Synthesis and Biological Screening of Structurally Modified Phaeosphaeride Analogues
Abstract
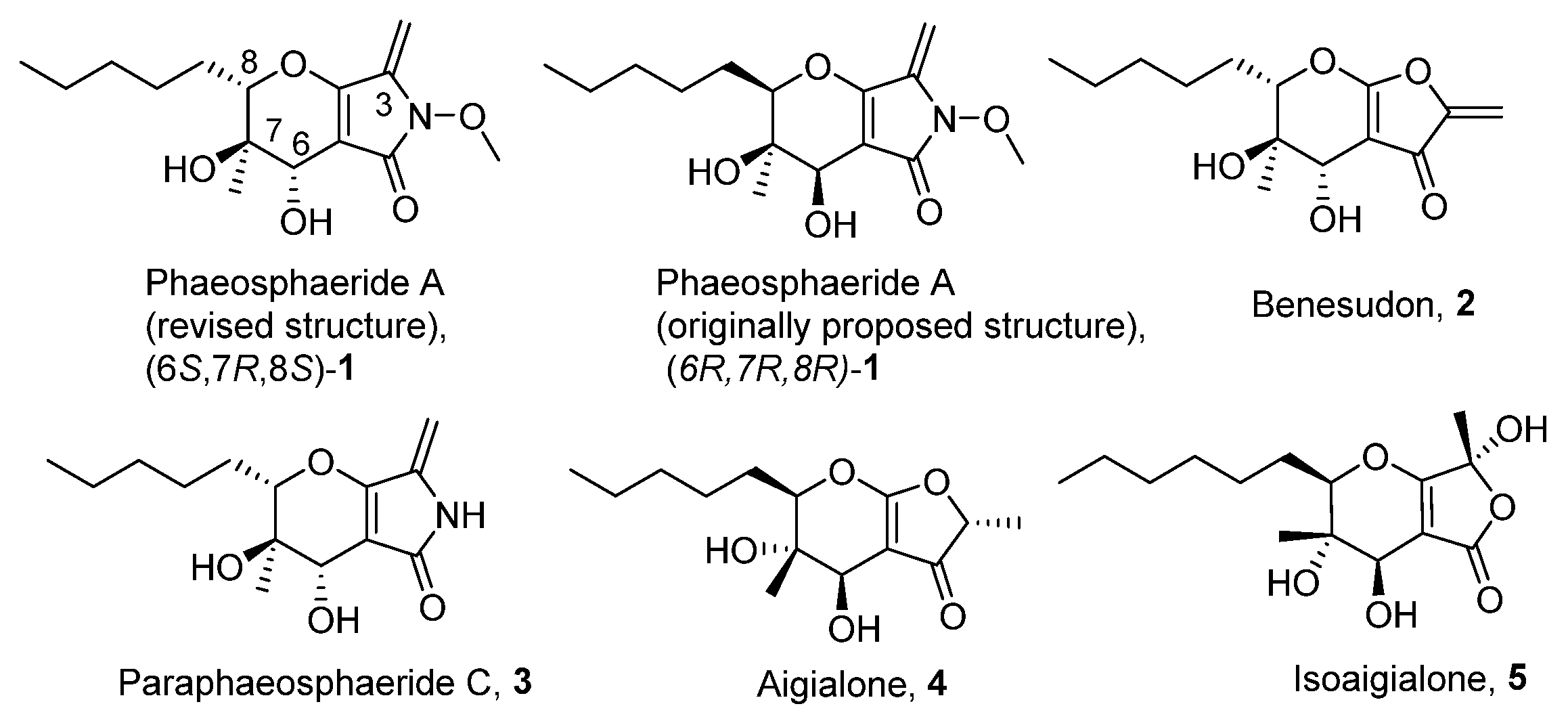
1. Introduction
2. Results
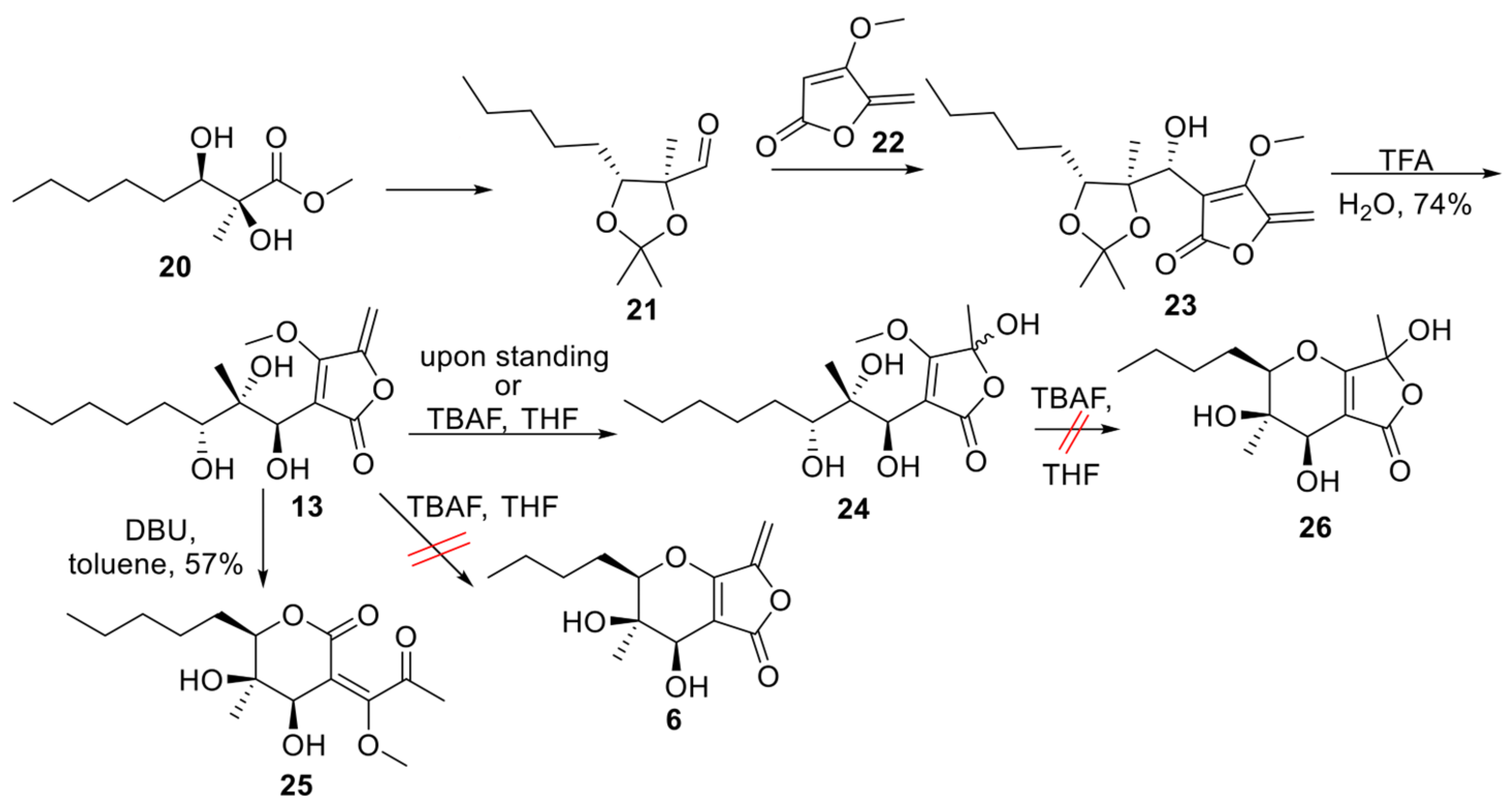
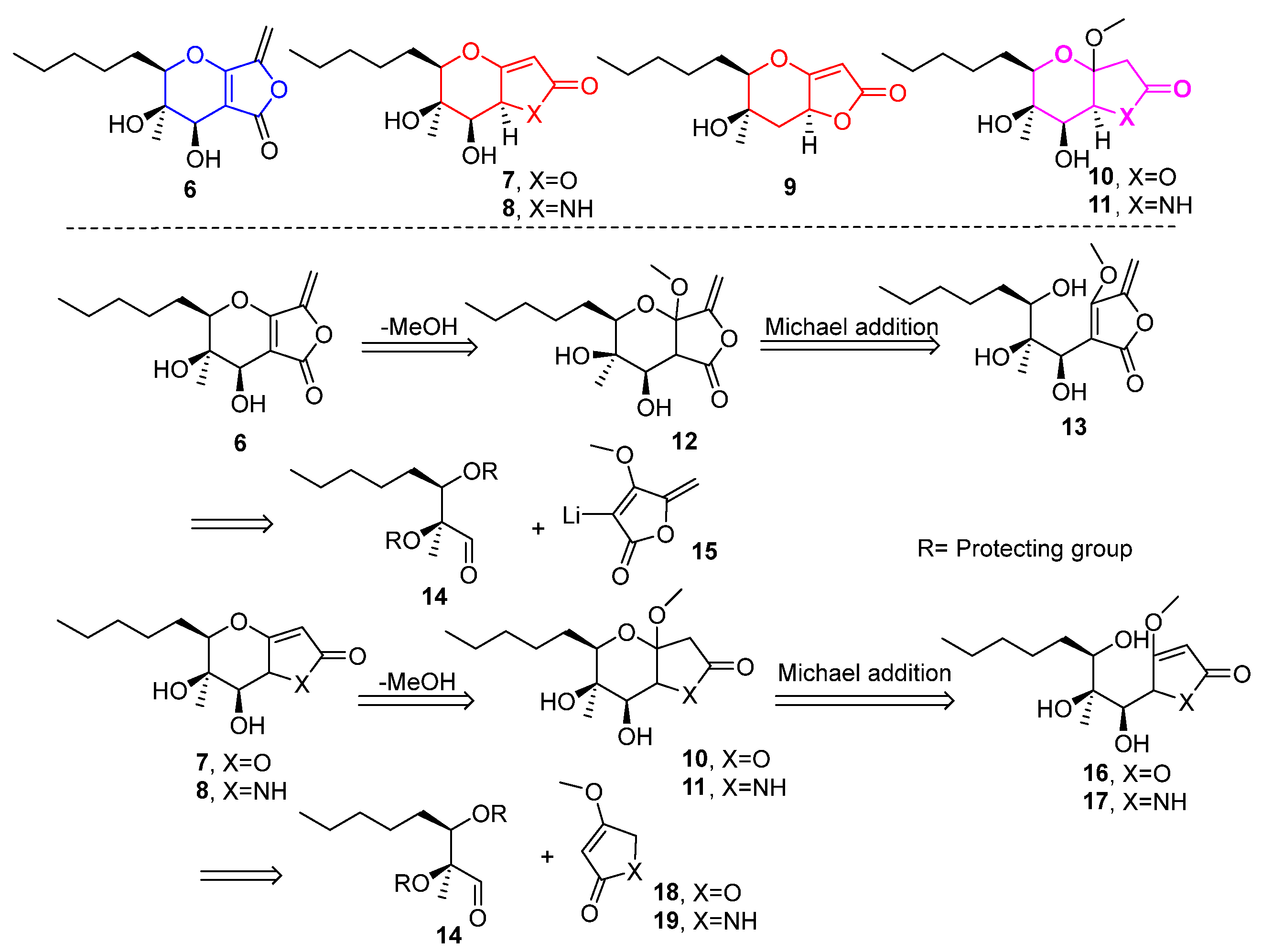
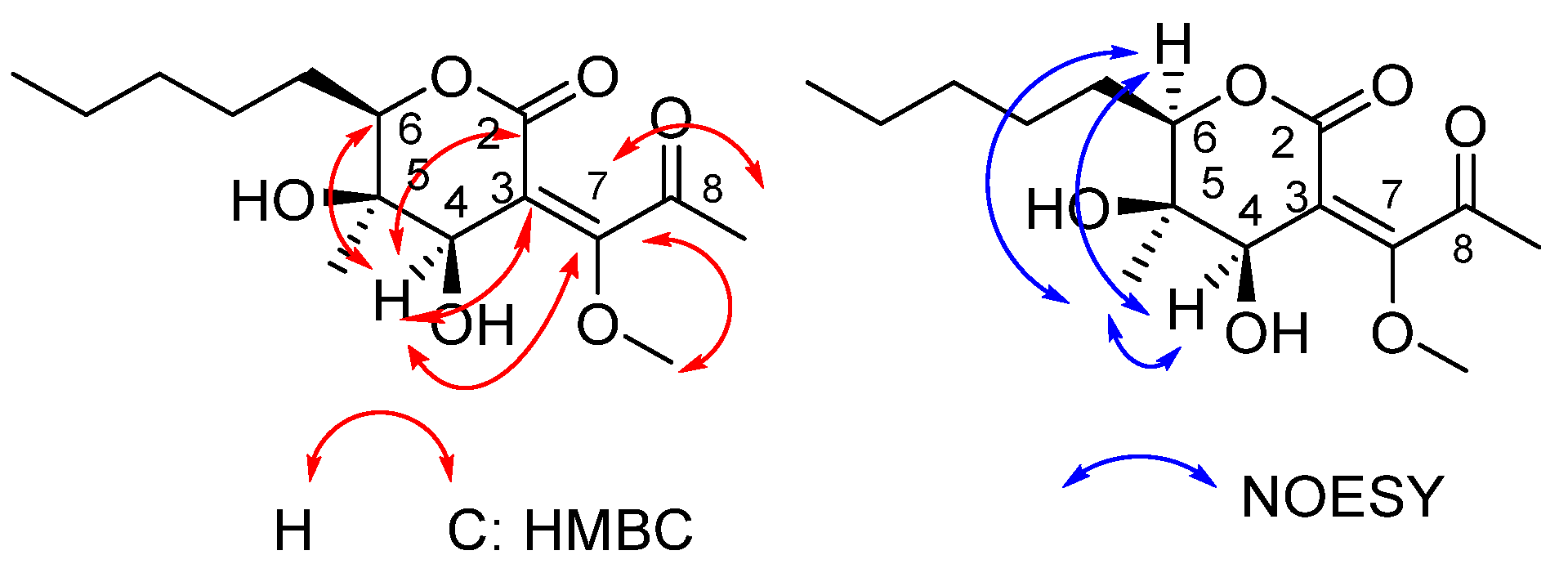
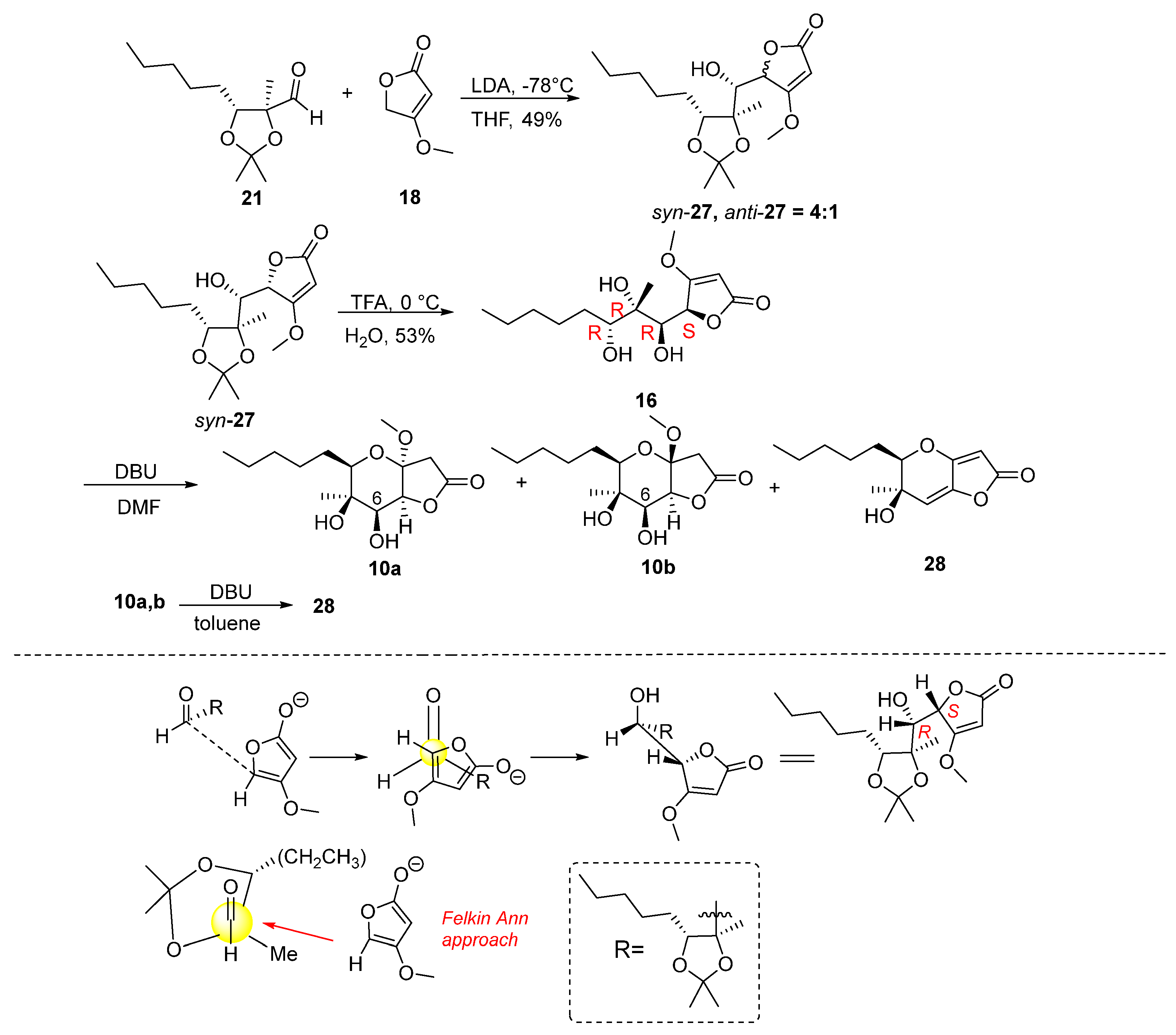
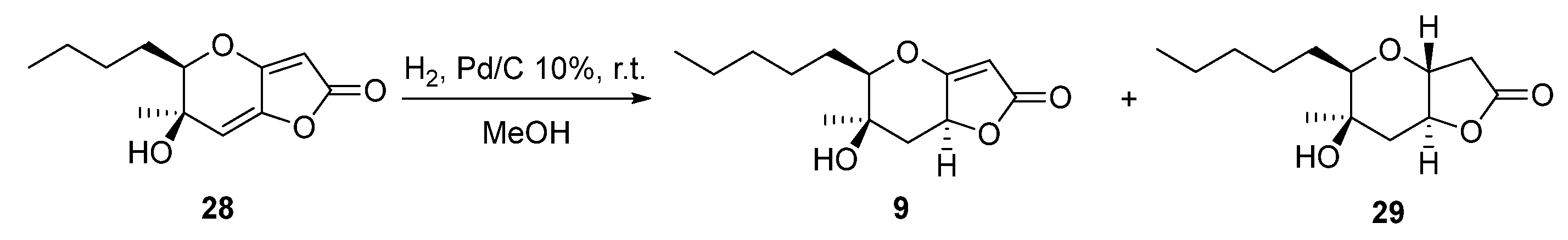
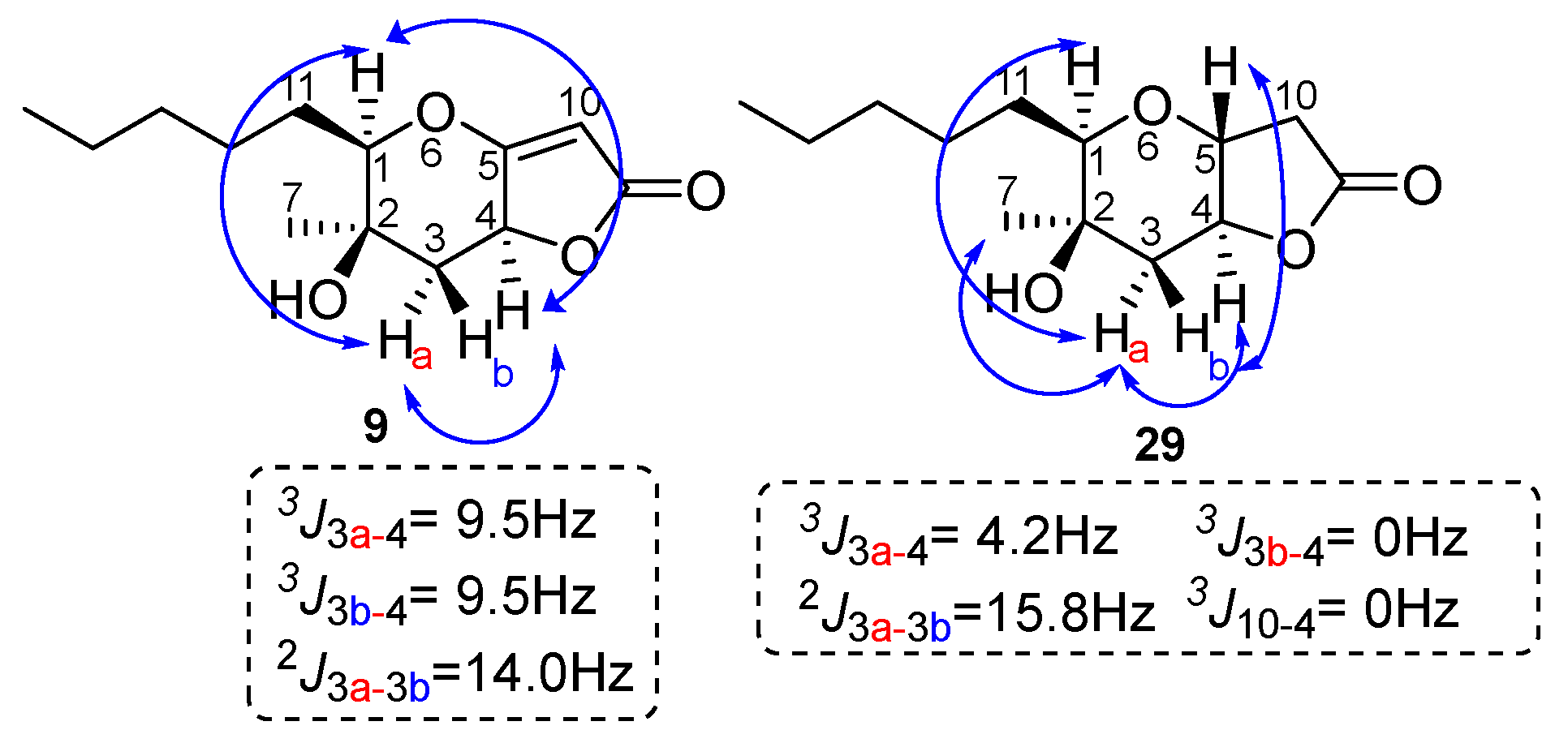
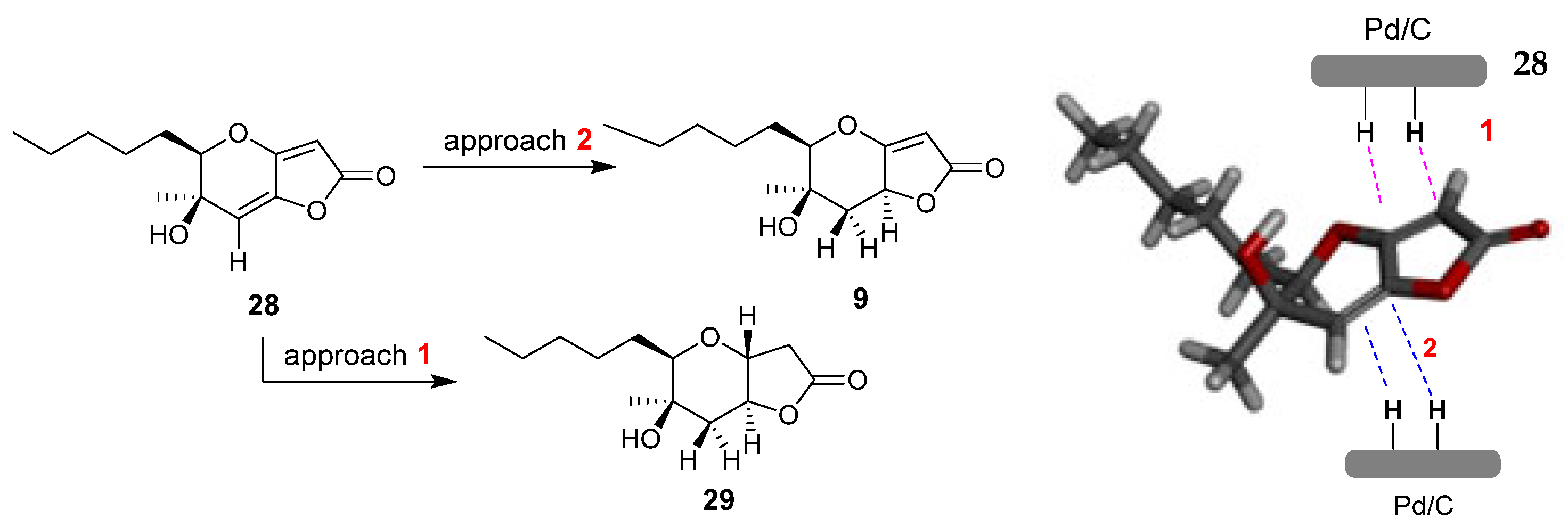
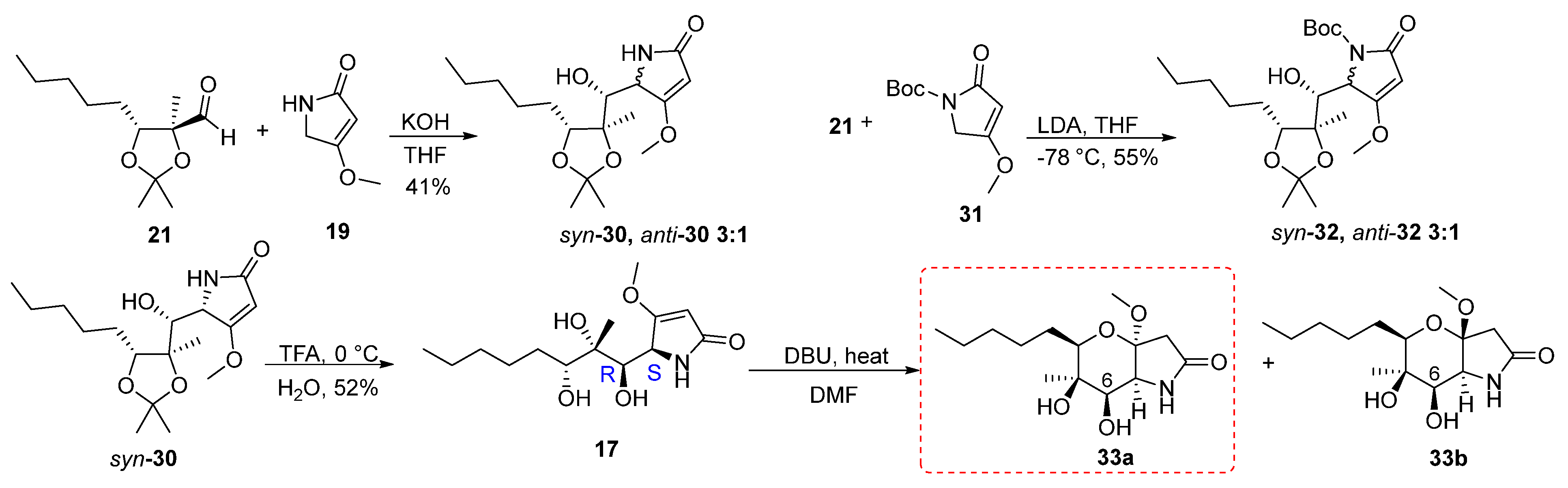
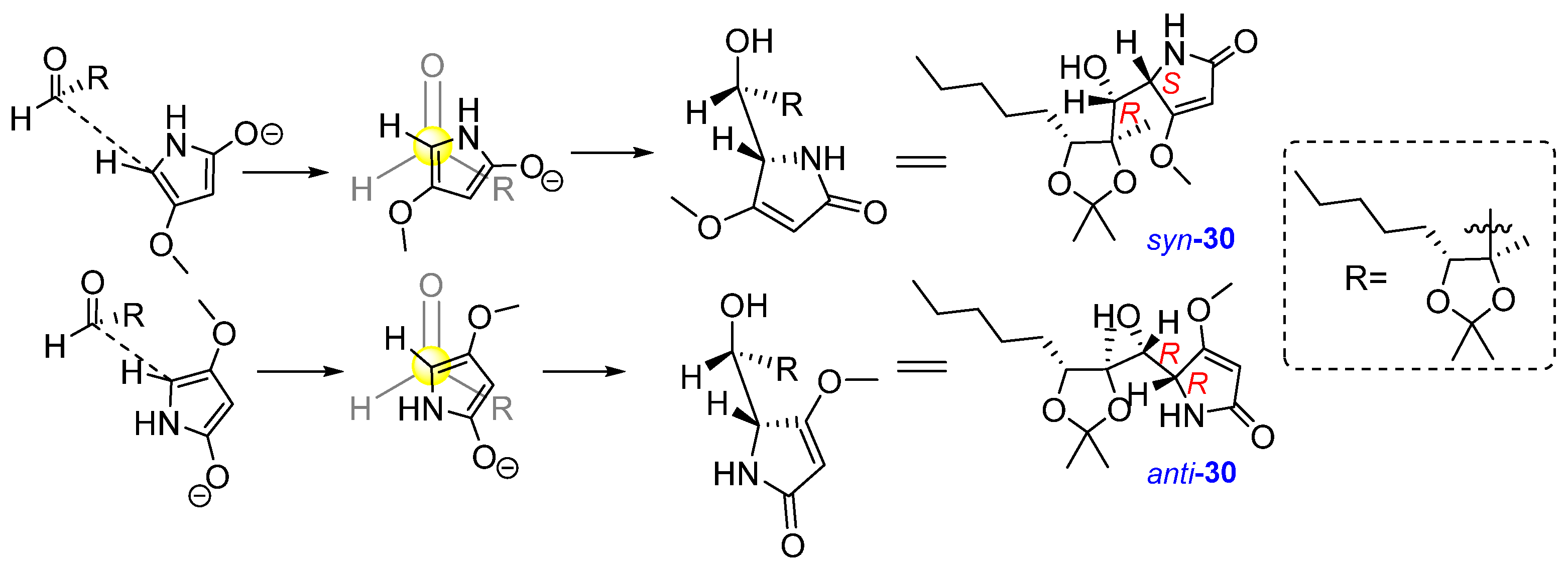
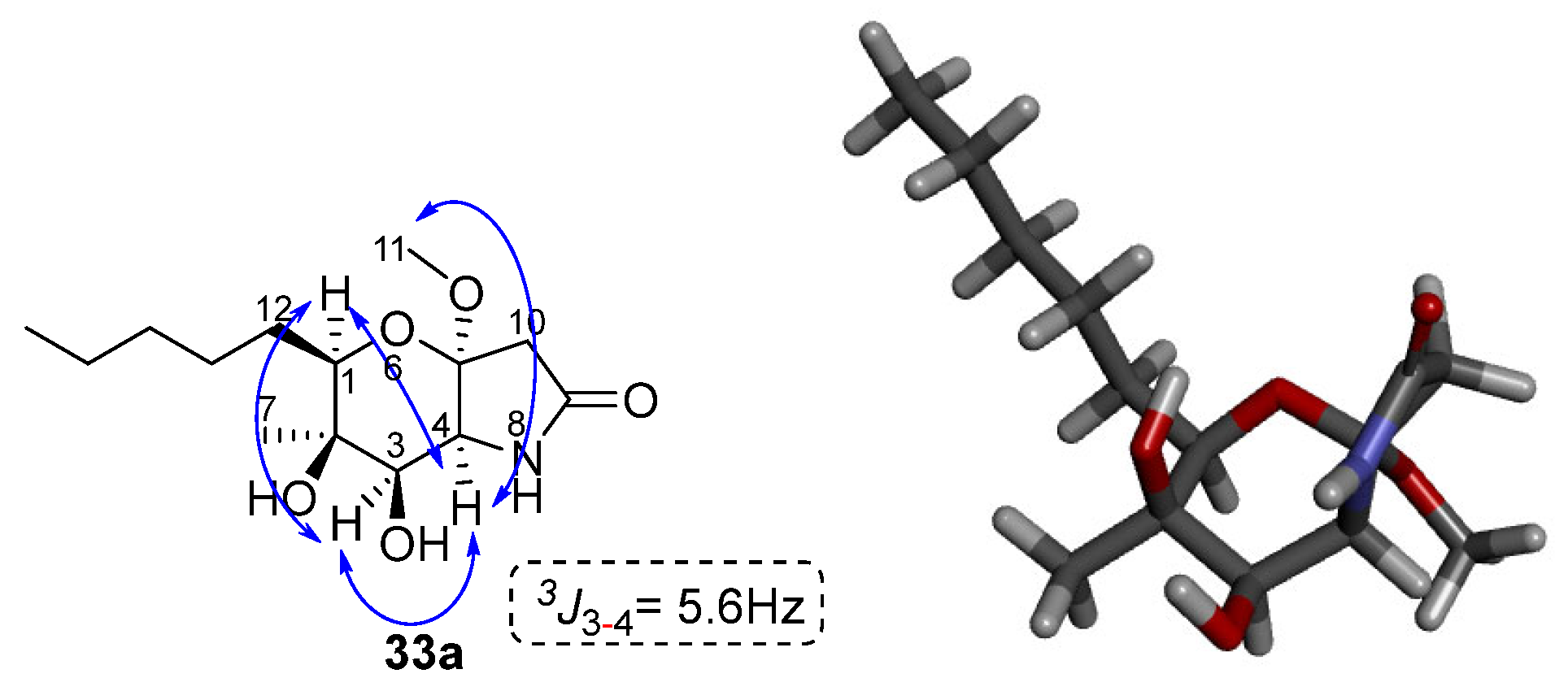
2.1. Synthesis of Phaeosphaeride Analogues
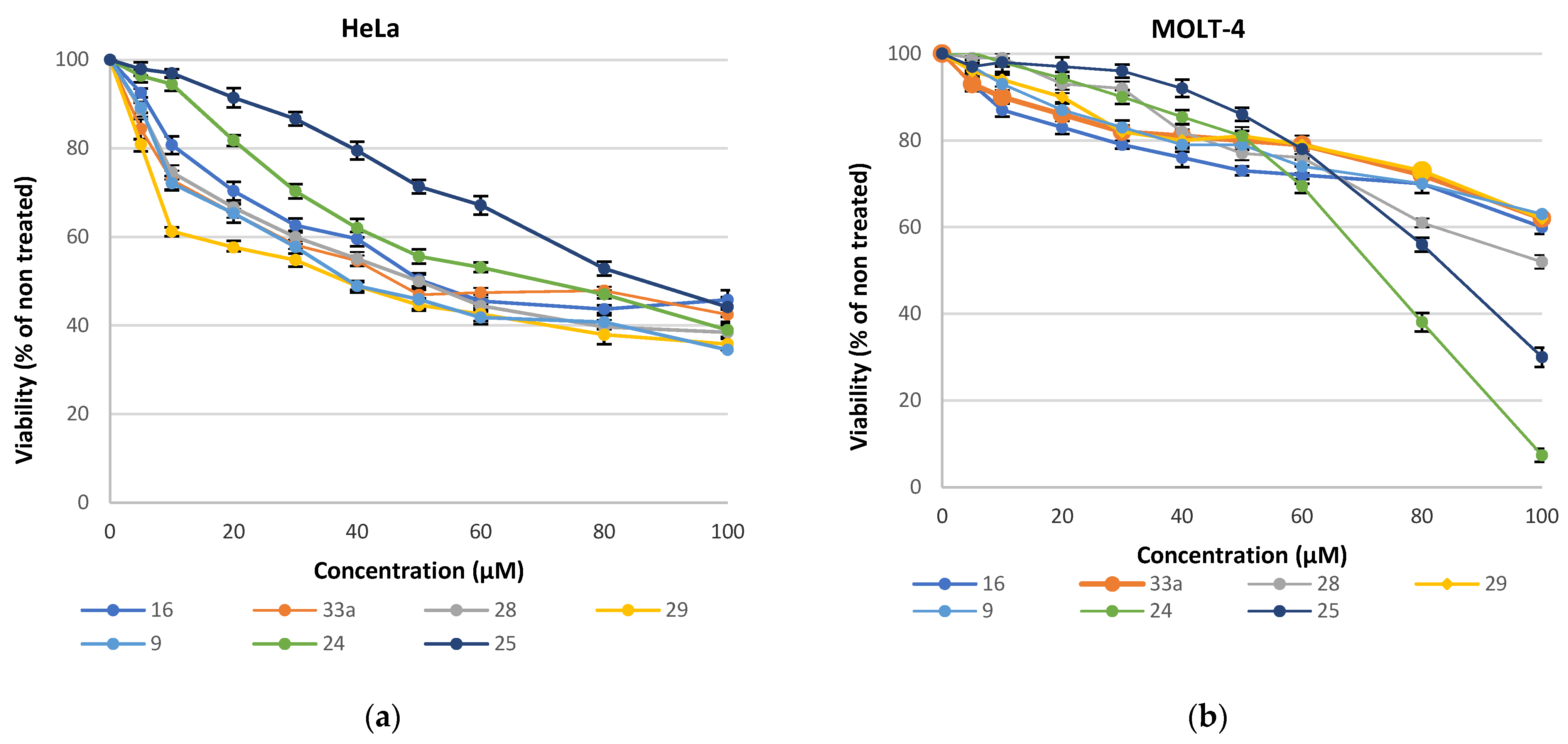
2.2. Cell Viability Studies
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| SAR | Structure Activity Relationships |
| DMEM | Dulbecco’s Modified Eagle Medium |
| TBAF | Tetrabutylammonium fluoride |
| DBU | 1,8-Diazabicyclo(5.4.0)undec-7-ene |
| TFA | Trifluoroacetic acid |
| pTSA | p-Toluenesulfonic acid |
References
- Butler, M.S.; Robertson, A.A.; Cooper, M.A. Natural product and natural product derived drugs in clinical trials. Nat. Prod. Rep. 2014, 31, 1612–1661. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T.; International Natural Product Sciences Taskforce. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Mathur, S.; Hoskins, C. Drug development: Lessons from nature. Biomed. Rep. 2017, 6, 612–614. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.A.P.; Thornburg, C.C.; Henrich, C.J.; Grkovic, T.; O’Keefe, B.R. Creating and screening natural product libraries. Nat. Prod. Rep. 2020, 37, 893–918. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, S.; Zhao, S.; Wang, X.; Liu, B.; Xu, H. Click Chemistry in Natural Product Modification. Front. Chem. 2021, 9, 774977. [Google Scholar] [CrossRef]
- Singh, K.; Gupta, J.K.; Chanchal, D.K.; Shinde, M.G.; Kumar, S.; Jain, D.; Almarhoon, Z.M.; Alshahrani, A.M.; Calina, D.; Sharifi-Rad, J.; et al. Natural products as drug leads: Exploring their potential in drug discovery and development. Naunyn Schmiedebergs Arch. Pharmacol. 2024, 398, 4673–4687. [Google Scholar] [CrossRef]
- Maloney, K.N.; Hao, W.; Xu, J.; Gibbons, J.; Hucul, J.; Roll, D.; Brady, S.F.; Schroeder, F.C.; Clardy, J. Phaeosphaeride A, an inhibitor of STAT3-dependent signaling isolated from an endophytic fungus. Org. Lett. 2006, 8, 4067–4070. [Google Scholar] [CrossRef]
- Chatzimpaloglou, A.; Yavropoulou, M.P.; Rooij, K.E.; Biedermann, R.; Mueller, U.; Kaskel, S.; Sarli, V. Total synthesis and biological activity of the proposed structure of phaeosphaeride A. J. Org. Chem. 2012, 77, 9659–9667. [Google Scholar] [CrossRef]
- Chatzimpaloglou, A.; Kolosov, M.; Eckols, T.K.; Tweardy, D.J.; Sarli, V. Synthetic and biological studies of phaeosphaerides. J. Org. Chem. 2014, 79, 4043–4054. [Google Scholar] [CrossRef]
- Kobayashi, K.; Okamoto, I.; Morita, N.; Kiyotani, T.; Tamura, O. Synthesis of the proposed structure of phaeosphaeride A. Org. Biomol. Chem. 2011, 9, 5825–5832. [Google Scholar] [CrossRef]
- Poluektova, E.; Tokarev, Y.; Sokornova, S.; Chisty, L.; Evidente, A.; Berestetskiy, A. Curvulin and Phaeosphaeride A from Paraphoma sp. VIZR 1.46 Isolated from Cirsium arvense as Potential Herbicides. Molecules 2018, 23, 2795. [Google Scholar] [CrossRef] [PubMed]
- Abzianidze, V.; Beltyukov, P.; Zakharenkova, S.; Moiseeva, N.; Mejia, J.; Holder, A.; Trishin, Y.; Berestetskiy, A.; Kuznetsov, V. Synthesis and Biological Evaluation of Phaeosphaeride A Derivatives as Antitumor Agents. Molecules 2018, 23, 3043. [Google Scholar] [CrossRef] [PubMed]
- Abzianidze, V.V.; Efimova, K.P.; Poluektova, E.V.; Trishin, Y.G.; Kuznetsov, V.A. Synthesis of natural phaeosphaeride A and semi-natural phaeosphaeride B derivatives. Mendeleev Commun. 2017, 27, 490–492. [Google Scholar] [CrossRef]
- Kobayashi, K.; Tanaka, K.; Kogen, H. Total Synthesis and Biological Evaluation of Phaeosphaerides. Catalysts 2018, 8, 206. [Google Scholar] [CrossRef]
- Li, C.S.; Ding, Y.; Yang, B.J.; Miklossy, G.; Yin, H.Q.; Walker, L.A.; Turkson, J.; Cao, S. A New Metabolite with a Unique 4-Pyranone-γ-Lactam-1,4-Thiazine Moiety from a Hawaiian-Plant Associated Fungus. Org. Lett. 2015, 17, 3556–3559. [Google Scholar] [CrossRef]
- Silva, G.H.; Zeraik, M.L.; de Oliveira, C.M.; Teles, H.L.; Trevisan, H.C.; Pfenning, L.H.; Nicolli, C.P.; Young, M.C.M.; Mascarenhas, Y.P.; Abreu, L.M.; et al. Lactone Derivatives Produced by a Phaeoacremonium sp., an Endophytic Fungus from Senna spectabilis. J. Nat. Prod. 2017, 80, 1674–1678. [Google Scholar] [CrossRef]
- Trenti, F.; Cox, R.J. Structural Revision and Biosynthesis of the Fungal Phytotoxins Phyllostictines A and B. J. Nat. Prod. 2017, 80, 1235–1240. [Google Scholar] [CrossRef]
- Hirayama, Y.; Matsunaga, M.; Fukao, A.; Kobayashi, K. Biological evaluation of signal transducer and activator of transcrip-tion 3 (STAT3) targeting by phaeosphaeride A and its analogs. Bioorg. Med. Chem. Lett. 2024, 114, 130004. [Google Scholar] [CrossRef]
- Karak, M.; Barbosa, L.C.; Acosta, J.A.; Sarotti, A.M.; Boukouvalas, J. Thermodynamically driven, syn-selective vinylogous aldol reaction of tetronamides. Org. Biomol. Chem. 2016, 14, 4897–4907. [Google Scholar] [CrossRef]
- Abzianidze, V.; Moiseeva, N.; Suponina, D.; Zakharenkova, S.; Rogovskaya, N.; Laletina, L.; Holder, A.A.; Krivorotov, D.; Bogachenkov, A.; Garabadzhiu, A.; et al. Natural Phaeosphaeride A Derivatives Overcome Drug Resistance of Tumor Cells and Modulate Signaling Pathways. Pharmaceuticals 2022, 15, 395. [Google Scholar] [CrossRef]
- Abzianidze, V.; Kadochnikov, V.; Suponina, S.D.; Skvortsov, V.N.; Beltyukov, P.P.; Babakov, N.V.; Krivorotov, V.D.; Barysheva, M.E.; Garabadzhiu, V.A. X-ray structure and in silico molecular docking of a natural phaeosphaeride A derivative for targets associated with kinase cascade. Mendeleev Commun. 2023, 33, 534. [Google Scholar] [CrossRef]
- Leonidis, G.; Koukiali, A.; Sigala, I.; Tsimaratou, K.; Beis, D.; Giannakouros, T.; Nikolakaki, E.; Sarli, V. Synthesis and Anti-Angiogenic Activity of Novel c(RGDyK) Peptide-Based JH-VII-139-1 Conjugates. Pharmaceutics 2023, 15, 381. [Google Scholar] [CrossRef]

| Entry | Reagent | Equiv. | Temperature (°C) | Time (h) | Yield |
|---|---|---|---|---|---|
| 1 | TBAF (1.6M) | 1.5 | 60 | 1 | 24 (20%) + complex mixture |
| 2 | TBAF (1.6M) | 1.5 | rt | overnight | 24 (11%) + complex mixture |
| 3 | DBU | 1.5 | rt | overnight | 25 (57%) |
| 4 | p-TSA | 1.5 | 60 | 0.5 | 24 (10%) + complex mixture |
| Position | δC (mult.) | δH (mult., J in Hz) | Position | δC (mult.) | δH (mult., J in Hz) |
|---|---|---|---|---|---|
| 1 | - | - | 9 | 31.7 | 1.64–1.45 (m, 2H) |
| 2 | 168.6 | - | 10 | 22.5 | 1.34–1.27 (m, 2H) |
| 3 | 106.9 | - | 11 | 14.0 | 0.89 (t, J = 6.7 Hz, 3H) |
| 4 | 70.1 | 4.90 (s, 1H) | 12 | 15.3 | 1.36 (s, 3H) |
| 5 | 90.4 | - | 13 | 198.3 | - |
| 6 | 76.0 | 3.47 (d, J = 9.7 Hz, 1H) | 14 | 30.7 | 2.51 (s, 3H) |
| 7 | 30.7 | 1.64–1.45 (m, 2H) | 15 | 164.8 | - |
| 8 | 25.9 | 1.52–1.43 (m, 2H) | 16 | 58.1 | 3.89 (s, 3H) |
| Entry | Equiv. | Temperature (°C) | Time (h) | Yield |
|---|---|---|---|---|
| 1 | 1.5 | 60 | 72 | 10a,b (69%), 28 (39%) |
| 2 | 1.5 | 60 | 24 | 10a,b (74%), 28 (12%) |
| 3 | 3 | 80 | 48 | 10a,b (46%), 28 (47%) |
| Entry | 1.5 Equiv. | Temperature (°C) | Solvent | Time (h) | Yield |
|---|---|---|---|---|---|
| 1 | TBAF | 60 | THF | 1 | complex mixture |
| 2 | TBAF | 60 | THF | 24 | complex mixture |
| 3 | DBU | rt | Toluene | 24 | no reaction |
| 4 | DBU | 60 | Toluene | 24 | 33a,b (86%) |
| Compound | HeLa (μΜ) | MOLT-4 (μΜ) | Compound | HeLa (μΜ) | MOLT-4 (μΜ) |
|---|---|---|---|---|---|
| 16 | 50.3 ± 2.1 | >100 | 25 | 88.6 ± 3.2 | 83.8 ± 2.9 |
| 24 | 57.9 ± 1.6 | 71.8 ± 2.4 | 9 | 43.5 ± 2.1 | >100 |
| 28 | 49.4 ± 2.4 | >100 | 29 | 39.1 ± 1.9 | >100 |
| 33a | 46.2 ± 3.7 | >100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rantzios, K.; Chatzimentor, O.-E.; Leonidis, G.; Giuliani, J.; Sigala, I.; Sarli, V. Synthesis and Biological Screening of Structurally Modified Phaeosphaeride Analogues. Molecules 2025, 30, 2016. https://doi.org/10.3390/molecules30092016
Rantzios K, Chatzimentor O-E, Leonidis G, Giuliani J, Sigala I, Sarli V. Synthesis and Biological Screening of Structurally Modified Phaeosphaeride Analogues. Molecules. 2025; 30(9):2016. https://doi.org/10.3390/molecules30092016
Chicago/Turabian StyleRantzios, Konstantinos, Oraia-Eirini Chatzimentor, George Leonidis, Jorgo Giuliani, Ioanna Sigala, and Vasiliki Sarli. 2025. "Synthesis and Biological Screening of Structurally Modified Phaeosphaeride Analogues" Molecules 30, no. 9: 2016. https://doi.org/10.3390/molecules30092016
APA StyleRantzios, K., Chatzimentor, O.-E., Leonidis, G., Giuliani, J., Sigala, I., & Sarli, V. (2025). Synthesis and Biological Screening of Structurally Modified Phaeosphaeride Analogues. Molecules, 30(9), 2016. https://doi.org/10.3390/molecules30092016








