Targeted Dereplication of H. patulum and H. hookeranium Extracts: Establishing MS/MS Fingerprints for the Identification of Polycyclic Polyprenylated Acylphloroglucinols
Abstract
1. Introduction
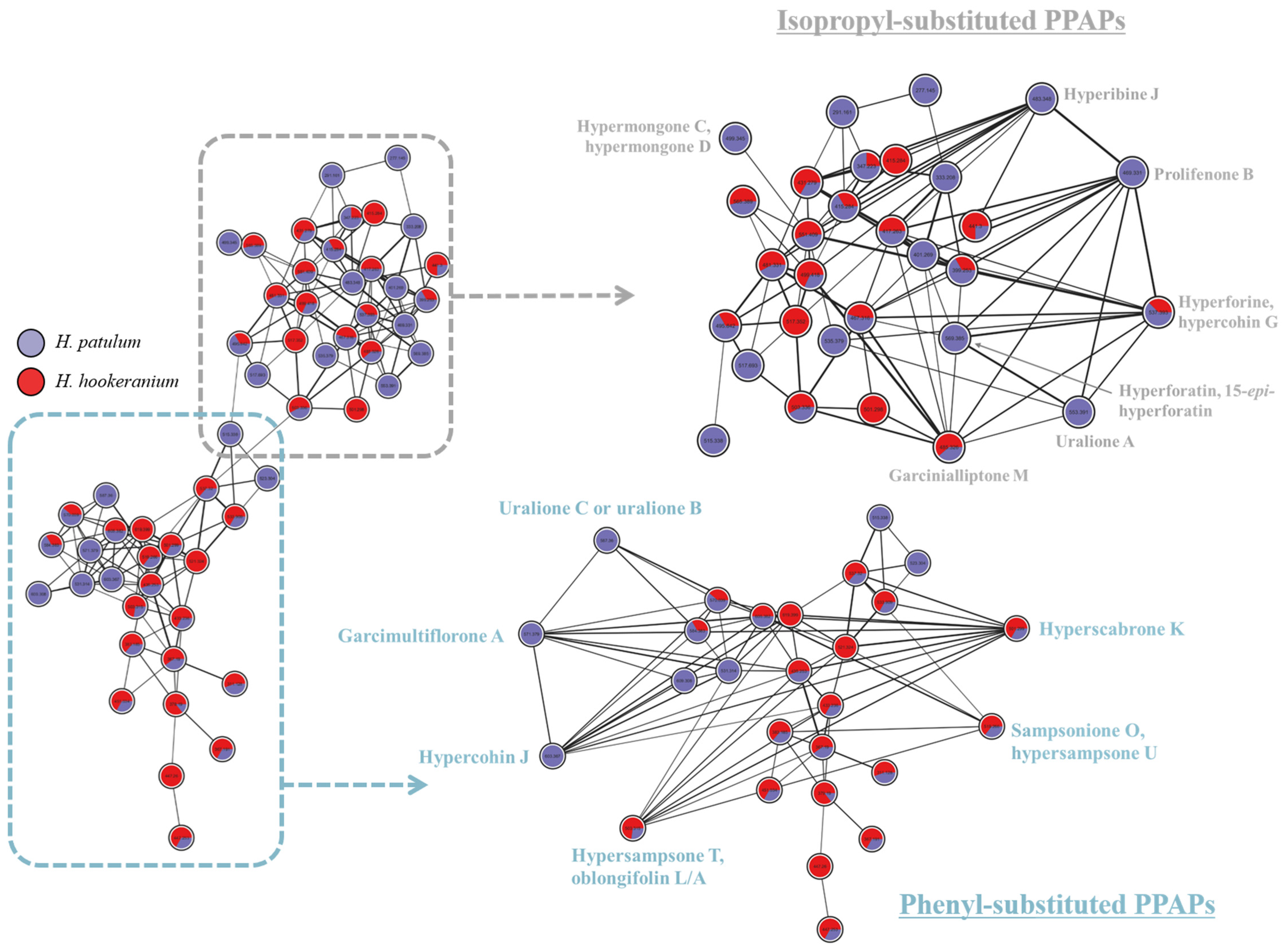
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals and General Procedures
3.2. Plant Material
3.3. Plant Extraction
3.4. UHPLC-ESI-Q-TOF-MS/MS
3.5. Molecular Network and Peak Annotation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robson, N.K.B. And Then Came Molecular Phylogenetics—Reactions to a Monographic Study of Hypericum (Hypericaceae). Phytotaxa 2016, 255, 181–198. [Google Scholar] [CrossRef]
- Zhang, R.; Ji, Y.; Zhang, X.; Kennelly, E.J.; Long, C. Ethnopharmacology of Hypericum Species in China: A Comprehensive Review on Ethnobotany, Phytochemistry and Pharmacology. J. Ethnopharmacol. 2020, 254, 112686. [Google Scholar] [CrossRef] [PubMed]
- Bridi, H.; Pustay, A.P.; Bordignon, S.A.d.L.; Picoli, S.U.; von Poser, G.L.; Ferraz, A.d.B.F. Antimicrobial Activity of Dimeric Acylphloroglucinols Isolated from Southern Brazilian Hypericum Species against to Resistant Bacterial. Nat. Prod. Res. 2022, 36, 6448–6452. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Ren, F.; Yan, X.; Wang, X.; Wu, T.; Li, N. Cytotoxic and Anti-Inflammatory Constituents from Roots of Hypericum beanii and the Antitumor Potential under the View of Cancer-Related Inflammation. Fitoterapia 2024, 172, 105745. [Google Scholar] [CrossRef]
- Ramalhete, N.; Machado, A.M.; Serrano, R.; Gomes, E.; Mota-Filipe, H.; Silva, O. Comparative Study on the in Vivo Antidepressant Activities of the Portuguese Hypericum foliosum, Hypericum androsaemum and Hypericum perforatum Medicinal Plants. Ind. Crops Prod. 2016, 82, 29–36. [Google Scholar] [CrossRef]
- Rezaie, A.; Dorostkar, K.R.; Pashazadeh, M.; Nejad, S.M. Study of Sedative and Anxiolytic Effects of Herbal Extract Hypericum perforatum in Comparison with Diazepam in Rats. Int. J. Infect. Dis. 2008, 12, e171. [Google Scholar] [CrossRef]
- Bridi, H.; Meirelles, G.d.C.; von Poser, G.L. Structural Diversity and Biological Activities of Phloroglucinol Derivatives from Hypericum Species. Phytochemistry 2018, 155, 203–232. [Google Scholar] [CrossRef]
- Cirak, C.; Seyis, F. Phenolic Constituents of Six Hypericum Species from Türkiye and Their Chemotaxonomic Relevance. S. Afr. J. Bot. 2023, 159, 596–604. [Google Scholar] [CrossRef]
- Manning, K.; Petrunak, E.; Lebo, M.; González-Sarrías, A.; Seeram, N.P.; Henry, G.E. Acylphloroglucinol and Xanthones from Hypericum ellipticum. Phytochemistry 2011, 72, 662–667. [Google Scholar] [CrossRef]
- Seyis, F.; Yurteri, E.; Özcan, A.; Cirak, C.; Yayla, F. Volatile Secondary Metabolites of Hypericum tetrapterum and Hypericum bithynicum. Biochem. Syst. Ecol. 2022, 105, 104542. [Google Scholar] [CrossRef]
- Yang, X.-W.; Grossman, R.B.; Xu, G. Research Progress of Polycyclic Polyprenylated Acylphloroglucinols. Chem. Rev. 2018, 118, 3508–3558. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and Community Curation of Mass Spectrometry Data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Morehouse, N.J.; Clark, T.N.; McMann, E.J.; van Santen, J.A.; Haeckl, F.P.J.; Gray, C.A.; Linington, R.G. Annotation of Natural Product Compound Families Using Molecular Networking Topology and Structural Similarity Fingerprinting. Nat. Commun. 2023, 14, 308. [Google Scholar] [CrossRef]
- Nothias, L.-F.; Nothias-Esposito, M.; Da Silva, R.; Wang, M.; Protsyuk, I.; Zhang, Z.; Sarvepalli, A.; Leyssen, P.; Touboul, D.; Costa, J.; et al. Bioactivity-Based Molecular Networking for the Discovery of Drug Leads in Natural Product Bioassay-Guided Fractionation. J. Nat. Prod. 2018, 81, 758–767. [Google Scholar] [CrossRef]
- Altay, A.; Yeniceri, E.; Taslimi, P.; Taskin-Tok, T.; Yilmaz, M.A.; Koksal, E. LC-MS/MS Analysis and Diverse Biological Activities of Hypericum scabrum L.: In Vitro and In Silico Research. S. Afr. J. Bot. 2022, 150, 940–955. [Google Scholar] [CrossRef]
- Peron, G.; Hošek, J.; Rajbhandary, S.; Pant, D.R.; Dall’Acqua, S. LC-MSn and HR-MS Characterization of Secondary Metabolites from Hypericum japonicum Thunb. Ex Murray from Nepalese Himalayan Region and Assessment of Cytotoxic Effect and Inhibition of NF-κB and AP-1 Transcription Factors in Vitro. J. Pharm. Biomed. Anal. 2019, 174, 663–673. [Google Scholar] [CrossRef]
- Alahmad, A.; Alghoraibi, I.; Zein, R.; Kraft, S.; Dräger, G.; Walter, J.-G.; Scheper, T. Identification of Major Constituents of Hypericum perforatum L. Extracts in Syria by Development of a Rapid, Simple, and Reproducible HPLC-ESI-Q-TOF MS Analysis and Their Antioxidant Activities. ACS Omega 2022, 7, 13475–13493. [Google Scholar] [CrossRef]
- Tatsis, E.C.; Boeren, S.; Exarchou, V.; Troganis, A.N.; Vervoort, J.; Gerothanassis, I.P. Identification of the Major Constituents of Hypericum perforatum by LC/SPE/NMR and/or LC/MS. Phytochemistry 2007, 68, 383–393. [Google Scholar] [CrossRef]
- Tocci, N.; Perenzoni, D.; Iamonico, D.; Fava, F.; Weil, T.; Mattivi, F. Extracts from Hypericum hircinum Subsp. Majus Exert Antifungal Activity Against a Panel of Sensitive and Drug-Resistant Clinical Strains. Front. Pharmacol. 2018, 9, 382. [Google Scholar] [CrossRef]
- Abdalla, M.A.; Famuyide, I.; Wooding, M.; McGaw, L.J.; Mühling, K.H. Secondary Metabolite Profile and Pharmacological Opportunities of Lettuce Plants Following Selenium and Sulfur Enhancement. Pharmaceutics 2022, 14, 2267. [Google Scholar] [CrossRef]
- Cottet, K.; Xu, B.; Coric, P.; Bouaziz, S.; Michel, S.; Vidal, M.; Lallemand, M.-C.; Broussy, S. Guttiferone A Aggregates Modulate Silent Information Regulator 1 (SIRT1) Activity. J. Med. Chem. 2016, 59, 9560–9566. [Google Scholar] [CrossRef] [PubMed]
- Henry, G.E.; Raithore, S.; Zhang, Y.; Jayaprakasam, B.; Nair, M.G.; Heber, D.; Seeram, N.P. Acylphloroglucinol Derivatives from Hypericum prolificum. J. Nat. Prod. 2006, 69, 1645–1648. [Google Scholar] [CrossRef] [PubMed]
- Mitsopoulou, K.P.; Vidali, V.P.; Maranti, A.; Couladouros, E.A. Isolation and Structure Elucidation of Hyperibine J [Revised Structure of Adhyperfirin (7-Deprenyl-13-Methylhyperforin)]: Synthesis of Hyperibone J. Eur. J. Org. Chem. 2015, 2015, 287–290. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Luo, J.; Zhou, Z.; Xue, G.; Kong, L. New Phloroglucinol Derivatives from the Whole Plant of Hypericum uralum. Fitoterapia 2017, 123, 59–64. [Google Scholar] [CrossRef]
- Li, H.; Meng, X.; Zhang, L.; Zhang, B.; Liu, X.; Fu, W.; Tan, H.; Lao, Y.; Xu, H. Oblongifolin C and Guttiferone K Extracted from Garcinia yunnanensis Fruit Synergistically Induce Apoptosis in Human Colorectal Cancer Cells in Vitro. Acta Pharmacol. Sin. 2017, 38, 252–263. [Google Scholar] [CrossRef]
- Wang, M.; Dong, Q.; Wang, H.; He, Y.; Chen, Y.; Zhang, H.; Wu, R.; Chen, X.; Zhou, B.; He, J.; et al. Oblongifolin M, an Active Compound Isolated from a Chinese Medical Herb Garcinia Oblongifolia, Potently Inhibits Enterovirus 71 Reproduction through Downregulation of ERp57. Oncotarget 2016, 7, 8797–8808. [Google Scholar] [CrossRef][Green Version]
- Zanoli, P. Role of Hyperforin in the Pharmacological Activities of St. John’s Wort. CNS Drug Rev. 2004, 10, 203–218. [Google Scholar] [CrossRef]
- Koeberle, A.; Rossi, A.; Bauer, J.; Dehm, F.; Verotta, L.; Northoff, H.; Sautebin, L.; Werz, O. Hyperforin, an Anti-Inflammatory Constituent from St. John’s Wort, Inhibits Microsomal Prostaglandin E2 Synthase-1 and Suppresses Prostaglandin E2 Formation in Vivo. Front. Pharmacol. 2011, 2, 7. [Google Scholar] [CrossRef]
- Schempp, C.M.; Pelz, K.; Wittmer, A.; Schöpf, E.; Simon, J.C. Antibacterial Activity of Hyperforin from St John’s Wort, against Multiresistant Staphylococcus aureus and Gram-Positive Bacteria. Lancet 1999, 353, 2129. [Google Scholar] [CrossRef]
- Ji, Y.; Zhang, R.; Bensalel, J.; Morcol, T.; Gu, R.; Gallego-Delgado, J.; Kennelly, E.J.; Long, C. Metabolomic and Chemometric Analyses of St. John’s Wort and Related Asian Hypericum Species Linked to Bioactivity. J. Ethnopharmacol. 2024, 329, 118163. [Google Scholar] [CrossRef]
- Chen, Q.; Di, L.; Zhang, Y.; Li, N. Chemical Constituents with Cytotoxic and Anti-Inflammatory Activity in Hypericum sampsonii and the Antitumor Potential under the View of Cancer-Related Inflammation. J. Ethnopharmacol. 2020, 259, 112948. [Google Scholar] [CrossRef] [PubMed]
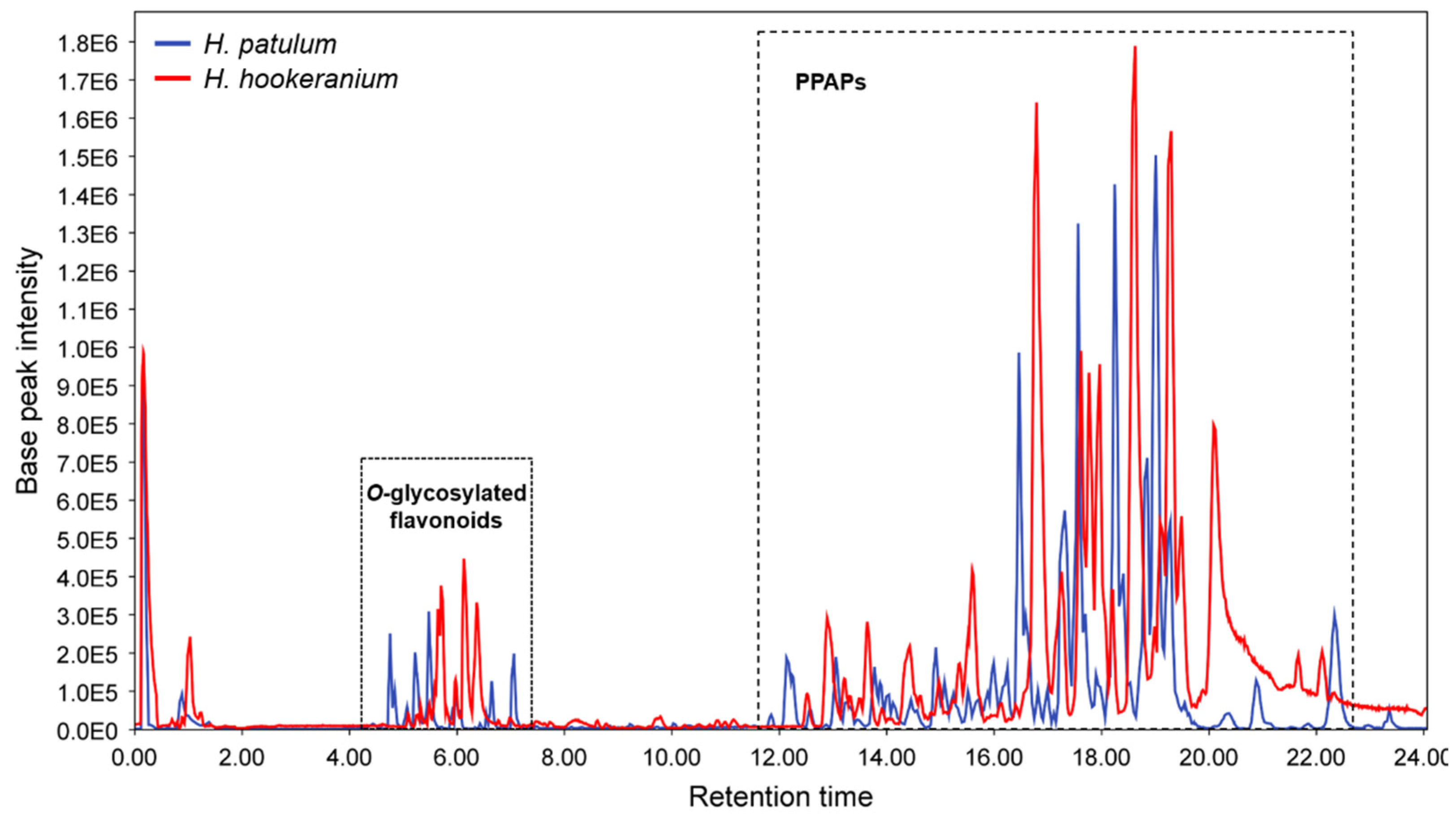

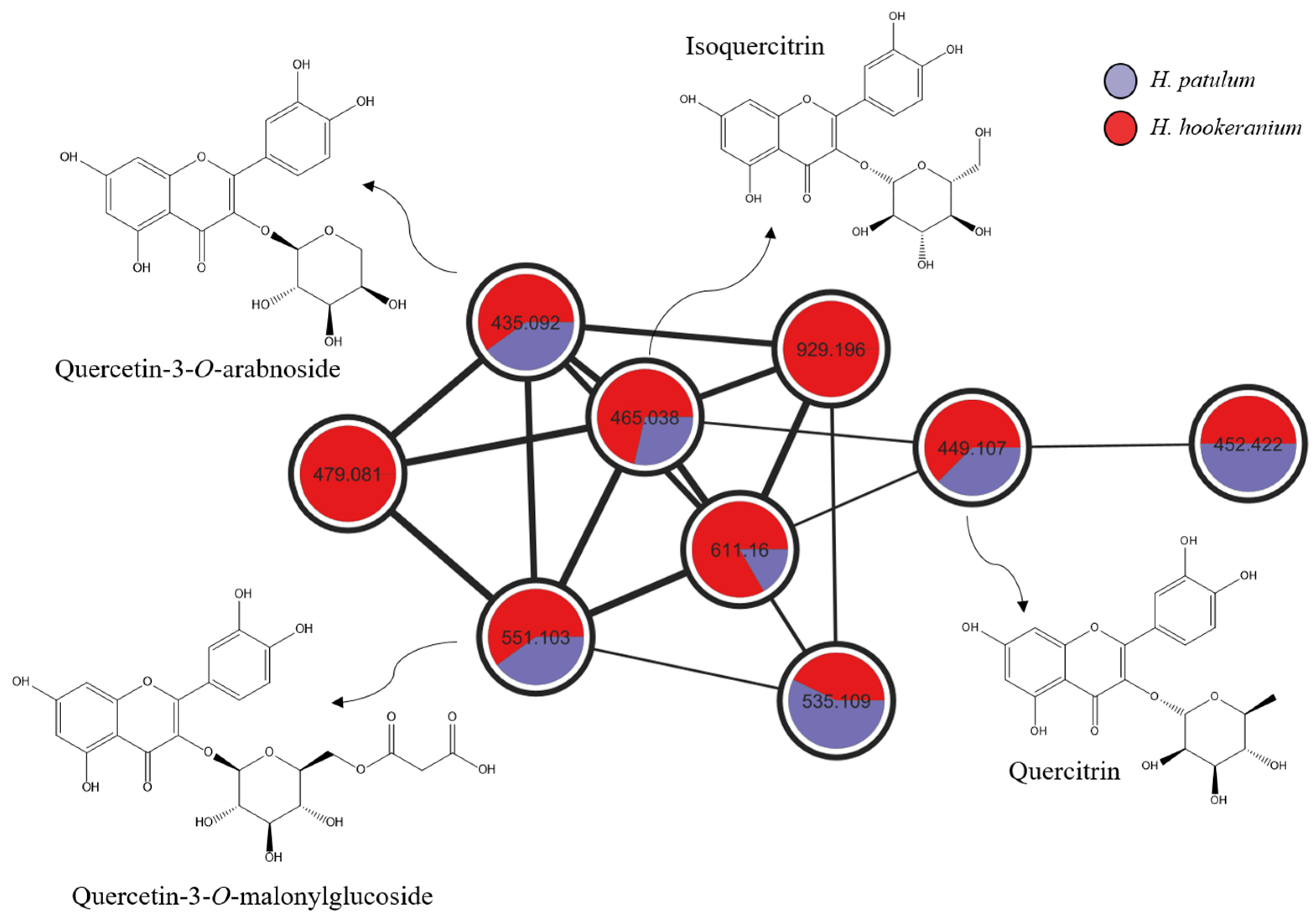
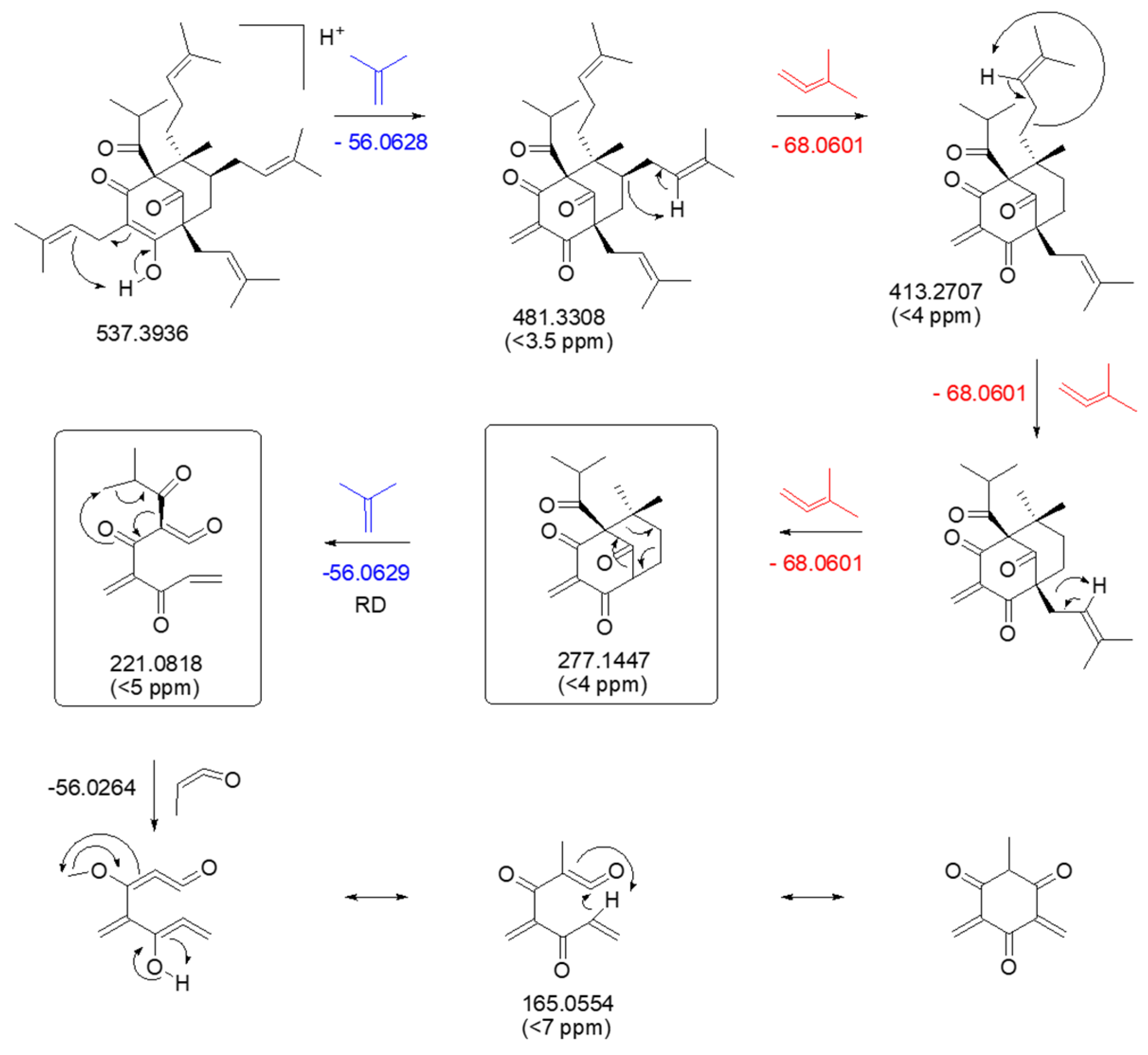

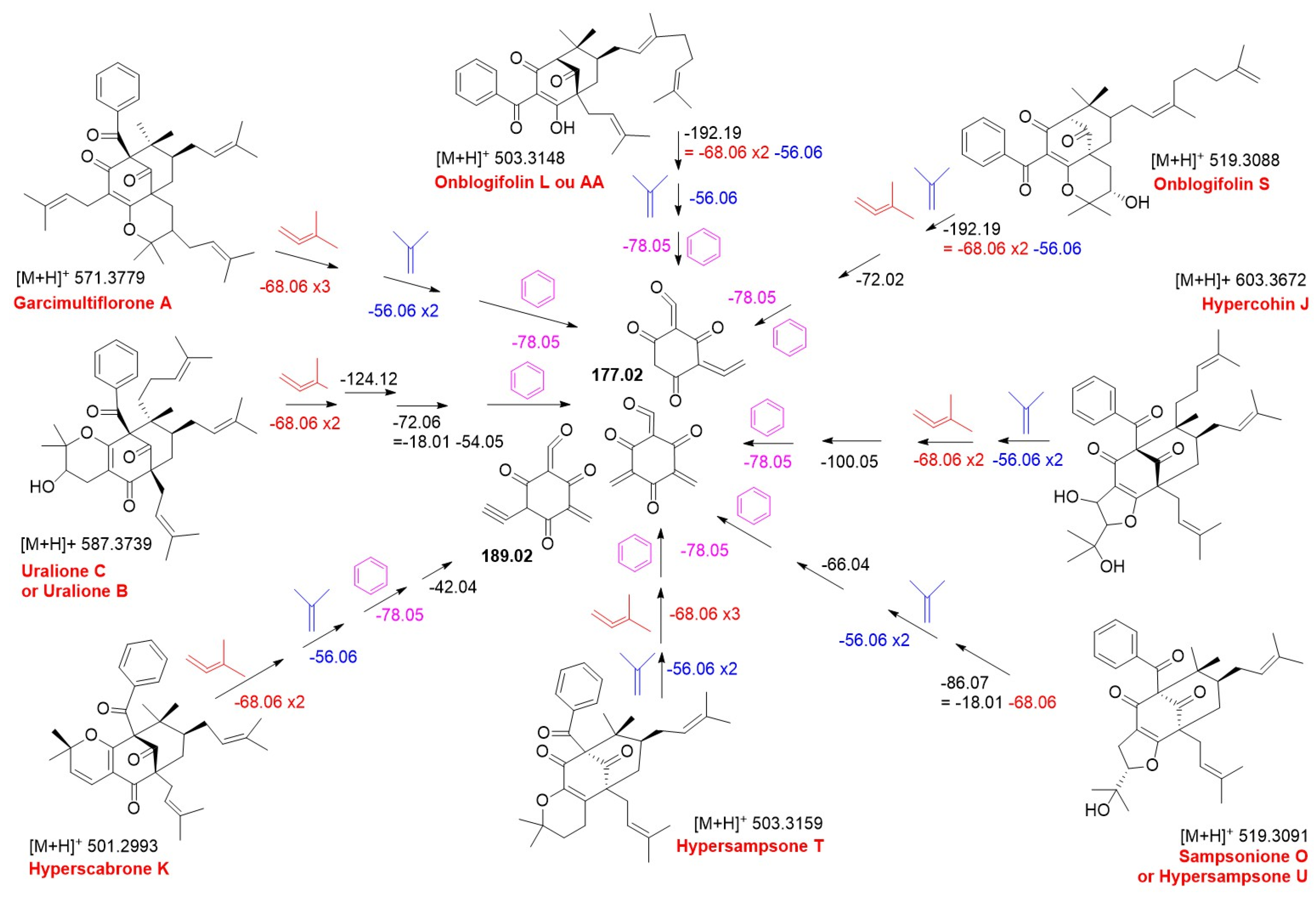
| RT (min) | Exact Mass | [M + H]+the | [M + H]+exp | Molecular Formula | Δ (ppm) | Compound Assignment | Cosine Score | Species | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 4.83/5.29 | 354.0951 | 355.1029 | 355.1048 | C16H18O9 | 5.35 | Chlorogenic acid | 0.90 | H. patulum, H. hookeranium | [17,18] |
| 4.81 | 578.1424 | 579.1502 | 579.1523 | C30H26O12 | 3.63 | Procyanidin B2 | 0.89 | H. patulum | [19] |
| 5.22/6.17 | 464.0955 | 465.1033 | 465.1051 | C21H20O12 | 3.87 | Isoquercitrin | 0.93 | H. patulum, H. hookeranium | [17,18] |
| 5.57/6.39 | 448.1006 | 449.1084 | 449.1090 | C21H20O11 | 1.33 | Quercitrin | 0.92 | H. patulum, H. hookeranium | [17,18] |
| 5.47/6.36 | 550.0959 | 551.1037 | 551.1057 | C24H22O15 | 3.63 | Quercetin-3-O-malonylglucoside | 0.92 | H. patulum, H. hookeranium | [20] |
| 5.44/6.31 | 434.0849 | 435.0927 | 435.0934 | C20H18O11 | 1.61 | Quercetin-3-O-arabnoside | 0.93 | H. patulum, H. hookeranium | [19] |
| 5.45 | 578.1424 | 579.1502 | 579.1477 | C30H26O12 | 4.32 | Procyanidin B1 | 0.78 | H. hookeranium | [19] |
| 6.34/6.62 | 302.0426 | 303.0505 | 303.0522 | C15H10O7 | 5.61 | Quercetin | 0.79 | H. patulum, H. hookeranium | [17,18] |
| RT (min) | Exact Mass | [M + H]+the | [M + H]+exp | Molecular Formula | Δ (ppm) | MS/MS Fragments | Putative Identification |
|---|---|---|---|---|---|---|---|
| 13.05 | 500.2927 | 501.3005 | 501.2994 | C33H40O4 | 2.19 | 433.2378, 365.1768, 309.1133, 231.0666, 189.0220 | Hyperscabrone K |
| 15.84 | 552.3815 | 553.3893 | 553.3916 | C35H52O5 | 4.15 | 485.3294, 427.2862, 361.2033, 293.1403, 235.0984 | Uralione A |
| 15.99 | 602.3607 | 603.3686 | 603.3672 | C38H50O6 | 2.28 | 479.2402, 445.2375, 379.1901, 311.1286, 255.0661, 177.0195 | Hypercohin J |
| 16.20 | 586.3658 | 587.3737 | 587.3739 | C38H50O5 | 0.43 | 519.3101, 395.1855, 327.1231, 309.1129, 255.0655, 177.0185 | Uralione C, Uralione B |
| 16.42 | 566.3971 | 567.4050 | 567.4046 | C36H54O5 | 0.70 | 499.3445, 443.2833, 375.2188, 307.1549, 217.0867 | Hyphenrone E |
| 16.46 | 518.3032 | 519.3111 | 519.3088 | C33H42O5 | 4.26 | 433.2396, 377.1756, 311.1283, 255.0652, 177.0190 | Sampsonione O, Hypersampsone U |
| 17.27 | 502.3083 | 503.3161 | 503.3159 | C33H42O4 | 0.40 | 447.2530, 379.1919, 311.1290, 255.0659, 177.0193 | Hypersampsone T |
| 17.56 | 484.3189 | 485.3267 | 485.3260 | C30H44O5 | 2.09 | 467.3212, 399.2554, 343.1917, 277.1448, 221.0817, 163.0404 | Garcinialliptone M |
| 17.61 | 568.3764 | 569.3842 | 569.3846 | C35H52O6 | 0.65 | 551.3711, 451.3711, 277.1447, 203.1810, 163.0480 | Hyperforatin, 15-epi-hyperforatin |
| 18.24 | 498.3345 | 499.3424 | 499.3404 | C31H46O5 | 3.86 | 413.2709, 357.2064, 275.1654, 219.1019, 163.0401 | Hypermongone C, Hypermongone D |
| 18.28 | 536.3866 | 537.3944 | 537.3932 | C35H52O4 | 2.11 | 481.3308, 413.2707, 277.1447, 221.0818, 165.0554 | Hyperforin, Hypercohin G |
| 18.40 | 468.3240 | 469.3318 | 469.3316 | C30H44O4 | 0.43 | 413.2696, 345.2064, 277.1447, 221.0818, 165.0554 | Prolifenone B |
| 19.01 | 570.3709 | 571.3787 | 571.3779 | C38H50O4 | 1.40 | 503.3243, 447.2563, 379.1930, 311.1294, 255.0662, 177.0195 | Garcimultiflorone A |
| 19.28 | 482.3396 | 483.3474 | 483.3480 | C31H46O4 | 1.09 | 427.2894, 359.2233, 291.1617, 235.0978, 163.0407 | Hyperibine J |
| RT (min) | Exact Mass | [M + H]+the | [M + H]+exp | Molecular Formula | Δ (ppm) | MS/MS Fragments | Putative Identification |
|---|---|---|---|---|---|---|---|
| 13.49 | 518.3032 | 519.3111 | 519.3088 | C33H42O5 | 4.43 | 327.1225, 255.0647, 231.0637, 177.0182 | Oblongifolin S |
| 13.64 | 500.2927 | 501.3005 | 501.2976 | C33H40O4 | 5.78 | 433.2335, 309.1100, 231.0638, 189.0179 | Hyperscabrone K |
| 14.39 | 518.3032 | 519.3111 | 519.3066 | C33H42O5 | 8.67 | 377.1747, 311.1279, 255.0647, 177.0182 | Sampsonione O isomer, Hypersampsone U isomer |
| 16.89 | 518.3032 | 519.3111 | 519.3085 | C33H42O5 | 5.01 | 377.1747, 311.1279, 255.0647, 177.0182 | Sampsonione O isomer, Hypersampsone U isomer |
| 17.55 | 502.3083 | 503.3161 | 503.3155 | C33H42O4 | 1.19 | 311.1279, 255.0647, 177.0182 | Oblongifolin L isomer, Oblongifolin A isomer |
| 17.76 | 502.3083 | 503.3161 | 503.3155 | C33H42O4 | 1.19 | 311.1279, 255.0647, 177.0182 | Oblongifolin L isomer, Oblongifolin A isomer |
| 17.91 | 484.3189 | 485.3267 | 485.3256 | C30H44O5 | 2.27 | 343.1898, 277.1435, 221.0799, 163.0388 | Garcinialliptone M |
| 18.67 | 536.3866 | 537.3944 | 537.3921 | C35H52O4 | 4.28 | 481.3294, 413.2678, 277.1435, 221.0799, 165.0540 | Hyperforin, hypercohin G |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dugay, A.; Souquet, F.; Hozain, D.; Pakora, G.A.; Buisson, D.; Amand, S.; Lallemand, M.-C.; de Oliveira Junior, R.G. Targeted Dereplication of H. patulum and H. hookeranium Extracts: Establishing MS/MS Fingerprints for the Identification of Polycyclic Polyprenylated Acylphloroglucinols. Molecules 2025, 30, 2531. https://doi.org/10.3390/molecules30122531
Dugay A, Souquet F, Hozain D, Pakora GA, Buisson D, Amand S, Lallemand M-C, de Oliveira Junior RG. Targeted Dereplication of H. patulum and H. hookeranium Extracts: Establishing MS/MS Fingerprints for the Identification of Polycyclic Polyprenylated Acylphloroglucinols. Molecules. 2025; 30(12):2531. https://doi.org/10.3390/molecules30122531
Chicago/Turabian StyleDugay, Annabelle, Florence Souquet, David Hozain, Gilles Alex Pakora, Didier Buisson, Séverine Amand, Marie-Christine Lallemand, and Raimundo Gonçalves de Oliveira Junior. 2025. "Targeted Dereplication of H. patulum and H. hookeranium Extracts: Establishing MS/MS Fingerprints for the Identification of Polycyclic Polyprenylated Acylphloroglucinols" Molecules 30, no. 12: 2531. https://doi.org/10.3390/molecules30122531
APA StyleDugay, A., Souquet, F., Hozain, D., Pakora, G. A., Buisson, D., Amand, S., Lallemand, M.-C., & de Oliveira Junior, R. G. (2025). Targeted Dereplication of H. patulum and H. hookeranium Extracts: Establishing MS/MS Fingerprints for the Identification of Polycyclic Polyprenylated Acylphloroglucinols. Molecules, 30(12), 2531. https://doi.org/10.3390/molecules30122531









