Influence of Enzymatic Acylation on the Stability and Antioxidant Properties of Cyanidin-3-O-Glucoside in Both Aqueous and Lipid Systems
Abstract
1. Introduction
2. Results and Discussion
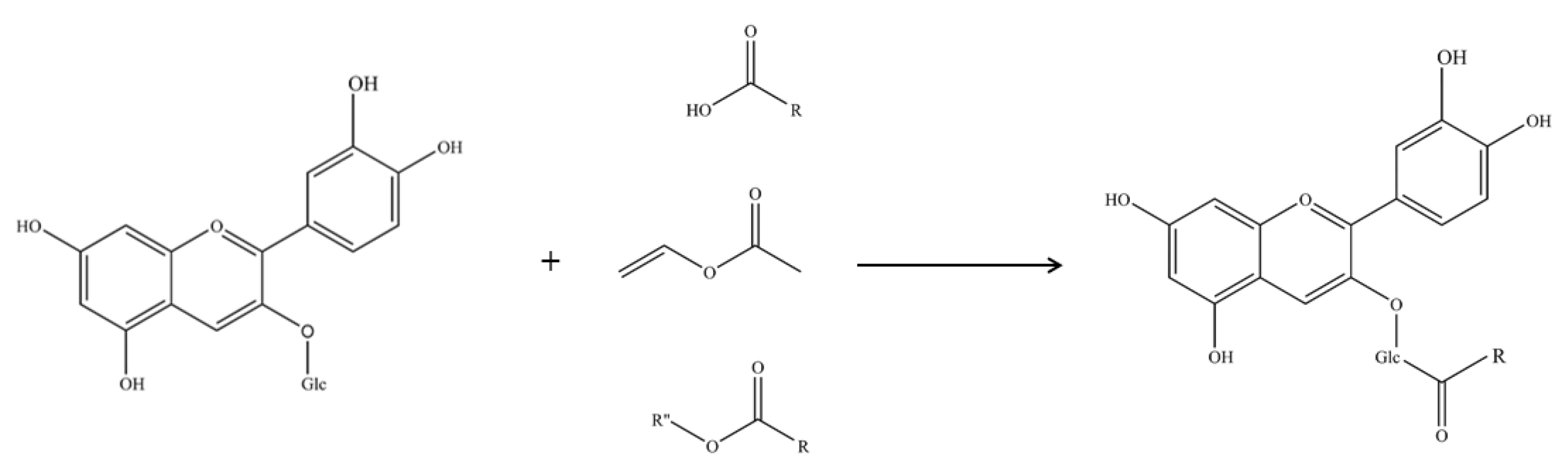
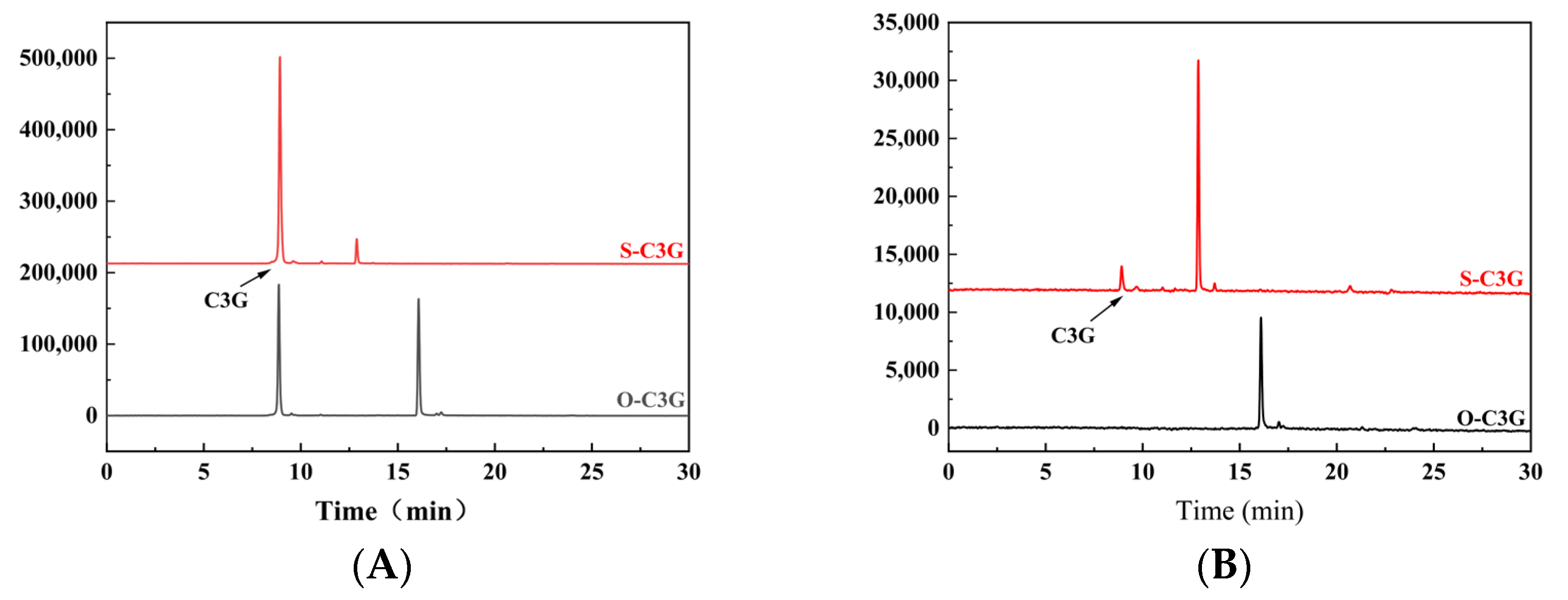
2.1. Enzymatic Acylation and Purification of Acylated Derivatives
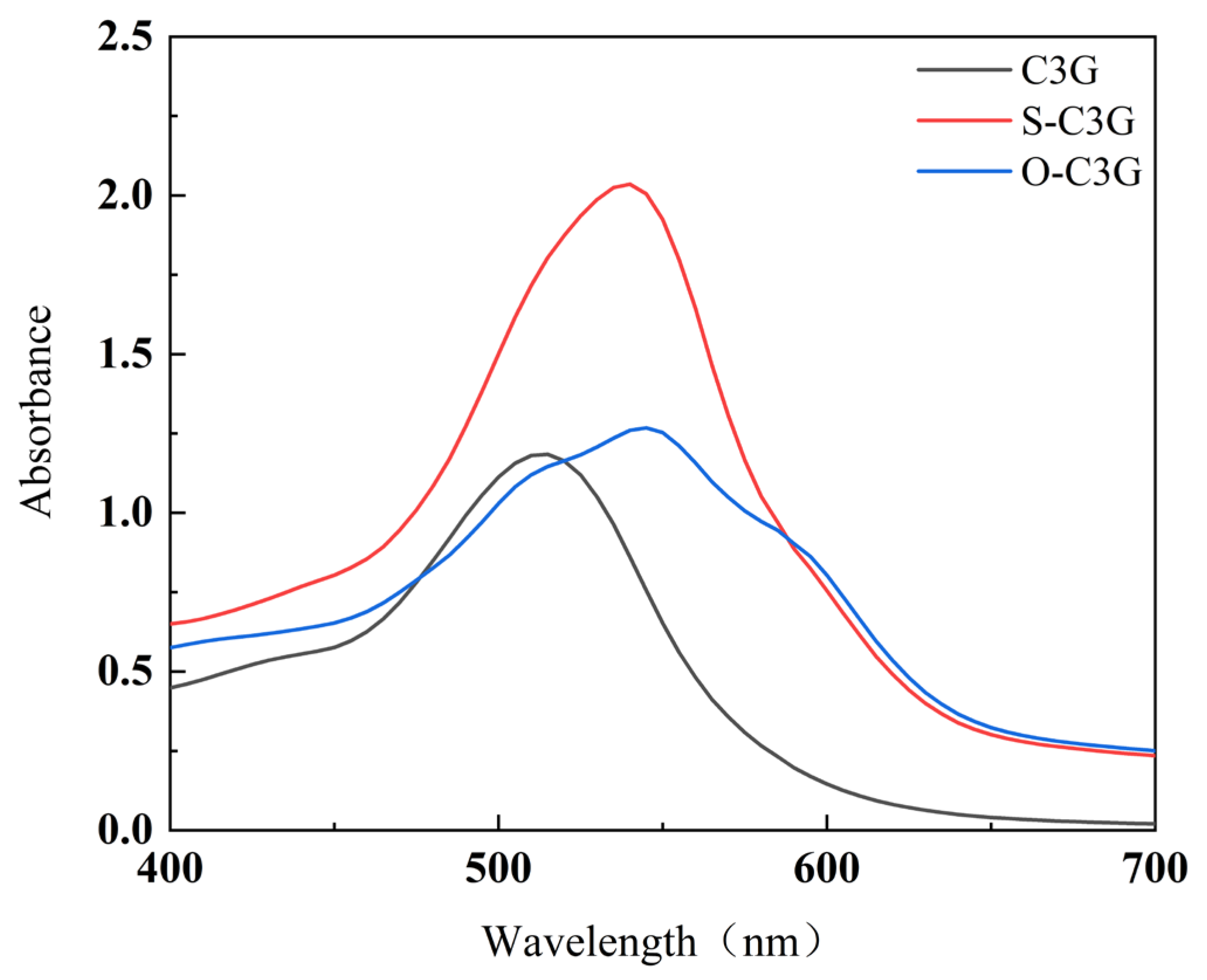
2.2. UV–Vis Analysis
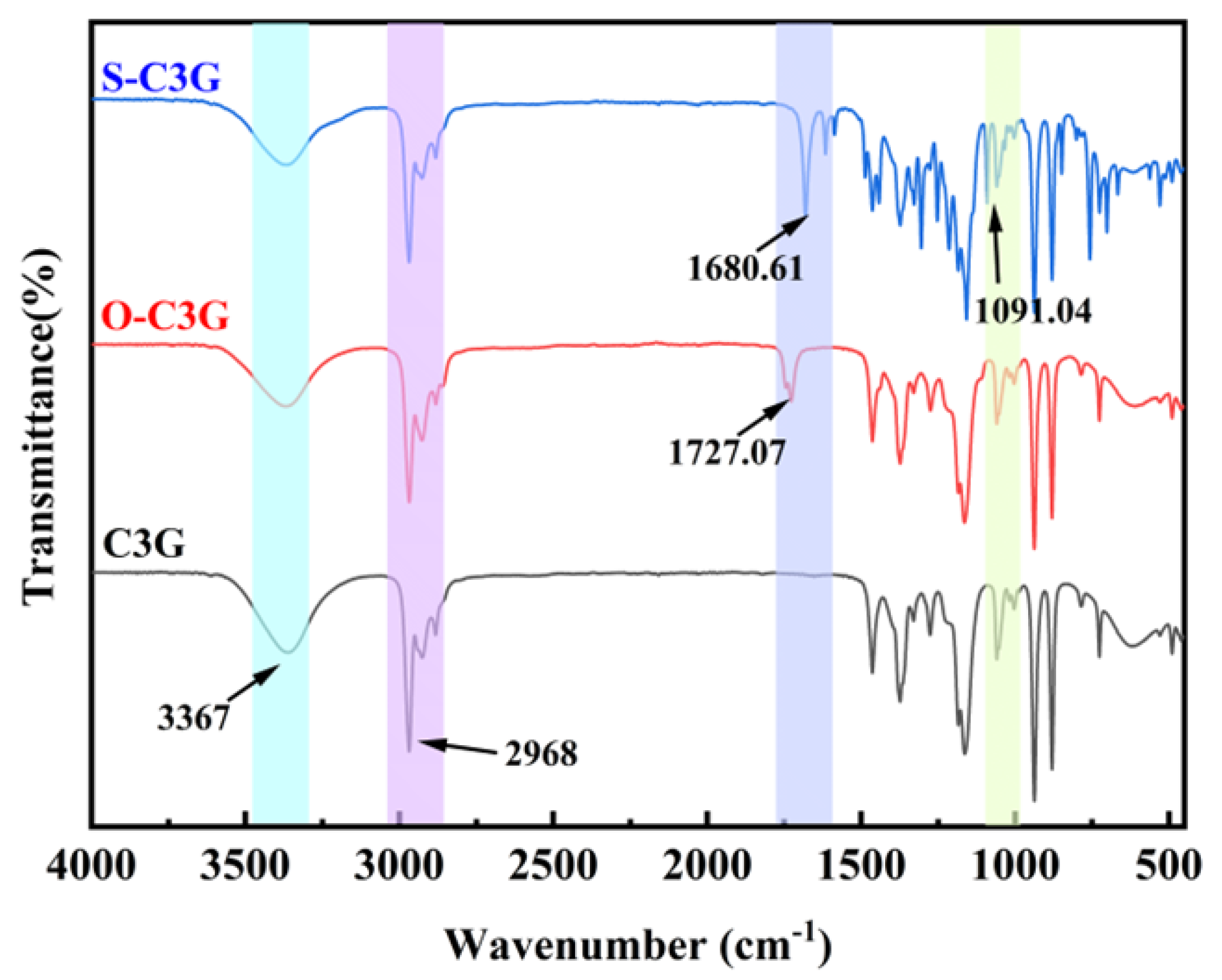
2.3. FTIR Analysis
2.4. Stability of C3G and Acylated C3Gs
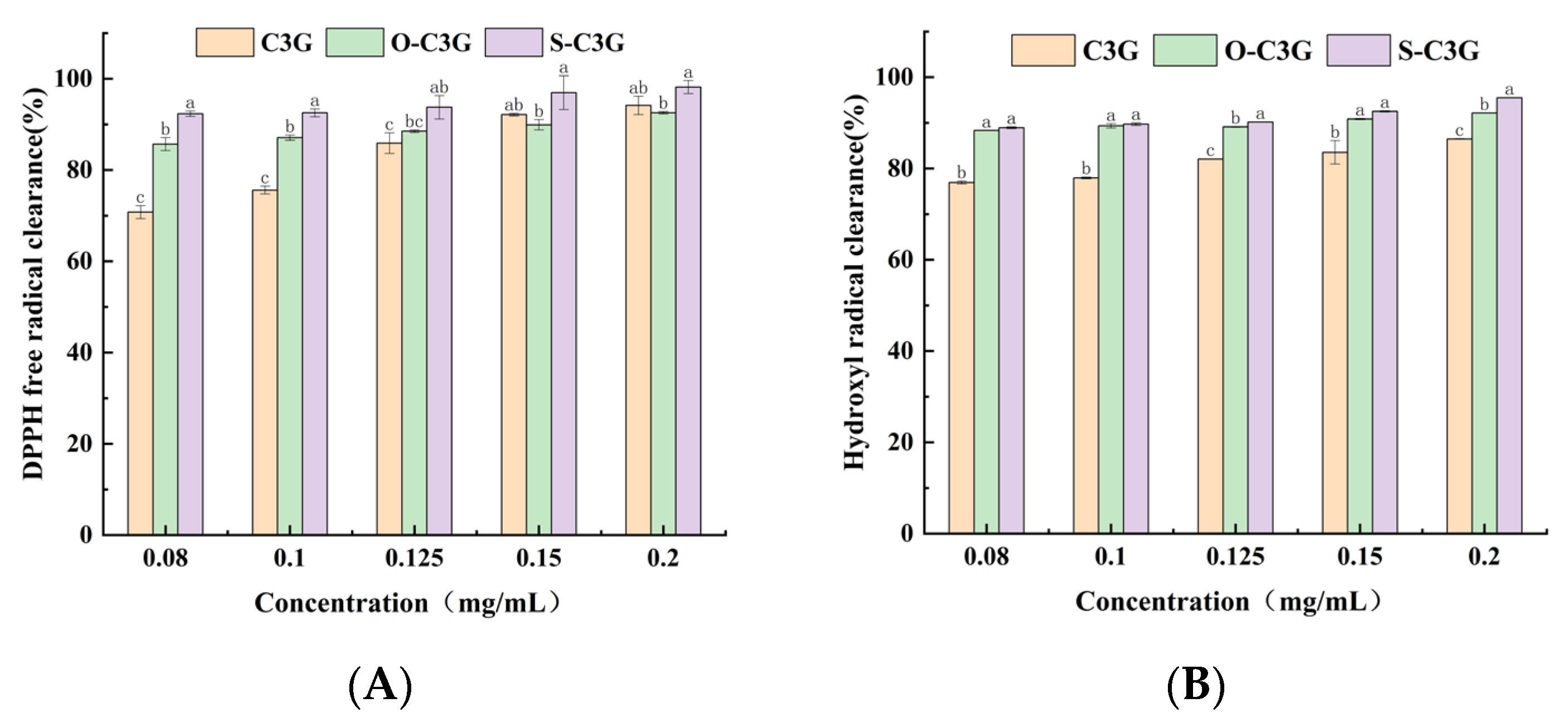
2.5. Antioxidant Properties in Aqueous Solution Systems
2.6. Antioxidant Properties in Lipid Systems
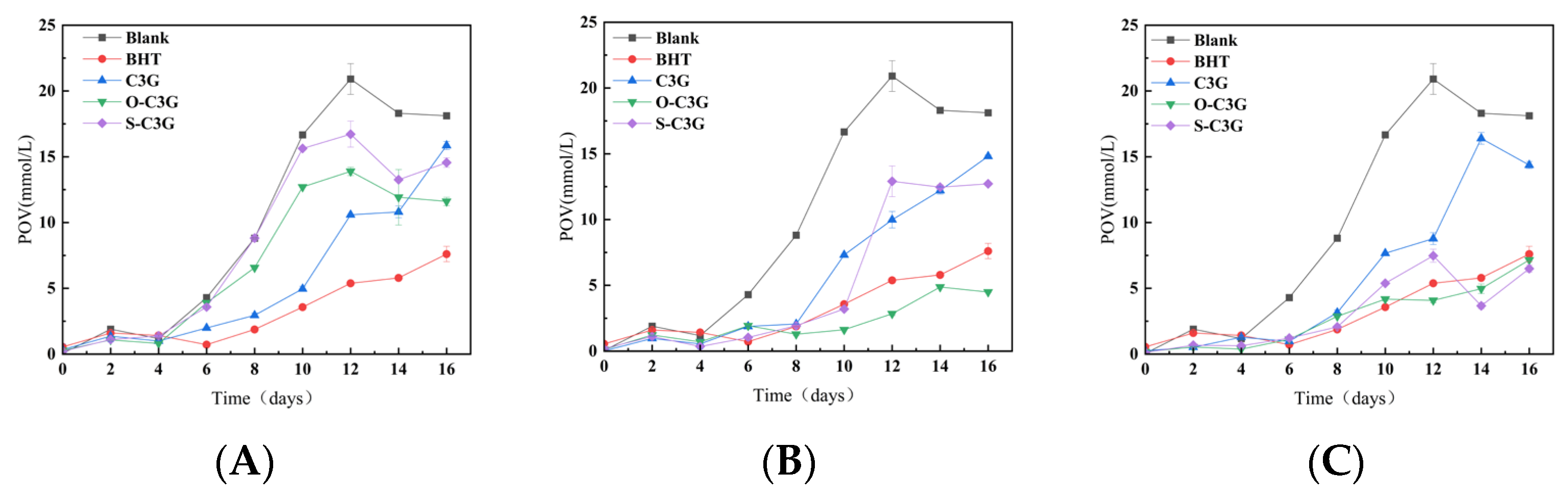
2.6.1. The Change of Peroxidation Value (POV)
2.6.2. The Change of Malondialdehyde Content (TBARS)
2.7. Molecular Structure Optimization and Orbital Analysis
2.8. Comparison of Antioxidant Properties in Different Systems
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Preparation of Enzymatic Acylated C3Gs
3.3. Structural Characterization of C3G and Acylated C3Gs
3.3.1. HPLC Analysis
3.3.2. UV–Vis Analysis
3.3.3. FTIR Analysis
3.4. The Stability of C3G and Acylated C3Gs
3.4.1. Determination of Thermostability
3.4.2. Determination of Photostability
3.5. Determination of Antioxidant Activity in Different Systems
3.5.1. Antioxidant Properties in Aqueous Solution Systems
3.5.2. Antioxidant Properties in Lipid Systems
3.6. Theoretical and Computational Studies
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| C3G | Cyanidin-3-O-glucoside |
| O-C3G | Cyanidin-3-O-glucoside n-octanoate acid acyl product |
| S-C3G | Cyanidin-3-O-glucoside salicyl acyl product |
| acylated C3Gs | Cyanidin-3-O-glucoside n-octanoate acid acyl product and cyanidin-3-O-glucoside salicyl acyl product |
References
- Kamiya, H.; Yanase, E.; Nakatsuka, S.-I. Novel Oxidation Products of Cyanidin 3-O-Glucoside with 2,2′-Azobis-(2,4-Dimethyl)Valeronitrile and Evaluation of Anthocyanin Content and Its Oxidation in Black Rice. Food Chem. 2014, 155, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yin, L.; Huang, L.; Tekliye, M.; Xia, X.; Li, J.; Dong, M. Composition, Antioxidant Activity, and Neuroprotective Effects of Anthocyanin-Rich Extract from Purple Highland Barley Bran and Its Promotion on Autophagy. Food Chem. 2021, 339, 127849. [Google Scholar] [CrossRef] [PubMed]
- Nankar, A.N.; Dungan, B.; Paz, N.; Sudasinghe, N.; Schaub, T.; Holguin, F.O.; Pratt, R.C. Quantitative and Qualitative Evaluation of Kernel Anthocyanins from Southwestern United States Blue Corn. J. Sci. Food Agric. 2016, 96, 4542–4552. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, W.; Soladoye, O.P. Towards Innovative Food Processing of Flavonoid Compounds: Insights into Stability and Bioactivity. LWT 2021, 150, 111968. [Google Scholar] [CrossRef]
- Grajeda-Iglesias, C.; Salas, E.; Barouh, N.; Baréa, B.; Figueroa-Espinoza, M.C. Lipophilization and MS Characterization of the Main Anthocyanins Purified from Hibiscus Flowers. Food Chem. 2017, 230, 189–194. [Google Scholar] [CrossRef]
- Sasaki, N.; Nishizaki, Y.; Ozeki, Y.; Miyahara, T. The Role of Acyl-Glucose in Anthocyanin Modifications. Molecules 2014, 19, 18747–18766. [Google Scholar] [CrossRef]
- Yang, W.; Kortesniemi, M.; Ma, X.; Zheng, J.; Yang, B. Enzymatic Acylation of Blackcurrant (Ribes Nigrum) Anthocyanins and Evaluation of Lipophilic Properties and Antioxidant Capacity of Derivatives. Food Chem. 2019, 281, 189–196. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, J.; Wang, Z.; Shen, R.; Cui, N.; Sun, A. Direct Acylation of Cyanidin-3-Glucoside with Lauric Acid in Blueberry and Its Stability Analysis. Int. J. Food Prop. 2015, 19, 1–12. [Google Scholar] [CrossRef]
- Luo, X.; Wang, R.; Wang, J.; Li, Y.; Luo, H.; Chen, S.; Zeng, X.; Han, Z. Acylation of Anthocyanins and Their Applications in the Food Industry: Mechanisms and Recent Research Advances. Foods 2022, 11, 2166. [Google Scholar] [CrossRef]
- Luo, X.-P.; Du, L.-H.; He, F.; Zhou, C.-H. Controllable Regioselective Acylation of Flavonoids Catalyzed by Lipase in Microreactors. J. Carbohydr. Chem. 2013, 32, 450–462. [Google Scholar] [CrossRef]
- Passicos, E.; Santarelli, X.; Coulon, D. Regioselective Acylation of Flavonoids Catalyzed by Immobilized Candida Antarctica Lipase under Reduced Pressure. Biotechnol. Lett. 2004, 26, 1073–1076. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Li, C.; Shao, P.; Jiang, L.; Chen, B.; Farag, M.A. Enzymatic Acylation of Cyanidin-3- O-Glucoside in Raspberry Anthocyanins for Intelligent Packaging: Improvement of Stability, Lipophilicity and Functional Properties. Curr. Res. Food Sci. 2022, 5, 2219–2227. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Liu, S.; Zhao, Z.; You, L.; Harrison, M.D.; Zhang, Z. Enzymatic Acylation of Cyanidin-3-Glucoside with Fatty Acid Methyl Esters Improves Stability and Antioxidant Activity. Food Chem. 2021, 343, 128482. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Jiang, X.; Huang, G.; Xin, X.; Attaribo, T.; Zhang, Y.; Zhang, N.; Gui, Z. Enhancement of Stability and Antioxidant Activity of Mulberry Anthocyanins Through Succinic Acid Acylation. Food Technol. Biotechnol. 2022, 60, 321–329. [Google Scholar] [CrossRef]
- Cruz, L.; Guimarães, M.; Araújo, P.; Évora, A.; de Freitas, V.; Mateus, N. Malvidin 3-Glucoside-Fatty Acid Conjugates: From Hydrophilic toward Novel Lipophilic Derivatives. J. Agric. Food Chem. 2017, 65, 6513–6518. [Google Scholar] [CrossRef]
- Liu, J.; Zhuang, Y.; Hu, Y.; Xue, S.; Li, H.; Chen, L.; Fei, P. Improving the Color Stability and Antioxidation Activity of Blueberry Anthocyanins by Enzymatic Acylation with P-Coumaric Acid and Caffeic Acid. LWT 2020, 130, 109673. [Google Scholar] [CrossRef]
- Saad, K.; Giridhar, P.; Shetty, N. Medium Composition Potentially Regulates the Anthocyanin Production from Suspension Culture of Daucus Carota. 3 Biotech 2018, 8, 134. [Google Scholar] [CrossRef]
- Trouillas, P.; Sancho-García, J.C.; De Freitas, V.; Gierschner, J.; Otyepka, M.; Dangles, O. Stabilizing and Modulating Color by Copigmentation: Insights from Theory and Experiment. Chem. Rev. 2016, 116, 4937–4989. [Google Scholar] [CrossRef]
- Mendoza, J.; Pina, F.; Basílio, N.; Guimarães, M.; de Freitas, V.; Cruz, L. Extending the Stability of Red and Blue Colors of Malvidin-3-Glucoside-Lipophilic Derivatives in the Presence of SDS Micelles. Dye. Pigment. 2018, 151, 321–326. [Google Scholar] [CrossRef]
- Fernandez-Aulis, F.; Torres, A.; Sanchez-Mendoza, E.; Cruz, L.; Navarro-Ocana, A. New Acylated Cyanidin Glycosides Extracted from Underutilized Potential Sources: Enzymatic Synthesis, Antioxidant Activity and Thermostability. Food Chem. 2020, 309, 125796. [Google Scholar] [CrossRef]
- Marathe, S.J.; Shah, N.N.; Bajaj, S.R.; Singhal, R.S. Esterification of Anthocyanins Isolated from Floral Waste: Characterization of the Esters and Their Application in Various Food Systems. Food Biosci. 2021, 40, 100852. [Google Scholar] [CrossRef]
- Sari, P.; Rusdianto, A.S.; Siswoyo, T.A. Stability and Antioxidant Activity of Acylated Jambolan (Syzygium Cumini) Anthocyanins Synthesized by Lipase-Catalyzed Transesterification. Int. Food Res. J. 2015, 22, 671–676. [Google Scholar]
- Kong, Y.; Wang, X.; Wu, Z.; Li, Y.; Xu, F.; Xie, F. Enzymatic Acylation of Black Rice Anthocyanins and Evaluation of Antioxidant Capacity and Stability of Their Derivatives. Foods 2023, 12, 4505. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, M.; Mateus, N.; de Freitas, V.; Cruz, L. Improvement of the Color Stability of Cyanidin-3-Glucoside by Fatty Acid Enzymatic Acylation. J. Agric. Food Chem. 2018, 66, 10003–10010. [Google Scholar] [CrossRef]
- Lin, Y.; Li, C.; Shi, L.; Wang, L. Anthocyanins: Modified New Technologies and Challenges. Foods 2023, 12, 1368. [Google Scholar] [CrossRef]
- Marquez-Rodriguez, A.S.; Guimarães, M.; Mateus, N.; de Freitas, V.; Ballinas-Casarrubias, M.L.; Fuentes-Montero, M.E.; Salas, E.; Cruz, L. Disaccharide Anthocyanin Delphinidin 3-O-Sambubioside from Hibiscus Sabdariffa L.: Candida Antarctica Lipase B-Catalyzed Fatty Acid Acylation and Study of Its Color Properties. Food Chem. 2021, 344, 128603. [Google Scholar] [CrossRef]
- Lv, X.; Li, L.; Lu, X.; Wang, W.; Sun, J.; Liu, Y.; Mu, J.; Ma, Q.; Wang, J. Effects of Organic Acids on Color Intensification, Thermodynamics, and Copigmentation Interactions with Anthocyanins. Food Chem. 2022, 396, 133691. [Google Scholar] [CrossRef]
- Sendri, N.; Singh, S.; Sharma, B.; Purohit, R.; Bhandari, P. Effect of Co-Pigments on Anthocyanins of Rhododendron Arboreum and Insights into Interaction Mechanism. Food Chem. 2023, 426, 136571. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, Y.; Sun, B.; Yang, Y.; Wang, S.; Feng, Z.; Li, J. The Structure of Anthocyanins and the Copigmentation by Common Micromolecular Copigments: A Review. Food Res. Int. 2024, 176, 113837. [Google Scholar] [CrossRef]
- Fei, P.; Zeng, F.; Zheng, S.; Chen, Q.; Hu, Y.; Cai, J. Acylation of Blueberry Anthocyanins with Maleic Acid: Improvement of the Stability and Its Application Potential in Intelligent Color Indicator Packing Materials. Dyes Pigment. 2021, 184, 108852. [Google Scholar] [CrossRef]
- Cai, J.; Zeng, F.; Zheng, S.; Huang, X.; Zhang, J.; Zhang, P.; Fei, P. Preparation of Lipid-Soluble Bilberry Anthocyanins through Acylation with Cinnamic Acids and Their Antioxidation Activities. J. Agric. Food Chem. 2020, 68, 7467–7473. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Liu, J.; Zhuang, Y.; Fei, P. Acylating Blueberry Anthocyanins with Fatty Acids: Improvement of Their Lipid Solubility and Antioxidant Activities. Food Chem. X 2022, 15, 100420. [Google Scholar] [CrossRef]
- Houghton, A.; Appelhagen, I.; Martin, C. Natural Blues: Structure Meets Function in Anthocyanins. Plants 2021, 10, 726. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Li, C.; Zhang, L.; Liu, Q.; Ou, S.; Zeng, X. Enzymatic Acylation of Anthocyanin Isolated from Black Rice with Methyl Aromatic Acid Ester as Donor: Stability of the Acylated Derivatives. J. Agric. Food Chem. 2016, 64, 1137–1143. [Google Scholar] [CrossRef]
- Cai, D.; Li, X.; Chen, J.; Jiang, X.; Ma, X.; Sun, J.; Tian, L.; Vidyarthi, S.K.; Xu, J.; Pan, Z.; et al. A Comprehensive Review on Innovative and Advanced Stabilization Approaches of Anthocyanin by Modifying Structure and Controlling Environmental Factors. Food Chem. 2022, 366, 130611. [Google Scholar] [CrossRef]
- Tang, R.; He, Y.; Fan, K. Recent Advances in Stability Improvement of Anthocyanins by Efficient Methods and Its Application in Food Intelligent Packaging: A Review. Food Biosci. 2023, 56, 103164. [Google Scholar] [CrossRef]
- Molaeafard, S.; Jamei, R.; Poursattar Marjani, A. Co-Pigmentation of Anthocyanins Extracted from Sour Cherry (Prunus Cerasus L.) with Some Organic Acids: Color Intensity, Thermal Stability, and Thermodynamic Parameters. Food Chem. 2021, 339, 128070. [Google Scholar] [CrossRef]
- Enaru, B.; Drețcanu, G.; Pop, T.D.; Stǎnilǎ, A.; Diaconeasa, Z. Anthocyanins: Factors Affecting Their Stability and Degradation. Antioxidants 2021, 10, 1967. [Google Scholar] [CrossRef]
- Zhao, C.-L.; Yu, Y.-Q.; Chen, Z.-J.; Wen, G.-S.; Wei, F.-G.; Zheng, Q.; Wang, C.-D.; Xiao, X.-L. Stability-Increasing Effects of Anthocyanin Glycosyl Acylation. Food Chem. 2017, 214, 119–128. [Google Scholar] [CrossRef]
- Tang, M.; Zhu, S.; Liu, Z.; Jiang, C.; Wu, Y.; Li, H.; Wang, B.; Wang, E.; Ma, J.; Wang, C. Tailoring π-Conjugated Systems: From π-π Stacking to High-Rate-Performance Organic Cathodes. Chem 2018, 4, 2600–2614. [Google Scholar] [CrossRef]
- Li, Z.; Teng, W.; Xie, X.; Bao, Y.; Xu, A.; Sun, Y.; Yang, B.; Tian, J.; Li, B. Enzymatic Acylation of Cyanidin-3-O-Glucoside with Aromatic and Aliphatic Acid Methyl Ester: Structure-Stability Relationships of Acylated Derivatives. Food Res. Int. 2024, 192, 114824. [Google Scholar] [CrossRef] [PubMed]
- Gamage, G.C.V.; Lim, Y.Y.; Choo, W.S. Sources and Relative Stabilities of Acylated and Nonacylated Anthocyanins in Beverage Systems. J. Food Sci. Technol. (JFST) 2022, 59, 831–845. [Google Scholar] [CrossRef] [PubMed]
- Hui, Y.; Wen, S.; Lihong, W.; Chuang, W.; Chaoyun, W. Molecular Structures of Nonvolatile Components in the Haihong Fruit Wine and Their Free Radical Scavenging Effect. Food Chem. 2021, 353, 129298. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Xu, H.; Zhang, H.; Wei, F.; Huang, L.; Ke, S.; Fu, J.; Jing, C.; Cheng, J.; Liu, S. Tuning Electron Delocalization of Hydrogen-Bonded Organic Framework Cathode for High-Performance Zinc-Organic Batteries. Nat. Commun. 2023, 14, 5235. [Google Scholar] [CrossRef]
- Tan, X.; Li, H.; Huang, W.; Ma, W.; Lu, Y.; Yan, R. Enzymatic Acylation Improves the Stability and Bioactivity of Lutein: Protective Effects of Acylated Lutein Derivatives on L-O2 Cells upon H2O2-Induced Oxidative Stress. Food Chem. 2023, 410, 135393. [Google Scholar] [CrossRef]
- Teng, H.; Mi, Y.; Cao, H.; Chen, L. Enzymatic Acylation of Raspberry Anthocyanin: Evaluations on Its Stability and Oxidative Stress Prevention. Food Chem. 2022, 372, 130766. [Google Scholar] [CrossRef]
- Sariburun, E.; Şahin, S.; Demir, C.; Türkben, C.; Uylaşer, V. Phenolic Content and Antioxidant Activity of Raspberry and Blackberry Cultivars. J. Food Sci. 2010, 75, C328–C335. [Google Scholar] [CrossRef]
- Zeng, F.; Zeng, H.; Ye, Y.; Zheng, S.; Zhuang, Y.; Liu, J.; Fei, P. Preparation of Acylated Blueberry Anthocyanins through an Enzymatic Method in an Aqueous/Organic Phase: Effects on Their Colour Stability and pH-Response Characteristics. Food Funct. 2021, 12, 6821–6829. [Google Scholar] [CrossRef]
- Nagpal, T.; Yadav, V.; Khare, S.K.; Siddhanta, S.; Sahu, J.K. Monitoring the Lipid Oxidation and Fatty Acid Profile of Oil Using Algorithm-Assisted Surface-Enhanced Raman Spectroscopy. Food Chem. 2023, 428, 136746. [Google Scholar] [CrossRef]
- Gao, L.; Sun, H.; Nagassa, M.; Li, X.; Pei, H.; Liu, S.; Gu, Y.; He, S. Edible Film Preparation by Anthocyanin Extract Addition into Acetylated Cassava Starch/Sodium Carboxymethyl Cellulose Matrix for Oxidation Inhibition of Pumpkin Seeds. Int. J. Biol. Macromol. 2024, 267, 131439. [Google Scholar] [CrossRef]
- Zhao, Y.; Kong, X.; Hu, X.; Sun, Y.; Jiang, N. Black Rice Anthocyanins as An Effective Antioxidant of Inhibition of Oil Oxidative Based on Molecular Modification. J. Oleo Sci. 2024, 73, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Strugała, P.; Dudra, A.; Gabrielska, J. Interaction between Mimic Lipid Membranes and Acylated and Nonacylated Cyanidin and Its Bioactivity. J. Agric. Food Chem. 2016, 64, 7414–7422. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tan, X.; Huang, W.; Zhu, X.; Yang, X.; Shen, Y.; Yan, R. Enzymatic Acylation of Flavonoids from Bamboo Leaves: Improved Lipophilicity and Antioxidant Activity for Oil-Based Foods. J. Agric. Food Chem. 2023, 71, 4817–4824. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Zhang, Q.; Vriesekoop, F.; Yuan, Q.; Liang, H. Preparation of Organogel with Tea Polyphenols Complex for Enhancing the Antioxidation Properties of Edible Oil. J. Agric. Food Chem. 2014, 62, 8379–8384. [Google Scholar] [CrossRef]
- Marteau, C.; Favier, D.; Nardello-Rataj, V.; Aubry, J.-M. Dramatic Solvent Effect on the Synergy between α-Tocopherol and BHT Antioxidants. Food Chem. 2014, 160, 190–195. [Google Scholar] [CrossRef]
- Sun, J.; Wei, Z.; Xue, C. Porous Gelatin Microspheres for High Loading and Improved Stability of Anthocyanins: Serving as Antioxidant Factors and Food Freshness Indicators. Food Hydrocoll. 2024, 148, 109494. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, Z.; Wang, W.; Ye, Q.; Xiao, J. Simulating the Behavior of Antioxidant to Explore the Mechanisms of Oxidative Stability in Pickering Emulsion. Food Chem. 2024, 447, 138291. [Google Scholar] [CrossRef]
- Ryu, K.S.; Shim, K.S.; Shin, D. Effect of Grape Pomace Powder Addition on TBARS and Color of Cooked Pork Sausages during Storage. Food Sci. Anim. Resour. 2014, 34, 200–206. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, X.; Zheng, J.; Herrera-Balandrano, D.D.; Zhang, X.; Huang, W.; Sui, Z. In Vivo Antioxidant Activity of Rabbiteye Blueberry (Vaccinium Ashei Cv. ’Brightwell’) Anthocyanin Extracts. J. Zhejiang Univ. Sci. B 2023, 24, 602–616. [Google Scholar] [CrossRef]
- Zhu, F.; Li, J.; Ma, Z.; Li, J.; Du, B. Structural Identification and in Vitro Antioxidant Activities of Anthocyanins in Black Chokeberry (Aronia Melanocarpa Lliot). eFood 2021, 2, 201–208. [Google Scholar] [CrossRef]
- Ardhaoui, M.; Falcimaigne, A.; Ognier, S.; Engasser, J.M.; Moussou, P.; Pauly, G.; Ghoul, M. Effect of Acyl Donor Chain Length and Substitutions Pattern on the Enzymatic Acylation of Flavonoids. J. Biotechnol. 2004, 110, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Wang, Y.; Xie, P.; Zhang, L.; Li, Y.; Zhou, J. Copigmentation Effects of Phenolics on Color Enhancement and Stability of Blackberry Wine Residue Anthocyanins: Chromaticity, Kinetics and Structural Simulation. Food Chem. 2019, 275, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Luan, H.; Tang, C.; Li, X.; Li, S.; Ding, H.; Yu, X.; Arneborg, N.; Yang, G.; Zhou, J. Enzymatic Acylation of Blueberry (Vaccinium Ashei) Pomace Anthocyanins and Determination of Their Cytotoxic Properties. Process Biochem. 2023, 130, 705–714. [Google Scholar] [CrossRef]
- Gui, H.; Dai, J.; Tian, J.; Jiang, Q.; Zhang, Y.; Ren, G.; Song, B.; Wang, M.; Saiwaidoula, M.; Dong, W.; et al. The Isolation of Anthocyanin Monomers from Blueberry Pomace and Their Radical-Scavenging Mechanisms in DFT Study. Food Chem. 2023, 418, 135872. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cheng, Z.; Gao, N.; Zhang, Y.; Wang, M.; Ren, G.; Song, B.; Liang, Q.; Bao, Y.; Tan, H.; et al. Effects of Sucrose Degradation Product Furfural on Cyanidin-3-O-Glucoside: Mechanism of Action, Stability, and Identification of Products in Sugar Solutions. Food Res. Int. 2023, 168, 112788. [Google Scholar] [CrossRef]
- Singh, S.; Sendri, N.; Sharma, B.; Kumar, P.; Sharma, A.; Tirpude, N.V.; Purohit, R.; Bhandari, P. Copigmentation Effect on Red Cabbage Anthocyanins, Investigation of Their Cellular Viability and Interaction Mechanism. Food Res. Int. 2025, 200, 115427. [Google Scholar] [CrossRef]
- Shaker, L.M.; Al-Amiery, A.A.; Abed, T.K.; Al-Azzawi, W.K.; Kadhum, A.A.H.; Sulaiman, G.M.; Mohammed, H.A.; Khan, M.; Khan, R.A. An Overview of the Density Functional Theory on Antioxidant Bioactivity Predictive Feasibilities: Insights from Natural Antioxidant Products. J. Mol. Struct. 2024, 1301, 137393. [Google Scholar] [CrossRef]
- Suarez-Jimenez, G.; Sánchez, M.; Yepiz-Plascencia, G.; Burgos-Hernández, A.; Brauer, J. In Vitro Antioxidant, Antimutagenic and Antiproliferative Activities of Collagen Hydrolysates of Jumbo Squid (Dosidicus Gigas) Byproducts. Food Sci. Technol. 2015, 35, 421–427. [Google Scholar] [CrossRef]
- Yamauchi, M.; Kitamura, Y.; Nagano, H.; Kawatsu, J.; Gotoh, H. DPPH Measurements and Structure—Activity Relationship Studies on the Antioxidant Capacity of Phenols. Antioxidants 2024, 13, 309. [Google Scholar] [CrossRef]
- Czerwińska, J.; Nowak, M.; Wojtczak, P.; Dziuban-Lech, D.; Cieśla, J.M.; Kołata, D.; Gajewska, B.; Barańczyk-Kuźma, A.; Robinson, A.R.; Shane, H.L.; et al. ERCC1-Deficient Cells and Mice Are Hypersensitive to Lipid Peroxidation. Free Radic. Biol. Med. 2018, 124, 79–96. [Google Scholar] [CrossRef]
- Slatter, D.A.; Bolton, C.H.; Bailey, A.J. The Importance of Lipid-Derived Malondialdehyde in Diabetes Mellitus. Diabetologia 2000, 43, 550–557. [Google Scholar] [CrossRef]
- Liu, R.; Xu, Y.; Zhang, T.; Gong, M.; Liu, R.; Chang, M.; Wang, X. Interactions between Liposoluble Antioxidants: A Critical Review. Food Res. Int. 2022, 155, 111104. [Google Scholar] [CrossRef] [PubMed]
- Laguerre, M.; Bayrasy, C.; Panya, A.; Weiss, J.; McClements, D.J.; Lecomte, J.; Decker, E.A.; Villeneuve, P. What Makes Good Antioxidants in Lipid-Based Systems? The next Theories beyond the Polar Paradox. Crit. Rev. Food Sci. Nutr. 2015, 55, 183–201. [Google Scholar] [CrossRef]
- Zhang, M.; Xin, X.; Lai, F.; Zhang, X.; Li, X.; Wu, H. Cellular Transport of Esculin and Its Acylated Derivatives in Caco-2 Cell Monolayers and Their Antioxidant Properties in Vitro. J. Agric. Food Chem. 2017, 65, 7424–7432. [Google Scholar] [CrossRef]
- Jeyaraj, E.J.; Lim, Y.Y.; Choo, W.S. Antioxidant, Cytotoxic, and Antibacterial Activities of Clitoria Ternatea Flower Extracts and Anthocyanin-Rich Fraction. Sci. Rep. 2022, 12, 14890. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, Y.; Deng, Y.; Yang, B.; Guo, R.; Jin, X.; Zhou, L. Preparation and Antioxidant Activity In Vitro of Fermented Tremella Fuciformis Extracellular Polysaccharides. Fermentation 2022, 8, 616. [Google Scholar] [CrossRef]
- Gan, L.-J.; Yang, D.; Shin, J.-A.; Kim, S.-J.; Hong, S.-T.; Lee, J.H.; Sung, C.-K.; Lee, K.-T. Oxidative Comparison of Emulsion Systems from Fish Oil-Based Structured Lipid versus Physically Blended Lipid with Purple-Fleshed Sweet Potato (Ipomoea Batatas L.) Extracts. J. Agric. Food Chem. 2012, 60, 467–475. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, Z.; Liu, M.; Lyu, J.; Rong, H.; Gan, L. Influence of Enzymatic Acylation on the Stability and Antioxidant Properties of Cyanidin-3-O-Glucoside in Both Aqueous and Lipid Systems. Molecules 2025, 30, 2015. https://doi.org/10.3390/molecules30092015
Ye Z, Liu M, Lyu J, Rong H, Gan L. Influence of Enzymatic Acylation on the Stability and Antioxidant Properties of Cyanidin-3-O-Glucoside in Both Aqueous and Lipid Systems. Molecules. 2025; 30(9):2015. https://doi.org/10.3390/molecules30092015
Chicago/Turabian StyleYe, Ziwei, Mingyun Liu, Jingmei Lyu, Han Rong, and Lujing Gan. 2025. "Influence of Enzymatic Acylation on the Stability and Antioxidant Properties of Cyanidin-3-O-Glucoside in Both Aqueous and Lipid Systems" Molecules 30, no. 9: 2015. https://doi.org/10.3390/molecules30092015
APA StyleYe, Z., Liu, M., Lyu, J., Rong, H., & Gan, L. (2025). Influence of Enzymatic Acylation on the Stability and Antioxidant Properties of Cyanidin-3-O-Glucoside in Both Aqueous and Lipid Systems. Molecules, 30(9), 2015. https://doi.org/10.3390/molecules30092015





