Stilbenes Against Alzheimer’s Disease: A Comprehensive Review of Preclinical Studies of Natural and Synthetic Compounds Combined with the Contributions of Developed Nanodrug Delivery Systems
Abstract
1. Introduction
2. Natural Sources for Stilbene Derivatives
3. Chemistry and Structural Classification of Stilbenes
- Group 1: Simple stilbenes
- Group 2: Prenylated and geranylated stilbenes
- Group 3: 2-phenyl-benzofuran derivatives
- Group 4: Carbon-substituted stilbenes (excluding prenylated and geranylated stilbenes) which have acyl, benzyl, and carboxyl groups [37].
3.1. Simple Stilbenes
3.2. Prenylated and Geranylated Stilbenes
3.3. 2-Phenyl-Benzofuran Derivatives
3.4. Carbon-Substituted Stilbenes
- Two individual units linked by only one C-C or C-O-C bond (with two connection points)
- Two individual units connected by two C-C or C-O-C bonds (with four connection points), often forming a ring. For instance, the distinct dihydrobenzofuran segment is typically formed by two units with a C-C and a C-O-C bond.
- Two individual units connected by three C-C or C-O-C bonds (with six connection points) create two rings.
- Two individual units connected by four C-C or C-O-C bonds (with eight connection points). This configuration is rare [7].
4. Structural Characteristics of Stilbene Derivatives
5. Biosynthesis of Stilbenes
6. Alzheimer Disease
6.1. Etiology of Alzheimer’s Disease
6.2. Pathophysiology of Alzheimer’s Disease
6.2.1. Amyloid-β Pathology
6.2.2. Tau Pathology
6.2.3. Neuroinflammation and Neuron Loss
6.3. Current Pharmacological Treatments
7. Potential Benefits and Limitations of Natural Stilbenes in AD
7.1. Potentitail Benefits
7.1.1. Resveratrol
7.1.2. Piceatannol (Astringenin)
7.1.3. Oxyresveratrol
7.1.4. Pterostilbene
7.1.5. Gnetol
7.1.6. Amurensin G
7.1.7. Miyabenol C
7.1.8. Mulberroside A
7.1.9. Polydatin
7.1.10. Rhapontigenin
7.1.11. Scirpusin A, B
7.1.12. ε-Viniferin
7.2. Limits and Difficulties of Natural Stilbene-Based Therapies
8. Synthetic Derivatives of Stilbenes and Their Neuroprotective Activity
9. Nanotechnology-Based Systems for Stilbenes Delivery on Neurodegenerative Disorders
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Akram, M.; Nawaz, A. Effects of medicinal plants on Alzheimer’s disease and memory deficits. Neural Regen Res. 2017, 12, 660–670. [Google Scholar] [CrossRef] [PubMed]
- Koul, B.; Farooq, U.; Yadav, D.; Song, M. Phytochemicals: A promising alternative for the prevention of Alzheimer’s disease. Life 2023, 13, 999. [Google Scholar] [CrossRef] [PubMed]
- John, O.O.; Amarachi, I.S.; Chinazom, A.P.; Adaeze, E.; Kale, M.B.; Umare, M.D.; Upaganlawar, A.B. Phytotherapy: A promising approach for the treatment of Alzheimer’s disease. Pharmacol. Res.-Mod. Chin. Med. 2022, 2, 100030. [Google Scholar] [CrossRef]
- Hapeshi, A.; Benarroch, J.M.; Clarke, D.J.; Waterfield, N.R. Iso-propyl stilbene: A life cycle signal? Microbiology 2019, 165, 516–526. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Mascarenhas, R.; Harish, H.M.; Gowda, Y.; Lakshmaiah, V.V.; Nagella, P.; Al-Mssallem, M.Q.; Alessa, F.M.; Almaghasla, M.I.; Rezk, A.A.S. Stilbenes, a versatile class of natural metabolites for inflammation-an overview. Molecules 2023, 28, 3786. [Google Scholar] [CrossRef]
- Teka, T.; Zhang, L.; Ge, X.; Li, Y.; Han, L.; Yan, X. Stilbenes: Source plants, chemistry, biosynthesis, pharmacology, application and problems related to their clinical application-A comprehensive review. Phytochemistry 2022, 197, 113128. [Google Scholar] [CrossRef]
- Shen, T.; Wang, X.-N.; Lou, H.-X. Natural stilbenes: An overview. Nat. Prod. Rep. 2009, 26, 916–935. [Google Scholar] [CrossRef]
- Rivière, C.; Pawlus, A.D.; Mérillon, J.M. Natural stilbenoids: Distribution in the plant kingdom and chemotaxonomic interest in Vitaceae. Nat. Prod. Rep. 2012, 29, 1317–1333. [Google Scholar] [CrossRef]
- Navarro-Orcajada, S.; Conesa, I.; Vidal-Sánchez, F.J.; Matencio, A.; Albaladejo-Maricó, L.; García-Carmona, F.; López-Nicolás, J.M. Stilbenes: Characterization, bioactivity, encapsulation and structural modifications. A review of their current limitations and promising approaches. Crit. Rev. Food Sci. Nutr. 2023, 63, 7269–7287. [Google Scholar] [CrossRef]
- Yu, X.; Jia, Y.; Ren, F. Multidimensional biological activities of resveratrol and its prospects and challenges in the health field. Front. Nutr. 2024, 11, 1408651. [Google Scholar] [CrossRef]
- Bayach, I. Non-Covalent Interactions in Natural Products. Ph.D. Thesis, Université de Limoges, Limoges, France, 2014. [Google Scholar]
- El Khawand, T.; Courtois, A.; Valls, J.; Richard, T.; Krisa, S. A review of dietary stilbenes: Sources and bioavailability. Phytochem Rev. 2018, 17, 1007–1029. [Google Scholar] [CrossRef]
- Li, Q.; Chen, L.; Liu, X.; Li, X.; Cao, Y.; Bai, Y.; Qi, F. Pterostilbene inhibits amyloid-β-induced neuroinflammation in a microglia cell line by inactivating the NLRP3/caspase-1 inflammasome pathway. J. Cell. Biochem. 2018, 119, 7053–7062. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; Zhu, Q.; Zhang, S.; Yang, D.O.; Lu, J.H. Resveratrol in experimental Alzheimer’s disease models: A systematic review of preclinical studies. Pharm. Res. 2019, 150, 104476. [Google Scholar] [CrossRef] [PubMed]
- Al-Edresi, S.; Alsalahat, I.; Freeman, S.; Aojula, H.; Penny, J. Resveratrol-mediated cleavage of amyloid β1-42 peptide: Potential relevance to Alzheimer’s disease. Neurobiol. Aging 2020, 94, 24–33. [Google Scholar] [CrossRef]
- Mahmoud Moustafa, E.; Rashed, E.R.; Rashed, R.R.; Omar, N.N. Piceatannol promotes hepatic and renal AMPK/SIRT1/PGC-1α mitochondrial pathway in rats exposed to reserpine or gamma-radiation. Int. J. Immunopathol. Pharmacol. 2021, 35, 20587384211016194. [Google Scholar] [CrossRef]
- Rahman, M.A.; Cho, Y.; Nam, G.; Rhim, H. Antioxidant compound, oxyresveratrol, inhibits APP production through the AMPK/ULK1/mTOR-mediated autophagy pathway in mouse cortical astrocytes. Antioxidants 2021, 10, 408. [Google Scholar] [CrossRef]
- Su, X.; Zhou, D.; Li, N. Bioactive stilbenes from plants. In Studies in Natural Products Chemistry; Rahman, A., Ed.; Elsevier: Bengaluru, India, 2022; Volume 73, pp. 265–403. [Google Scholar] [CrossRef]
- Li, X.M.; Lin, M.; Wang, Y.H.; Liu, X. Four new stilbenoids from the lianas of Gnetum montanum f. megalocarpum. Planta Med. 2004, 70, 160–165. [Google Scholar] [CrossRef]
- Yao, C.S.; Lin, M.; Liu, X.; Wang, Y.H. Stilbene derivatives from Gnetum cleistostachyum. J. Asian Nat. Prod. Res. 2005, 7, 131–137. [Google Scholar] [CrossRef]
- Murata, H.; Tanaka, T.; Iliya, I.; Furasawa, M.; Ito, T.; Nakaya, K.I.; Iinuma, M. New resveratrol dimer glucosides and trimers in stem and root of Welwitschia mirabilis. Heterocycles 2004, 63, 1821. [Google Scholar] [CrossRef]
- Li, S.H.; Niu, X.M.; Zahn, S.; Gershenzon, J.; Weston, J.; Schneider, B. Diastereomeric stilbene glucoside dimers from the bark of Norway spruce (Picea abies). Phytochemistry 2008, 69, 772–782. [Google Scholar] [CrossRef]
- Tanaka, T.; Iliya, I.; Ito, T.; Furusawa, M.; Nakaya, K.I.; Iinuma, M.; Shirataki, Y.; Matsuura, N.; Ubukata, M.; Murata, J.; et al. Stilbenoids in lianas of Gnetum parvifolium. Chem. Pharm. Bull. 2001, 49, 858–862. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, H.; Takahashi, N.; Oshima, Y. Novel aromatics bearing 4-O-methylglucose unit isolated from the oriental crude drug Bombyx Batryticatus. Tetrahedron Lett. 2004, 45, 367–370. [Google Scholar] [CrossRef]
- Eleftherianos, I.; Boundy, S.; Joyce, S.A.; Aslam, S.; Marshall, J.W.; Cox, R.J.; Simpson, T.J.; Clarke, D.J.; Constant, R.H.F.; Reynolds, S.E. An antibiotic produced by an insect-pathogenic bacterium suppresses host defenses through phenoloxidase inhibition. Proc. Natl. Acad. Sci. USA 2007, 104, 2419–2424. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.N.; Siji, J.V.; Rajasekharan, K.N.; Nambisan, B.; Mohandas, C. Bioactive stilbenes from a Bacillus sp. N strain associated with a novel rhabditid entomopathogenic nematode. Lett. Appl. Microbiol. 2012, 54, 410–417. [Google Scholar] [CrossRef]
- Shi, J.; Zeng, Q.; Liu, Y.; Pan, Z. Alternaria sp. MG1, a resveratrol-producing fungus: Isolation, identification, and optimal cultivation conditions for resveratrol production. Appl. Microbiol. Biotechnol. 2012, 95, 369–379. [Google Scholar] [CrossRef]
- Wang, J.; Cox, D.G.; Ding, W.; Huang, G.; Lin, Y.; Li, C. Three new resveratrol derivatives from the mangrove endophytic fungus Alternaria sp. Marine Drugs 2014, 12, 2840–2850. [Google Scholar] [CrossRef]
- Dubrovina, A.S.; Kiselev, K.V. Regulation of stilbene biosynthesis in plants. Planta 2017, 246, 597–623. [Google Scholar] [CrossRef]
- Tian, B.; Liu, J. Resveratrol: A review of plant sources, synthesis, stability, modification and food application. J. Sci. Food Agric. 2020, 100, 1392–1404. [Google Scholar] [CrossRef]
- Mattio, L.M.; Catinella, G.; Dallavalle, S.; Pinto, A. Stilbenoids: A natural arsenal against bacterial pathogens. Antibiotics 2020, 9, 336. [Google Scholar] [CrossRef]
- Rimando, A.M.; Kalt, W.; Magee, J.B.; Dewey, J.; Ballington, J.R. Resveratrol, pterostilbene, and piceatannol in vaccinium berries. J. Agric. Food Chem. 2004, 52, 4713–4719. [Google Scholar] [CrossRef]
- Guebailia, H.A.; Chira, K.; Richard, T.; Mabrouk, T.; Furiga, A.; Vitrac, X.; Monti, J.P.; Delaunay, J.C.; Mérillon, J.P. Hopeaphenol: The first resveratrol tetramer in wines from North Africa. J. Agric. Food Chem. 2006, 54, 9559–9564. [Google Scholar] [CrossRef] [PubMed]
- Moss, R.; Mao, Q.; Taylor, D.; Saucier, C. Investigation of monomeric and oligomeric wine stilbenoids in red wines by ultra-high-performance liquid chromatography/electrospray ionization quadrupole time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2013, 27, 1815–1827. [Google Scholar] [CrossRef] [PubMed]
- Arraki, K.; Renouf, É.; Waffo-Téguo, P.; Mérillon, J.M.; Richard, T.; Decendit, A. Identification and quantification of stilbenes in some Tunisian red wines using UPLC-MS and HPLC-DAD. OENO One 2017, 51, 231–236. [Google Scholar] [CrossRef]
- Oh, W.Y.; Gao, Y.; Shahidi, F. Stilbenoids: Chemistry, occurrence, bioavailability and health effects-a review. J. Food Bioact. 2021, 13, 20–31. [Google Scholar] [CrossRef]
- Hădărugă, N.G.; Hădărugă, D.I. Stilbenes and its derivatives and glycosides. In Handbook of Food Bioactive Ingredients; Jafari, S.M., Rashidinejad, A., Simal-Gandara, J., Eds.; Springer Nature: Cham, Switzerland, 2023; pp. 487–544. [Google Scholar]
- Ziaullah; Rupasinghe, H.P.V. Application of nmr spectroscopy in plant polyphenols associated with human health. In Applications of NMR Spectroscopy; Rahman, A., Choudhary, M.I., Eds.; Bentham Science Publishers: Sharjah, United Arab Emirates, 2015; pp. 3–92. [Google Scholar]
- Duta-Bratu, C.G.; Nitulescu, G.M.; Mihai, D.P.; Olaru, O.T. Resveratrol and other natural oligomeric stilbenoid compounds and their therapeutic applications. Plants 2023, 12, 2935. [Google Scholar] [CrossRef]
- Niesen, D.B.; Hessler, C.; Seeram, N.P. Beyond resveratrol: A review of natural stilbenoids identified from 2009–2013. J. Berry Res. 2013, 3, 181–196. [Google Scholar] [CrossRef]
- Solladié, G.; Pasturel-Jacopé, Y.; Maignan, J. A re-investigation of resveratrol synthesis by Perkins reaction. Application to the synthesis of aryl cinnamic acids. Tetrahedron 2003, 59, 3315–3321. [Google Scholar] [CrossRef]
- Saraswati, S.V.; Thomas, N.F.; Weber, J.F. Strategies and methods for the syntheses of natural oligomeric stilbenoids and analogues. Curr. Org. Chem. 2012, 16, 605–662. [Google Scholar] [CrossRef]
- Mei, Y.Z.; Liu, R.X.; Wang, D.P.; Wang, X.; Dai, C.C. Biocatalysis and biotransformation of resveratrol in microorganisms. Biotechnol. Lett. 2015, 37, 9–18. [Google Scholar] [CrossRef]
- Mendonça, E.L.S.S.; Xavier, J.A.; Fragoso, M.B.T.; Silva, M.O.; Escodro, P.B.; Oliveira, A.C.M.; Tucci, P.; Saso, L.; Goulart, M.O.F. E-Stilbenes: General chemical and biological aspects, potential pharmacological activity based on the Nrf2 pathway. Pharmaceuticals 2024, 17, 232. [Google Scholar] [CrossRef]
- Bo, S.; Chang, S.K.; Zhu, H.; Jiang, Y.; Yang, B. Naturally occurring prenylated stilbenoids: Food sources, biosynthesis, applications and health benefits. Crit. Rev. Food Sci. Nutr. 2023, 63, 8083–8106. [Google Scholar] [CrossRef] [PubMed]
- Sobolev, V.S.; Neff, S.A.; Gloer, J.B. New dimeric stilbenoids from fungal-challenged peanut (Arachis hypogaea) seeds. J. Agric. Food Chem. 2010, 58, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Sobolev, V.S. Production of phytoalexins in peanut (Arachis hypogaea) seed elicited by selected microorganisms. J. Agric. Food Chem. 2013, 61, 1850–1858. [Google Scholar] [CrossRef]
- Sobolev, V.S.; Krausert, N.M.; Gloer, J.B. New monomeric stilbenoids from peanut (Arachis hypogaea) seeds challenged by an Aspergillus flavus Strain. J. Agric. Food Chem. 2016, 64, 579–584. [Google Scholar] [CrossRef]
- De Bruijn, W.J.C.; Araya-Cloutier, C.; Bijlsma, J.; de Swart, A.; Sanders, M.G.; de Waard, P.; Gruppen, H.; Vincken, J.P. Antibacterial prenylated stilbenoids from peanut (Arachis hypogaea). Phytoche. Lett. 2018, 28, 13–18. [Google Scholar] [CrossRef]
- Boonlaksiri, C.; Oonanant, W.; Kongsaeree, P.; Kittakoop, P.; Tanticharoen, M.; Thebtaranonth, Y. An antimalarial stilbene from Artocarpus integer. Phytochemistry 2000, 54, 415–417. [Google Scholar] [CrossRef]
- Hakim, E.H.; Ulinnuha, U.Z.; Syah, Y.M.; Ghisalberti, E.L. Artoindonesianins N and O, new prenylated stilbene and prenylated arylbenzofuran derivatives from Artocarpus gomezianus. Fitoterapia 2002, 73, 597–603. [Google Scholar] [CrossRef]
- Jayasinghe, L.; Rupasinghe, G.K.; Hara, N.; Fujimoto, Y. Geranylated phenolic constituents from the fruits of Artocarpus nobilis. Phytochemistry 2006, 67, 1353–1358. [Google Scholar] [CrossRef]
- Hošek, J.; Leláková, V.; Bobál, P.; Pížová, H.; Gazdová, M.; Malaník, M.; Jakubczyk, K.; Veselý, O.; Landa, P.; Temml, V.; et al. Prenylated stilbenoids affect inflammation by inhibiting the NF-κB/AP-1 signaling pathway and cyclooxygenases and lipoxygenase. J. Nat. Prod. 2019, 82, 1839–1848. [Google Scholar] [CrossRef]
- Ioset, J.R.; Marston, A.; Gupta, M.P.; Hostettmann, K. Five new prenylated stilbenes from the root bark of Lonchocarpus chiricanus. J. Nat. Prod. 2001, 64, 710–715. [Google Scholar] [CrossRef]
- Mabry, T.J. Selected topics from forty years of natural products research: Betalains to flavonoids, antiviral proteins, and neurotoxic nonprotein amino acids. J. Nat. Prod. 2001, 64, 1596–1604. [Google Scholar] [CrossRef] [PubMed]
- Sung, S.H.; Kim, E.S.; Lee, K.Y.; Lee, M.K.; Kim, Y.C. A new neuroprotective compound of Ligustrum japonicum leaves. Planta Med. 2006, 72, 62–64. [Google Scholar] [CrossRef] [PubMed]
- Van Der Kaaden, J.E.; Hemscheidt, T.K.; Mooberry, S.L. Mappain, a new cytotoxic prenylated stilbene from Macaranga mappa. J. Nat. Prod. 2001, 64, 103–105. [Google Scholar] [CrossRef]
- Beutler, J.A.; Shoemaker, R.H.; Johnson, T.; Boyd, M.R. Cytotoxic geranyl stilbenes from Macaranga schweinfurthii. J. Nat. Prod. 1998, 61, 1509–1512. [Google Scholar] [CrossRef]
- Beutler, J.A.; Jato, J.; Cragg, G.M.; Boyd, M.R. Schweinfurthin D, a cytotoxic stilbene from Macaranga schweinfurthii. Nat. Prod. Lett. 2000, 14, 399–404. [Google Scholar] [CrossRef]
- Pacher, T.; Seger, C.; Engelmeier, D.; Vajrodaya, S.; Hofer, O.; Greger, H. Antifungal stilbenoids from Stemona collinsae. J. Nat. Prod. 2002, 65, 820–827. [Google Scholar] [CrossRef]
- Kyekyeku, J.O.; Kusari, S.; Adosraku, R.K.; Zühlke, S.; Spiteller, M. Prenylated 2-arylbenzofuran derivatives with potent antioxidant properties from Chlorophora regia (Moraceae). Fitoterapia 2016, 108, 41–47. [Google Scholar] [CrossRef]
- Thuy, P.T.; Van Trang, N.; Son, N.T. Antioxidation of 2-phenylbenzofuran derivatives: Structural-electronic effects and mechanisms. RSC Adv. 2020, 10, 6315–6332. [Google Scholar] [CrossRef]
- Xiao, K.; Zhang, H.J.; Xuan, L.J.; Zhang, J.; Xu, Y.M.; Bai, D.L. Stilbenoids: Chemistry and bioactivities. In Studies in Natural Products Chemistry; Rahman, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2008; Volume 34, pp. 453–646. [Google Scholar]
- Shen, T.; Xie, C.; Wang, X.; Lou, H.X. Stilbenoids. In Natural Products; Ramawat, K.G., Mérillon, J.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1901–1949. [Google Scholar] [CrossRef]
- Gabaston, J.; Valls Fonayet, J.; Franc, C.; Waffo-Teguo, P.; de Revel, G.; Hilbert, G.; Gomès, E.; Richard, T.; Mérillon, J.M. Characterization of stilbene composition in grape berries from wild Vitis species in year-to-year harvest. J. Agric. Food Chem. 2020, 68, 13408–13417. [Google Scholar] [CrossRef]
- Shi, Y.; Zhao, X.; Wang, C.; Wang, Y.; Zhang, S.; Li, P.; Feng, X.; Jin, B.; Yuan, M.; Cui, S.; et al. Ultrafast nonadiabatic photoisomerization dynamics mechanism for the UV photoprotection of stilbenoids in grape skin. Chem. Asian J. 2020, 15, 1478–1483. [Google Scholar] [CrossRef]
- Cai, C.; Liu, M.; Yan, H.; Zhao, Y.; Shi, Y.; Guo, Q.; Pei, W.; Han, J.; Wang, Z. A combined calorimetric, spectroscopic and molecular dynamic simulation study on the inclusion complexation of (E)-piceatannol with hydroxypropyl-β-cyclodextrin in various alcohol + water cosolvents. J. Chem. Thermodyn. 2019, 132, 341–351. [Google Scholar] [CrossRef]
- He, J.; Zheng, Z.P.; Zhu, Q.; Guo, F.; Chen, J. Encapsulation mechanism of oxyresveratrol by β-cyclodextrin and hydroxypropyl-β-cyclodextrin and computational analysis. Molecules 2017, 22, 1801. [Google Scholar] [CrossRef] [PubMed]
- López-Nicolás, J.M.; Rodríguez-Bonilla, P.; García-Carmona, F. Complexation of pinosylvin, an analogue of resveratrol with high antifungal and antimicrobial activity, by different types of cyclodextrins. J. Agric. Food Chem. 2009, 57, 10175–10180. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Caruso, F.; Opazo, C.; Salciccioli, F. Crystal and molecular structure of piceatannol; scavenging features of resveratrol and piceatannol on hydroxyl and peroxyl radicals and docking with transthyretin. J. Agric. Food Chem. 2008, 56, 10557–10566. [Google Scholar] [CrossRef]
- Papuc, C.; Goran, G.V.; Predescu, C.N.; Nicorescu, V.; Stefan, G. Plant polyphenols as antioxidant and antibacterial agents for shelf-life extension of meat and meat products: Classification, structures, sources, and action mechanisms. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1243–1268. [Google Scholar] [CrossRef]
- Mattio, L.; Catinella, G.; Iriti, M.; Vallone, L. Inhibitory activity of stilbenes against filamentous fungi. Ital. J. Food Saf. 2021, 10, 8461. [Google Scholar] [CrossRef]
- De Filippis, B.; Ammazzalorso, A.; Fantacuzzi, M.; Giampietro, L.; Maccallini, C.; Amoroso, R. Anticancer activity of stilbene-based derivatives. ChemMedChem 2017, 12, 558–570. [Google Scholar] [CrossRef]
- Muller, A.G.; Sarker, S.D.; Saleem, I.Y.; Hutcheon, G.A. Delivery of natural phenolic compounds for the potential treatment of lung cancer. DARU J. Pharm. Sci. 2019, 27, 433–449. [Google Scholar] [CrossRef]
- Shimoda, K.; Kubota, N.; Uesugi, D.; Kobayashi, Y.; Hamada, H.; Hamada, H. Glycosylation of stilbene compounds by cultured plant cells. Molecules 2020, 25, 1437. [Google Scholar] [CrossRef]
- Valletta, A.; Iozia, L.M.; Leonelli, F. Impact of environmental factors on stilbene biosynthesis. Plants 2021, 10, 90. [Google Scholar] [CrossRef]
- Hoshino, Y.; Gaucher, E.A. On the origin of isoprenoid biosynthesis. Mol. Biol. Evol. 2018, 35, 2185–2197. [Google Scholar] [CrossRef] [PubMed]
- Lleó, A. Alzheimer’s disease: An ignored condition. Med. Clin. 2018, 150, 432–433. [Google Scholar] [CrossRef] [PubMed]
- Wanleenuwat, P.; Iwanowski, P.; Kozubski, W. Alzheimer’s dementia: Pathogenesis and impact of cardiovascular risk factors on cognitive decline. Postgrad. Med. 2019, 131, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Vermunt, L.; Sikkes, S.A.M.; van den Hout, A.; Handels, R.; Bos, I.; van der Flier, W.M.; Kern, S.; Ousset, P.J.; Maruff, P.; Skoog, I.; et al. Alzheimer Disease Neuroimaging Initiative; AIBL Research Group; ICTUS/DSA study groups. Duration of preclinical, prodromal, and dementia stages of Alzheimer’s disease in relation to age, sex, and APOE genotype. Alzheimer’s Dement. 2019, 15, 888–898. [Google Scholar] [CrossRef]
- Takizawa, C.; Thompson, P.L.; van Walsem, A.; Faure, C.; Maier, W.C. Epidemiological and economic burden of Alzheimer’s disease: A systematic literature review of data across Europe and the United States of America. J. Alzheimer’s Dis. 2015, 43, 1271–1284. [Google Scholar] [CrossRef]
- Tiwari, S.; Atluri, V.; Kaushik, A.; Yndart, A.; Nair, M. Alzheimer’s disease: Pathogenesis, diagnostics, and therapeutics. Int. J. Nanomed. 2019, 14, 5541–5554. [Google Scholar] [CrossRef]
- Prince, M.; Bryce, R.; Albanese, E.; Wimo, A.; Ribeiro, W.; Ferri, C.P. The global prevalence of dementia: A systematic review and metaanalysis. Alzheimer’s Dement. 2013, 9, 63–75. [Google Scholar] [CrossRef]
- Niu, H.; Álvarez-Álvarez, I.; Guillén-Grima, F.; Aguinaga-Ontoso, I. Prevalence and incidence of Alzheimer’s disease in Europe: A meta-analysis. Neurologia 2017, 32, 523–532. [Google Scholar] [CrossRef]
- Jordan, B. Life expectancy curves reveal major demographic events. Med. Sci. 2017, 33, 355–362. [Google Scholar] [CrossRef][Green Version]
- Garre-Olmo, J. Epidemiology of Alzheimer’s disease and other dementias. Rev. Neurol. 2018, 66, 377–386. [Google Scholar] [CrossRef]
- García-Morales, V.; González-Acedo, A.; Melguizo-Rodríguez, L.; Pardo-Moreno, T.; Costela-Ruiz, V.J.; Montiel-Troya, M.; Ramos-Rodríguez, J.J. Current understanding of the physiopathology, diagnosis and therapeutic approach to Alzheimer’s disease. Biomedicines 2021, 9, 1910. [Google Scholar] [CrossRef] [PubMed]
- Shieh, J.C.; Huang, P.T.; Lin, Y.F. Alzheimer’s disease and diabetes: Insulin signaling as the bridge linking two pathologies. Mol. Neurobiol. 2020, 57, 1966–1977. [Google Scholar] [CrossRef] [PubMed]
- St George-Hyslop, P.H.; Tanzi, R.E.; Polinsky, R.J.; Haines, J.L.; Nee, L.; Watkins, P.C.; Myers, R.H.; Feldman, R.G.; Pollen, D.; Drachman, D.; et al. The genetic defect causing familial Alzheimer’s disease maps on chromosome 21. Science 1987, 235, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Yamada, M. Risk factors for Alzheimer’s disease. Brain Nerve 2010, 62, 679–690. [Google Scholar] [CrossRef]
- De Leeuw, F.E.; de Groot, J.C.; Oudkerk, M.; Witteman, J.C.; Hofman, A.; van Gijn, J.; Breteler, M.M.B. Hypertension and cerebral white matter lesions in a prospective cohort study. Brain 2002, 125, 765–772. [Google Scholar] [CrossRef]
- Reitz, C.; Tang, M.X.; Manly, J.; Mayeux, R.; Luchsinger, J.A. Hypertension and the risk of mild cognitive impairment. Arch. Neurol. 2007, 64, 1734–1740. [Google Scholar] [CrossRef]
- Arvanitakis, Z.; Schneider, J.A.; Wilson, R.S.; Bienias, J.L.; Kelly, J.F.; Evans, D.A.; Bennett, D.A. Statins, incident Alzheimer disease, change in cognitive function, and neuropathology. Neurology 2008, 70 Pt. 2, 1795–1802. [Google Scholar] [CrossRef]
- Sensi, S.L. Alzheimer’s Disease, time to turn the tide. Aging 2018, 10, 2537–2538. [Google Scholar] [CrossRef]
- Infante-Garcia, C.; Ramos-Rodriguez, J.J.; Galindo-Gonzalez, L.; Garcia-Alloza, M. Long-term central pathology and cognitive impairment are exacerbated in a mixed model of Alzheimer’s disease and type 2 diabetes. Psychoneuroendocrinology 2016, 65, 15–25. [Google Scholar] [CrossRef]
- Kumar Thakur, A.; Kamboj, P.; Goswami, K.; Ahuja, K. Pathophysiology and management of Alzheimer’s disease: An overview. J. Analyt. Pharm. Res. 2018, 9, 226–235. [Google Scholar] [CrossRef]
- Abubakar, M.B.; Sanusi, K.O.; Ugusman, A.; Mohamed, W.; Kamal, H.; Ibrahim, N.H.; Khoo, C.S.; Kumar, J. Alzheimer’s disease: An update and insights into pathophysiology. Front. Aging Neurosci. 2022, 14, 742408. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.N.; Maass, A.; Harrison, T.M.; Baker, S.L.; Jagust, W.J. Cortical tau deposition follows patterns of entorhinal functional connectivity in aging. eLife 2019, 8, e49132. [Google Scholar] [CrossRef]
- Jagust, W. Imaging the evolution and pathophysiology of Alzheimer disease. Nat. Rev. Neurosci. 2018, 19, 687–700. [Google Scholar] [CrossRef]
- Shah, S.; Lee, S.F.; Tabuchi, K.; Hao, Y.H.; Yu, C.; LaPlant, Q.; Ball, H.; Dann III, C.E.; Südhof, T.; Yu, G. Nicastrin functions as a gamma-secretase-substrate receptor. Cell 2005, 122, 435–447. [Google Scholar] [CrossRef]
- De-Paula, V.J.; Radanovic, M.; Diniz, B.S.; Forlenza, O.V. Alzheimer’s disease. In Protein Aggregation and Fibrillogenesis in Cerebral and Systemickura Amyloid Disease; Harris, J.R., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 329–352. [Google Scholar]
- Staderini, M.; Martín, M.A.; Bolognesi, M.L.; Menéndez, J.C. Imaging of β-amyloid plaques by near infrared fluorescent tracers: A new frontier for chemical neuroscience. Chem. Soc. Rev. 2015, 44, 1807–1819. [Google Scholar] [CrossRef]
- Serrano-Pozo, A.; Frosch, M.P.; Masliah, E.; Hyman, B.T. Neuropathological alterations in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2011, 1, a006189. [Google Scholar] [CrossRef]
- Takahashi, R.H.; Nagao, T.; Gouras, G.K. Plaque formation and the intraneuronal accumulation of β-amyloid in Alzheimer’s disease. Pathol. Int. 2017, 67, 185–193. [Google Scholar] [CrossRef]
- Ding, Y.; Zhao, J.; Zhang, X.; Wang, S.; Viola, K.L.; Chow, F.E.; Zhang, Y.; Lippa, C.; Klein, W.L.; Gong, Y. Amyloid beta oligomers target to extracellular and intracellular neuronal synaptic proteins in Alzheimer’s disease. Front. Neurol. 2019, 10, 1140. [Google Scholar] [CrossRef]
- Cheng, X.; Wu, J.; Geng, M.; Xiong, J. Role of synaptic activity in the regulation of amyloid beta levels in Alzheimer’s disease. Neurobiol. Aging 2014, 35, 1217–1232. [Google Scholar] [CrossRef]
- Spires-Jones, T.L.; Hyman, B.T. The intersection of amyloid beta and tau at synapses in Alzheimer’s disease. Neuron 2014, 82, 756–771. [Google Scholar] [CrossRef]
- De Wilde, M.C.; Overk, C.R.; Sijben, J.W.; Masliah, E. Meta-analysis of synaptic pathology in Alzheimer’s disease reveals selective molecular vesicular machinery vulnerability. Alzheimer’s Dement. 2016, 12, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Urbanc, B.; Cruz, L.; Le, R.; Sanders, J.; Ashe, K.H.; Duff, K.; Stanley, H.E.; Irizarry, M.C.; Hyman, B.T. Neurotoxic effects of thioflavin S-positive amyloid deposits in transgenic mice and Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2002, 99, 13990–13995. [Google Scholar] [CrossRef]
- Iaccarino, L.; Tammewar, G.; Ayakta, N.; Baker, S.L.; Bejanin, A.; Boxer, A.L.; Gorno-Tempini, M.L.; Janabi, M.; Kramer, J.H.; Lazaris, A.; et al. Local and distant relationships between amyloid, tau and neurodegeneration in Alzheimer’s disease. Neuroimage. Clin. 2017, 17, 452–464. [Google Scholar] [CrossRef]
- Greenberg, S.M.; Bacskai, B.J.; Hernandez-Guillamon, M.; Pruzin, J.; Sperling, R.; van Veluw, S.J. Cerebral amyloid angiopathy and Alzheimer disease—One peptide, two pathways. Nat. Rev. Neurol 2020, 16, 30–42. [Google Scholar] [CrossRef]
- Chantran, Y.; Capron, J.; Alamowitch, S.; Aucouturier, P. Anti-Aβ antibodies and cerebral amyloid angiopathy complications. Front. Immunol. 2019, 10, 1534. [Google Scholar] [CrossRef]
- Vázquez-Costa, J.F.; Baquero-Toledo, M.; Sastre-Bataller, I.; Mas-Estellés, F.; Vílchez-Padilla, J.J. Inflammatory amyloid angiopathy. Neurologia 2014, 29, 254–256. [Google Scholar] [CrossRef]
- Van Veluw, S.J.; Reijmer, Y.D.; van der Kouwe, A.J.; Charidimou, A.; Riley, G.A.; Leemans, A.; Bacskai, B.J.; Frosch, M.P.; Viswanathan, A.; Greenberg, S.M. Histopathology of diffusion imaging abnormalities in cerebral amyloid angiopathy. Neurology 2019, 92, e933–e943. [Google Scholar] [CrossRef]
- Nalivaeva, N.N.; Belyaev, N.D.; Zhuravin, I.A.; Turner, A.J. The Alzheimer’s amyloid-degrading peptidase, neprilysin: Can we control it? Int. J. Alzheimers Dis. 2012, 2012, 383796. [Google Scholar] [CrossRef]
- Klein, C.; Patte-Mensah, C.; Taleb, O.; Bourguignon, J.J.; Schmitt, M.; Bihel, F.; Maitre, M.; Mensah-Nyagan, A.G. The neuroprotector kynurenic acid increases neuronal cell survival through neprilysin induction. Neuropharmacology 2013, 70, 254–260. [Google Scholar] [CrossRef]
- Toombs, J.; Zetterberg, H. Untangling the tau microtubule-binding region. Brain 2021, 144, 359–362. [Google Scholar] [CrossRef]
- Avila, J. Tau phosphorylation and aggregation in Alzheimer’s disease pathology. FEBS Lett. 2006, 580, 2922–2927. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.X.; Grundke-Iqbal, I.; Iqbal, K. Targeting tau protein in Alzheimer’s disease. Drugs Aging 2010, 27, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Woodhouse, A.; West, A.K.; Chuckowree, J.A.; Vickers, J.C.; Dickson, T.C. Does beta-amyloid plaque formation cause structural injury to neuronal processes? Neurotox. Res. 2005, 7, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Calsolaro, V.; Edison, P. Neuroinflammation in Alzheimer’s disease: Current evidence and future directions. Alzheimer’s Dement. 2016, 12, 719–732. [Google Scholar] [CrossRef]
- Lyman, M.; Lloyd, D.G.; Ji, X.; Vizcaychipi, M.P.; Ma, D. Neuroinflammation: The role and consequences. Neurosci. Res. 2014, 79, 1–12. [Google Scholar] [CrossRef]
- Wes, P.D.; Sayed, F.A.; Bard, F.; Gan, L. Targeting microglia for the treatment of Alzheimer’s disease. Glia 2016, 64, 1710–1732. [Google Scholar] [CrossRef]
- Hierro-Bujalance, C.; Bacskai, B.J.; Garcia-Alloza, M. In vivo imaging of microglia with multiphoton microscopy. Front. Aging Neurosci. 2018, 10, 218. [Google Scholar] [CrossRef]
- Sankar, S.B.; Infante-Garcia, C.; Weinstock, L.D.; Ramos-Rodriguez, J.J.; Hierro-Bujalance, C.; Fernandez-Ponce, C.; Wood, L.B.; Garcia-Alloza, M. Amyloid beta and diabetic pathology cooperatively stimulate cytokine expression in an Alzheimer’s mouse model. J. Neuroinflamm. 2020, 17, 38. [Google Scholar] [CrossRef]
- Hickman, S.E.; Allison, E.K.; El Khoury, J. Microglial dysfunction and defective beta-amyloid clearance pathways in aging Alzheimer’s disease mice. J. Neurosci. 2008, 28, 8354–8360. [Google Scholar] [CrossRef]
- Ionescu-Tucker, A.; Cotman, C.W. Emerging roles of oxidative stress in brain aging and Alzheimer’s disease. Neurobiol. Aging 2021, 107, 86–95. [Google Scholar] [CrossRef]
- Kim, T.S.; Pae, C.U.; Yoon, S.J.; Jang, W.Y.; Lee, N.J.; Kim, J.J.; Lee, S.J.; Lee, C.; Paik, I.H.; Lee, C.U. Decreased plasma antioxidants in patients with Alzheimer’s disease. Int. J. Geriatr. Psychiatry 2006, 21, 344–348. [Google Scholar] [CrossRef]
- Williamson, J.D.; Pajewski, N.M.; Auchus, A.P.; Bryan, R.N.; Chelune, G.; Cheung, A.K. Effect of intensive vs standard blood pressure control on probable dementia: A randomized clinical trial. JAMA 2019, 321, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Andrieu, S.; Guyonnet, S.; Coley, N.; Cantet, C.; Bonnefoy, M.; Bordes, S.; Bories, L.; Cufi, M.-N.; Dantoine, T. Effect of long-term omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): A randomised, placebo controlled trial. Lancet Neurol. 2017, 16, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Delrieu, J.; Payoux, P.; Carrié, I.; Cantet, C.; Weiner, M.; Vellas, B.; Andrieu, S. Multidomain intervention and/or omega-3 in nondemented elderly subjects according to amyloid status. Alzheimers Dement. 2019, 15, 1392–1401. [Google Scholar] [CrossRef]
- Moll van Charante, E.P.; Richard, E.; Eurelings, L.S.; van Dalen, J.W.; Ligthart, S.A.; van Bussel, E.F.; Hoevenaar-Blom, M.P.; Vermeulen, M.; van Gool, W.A. Effectiveness of a 6-year multidomain vascular care intervention to prevent dementia (preDIVA): A cluster-randomised controlled trial. Lancet 2016, 388, 797–805. [Google Scholar] [CrossRef]
- Ngandu, T.; Lehtisalo, J.; Solomon, A.; Levälahti, E.; Ahtiluoto, S.; Antikainen, R.; Backman, L.; Hanninen, T.; Jula, A.; Laatikainen, T. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): A randomised controlled trial. Lancet 2015, 385, 2255–2263. [Google Scholar] [CrossRef]
- Scheltens, P.; De Stroope, B.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef]
- Colin, M.; Dujardin, S.; Schraen-Maschke, S.; Meno-Tetang, G.; Duyckaerts, C.; Courade, J.P.; Buee, L. From the prion-like propagation hypothesis to therapeutic strategies of anti-tau immunotherapy. Acta Neuropathol. 2020, 139, 3–25. [Google Scholar] [CrossRef]
- Tolar, M.; Abushakra, S.; Hey, J.A.; Porsteinsson, A.; Sabbagh, M. Aducanumab, gantenerumab, BAN2401, and ALZ-801-the frst wave of amyloid-targeting drugs for Alzheimer’s disease with potential for nar term approval. Alzheimers Res. Ther. 2020, 12, 95. [Google Scholar] [CrossRef]
- Panza, F.; Lozupone, M.; Logroscino, G.; Imbimbo, B.P. A critical appraisal of amyloid-β-targeting therapies for Alzheimer diease. Nat. Rev. Neurol. 2019, 15, 73–88. [Google Scholar] [CrossRef]
- Shi, M.; Chu, F.; Zhu, F.; Zhu, J. Impact of anti-amyloid-β monoclonal antibodies on the pathology and clinical profile of Alzeimer’s disease: A focus on aducanumab and lecanemab. Front. Aging Neurosci. 2022, 14, 870517. [Google Scholar] [CrossRef] [PubMed]
- Olivares, D.; Deshpande, V.K.; Shi, Y.; Lahiri, D.K.; Greig, N.H.; Rogers, J.T.; Huang, X. N-methyl D-aspartate (NMDA) receptor antagonists and memantine treatment for Alzheimer’s disease, vascular dementia and Parkinson’s disease. Curr. Alzheimer Res. 2012, 9, 746–758. [Google Scholar] [CrossRef] [PubMed]
- Sevigny, J.; Chiao, P.; Bussière, T.; Weinreb, P.H.; Williams, L.; Maier, M.; Dunstan, R.; Salloway, S.; Chen, T.; Ling, Y.; et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature 2016, 537, 50–56. [Google Scholar] [CrossRef]
- Illes-Toth, E.; Rempel, D.L.; Gross, M.L. Exploration of resveratrol as a potent modulator of α-synuclein fibril formation. ACS. Chem. Neurosci. 2024, 15, 503–516. [Google Scholar] [CrossRef]
- Regitz, C.; Fitzenberger, E.; Mahn, F.L.; Dußling, L.M.; Wenzel, U. Resveratrol reduces amyloid-beta (Aβ1–42)-induced paralysis through targeting proteostasis in an Alzheimer model of Caenorhabditis elegans. Eur. J. Nutr. 2016, 55, 741–747. [Google Scholar] [CrossRef]
- Naslund, J.; Karlstrom, A.R.; Tjernberg, L.O.; Schierhorn, A.; Terenius, L.; Nordstedt, C. High-resolution separation of amyloid beta-peptides: Structural variants present in Alzheimer’s disease amyloid. J. Neurochem. 1996, 67, 294–301. [Google Scholar] [CrossRef]
- Choi, C.W.; Choi, Y.H.; Cha, M.R.; Kim, Y.S.; Yon, G.H.; Hong, K.S.; Park, W.K.; Kim, Y.H.; Ryu, S.Y. In vitro BACE-1 inhibitory activity of resveratrol oligomers from the seed extract of Paeonia lactiflora. Planta Med. 2011, 77, 374–376. [Google Scholar] [CrossRef]
- Koukoulitsa, C.; Villalonga-Barber, C.; Csonka, R.; Alexi, X.; Leonis, G.; Dellis, D.; Hamelink, E.; Belda, O.; Steele, B.R.; Micha-Screttas, M.; et al. Biological and computational evaluation of resveratrol inhibitors against Alzheimer’s disease. J. Enzyme Inhib. Med. Chem. 2016, 31, 67–77. [Google Scholar] [CrossRef]
- Guo, J.P.; Yu, S.; McGeer, P.L. Simple in vitro assays to identify amyloid-β aggregation blockers for Alzheimer’s disease therapy. J. Alzheimer’s Dis. 2010, 19, 1359–1370. [Google Scholar] [CrossRef]
- Porquet, D.; Casadesús, G.; Bayod, S.; Vicente, A.; Canudas, A.M.; Vilaplana, J.; Pelegrí, C.; Sanfeliu, C.; Camins, A.; Pallàs, M.; et al. Dietary resveratrol prevents Alzheimer’s markers and increases life span in SAMP8. Age 2013, 35, 1851–1865. [Google Scholar] [CrossRef]
- Kim, D.; Nguyen, M.D.; Dobbin, M.M.; Fischer, A.; Sananbenesi, F.; Rodgers, J.T.; Delalle, I.; Baur, J.A.; Sui, G.; Armour, S.M.; et al. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis. EMBO J. 2007, 26, 3169–3179. [Google Scholar] [CrossRef] [PubMed]
- Porquet, D.; Griñán-Ferré, C.; Ferrer, I.; Camins, A.; Sanfeliu, C.; Del Valle, J.; Pallàs, M. Neuroprotective role of trans-resveratrol in a murine model of familial Alzheimer’s disease. J. Alzheimers Dis. 2014, 42, 1209–1220. [Google Scholar] [CrossRef]
- Cohen, H.Y.; Miller, C.; Bitterman, K.J.; Wall, N.R.; Hekking, B.; Kessler, B.; Howitz, K.T.; Gorospe, M.; de Cabo, R.; Sinclair, D.A. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science 2004, 305, 390–392. [Google Scholar] [CrossRef]
- Baur, J.A.; Pearson, K.J.; Price, N.L.; Jamieson, H.A.; Lerin, C.; Kalra, A.; Prabhu, V.V.; Allard, J.S.; Lopez-Lluch, G.; Lewis, K.; et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006, 444, 337–342. [Google Scholar] [CrossRef]
- Park, H.; Kam, T.I.; Kim, Y.; Choi, H.; Gwon, Y.; Kim, C.; Koh, J.Y.; Jung, Y.K. Neuropathogenic role of adenylate kinase-1 in Aβ-mediated tau phosphorylation via AMPK and GSK3β. Hum. Mol. Genet. 2012, 21, 2725–2737. [Google Scholar] [CrossRef]
- Park, S.J.; Ahmad, F.; Philp, A.; Baar, K.; Williams, T.; Luo, H.; Ke, H.; Rehmann, H.; Taussig, R.; Brown, A.L.; et al. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell 2012, 148, 421–433. [Google Scholar] [CrossRef]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Tang, L.; Zhang, N.; Fan, D. Protective effects of resveratrol through the up-regulation of SIRT1 expression in the mutant hSOD1-G93A-bearing motor neuron-like cell culture model of amyotrophic lateral sclerosis. Neurosci. Lett. 2011, 503, 250–255. [Google Scholar] [CrossRef]
- Price, N.L.; Gomes, A.P.; Ling, A.J.; Duarte, F.V.; Martin-Montalvo, A.; North, B.J.; Agarwal, B.; Ye, L.; Ramadori, G.; Teodoro, J.S.; et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012, 15, 675–690. [Google Scholar] [CrossRef]
- Du, L.L.; Xie, J.Z.; Cheng, X.S.; Li, X.H.; Kong, F.L.; Jiang, X.; Ma, Z.W.; Wang, J.Z.; Chen, C.; Zhou, X.W. Activation of sirtuin 1 attenuates cerebral ventricular streptozotocin-induced tau hyperphosphorylation and cognitive injuries in rat hippocampi. Age 2014, 36, 613–623. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, Y.; Li, J.; Zhang, C. Resveratrol ameliorates spatial learning memory impairment induced by Aβ(1-42) in rats. Neuroscience 2017, 344, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, B.; Milbrandt, J. Resveratrol stimulates AMP kinase activity in neurons. Proc. Natl. Acad. Sci. USA 2007, 104, 7217–7222. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Luo, A.; Gao, J.; Tang, X.; Zhao, Y.; Zhou, B.; Zhou, Z.; Li, S. The role of SIRT1 in neuroinflammation and cognitive dysfunction in aged rats after anesthesia and surgery. Am. J. Transl. Res. 2019, 11, 1555–1568. [Google Scholar]
- Li, L.; Sun, Q.; Li, Y.; Yang, Y.; Yang, Y.; Chang, T.; Man, M.; Zheng, L. Overexpression of SIRT1 induced by resveratrol and inhibitor of miR-204 suppresses activation and proliferation of microglia. J. Mol. Neurosci. 2015, 56, 858–867. [Google Scholar] [CrossRef]
- Howitz, K.T.; Bitterman, K.J.; Cohen, H.Y. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 2003, 425, 191–196. [Google Scholar] [CrossRef]
- Melzig, M.F.; Escher, F. Induction of neutral endopeptidase and angiotensin-converting enzyme activity of SK-N-SH cells in vitro by quercetin and resveratrol. Pharmazie 2002, 57, 556–558. [Google Scholar]
- Coppa, T.; Lazze, M.C.; Cazzalini, O.; Perucca, P.; Pizzala, R.; Bianchi, L.; Stivala, L.A.; Forti, L.; Maccario, C.; Vannini, V.; et al. Structure-activity relationship of resveratrol and its analogue, 4,4’-dihydroxy-trans-stilbene, toward the endothelin axis in human endothelial cells. J. Med. Food 2011, 14, 1173–1180. [Google Scholar] [CrossRef]
- El-Sayed, N.S.; Bayan, Y. Possible role of resveratrol targeting estradiol and neprilysin pathways in lipopolysaccharide model of Alzheimer disease. Adv. Exp. Med. Biol. 2015, 822, 107–118. [Google Scholar] [CrossRef]
- Melchor, J.P.; Pawlak, R.; Strickland, S. The tissue plasminogen activator-plasminogen proteolytic cascade accelerates amyloid-β (Aβ) degradation and inhibits Aβ-induced neurodegeneration. J. Neurosci. 2003, 23, 8867–8871. [Google Scholar] [CrossRef]
- Abou-Agag, L.H.; Aikens, M.L.; Tabengwa, E.M.; Benza, R.L.; Shows, S.R.; Grenett, H.E.; Booyse, F.M. Polyphyenolics increase t-PA and u-PA gene transcription in cultured human endothelial cells. Alcohol. Clin. Exp. Res. 2001, 25, 155–162. [Google Scholar] [CrossRef]
- Cherny, R.A.; Atwood, C.S.; Xilinas, M.E.; Gray, D.N.; Jones, W.D.; McLean, C.A.; Barnham, K.J.; Volitakis, I.; Fraser, F.W.; Kim, Y.; et al. Treatment with a copper-zinc chelator markedly and rapidly inhibits beta-amyloid accumulation in Alzheimer’s disease transgenic mice. Neuron 2001, 30, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Belguendouz, L.; Fremont, L.; Linard, A. Resveratrol inhibits metal ion-dependent and independent peroxidation of porcine low-density lipoproteins. Biochem. Pharmacol. 1997, 53, 1347–1355. [Google Scholar] [CrossRef] [PubMed]
- Frémont, L.; Belguendouz, L.; Delpal, S. Antioxidant activity of resveratrol and alcohol-free wine polyphenols related to LDL oxidation and polyunsaturated fatty acids. Life Sci. 1999, 64, 2511–2521. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.Y.; Kwon, S.H.; Seong, Y.H.; Bae, K.; Hur, J.M.; Lee, Y.Y.; Suh, D.Y.; Song, K.S. β-secretase (BACE1)-inhibiting stilbenoids from Smilax Rhizoma. Phytomedicine 2007, 14, 403–408. [Google Scholar] [CrossRef]
- Marambaud, P.; Zhao, H.; Davies, P. Resveratrol promotes clearance of Alzheimer’s disease amyloid-beta peptides. J. Biol. Chem. 2005, 280, 37377–37382. [Google Scholar] [CrossRef]
- Vingtdeux, V.; Giliberto, L.; Zhao, H.; Chandakkar, P.; Wu, Q.; Simon, J.E.; Janle, E.M.; Lobo, J.; Ferruzzi, M.G.; Davies, P.; et al. AMP-activated protein kinase signaling activation by resveratrol modulates amyloid-beta peptide metabolism. J. Biol. Chem. 2010, 285, 9100–9113. [Google Scholar] [CrossRef]
- Karuppagounder, S.S.; Pinto, J.T.; Xu, H.; Chen, H.L.; Beal, M.F.; Gibson, G.E. Dietary supplementation with resveratrol reduces plaque pathology in a transgenic model of Alzheimer’s disease. Neurochem. Int. 2009, 54, 111–118. [Google Scholar] [CrossRef]
- Wang, J.; Ho, L.; Zhao, Z.; Seror, I.; Humala, N.; Dickstein, D.L.; Thiyagarajan, M.; Percival, S.S.; Talcott, S.T.; Pasinetti, G.M. Moderate consumption of Cabernet Sauvignon attenuates Abeta neuropathology in a mouse model of Alzheimer’s disease. FASEB J. 2006, 20, 2313–2320. [Google Scholar] [CrossRef]
- Luchsinger, J.A.; Tang, M.X.; Siddiqui, M.; Shea, S.; Mayeux, R. Alcohol intake and risk of dementia. J. Am. Geriatr. Soc. 2004, 52, 540–546. [Google Scholar] [CrossRef]
- Ho, L.; Chen, L.H.; Wang, J.; Zhao, W.; Talcott, S.T.; Ono, K.; Teplow, D.; Humala, N.; Cheng, A.; Percival, S.S.; et al. Heterogeneity in red wine polyphenolic contents differentially influences Alzheimer’s disease-type neuropathology and cognitive deterioration. J. Alzheimers Dis. 2009, 16, 59–72. [Google Scholar] [CrossRef]
- Miyazawa, K.; Fukunaga, H.; Tatewaki, Y.; Takano, Y.; Yamamoto, S.; Mutoh, T.; Taki, Y. Alzheimer’s disease and specialized pro-resolving lipid mediators: Do MaR1, RvD1, and NPD1 show promise for prevention and treatment? Int. J. Mol. Sci. 2020, 21, 5783. [Google Scholar] [CrossRef] [PubMed]
- Conte, A.; Pellegrini, S.; Tagliazucchi, D. Synergistic protection of PC12 cells from beta-amyloid toxicity by resveratrol and catechin. Brain Res. Bull. 2003, 62, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.H.; Surh, Y.J. Protective effect of resveratrol on beta-amyloid-induced oxidative PC12 cell death. Free Rad. Biol. Med. 2003, 34, 1100–1110. [Google Scholar] [CrossRef] [PubMed]
- Misiti, F.; Sampaolese, B.; Mezzogori, D.; Orsini, F.; Pezzotti, M.; Giardina, B.; Clementi, M.E. Protective effect of rhubarb derivatives on amyloid beta (1–42) peptide-induced apoptosis in IMR-32 cells: A case of nutrigenomic. Brain Res. Bull. 2006, 71, 29–36. [Google Scholar] [CrossRef]
- Schweiger, S.; Matthes, F.; Posey, K.; Kickstein, E.; Weber, S.; Hettich, M.M.; Pfurtscheller, S.; Ehninger, D.; Schneider, R.; Krauß, S. Resveratrol induces dephosphorylation of Tau by interfering with the MID1-PP2A complex. Sci. Rep. 2017, 7, 13753. [Google Scholar] [CrossRef]
- Sun, J.; Wei, C.; Liu, Y.; Xie, W.; Xu, M.; Zhou, H.; Liu, J. Progressive release of mesoporous nano-selenium delivery system for the multi-channel synergistic treatment of Alzheimer’s disease. Biomaterials 2019, 197, 417–431. [Google Scholar] [CrossRef]
- Rao, Y.L.; Ganaraja, B.; Suresh, P.K.; Joy, T.; Ullal, S.D.; Manjrekar, P.A.; Murlimanju, B.V.; Gaurav Sharma, B.; Massand, A.; Agrawal, A. Outcome of resveratrol and resveratrol with donepezil combination on the β-amyloid plaques and neurofibrillary tangles in Alzheimer’s disease. 3 Biotech 2024, 14, 190. [Google Scholar] [CrossRef]
- Sharma, S.; Goyal, H.; Joshi, S.; Nehru, B.; Saini, A. Molecular interactions of resveratrol with Aβ 42 peptide and fibril during in-vitro Aβ 42 aggregation. Adv. Redox Res. 2023, 7, 100060. [Google Scholar] [CrossRef]
- Sie, Y.Y.; Chen, L.C.; Li, C.J.; Yuan, Y.H.; Hsiao, S.H.; Lee, M.H.; Wang, C.C.; Hou, W.C. Inhibition of acetylcholinesterase and amyloid-β aggregation by piceatannol and analogs: Assessing in vitro and in vivo impact on a murine model of scopolamine-induced memory impairment. Antioxidants 2023, 12, 1362. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, K.W.; Lee, H.J. Protective effects of piceatannol against beta-amyloid-induced neuronal cell death. Ann. N. Y. Acad. Sci. 2007, 1095, 473–482. [Google Scholar] [CrossRef]
- Choi, B.; Kim, S.; Jang, B.G.; Kim, M.J. Piceatannol, a natural analogue of resveratrol, effectively reduces beta-amyloid levels via activation of alpha-secretase and matrix metalloproteinase-9. J. Funct. Foods 2016, 23, 124–134. [Google Scholar] [CrossRef]
- Kawakami, S.; Kinoshita, Y.; Maruki-Uchida, H.; Yanae, K.; Sai, M.; Ito, T. Piceatannol and its metabolite, isorhapontigenin, induce SIRT1 expression in THP-1 human monocytic cell line. Nutrients 2014, 6, 4794–4804. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.J.; Zhang, W.Q.; Liu, J.J.; Cui, Y.; Cui, J.Z. Piceatannol protects against cerebral ischemia/reperfusion induced apoptosis and oxidative stress via the Sirt1/FoxO1 signaling pathway. Mol. Med. Rep. 2020, 22, 5399–5411. [Google Scholar] [CrossRef]
- Liu, J.; Mai, P.; Yang, Z.; Wang, Z.; Yang, W.; Wang, Z. Piceatannol protects PC-12 cells against oxidative damage and mitochondrial dysfunction by ınhibiting autophagy via SIRT3 pathway. Nutrients 2023, 15, 2973. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Preeti, K.; Kumar, R.; Kumar Khatri, D.; Bala Singh, S. Piceatannol promotes neuroprotection by inducing mitophagy and mitobiogenesis in the experimental diabetic peripheral neuropathy and hyperglycemia-induced neurotoxicity. Int. Immunopharmacol. 2023, 116, 109793. [Google Scholar] [CrossRef]
- Hosoda, R.; Hamada, H.; Uesugi, D.; Iwahara, N.; Nojima, I.; Horio, Y.; Kuno, A. Different antioxidative and antiapoptotic effects of piceatannol and resveratrol. J. Pharmacol. Exp. Ther. 2021, 376, 385–396. [Google Scholar] [CrossRef]
- Rivière, C.; Richard, T.; Quentin, L.; Krisa, S.; Mérillon, J.M.; Monti, J.P. Inhibitory activity of stilbenes on Alzheimer’s beta-amyloid fibrils in vitro. Bioorg. Med. Chem. 2007, 15, 1160–1167. [Google Scholar] [CrossRef]
- Dos Santos, L.C.; Mendiola, J.A.; Sánchez-Camargo, A.D.P.; Álvarez-Rivera, G.; Viganó, J.; Cifuentes, A.; Ibáñez, E.; Martínez, J. Selective extraction of piceatannol from Passiflora edulis by-products: Application of HSPs strategy and inhibition of neurodegenerative enzymes. Int. J. Mol. Sci. 2021, 22, 6248. [Google Scholar] [CrossRef]
- Sato, A.; Tagai, N.; Ogino, Y.; Uozumi, H.; Kawakami, S.; Yamamoto, T.; Tanuma, S.I.; Maruki-Uchida, H.; Mori, S.; Morita, M. Passion fruit seed extract protects beta-amyloid-induced neuronal cell death in a differentiated human neuroblastoma SH-SY5Y cell model. Food Sci. Nutr. 2022, 10, 1461–1468. [Google Scholar] [CrossRef]
- Fu, Z.; Yang, J.; Wei, Y.; Li, J. Effects of piceatannol and pterostilbene against β-amyloid-induced apoptosis on the PI3K/Akt/Bad signaling pathway in PC12 cells. Food Funct. 2016, 7, 1014–1023. [Google Scholar] [CrossRef]
- Wen, H.; Fu, Z.; Wei, Y.; Zhang, X.; Ma, L.; Gu, L.; Li, J. Antioxidant activity and neuroprotective activity of stilbenoids in rat primary cortex neurons via the PI3K/Akt signalling pathway. Molecules 2018, 23, 2328. [Google Scholar] [CrossRef]
- Xiong, L.; Xiang, D.; Yuan, F.; Tong, H.; Yang, R.; Zhou, L.; Xu, B.; Deng, C.; Li, X. Piceatannol-3’-O-β-D-glucopyranoside attenuates colistin-induced neurotoxicity by suppressing oxidative stress via the NRF2/HO-1 pathway. Biomed. Pharmacother. 2023, 161, 114419. [Google Scholar] [CrossRef] [PubMed]
- Likhitwitayawuid, K. Oxyresveratrol: Sources, productions, biological activities, pharmacokinetics, and delivery systems. Molecules 2021, 26, 4212. [Google Scholar] [CrossRef] [PubMed]
- Hankittichai, P.; Lou, H.J.; Wikan, N.; Smith, D.R.; Potikanond, S.; Nimlamool, W. Oxyresveratrol inhibits IL-1β-induced inflammation via suppressing AKT and ERK1/2 activation in human microglia, HMC3. Int. J. Mol. Sci. 2020, 21, 6054. [Google Scholar] [CrossRef]
- Ban, J.Y.; Jeon, S.Y.; Nguyen, T.T.; Bae, K.; Song, K.S.; Seong, Y.H. Neuroprotective effect of oxyresveratrol from smilacis chinae rhizome on amyloid Beta protein (25-35)-induced neurotoxicity in cultured rat cortical neurons. Biol. Pharm. Bull. 2006, 29, 2419–2424. [Google Scholar] [CrossRef]
- Andrabi, S.A.; Spina, M.G.; Lorenz, P.; Ebmeyer, U.; Wolf, G.; Horn, T.F. Oxyresveratrol (trans-2,3′,4,5′-tetrahydroxystilbene) is neuroprotective and inhibits the apoptotic cell death in transient cerebral ischemia. Brain Res. 2004, 1017, 98–107. [Google Scholar] [CrossRef]
- Mahamud, N.; Songvut, P.; Muangnoi, C.; Rodsiri, R.; Dahlan, W.; Tansawat, R. Untargeted metabolomics reveal pathways associated with neuroprotective effect of oxyresveratrol in SH-SY5Y cells. Sci. Rep. 2023, 13, 20385. [Google Scholar] [CrossRef]
- Blasko, I.; Veerhuis, R.; Stampfer-Kountchev, M.; Saurwein-Teissl, M.; Eikelenboom, P.; Grubeck-Loebenstein, B. Costimulatory effects of interferon-gamma and interleukin-1beta or tumor necrosis factor α on the synthesis of Aβ1-40 and Aβ1-42 by human astrocytes. Neurobiol. Dis. 2000, 7 Pt B, 682–689. [Google Scholar] [CrossRef]
- Lesné, S.; Docagne, F.; Gabriel, C.; Liot, G.; Lahiri, D.K.; Buée, L.; Plawinski, L.; Delacourte, A.; MacKenzie, E.T.; Buisson, A.; et al. Transforming growth factor-β1 potentiates amyloid-β heneration in astrocytes and in transgenic mice. J. Biol. Chem. 2003, 278, 18408–18418. [Google Scholar] [CrossRef]
- Hasriadi, H.; Wong-On, M.; Lapphanichayakool, P.; Limpeanchob, N. Naueroprotective effect of Artocarpus Lakoocha extract and oxyresveratrolk against hydrogen peroxide-induced toxicity in SH-SY5Y cells. Int. J. Pharm. Pharmaceutic. Sci. 2017, 9, 229–233. [Google Scholar] [CrossRef]
- Yang, X.; Qiang, X.; Li, Y.; Luo, L.; Xu, R.; Zheng, Y.; Cao, Z.; Tan, Z.; Deng, Y. Pyridoxine-resveratrol hybrids Mannich base derivatives as novel dual inhibitors of AChE and MAO-B with antioxidant and metal-chelating properties for the treatment of Alzheimer’s disease. Bioorg. Chem. 2017, 71, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liu, H.; Yue, L.; Li, X.; Zhao, L.; Yang, X.; Wang, X.; Yang, Y.; Qu, Y. Neuroprotective effects of pterostilbene against oxidative stress injury: Involvement of nuclear factor erythroid 2-related factor 2 pathway. Brain Res. 2016, 1643, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Deng, R.; Li, E.T.S.; Shen, J.; Wang, M. Pinosylvin provides neuroprotection against cerebral ischemia and reperfusion injury through enhancing PINK1/Parkin mediated mitophagy and Nrf2 pathway. J. Funct. Foods 2020, 71, 104019. [Google Scholar] [CrossRef]
- Chang, J.; Rimando, A.; Pallas, M.; Camins, A.; Porquet, D.; Reeves, J.; Shukitt-Hale, B.; Smith, M.A.; Joseph, J.A.; Casadesus, G. Low-dose pterostilbene, but not resveratrol, is a potent neuromodulator in aging and Alzheimer’s disease. Neurobiol. Aging 2012, 33, 2062–2071. [Google Scholar] [CrossRef]
- Naik, B.; Nirwane, A.; Majumdar, A. Pterostilbene ameliorates intracerebroventricular streptozotocin induced memory decline in rats. Cogn. Neurodyn. 2017, 11, 35–49. [Google Scholar] [CrossRef]
- Li, Q.; Li, X.; Tian, B.; Chen, L. Protective effect of pterostilbene in a streptozotocin-induced mouse model of Alzheimer’s disease by targeting monoamine oxidase B. J. Appl. Toxicol. 2022, 42, 1777–1786. [Google Scholar] [CrossRef]
- Xu, J.; Liu, J.; Li, Q.; Mi, Y.; Zhou, D.; Meng, Q.; Chen, G.; Li, N.; Hou, Y. Pterostilbene alleviates Aβ1-42 -induced cognitive dysfunction via inhibition of oxidative stress by activating Nrf2 signaling pathway. Mol. Nutr. Food Res. 2021, 65, e2000711. [Google Scholar] [CrossRef]
- Joseph, J.A.; Fisher, D.R.; Cheng, V.; Rimando, A.M.; Shukitt-Hale, B. Cellular and behavioral effects of stilbene resveratrol analogues: Implications for reducing the deleterious effects of aging. J. Agr. Food Chem. 2008, 56, 10544–10551. [Google Scholar] [CrossRef]
- La Spina, M.; Sansevero, G.; Biasutto, L.; Zoratti, M.; Peruzzo, R.; Berardi, N.; Sale, A.; Azzolini, M. Pterostilbene ımproves cognitive performance in aged rats: An in vivo study. Cell. Physiol. Biochem. 2019, 52, 232–239. [Google Scholar] [CrossRef]
- Zhu, L.; Lu, F.; Zhang, X.; Liu, S.; Mu, P. SIRT1 is involved in the neuroprotection of pterostilbene against amyloid β 25-35-induced cognitive deficits in mice. Front. Pharmacol. 2022, 13, 877098. [Google Scholar] [CrossRef]
- Sermboonpaisarn, T.; Sawasdee, P. Potent and selective butyrylcholinesterase inhibitors from Ficus foveolata. Fitoterapia 2012, 83, 780–784. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, R.; Park, Y.U.; Lee, T.H.; Kim, S.Y. Gnetol, a resveratrol derivative ameliorates malathion-induced neurotoxicity through modulating lysosomal membrane permeabilization in N2a cells. J. Alzheimer’s Dis. Parkinson. 2017, 7. [Google Scholar] [CrossRef]
- Oh, W.K.; Cho, K.B.; Hien, T.T.; Kim, T.H.; Kim, H.S.; Dao, T.T.; Han, H.K.; Kwon, S.M.; Ahn, S.G.; Yoon, J.H.; et al. Amurensin G, a potent natural SIRT1 inhibitor, rescues doxorubicin responsiveness via down-regulation of multidrug resistance 1. Mol. Pharmacol. 2010, 78, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Maurice, T.; Lockhart, B.P.; Privat, A. Amnesia induced in mice by centrally administered beta-amyloid peptides involves cholinergic dysfunction. Brain Res. 1996, 706, 181–193. [Google Scholar] [CrossRef]
- Francis, P.T.; Palmer, A.M.; Snape, M.; Wilcock, G.K. The cholinergic hypothesis of Alzheimer’s disease: A review of progress. J. Neurol. Neurosurg. Psychiatry 1999, 66, 137–147. [Google Scholar] [CrossRef]
- Van Marum, R.J. Current and future therapy in Alzheimer’s disease. Fundam. Clin. Pharmacol. 2008, 22, 265–274. [Google Scholar] [CrossRef]
- Ha, T.; Kim, H.; Thuong, P.T.; Ngoc, T.M.; Lee, I.; Hung, N.D.; Bae, K. Antioxidant and lipoxygenase inhibitory activity of oligostilbenes from the leaf and stem of Vitis amurensis. J. Ethnopharmacol. 2009, 125, 304–309. [Google Scholar] [CrossRef]
- Jakab, M.; Lach, S.; Bacová, Z.; Langelüddecke, C.; Strbák, V.; Schmidt, S.; Iglseder, E.; Paulmichl, M.; Geibel, J.; Ritter, M. Resveratrol inhibits electrical activity and insulin release from insulinoma cells by block of voltage-gated Ca+ channels and swelling-dependent Cl- currents. Cell Physiol. Biochem. 2008, 22, 567–578. [Google Scholar] [CrossRef]
- Lee, B.Y.; Ban, J.Y.; Seong, Y.H. Chronic stimulation of GABAA receptor with muscimol reduces amyloid beta protein (25-35)-induced neurotoxicity in cultured rat cortical cells. Neurosci. Res. 2005, 52, 347–356. [Google Scholar] [CrossRef]
- Ruan, C.J.; Si, J.Y.; Zhang, L.; Chen, D.H.; Du, G.H.; Sun, L. Protective effect of stilbenes containing extract-fraction from Cajanus cajan L. on Aβ25–35-induced cognitive deficits in mice. Neurosci. Lett. 2009, 467, 159–163. [Google Scholar] [CrossRef]
- Kontush, A. Amyloid-beta: An antioxidant that becomes a pro-oxidant and critically contributes to Alzheimer’s disease. Free Radic. Biol. Med. 2001, 31, 1120–1131. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.A.; Richey Harris, P.L.; Sayre, L.M.; Beckman, J.S.; Perry, G. Widespread peroxynitrite-mediated damage in Alzheimer’s disease. J. Neurosci. 1997, 17, 2653–2657. [Google Scholar] [CrossRef] [PubMed]
- Sano, M.; Ernesto, C.; Thomas, R.G.; Klauber, M.R.; Schafer, K.; Grundman, M.; Woodbury, P.; Growdon, J.; Cotman, C.W.; Pfeiffer, E.; et al. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer’s disease. N. Engl. J. Med. 1997, 336, 1216–1222. [Google Scholar] [CrossRef]
- Yamada, K.; Tanaka, T.; Han, D.; Senzaki, K.; Kameyama, T.; Nabeshima, T. Protective effects of idebenone and α-tocopherol on β-amyloid-(1-42)-induced learning and memory deficits in rats: Implication of oxidative stress in β-amyloid-induced neurotoxicity in vivo. Eur. J. Neurosci. 1999, 11, 83–90. [Google Scholar] [CrossRef]
- Wenk, G.L. Neuropathologic changes in Alzheimer’s disease: Potential targets for treatment. J. Clin. Psychiatr. 2006, 67 (Suppl. 3), 3–23. [Google Scholar]
- Solntseva, E.I.; Bukanova, J.V.; Marchenko, E.; Skrebitsky, V.G. Donepezil is a strong antagonist of voltage-gated calcium and potassium channels in molluscan neurons. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2007, 144, 319–326. [Google Scholar] [CrossRef]
- Chaher, N.; Arraki, K.; Dillinseger, E.; Temsamani, H.; Bernillon, S.; Pedrot, E.; Delaunay, J.C.; Mérillon, J.M.; Monti, J.P.; Izard, J.C.; et al. Bioactive stilbenes from Vitis vinifera grapevine shoots extracts. J. Sci. Food Agric. 2014, 94, 951–954. [Google Scholar] [CrossRef]
- Jin, Q.; Han, X.H.; Hong, S.S.; Lee, C.; Choe, S.; Lee, D.; Kim, Y.; Hong, J.T.; Lee, M.K.; Hwang, B.Y. Antioxidative oligostilbenes from Caragana sinica. Bioorg. Med. Chem. Lett. 2012, 22, 973–976. [Google Scholar] [CrossRef]
- Yang, J.B.; Wang, A.G.; Ji, T.F.; Su, Y.L. Two new oligostilbenes from the stem of Parthenocissus quinquefolia. J. Asian Nat. Prod. Res. 2014, 16, 275–280. [Google Scholar] [CrossRef]
- Tanaka, T.; Ito, T.; Iinuma, M.; Ohyama, M.; Ichise, M.; Tateishi, Y. Stilbene oligomers in roots of Sophora davidii. Phytochemistry 2000, 53, 1009–1014. [Google Scholar] [CrossRef]
- Wang, C.K.; Chen, L.G.; Wen, C.L.; Hou, W.C.; Hung, L.F.; Yen, S.J.; Shen, Y.J.; Lin, S.Y.; Liang, Y.C. Neuroprotective activity of Vitis thunbergii var. taiwaniana extracts in vitro and in vivo. J. Med. Food 2010, 13, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Koo, E.H. Biology and pathophysiology of the amyloid precursor protein. Mol. Neurodegener. 2011, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.W.; Thompson, R.; Zhang, H.; Xu, H. APP processing in Alzheimer’s disease. Mol. Brain 2011, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Hussain, I.; Powell, D.; Howlett, D.R.; Tew, D.G.; Meek, T.D.; Chapman, C.; Gloger, I.S.; Murphy, K.E.; Southan, C.D.; Ryan, D.M.; et al. Identification of a novel aspartic protease (Asp 2) as β-secretase. Mol. Cell. Neurosci. 1999, 14, 419–427. [Google Scholar] [CrossRef]
- Lin, X.; Koelsch, G.; Wu, S.; Downs, D.; Dashti, A.; Tang, J. Human aspartic protease memapsin 2 cleaves the beta-secretase site of beta-amyloid precursor protein. Proc. Natl. Acad. Sci. USA 2000, 97, 1456–1460. [Google Scholar] [CrossRef]
- Yang, L.B.; Lindholm, K.; Yan, R.; Citron, M.; Xia, W.; Yang, X.L.; Beach, T.; Sue, L.; Wong, P.; Price, D.; et al. Elevated β-secretase expression and enzymatic activity detected in sporadic Alzheimer’s disease. Nat. Med. 2003, 9, 3–4. [Google Scholar] [CrossRef]
- Johnston, J.A.; Liu, W.W.; Todd, S.A.; Coulson, D.T.R.; Murphy, S.; Irvine, G.B.; Passmore, A.P. Expression and activity of β-site amyloid precursor protein cleaving enzyme in Alzheimer’s disease. Biochem. Soc. Trans. 2005, 33, 1096–1100. [Google Scholar] [CrossRef]
- Lammich, S.; Kojro, E.; Postina, R.; Gilbert, S.; Pfeiffer, R.; Jasionowski, M.; Haass, C.; Fahrenholz, F. Constitutive and regulated α-secretase cleavage of Alzheimer’s amyloid precursor protein by a disintegrin metalloprotease. Proc. Natl. Acad. Sci. USA 1999, 96, 3922–3927. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, Q.; Zhang, Y.W.; Xu, H. Proteolytic processing of Alzheimer’s β-amyloid precursor protein. J. Neurochem. 2012, 120, 9–21. [Google Scholar] [CrossRef]
- Hu, J.; Lin, T.; Gao, Y.; Xu, J.; Jiang, C.; Wang, G.; Bu, G.; Xu, H.; Chen, H.; Zhang, Y.W. The resveratrol trimer miyabenol C inhibits β-secretase activity and β-amyloid generation. PLoS ONE 2015, 10, e0115973. [Google Scholar] [CrossRef]
- Du, Q.H.; Peng, C.; Zhang, H. Polydatin: A review of pharmacology and pharmacokinetics. Pharm. Biol. 2013, 51, 1347–1354. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, S.; Gravandi, M.M.; Abdian, S.; Akkol, E.K.; Farzaei, M.H.; Sobarzo-Sánchez, E. The neuroprotective role of polydatin: Neuropharmacological mechanisms, molecular targets, therapeutic potentials, and clinical perspective. Molecules. 2021, 26, 5985. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Wang, K.; Sheng, S.; Cui, J. Polydatin ameliorates chemotherapy-induced cognitive impairment (chemobrain) by inhibiting oxidative stress, inflammatory response, and apoptosis in rats. Biosci. Biotechnol. Biochem. 2020, 84, 1201–1210. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, Y.; Xun, Y.; Liu, H.; Wei, C.; Wang, H.; Yang, X.; Yuan, S.; Liu, N.; Xiang, S. Polydatin protects neuronal cells from hydrogen peroxide damage by activating CREB/Ngb signaling. Mol. Med. Rep. 2022, 25, 9. [Google Scholar] [CrossRef]
- Tang, K.S. Protective Effects of Polydatin Against Dementia-Related Disorders. Curr. Neuropharmacol. 2021, 19, 127–135. [Google Scholar] [CrossRef]
- Clementi, M.E.; Pezzotti, M.; Orsini, F.; Sampaolese, B.; Mezzogori, D.; Grassi, C.; Giardina, B.; Misiti, F. Alzheimer’s amyloid β-peptide (1–42) induces cell death in human neuroblastoma via bax/bcl-2 ratio increase: An intriguing role for methionine 35. Biochem. Biophys. Res. Commun. 2006, 342, 206–213. [Google Scholar] [CrossRef]
- Cao, G.; Minami, M.; Pei, W.; Yan, C.; Chen, D.; O’Horo, C.; Graham, S.H.; Chen, J. Intracellular Bax translocation after transient cerebral ischemia: Implications for a role of the mitochondrial apoptotic signaling pathway in ischemic neuronal death. J. Cereb. Blood Flow. Metab. 2001, 21, 321–333. [Google Scholar] [CrossRef]
- Orallo, F. Comparative studies of the antioxidant effects of cis- and trans- resveratrol. Curr. Med. Chem. 2006, 13, 87–98. [Google Scholar] [CrossRef]
- Park, E.K.; Choo, M.K.; Yoon, H.K.; Kim, D.H. Antithrombotic and antiallergic activities of rhaponticin from Rhei Rhizoma are activated by human intestinal bacteria. Arch. Pharm. Res. 2002, 25, 528–533. [Google Scholar] [CrossRef]
- Mostefa, N.; Djebli, N.; Khanh, P.N.; Ha, N.X.; Anh, H.T.N.; Ha, V.T.; Huong, T.T.; Anh, D.V.; Cuong, N.M. Anti-Alzheimer’s activity of polyphenolic stilbene-rich acetone fraction of the oil-removed seeds of Passiflora edulis: In vivo and in silico studies. Chem. Biodivers. 2023, 20, e202201051. [Google Scholar] [CrossRef]
- Rivière, C.; Papastamoulis, Y.; Fortin, P.Y.; Delchier, N.; Andriamanarivo, S.; Waffo-Teguo, P.; Kapche, G.D.; Amira-Guebalia, H.; Delaunay, J.C.; Mérillon, J.M.; et al. New stilbene dimers against amyloid fibril formation. Bioorg. Med. Chem. Lett. 2010, 20, 3441–3443. [Google Scholar] [CrossRef] [PubMed]
- Fuloria, S.; Sekar, M.; Khattulanuar, F.S.; Gan, S.H.; Rani, N.N.I.M.; Ravi, S.; Subramaniyan, V.; Jeyabalan, S.; Begum, M.Y.; Chidambaram, K.; et al. Chemistry, biosynthesis and pharmacology of viniferin: Potential resveratrol-derived molecules for new drug discovery, development and therapy. Molecules 2022, 27, 5072. [Google Scholar] [CrossRef] [PubMed]
- Richard, T.; Pawlus, A.D.; Iglésias, M.L.; Pedrot, E.; Waffo-Teguo, P.; Mérillon, J.M.; Monti, J.P. Neuroprotective properties of resveratrol and derivatives. Ann. N. Y. Acad. Sci. 2011, 1215, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Richard, T.; Papastamoulis, Y.; Waffo-Teguo, P.; Monti, J.P. 3D NMR structure of a complex between the amyloid beta peptide (1–40) and the polyphenol ε-viniferin glucoside: Implications in Alzheimer’s disease. Biochim. Biophys. Acta. 2013, 1830, 5068–5074. [Google Scholar] [CrossRef]
- Greene, L.A.; Tischler, A.S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc. Natl Acad. Sci. USA 1976, 73, 2424–2428. [Google Scholar] [CrossRef]
- Capiralla, H.; Vingtdeux, V.; Zhao, H.; Sankowski, R.; Al-Abed, Y.; Davies, P.; Marambaud, P. Resveratrol mitigates lipopolysaccharide-and Aβ-mediated microglial inflammation by inhibiting the TLR4/NF-κB/STAT signaling cascade. J. Neurochem. 2012, 120, 461–472. [Google Scholar] [CrossRef]
- Richard, T.; Poupard, P.; Nassra, M.; Papastamoulis, Y.; Iglésias, M.L.; Krisa, S.; Waffo-Teguo, P.; Mérillon, J.M.; Monti, J.P. Protective effect of ε-viniferin on β-amyloid peptide aggregation investigated by electrospray ionization mass spectrometry. Bioorg. Med. Chem. 2011, 19, 3152–3155. [Google Scholar] [CrossRef]
- Sung, S.H.; Kang, S.Y.; Lee, K.Y.; Park, M.J.; Kim, J.H.; Park, J.H.; Kim, Y.C.; Kim, J.; Kim, Y.C. (+)-Alpha-viniferin, a stilbene trimer from Caragana chamlague, inhibits acetylcholinesterase. Biol. Pharm. Bull. 2002, 25, 125–127. [Google Scholar] [CrossRef]
- Wiciński, M.; Domanowska, A.; Wódkiewicz, E.; Malinowski, B. Neuroprotective properties of resveratrol and its derivatives-influence on potential mechanisms leading to the development of Alzheimer’s disease. Int. J. Mol. Sci. 2020, 21, 2749. [Google Scholar] [CrossRef]
- Praticò, D.; Uryu, K.; Leight, S.; Trojanoswki, J.Q.; Lee, V.M. Increased lipid peroxidation precedes amyloid plaque formation in an animal model of Alzheimer amyloidosis. J. Neurosci. 2001, 21, 4183–4187. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Lauderback, C.M. Lipid peroxidation and protein oxidation in Alzheimer’s disease brain: Potential causes and consequences involving amyloid beta-peptide-associated free radical oxidative stress. Free Radic. Biol. Med. 2002, 32, 1050–1060. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef] [PubMed]
- Saso, L.; Firuzi, O. Pharmacological applications of antioxidants: Lights and shadows. Curr. Drug Targets 2014, 15, 1177–1199. [Google Scholar] [CrossRef] [PubMed]
- Jeřábek, J.; Uliassi, E.; Guidotti, L.; Korábečný, J.; Soukup, O.; Sepsova, V.; Hrabinova, M.; Kuča, K.; Bartolini, M.; Peña-Altamira, L.E.; et al. Tacrine-resveratrol fused hybrids as multi-target-directed ligands against Alzheimer’s disease Eur. J. Med. Chem. 2017, 127, 250–262. [Google Scholar] [CrossRef]
- Arbo, B.D.; André-Miral, C.; Nasre-Nasser, R.G.; Schimith, L.E.; Santos, M.G.; Costa-Silva, D.; Muccillo-Baisch, A.L.; Hort, M.A. Resveratrol derivatives as potential treatments for Alzheimer’s and Parkinson’s disease. Front. Aging Neurosci. 2020, 12, 103. [Google Scholar] [CrossRef]
- Tang, Y.W.; Shi, C.J.; Yang, H.L.; Cai, P.; Liu, Q.H.; Yang, X.L.; Kong, L.Y.; Wang, X.B. Synthesis and evaluation of isoprenylation-resveratrol dimer derivatives against Alzheimer’s disease. Eur. J. Med. Chem. 2019, 163, 307–319. [Google Scholar] [CrossRef]
- Li, S.Y.; Wang, X.B.; Kong, L.Y. Design, synthesis and biological evaluation of imine resveratrol derivatives as multi-targeted agents against Alzheimer’s disease. Eur. J. Med. Chem. 2014, 71, 36–45. [Google Scholar] [CrossRef]
- Lu, C.; Guo, Y.; Yan, J.; Luo, Z.; Luo, H.B.; Yan, M.; Huang, L.; Li, X. Design, synthesis, and evaluation of multitarget-directed resveratrol derivatives for the treatment of Alzheimer’s disease. J. Med. Chem. 2013, 56, 5843–5859. [Google Scholar] [CrossRef]
- Chao, J.; Li, H.; Cheng, K.W.; Yu, M.S.; Chang, R.C.C.; Wang, M. Protective effects of pinostilbene, a resveratrol methylated derivative, against 6-hydroxydopamine-ınduced neurotoxicity in SH-SY5Y cells. J. Nutr. Biochem. 2010, 21, 482–489. [Google Scholar] [CrossRef]
- Belmonte-Reche, E.; Peñalver, P.; Caro-Moreno, M.; Mateos-Martín, M.L.; Adán, N.; Delgado, M.; González-Rey, E.; Morales, J.C. Silyl resveratrol derivatives as potential therapeutic agents for neurodegenerative and neurological diseases. Eur. J. Med. Chem. 2021, 223, 113655. [Google Scholar] [CrossRef]
- Yuan, W.; Shang, Z.; Qiang, X.; Tan, Z.; Deng, Y. Synthesis of pterostilbene and resveratrol carbamate derivatives as potential dual cholinesterase inhibitors and neuroprotective agents. Res. Chem. Intermed. 2013, 40, 787–800. [Google Scholar] [CrossRef]
- Jung, J.C.; Lim, E.; Lee, Y.; Kang, J.M.; Kim, H.; Jang, S.; Oh, S.; Jung, M. Synthesis of novel trans-stilbene derivatives and evaluation of their potent antioxidant and neuroprotective effects. Eur. J. Med. Chem. 2009, 44, 3166–3174. [Google Scholar] [CrossRef] [PubMed]
- Mlakić, M.; Fodor, L.; Odak, I.; Horváth, O.; Lovrić, M.J.; Barić, D.; Milašinović, V.; Molčanov, K.; Marinić, Ž.; Lasić, Z.; et al. Resveratrol-maltol and resveratrol-thiophene hybrids as cholinesterase inhibitors and antioxidants: Synthesis, biometal chelating capability and crystal structure. Molecules 2022, 27, 6379. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.S.; Liu, Y.; Hou, J.W.; Yang, J.; Zhang, X.Y.; Zhao, Y.; Xie, S.S.; Ding, Y.; Zhang, T. Design, synthesis and evaluation of resveratrol-indazole hybrids as novel monoamine oxidases inhibitors with amyloid-β aggregation inhibition. Bioorg. Chem. 2018, 76, 130–139. [Google Scholar] [CrossRef]
- Mellado, M.; González, C.; Mella, J.; Aguilar, L.F.; Celik, I.; Borges, F.; Uriarte, E.; Delogu, G.; Viña, D.; Matos, M.J. Coumarin-Resveratrol-Inspired Hybrids as Monoamine Oxidase B Inhibitors: 3-Phenylcoumarin versus trans-6-Styrylcoumarin. Molecules 2022, 27, 928. [Google Scholar] [CrossRef]
- Agbo, E.N.; Gildenhuys, S.; Choong, Y.S.; Mphahlele, M.J.; More, G.K. Synthesis of furocoumarin-stilbene hybrids as potential multifunctional drugs against multiple biochemical targets associated with Alzheimer’s disease. Bioorg Chem. 2020, 101, 103997. [Google Scholar] [CrossRef]
- Martínez, A. Synthesis, in vitro activity, and molecular docking of caffeic acid and resveratrol derivatives against Alzheimer’s disease-related enzymes. Med. Chem. Res. 2024, 33, 1681–1697. [Google Scholar] [CrossRef]
- Cheng, G.; Xu, P.; Zhang, M.; Chen, J.; Sheng, R.; Ma, Y. Resveratrol-maltol hybrids as multi-target-directed agents for Alzheimer’s disease. Bioorg. Med. Chem. 2018, 26, 5759–5765. [Google Scholar] [CrossRef]
- Carradori, S.; Fantacuzzi, M.; Ammazzalorso, A.; Angeli, A.; De Filippis, B.; Galati, S.; Petzer, A.; Petzer, J.P.; Poli, G.; Tuccinardi, T.; et al. Resveratrol analogues as dual ınhibitors of monoamine oxidase b and carbonic anhydrase VII: A new multi-target combination for neurodegenerative diseases? Molecules 2022, 27, 7816. [Google Scholar] [CrossRef]
- Puksasook, T.; Kimura, S.; Tadtong, S.; Jiaranaikulwanitch, J.; Pratuangdejkul, J.; Kitphati, W.; Suwanborirux, K.; Saito, N.; Nukoolkarn, V. Semisynthesis and biological evaluation of prenylated resveratrol derivatives as multi-targeted agents for Alzheimer’s disease. J. Nat. Med. 2017, 71, 665–682. [Google Scholar] [CrossRef]
- Pan, L.F.; Wang, X.B.; Xie, S.S.; Li, S.Y.; Kong, L.Y. Multitarget-directed resveratrol derivatives: Anti-cholinesterases, anti-β-amyloid aggregation and monoamine oxidase inhibition properties against Alzheimer’s disease. Med. Chem. Comm. 2014, 5, 609–616. [Google Scholar] [CrossRef]
- Mlakić, M.; Odak, I.; Barić, D.; Talić, S.; Šagud, I.; Štefanić, Z.; Molčanov, K.; Lasić, Z.; Kovačević, B.; Škorić, I. New resveratrol analogs as improved biologically active structures: Design, synthesis and computational modeling. Bioorg. Chem. 2024, 143, 106965. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.C.; Tsai, W.J.; Ueng, Y.F.; Tzeng, T.T.; Chen, H.L.; Zhu, P.R.; Huang, C.H.; Shiao, Y.J.; Li, W.T. Neuroprotective and antineuroinflammatory effects of hydroxyl-functionalized stilbenes and 2-arylbenzo[b]furans. J. Med. Chem. 2017, 60, 4062–4073. [Google Scholar] [CrossRef] [PubMed]
- Firdoos, S.; Dai, R.; Tahir, R.A.; Khan, Z.Y.; Li, H.; Zhang, J.; Ni, J.; Quan, Z.; Qing, H. In silico identification of novel stilbenes analogs for potential multi-targeted drugs against Alzheimer’s disease. J. Mol. Model. 2023, 29, 209. [Google Scholar] [CrossRef]
- Nagumo, M.; Ninomiya, M.; Oshima, N.; Itoh, T.; Tanaka, K.; Nishina, A.; Koketsu, M. Comparative analysis of stilbene and benzofuran neolignan derivatives as acetylcholinesterase inhibitors with neuroprotective and anti-inflammatory activities. Bioorg. Med. Chem. Lett. 2019, 29, 2475–2479. [Google Scholar] [CrossRef]
- Braymer, J.J.; Choi, J.S.; DeToma, A.S.; Wang, C.; Nam, K.; Kampf, J.W.; Ramamoorthy, A.; Lim, M.H. Development of bifunctional stilbene derivatives for targeting and modulating metal-amyloid-β species. Inorg. Chem. 2011, 50, 10724–10734. [Google Scholar] [CrossRef]
- Yu, Z.; Guo, W.; Patel, S.; Cho, H.J.; Sun, L.; Mirica, L.M. Amphiphilic stilbene derivatives attenuate the neurotoxicity of soluble Aβ42 oligomers by controlling their interactions with cell membranes. Chem. Sci. 2022, 13, 12818–12830. [Google Scholar] [CrossRef]
- Liu, W.G.; Zhao, J.Y.; Zhang, H.; Liu, X.Y.; Guo, X.L. MSTMP, a stilbene derivative, protects SH-SY5Y cells against oxidative stress. Can. J. Neurol. Sci. 2014, 41, 382–388. [Google Scholar] [CrossRef]
- Azmi, M.N.; Sian, T.A.; Suhaimi, M.; Kamarudin, M.N.A.; Din, M.F.M.; Nafiah, M.A.; Thomas, N.F.; Kadir, H.A.; Awang, K. Synthesis of indolostilbenes via FeCl3-promoted oxidative cyclisation and their biological effects on NG108-15 cell viability and H2O2-induced cytotoxicity. J. Phys. Sci. 2021, 32, 69–89. [Google Scholar] [CrossRef]
- Patel, D.V.; Patel, N.R.; Kanhed, A.M.; Teli, D.M.; Patel, K.B.; Joshi, P.D.; Patel, S.P.; Gandhi, P.M.; Chaudhary, B.N.; Prajapati, N.K.; et al. Novel carbazole-stilbene hybrids as multifunctional anti-Alzheimer agents. Bioorg. Chem. 2020, 101, 103977. [Google Scholar] [CrossRef]
- Zheng, Y.; Qiang, X.; Xu, R.; Song, Q.; Tian, C.; Liu, H.; Li, W.; Tan, Z.; Deng, Y. Design, synthesis and evaluation of pterostilbene β-amino alcohol derivatives as multifunctional agents for Alzheimer’s disease treatment. Bioorg. Chem. 2018, 78, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Alotaibi, S.H.; Wei, Y.; Lone, A.M. Preventive effect of 3,3′-dimethoxy-4,4′-dihydroxystilbene triazole against Alzheimer’s disease by inhibition of neuronal apoptosis. ChemistrySelect 2023, 8, e202204087. [Google Scholar] [CrossRef]
- Hilt, S.; Liu, R.; Maezawa, I.; Rojalin, T.; Aung, H.H.; Budamagunta, M.; Slez, R.; Gong, Q.; Carney, R.P.; Voss, J.C. Novel stilbene-nitroxyl hybrid compounds display discrete modulation of amyloid beta toxicity and structure. Front. Chem. 2022, 10, 896386. [Google Scholar] [CrossRef]
- Jia, J.; Cui, M.; Dai, J.; Wang, X.; Ding, Y.S.; Jia, H.; Liu, B. 99mTc-labeled benzothiazole and stilbene derivatives as ımaging agents for Aβ plaques in cerebral amyloid angiopathy. Med. Chem. Comm. 2014, 5, 153–158. [Google Scholar] [CrossRef]
- Ono, M.; Wilson, A.; Nobrega, J.; Westaway, D.; Verhoeff, P.; Zhuang, Z.P.; Kung, M.P.; Kung, H.F. 11C-labeled stilbene derivatives as Aβ-aggregate-specific PET imaging agents for Alzheimer’s disease. Nucl. Med. Biol. 2003, 30, 565–571. [Google Scholar] [CrossRef]
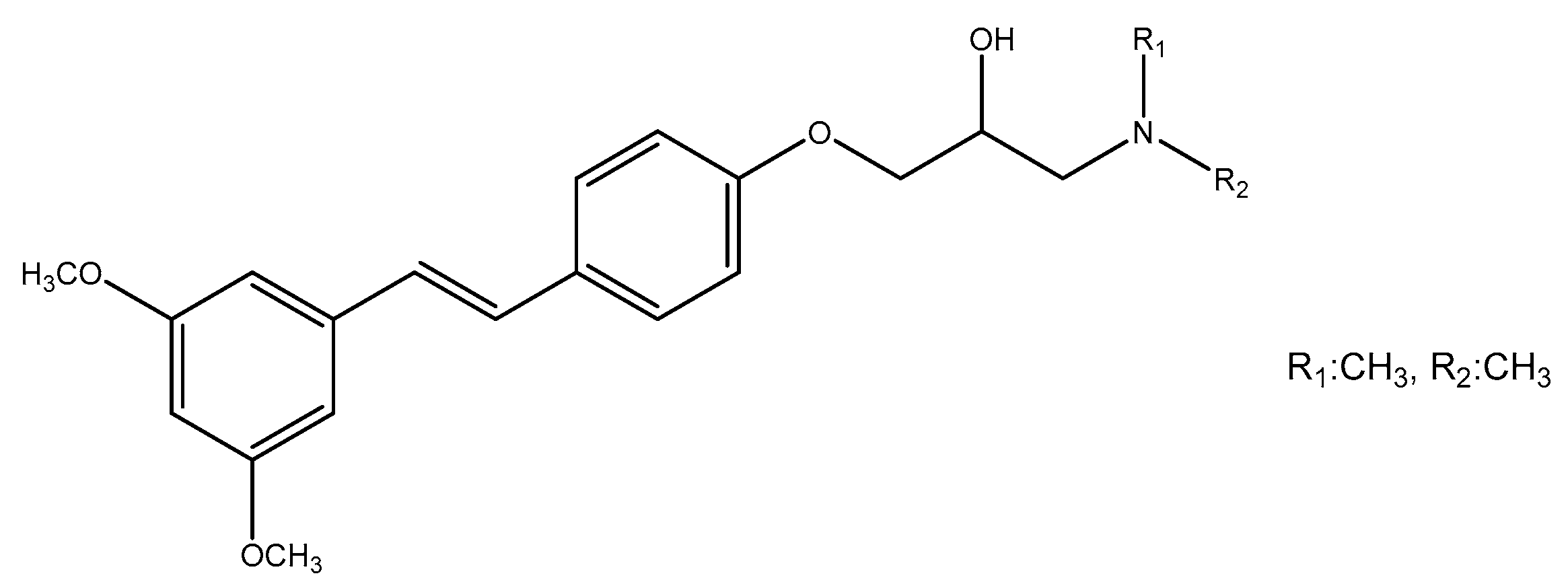
- Villalonga-Barber, C.; Meligova, A.K.; Alexi, X.; Steele, B.R.; Kouzinos, C.E.; Screttas, C.G.; Katsanou, E.S.; Micha-Screttas, M.; Alexis, M.N. New hydroxystilbenoid derivatives endowed with neuroprotective activity and devoid of interference with estrogen and aryl hydrocarbon receptor-mediated transcription. Bioorg. Med. Chem. 2011, 19, 339–351. [Google Scholar] [CrossRef]
- Cui, M.; Tang, R.; Zhang, J.; Zhang, X.; Li, Z.; Jia, H.; Liu, B. Carbon-11 labeled stilbene derivatives from natural products for the imaging of Aβ plaques in the brain. Radiochim. Acta 2014, 102, 185–192. [Google Scholar] [CrossRef]
- Anand, S.; Sowbhagya, R.; Ansari, M.A.; Alzohairy, M.A.; Alomary, M.N.; Almalik, A.I.; Ahmad, W.; Tripathi, T.; Elderdery, A.Y. Polyphenols and their nanoformulations: Protective effects against human diseases. Life 2022, 12, 1639. [Google Scholar] [CrossRef]
- Ehsanifard, Z.; Motaqi, M.; Hosseini, S.; Hasani, F. Effects of curcuminoids and resveratrol in micro and nanoformulations on brain-derived neurotrophic factor: A brief review. Micro. Nano. Bio. Aspects 2023, 2, 35–40. [Google Scholar] [CrossRef]
- Vesely, O.; Baldovska, S.; Kolesarova, A. Enhancing bioavailability of nutraceutically used resveratrol and other stilbenoids. Nutrients 2021, 13, 3095. [Google Scholar] [CrossRef]
- Grabska-Kobyłecka, I.; Szpakowski, P.; Król, A.; Książek-Winiarek, D.; Kobyłecki, A.; Głąbiński, A.; Nowak, D. Polyphenols and their impact on the prevention of neurodegenerative diseases and development. Nutrients 2023, 15, 3454. [Google Scholar] [CrossRef] [PubMed]
- Moradi, S.Z.; Momtaz, S.; Bayrami, Z.; Farzaei, M.H.; Abdollahi, M. Nanoformulations of herbal extracts in treatment of neurodegenerative disorders. Front. Bioeng. Biotechnol. 2020, 8, 238. [Google Scholar] [CrossRef] [PubMed]
- Farzaei, M.H.; Rahimi, R.; Nikfar, S.; Abdollahi, M. Effect of resveratrol on cognitive and memory performance and mood: A meta-analysis of 225 patients. Pharmacol. Res. 2018, 128, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Min, J.B.; Kim, E.S.; Lee, J.S.; Lee, H.G. Preparation, characterization, and cellular uptake of resveratrol-loaded trimethyl chitosan nanoparticles. Food Sci. Biotechnol. 2018, 27, 441–450. [Google Scholar] [CrossRef]
- Summerlin, N.; Soo, E.; Thakur, S.; Qu, Z.; Jambhrunkar, S.; Popat, A. Resveratrol nanoformulations: Challenges and opportunities. Int. J. Pharm. 2015, 479, 282–290. [Google Scholar] [CrossRef]
- Fonseca-Santos, B.; Chorilli, M. The uses of resveratrol for neurological diseases treatment and insights for nanotechnology based-drug delivery systems. Int. J. Pharm. 2020, 589, 119832. [Google Scholar] [CrossRef]
- Loureiro, J.A.; Andrade, S.; Duarte, A.; Neves, A.R.; Queiroz, J.F.; Nunes, C.; Sevin, E.; Fenart, L.; Gosselet, F.; Coelho, M.A.; et al. Resveratrol and grape extract-loaded solid lipid nanoparticles for the treatment of Alzheimer’s disease. Molecules 2017, 22, 277. [Google Scholar] [CrossRef]
- Frozza, R.L.; Bernardi, A.; Paese, K.; Hoppe, J.B.; da Silva, T.; Battastini, A.M.; Pohlmann, A.R.; Guterres, S.S.; Salbego, C. Characterization of trans-resveratrol-loaded lipid-core nanocapsules and tissue distribution studies in rats. J. Biomed. Nanotechnol. 2010, 6, 694–703. [Google Scholar] [CrossRef]
- Frozza, R.L.; Bernardi, A.; Hoppe, J.B.; Meneghetti, A.B.; Matté, A.; Battastini, A.M.; Pohlmann, A.R.; Guterres, S.S.; Salbego, C. Neuroprotective effects of resveratrol against aβ administration in rats are improved by lipid-core nanocapsules. Mol. Neurobiol. 2013, 47, 1066–1080. [Google Scholar] [CrossRef]
- Lu, W.; Shi, Y.; Jackson, A.C.; Bjorgan, K.; During, M.J.; Sprengel, R.; Seeburg, P.H.; Nicoll, R.A. Subunit composition of synaptic AMPA receptors revealed by a single-cell genetic approach. Neuron 2009, 62, 254–268. [Google Scholar] [CrossRef]
- Abozaid, O.A.R.; Sallam, M.W.; El-Sonbaty, S.; Aziza, S.; Emad, B.; Ahmed, E.S.A. Resveratrol-Selenium nanoparticles alleviate neuroinflammation and neurotoxicity in a rat model of Alzheimer’s Disease by regulating sirt1/miRNA-134/GSK3β Expression. Biol. Trace Elem. Res. 2022, 200, 5104–5114. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, W.; Chen, J.; Wang, N.; Zheng, G. A comparative study of resveratrol and resveratrol-functional selenium nanoparticles: Inhibiting amyloid β aggregation and reactive oxygen species formation properties. J. Biomed. Mater. Res. A 2018, 106, 3034–3041. [Google Scholar] [CrossRef] [PubMed]
- Davidov-Pardo, G.; McClements, D.J. Nutraceutical delivery systems: Resveratrol encapsulation in grape seed oil nanoemulsions formed by spontaneous emulsification. Food Chem. 2015, 167, 205–212. [Google Scholar] [CrossRef]
- Gupta, D.K.; Ahad, A.; Waheed, A.; Aqil, M.; Al-Jenoobi, F.I.; Al-Mohizea, A.M. Bilosomes: A novel platform for drug delivery. In Systems of Nanovesicular Drug Delivery; Nayak, A.M., Hasnain, M.S., Aminabhavi, T.M., Torchilin, V.P., Eds.; Academic Press: London, UK, 2022; pp. 293–309. [Google Scholar]
- Abbas, H.; Gad, H.A.; Khattab, M.A.; Mansour, M. The tragedy of Alzheimer’s Disease: Towards better management via resveratrol-loaded oral bilosomes. Pharmaceutics 2021, 13, 1635. [Google Scholar] [CrossRef]
- Rajput, A.P.; Butani, S.B. Resveratrol anchored nanostructured lipid carrier loaded in situ gel via nasal route: Formulation, optimization and in vivo characterization. J. Drug Deliv. Sci. Tech. 2019, 51, 214–223. [Google Scholar] [CrossRef]
- Pangeni, R.; Sharma, S.; Mustafa, G.; Ali, J.; Baboota, S. Vitamin E loaded resveratrol nanoemulsion for brain targeting for the treatment of Parkinson’s disease by reducing oxidative stress. Nanotechnology 2014, 25, 485102. [Google Scholar] [CrossRef]
- Nasr, M. Development of an optimized hyaluronic acid-based lipidic nanoemulsion co-encapsulating two polyphenols for nose to brain delivery. Drug Deliv. 2016, 23, 1444–1452. [Google Scholar] [CrossRef]
- Kaul, S.; Gulati, N.; Verma, D.; Mukherjee, S.; Nagaich, U. Role of nanotechnology in cosmeceuticals: A review of recent advances. J. Pharm. 2018, 2018, 3420204. [Google Scholar] [CrossRef]
- Ahirrao, M.; Shrotriya, S. In vitro and in vivo evaluation of cubosomal in situ nasal gel containing resveratrol for brain targeting. Drug Dev. Ind. Pharm. 2017, 43, 1686–1693. [Google Scholar] [CrossRef]
- Lakshmi, S.; Varija Raghu, S.; Elumalai, P.; Sivan, S. Alkoxy glycerol enhanced activity of oxyresveratrol in Alzheimer’s disease by rescuing tau protein. Neurosci. Lett. 2021, 759, 135981. [Google Scholar] [CrossRef]
- Agarwal, T.; Manandhar, S.; Kumar, B.H.; Famurewa, A.C.; Gurram, P.C.; Suggala, R.S.; Sankhe, R.; Mudgal, J.; Pai, K.S.R. Oxyresveratrol-β-cyclodextrin mitigates streptozotocin-induced Alzheimer’s model cognitive impairment, histone deacetylase activity in rats: In silico & in vivo studies. Sci. Rep. 2024, 14, 9897. [Google Scholar] [CrossRef]
- Baek, M.K.; Lee, J.H.; Cho, Y.H.; Kim, H.H.; Lee, G.W. Self-microemulsifying drug-delivery system for improved oral bioavailability of pranlukast hemihydrate: Preparation and evaluation. Int. J. Nanomed. 2013, 8, 167–176. [Google Scholar] [CrossRef][Green Version]
- Sangsen, Y.; Sooksawate, T.; Likhitwitayawuid, K.; Sritularak, B.; Wiwattanapatapee, R. A self-microemulsifying formulation of oxyresveratrol prevents amyloid beta protein-ınduced neurodegeneration in mice. Planta Med. 2018, 84, 820–828. [Google Scholar] [CrossRef]
- Liu, J.; Xu, J.; Jia, L.; Zhou, Y.; Fu, Q.; Wang, Y.; Mu, D.; Wang, D.; Li, N.; Hou, Y. Pterostilbene nanoemulsion promotes Nrf2 signaling pathway to downregulate oxidative stress for treating Alzheimer’s disease. Int. J. Pharm. 2024, 655, 124002. [Google Scholar] [CrossRef]
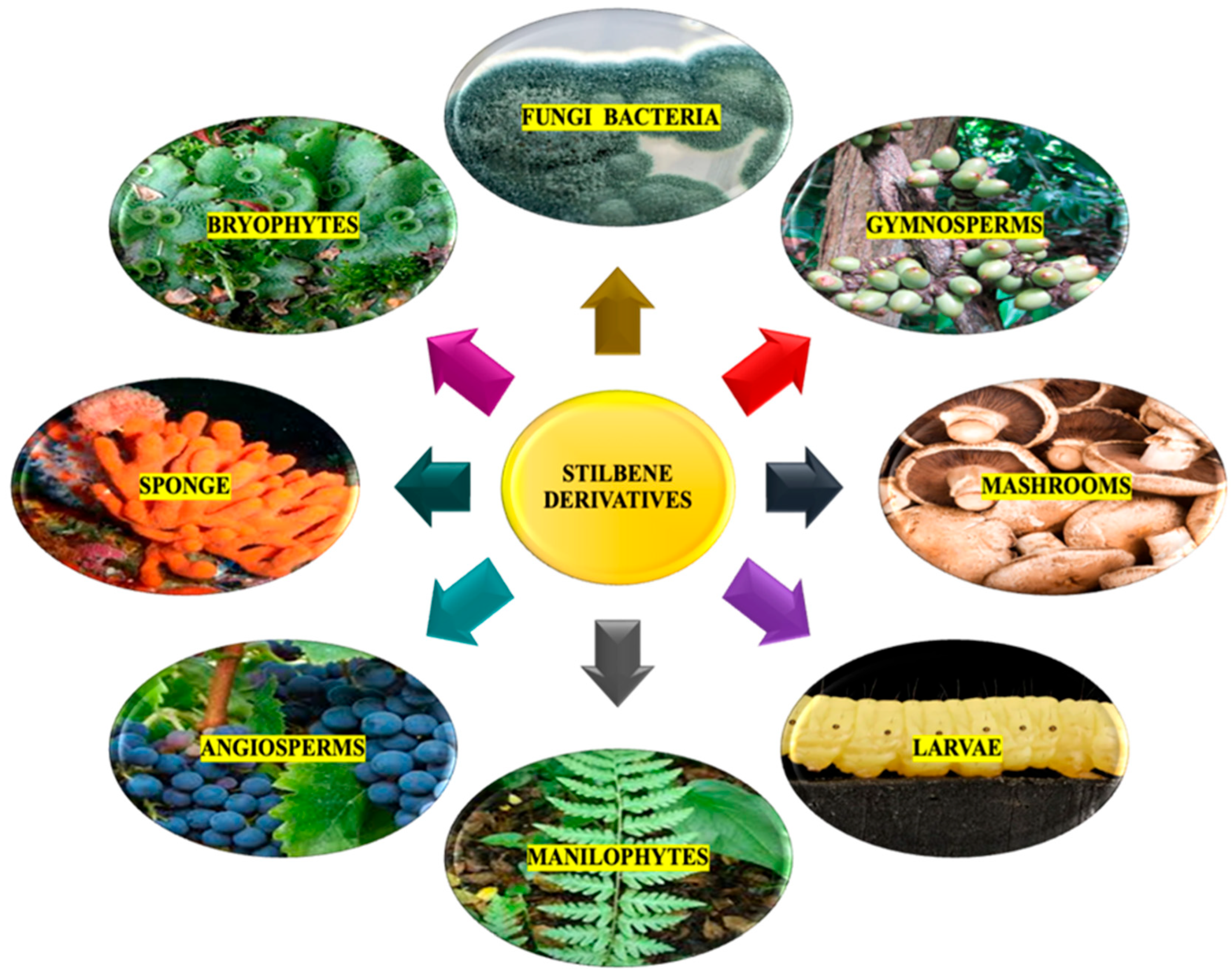

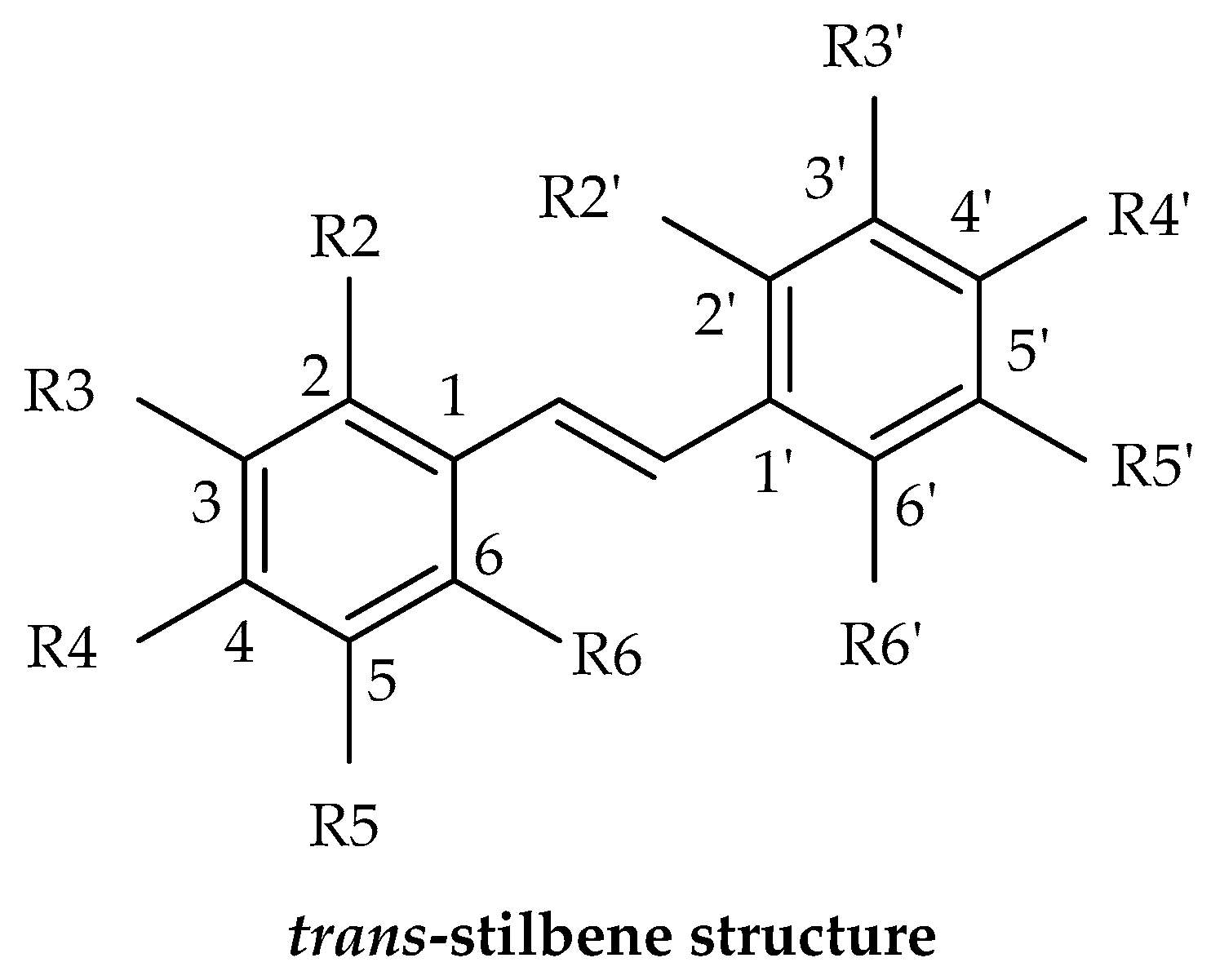



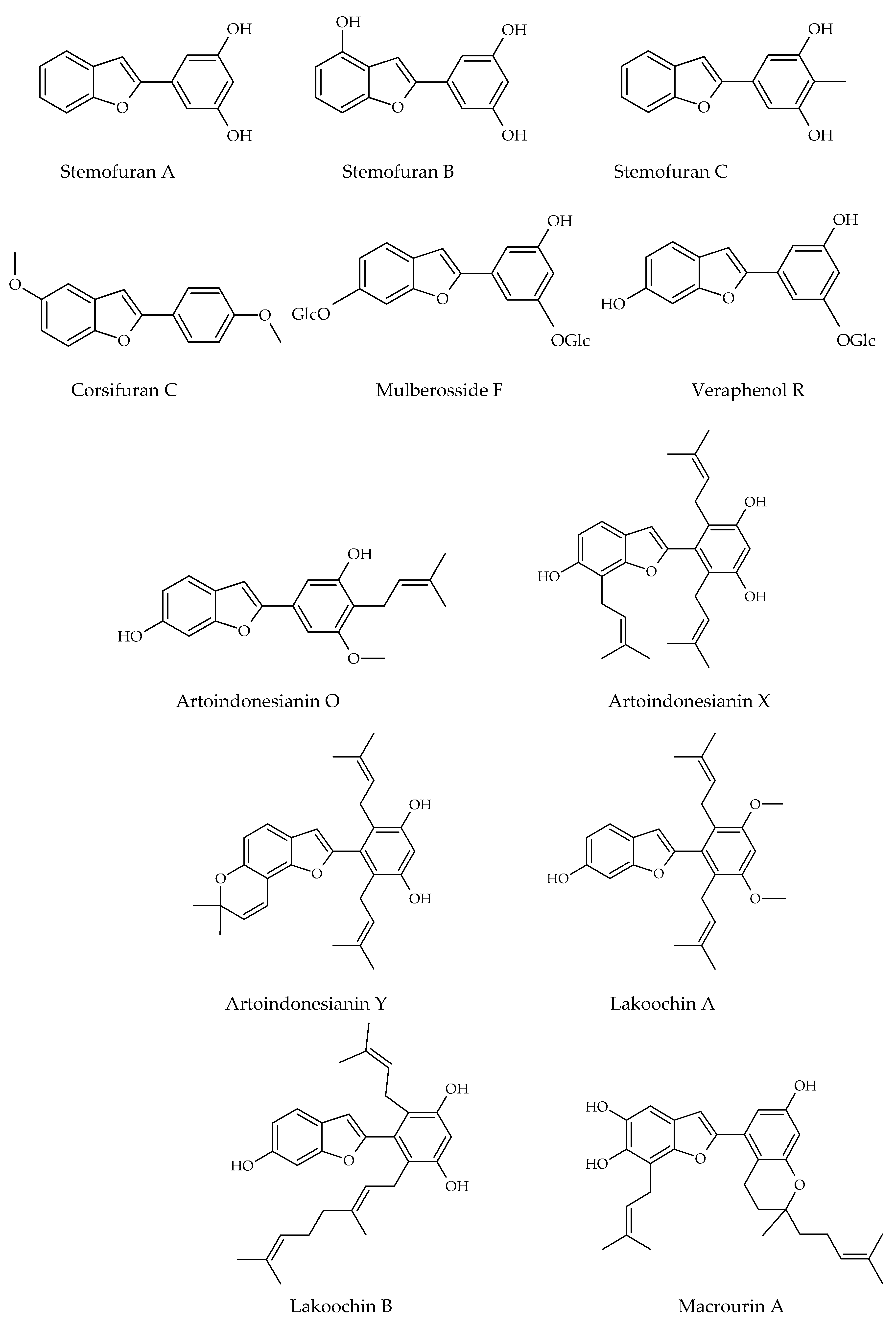
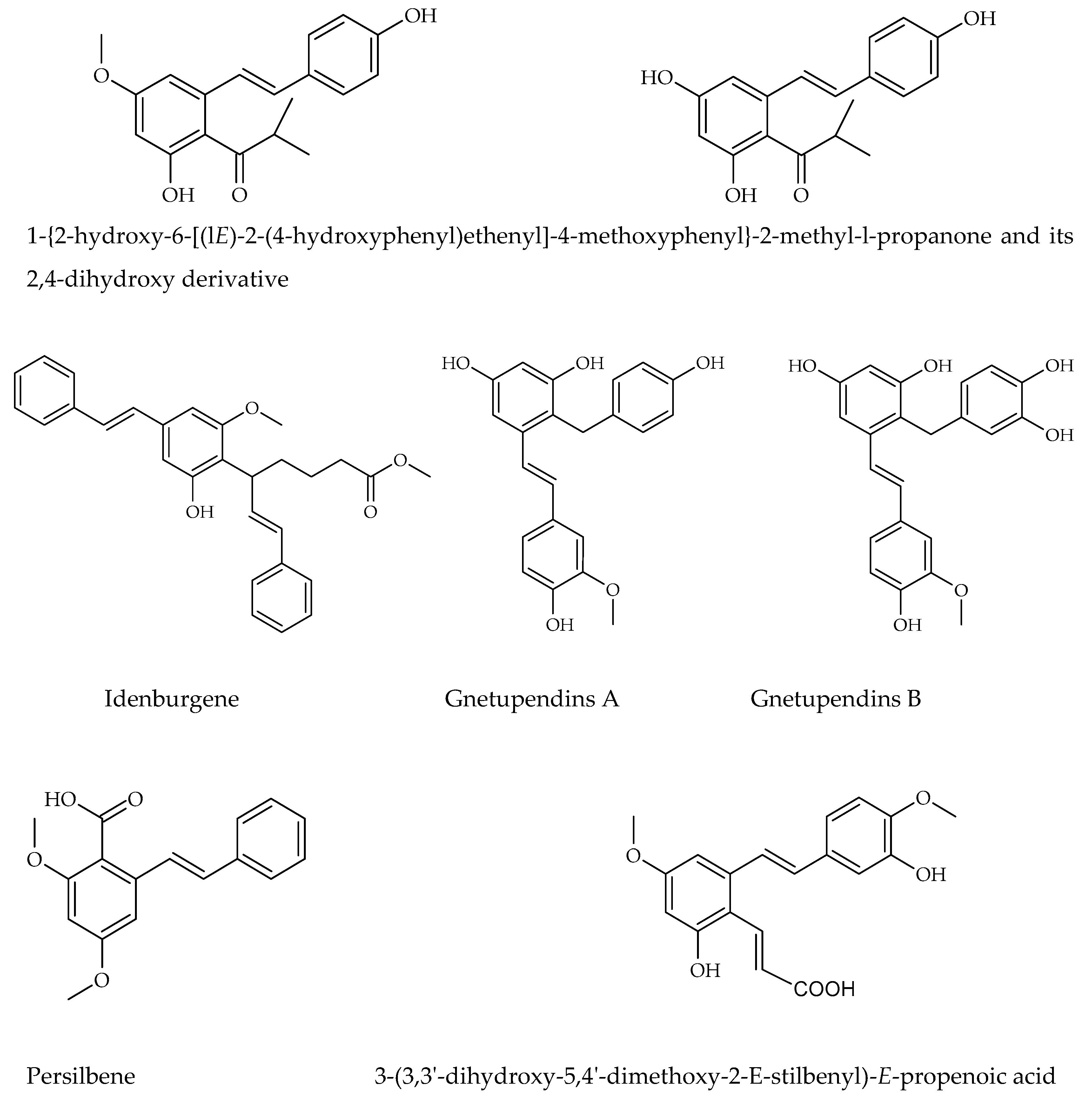
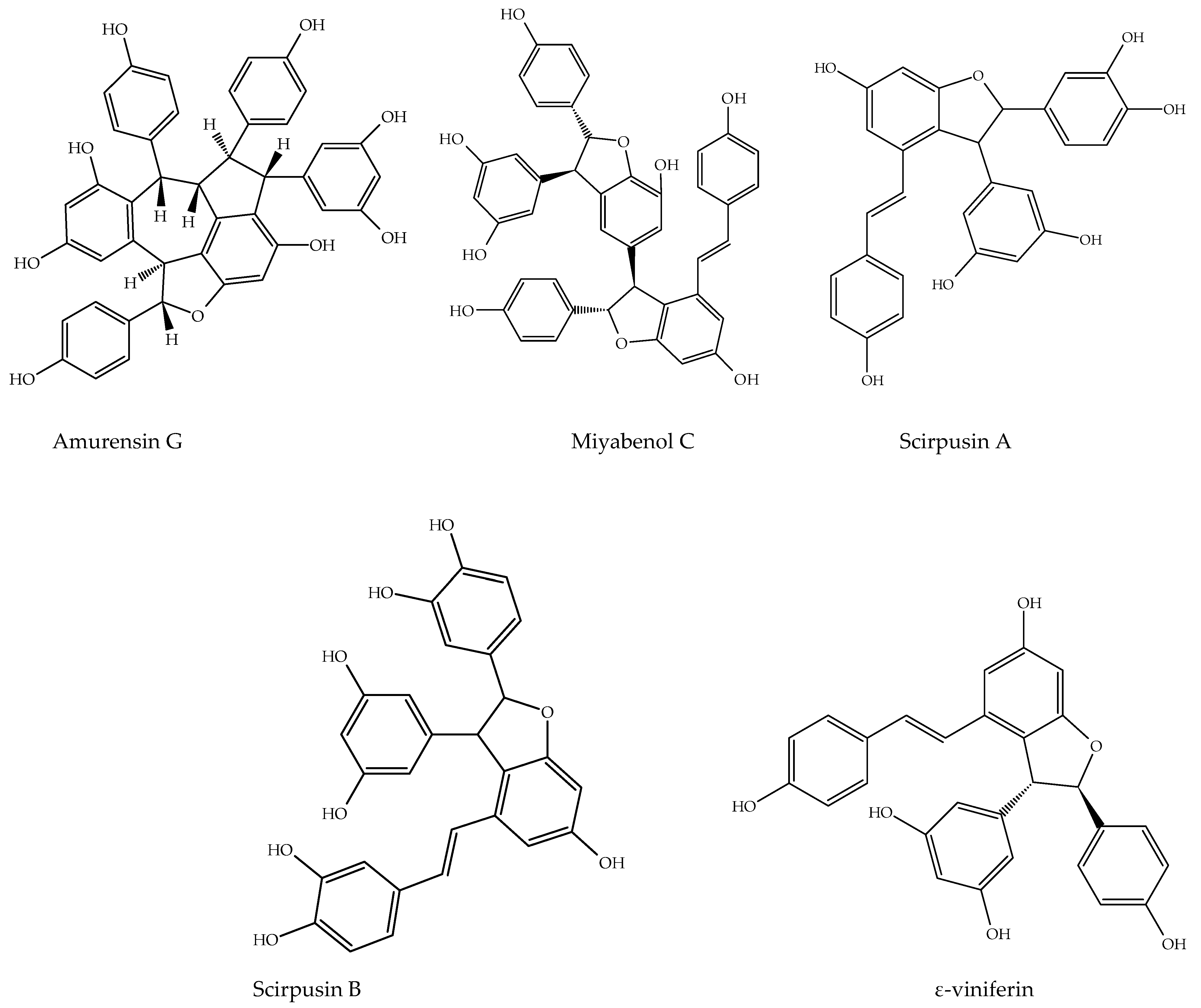
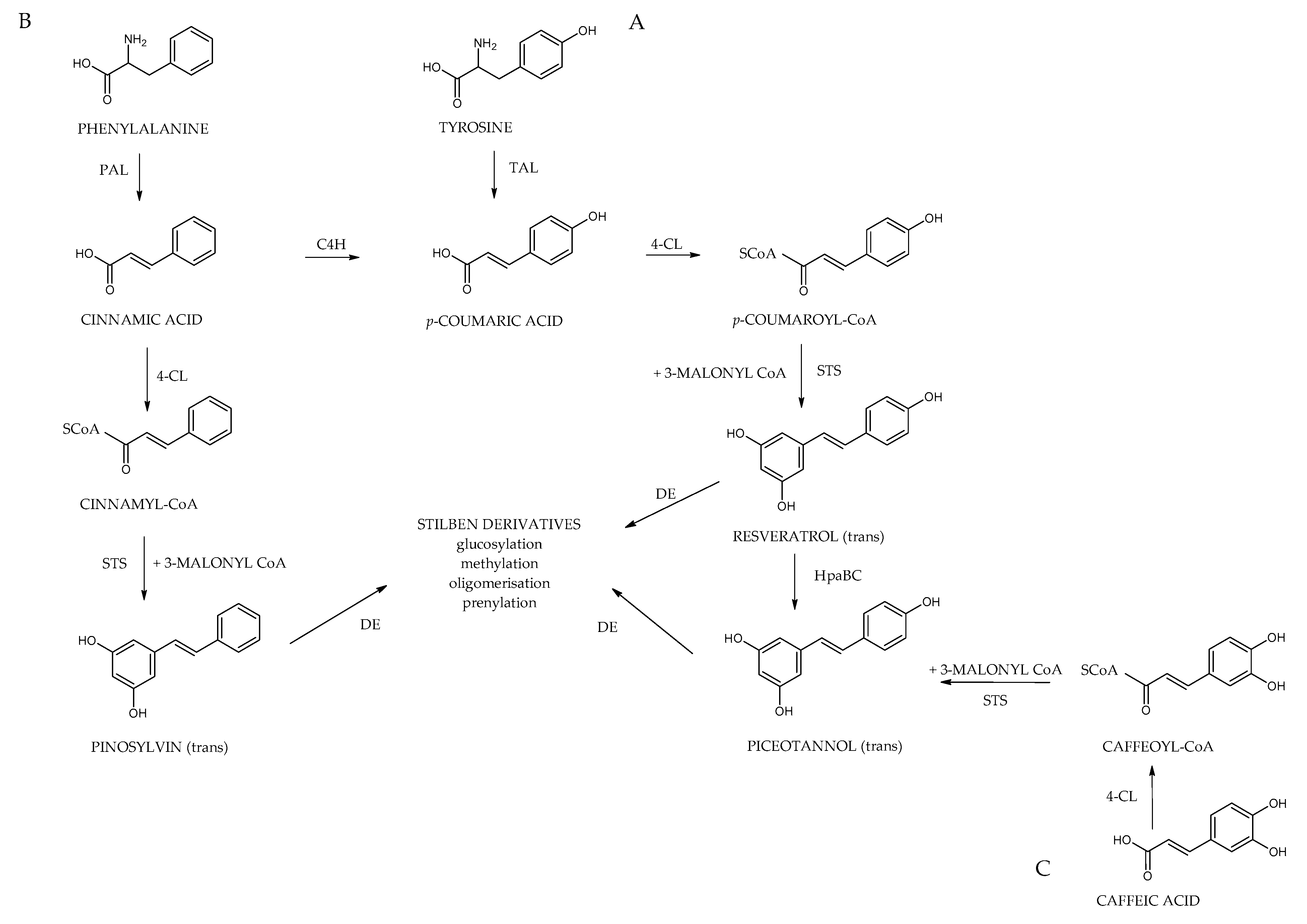


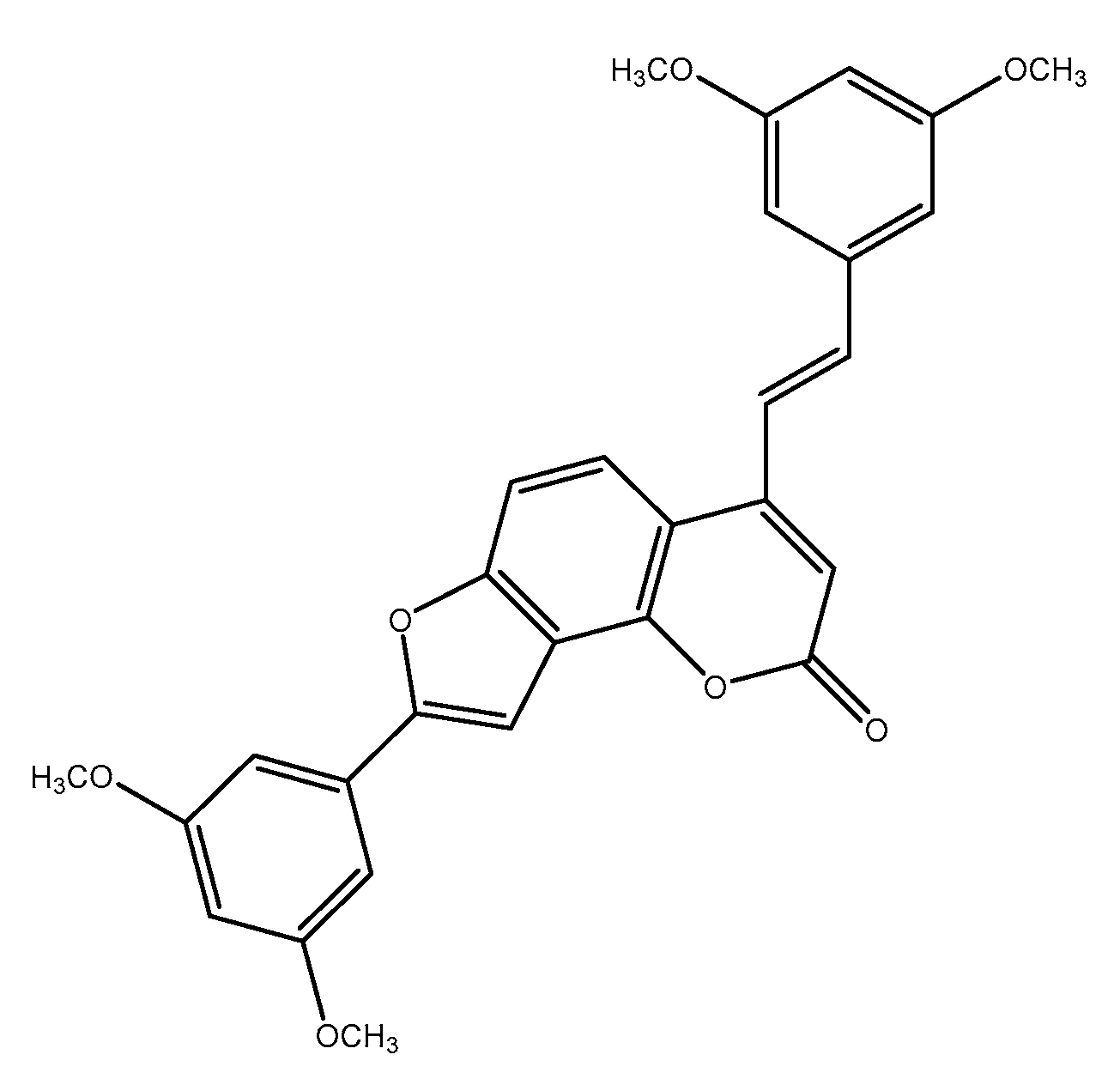
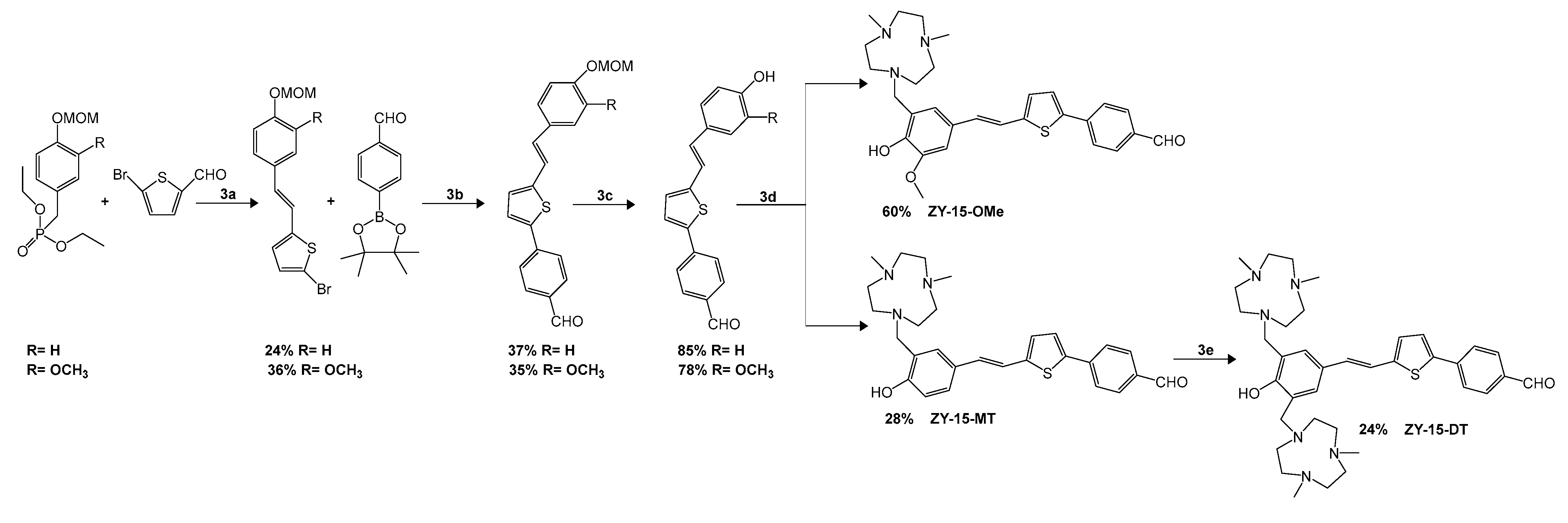
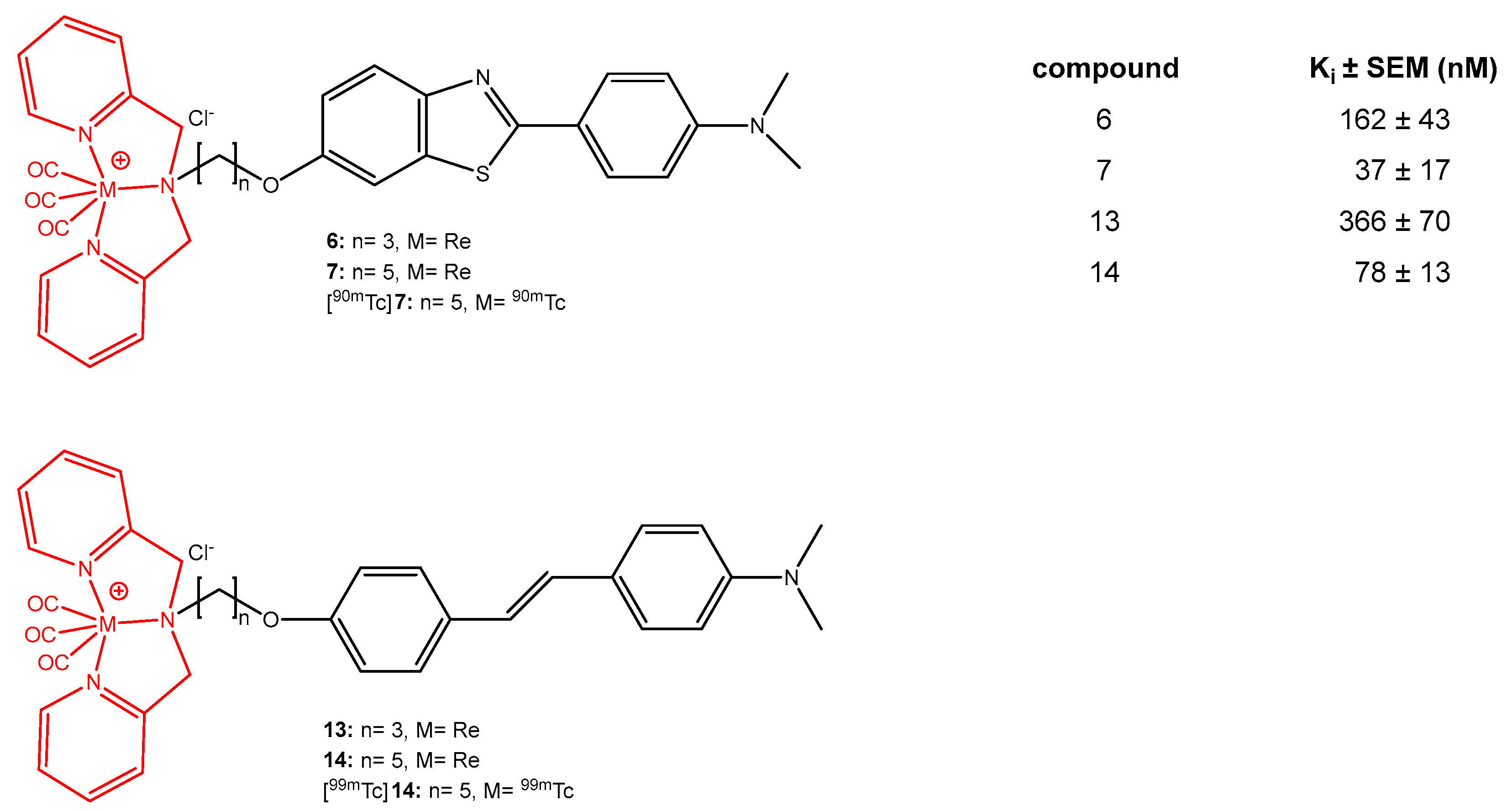
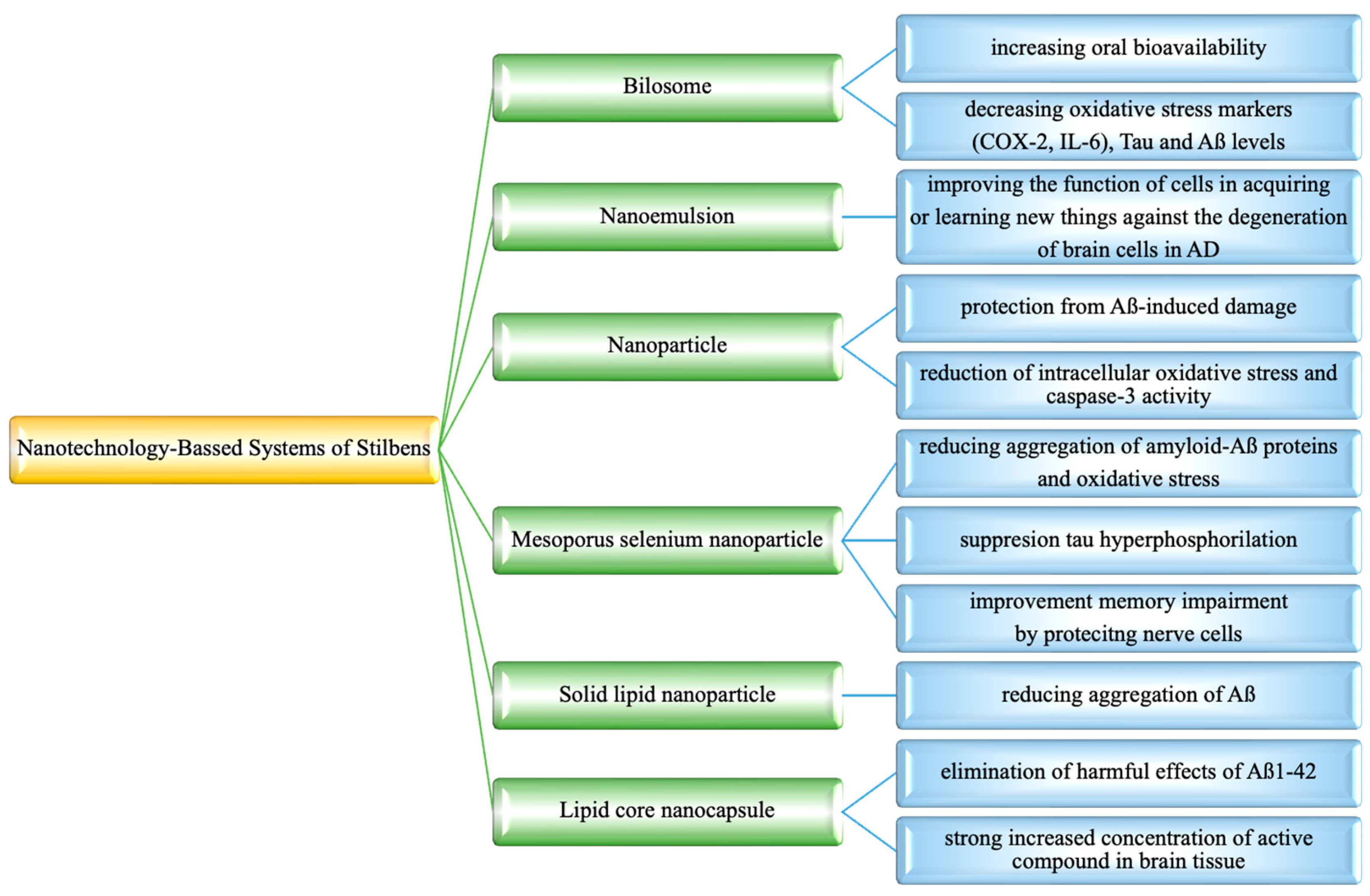
| Model | Concentration/Dose | Key Findings | Reference |
|---|---|---|---|
| In vitro (Aβ42 aggregation inhibition) | 50, 100 µM | Prevented sufuranyl free radical formation and cross β-sheet structures Decreased secondary structure content Decline in unordered nontoxic aggregates | [185] |
| In vitro (Aβ aggregation inhibition, microplate assay, tissue-based assay) | 40.8 nM | Blocked Aβ1-42-HiLyte488 molecules Aβ1-42 aggregation reduced by 50% | [146] |
| In vitro (BACE-1 inhibition, oxytosis inhibition) | IC50: 28 µM EC50: 4.667 µM | Inhibited BACE-1 Declined oxytosis | [145] |
| In vitro (BACE 1 inhibition) | 11.9 µM | Inhibited β-site APP-cleaving enzyme 1 (BACE-1) | [144] |
| In vitro (Caenorhabditis elegans AD model) | 100 µM | Mitigated Aβ toxicity Reduced Aβ aggregation and lysosomes amount Activated proteasomal degradation | [142] |
| In vitro (HEK293 cells) | 20–40 µM | Lowered Aβ40 and Aβ42 Activated AMPK Inhibited mTOR | [172] |
| Reduced secreted Aβ levels, activated AMPK, induced autophagy | [173] | ||
| 100 µM | Inhibited MID1-α4 complex Reduced MID1 transcript and protein level Increased PP2A activity and dephosphorylates Tau at PP2A-sensitive locations | [182] | |
| In vitro (HUVEC cells) | 30 µM | Reduced endothelin-1 secretion and mRNA levels | [164] |
| 10 µM | Increased t-PA and u-PA antigen levels Increased expression of surface-localized fibrinolytic activity | [167] | |
| In vitro (IMR-32 cells) | 30 µM | Upregulated Bcl-2, downregulated bax, protective against Aβ toxicity | [181] |
| In vitro (N9 cell line, LPS stimulated) | 15 and 30 μM | Inhibited MMP-9, iNOS, IL-1β, and IL-6 Overexpressed SIRT1 | [161] |
| In vitro (Neuro2a cells) | 10 μM | Resveratrol activates AMPK independently of SIRT1, promotes neurite outgrowth DecreaseD PGC-1α acetylation Increased PGC-1α activity | [159] |
| In vitro (PC12 cells, Aβ25–35 induced) | 25 μM | Deceased ROI formation Inhibited PARP cleavage and JNK phosphorylation Increased expression of Bcl-XL | [180] |
| In vitro (PC12 cells, Aβ induced) | Combinations 50 μM catechin and 10 μM resveratrol or 25 μM resveratrol and 10 μM catechin | Protective against ROS toxicity and Aβ toxicity | [179] |
| In vitro (Thioflavin-T assay SH-SY5Y human neuroblastoma cells, Aβ1-42 induced) | Molar ratios of 1:0.01, 1:0.1, 1:1, 1:10, and 1:100 1 µM | Cleaved Aβ1-42 peptide Reduced toxicity of Aβ1-42 | [15] |
| In vivo (C57Bl/6J mice) | 200–400 mg/kg/day for 15 weeks High fat diet with resveratrol | Increased PGC-1α protein Improved mitochondrial function Effective against obesity and insulin resistance | [154] |
| In vivo (C57BL/6J SIRT 1 knockout mice) | 25–30 mg/kg/day and 215–230 mg/kg of body weight/day with high fat diet | Activated AMPK in a SIRT1-independent manner Improved mitochondrial function | [156] |
| In vivo (LPS stimulated mice) | 4 mg/kg i.p. 7 days | Improved estrogen level Increased NEP level | [165] |
| In vivo (p25 transgenic mice (model of AD and tauopathies) | 5 μg/μL for 3 weeks intracerebroventricular injection | Reduced neurodegeneration Enhanced learning Lower caspase-3 levels Decreased astrogliosis in hippocampal regions Inhibited SIRT 1 substrates PGC-1alpha and p53 acetylation | [148] |
| In vivo (PS19 mice) | 40 mg/kg for 5 weeks | Reduced tau phosphorylation, neuroinflammation, and synapse loss, Restored cognitive deficits | [183] |
| In vivo (SAMP8 mice) | 1 g/kg for 7 months supplemented with trans-resveratrol diet | Reduced tau phosphorylation by inhibiting CDK5 and GSK3β Activated AMPK pathways and SIRT1 Increased ADAM10 expression Reduces Aβ burden | [147] |
| In vivo (Tg19959 mice) | 1 g/kg resveratrol-supplemented diet for seven months | Inhibited tau hyperphosphorylation and kinase activities such as CDK5, GSK3β No effect on JNK | [147] |
| 0.2% resveratrol with diet | Reduced plaque formation and Aβ deposition No significant changes on SIRT1 activation or APP processing | [174] | |
| In vivo (Tg2576 mice) | 0.2 mg/L in Cabernet Sauvignon | Positive effect on APP processing, preventing Aβ peptide generation | [175] |
| In vivo (Tg6799 mice) | 60 mg/kg for 60 days oral gavage | Decreased amyloid plaques Reduced Aβ42 and Aβ40 levels in the hippocampus Decreased APP, sAPPα, sAPPβ, and BACE1 (β-secretase) expression levels | [14] |
| In vivo (Wild-type C57BL/6 mice) | 25 mg/kg i.p. for 2 weeks | Reduced tau phosphorylation dephosphorylation of Tau in cortical neurons | [182] |
| Lifespan determination using PSY316AT MATα HEK 293 cells | 100–200 µM 0.5 µM | Lowered Michaelis constant of SIRT1 Increases cell survival Stimulated SIRT1-dependent deacetylation of p53 Stimulated Sir2, Increased DNA stability | [162] |
| Model | Concentration/Dose | Key Findings | Reference |
|---|---|---|---|
| In vitro (PC12 cells, Aβ25-35-induced) | 10, 20 µM | Inhibited internucleosomal DNA fragmentation, nucleus condensation, PARP cleavage, caspase-3 Reduced ROS formation | [187] |
| In vitro (Neuro2a cells, Hek 293 APPsw cells) | 10–80 µM (oxyresveratrol), 2.5–20 µM (piceatannol) | Prevented sAPP secretion, decreased γ-secretase activity, increased α-secretase activity Activated MMP-9 Reduced Aβ1–40 and Aβ1–42 levels | [188] |
| In vitro (THP-1 cells) | 6–10 µM | Increased SIRT1 protein expression in a concentration-dependent manner | [189] |
| In vitro (PC-12 cells, H2O2-induced) | 5–20 µM | Improved mitochondrial function Increased TFAM, PGC-1α Restored SIRT3 expression | [191] |
| In vitro (Neuro2a cells, high glucose-induced) | 5, 10 µM | Reduced mitochondrial superoxide production Stabilized mitochondrial membrane potential | [192] |
| In vitro (PC12 cells, Aβ-induced) | 25 µM | Enhanced phosphorylation of Akt and Bad Inhibited Bcl-2/Bax expression Suppressed cleavage of caspase-9, caspase-3 and PARP | [197] |
| In vitro (ndSH-SY5Y cells, Aβ-induced) | 1 µM | Suppressed Aβ-induced neurite fragmentation and neuronal cell death | [196] |
| In vitro (Rat primary cortical neurons, Aβ25-35-induced) | 20, 50 µM | Promoted cell survival, reduced ROS levels Activated PI3K/Akt pathway Inhibited caspase-9, caspase-3, and PARP cleavage | [198] |
| In vitro (N2a Cells, Colistin-induced) | 5, 50 µM | Suppressed ROS, activated NRF2/HO-1 pathway Protected against apoptosis | [199] |
| In vitro (Antimycin A-induced ROS model, C2C12 cells) | 10, 30 and 50 µM | Reduced ROS, inhibited apoptosis more effectively than resveratrol Induced heme oxygenase-1 (HO1) expression Prevention from mitochondrial ROS-induced cell death via SIRT1-dependent and -independent pathways | [193] |
| In vitro (DPPH, AChE inhibition, amyloid aggregation) | IC50: 40.2 µM DPPH 271.74 µM AChE Inhibition 0.48 µM amyloid aggregation | Piceatannol most active in DPPH scavenging, AChE inhibition, and Aβ peptide aggregation | [186] |
| In vivo (Rat, γ-radiation/reserpine-induced) | 10 mg/kg BW/day (oral) | Enhanced mitochondrial biogenesis via SIRT1/p38-AMPK Reduced oxidative stress, inflammation, and apoptosis | [16] |
| In vivo (CIRI mouse model) | 10, 20 mg/kg/day (oral) | Protected hippocampus neurons Improved neurological function Reduced ROS, Bax, and caspase 3 | [190] |
| Model | Concentration/Dose | Key Findings | Reference |
|---|---|---|---|
| In vitro (BACE1 inhibition) | 1.5 × 105 M | Oxyresveratrol was a specific BACE1 inhibitor with reduced effect on other proteases | [171] |
| In vitro (BV-2 cells, LPS-induced) | 10 µM | Reduced inflammation via MAPKs and NF-κB signaling Inhibited pro-inflammatory mediators | [190] |
| In vitro (Cortical astrocyte and neuron cultures) | 10 µM | Lower p62 expression and p62/SQSTM1 levels Increased the expression of lysosome-associated membrane protein 1 (LAMP1) Attenuated APP production Regulating AMPK/ULK1/mTOR-dependent autophagy induction | [17] |
| In vitro (HMC3 cells, IL-1β-induced) | 10, 20, 40 µM | Suppressed PI3K/AKT/p70S6K pathway activation Reduced IL-6 and MCP-1 release in microglial cells | [201] |
| In vitro (Rat cortical neurons, Aβ25-35-induced) | 1, 10 µM | Inhibited [Ca2+]c increase, glutamate release, ROS production Prevented apoptosis | [202] |
| In vitro (SH-SY5Y cells, H2O2-induced) | 5–100 µM | Neuroprotective effect by mitigating oxidative stress, Reducing ROS Preventing lipid peroxidation | [207] |
| In vitro (SH-SY5Y cells, rotenone-induced) | 10, 20 µM | Increased cell viability, GSH levels Decreased MMP, ROS, Bax, cytochrome C, and caspase-3 activity | [204] |
| In vivo (MCAO rat, I/R damage) | 10, 20 mg/kg (oral) | Reduced brain infarct volume Improved neurological impairments Blocked caspase-3 activation and cytochrome C release | [203] |
| Model | Concentration/Dose | Key Findings | Reference |
|---|---|---|---|
| In vitro (Neuroblastoma cells, SH-SY5Y) | 2.5, 5, or 10 μM | Reduced ROS Delayed cell death Improved mitochondrial function Increased Nrf2, HO-1, and GST levels | [208] |
| In vitro (HT22 cell neurons, glutamate-induced) | 1, 3 or 10 μM | Reduced oxidative stress through Nrf2 activation Decreased glutamate-induced apoptosis Increased GSH levels and SOD enzymatic activity Enhanced HO-1 expression and reversed NQO1 downregulation | [209] |
| In vitro (PC12 cells, H2O2-induced) | 10 μM | Pinosylvin most effective in reducing cell death Improving mitochondrial function Activating Nrf2/PINK1/Parkin pathway | [210] |
| In vitro (PC12 cells, Aβ25-35-induced) | 25, 50 μM | Increased cell viability, reduced apoptosis and ROS Activated PI3K/Akt pathway | [197] |
| In vitro (BV-2 cells, Aβ1−42-induced) | 5, 10 μM | Suppressed iNOS/NO Reduced NLRP3/caspase-1 inflammasome activation Decreased TNF-α, IL-1β, IL-6 | [13] |
| In vitro (Isolated mouse neurons, Aβ25-35-induced) | 2 μM | Inhibited mitochondrial apoptosis Increased neural plasticity Slowed neuronal loss via SIRT1/Nrf2-mediated mechanisms | [217] |
| In vivo (SAMP8 mice) | 120 mg/kg diet | Restored cognitive performance Rduced Tau phosphorylation No significant effect on SIRT1 or acetylated p53 | [211] |
| In vivo (Rat, streptozotocin-induced memory loss | 10, 30, 50 mg/kg (oral) | Improved memory Increased cholinergic transmission Enhanced brain antioxidant activity (SOD, catalase, GSH) Reduced nitrite, lipid peroxides | [212] |
| In vivo (Rat, streptozotocin-induced) | 20 mg/kg/day (5 weeks) | Reduced Aβ1−42 accumulation, tau hyperphosphorylation, neuronal apoptosis, inflammation Increased SOD and GSH | [213] |
| In vivo (Mice, Aβ1-42-induced) | 10, 20, 40 mg/kg (oral) | Mitigated neuron loss Reduced ROS, increased antioxidant genes Promoted Nrf2 translocation | [214] |
| In vivo (Rat, Morris water maze) | 40, 160 mg/kg diet | Enhanced cognitive performance and working memory Reversed dopamine release | [215] |
| In vivo (Aged rat, working memory test) and two declarative memory tests | 22.5 mg/kg (oral) | Reversed aging effects on memory Increased REST, PSD-95, mitochondrial porin1, CREB phosphorylation | [216] |
| In vivo (Male KM mice, Aβ25-35-induced) | 40 mg/kg (oral) | Increased expression of SIRT1 Enhanced learning–memory, working-memory, spatial learning-memory Increased NeuN, PSD-95, and SYN-1 proteins Increased Bcl2/Bax | [217] |
| Compound(s) | Target Action | Specific Effects | Concentration | Reference |
|---|---|---|---|---|
| 2-methoxy-5-(2,3,4-trimethoxypheny) styrylpyridine | Reduction MDA, Increasing SOD activity and gSH levels, Inhibition of NO | Decrease lipid peroxidation and nitric oxide content by inhibiting inducible NOS (iNOS) expression and activity Increase free radical scavenging activity Restore endogenous antioxidation Inhibit apoptosis through caspase-3 and caspase-9 inhibition Protect cell membrane integrity | 25–100 μmol/L | [295] |
| 3,3′-dimethoxy-4,4′-dihydroxystilbene triazole (DMDHSB) | Aβ-induced neuronal damage protection | Reverses up-regulation of iNOS production Prevents Aβ treatment-associated increase in NF-kB nuclear translocation | 8 μM | [299] |
| Amphiphilic compounds | Targeting AβO | Disrupt interactions between AβO and cell membranes | 10 mM | [294] |
| Carbazole-stilbene hybrids | ChE inhibition, Aβ aggregation inhibition, Antioxidant, Metal chelation | Potent inhibitory activity against AChE and BChE Significant inhibition of self-mediated Aβ1-42 aggregation | IC50: 2.64 μM (for AChE), 1.29 μM (for BChE) | [297] |
| Coumarin-resveratrol-inspired hybrids | MAO-B inhibition | Significant potency as MAO-B inhibitors, high selectivity | pIC50: 6.959 (for trans-6-Styrylcoumarin) | [282] |
| Furocoumarin-stilbene hybrids | AChE, BChE, β-secretase, COX-2, and LOX-5 inhibition, Antioxidant | Significant anticholinesterase activity Effective inhibition of β-secretase, COX-2, and LOX-5 Strong antioxidant properties | 6.8 µM (DPPH) | [283] |
| Hydroxyl-functionalized stilbenes, 2-arylbenzo[b]furans | Neuroprotection against Aβ and glutamate-induced toxicity | Protect neurons from Aβ-mediated and glutamate-induced toxicity Anti-neuroinflammatory effects | 50 μM | [290] |
| Pinostilbene | 6-hydroxydopamine-induced neurotoxicity protection | Reduces LDH release and caspase-3 activity | 1 to 10 μM | [276] |
| Pterostilbene β-amino alcohol derivatives | AChE and BChE inhibition, Antioxidant, Neuroprotective | Potent inhibitory effect on EeAChE, antioxidant properties Neuroprotective against H2O2-induced injury | IC50: 24.04 μM (5f for AChE), 40.23% (Aβ1-42 aggregation) | [298] |
| Pterostilbene/ Resveratrol Carbamate derivative | Inhibition of AChE and BChE, enhancing cholinergic neurotransmission | Dual ChE inhibitor Neuroprotective | 6.3 µM (AChE, Rivastigmine Tartrate), 1.37 µM (BChE, Rivastigmine Tartrate) | [278] |
| Pyridoxine-resveratrol hybrids | Dual inhibition of AChE and MAO-B | High potency in inhibiting AChE and MAO-B | IC50: 2.11 μM (7d), 1.56 μM (8b) for AChE; 2.68 μM (7e) for MAO-B | [208] |
| Rhenium complexes | Targeting Aβ plaques in cerebral amyloid angiopathy | Moderate affinity towards Aβ1-42 aggregates, intense labeling of Ab plaques, potential as SPECT imaging agents | Binding affinity with a Ki value of 37 Nm (complex 7), Ki value of 78 Nm (complex 14) | [301] |
| Stilbene, Benzofuran neolignan derivatives | AChE inhibition, Neuroprotectiv, Anti-inflammatory | Inhibit AChE and NO production Protect against cell damage | IC50: 39.3 ± 1.2 μM (Grossamide), 29.8 ± 0.9 μM (Boehmenan) | [292] |
| Tacrine-resveratrol hybrid | AChE inhibition, Aβ aggregation inhibitionA | Potent inhibition of hAChE Impedes Aβ self-aggregation | IC50: 0.80 μM (5 for hAChE) | [271] |
| Trans-stilbene derivative | Neuroprotection without significant interference with estrogen receptor or aryl hydrocarbon receptor signaling | Neuroprotective | EC:12 µM | [303] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Küpeli Akkol, E.; Karatoprak, G.Ş.; Dumlupınar, B.; Bahadır Acıkara, Ö.; Arıcı, R.; Yücel, Ç.; Aynal, L.C.; Sobarzo Sánchez, E. Stilbenes Against Alzheimer’s Disease: A Comprehensive Review of Preclinical Studies of Natural and Synthetic Compounds Combined with the Contributions of Developed Nanodrug Delivery Systems. Molecules 2025, 30, 1982. https://doi.org/10.3390/molecules30091982
Küpeli Akkol E, Karatoprak GŞ, Dumlupınar B, Bahadır Acıkara Ö, Arıcı R, Yücel Ç, Aynal LC, Sobarzo Sánchez E. Stilbenes Against Alzheimer’s Disease: A Comprehensive Review of Preclinical Studies of Natural and Synthetic Compounds Combined with the Contributions of Developed Nanodrug Delivery Systems. Molecules. 2025; 30(9):1982. https://doi.org/10.3390/molecules30091982
Chicago/Turabian StyleKüpeli Akkol, Esra, Gökçe Şeker Karatoprak, Berrak Dumlupınar, Özlem Bahadır Acıkara, Reyhan Arıcı, Çiğdem Yücel, Leyli Can Aynal, and Eduardo Sobarzo Sánchez. 2025. "Stilbenes Against Alzheimer’s Disease: A Comprehensive Review of Preclinical Studies of Natural and Synthetic Compounds Combined with the Contributions of Developed Nanodrug Delivery Systems" Molecules 30, no. 9: 1982. https://doi.org/10.3390/molecules30091982
APA StyleKüpeli Akkol, E., Karatoprak, G. Ş., Dumlupınar, B., Bahadır Acıkara, Ö., Arıcı, R., Yücel, Ç., Aynal, L. C., & Sobarzo Sánchez, E. (2025). Stilbenes Against Alzheimer’s Disease: A Comprehensive Review of Preclinical Studies of Natural and Synthetic Compounds Combined with the Contributions of Developed Nanodrug Delivery Systems. Molecules, 30(9), 1982. https://doi.org/10.3390/molecules30091982








