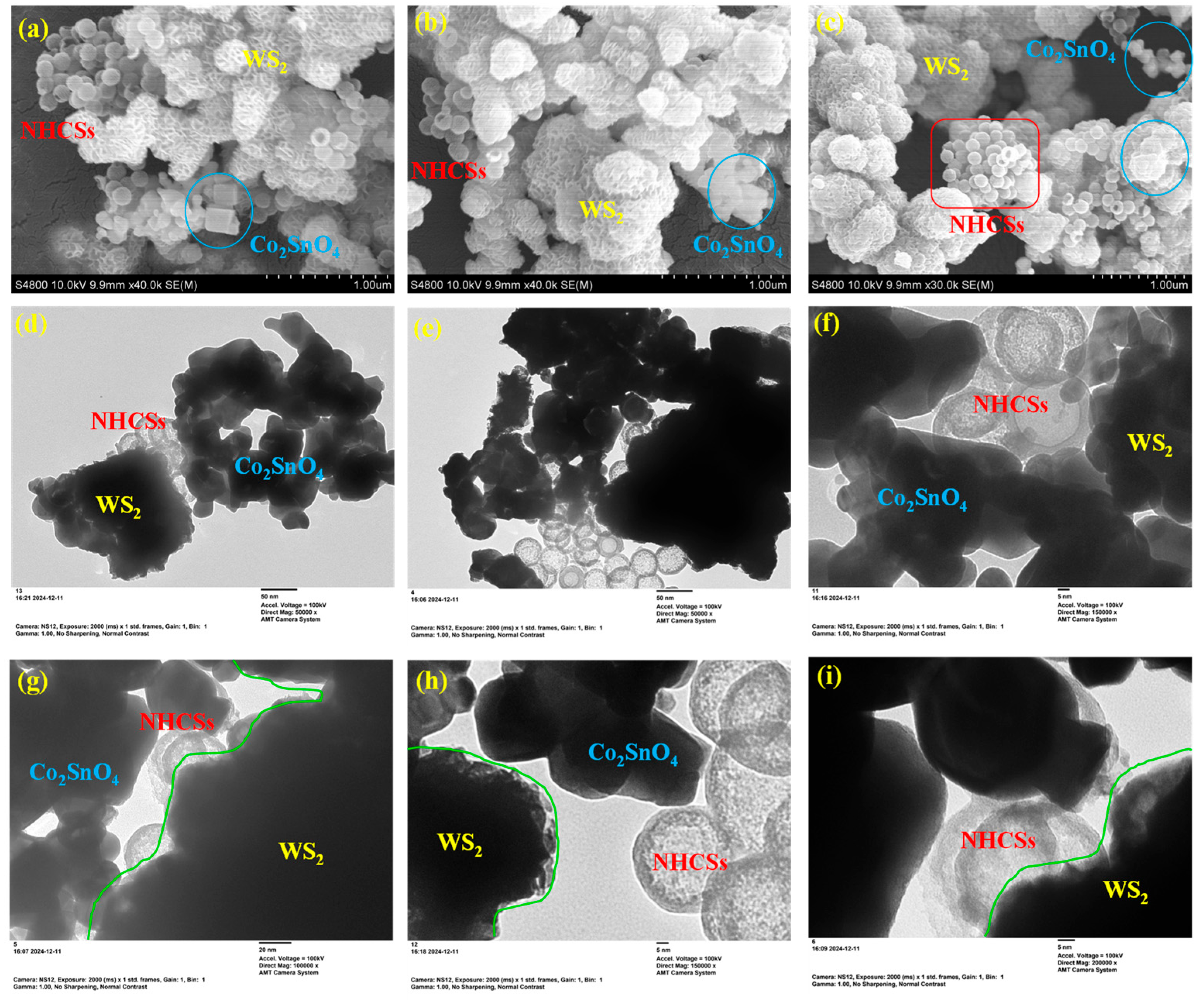
2.3. Morphology Structure Analysis
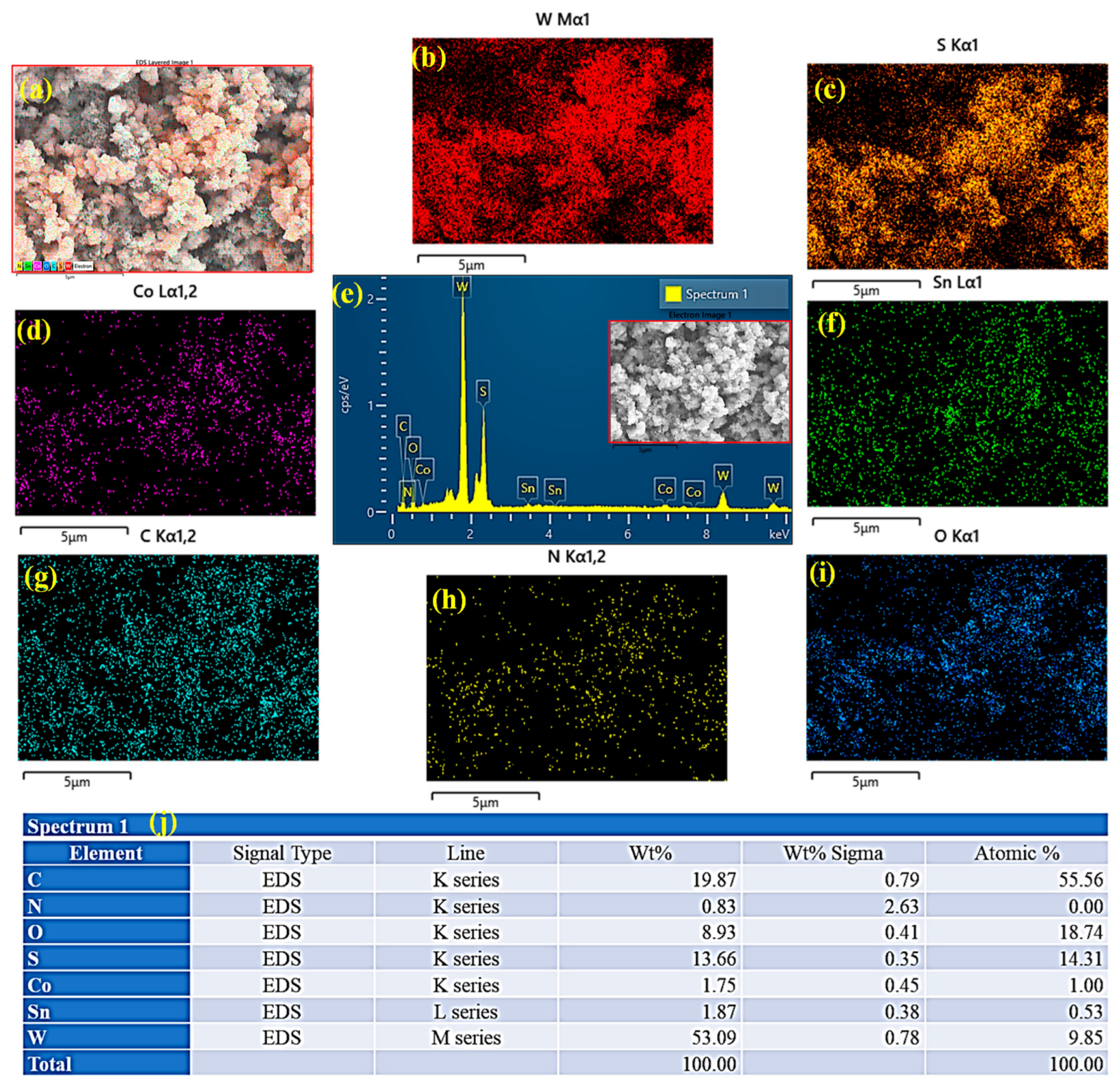
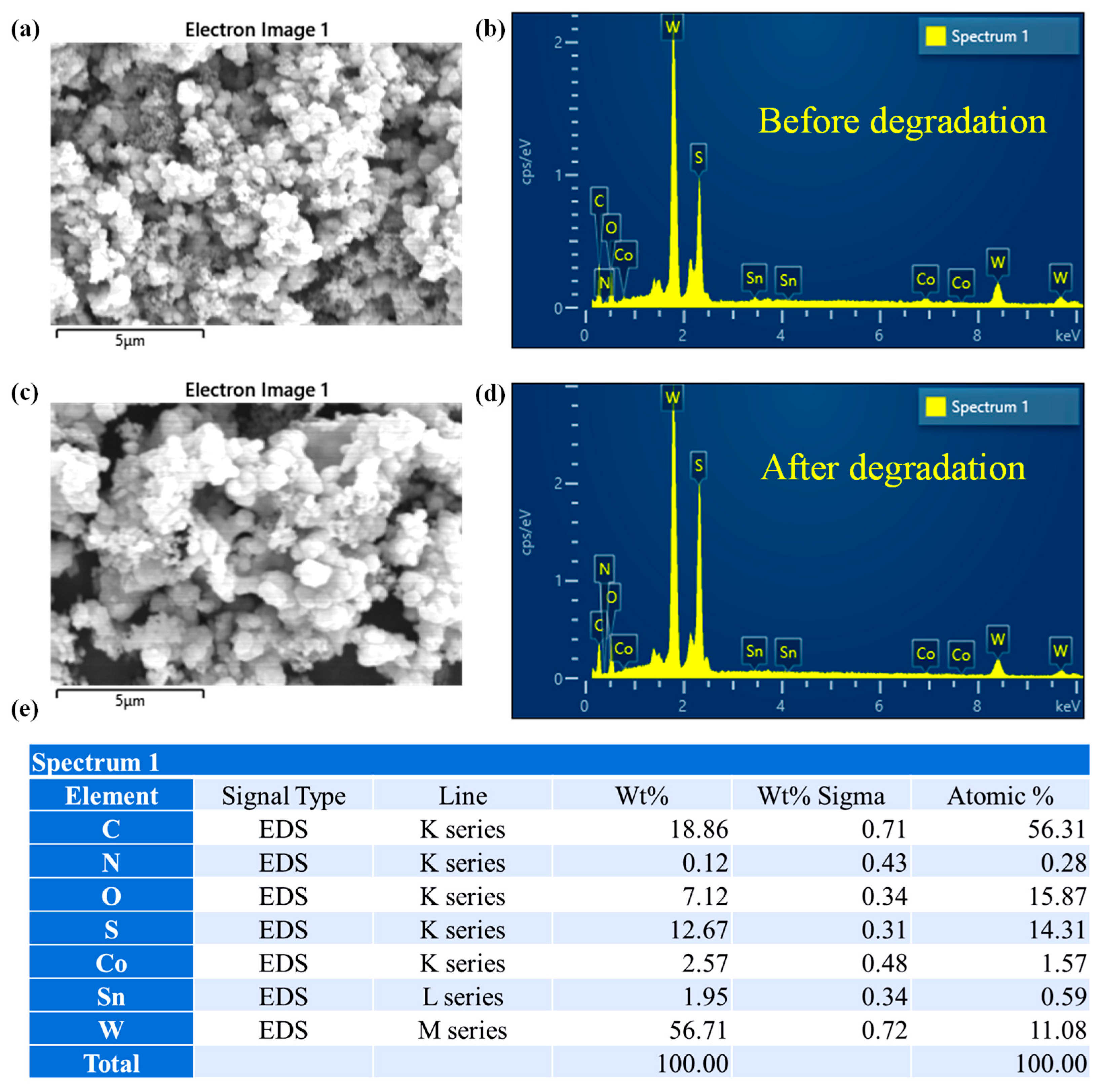
2.4. Elemental Composition Analysis
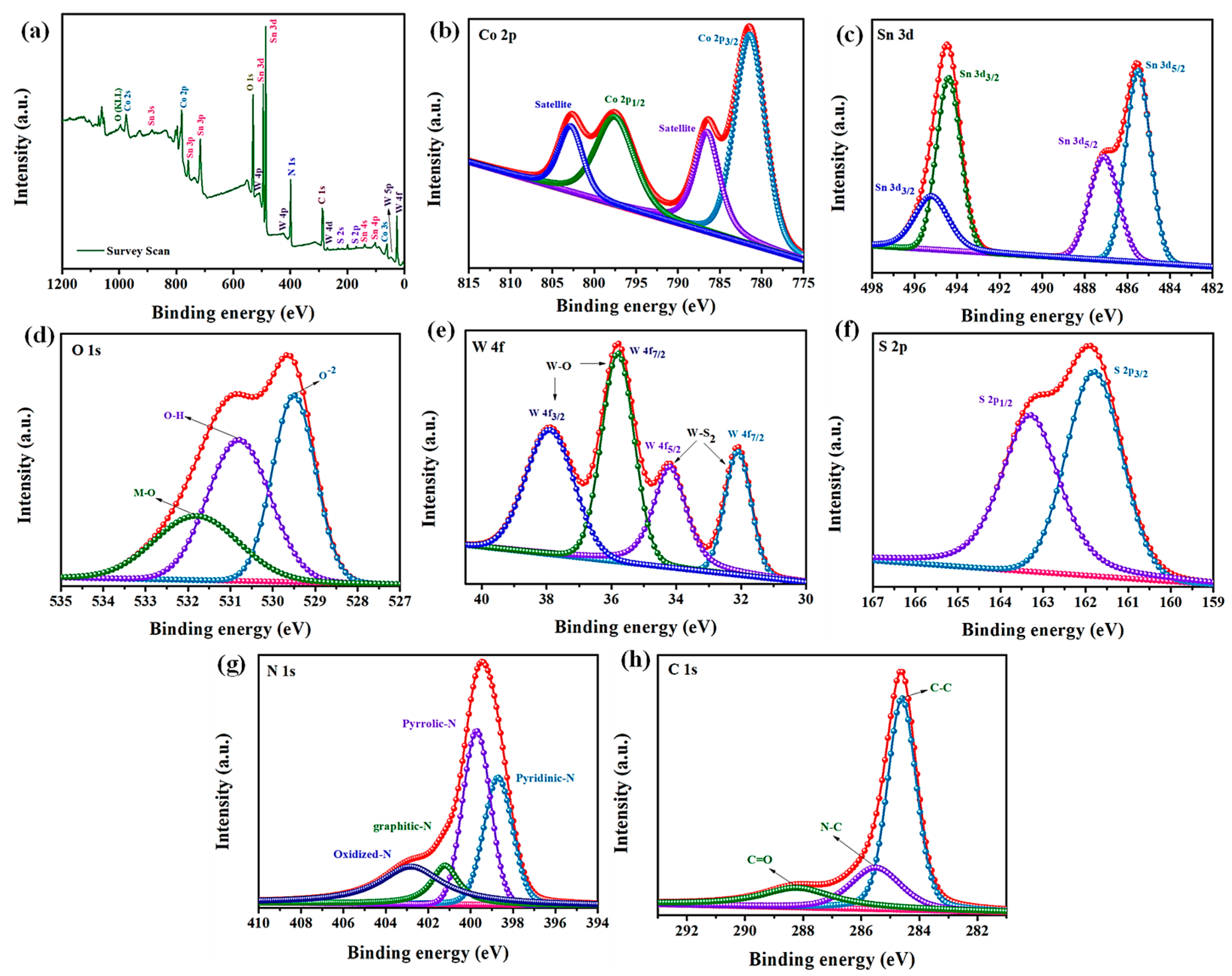
2.5. XPS Analysis
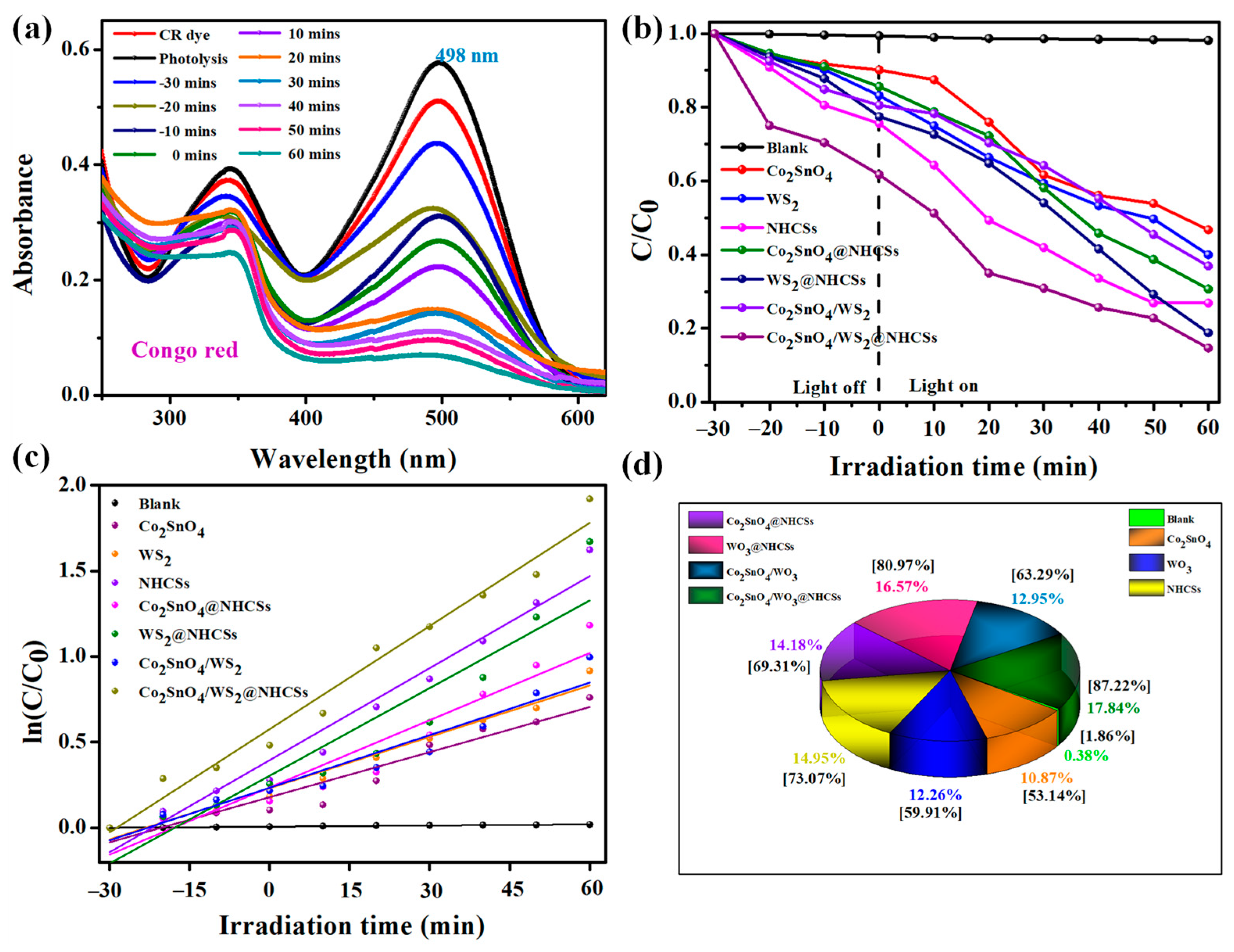
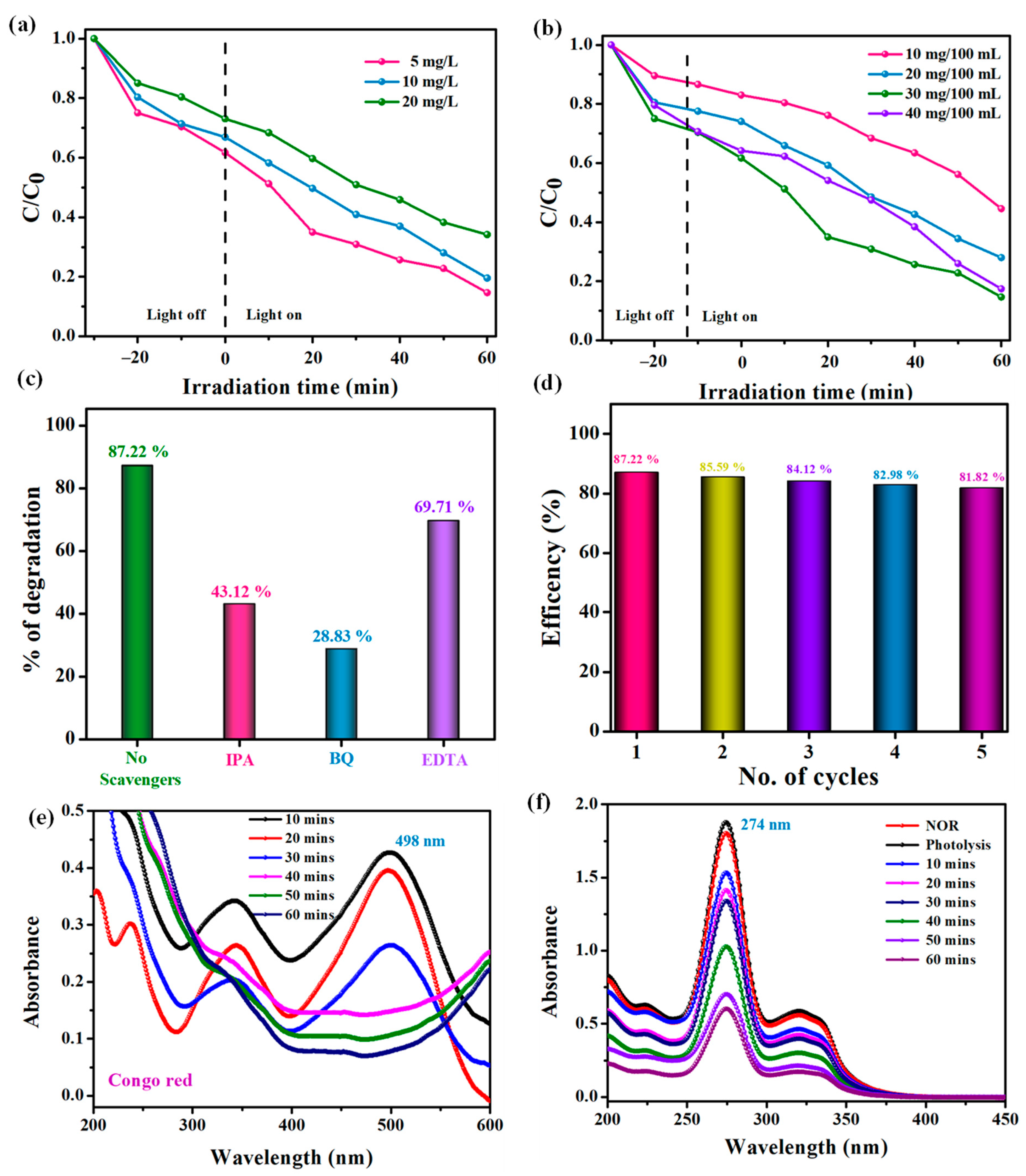
2.6. Photocatalytic Degradation Performance of CR Dye
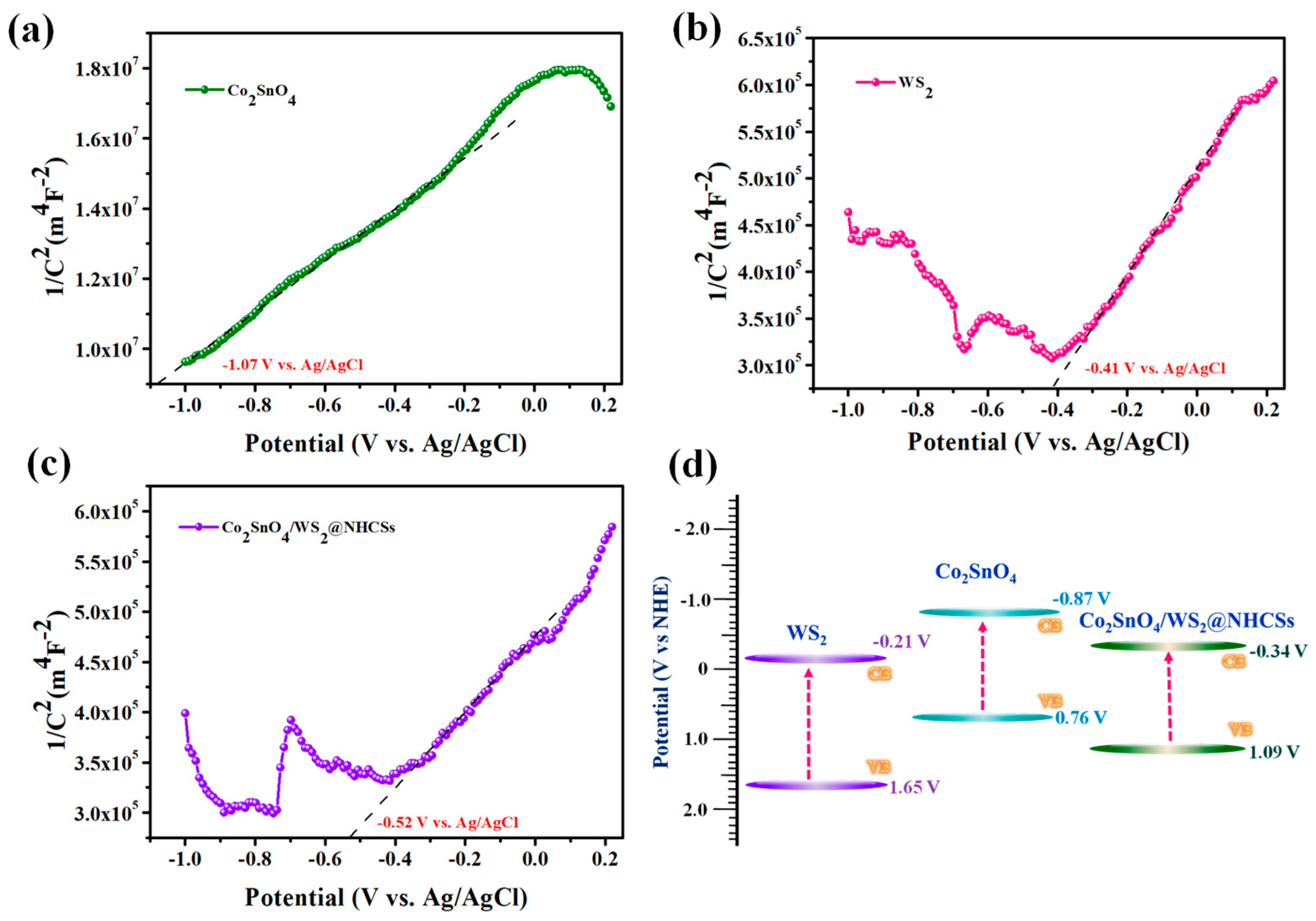
2.7. Mott–Schottky Plot Analysis
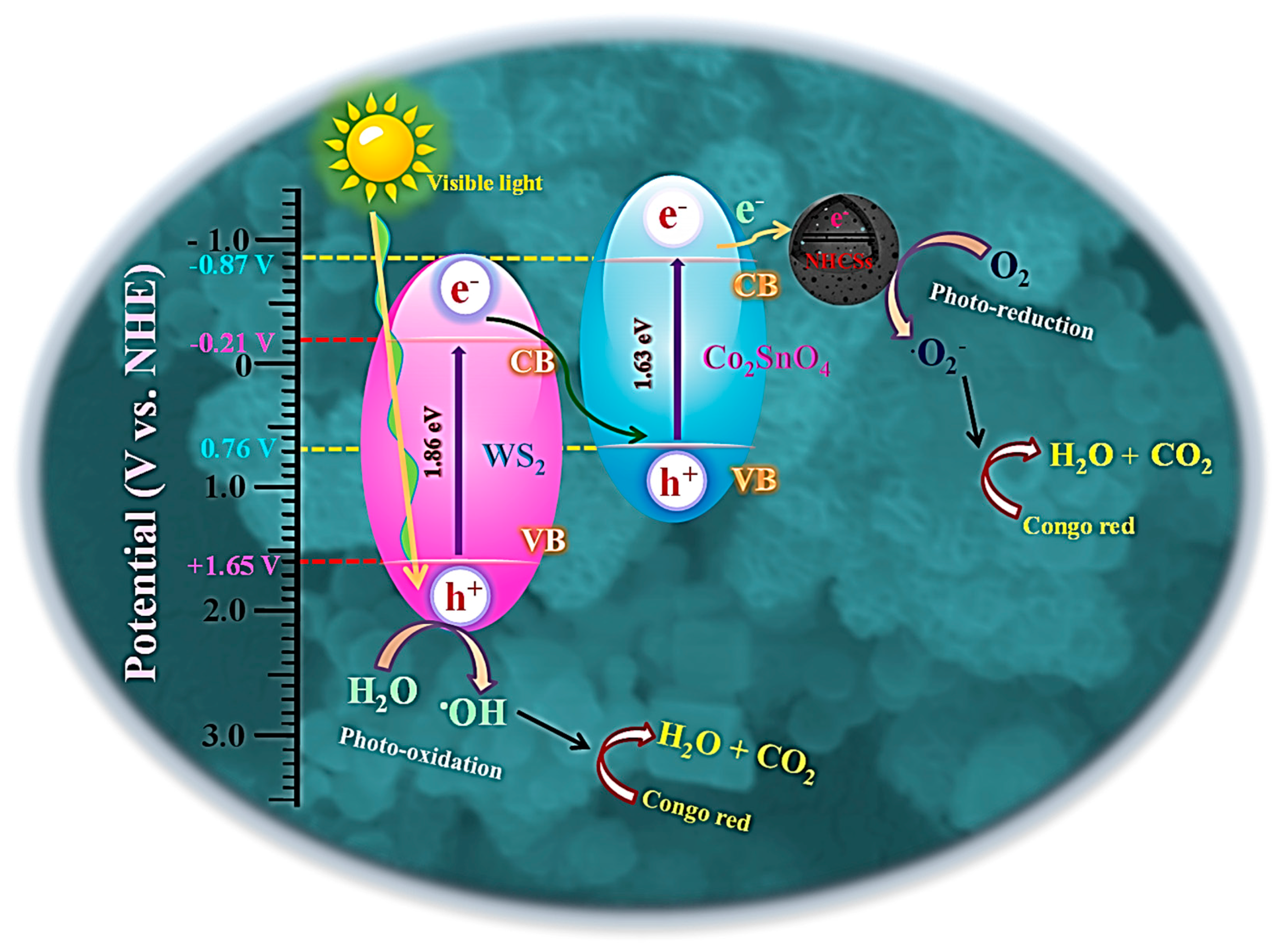
2.8. Photocatalytic Degradation Mechanism
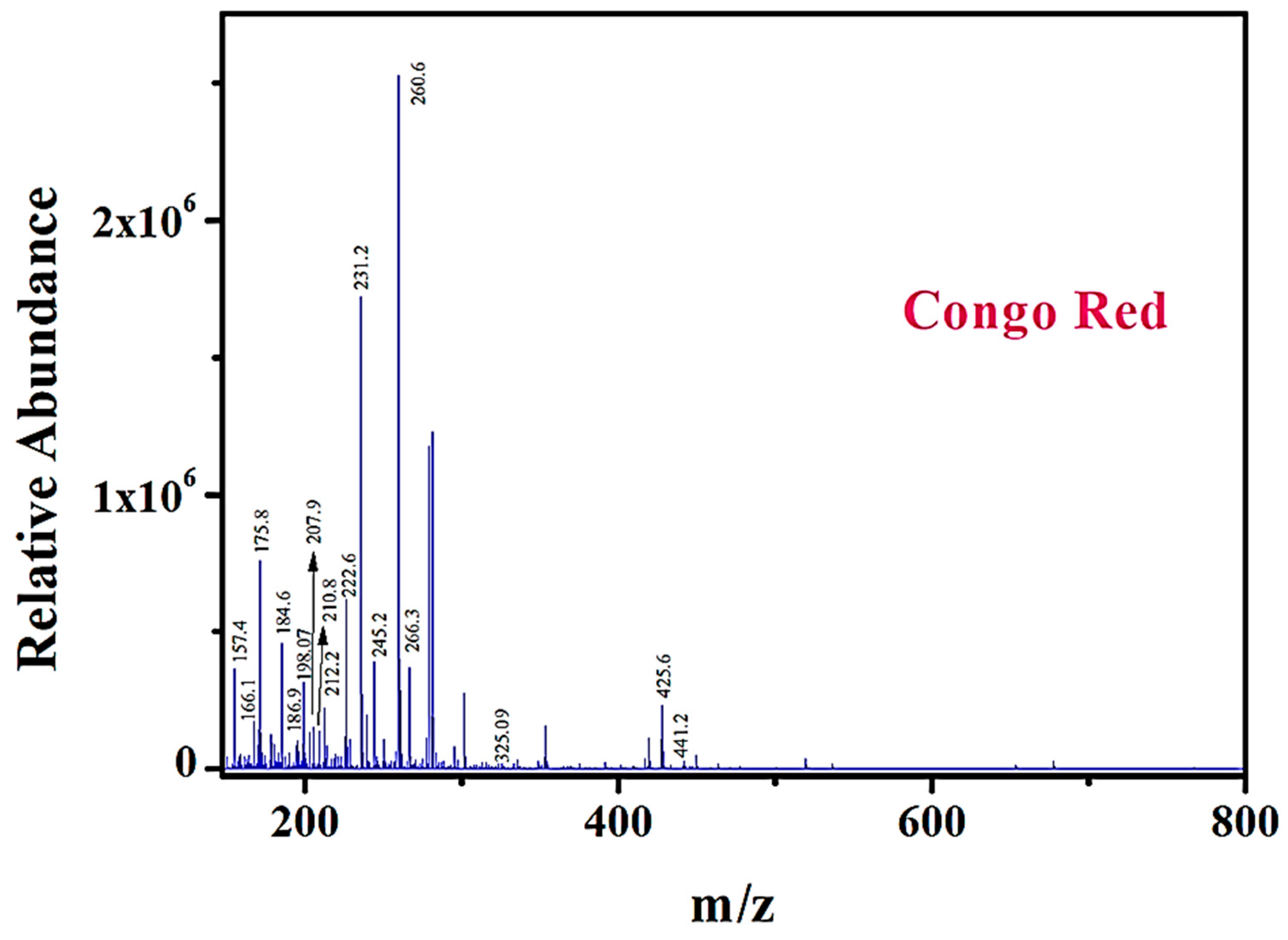
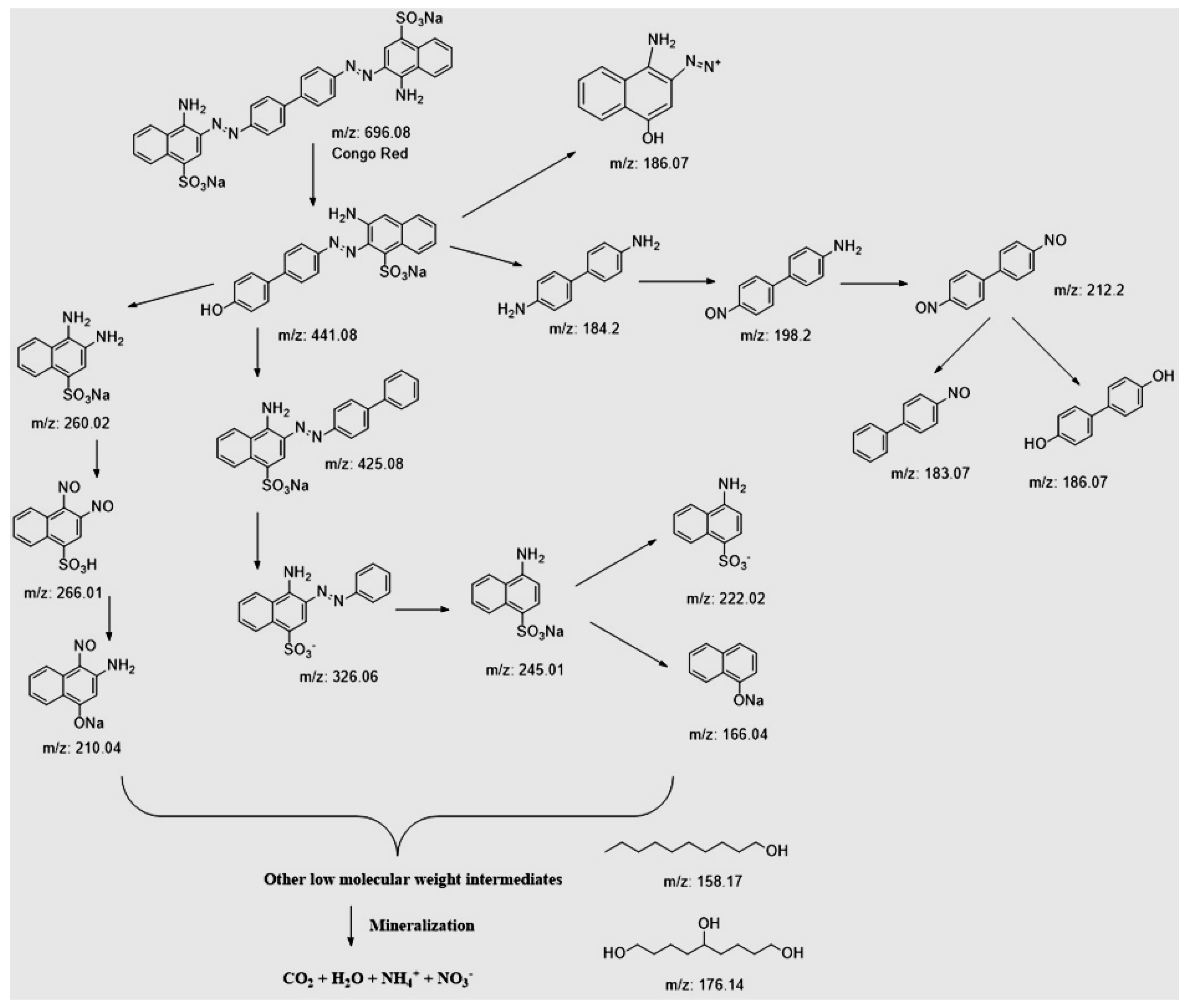
2.9. Photocatalytic Degradation Pathway Analysis
3. Materials and Methods
3.1. Material Usage
3.2. Synthesis of Co2SnO4
3.3. Synthesis of WS2
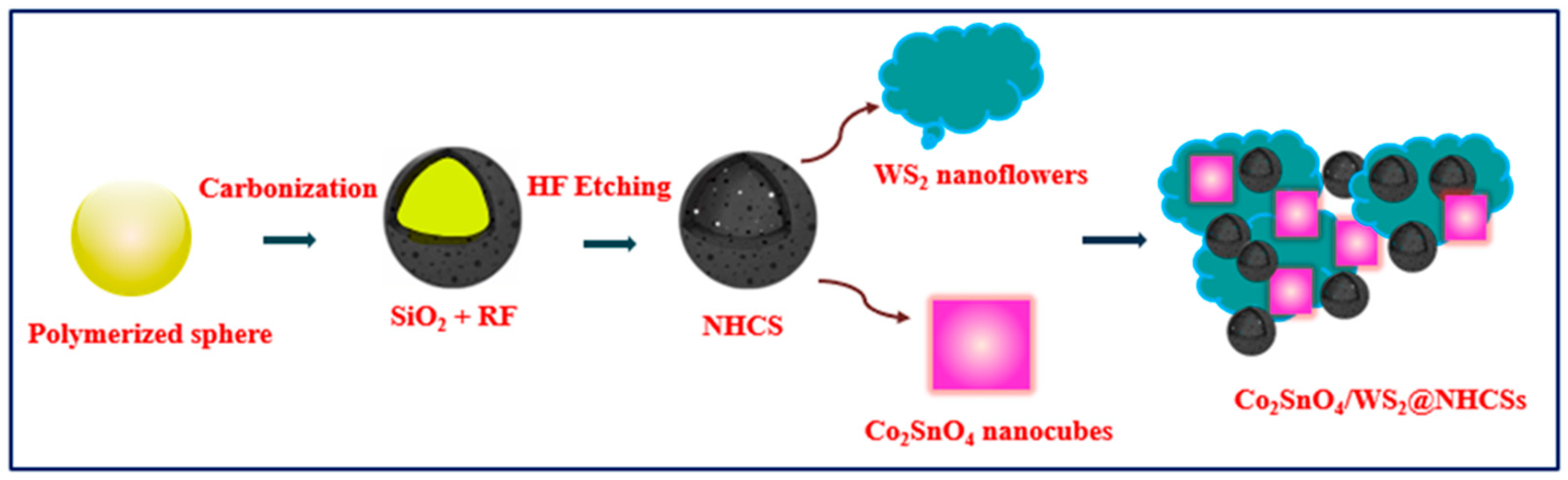
3.4. Synthesis of NHCSs
3.5. Synthesis of Co2SnO4/WS2@NHCSs
3.6. Characterization Techniques
3.7. Photodegradation Process
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shen, C.H.; Wen, X.J.; Fei, Z.H.; Liu, Z.T.; Mu, Q.M. Visible-light-driven activation of peroxymonosulfate for accelerating ciprofloxacin degradation using CeO2/Co3O4 p-n heterojunction photocatalysts. Chem. Eng. J. 2020, 391, 123612. [Google Scholar] [CrossRef]
- Xing, Z.; Zhang, J.; Cui, J.; Yin, J.; Zhao, T.; Kuang, J.; Xiu, Z.; Wan, N.; Zhou, W. Recent advances in floating TiO2-based photocatalysts for environmental application. Appl. Catal. B Environ. 2018, 225, 452–467. [Google Scholar] [CrossRef]
- Ashraf, W.; Fatima, T.; Srivastava, K.; Khanuja, M. Superior photocatalytic activity of tungsten disulfide nanostructures: Role of morphology and defects. Appl. Nanosci. 2019, 9, 1515–1529. [Google Scholar] [CrossRef]
- Ashraf, W.; Bansal, S.; Singh, V.; Barman, S.; Khanuja, M. BiOCl/WS2 hybrid nanosheet (2D/2D) heterojunctions for visible-light-driven photocatalytic degradation of organic/inorganic water pollutants. RSC Adv. 2020, 10, 25073–25088. [Google Scholar] [CrossRef]
- Su, F.; Li, P.; Huang, J.; Gu, M.; Liu, Z.; Xu, Y. Photocatalytic degradation of organic dye and tetracycline by ternary Ag2O/AgBr–CeO2 photocatalyst under visible-light irradiation. Sci. Rep. 2021, 11, 1–13. [Google Scholar] [CrossRef]
- Saeed, M.; Muneer, M.; Akram, N.; Haq, A.U.; Afzal, N.; Hamayun, M. Synthesis and characterization of silver loaded alumina and evaluation of its photo catalytic activity on photo degradation of methylene blue dye. Chem. Eng. Res. Des. 2019, 148, 218–226. [Google Scholar] [CrossRef]
- Saeed, M.; Adeel, S.; Ilyas, M.; Shahzad, M.A.; Usman, M.; Haq, E.; Hamayun, M. Oxidative degradation of methyl orange catalyzed by lab prepared nickel hydroxide in aqueous medium. Desalin. Water Treat. 2016, 57, 12804–12813. [Google Scholar] [CrossRef]
- Su, X.H.; Ling, W.L.; Teng, T.T.; Wong, Y.S. Combination and hybridization of treatments in dye wastewater treatment: A review. J. Environ. Chem. Eng. 2016, 4, 3618–3631. [Google Scholar] [CrossRef]
- Ru, M.; Lang, K.; Xiao, X.; Wang, H.; Meng, H.; Jiang, B. Efficient electrocatalytic degradation of tetracycline using NiCo2O4/MnO2 composite electrode: Synergistic enhancement mechanisms and environmental toxicity assessment. J. Environ. Chem. Eng. 2025, 13, 115947. [Google Scholar] [CrossRef]
- Suppuraj, P.; Srishankar, J.; Sobana, N.; Surya, C.; Thirunarayanan, G.; Thirugnanam, R.; Muthuvel, I. A proficient hydrothermally designed Au-TiO2/ZnFe2O4 composite for ciprofloxacin removal in wastewater under natural solar light. Inorg. Chem. Commun. 2023, 156, 111317. [Google Scholar]
- Guan, G.; Ye, E.; You, M.; Li, Z. Hybridized 2D nanomaterials toward highly efficient photocatalysis for degrading pollutants: Current status and future perspectives. Small 2020, 16, 1907087. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.M.; Aziz, F.; Mohtar, S.S.; Mhamad, S.A.; Ahmadu, B.; Nasir, M.U.; Muhammad, K.Y.; Aziz, M. A review of research trends on the usage of photocatalysis for wastewater treatment: Bibliometric analysis. Sustain. Water Resour. Manag. 2023, 9, 88. [Google Scholar] [CrossRef] [PubMed]
- Al-Nuaim, M.A.; Alwasiti, A.A.; Shnain, Z.Y. The photocatalytic process in the treatment of polluted water. Chem. Pap. 2023, 77, 677–701. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Gao, X.; Zou, J.; Pang, F. Research on Photocatalytic Wastewater Treatment Reactors: Design, Optimization, and Evaluation Criteria. Catalysts 2023, 13, 974. [Google Scholar] [CrossRef]
- Paul, D.; Devaprakasam, D. A novel approach to enhance performance, stability and longevity of the solar cells by mitigating UV-induced degradation with monolayer − Boosted nano-TiO2 coatings. Solar Energy 2025, 285, 113118. [Google Scholar] [CrossRef]
- González-Ballesteros, N.; Martins, P.M.; Tavares, C.J.; Lanceros-Méndez, S. Quercetin-mediated green synthesis of Au/TiO2 nanocomposites for the photocatalytic degradation of antibiotic ciprofloxacin. J. Ind. Eng. Chem. 2025, 143, 526–537. [Google Scholar] [CrossRef]
- Etshindo, L.A.; Sousa, C.; Tamiasso-Martinhon, P.; Colaço, M.V.; Camara, A.R.; Rocha, A.S. SnO2-TiO2 materials for photocatalytic degradation of cationic dye under UV and visible light and a chitosan composite film investigation. Catal. Today 2025, 444, 114995. [Google Scholar] [CrossRef]
- Joseph, A.; Aneesh, P.M. Efficient degradation of methylene blue: A comparative study using hydrothermally synthesized SnS2, WS2 and VS2 nanostructures. Mater. Res. Bull. 2022, 146, 111623. [Google Scholar] [CrossRef]
- Choi, W.; Cho, M.Y.; Konar, A.; Lee, J.H.; Cha, G.B.; Hong, S.C.; Kim, S.; Kim, J.; Jena, D.; Joo, J. High-detectivity multilayer MoS2 phototransistors with spectral response from ultraviolet to infrared. Adv. Mater. 2012, 24, 5832–5836. [Google Scholar] [CrossRef]
- Rahman, A.; Khan, M.M. Chalcogenides as photocatalysts. New J. Chem. 2021, 45, 19622–19635. [Google Scholar] [CrossRef]
- Dang, H.; Chen, L.; Chen, L.; Yuan, M.; Yan, Z.; Li, M. Hydrothermal synthesis of 1T-WS2 nanosheets with excellent adsorption performance for dye removal from wastewater. Mater. Lett. 2019, 254, 42–45. [Google Scholar] [CrossRef]
- Sarma, G.V.S.S.; Chavali, M.; Nikolova, M.P.; Enamala, M.K.; Kuppan, C. Basic Principles, Fundamentals, and Mechanisms of Chalcogenide-Based Nanomaterials in Photocatalytic Reactions. In Chalcogenide-Based Nanomaterials as Photocatalysts; Elsevier: Amsterdam, The Netherlands, 2021; pp. 77–103. [Google Scholar]
- Akhundi, A.; Habibi-Yangjeh, A.; Abitorabi, M.; Rahim Pouran, S. Review on photocatalytic conversion of carbon dioxide to value-added compounds and renewable fuels by graphitic carbon nitride-based photocatalysts. Catal. Rev. 2019, 61, 595–628. [Google Scholar] [CrossRef]
- Fergani, S.; Hanane, Z.; Adel, S.; Souad, T.; Amel, B.; Khaldou, B. Co2SnO4@Co3O4-SnO2 composite for enhanced peroxymonosulfate activation: Catalytic degradation of diclofenac, mechanism and degradation pathways. Reac. Kinet. Mech. Cat. 2023, 136, 1033–1048. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Nguyen, X.H.; Nguyen, V.L.; Pham, V.T.; Thai, V.N.; Luu, T.L.; Tap, V.H.; Le, H.N. Enhanced degradation of cefalexin using Co2SnO4@rGO as an effective peroxymonosulfate activator in hybrid ozonation system. J. Environ. Chem. Eng. 2023, 11, 110076. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, L.; An, C.; Wang, M. Constructing Z-Scheme 3D WO3@Co2SnO4 Heterojunction as Dual-Photocathode for Production of H2O2 and In-Situ Degradation of Organic Pollutants. Water 2024, 16, 406. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, Y.; Yang, Y.; Wang, Y.; Qin, C.; Liang, R.; Chen, X.; Ye, Z.; Zhu, L. Carbon Sphere Template Derived Hollow Nanostructure for Photocatalysis and Gas Sensing. Nanomaterials 2020, 10, 378. [Google Scholar] [CrossRef]
- Ouyang, H.; Huang, H.; Wang, H.; Zheng, Z. The morphology evolution of nitrogen-doped carbon quantum dots/hollow TiO2 composites and their applications in photocatalysis. J. Mater. Sci. 2020, 55, 976–989. [Google Scholar] [CrossRef]
- Cheng, C.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. ZnIn2S4 grown on nitrogen-doped hollow carbon spheres: An advanced catalyst for Cr(VI) reduction. J. Hazard. Mater. Mater. 2020, 391, 122205. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, X.; Chen, J.; Wang, X.; Wang, D.; Mao, Z. Templated transformation of g-C3N4 nanosheets into nitrogen-doped hollow carbon sphere with tunable nitrogen-doping properties for application in Li-ions batteries. Carbon 2020, 168, 458–467. [Google Scholar] [CrossRef]
- Dehkordi, A.B.; Badiei, A. Insight into the activity of TiO2@nitrogen-doped hollow carbon spheres supported on g-C3N4 for robust photocatalytic performance. Chemosphere 2022, 288, 132392. [Google Scholar] [CrossRef]
- Shao, B.; Liu, X.; Liu, Z.; Zeng, G.; Zhang, W.; Liang, Q.; Liu, Y.; He, Q.; Yuan, X.; Wang, D.; et al. Synthesis and characterization of 2D/0D g-C3N4/CdS-nitrogen doped hollow carbon spheres (NHCs) composites with enhanced visible light photodegradation activity for antibiotic. Chem. Eng. J. 2019, 374, 479–493. [Google Scholar] [CrossRef]
- Liu, S.; Ke, J.; Sun, H.; Liu, J.; Tade, M.O.; Wang, S. Size dependence of uniformed carbon spheres in promoting graphitic carbon nitride toward enhanced photocatalysis. Appl. Catal. B 2017, 204, 358–364. [Google Scholar] [CrossRef]
- Mcevoy, J.G.; Cui, W.; Zhang, Z. Synthesis and characterization of Ag/AgCl–activated carbon composites for enhanced visible light photocatalysis. Appl. Catal. B 2014, 144, 702–712. [Google Scholar] [CrossRef]
- Sanati, S.; Abazari, R.; Albero, J.; Morsali, A.; García, H.; Liang, Z.; Zou, R. Metal–organic framework derived bimetallic materials for electrochemical energy storage. Angew. Chem. Int. Ed. 2021, 60, 11048–11067. [Google Scholar] [CrossRef]
- Wu, Q.; Yang, L.; Wang, X.; Hu, Z. Carbon-Based Nanocages: A new platform for advanced energy storage and conversion. Adv. Mater. 2020, 32, 1904177. [Google Scholar] [CrossRef]
- Du, J.; Liu, L.; Hu, Z.; Yu, Y.; Zhang, Y.; Hou, S.; Chen, A. Raw-cotton-derived N-doped carbon fiber aerogel as an efficient electrode for electrochemical capacitor. ACS Sustain. Chem. Eng. 2018, 6, 4008–4015. [Google Scholar] [CrossRef]
- Ranjithkumar, R.; Youk, J.H. Nitrogen-doped hollow carbon spheres decorated with NiCo alloy nanoparticles for high-performance supercapacitor electrode. Mater. Today Chem. 2024, 36, 101939. [Google Scholar] [CrossRef]
- Zhou, T.; Zhou, Y.; Ma, R.; Zhou, Z.; Liu, G.; Liu, Q.; Zhu, Y.; Wang, J. Nitrogen-doped hollow mesoporous carbon spheres as a highly active and stable metal-free electrocatalyst for oxygen reduction. Carbon 2017, 114, 177–186. [Google Scholar] [CrossRef]
- Arunpandian, M.; Oh, T.H.; Selvakumar, K.; Sriram, G. Fabrication of novel N-rich g-C3N5 decorated cubic spinel Co2SnO4 hybrids: Studies on morphology, degradation efficacy, pathway and mechanism. J. Environ. Chem. Eng. 2024, 12, 113961. [Google Scholar] [CrossRef]
- Joseph, A.; Tadi, K.K.; Anju, K.S.; Aneesh, P.M. Structural, optical, magnetic and electrochemical properties of hydrothermally synthesized WS2 nanoflakes. J. Mater. Res. 2021, 36, 884–895. [Google Scholar] [CrossRef]
- Huang, F.; Jian, J.; Wu, R. Few-layer thick WS2 nanosheets produced by intercalation/exfoliation route. J. Mater. Sci. 2016, 51, 10160–10165. [Google Scholar] [CrossRef]
- Shi, L.; Wang, X.; Lu, L.; Yang, X.; Wu, X. Preparation of TiO2/polyaniline nanocomposite from a lyotropic liquid crystalline solution. Synth. Met. 2009, 159, 2525–2529. [Google Scholar] [CrossRef]
- Khataee, A.; Eghbali, P.; Irani-Nezhad, M.H.; Hassani, A. Sonochemical synthesis of WS2 nanosheets and its application in sonocatalytic removal of organic dyes from water solution. Ultrason. Sonochem. 2018, 48, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Vattikuti, S.P.; Byon, C.; Chitturi, V. Selective hydrothermally synthesis of hexagonal WS2 platelets and their photo catalytic performance under visible light irradiation Superlattice. Superlattices Microst. 2016, 94, 39–50. [Google Scholar] [CrossRef]
- Hazarika, S.J.; Mohanta, D. Inorganic fullerene-type WS2 nanoparticles: Processing, characterization and its photocatalytic performance on malachite green. Appl. Phys. A 2017, 123, 381. [Google Scholar] [CrossRef]
- Guan, D.; Xu, H.; Huang, Y.C.; Jing, C.; Tsujimoto, Y.; Xu, X.; Lin, Z.; Tang, J.; Wang, Z.; Sun, X.; et al. Operando Studies Redirect Spatiotemporal Restructuration of Model Coordinated Oxides in Electrochemical Oxidation. Adv. Mater. 2025, 37, 2413073. [Google Scholar] [CrossRef]
- Wu, B.; Zhang, S.; Yao, F.; Zhang, F.; Xu, S. Synergistic lithium storage of a multi-component Co2SnO4/Co3O4/Al2O3/C composite from a single-source precursor. RSC Adv. 2015, 5, 69932–69938. [Google Scholar] [CrossRef]
- Maa, H.; Yang, J.; Gao, X.; Liu, Z.; Liu, X.; Xu, Z. Removal of chromium (VI) from water by porous carbon derived from corn straw: Influencing factors, regeneration and mechanism. J. Hazard. Mater. 2019, 369, 550–560. [Google Scholar] [CrossRef]
- Fatima, T.; Husain, S.; Khanuja, M. Superior photocatalytic and electrochemical activity of novel WS2/PANI nanocomposite for the degradation and detection of pollutants: Antibiotic, heavy metal ions, and dyes. J. chem. Eng. Adv. 2022, 12, 100373. [Google Scholar] [CrossRef]
- Ali, M.B.; Barras, A.; Addad, A.; Sieber, B.; Elhouichet, H.; Férid, M.; Szunerits, S.; Boukherroub, R. Co2SnO4 nanoparticles as a high-performance catalyst for oxidative degradation of rhodamine B dye and pentachlorophenol by activation of peroxymonosulfate. Phys. Chem. Chem. Phys. 2017, 19, 6569–6578. [Google Scholar] [CrossRef]
- Joshi, S.; Ippolito, S.J.; Sunkara, M.V. Convenient architectures of Cu2O/SnO2 type II p–n heterojunctions and their application in visible light catalytic degradation of rhodamine B. RSC Adv. 2016, 6, 43672–43684. [Google Scholar] [CrossRef]
- Guo, X.; Xiao, Q.; Yang, T.; Liu, Y.; Jin, Z. Construction of S-Scheme Co2SnO4/graphdiyne heterojunction to promote carrier transfer for efficiently photocatalytic hydrogen evolution characterized with in situ XPS. Sep. Purif. Technol. 2023, 325, 124764. [Google Scholar] [CrossRef]
- Zhao, Z.; Du, Z.; Huang, X.; Jiang, C. 1D–2D heterostructured silicon carbide fibers@ WS2 with high efficiency and broad bandwidth for microwave absorption performance. Ceram. Int. 2023, 49, 9916–9923. [Google Scholar] [CrossRef]
- Perrozzi, F.; Emamjomeh, S.; Paolucci, V.; Taglieri, G.; Ottaviano, L.; Cantalini, C. Thermal stability of WS2 flakes and gas sensing properties of WS2/WO3 composite to H2, NH3 and NO2. Sens. Actuators B 2017, 243, 812–822. [Google Scholar] [CrossRef]
- Liu, S.; Shen, B.; Niu, Y.; Xu, M. Fabrication of WS2-nanoflowers@rGO composite as an anode material for enhanced electrode performance in lithium-ion batteries. J. Colloid Interface Sci. 2017, 488, 20–25. [Google Scholar] [CrossRef]
- Li, J.; Yin, Y.; Liu, E.; Ma, Y.; Wan, J.; Fan, J.; Hu, X. In situ growing Bi2MoO6 on g-C3N4 nanosheets with enhanced photocatalytic hydrogen evolution and disinfection of bacteria under visible light irradiation. J. Hazard. Mater. 2017, 321, 183–192. [Google Scholar] [CrossRef]
- Paul, A.; Dhar, S.S. Designing Cu2V2O7/CoFe2O4/g-C3N4 ternary nanocomposite: A high performance magnetically recyclable photocatalyst in the reduction of 4- nitrophenol to 4-aminophenol. J. Solid State Chem. 2020, 290, 121563. [Google Scholar] [CrossRef]
- Shahabuddin, S.; Mehmood, S.; Ahmad, I.; Sridewi, N. Synthesis and Characterization of 2D-WS2 Incorporated Polyaniline Nanocomposites as Photo Catalyst for Methylene Blue Degradation. Nanomaterials 2022, 12, 2090. [Google Scholar] [CrossRef]
- Ashraf, W.; Parvez, S.H.; Khanuja, M. Synthesis of highly efficient novel two-step spatial 2D photocatalyst material WS2/ZnIn2S4 for degradation/reduction of various toxic pollutants. Environ. Res. 2023, 236, 116715. [Google Scholar] [CrossRef]
- Huang, H.; Lei, Y.; Bai, L.; Liang, Y.; Yang, H. Morphology-dependent quasi 2D/2D point-flat-plate ternary CdS/MoS2/WS2 heterojunction with improved visible photocatalytic degradation of tetracycline. Colloids Surf. A Physicochem. Eng. Asp. 2023, 657, 130558. [Google Scholar] [CrossRef]
- Wang, G.; Dou, K.; Cao, H.; Du, R.; Liu, J.; Tsidaeva, N.; Wang, W. Designing Z-scheme CdS/WS2 heterojunctions with enhanced photocatalytic degradation of organic dyes and photoreduction of Cr (VI): Experiments, DFT calculations and mechanism. Sep. Purif. Technol. 2022, 291, 120976. [Google Scholar] [CrossRef]
- Wen, X.J.; Niu, C.G.; Zhang, L.; Liang, C.; Zeng, G.M. A novel Ag2O/CeO2 heterojunction photocatalysts for photocatalytic degradation of enrofloxacin: Possible degradation pathways, mineralization activity and an in-depth mechanism insight. Appl. Catal. B Environ. 2018, 221, 701–714. [Google Scholar] [CrossRef]
- Ismael, M.; Wark, M. Photocatalytic activity of CoFe2O4/g-C3N4 nanocomposite toward degradation of different organic pollutants and their inactivity toward hydrogen production: The role of the conduction band position. FlatChem 2022, 32, 100337. [Google Scholar] [CrossRef]
- Ranjith Kumar, D.; Ranjith, K.S.; Haldorai, Y.; Kandasami, A.; Rajendra Kumar, R.T. Nitrogen-implanted ZnO nanorod arrays for visible light photocatalytic degradation of a pharmaceutical drug acetaminophen. ACS Omega 2019, 4, 11973–11979. [Google Scholar] [CrossRef]
- Thomas, M.; Naikoo, G.A.; Sheikh, M.; Bano, M.; Khan, F. Effective photocatalytic degradation of Congo red dye using alginate/carboxymethyl cellulose/TiO2 nanocomposite hydrogel under direct sunlight irradiation. J. Photochem. Photobiol. A 2016, 327, 33–43. [Google Scholar] [CrossRef]
- Liu, C.; Wang, J.; Li, J.; Zeng, M.; Luo, R.; Shen, J.; Sun, X.; Han, W.; Wang, L. Synthesis of N-doped hollow-structured mesoporous carbon nanospheres for high-performance supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 7194–7204. [Google Scholar] [CrossRef]
| Degradation Efficiency (%) | Apparent Rate Constants k (min−1) | |||
|---|---|---|---|---|
| CR | R2 | k | ||
| 1 | Without Catalyst | 0.9656 | 0.9656 | 2.187 × 10−4 |
| 2 | Co2SnO4 | 0.9221 | 0.9221 | 0.0087 |
| 3 | WS2 | 0.9739 | 0.9739 | 0.0099 |
| 4 | NHCSs | 0.9648 | 0.9648 | 0.0179 |
| 5 | Co2SnO4@NHCSs | 0.9109 | 0.9109 | 0.0131 |
| 6 | WS2@NHCSs | 0.8751 | 0.8751 | 0.0171 |
| 7 | Co2SnO4/WS2 | 0.9259 | 0.9259 | 0.0102 |
| 8 | Co2SnO4/WS2@NHCSs | 0.9769 | 0.9769 | 0.0201 |
| S. No | Catalyst | Wt. of Catalyst (g/L) | Organic Pollutant | Light Source | % of deg. | Deg. Time (min) | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | WS2/BiOCl | 0.05 | MG | Visible light | 94 | 180 | [4] |
| 2 | WS2 | 0.1 | BV 10 | Ultrasonic | 94.01 | 150 | [44] |
| 3 | WS2/Polyaniline | 0.02 | MB | UV light | 93 | 90 | [59] |
| 4 | WS2/ZnIn2S4 | 0.025 | MG | UV light | 90 | 5 | [60] |
| 5 | CdS/MoS2/WS2 | 0.01 | RhB | Visible light | 90 | 30 | [61] |
| 6 | CdS/WS2 | 0.03 | MB | Visible light | 91 | 90 | [62] |
| 7 | WS2/ZnO | 0.05 | MB | Visible light | 80.5 | 120 | [26] |
| 8 | Co2SnO4/WS2@NHCSs | 0.03 | CR | Visible light | 87.22 | 60 | This Work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arunpandian, M.; Oh, T.H. Nitrogen-Doped Hollow Carbon Spheres-Decorated Co2SnO4/WS2 Heterostructures with Improved Visible-Light Photocatalytic Degradation of Organic Dye. Molecules 2025, 30, 2081. https://doi.org/10.3390/molecules30092081
Arunpandian M, Oh TH. Nitrogen-Doped Hollow Carbon Spheres-Decorated Co2SnO4/WS2 Heterostructures with Improved Visible-Light Photocatalytic Degradation of Organic Dye. Molecules. 2025; 30(9):2081. https://doi.org/10.3390/molecules30092081
Chicago/Turabian StyleArunpandian, Muthuraj, and Tae Hwan Oh. 2025. "Nitrogen-Doped Hollow Carbon Spheres-Decorated Co2SnO4/WS2 Heterostructures with Improved Visible-Light Photocatalytic Degradation of Organic Dye" Molecules 30, no. 9: 2081. https://doi.org/10.3390/molecules30092081
APA StyleArunpandian, M., & Oh, T. H. (2025). Nitrogen-Doped Hollow Carbon Spheres-Decorated Co2SnO4/WS2 Heterostructures with Improved Visible-Light Photocatalytic Degradation of Organic Dye. Molecules, 30(9), 2081. https://doi.org/10.3390/molecules30092081







