Modification and Application of Natural Clinoptilolite and Mordenite from Almaty Region for Drinking Water Purification
Abstract
1. Introduction
Practical Implications and Recommendations
- -
- Industrial Applications: The superior degree of removal of Pb2+, Cd2+, and As3+ by acid-modified clinoptilolite and mordenite makes them suitable materials for the treatment of industrial wastewaters.
- -
- Cost-Effective Solution: For the same applications, modified zeolites are economically advantageous and environmentally sustainable compared to activated carbon and membrane filtration, generating very little secondary waste.
- -
- Sustainable Water Purification: Due to their remarkable reusability (retaining over 80% of adsorption capacity after five cycles), modified zeolites can be relied on for long-term applications in remote areas.
- -
- Future Research Directions: Researching further impurities such as organic waste products and nitrates will further increase the applicability of these modified zeolites.
2. Results
2.1. Structural and Chemical Changes After Acid Modification
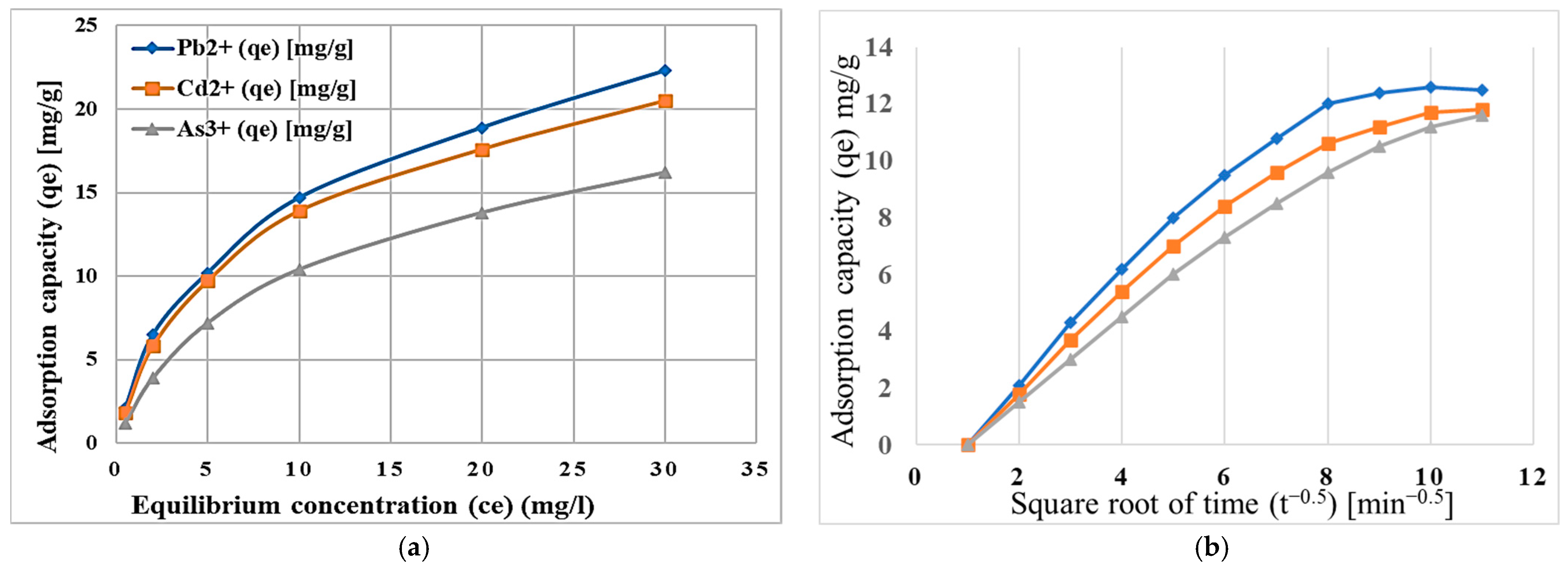
2.2. Heavy Metal and Contaminant Removal Efficiency
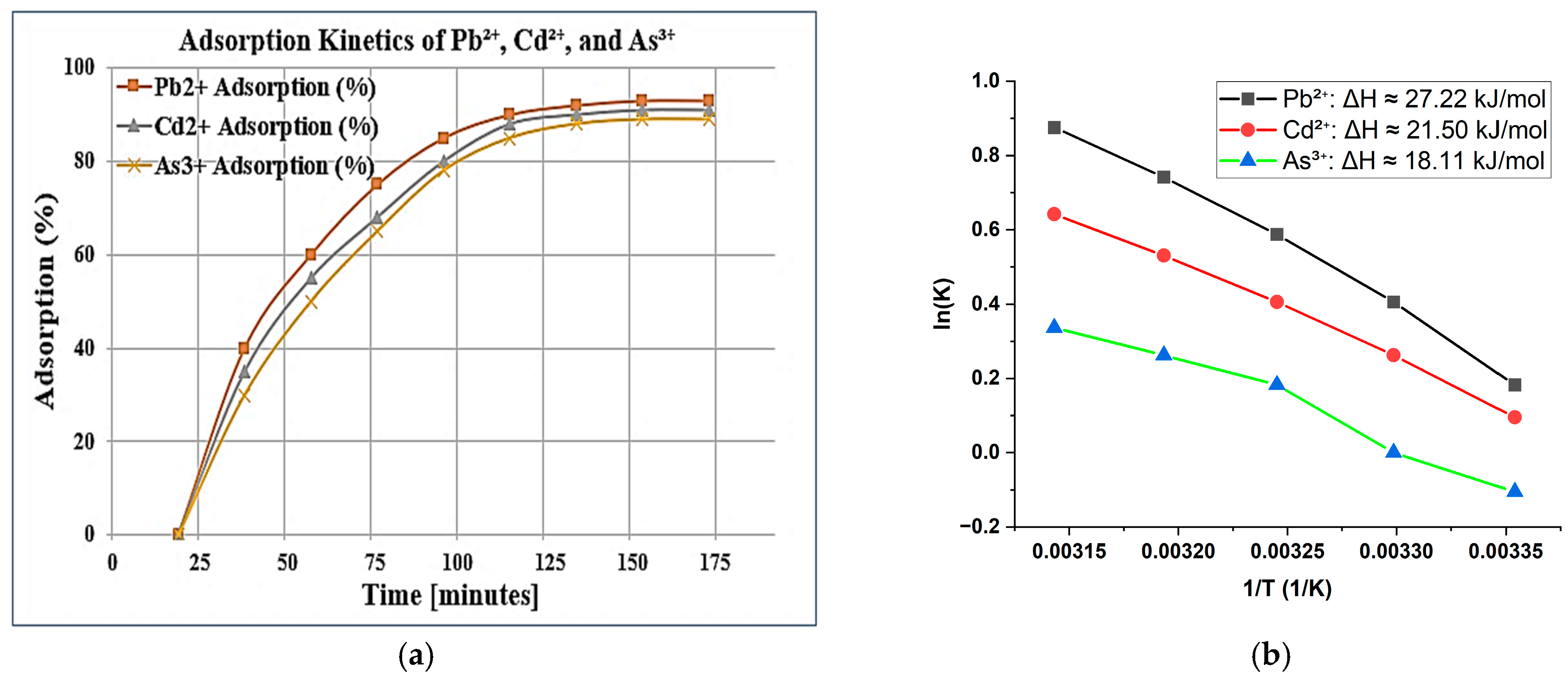
2.3. Kinetics and Thermodynamics of Heavy Metal Adsorption
Statistical Error Analysis
2.4. Effect of pH, Contact Time, and Temperature on Adsorption
Cd2+: ΔH ≈ 21.50 kJ/mol
As3+: ΔH ≈ 18.11 kJ/mol
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shi, J.; Yang, Z.; Dai, H.; Lu, X.; Peng, L.; Tan, X.; Shi, L.; Fahim, R. Preparation and application of modified zeolites as adsorbents in wastewater treatment. Water Sci. Technol. 2018, 2017, 621–635. [Google Scholar] [CrossRef]
- Mohd Zuhan, M.K.N.; Azhari, S.; Tamar Jaya, M.A. Modified zeolite as purification material in wastewater treatment: A review. Sci. Res. J. 2021, 18, 177–213. [Google Scholar]
- Muscarella, S.M.; Badalucco, L.; Cano, B.; Laudicina, V.A.; Mannina, G. Ammonium adsorption, desorption and recovery by acid and alkaline treated zeolite. Bioresour. Technol. 2021, 341, 125812. [Google Scholar] [CrossRef] [PubMed]
- Bahmanzadegan, F.; Ghaemi, A. Modification and functionalization of zeolites to improve the efficiency of CO2 adsorption: A review. Case Stud. Chem. Environ. Eng. 2023, 9, 100564. [Google Scholar] [CrossRef]
- Fu, H.; Li, Y.; Yu, Z.; Shen, J.; Li, J.; Zhang, M.; Ding, T.; Xu, L.; Lee, S.S. Ammonium removal using a calcined natural zeolite modified with sodium nitrate. J. Hazard. Mater. 2020, 393, 122481. [Google Scholar] [CrossRef]
- Pan, M.; Zhang, M.; Zou, X.; Zhao, X.; Deng, T.; Chen, T.; Huang, X. The investigation into the adsorption removal of ammonium by natural and modified zeolites: Kinetics, isotherms, and thermodynamics. Water SA 2019, 45, 648–656. [Google Scholar] [CrossRef]
- Lv, Y.; Ma, B.; Liu, Y.; Wang, C.; Chen, Y. Adsorption behavior and mechanism of mixed heavy metal ions by zeolite adsorbent prepared from lithium leach residue. Microporous Mesoporous Mater. 2022, 329, 111553. [Google Scholar] [CrossRef]
- Bacakova, L.; Vandrovcova, M.; Kopova, I.; Jirka, I. Applications of zeolites in biotechnology and medicine—A review. Biomater. Sci. 2018, 6, 974–989. [Google Scholar] [CrossRef]
- Kuldeyev, E.; Seitzhanova, M.; Tanirbergenova, S.; Tazhu, K.; Doszhanov, E.; Mansurov, Z.; Azat, S.; Nurlybaev, R.; Berndtsson, R. Modifying natural zeolites to improve heavy metal adsorption. Water 2023, 15, 2215. [Google Scholar] [CrossRef]
- Abedi, T.; Mojiri, A. Constructed wetland modified by biochar/zeolite addition for enhanced wastewater treatment. Environ. Technol. Innov. 2019, 16, 100472. [Google Scholar] [CrossRef]
- Moradi, M.; Karimzadeh, R.; Moosavi, E.S. Modified and ion exchanged clinoptilolite for the adsorptive removal of sulfur compounds in a model fuel: New adsorbents for desulfurization. Fuel 2018, 217, 467–477. [Google Scholar] [CrossRef]
- Margeta, K.; Farkaš, A. Introductory chapter: Zeolites-from discovery to new applications on the global market. In Zeolites-New Challenges; IntechOpen: London, UK, 2020; Available online: https://www.intechopen.com/chapters/72633 (accessed on 27 March 2025).
- Mansurov, Z.A.; Velasco, L.F.; Lodewyckx, P.; Doszhanov, E.O.; Azat, S. Modified Carbon Sorbents Based on Walnut Shell for Sorption of Toxic Gases. J. Eng. Phys. Thermophys. 2022, 95, 1383–1392. [Google Scholar] [CrossRef]
- Rashed, M.N.; Palanisamy, P.N. Introductory chapter: Adsorption and ion exchange properties of zeolites for treatment of polluted water. In Zeolites and Their Applications; IntechOpen: London, UK, 2018; Available online: https://www.intechopen.com/chapters/61328 (accessed on 27 March 2025).
- Doszhanov, Y.; Atamanov, M.; Jandosov, J.; Saurykova, K.; Bassygarayev, Z.; Orazbayev, A.; Turganbay, S.; Sabitov, A. Preparation of Granular Organic Iodine and Selenium Complex Fertilizer Based on Biochar for Biofortification of Parsley. Scientifica 2024, 2024, 6601899. [Google Scholar] [CrossRef]
- Seitzhanova, M.; Azat, S.; Yeleuov, M.; Taurbekov, A.; Mansurov, Z.; Doszhanov, E.; Berndtsson, R. Production of Graphene Membranes from Rice Husk Biomass Waste for Improved Desalination. Nanomaterials 2024, 14, 224. [Google Scholar] [CrossRef]
- Nayak, Y.N.; Nayak, S.; Nadaf, Y.F.; Shetty, N.S.; Gaonkar, S.L. Zeolite catalyzed friedel-crafts reactions: A review. Lett. Org. Chem. 2020, 17, 491–506. [Google Scholar] [CrossRef]
- Chen, X.; Shen, B.; Sun, H. Ion-exchange modified zeolites X for selective adsorption desulfurization from Claus tail gas: Experimental and computational investigations. Microporous Mesoporous Mater. 2018, 261, 227–236. [Google Scholar] [CrossRef]
- Jiang, N.; Shang, R.; Heijman, S.G.; Rietveld, L.C. High-silica zeolites for adsorption of organic micro-pollutants in water treatment: A review. Water Res. 2018, 144, 145–161. [Google Scholar] [CrossRef] [PubMed]
- Razmakhnin, K.K. On the question of using natural zeolites to increase the environmental safety of mining regions. IOP Conf. Ser. Earth Environ. Sci. 2022, 991, 012039. [Google Scholar] [CrossRef]
- Kianfar, E.; Mahler, A. Zeolites: Properties, applications, modification, and selectivity. In Zeolites: Advances in Research and Applications; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2020; Chapter 1; Available online: https://www.researchgate.net/publication/337928117_Zeolites_Properties_Applications_Modification_and_Selectivity (accessed on 27 March 2025).
- Huntley, G.M.; Luck, R.L.; Mullins, M.E.; Newberry, N.K. Hydrochloric acid modification and lead removal studies on naturally occurring zeolites from Nevada, New Mexico, and Arizona. Processes 2021, 9, 1238. [Google Scholar] [CrossRef]
- Eyde, D.T.; Olegario, E.M. Zeolites. Min. Eng. 2020, 72, 93. [Google Scholar]
- Cieśla, J.; Franus, W.; Franus, M.; Kedziora, K.; Gluszczyk, J.; Szerement, J.; Jozefaciuk, G. Environmental-friendly modifications of zeolite to increase its sorption and anion exchange properties, physicochemical studies of the modified materials. Materials 2019, 12, 3213. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Meier, S.; Saravanamurugan, S.; Riisager, A. Modification of commercial Y zeolites by alkaline treatment for improved performance in the isomerization of glucose to fructose. Mol. Catal. 2021, 510, 111686. [Google Scholar] [CrossRef]
- Wang, C.; Leng, S.; Guo, H.; Cao, L.; Huang, J. Acid and alkali treatments for regulation of hydrophilicity/hydrophobicity of natural zeolite. Appl. Surf. Sci. 2019, 478, 319–326. [Google Scholar] [CrossRef]
- Wen, J.; Dong, H.; Zeng, G. Application of zeolite in removing salinity/sodicity from wastewater: A review of mechanisms, challenges and opportunities. J. Clean. Prod. 2018, 197, 1435–1446. [Google Scholar] [CrossRef]
- Pérez-Botella, E.; Valencia, S.; Rey, F. Zeolites in adsorption processes: State of the art and future prospects. Chem. Rev. 2022, 122, 17647–17695. [Google Scholar] [CrossRef] [PubMed]
- de Magalhães, L.F.; da Silva, G.R.; Peres, A.E.C. Zeolite application in wastewater treatment. Adsorpt. Sci. Technol. 2022, 2022, 4544104. [Google Scholar] [CrossRef]
- Yasir, A.; Janabi, N. Discovered a new type of zeolite and tested on some chemical properties of soil and plant yield. Plant Arch. 2020, 20 (Suppl. 1), 1978–1982. [Google Scholar]
- Cha, Y.H.; Mun, S.; Lee, K.B. Development of modified zeolite for adsorption of mixed sulfur compounds in natural gas by combination of ion exchange and impregnation. Appl. Surf. Sci. 2023, 619, 156634. [Google Scholar] [CrossRef]
- Guida, S.; Potter, C.; Jefferson, B.; Soares, A. Preparation and evaluation of zeolites for ammonium removal from municipal wastewater through ion exchange process. Sci. Rep. 2020, 10, 12426. [Google Scholar] [CrossRef]
- Krstić, V. Role of zeolite adsorbent in water treatment. In Handbook of Nanomaterials for Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2021; pp. 417–481. [Google Scholar] [CrossRef]
- Derbe, T.; Temesgen, S.; Bitew, M. A short review on synthesis, characterization, and applications of zeolites. Adv. Mater. Sci. Eng. 2021, 2021, 6637898. [Google Scholar] [CrossRef]
- Lu, W.; Zhang, C.; Su, P.; Wang, X.; Shen, W.; Quan, B.; Shen, Z.; Song, L. Research progress of modified natural zeolites for removal of typical anions in water. Environ. Sci. Water Res. Technol. 2022, 8, 2170–2189. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption kinetics and isotherm models of heavy metals by various adsorbents: An overview. Crit. Rev. Environ. Sci. Technol. 2023, 53, 1837–1865. [Google Scholar] [CrossRef]
- Ongarbayev, Y.; Baigulbayeva, M.; Ualieva, P.; Abdieva, G.; Tileuberdi, Y. Carbonized Sorbents of Shungite and Rice Husk for Purification of Petroleum Contaminated Soils. J. Ecol. Eng. 2022, 23, 16–25. [Google Scholar] [CrossRef]
- Sabitov, A.; Atamanov, M.; Doszhanov, O.; Saurykova, K.; Tazhu, K.; Kerimkulova, A.; Orazbayev, A.; Doszhanov, Y. Surface Characteristics of Activated Carbon Sorbents Obtained from Biomass for Cleaning Oil-Contaminated Soils. Molecules 2024, 29, 3786. [Google Scholar] [CrossRef]
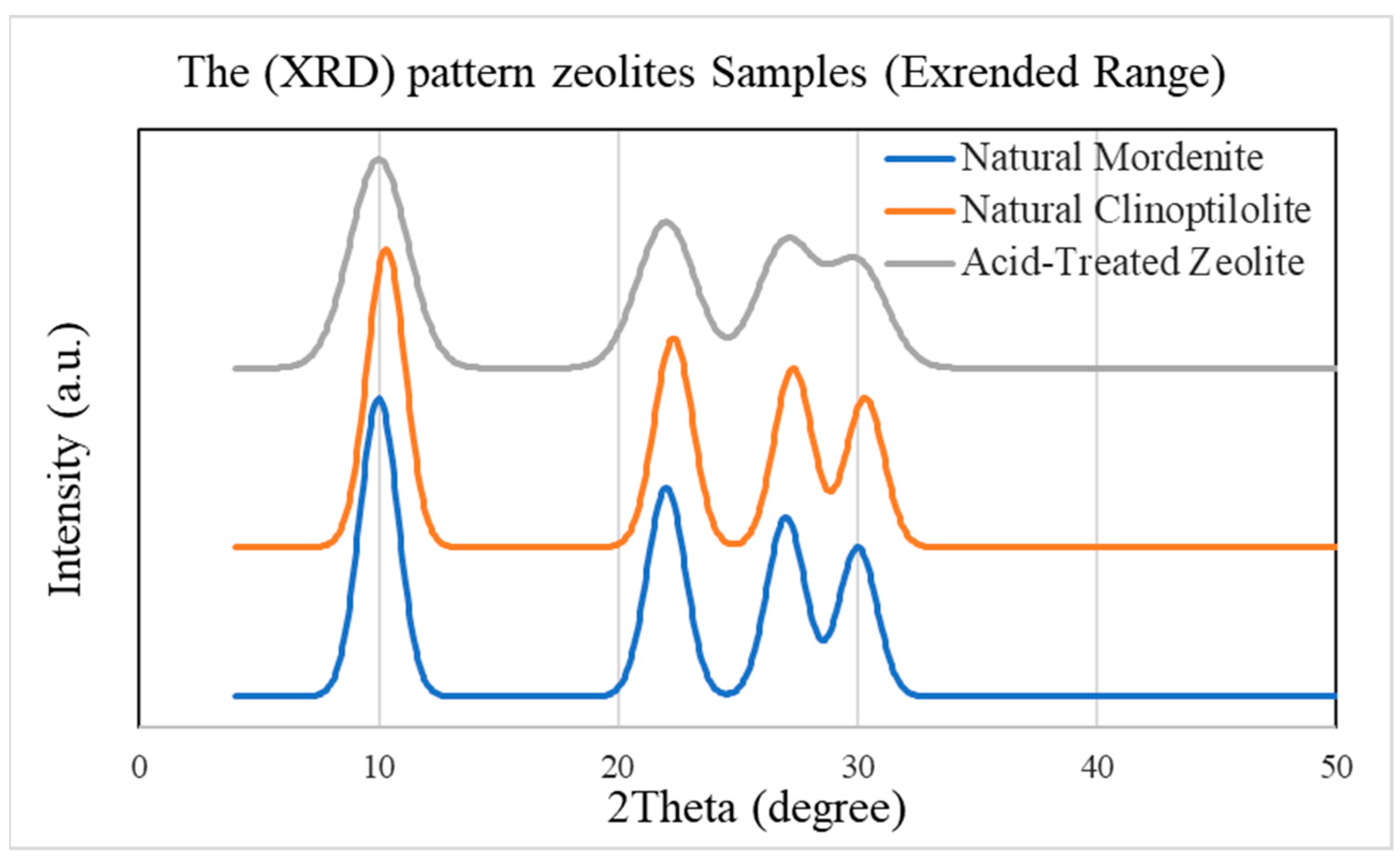
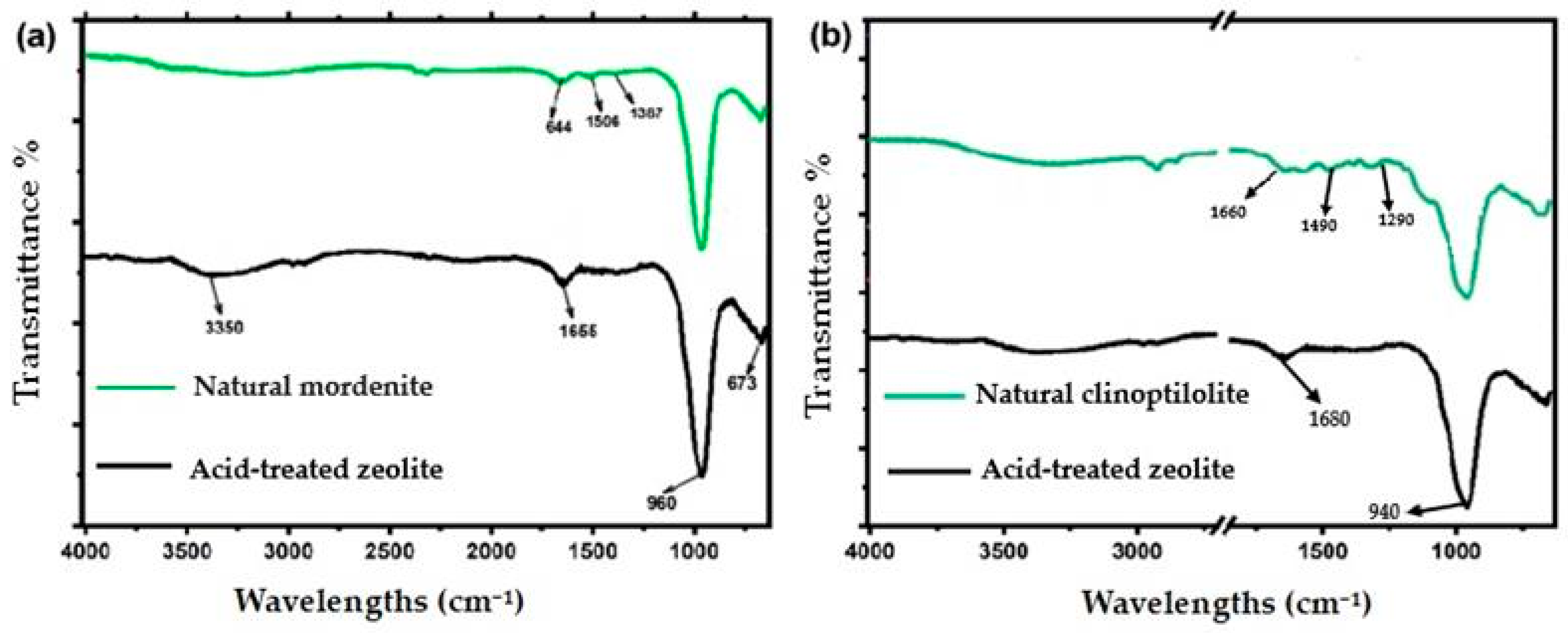
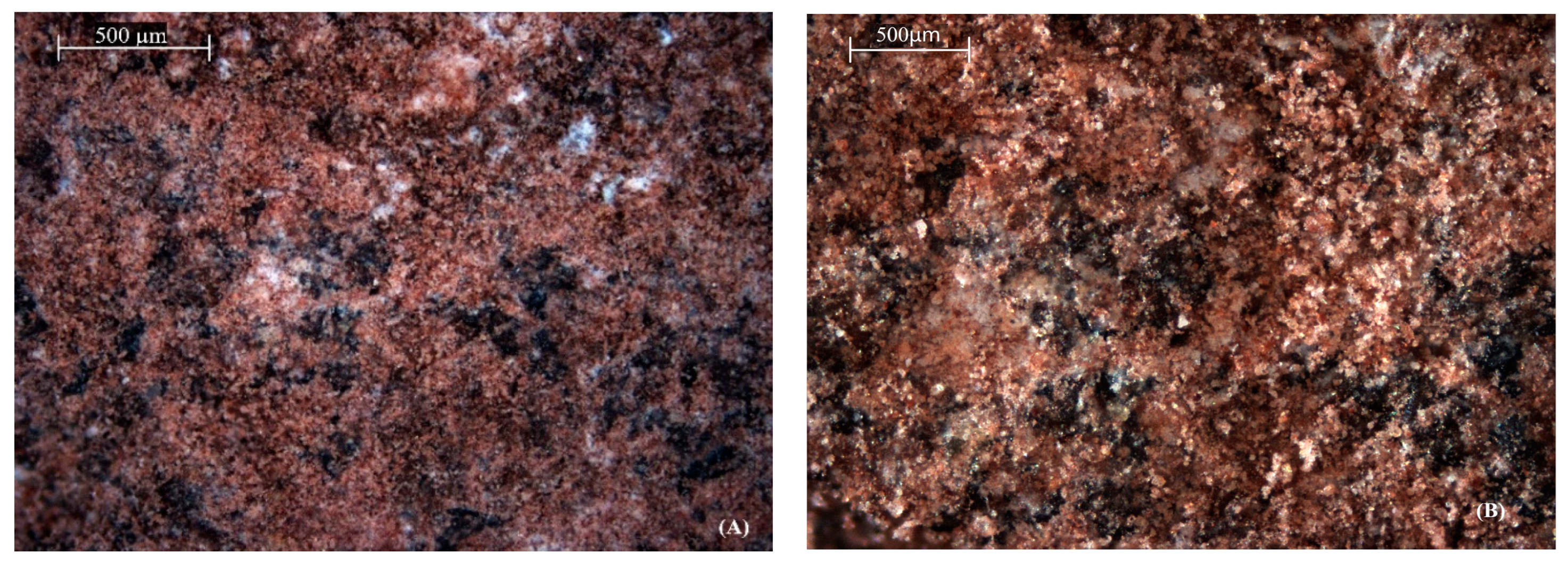

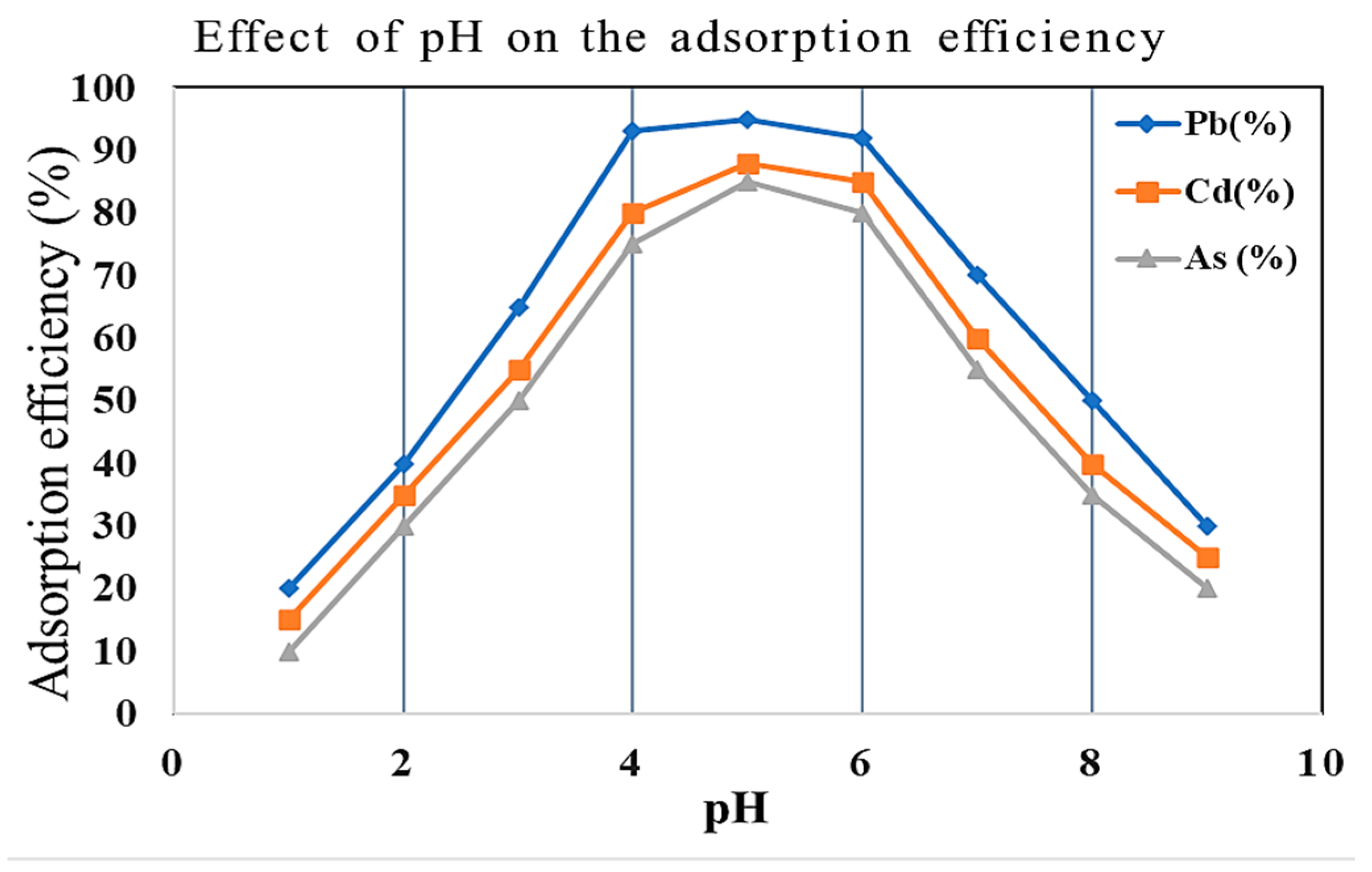
| Metal Ion | Zeolite Type | Removal Before (%) | Removal After (%) | Δ Efficiency (%) | Adsorption Nature | Observed Trend |
|---|---|---|---|---|---|---|
| Pb2+ | Clinoptilolite | 64% | 94% | +30% | Highly Favorable | Most efficiently adsorbed in both zeolites |
| Cd2+ | Clinoptilolite | 55.5% | 86% | +30.5% | Spontaneous | Moderate performance, improved after modification |
| As3+ | Clinoptilolite | 51% | 84% | +33% | Spontaneous | Least adsorbed before treatment, significantly improved |
| Pb2+ | Mordenite | 72% | 95% | +23% | Highly Favorable | Highest overall efficiency |
| Cd2+ | Mordenite | 57% | 90% | +33% | Spontaneous | Strong performance after modification |
| As3+ | Mordenite | 52.5% | 87% | +34.5% | Spontaneous | Most improved among all ions |
| Metal Ion | ΔG (kJ/mol) | ΔH (kJ/mol) | ΔS (J/mol·K) |
|---|---|---|---|
| Pb2+ | −5.23 | 18.4 | 78.5 |
| Cd2+ | −4.89 | 15.6 | 62.7 |
| As3+ | −3.12 | 12.1 | 55.2 |
| Metal Ion | Mean Adsorption Capacity (mg/g) | Standard Deviation | Relative Error (%) |
|---|---|---|---|
| Pb2+ | 94 | 2.5 | 2.66 |
| Cd2+ | 86 | 2.0 | 2.33 |
| As3+ | 84 | 1.8 | 2.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zahid, M.; Doszhanov, Y.; Saurykova, K.; Ahmadi, N.; Bolatova, D.; Kurmanbayeva, M.; Aydarbek, A.; Ihsas, R.; Seitzhanova, M.; Akhmetzhanova, D.; et al. Modification and Application of Natural Clinoptilolite and Mordenite from Almaty Region for Drinking Water Purification. Molecules 2025, 30, 2021. https://doi.org/10.3390/molecules30092021
Zahid M, Doszhanov Y, Saurykova K, Ahmadi N, Bolatova D, Kurmanbayeva M, Aydarbek A, Ihsas R, Seitzhanova M, Akhmetzhanova D, et al. Modification and Application of Natural Clinoptilolite and Mordenite from Almaty Region for Drinking Water Purification. Molecules. 2025; 30(9):2021. https://doi.org/10.3390/molecules30092021
Chicago/Turabian StyleZahid, Mudasir, Yerlan Doszhanov, Karina Saurykova, Noorahmad Ahmadi, Didar Bolatova, Meruyert Kurmanbayeva, Akbope Aydarbek, Rahmuddin Ihsas, Makpal Seitzhanova, Dana Akhmetzhanova, and et al. 2025. "Modification and Application of Natural Clinoptilolite and Mordenite from Almaty Region for Drinking Water Purification" Molecules 30, no. 9: 2021. https://doi.org/10.3390/molecules30092021
APA StyleZahid, M., Doszhanov, Y., Saurykova, K., Ahmadi, N., Bolatova, D., Kurmanbayeva, M., Aydarbek, A., Ihsas, R., Seitzhanova, M., Akhmetzhanova, D., Kerimkulova, A., & Doszhanov, O. (2025). Modification and Application of Natural Clinoptilolite and Mordenite from Almaty Region for Drinking Water Purification. Molecules, 30(9), 2021. https://doi.org/10.3390/molecules30092021







