Abstract
The discovery of bioactive natural products is often challenged by the complexity of isolating and characterizing active compounds within diverse mixtures. Previously, we introduced a 1H NMR-based weighted gene correlation network analysis (WGCNA) approach to identify spectral features linked to growth inhibitory activity of Piper (Piperaceae) leaf extracts against model plant, fungal, and bacterial organisms. This method enabled us to prioritize specific spectral features linked to bioactivity, offering a targeted approach to natural product discovery. In this study, we validate the predictive capacity of the WGCNA by isolating the compounds responsible for the bioactivity-associated resonances and confirming their antifungal efficacy. Using growth inhibition assays, we verified that the isolated compounds, including three novel antifungal agents, exhibited significant bioactivity. Notably, one of these compounds contains a rare imidazolium heterocyclic motif, marking a new structural class in Piper. These findings substantiate the 1H NMR-based WGCNA as a reliable tool for identifying structural types associated with biological activity, streamlining the process of discovering bioactive natural products in complex extracts.
1. Introduction
Natural products have long been a cornerstone of drug discovery, providing structurally diverse bioactive compounds [1]. However, traditional bioassay-guided fractionation can be slow and inefficient, often leading to the repeated isolation of known compounds. Advances in metabolomics and network-based analytical approaches now offer more targeted strategies for identifying novel bioactive molecules and include the use of NMR-based metabolomic approaches [2,3,4,5,6]. Previously, we introduced a 1H NMR-based weighted gene co-expression network analysis (WGCNA) approach to identify spectral features linked to antifungal activity in Piper (Piperaceae) extracts. This method enabled us to correlate specific 1H NMR resonances with bioactivity, effectively prioritizing candidate compounds for isolation [7]. While WGCNA demonstrated strong predictive power, direct validation through isolation, structural characterization, and verification of the bioactivity of purified target compounds was necessary.
The Piper genus (Piperacace), comprising over 2500 species with a pantropical distribution, has been widely studied in chemical ecology and bioactive molecule discovery due to its rich phytochemical diversity and role in plant defense mechanisms [8,9,10,11,12]. Our research has focused on Piper as a model for understanding chemically mediated ecological interactions, particularly in plant–pathogen and plant–herbivore relationships [13,14,15,16,17,18]. Known for producing biologically active secondary metabolites, including antifungal amides and phenylpropanoids, Piper serves as an ideal system for studying how secondary metabolites contribute to ecological resilience and potential applications.
In this study, we aimed to evaluate the predictive power of 1H NMR-based WGCNA by isolating and structurally characterizing bioactive compounds corresponding to antifungal activity-associated spectral features. By targeting resonances affiliated with the specific modules identified in the WGCNA analysis, we successfully isolated three antifungal compounds, including a rare imidazolium-containing natural product. Growth-inhibition assays confirmed their antifungal efficacy, directly linking the predicted spectral features to functional bioactive molecules. These findings substantiate WGCNA as a viable method for identifying bioactive compounds in complex natural product mixtures, streamlining the discovery of biologically relevant metabolites.
2. Results
2.1. Target Identification for Isolation, Characterization and Biological Verification
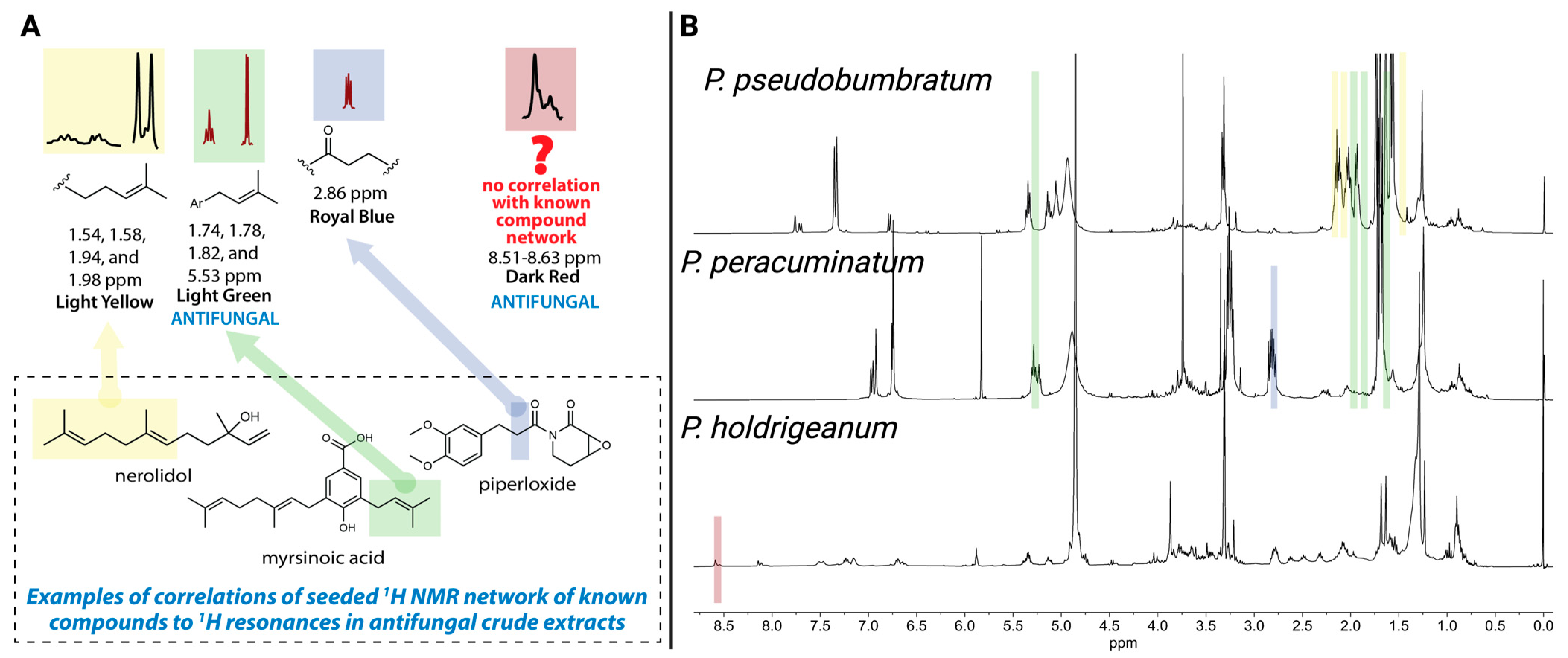
In our previous study of 1H NMR spectra from methanolic extracts of 30 Piper species, we used network analysis to identify spectral modules correlated with antifungal activity [7,19]. Two modules showed significant correlations with Saccharomyces cerevisiae inhibition: a light green module linked to P. peracuminatum and P. pseudobumbratum and a dark red module associated with P. holdridgeanum. Structural annotation of these modules, aided by a library of known Piper compounds, indicated that the light green module matched spectral features of prenylated aryl acids such as myrsinoic acid, reflecting the dominant prenylated phenol resonances present in both P. peracuminatum and P. pseudobumbratum; however, the specific structures of the active compounds remained unknown (Figure 1). The dark red module contained an unusual signal (δH 8.53–8.62) with no match to known compounds, suggesting a novel scaffold in P. holdridgeanum that was not represented by the group of known Piper compounds (Figure 1). Importantly, this signal originated from a minor component of the extract, unlike the other two active extracts. These spectral insights guided our targeted isolation of antifungal compounds from these three species, resulting in the isolation of the active compounds that contain these resonances and validating the predictive power of the 1H NMR network-based approach.
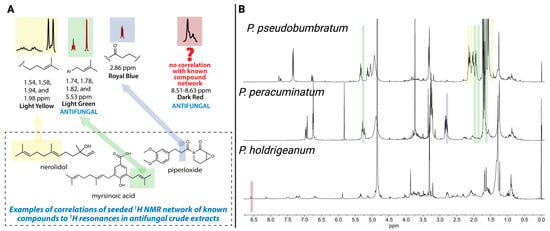
Figure 1.
(A) Identification of target 1H NMR resonances and predicted structural motifs based on previous studies [7,19]. (B) Crude 1H NMR spectra of methanolic extracts from three Piper species with confirmed antifungal activity. Colored module overlays (light yellow, light green, royal blue, and dark red) indicate chemical shift regions correlated with specific extracts. The dark red module (δH 8.61–8.53); P. holdridgeanum) and light green module (δH 5.33, 1.82, 1.78, 1.74); P.dobumbratum and P. peracuminatum) were significantly associated with antifungal activity and were targeted for isolation. Created in BioRender. Jeffrey, C. (2025) https://BioRender.com/9k9pvls.
2.2. Compound Isolation Guided by Targeted 1H NMR Resonances
To isolate the compounds that correlated to the bioactivity, we adopted a general isolation protocol that minimized the number of fractions and the necessity of repeated bioactivity screening by tracking the target resonances through 1H NMR analysis of semi-pure and pure fractions. The crude methanolic extracts were pre-fractionated using a C18 column eluted with 50–100% v/v acetone/H2O. Fractions were analyzed by 1H NMR, and those containing the target resonances were then prioritized for further purification via reverse-phase preparatory chromatography using C18.
2.3. Structure Determination of Target Compounds
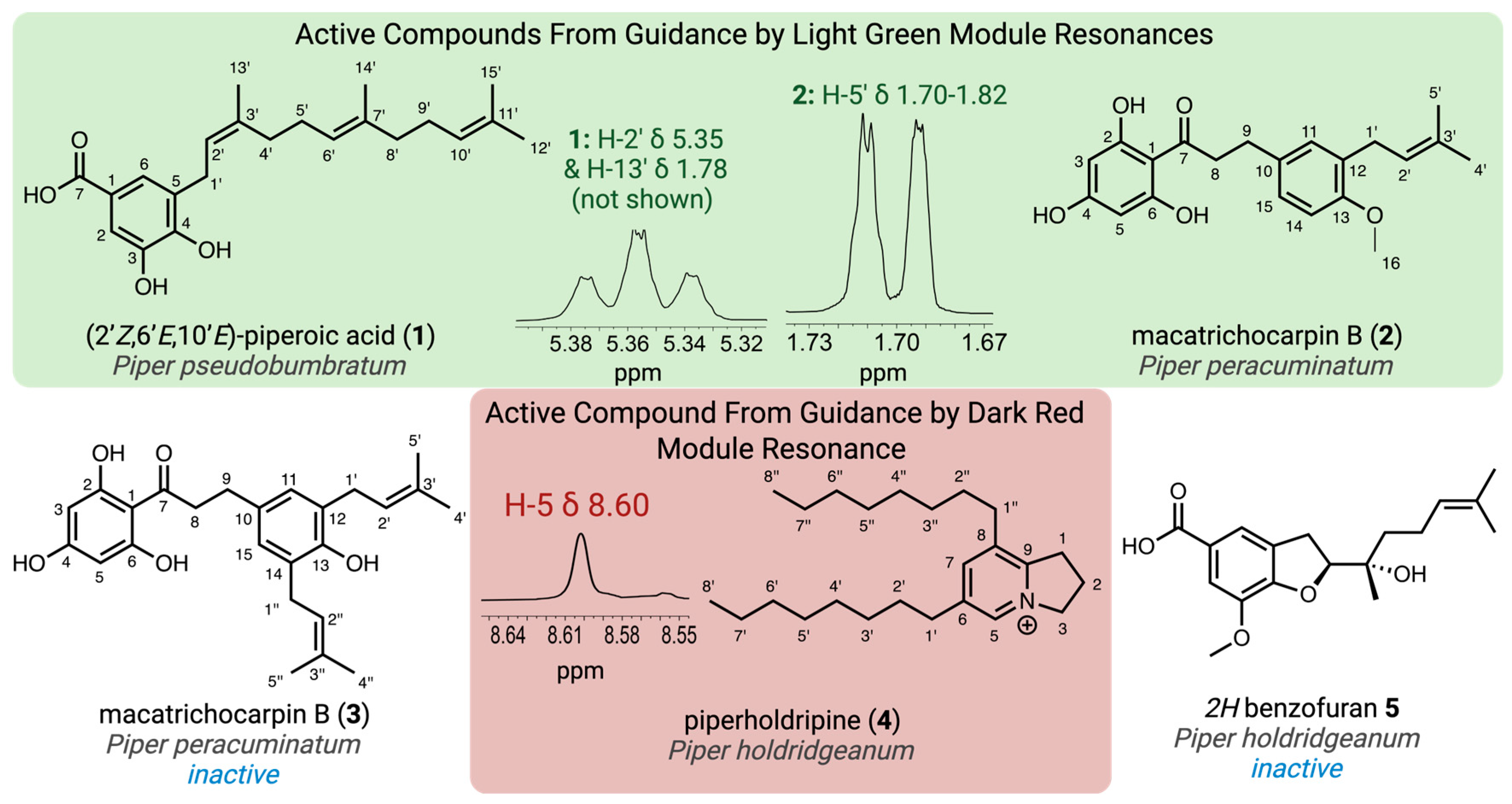
The isolated compounds were screened against S. cereviseae to confirm inhibitory activity and subsequently analyzed using various 2D NMR techniques for structural characterization. 1H NMR guided isolation from P. pseudobumbratum, P. peracuminatum, and P. holdridgeanum resulted in the purification of three compounds (1, 2, and 4, Figure 2) that contained the target resonances and demonstrated antifungal activity consistent with the inhibitory activity of the crude extracts. During the process of the 1H NMR targeted isolation, additional major compounds were purified and characterized from the focal species; however, they did not demonstrate antifungal activity (3 and 5, Figure 2).
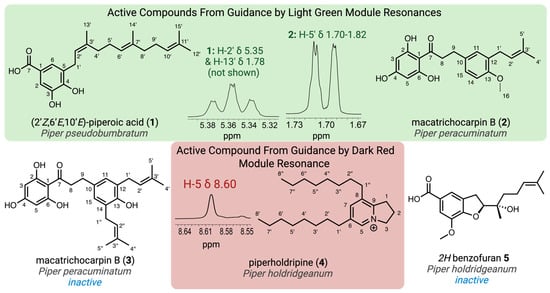
Figure 2.
Structures isolated from the crude extracts using the 1H NMR targeted approach along with the specific resonances that guided the isolation from the light green and dark red modules. Created in BioRender. Jeffrey, C. (2025) https://BioRender.com/tou5huv.
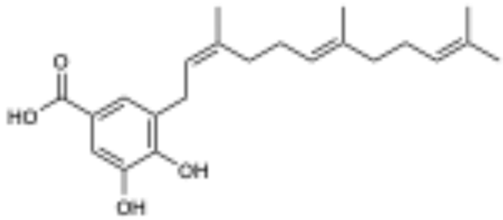
Initial fractionation of the P. pseudobumbratum extract yielded the target compound at high concentration from the chromatographic fraction eluted with 50% acetone/water on C18. Further purification of this extract provided 35 mg of compound 1 with an ESI-HRMS m/z 357.2009 (calculated for C22H30O4, 357.2071 [M − H]−), which displayed the signature resonances from module light green. Analysis of the 1H NMR data suggested the presence of a 1,2,3,5-tetrasubstitued aromatic ring with a single farnesyl substituent, which was characterized by the presence of three vinylic resonances (δH 5.07, 5.16 and 5.36) and the single benzylic methylene (δH 3.33) (Table 1). From the 13C NMR data, we concluded that one of the ring substituents was a carboxylic acid (δC 170.7 ppm), with the other two assigned as phenols given the presence of two oxygenated aryl carbons (δC 149.4 and 145.4). Finally, we concluded that both aromatic protons H-2 and H-6 were ortho to the carboxylic acid since they displayed 1H{13C} HMBC correlations with this quaternary carbon (C-7) and one of the hydroxylated carbons (C-4). The observed chemical shift values at δH 7.32 and 7.35 and meta coupling constant (J = 2 Hz) were consistent with this substitution pattern. NOESY data confirmed the configuration of the alkene groups, and the final structure was determined as (2’Z,6’E,10’E)-piperoic acid (1), which is a new isomeric form of the equivalent (2’E,6’Z,10’E) compound isolated from P. auritum [20].

Table 1.
1H and 13C NMR data and assignments for (2’Z,6’E,10’E)-piperoic acid (1).
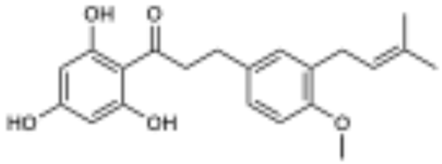
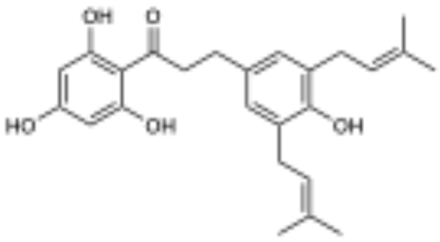
Solid-phase partitioning of the P. peracuminatum extract led to the recovery of the target peaks in the fractions eluted with 50% and 60% acetone/water. These fractions were pooled based on the targeted resonances for purification, and the resulting collections yielded two novel compounds–2 (20 mg) and 3 (16 mg)–with proximal eluting times that contained the target resonances. Compound 2 resulted in an ESIHRMS m/z 355.1560 (calculated for C21H24O5, 355.1551 [M − H]−), and compound 3 yielded an ESIHRMS m/z 409.2030 (calculated for C25H30O5, 409.2020 [M − H]−). Both compounds also had the signature peak from module royal blue (δH 2.86, H-9), which was confirmed to be part of a propenone moiety based on its 1H{1H} COSY correlations with the methylene resonance at ~δH 3.25 (H-8) and on the 1H{13C} HMBC peaks of both methylene resonances with the ketone resonance at δC 205.9 (C-7) (Table 2). The peak from H-9 was present at an integration ratio of 1:1 with the vinylic resonance (~δH 3.25, H-2’) in compound 3, which led us to the conclusion that this compound possessed two equivalent prenyl groups (Table 3). The most distinctive proton peak patterns for the two compounds were verified in the aromatic region, where compound 2 displayed three diagnostic aryl-H resonances for a 1,2,4-trisubstituted ring while 3 contained a singlet indicative of a symmetric 1,2,3,4-tetrasubstituted ring. We confirmed from the 1H{13C} HMBC correlations between these aromatic protons and the carbons C-9 and C-1’ that the prenyl groups were implicated in the symmetry of the aromatic ring of 3 and that in both compounds, the propenone moiety was attached to the ring through the β-carbonyl carbon and meta position relative to the prenyl groups. The presence of an oxygenated aryl carbon (C-13) in both compounds led to the conclusion that the remaining substituent was a hydroxyl group in compound 3 and a methoxy group in 2 (δH 3.78, H-16). Finally, we identified the singlet at δH 5.86 as being part of an acylphloroglucinol aromatic moiety, which was connected to the propenone linker through the carbonyl carbon (1H{13C} HMBC, C-7, and H-3/5), thus defining the structures of macatrichocarpin B (2), which was previously isolated from Macaranga trichocarpa, and its 14-prenylated derivative (3) [21].

Table 2.
1H and 13C NMR assignments for macatrichocarpin B (2).

Table 3.
1H and 13C NMR assignments for 14-prenylmacatrichocarpin B (3).
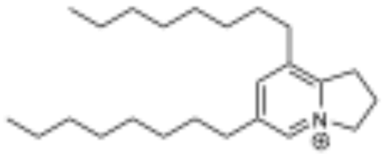
Initial attempts to purify the bioactive component(s) from P. holdrigeanum were met with failure, only resulting in the isolation of a methylated carboxylic acid derivative of a 2H-benzofuran previously isolated from Waltheria indica (5; see supporting information for the details of the structure determination) that did not contain the targeted resonances from module dark red and demonstrated no antifungal activity against S. cerevisiae [22]. We found that silica gel chromatography was not amenable to the isolation of the compound containing the targeted resonances and speculated that the lack of a strong chromophore also limited the application of traditional isolation methods using reverse-phase media with UV detection. By employing reverse-phase prep-HPLC with MSD detection to partition the crude extract, we recovered 10 fractions containing varying concentrations of the compound(s) exhibiting the target resonances (δH 8.61). All these fractions exhibited potent antifungal activity against S. cerevisiae and contained a component with m/z 344.3320 corresponding to C24H42N+ (calculated for 344.3312 [M]+). Further purification of the pooled fractions led to a highly enriched fraction of the target compound containing the singlet (δH 8.61) identified by module dark red along with a propenyl system [δH 4.83 (t), 2.50 (pent), and 3.47 (t), coupled via COSY] and two long-chain aliphatic substituents. The diagnostic 1H NMR resonances were assigned to the 5 and 7 positions of the indolizinium core, and 13C NMR analysis further demonstrated the presence of five aromatic carbon resonances (Table 4). HMBC analysis confirmed the connectivity of the substituents to the aromatic ring, which supported the assignment of the novel C6,C8-bis-octyl substituted 2,3-dihydro-1H-indolizidinium alkaloid, piperholdripine (4), as the principal bioactive compound from Piper holdridgeanum and represents a plant-derived indolizinium compound that demonstrates antimicrobial activity (Figure 3) [23,24,25,26].

Table 4.
1H and 13C NMR assignments for piperholdripine (4).
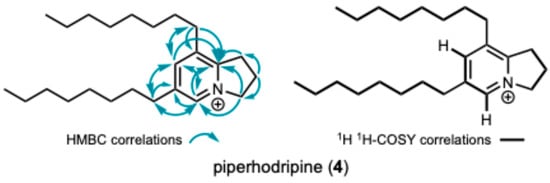
Figure 3.
Key 1H, 1H COSY, and 1H, 13C HMBC correlations leading to the assignment of the structure of piperholdripine (4).
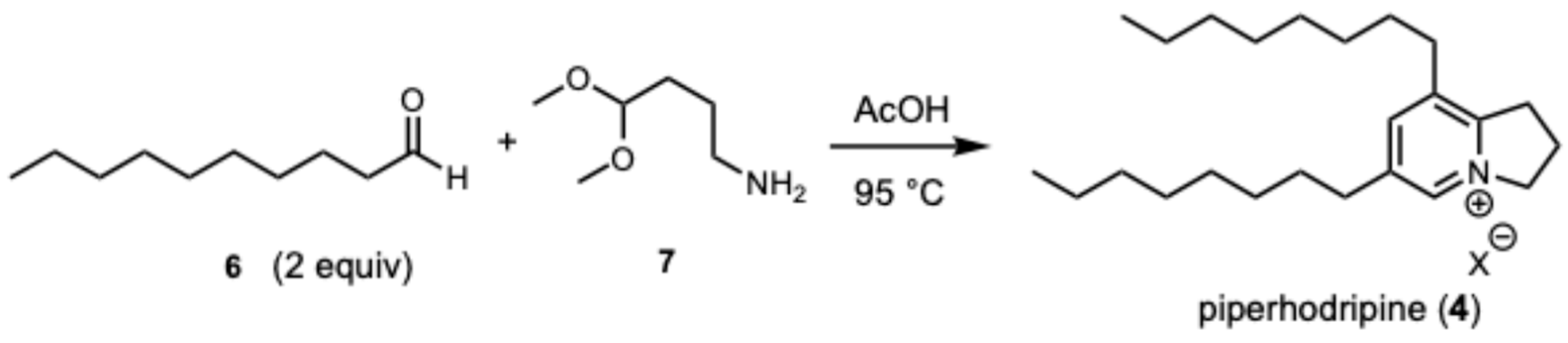
To verify the structure of piperholdripine (3), we pursued a confirmatory synthesis using methods reported by Snider [27]. Heating a solution of decanal (6) with 4-aminobutanal dimethyl acetal (7) in acetic acid at 80 °C provided piperholdripine (4) in 17% yield after isolation using flash reverse-phase chromatography (C-18, acetonitrile/water) which was spectroscopically found to be identical to the isolated compound (Scheme 1).
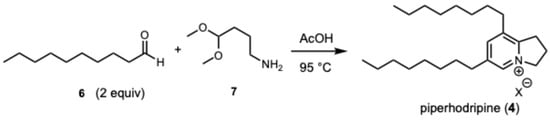
Scheme 1.
A confirmatory synthesis of piperholdripine (4) from decanal and 4-aminobutanal dimethyl acetal.
2.4. Bioactivity of the Isolated Compounds
The purified compounds were subjected to S. cerevisiae growth inhibition assays to investigate their antifungal properties. We first assayed the isolated compounds from P. pseudobubratum and P. peracuminatum at a standard concentration of 100 µM in S. cerevisiae growth inhibition assays as described in the supporting information (Figure 4A,B) [28]. (2’Z,6’E,10’E)-Piperoic acid (1) from P. pseudobumbratum inhibited S. cerevisiae growth by 25% compared to untreated or solvent controls. In contrast, macatrichocarpin B (2) inhibited S. cerevisiae growth by 75%, and cells treated with this compound exhibited markedly extended lag phase growth compared to untreated cells. Dose–response analysis of cells treated with macatrichocarpin B (2) indicated an IC50 of 10.5 µM (Figure 4C). 14-prenyl macatrichocarpin B (3) was less active at 100 µM compared to macatrichocarpin B (2), displaying only 40% growth inhibition compared to untreated controls. These results suggest that the prenyl substitution captured by module light green is a determining structural feature for compound activity in the investigated extracts, which is further supported by reports that identify prenylated benzoic acid derivatives with antifungal activity across several species of Piper [20,29,30]. It is noteworthy that although this structural feature was correlated with increased inhibitory activities of compounds 1 and 2, the presence of a second prenyl unit in 5-prenyl macatrichocarpin B (3) resulted in significantly reduced potency, suggesting a structural specificity affiliated with S. cerevisiae inhibition. Moreover, the enhanced inhibitory effect of macatrichocarpin B (2) was associated with another module (royal blue) that represented part of the dihydrochalcone motif, which is also present in antifungal compounds isolated from P. mollicomum and P. aduncum, and that can be postulated as an important structural determinant for yeast inhibition. Conversely, the module that was most strongly associated with P. pseudobumbratum (light yellow) was not correlated with inhibitory activity as it represented structural features of an elongated farnesyl group that were not as determining for inhibitory activity [31,32,33]. This is a minus sign or at least this is the minus sign that graphpad prism uses and can’t really be changed in the program.
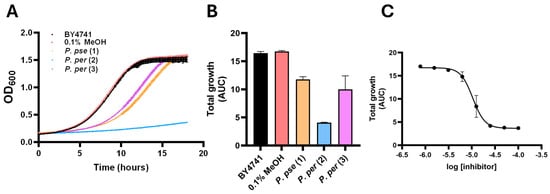
Figure 4.
Antifungal activity of P. pericuminatum and pseudobumbaratum compounds. Purified compounds were assayed against S. cerevisiae BY4741 as described in Materials and Methods at a concentration of 100 µM. S. cerevisiae treated with 0.1% MeOH served as a solvent control. (A) Representative growth curves of S. cerevisiae BY4741 treated 100 µM (2’Z,6’E,10’E)-piperoic acid (1) (orange), macatrichocarpin (2) (blue), or 5-prenyl macatrichocarpin (3) (purple) compared to 0.1% MeOH (red) and untreated (black) growth curves. Error bars represent SEM (n = 3). (B) AUC values of integrated growth curves are shown for similar experiments. Error bars represent SEM (n = 3). (C) Macatrichocarpin (2) was assayed at decreasing concentrations to establish a dose–response relationship. Error bars represent SEM (n = 3).
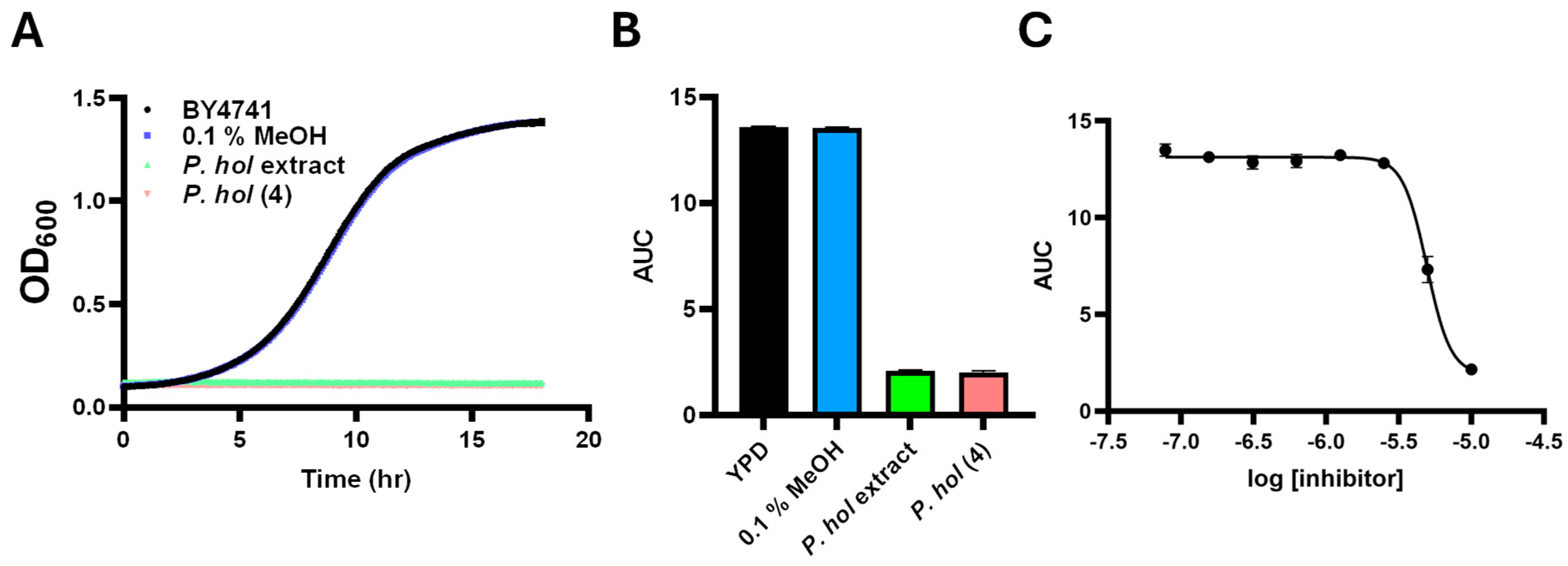
The antifungal activity of P. holdridgeanum extracts (Figure 5A,B) revealed an inhibitory effect comparable to that observed for P. peracuminatum but originating from a compound that was present at a considerably lower concentration according to the target resonances depicted by the network analysis. Piperholdripine (4), originating from isolation and synthesis, demonstrated strong antifungal activity, inhibiting S. cerevisiae growth by greater than 80% compared to untreated and solvent controls (Figure 5A,B). Dose–response analysis of piperholdripine (4) growth inhibition activity indicated an IC50 of 4.9 µM (Figure 5C). This compound did not inhibit the growth of the model bacterium Escherichia coli, demonstrating growth inhibition selectivity toward S. cerevisiae.
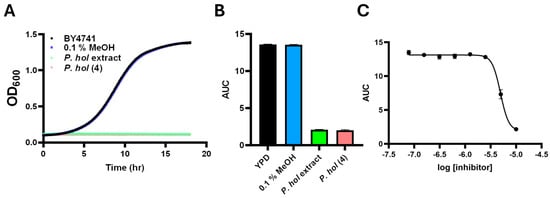
Figure 5.
Growth inhibition analysis of purified piperholdripine (4): S. cerevisiae BY4741 was grown in YPD medium supplemented with 80 µg/mL P. holdrigeanum extract, 100 µM purified compound 4, or 0.1% MeOH as a negative control for 18 h at 30°. (A) Representative growth curves of samples are shown. (B) Calculated and plotted growth curves. Error bars represent SEM (n = 3 biological replicates, 9 technical replicates). (C) Dose–response curves of S. cerevisiae cultures subjected to decreasing concentrations of piperholdripine (4).
3. Discussion
In summary, we verified that the 1H NMR network led to the identification of compounds (including two new molecules), which were found to be responsible for the bioactivity observed for the crude leaf extracts (Table 5). The light green module directed the isolation toward prenylated phenols and led to the isolation of iso-piperoic acid (1) and macatrichocarpin B (2), which both demonstrated inhibitory activity against S. cerevisiae. The inhibitory activity of iso-piperoic acid (1) was the lowest among the isolated compounds and lower than that of the crude extract (28.5% vs. 63.2% inhibition). However, direct quantitative comparison is challenging due to the unknown concentration of active component(s) within the crude mixture. Furthermore, potential synergistic effects or contributions from unidentified minor constituents may enhance the bioactivity of the crude extract, and further investigation is needed to fully assess these factors. The activity of macatrichocarpin B (2) was found to inhibit S. cerevisiae (IC50 = 10.9 μM) at a much closer level to the crude extract (75.3 vs. 98.1% inhibition). In addition to the light green module resonances, the dark red module that was represented by a resonance (δH 8.6) in the crude extract of Piper holdridgeanum and correlated with inhibitory activity against S. cerevisiae led to the isolation and characterization of piperholdripine (4). Piperholdripine (4) is an indolizinium natural product that represents a new compound that has not been observed across the Piper genus. The pure compound demonstrated the most potent inhibitory activity (IC50 = 4.9 μM) against S. cerevisiae in this study and close to that observed for the crude extract (85.3 vs. 95.8% inhibition).

Table 5.
Growth-inhibition activity of crude extracts and isolated compounds against S. cerevisiae.
Given the validation of the 1H NMR approach, we predict that the evaluation of a larger library of Piper extracts and integration of a larger set of known compounds using this methodology may further elucidate the structural effects (e.g., elongation and functionalization of the prenyl chain) in modulating the antifungal activities of these compounds and to guide the isolation of novel molecular structures associated with biological activity. Complementarily, the application of this approach with yeast deletion collections could provide a powerful method to identify the specific mechanisms of action of a compound [34]. Gene knockouts that result in altered drug resistance may offer direct clues to structure-cellular target affiliations and thus help to discern whether varying levels of inhibition result from differentiated compound-specificity towards a common target or if these compounds target distinct cellular processes. The refined structural information obtained from the chemical modules can then be evaluated against susceptible/resistant phenotypes to highlight the role of specific molecule motifs in dictating biological activity and be used to guide the isolation of bioactive constituents of a mixture based upon 1H NMR resonances.
4. Materials and Methods
4.1. Compound Isolation
For the isolation of target compounds, we sequentially and extensively extracted 6 g of oven-dried leaf material with n-hexane, acetone, and methanol at room temperature under mechanical agitation [7]. For all three focal Piper species, the acetone fractions displayed the target peaks upon 1H NMR analysis, so we pre-fractionated 200 mg of these extracts using preparative reverse-phase medium pressure liquid chromatography (RP-MPLC; 10 g column, FC-C18 60 μm, 2.5 cm × 8 cm), eluted with acetone/H2O gradient starting at 50% v/v and increasing by discrete increments of 10% v/v. The stationary phase was pre-conditioned with 50% acetone/H2O, and then the extracts were suspended in approximately 1 mL of mobile phase and injected into the column. Two samples of 15 mL were collected for each eluent mixture, then dried under reduced pressure and analyzed by 1H NMR. Fractions containing the target resonances were then further purified using RP-MPLC (5 g, FC-C18 60 μm, 1.5 cm × 5 cm, Yamazen), eluting with an appropriate linear gradient of acetone/H2O, and monitored by thin layer chromatography and UV absorption at 256 nm. Compound 1 was recovered in the 50% fractions of the P. pseudobumbratum extract and purified using the aforementioned chromatographic setup with a 30–60% v/v acetone/H2O gradient. Compounds 2 and 3 were obtained as a co-eluting mixture from the second 50% and the first 60% fractions of the P. peracuminatum extract and were purified in a similar manner using a 40–70% acetone/H2O gradient. The identity and purity of the isolated compounds were confirmed by 1H NMR analysis.
Extraction of dried leaves of Piper holdrigeanum with methanol provided 5.1 g of crude extract. Piper holdripine was isolated using preparative HPLC chromatographic separation, which was performed using an Agilent Infinity II Preparative LC system paired with a 6140 quadrupole MSD and variable wavelength detection. The HPLC with a gradient elution method of water and acetonitrile containing 0.1% formic acid using an Agilent InfinityLab Poroshell 120 4 SB-C18 column (21.1 × 150 mm, 25 mL/min) and a gradient starting at 70:30 water/acetonitrile and linearly increasing to 17:83 water/acetonitrile over 20 min followed by step increase to 100% acetonitrile for 10 min Agilent. This method resulted in 10.5 mg of a partially purified fraction containing the target compound. The resulting sample was further purified using the Agilent, a semi-preparative HPLC column (Agilent 5 Prep-C18 250x10mm, 8mL/min flow rate) using the identical water/acetonitrile gradient previously described, resulting in the purified sample of piperholdripine (4), which eluted as a broad peak eluting at approximately 13.2 min (0.8 mg, 344.4 m/z).
4.2. Spectroscopic Analysis
Purified compounds were resuspended in methanol-d4 containing 0.03% TMS to an approximate concentration of 10 mg/mL and subjected to complete structural characterization. One-dimensional and two-dimensional NMR analysis was conducted on a Varian 400-MR or a Varian 500 MHz spectrometer using the following number of transients: 1H: 128; 13C: 15,000; gCOSY: 4 × 128; gHSQC: 4 × 256; and gHMBC: 8 × 512. Optimal pulse widths (pw90), delay, and acquisition times were experimentally determined for each compound. In cases where the solvent residual peaks compromised the characterization of the compounds, samples were also prepared and analyzed using acetonitrile-d3 to resolve the assignments when necessary. High-resolution mass spectra for compounds 2–4 were performed on an Agilent 1200 series HPLC coupled to an Agilent 6230B TOFMS. The HPLC was run in a flow injection using an isocratic mobile phase consisting of 0.1% formic acid in acetonitrile with a flow rate of 0.4 mL/min. An amount of 1 μL of sample was injected into the mobile phase stream, and the mass spectrum was collected for 1 min. The sample was analyzed in position ion mode on the TOMFS with the following source conditions: 325 °C temperature; nitrogen gas flow of 5 l/min at a pressure of 20 psi; Vcap = 3500 V; and a fragmentor set at 175 V, with a skimmer voltage of 65 V, and an Oct RF Vpp of 750 V. HRMS was recorded for compound 1 by injecting (10.00 µL) on an Agilent 1290 Infinity II UPLC coupled to an Agilent 6560 Ion Mobility Quadrupole Time-of-Flight (IM-QTOF) mass spectrometer via a Jet Stream electrospray ionization source (ESI-TOF; gas temperature: 300 °C; flow: 11 L/m; nebulizer pressure: 35 psig; sheath gas temp: 300 °C; sheath gas flow: 12 L/m; VCap: 3500 V; nozzle voltage: 500 V; fragmentor: 350 V; skimmer: 65 V; octopole: 750 V; ion polarity: negative). All organic solvents and mobile phase modifiers were Fisher Optima grade. Mobile phase solvents consisted of 18 MΩ water (solvent A) and 99% acetonitrile (aq, solvent B), both modified by adding 0.1% formic acid.
(2’Z,6’E,10’E)-piperoic acid (1): 1H NMR (400 MHz, CDCl3): δ 7.32 (d, J = 2.0 Hz, 1H), 7.35 (dt, J = 2.0, 0.5 Hz, 1H), 3.33 (br d, J = 7.1 Hz, 2H), 5.36 (br t, J = 7.4 Hz, 1H), 2.21–2.15 (m, 2H), 2.16–2.09 (m, 2H), 5.16 (br t, J = 7.4 Hz, 1H), 1.98–1.92 (m, 2H), 2.08–2.01 (m, 2H), 5.07 (m, 1H), 1.65 (br s, 3H), 1.75 (br q, J = 1.2 Hz, 3H), 1.60 (br s, 3H), 1.58 (br s, 3H); 13C NMR (101 MHz, CDCl3): δ 122.2, 115.1, 145.4, 149.4, 137.3, 124.1, 170.7, 28.8, 124.0, 129.2, 32.9, 27.8, 125.3, 136.2, 40.8, 27.8, 125.5, 132.0, 25.9, 23.8, 16.1, and 17.7. HRMS (ESI negative) m/z [M − H]− calcd for C22H30O4, 357.2071; found 357.2079.
macatrichocarpin B (2): 1H NMR (400 MHz, CDCl3): δ 7.00 (dd, J = 8.2, 2.3 Hz, 1H), 6.99 (d, J = 2.0 Hz, 1H), 6.79 (d, J = 8.3 Hz, 1H), 5.86 (s, 2H), 5.25 (m, 1H), 3.78 (s, 3H), 3.27–3.24 (m, 2H), 3.25 (br d, J = 7.4 Hz, 2H), 2.85 (dd, J = 8.2, 6.7 Hz, 2H), 1.71 (br s, 3H), 1.69 (br s, 3H); 13C NMR (101 MHz, CDCl3): δ 205.9, 165.2, 164.9, 156.6, 134.7, 133.0, 130.6, 130.5, 127.7, 123.7, 111.6, 105.3, 95.9, 56.1, 46.7, 30.5, 29.3, 25.9, and 17.8. HRMS (ESI negative) m/z: [M − H]− calcd for C21H24O5, 355.1551;found 355.1560.
14-prenylmacatrichocarpin B (3):1H NMR (400 MHz, CDCl3): 7.35 (dt, J = 2.0, 0.5 Hz, 1H), 7.32 (d, J = 2.0 Hz, 1H), 5.36 (br t, J = 7.4 Hz, 1H), 5.16 (br t, J = 7.4 Hz, 1H), 5.07 (m, 1H), 3.33 (br d, J = 7.1 Hz, 2H), 2.21–2.15 (m, 2H), 2.16–2.09 (m, 2H), 2.08–2.01 (m, 2H), 1.98–1.92 (m, 2H), 1.75 (br q, J = 1.2 Hz, 3H), 1.65 (br s, 3H), 1.60 (br s, 3H), 1.58 (br s, 3H); 13C NMR (101 MHz, CDCl3): δ 170.7, 149.4, 145.4, 137.3, 136.2, 132.0, 129.2, 125.5, 125.3, 124.1, 124.0, 122.2, 115.1, 40.8, 32.9, 28.8, 27.8, 27.8, 25.9, 23.8, 17.7, and 16.1. HRMS (ESI negative) m/z: [M − H]− calcd for C25H30O5, 409.2020; found 409.2030.
Piperholdripine (4): 1H NMR (500 MHz, CDCl3): δ 8.60 (br s, 1H), 8.17 (br s, 1H), 4.83 (br t, J = 7.8 Hz, 2H), 3.47 (br t, J = 7.8 Hz, 2H), 2.80 (br t, J = 7.3 Hz, 4H), 2.50 (br pent, J = 7.8 Hz, 2H), 1.66–1.72 (m, 4H), 1.34–1.44 (m, 8H), 1.26–1.36 (m, 12H), 0.90 (m, 6H); 13C NMR (125 MHz, CDCl3): δ 156.6, 145.9, 142.9, 140.7, 138.8, 60.5, 33.2, 33.0, 32.7, 31.7, 31.6, 30.2, 30.1, 23.7, 22.1, 14.4. HRMS (ESI positive) m/z: [M]+ calcd for C24H42N+; found 344.3320.
4.3. Network Analysis
Network analysis of the crude 1H NMR spectra was performed according to previously reported methodology [7,19]. To facilitate the characterization of bioactive molecular targets from the crude extracts, we seeded the network analysis with spectra collected from a library of prepared mixtures of known compounds that broadly represent metabolite classes encountered in plant extracts. After the modules were calculated for the combined set of mixtures and extracts, we calculated the Pearson correlations between module eigenvalues and bioassay data. We then processed a parallel analysis to assign module correlations with compound concentrations from the mixtures. By comparing these results, we were then able to estimate the structural elements associated with the bioactive targets present in the crude extracts. Moreover, by referring to the modules that demonstrated a significant correlation with bioactivity, we identified diagnostic peaks in the 1H-NMR spectrum that were used as a guide for the isolation of the bioactive compounds.
4.4. Synthesis of Piperholdripine
Decanal (2.5 mL, 15.0 mmol, 2.0 equiv) was added to a solution of 4-aminobutanal dimethyl acetal (1.2 mL, 7.5 mmol, 1.0 equiv) in acetic acid (30 mL, 0.25 M). The solution was heated to 95 °C and stirred for 48 hr. After cooling, the reaction solution was made basic (pH~12) with 10M NaOH (70 mL), and the aqueous layer was washed with hexanes and extracted with chloroform. The combined chloroform layers were dried over MgSO4, filtered, and the solvent was evaporated under reduced pressure. Purification using solid-phase extraction on C18 (42g SepPack) with step elution with H2O in CH3CN (100%,75%, 50%, 0%) provided piperholdripine (4, 457 mg, 1.33 mmol, 17%) in the 50% fraction.
4.5. Bioassays
Crude methanolic extracts were prepared following standard procedures [7] and assayed against Saccharomyces cerevisiae BY4741. S. cerevisiae cells were initially grown on solid YPD medium (2% [w/v] peptone, 1% [w/v] yeast extract, 2% [w/v] glucose) and incubated for 2 days at 30 °C. A single colony was used to inoculate a 10 mL YPD culture, which was incubated at 30 °C for 18 h with shaking. Saturated cultures were diluted 100-fold into liquid YPD, and extracts or purified compounds were diluted into these cultures at indicated concentrations. Samples were arrayed into Honeycomb 100-well plates (Growth Curves USA) and assayed in a Bioscreen C plate reader (Growth Curves USA). OD600 readings were taken at 5 min intervals for 18 h with 30 s of shaking before each reading, as previously described. Following the identification of inhibitory targets, the assays were repeated with the purified compounds, initially at a concentration of 100 μM, and then at serial dilutions to calculate the half-maximal inhibitory concentration (IC50). Area Under Curve (AUC) values were plotted against the log of the inhibitor concentration, and IC50 was calculated in GraphPad Prism using the log(inhibitor) vs. response variable slope model.
5. Conclusions
This study validates the 1H NMR-based weighted gene co-expression network analysis (WGCNA) approach for identifying bioactive compounds in complex natural product extracts. By isolating and characterizing the compounds responsible for specific bioactivity-associated resonances identified in our previous network analysis, we confirmed that the resonances correlated with antifungal activity indeed correspond to biologically active compounds. This targeted approach led to the discovery of two novel antifungal compounds, including one with a rare imidazolium heterocyclic motif, thereby introducing a new structural class within the Piper genus. These findings support the WGCNA method as a reliable tool for natural product discovery, allowing for the efficient identification of 1H NMR spectral features linked to bioactivity. This not only streamlines the process of isolating bioactive compounds but also highlights the potential of network-based analyses in guiding the discovery of natural products with therapeutic relevance. Future applications of this approach may extend its utility across other bioactivity assays, further enhancing its role in identifying ecologically relevant and bioactive compounds from crude extracts.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules30092020/s1, Figure S1: Growth-inhibition activity of Piper extracts against S. cerevisiae; Figure S2: 1H NMR Spectrum (400 MHz) of 1 in CD3OD; Figure S3: 13C NMR Spectrum (101 MHz) of 1 in CD3OD; Figure S4: 1H-1H COSY NMR Spectrum (400 MHz) of 1 in CD3OD; Figure S5: HSQC NMR Spectrum (400/101 MHz) of 1 in CD3OD; Figure S6: HMBC NMR Spectrum (400/101 MHz) of 1 in CD3OD; Figure S7: 1H-1H NOESY NMR Spectrum (400 MHz) of 1 in CD3OD; Figure S8: 1H NMR Spectrum (400 MHz) of 2 in CD3OD; Figure S9: 1H NMR Spectrum (400 MHz) of 2 in CD3CN; Figure S10: 13C NMR Spectrum (101 MHz) of 2 in CD3CN; Figure S11: 1H-1H COSY NMR Spectrum (400 MHz) of 2 in CD3CN; Figure S12: HSQC NMR Spectrum (400/101 MHz) of 2 in CD3CN; Figure S13: HMBC NMR Spectrum (400/101 MHz) of 2 in CD3CN; Figure S14: 1H NMR Spectrum (400 MHz) of 3 in CD3CN; Figure S15: 13C NMR Spectrum (101 MHz) of 3 in CD3CN; Figure S16: 1H-1H COSY NMR Spectrum (400 MHz) of 3 in CD3CN; Figure S17: HSQC NMR Spectrum (400/101 MHz) of 3 in CD3CN; Figure S18: HMBC NMR Spectrum (400/101 MHz) of 3 in CD3CN; Figure S19: 1H NMR Spectrum (400 MHz) of 4 in CD3OD; Figure S20: Expanded 1H NMR Spectrum (400 MHz) of 4 in CD3OD, highlighting peaks in the 0–3.5 ppm region; Figure S21: 13C NMR Spectrum (101 MHz) of 4 in CD3OD; Figure S22: 1H-1H COSY NMR Spectrum (400 MHz) of 4 in CD3OD; Figure S23: HSQC NMR Spectrum (400/101 MHz) of 4 in CD3OD; Figure S24: HMBC NMR Spectrum (400/101 MHz) of 4 in CD3OD; Figure S25: 1H NMR Spectrum (400 MHz) of synthesized 4 in CD3OD; Figure S26: 13C NMR Spectrum (101 MHz) of synthesized 4 in CD3OD; Figure S27: 1H-1H COSY NMR Spectrum (400 MHz) of synthesized 4 in CD3OD; Figure S28: HSQC NMR Spectrum (400/101 MHz) of synthesized 4 in CD3OD; Figure S29: HMBC NMR Spectrum (400/101 MHz) of synthesized 4 in CD3OD; Figure S30: 1H NMR Spectrum (400 MHz) of 5 in CDCl3; Figure S31: 13C NMR Spectrum (101 MHz) of 5 in CDCl3.
Author Contributions
Conceptualization, C.S.J., C.D.D., I.S.W., L.A.D., L.A.R. and C.R.O.; methodology and validation, all authors.; writing—original draft preparation, C.S.J. and C.R.O.; writing—review and editing, all authors; funding acquisition, L.A.R., L.A.D. and C.S.J. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by grants from the NSF (EN-2133818) and by the Hitchcock Center for Chemical-Ecology fund. C.O. was supported by a Hitchcock Center for Chemical-Ecology graduate fellowship.
Institutional Review Board Statement
This study did not require ethical approval.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw NMR data for compounds 1–5 are deposited into the Natural Products Magnetic Resonance Database (NP-MRD; www.np-mrd.org), accessed on 10 May 2025.
Acknowledgments
Collection and export permits were granted by Sistema Nacional De Areas De Conservación (SINAC), Costa Rica (113-DGVS-2016). The authors thank Eric J. Tepe and Humberto Garcia for their identification and collection of the plant tissue. The authors thank Zachary D. Ledvina for assistance with acquiring HRMS data for compound 1. The graphical abstract was Created in BioRender. Jeffrey, C. (2025) https://BioRender.com/ni0bcvn.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Orhan, I.E.; Banach, M.; Rollinger, J.M.; Barreca, D.; Weckwerth, W.; Bauer, R.; Bayer, E.A.; et al. Natural Products in Drug Discovery: Advances and Opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Gaudêncio, S.P.; Pereira, F. Dereplication: Racing to Speed up the Natural Products Discovery Process. Nat. Prod. Rep. 2015, 32, 779–810. [Google Scholar] [CrossRef]
- Hight, S.K.; Clark, T.N.; Kurita, K.L.; McMillan, E.A.; Bray, W.; Shaikh, A.F.; Khadilkar, A.; Haeckl, F.P.J.; Carnevale-Neto, F.; La, S.; et al. High-Throughput Functional Annotation of Natural Products by Integrated Activity Profiling. Proc. Natl. Acad. Sci. USA 2022, 119, e2208458119. [Google Scholar] [CrossRef] [PubMed]
- Najmi, A.; Javed, S.A.; Al Bratty, M.; Alhazmi, H.A. Modern Approaches in the Discovery and Development of Plant-Based Natural Products and Their Analogues as Potential Therapeutic Agents. Molecules 2022, 27, 349. [Google Scholar] [CrossRef] [PubMed]
- Jahangir, M.; Nuringtyas, T.R.; Ali, K.; Wilson, E.G.; Choi, Y.H.; Verpoorte, R. NMR-Based Metabolomics: Understanding Plant Chemistry and Identification of Biologically Active Compounds. In NMR-Based Metabolomics; Keun, H.C., Ed.; The Royal Society of Chemistry: London, UK, 2018; ISBN 978-1-84973-643-5. [Google Scholar]
- Edison, A.S.; Colonna, M.; Gouveia, G.J.; Holderman, N.R.; Judge, M.T.; Shen, X.; Zhang, S. NMR: Unique Strengths That Enhance Modern Metabolomics Research. Anal. Chem. 2021, 93, 478–499. [Google Scholar] [CrossRef]
- Richards, L.A.; Oliveira, C.; Dyer, L.A.; Rumbaugh, A.; Urbano-Muñoz, F.; Wallace, I.S.; Dodson, C.D.; Jeffrey, C.S. Shedding Light on Chemically Mediated Tri-Trophic Interactions: A 1H-NMR Network Approach to Identify Compound Structural Features and Associated Biological Activity. Front. Plant Sci. 2018, 9, 1155. [Google Scholar] [CrossRef]
- Carsono, N.; Tumilaar, S.G.; Kurnia, D.; Latipudin, D.; Satari, M.H. A Review of Bioactive Compounds and Antioxidant Activity Properties of Piper Species. Molecules 2022, 27, 6774. [Google Scholar] [CrossRef]
- Kumar, B.; Tiwari, S.; Bajpai, V.; Singh, B. Phytochemistry of Plants of Genus Piper; CRC Press: Boca Raton, FL, USA, 2020; ISBN 978-1-003-01487-4. [Google Scholar]
- Martha Perez Gutierrez, R.; Maria Neira Gonzalez, A.; Hoyo-Vadillo, C. Alkaloids from Piper: A Review of Its Phytochemistry and Pharmacology. Mini-Rev. Med. Chem. 2013, 13, 163–193. [Google Scholar] [CrossRef]
- Xu, W.-H.; Li, X.-C. Antifungal Compounds from Piper Species. Curr. Bioact. Compd. 2011, 7, 262–267. [Google Scholar] [CrossRef]
- Parmar, V.S.; Jain, S.C.; Bisht, K.S.; Jain, R.; Taneja, P.; Jha, A.; Tyagi, O.D.; Prasad, A.K.; Wengel, J.; Olsen, C.E.; et al. Phytochemistry of the Genus Piper. Phytochemistry 1997, 46, 597–673. [Google Scholar] [CrossRef]
- Philbin, C.S.; Dyer, L.A.; Jeffrey, C.S.; Glassmire, A.E.; Richards, L.A. Structural and Compositional Dimensions of Phytochemical Diversity in the Genus Piper Reflect Distinct Ecological Modes of Action. J. Ecol. 2022, 110, 57–67. [Google Scholar] [CrossRef]
- de Oliveira, C.R., Jr.; Ledvina, Z.D.; Leonard, M.D.; Odoh, S.O.; Dodson, C.D.; Jeffrey, C.S. Isolation of New Neolignans and an Unusual Meroterpenoid from Piper Cabagranum. Front. Nat. Prod. 2024, 2, 1332436. [Google Scholar] [CrossRef]
- Uckele, K.A.; Jahner, J.P.; Tepe, E.J.; Richards, L.A.; Dyer, L.A.; Ochsenrider, K.M.; Philbin, C.S.; Kato, M.J.; Yamaguchi, L.F.; Forister, M.L.; et al. Phytochemistry Reflects Different Evolutionary History in Traditional Classes versus Specialized Structural Motifs. Sci. Rep. 2021, 11, 17247. [Google Scholar] [CrossRef] [PubMed]
- Richards, L.A.; Dyer, L.A.; Forister, M.L.; Smilanich, A.M.; Dodson, C.D.; Leonard, M.D.; Jeffrey, C.S. Phytochemical Diversity Drives Plant-Insect Community Diversity. Proc. Natl. Acad. Sci. USA 2015, 112, 10973–10978. [Google Scholar] [CrossRef] [PubMed]
- Gaia, A.M.; Yamaguchi, L.F.; Jeffrey, C.S.; Kato, M.J. Age-Dependent Changes from Allylphenol to Prenylated Benzoic Acid Production in Piper Gaudichaudianum Kunth. Phytochemistry 2014, 106, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Jeffrey, C.S.; Leonard, M.D.; Glassmire, A.E.; Dodson, C.D.; Richards, L.A.; Kato, M.J.; Dyer, L.A. Antiherbivore Prenylated Benzoic Acid Derivatives from Piper Kelleyi. J. Nat. Prod. 2014, 77, 148–153. [Google Scholar] [CrossRef]
- de Oliveira, C.R. A 1H NMR Network Approach for the Study of Specialized Metabolites: Development and Applications. Ph.D. Thesis, University of Nevada, Reno, Reno, NV, USA, 2021. [Google Scholar]
- Ampofo, S.A.; Roussis, V.; Wiemer, D.F. New Prenylated Phenolics from Piper Auritum. Phytochemistry 1987, 26, 2367–2370. [Google Scholar] [CrossRef]
- Syah, Y.M.; Hakim, E.H.; Achmad, S.A.; Hanafi, M.; Ghisalberti, E.L. Isoprenylated Flavanones and Dihydrochalcones from Macaranga Trichocarpa. Nat. Prod. Commun. 2009, 4, 1934578X0900400115. [Google Scholar] [CrossRef]
- Monteillier, A.; Cretton, S.; Ciclet, O.; Marcourt, L.; Ebrahimi, S.N.; Christen, P.; Cuendet, M. Cancer Chemopreventive Activity of Compounds Isolated from Waltheria Indica. J. Ethnopharmacol. 2017, 203, 214–225. [Google Scholar] [CrossRef]
- Zhang, J.; Morris-Natschke, S.L.; Ma, D.; Shang, X.; Yang, C.; Liu, Y.; Lee, K. Biologically Active Indolizidine Alkaloids. Med. Res. Rev. 2021, 41, 928–960. [Google Scholar] [CrossRef]
- Michael, J.P. Indolizidine and Quinolizidine Alkaloids. Nat. Prod. Rep. 2007, 24, 191. [Google Scholar] [CrossRef]
- Klausmeyer, P.; Chmurny, G.N.; McCloud, T.G.; Tucker, K.D.; Shoemaker, R.H. A Novel Antimicrobial Indolizinium Alkaloid from Aniba panurensis. J. Nat. Prod. 2004, 67, 1732–1735. [Google Scholar] [CrossRef] [PubMed]
- Damu, A.G.; Kuo, P.-C.; Shi, L.-S.; Li, C.-Y.; Kuoh, C.-S.; Wu, P.-L.; Wu, T.-S. Phenanthroindolizidine Alkaloids from the Stems of Ficus Septica. J. Nat. Prod. 2005, 68, 1071–1075. [Google Scholar] [CrossRef] [PubMed]
- Snider, B.B.; Neubert, B.J. Syntheses of Ficuseptine, Juliprosine, and Juliprosopine by Biomimetic Intramolecular Chichibabin Pyridine Syntheses. Org. Lett. 2005, 7, 2715–2718. [Google Scholar] [CrossRef] [PubMed]
- Roberson, M.G.; Smith, D.K.; White, S.M.; Wallace, I.S.; Tucker, M.J. Interspecies Bombolitins Exhibit Structural Diversity upon Membrane Binding, Leading to Cell Specificity. Biophys. J. 2019, 116, 1064–1074. [Google Scholar] [CrossRef]
- Lago, J.H.G.; Ramos, C.S.; Casanova, D.C.C.; Morandim, A.D.A.; Bergamo, D.C.B.; Cavalheiro, A.J.; Bolzani, V.D.S.; Furlan, M.; Guimarães, E.F.; Young, M.C.M.; et al. Benzoic Acid Derivatives from Piper Species and Their Fungitoxic Activity against Cladosporium cladosporioides and C. sphaerospermum. J. Nat. Prod. 2004, 67, 1783–1788. [Google Scholar] [CrossRef]
- Danelutte, A.P.; Lago, J.H.G.; Young, M.C.M.; Kato, M.J. Antifungal Flavanones and Prenylated Hydroquinones from Piper Crassinervium Kunth. Phytochemistry 2003, 64, 555–559. [Google Scholar] [CrossRef]
- Lago, J.H.G.; Young, M.C.M.; Reigada, J.B.; Soares, M.G.; Roesler, B.P.; Kato, M.J. Antifungal Derivatives from Piper Mollicomum and P. Lhotzkyanum (Piperaceae). Quím. Nova 2007, 30, 1222–1224. [Google Scholar] [CrossRef]
- Orjala, J.; Wright, A.D.; Behrends, H.; Folkers, G.; Sticher, O.; Rüegger, H.; Rali, T. Cytotoxic and Antibacterial Dihydrochalcones from Piper Aduncum. J. Nat. Prod. 1994, 57, 18–26. [Google Scholar] [CrossRef]
- Dal Picolo, C.R.; Bezerra, M.P.; Gomes, K.S.; Passero, L.F.D.; Laurenti, M.D.; Martins, E.G.A.; Sartorelli, P.; Lago, J.H.G. Antileishmanial Activity Evaluation of Adunchalcone, a New Prenylated Dihydrochalcone from Piper aduncum L. Fitoterapia 2014, 97, 28–33. [Google Scholar] [CrossRef]
- Giaever, G.; Nislow, C. The Yeast Deletion Collection: A Decade of Functional Genomics. Genetics 2014, 197, 451–465. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).