Mechanistic Advances in Hypoglycemic Effects of Natural Polysaccharides: Multi-Target Regulation of Glycometabolism and Gut Microbiota Crosstalk
Abstract
1. Introduction
2. Structure–Activity Relationships
2.1. Molecular Mass
2.2. Branching Complexity
2.3. Chemical Modification
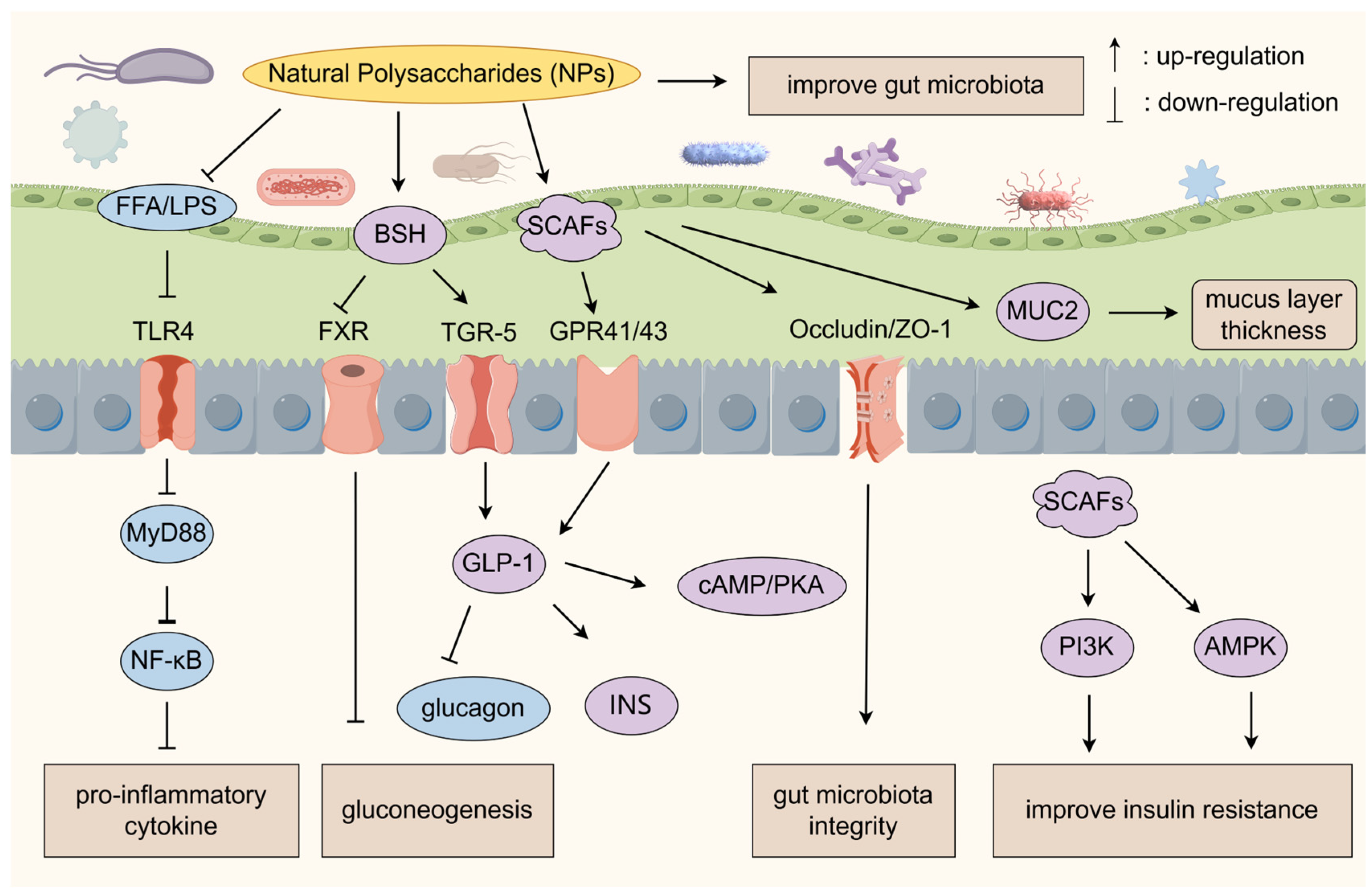
3. Regulation of Gut Microbiota
3.1. Gut–Liver Axis Regulatory Network
3.2. Gut Microbiota–Immunity Axis Regulatory Network
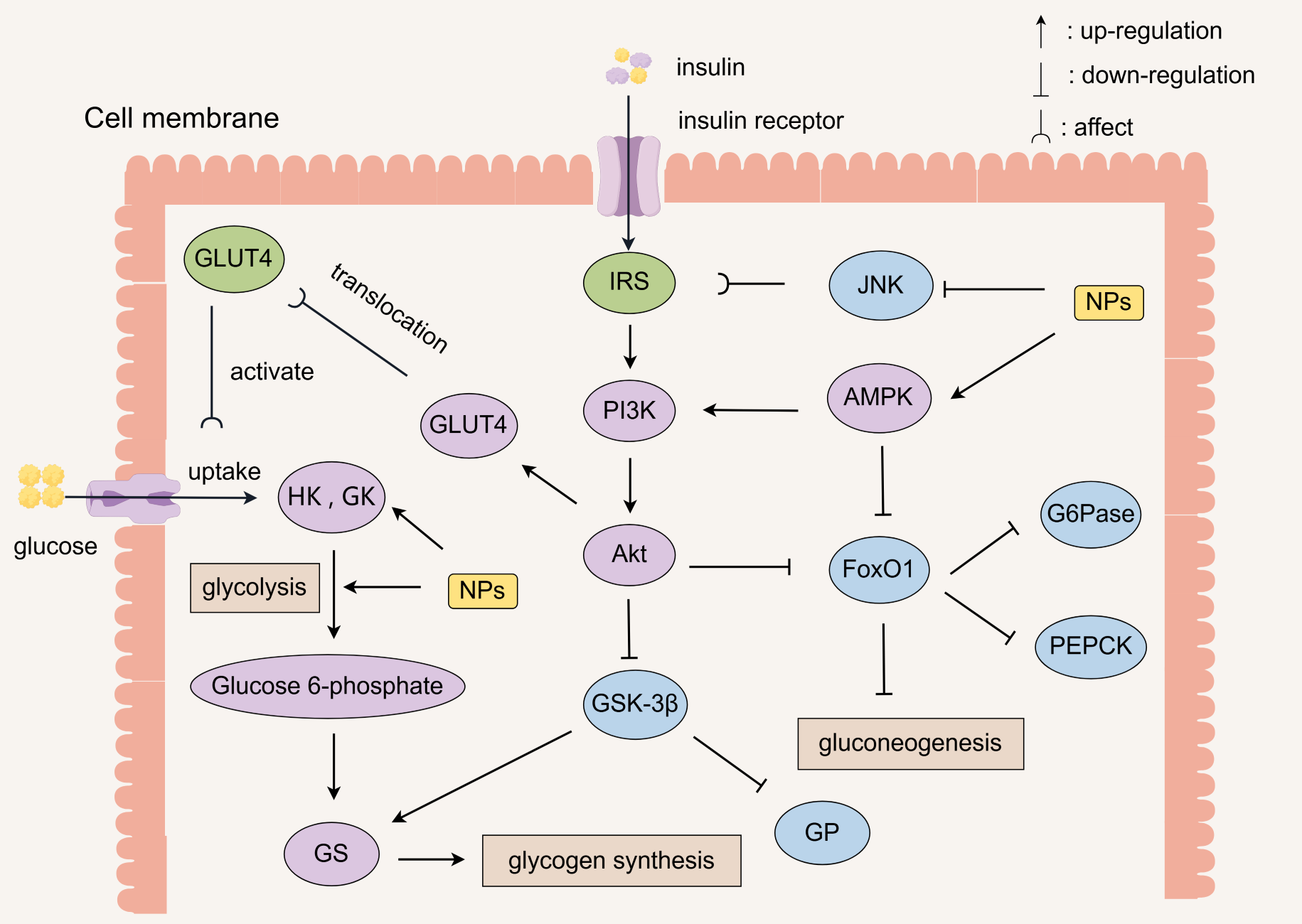
4. Regulation of Metabolism
4.1. Enhanced Insulin Signaling
4.2. Improvement of Insulin Resistance
4.3. Inhibition of Sugar Digestion
4.4. Regulation of Key Enzyme Activities
4.5. Inhibition of Gluconeogenesis
4.6. Improvement of Glucose Tolerance
4.7. Protecting Pancreatic Beta Cells
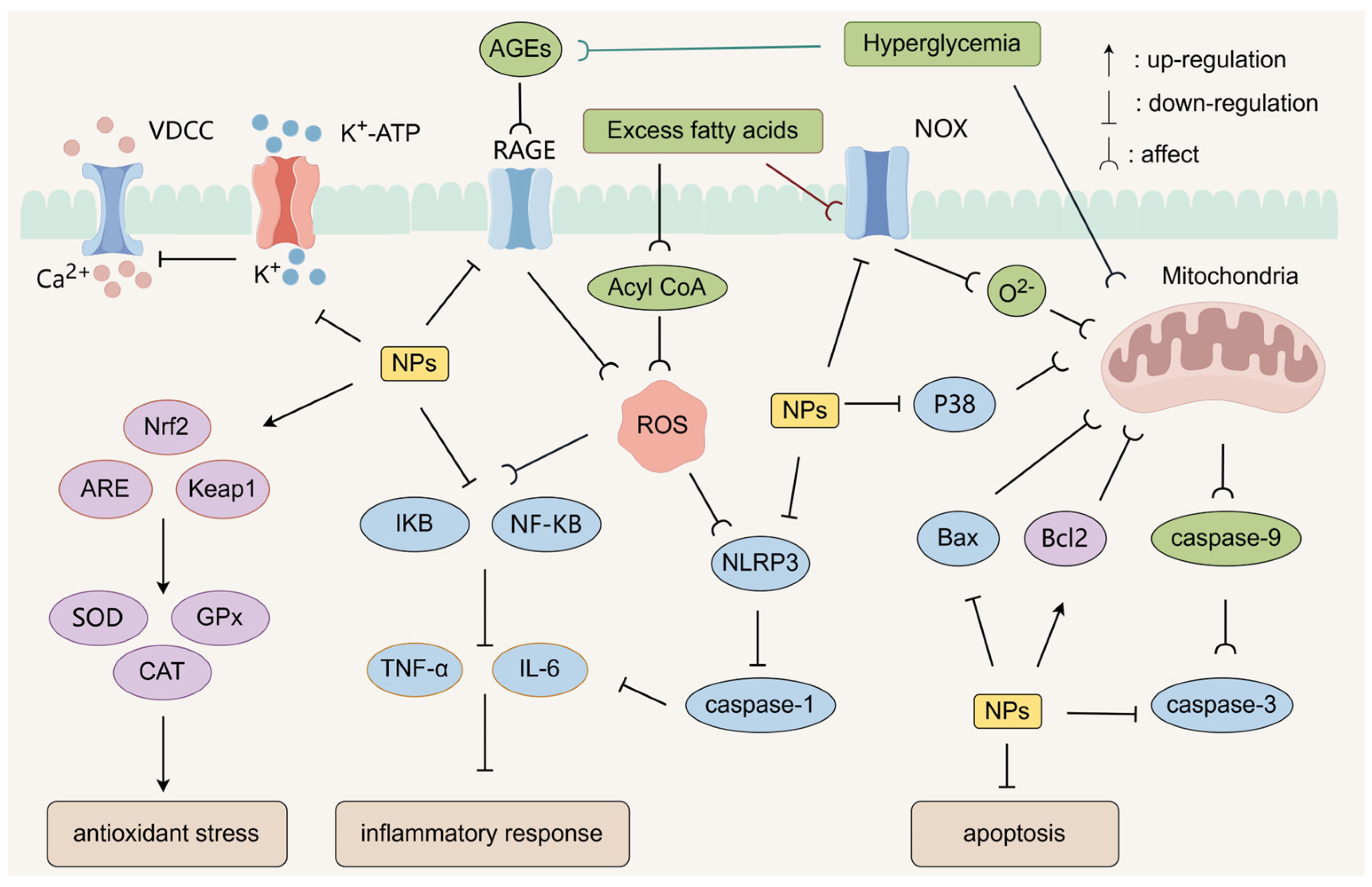
4.8. Antioxidant Stress
5. Regulation of Immunity
6. Clinical Application Strategies
6.1. Delivery System
6.2. Design of Combination Drug Programs
6.3. Individualized Treatment Strategies
7. Discussion
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AC | Gαs-adenylate cyclase |
| ACC | Acetyl coenzyme A carboxylase |
| Acetyl-CoA | Acetyl-coenzyme A |
| Acyl CoA | Acyl coenzyme A |
| AGEs | Advanced glycation end-products |
| Akt | Protein kinase B |
| AMPK | AMP-Activated Protein Kinase |
| AP-1 | Activator Protein 1 |
| ARE | Antioxidant Response Element |
| ASC | Apoptosis-associated speck-like protein containing a card |
| BAs | Bile acids |
| BCAAs | Branched-chain amino acids |
| Bcl-2 | B-cell lymphoma-2 protein |
| BSH | Bile salt hydrolase |
| CamkII | Calmodulin-dependent Protein Kinase II |
| CaMKKβ | Calmodulin-dependent Protein Kinase β |
| CAT | Catalase |
| COX-2 | Cyclooxygenase-2 |
| CPT-1 | Carnitine palmitoyltransferase-1 |
| CREB | cAMP response element-binding protein |
| CYP7A1 | Cholesterol 7α-hydroxylase |
| Dectin-1 | Dendritic cell-associated C-type lectin-1 |
| FBPase | Fructose-1,6-bisphosphatase |
| FFA | Free fatty acid |
| FFAR | Free fatty acid receptors |
| FoxO1 | Forkhead box protein O1 |
| FXR | Farnesoid X Receptor |
| G6Pase | Glucose-6-phosphatase |
| GK | Glucokinase |
| GLP-1 | Glucagon-like peptide-1 |
| GLUT | Glucose Transporter |
| GLUT2 | Glucose Transporter 2 |
| GLUT4 | Glucose Transporter 4 |
| GP | Glycogen phosphorylase |
| GPCRs | G protein-coupled receptors |
| GPR41 | Free Fatty Acid Receptor 3 |
| GPR43 | Free Fatty Acid Receptor 2 |
| GPx | Glutathione peroxidase |
| GS | Glycogen synthase |
| GSH | Glutathione |
| GSK-3β | Glycogen synthase kinase 3β |
| HDACs | Histone deacetylases |
| HK | Hexokinase |
| HMG-CoA | 3-hydroxy-3-methylglutaryl coenzyme A |
| IFN-γ | Interferon γ |
| IL-18 | Interleukin-18 |
| IL-1β | Interleukin-1β |
| IL-6 | Interleukin-6 |
| iNOS | Inducible nitric oxide synthase |
| INS | Insulin |
| INSR | Insulin receptor |
| IR | Insulin resistance |
| IRS | Insulin receptor substrate |
| IκBα | Inhibitor of nuclear factor kappa B alpha |
| LDLR | Low-Density Lipoprotein Receptor |
| LKB1 | Liver Kinase B1 |
| LPS | Lipopolysaccharides |
| MAPK | Mitogen-activated protein kinase |
| MDA | Malondialdehyde |
| MKP-1 | Mitogen-activated protein kinase phosphatase-1 |
| MUC2 | Mucin 2 |
| MyD88 | Myeloid differentiation factor 88 |
| NADPH | Reduced nicotinamide adenine dinucleotide phosphate |
| NF-κB | Nuclear factor κB |
| NOX | NADPH oxidase |
| NOX-1 | NADPH oxidase 1 |
| NPs | Natural polysaccharides |
| Nrf2 | Nuclear factor e2-related factor 2 |
| p-Akt | Phosphorylated Akt |
| p-AMPK | Phosphorylated AMP-activated protein kinase |
| PARP | Poly (ADP-ribose) polymerase |
| PC | Pyruvate carboxylase |
| PDX-1 | Pancreatic and duodenal homeobox 1 |
| PEPCK | Phosphoenolpyruvate carboxykinase |
| p-FoxO1 | Phosphorylated forkhead box protein o1 |
| PGC-1α | Peroxisome proliferator-activated receptor γ coactivator-1α |
| PI3K | Phosphatidylinositol 3-kinase |
| p-IRS | Phosphorylated Insulin Receptor Substrate |
| p-IRS-1 | Phosphorylated Insulin Receptor Substrate 1 |
| p-IRS-2 | Phosphorylated Insulin Receptor Substrate 2 |
| p-JNK | Phosphorylated JNK |
| PK | Pyruvate kinase |
| PKA | Protein Kinase A |
| PKC | Protein Kinase C |
| PKCθ | Protein Kinase C theta |
| p-P38 | Phosphorylated p38 MAPK |
| PPAR-α | Peroxisome Proliferator-Activated Receptor α |
| PPAR-γ | Peroxisome Proliferator-Activated Receptor γ |
| PTP1B | Protein tyrosine phosphatase 1B |
| RAGE | Receptor for Advanced Glycation End-products |
| ROS | Reactive oxygen species |
| SCFAs | Short-chain fatty acids |
| SGLT-1 | Sodium-Glucose Cotransporter 1 |
| Sirt1 | Sirtuin 1 |
| SOD | Superoxide dismutase |
| SREBP-1 | Sterol Regulatory Element-Binding Protein-1 |
| T1D | Type 1 diabetes |
| T2D | Type 2 diabetes |
| TGR5 | G protein-coupled receptor 5 |
| TLR4 | Toll-like receptor 4 |
| TNF-α | Tumor necrosis factor-α |
| TUDCA | Tauroursodeoxycholic Acid |
| VDCC | Voltage-dependent calcium channels |
| ZO-1 | Zonula occludens-1 |
References
- Zhou, B.; Rayner, A.W.; Gregg, E.W.; Sheffer, K.E.; Carrillo-Larco, R.M.; Bennett, J.E.; Shaw, J.E.; Paciorek, C.J.; Singleton, R.K.; Pires, A.B.; et al. Worldwide trends in diabetes prevalence and treatment from 1990 to 2022: A pooled analysis of 1108 population-representative studies with 141 million participants. Lancet 2024, 404, 2077–2093. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xie, J.C.; Zhang, Z.M.; Xia, B.H.; Li, Y.M.; Lin, Y.; Li, M.J.; Wu, P.; Lin, L.M. Recent advances in medicinal and edible homologous plant polysaccharides: Preparation, structure and prevention and treatment of diabetes. Int. J. Biol. Macromol. 2024, 258, 128873. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.T.; Zhang, Y.K.; Zheng, M.Z.; Ye, Y.X.; Shi, M.J.; Wang, X.; Cao, L.Y.; Wang, L. Polysaccharides in medicinal and food homologous plants regulate intestinal flora to improve type 2 diabetes: Systematic review. Phytomedicine 2024, 134, 156027. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, S.T.; Guo, F.C.; Gong, Y.P.; Chen, T.B.; Shaw, C.; Jiang, R.C.; Huang, F.; Lin, D. 1H-NMR-based metabolomics reveals the preventive effect of Enteromorpha prolifera polysaccharides on diabetes in zucker diabetic fatty rats. Food Sci. Nutr. 2024, 12, 4049–4062. [Google Scholar] [CrossRef]
- Yang, B.; Yang, R.Y.; Zhang, X.Y.; Wang, W.J.; Kan, J.Q. Hovenia dulcis (guaizao) polysaccharide ameliorates hyperglycemia through multiple signaling pathways in rats with type 2 diabetes mellitus. Int. J. Biol. Macromol. 2025, 285, 138338. [Google Scholar] [CrossRef]
- Li, M.; Law, D.; Zhu, S.; Najm, A.; Fazry, S.; Othman, B.A. Extraction, purification, characterization and antidiabetic mechanisms of plant polysaccharides: A critical review. Turk. J. Biochem. 2024, 49, 596–611. [Google Scholar] [CrossRef]
- Matsathit, U.; Sermwittayawong, D.; Komolkriengkrai, M.; Khimmaktong, W. Glucan-rich polysaccharides obtained from split gill mushroom [Schizophyllum commune (fr.)] Ameliorate hyperglycemia by enhancing insulin and glut2 pancreas in type 2 diabetic rats. Histol. Histopathol. 2025, 40, 191–203. [Google Scholar] [CrossRef]
- Lu, X.X.; Qin, L.; Guo, M.; Geng, J.J.; Dong, S.T.; Wang, K.; Xu, H.; Qu, C.F.; Miao, J.L.; Liu, M. A novel alginate from Sargassum seaweed promotes diabetic wound healing by regulating oxidative stress and angiogenesis. Carbohydr. Polym. 2022, 289, 119437. [Google Scholar] [CrossRef]
- Su, L.; Xin, C.X.; Yang, J.T.; Dong, L.R.; Mei, H.; Dai, X.J.; Wang, Q. A polysaccharide from inonotus obliquus ameliorates intestinal barrier dysfunction in mice with type 2 diabetes mellitus. Int. J. Biol. Macromol. 2022, 214, 312–323. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, J.; Li, H.; Song, H. Advances in dietary polysaccharides as hypoglycemic agents: Mechanisms, structural characteristics, and innovative applications. Crit. Rev. Food. Sci. Nutr. 2023, 65, 1383–1403. [Google Scholar] [CrossRef]
- Salamone, D.; Rivellese, A.A.; Vetrani, C. The relationship between gut microbiota, short-chain fatty acids and type 2 diabetes mellitus: The possible role of dietary fibre. Acta Diabetol. 2021, 58, 1131–1138. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.R.; Huang, G.L.; Huang, H.L. Extraction, structure, activity and application of konjac glucomannan. Ultrason. Sonochem. 2025, 116, 107315. [Google Scholar] [CrossRef]
- Guo, W.H.; Yun, J.M.; Wang, B.; Xu, S.Y.; Ye, C.G.; Wang, X.R.; Qu, Y.L.; Zhao, F.Y.; Yao, L. Comparative study on physicochemical properties and hypoglycemic activities of intracellular and extracellular polysaccharides from submerged fermentation of Morchella esculenta. Int. J. Biol. Macromol. 2024, 278, 134759. [Google Scholar] [CrossRef]
- Lv, T.T.; Liu, X.; Tao, J.; Zhang, Y.J.; Xie, Q.; Meng, X.; Liu, X. Ultrasound-assisted enzymatic extraction of polysaccharides from paulownia flowers: Process optimization, structural characterization, antioxidant and hypoglycemic activities. Microchem J. 2024, 199, 109940. [Google Scholar] [CrossRef]
- Takahashi, I. Importance of heparan sulfate proteoglycans in pancreatic islets and β-cells. Int. J. Mol. Sci. 2022, 23, 12082. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Xu, W.; Fu, S.; Li, S.; Fu, X.; Tan, C.; Wang, P.; Dou, Z.; Chen, C. The effect of the molecular weight of blackberry polysaccharides on gut microbiota modulation and hypoglycemic effect in vivo. Food Funct. 2024, 15, 8586–8603. [Google Scholar] [CrossRef]
- Deng, J.; Zou, X.; Liang, Y.; Zhong, J.; Zhou, K.; Zhang, J.; Zhang, M.; Wang, Z.; Sun, Y.; Li, M. Hypoglycemic effects of different molecular weight konjac glucomannans via intestinal microbiota and SCFAs mediated mechanism. Int. J. Biol. Macromol. 2023, 234, 122941. [Google Scholar] [CrossRef]
- Huang, R.F.; Xu, L.L.; Chen, T.X.; Li, K.; Xia, J.Y.; Wang, H.S. Epimedii folium polysaccharide ameliorated glucose metabolic disorder in type 2 diabetic mice by regulating the SIRT1/PPARγ signaling pathway. Indian J. Pharm. Educ. Res. 2022, 56, S274–S280. [Google Scholar] [CrossRef]
- Zhao, C.; Zhao, H.; Zhang, C.C.; Yang, X.H.; Chen, K.; Xue, Y.; Li, Q.; Deng, S.Y.; Cai, H.Z. Impact of Lycium barbarum polysaccharide on the expression of glucagon-like peptide 1 in vitro and in vivo. Int. J. Biol. Macromol. 2023, 224, 908–918. [Google Scholar] [CrossRef]
- Ke, B.; Ke, X.; Wan, X.S.; Yang, Y.B.; Huang, Y.J.; Qin, J.; Hu, C.H.; Shi, L. Astragalus polysaccharides attenuates TNF-α-induced insulin resistance via suppression of miR-721 and activation of PPAR-γ and PI3K/AKT in 3T3-L1 adipocytes. Am. J. Transl. Res. 2017, 9, 2195–2206. [Google Scholar]
- Suo, A.; Fan, G.; Wu, C.; Li, T.; Li, X.; Zhou, D.; Cong, K.; Cheng, X.; Sun, W. Efficient degradation and enhanced α-glucosidase inhibitory activity of apricot polysaccharides through non-thermal plasma assisted non-metallic Fenton reaction. Int. J. Biol. Macromol. 2024, 266, 131103. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.Y.; Hou, L.K.; Guo, J.H.; Sun, J.C.; Nong, Q.N.; Wang, Y.F.; Hu, S.W.; Zhao, W.J.; Tan, J.; Liu, X.F.; et al. The structures of two polysaccharides from Fructus Corni and their protective effect on insulin resistance. Carbohydr. Polym. 2025, 353, 123290. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Yang, Z.; Yuan, J.; Zuo, S.; Li, Z.; Yang, K.; Wang, S.; Li, J.; Zhu, L.; Zhang, Y. Preparation and characteristics of pumpkin polysaccharides and their effects on abnormal glucose metabolism in diabetes mice. Food Biosci. 2023, 54, 102792. [Google Scholar] [CrossRef]
- Huang, X.Z.; Wen, Y.X.; Chen, Y.H.; Liu, Y.Y.; Zhao, C. Structural characterization of Euglena gracilis polysaccharide and its in vitro hypoglycemic effects by alleviating insulin resistance. Int. J. Biol. Macromol. 2023, 236, 123984. [Google Scholar] [CrossRef]
- Geng, X.Q.; Pan, L.C.; Sun, H.Q.; Ren, Y.Y.; Zhu, Z.Y. Structural characterization of a polysaccharide from Abelmoschus esculentus L. Moench (okra) and its hypoglycemic effect and mechanism on type 2 diabetes mellitus. Food Funct. 2022, 13, 11973–11985. [Google Scholar] [CrossRef]
- Li, X.; Liu, H.; Yang, H.R.; Zeng, Y.J. Hypoglycemic effects of novel panax notoginseng polysaccharide in mice with diet-induced obesity. Foods 2022, 11, 3101. [Google Scholar] [CrossRef]
- Ning, Y.; Cui, B.; Yuan, C. Decreasing the digestibility of debranched corn starch by encapsulation with konjac glucomannan. Food Hydrocoll. 2020, 107, 105966. [Google Scholar] [CrossRef]
- Chen, R.X.; Xu, J.X.; Wu, W.H.; Wen, Y.X.; Lu, S.Y.; El-Seedi, H.R.; Zhao, C. Structure-immunomodulatory activity relationships of dietary polysaccharides. Curr. Res. Food Sci. 2022, 5, 1330–1341. [Google Scholar] [CrossRef]
- Zhang, D.; Jian, Y.; Zhang, Y.; Li, Y.; Gu, L.; Sun, H.; Liu, M.; Zhou, H.; Wang, Y.; Xu, Z. Short-chain fatty acids in diseases. Cell Commun. Signal. 2023, 21, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Li, W.; Zhang, L.; Eliyas, N. Effects of chemical modification on the structure and biological activities of polysaccharides extracted from Inonotus obliquus by microwave. Chem. Biodivers. 2024, 21, e202400783. [Google Scholar] [CrossRef]
- Fan, Y.M.; Huang, G.L. Preparation, structural analysis and antioxidant activity of polysaccharides and their derivatives from Pueraria lobata. Chem. Biodivers. 2023, 20, e202201253. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Y.; Li, T.H.; Zhang, S.S.; Tang, C.; Kang, W.Y. Research progress on glucose-regulating mechanism of food-derived polysaccharides. Food Biosci. 2025, 63, 105679. [Google Scholar] [CrossRef]
- Zhu, Q.Y.; Lin, L.Z.; Zhao, M.M. Sulfated fucan/fucosylated chondroitin sulfate-dominated polysaccharide fraction from low-edible-value sea cucumber ameliorates type 2 diabetes in rats: New prospects for sea cucumber polysaccharide based-hypoglycemic functional food. Int. J. Biol. Macromol. 2020, 159, 34–45. [Google Scholar] [CrossRef]
- Chen, L.; Ge, P.P.; Zeng, T.; Zhao, L.L.; Wong, K.H.; Shi, G.Y.; Cheung, P.; Ding, Z.Y. Sulfation of hyperbranched β-glucan from mushroom Pleurotus tuber-regium enhances α-glucosidase inhibition and anti-glycation properties. Food Biosci. 2025, 63, 105578. [Google Scholar] [CrossRef]
- Li, C.; Liu, Y.; Zhang, X.; Liu, H.; Zhang, H.; Zhang, X.; Zhang, X.; Ma, X.; Wang, B.; Xue, T.; et al. A novel polysaccharide of undaria pinnatifida: Structural characterization, carboxymethylation and hypoglycemic activity in vivo. Food Biosci. 2024, 60, 104479. [Google Scholar] [CrossRef]
- Gao, W.L.; Zheng, Z.; Wang, X.H.; Wang, L.; Zhang, N.; Liu, H.Y.; Cong, X.; Li, S.Y.; Zhu, Z.Z. Protective effects of different selenium green tea polysaccharides on the development of type 2 diabetes in mice. Foods 2023, 12, 4190. [Google Scholar] [CrossRef]
- Omer, A.B.; Altayb, H.N.; Al-Abbasi, F.A.; Gupta, G.; Ahmed, M.M.; Alghamdi, A.M.; Alzarea, S.I.; Sayyed, N.; Nadeem, M.S.; Kazmi, I. Acemannan ameliorates STZ-activated diabetes by attenuating high glucose via inhibiting inflammatory cytokines and apoptosis pathway. Int. J. Biol. Macromol. 2023, 253, 127127. [Google Scholar] [CrossRef]
- He, M.Y.; Tang, S.X.; Xu, T.T.; Yuan, Y.N.; Wu, T.; Pan, S.Y.; Xu, X.Y. Acetylation of the polysaccharide from Houttuynia cordata rhizome and their α-glucosidase inhibition mechanism. J. Food Sci. 2024, 89, 2672–2683. [Google Scholar] [CrossRef]
- Tao, W.; Liu, D.G.; Guo, Z.Q.; Han, P.F.; Ma, Y.; Wu, M.C.; Zhang, R.; He, J.R. Physicochemical properties, structural characterization, and antidiabetic activity of selenylated low molecular weight apple pectin in HFD/STZ-induced type 2 diabetic mice. Carbohydr. Polym. 2025, 348, 122790. [Google Scholar] [CrossRef]
- Hu, H.J.; Sun, W.J.; Zhang, L.F.; Zhang, Y.; Kuang, T.T.; Qu, D.S.; Lian, S.T.; Hu, S.S.; Cheng, M.; Xu, Y.P.; et al. Carboxymethylated Abrus cantoniensis polysaccharide prevents CTX-induced immunosuppression and intestinal damage by regulating intestinal flora and butyric acid content. Int. J. Biol. Macromol. 2024, 261, 129590. [Google Scholar] [CrossRef]
- Xue, H.K.; Tang, Y.Q.; Zha, M.; Xie, K.F.; Tan, J.Q. The structure-function relationships and interaction between polysaccharides and intestinal microbiota: A review. Int. J. Biol. Macromol. 2025, 291, 139063. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.T.; Cheong, K.L. Recent advances in marine algae oligosaccharides: Structure, analysis, and potential prebiotic activities. Crit. Rev. Food. Sci. Nutr. 2022, 62, 7703–7717. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Gao, Y.; Su, Y.; Li, J.; Ren, W.C.; Wang, Q.H.; Kuang, H.X. Probiotics with anti-type 2 diabetes mellitus properties: Targets of polysaccharides from traditional Chinese medicine. Chin. J. Nat. Med. 2022, 20, 641–655. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Yuan, M.; Zhu, X.Q.; Lin, D.N.; Li, X.Y.; Huang, L.; Chen, H.Y.; Rui, W. Evidence of honey-processed Astragalus polysaccharides improving intestinal immune function in spleen Qi deficiency mice integrated with microbiomics and metabolomics analysis. J. Sci. Food. Agric. 2025, 105, 2158–2168. [Google Scholar] [CrossRef]
- Li, J.R.; Zhao, J.Y.; Tian, C.X.; Dong, L.S.; Kang, Z.Z.; Wang, J.S.; Zhao, S.; Li, M.; Tong, X.L. Mechanisms of regulation of glycolipid metabolism by natural compounds in plants: Effects on short-chain fatty acids. Nutr. Metab. 2024, 21, 1–15. [Google Scholar] [CrossRef]
- Song, Q.Y.; Wu, H.K.; Weng, S.T.; Wang, Y.; Kong, L.Q.; Liu, Z.Q.; Zhang, K.P. Artemisia argyi polysaccharide ameliorates hyperglycemia through modulating gut microbiota and serum metabolism in type 2 diabetic mice. J. Funct. Foods 2024, 122, 106525. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Y.Y.; Wei, L.; Yang, Y.H.; Wang, B.T.; Liu, C.P.; Bai, J.Y.; Wang, C. In vitro fermentation characteristics of fucoidan and its regulatory effects on human gut microbiota and metabolites. Food Chem. 2025, 465, 141998. [Google Scholar] [CrossRef]
- Liu, C.; Miao, Y.; Zhao, J.W.; Yang, S.J.; Cheng, S.H.; Zhou, W.J.; Guo, W.K.; Li, A.L. In vitro simulated digestion of different heat treatments sweet potato polysaccharides and effects on human intestinal flora. Food Chem. 2025, 463, 141190. [Google Scholar] [CrossRef]
- Zhang, H.; Li, H.Z.; Pan, B.C.; Zhang, S.F.; Su, X.H.; Sun, W.J.; Zhang, T.Y.; Zhang, Z.Y.; Lv, S.Q.; Cui, H.T. Integrated 16s rRNA sequencing and untargeted metabolomics analysis to reveal the protective mechanisms of Polygonatum sibiricum polysaccharide on type 2 diabetes mellitus model rats. Curr. Drug Metab. 2023, 24, 270–282. [Google Scholar] [CrossRef]
- Tong, A.; Wang, D.; Liu, X.; Li, Z.; Zhao, R.; Liu, B.; Zhao, C. The potential hypoglycemic competence of low molecular weight polysaccharides obtained from Laminaria japonica. Foods 2023, 12, 3809. [Google Scholar] [CrossRef]
- Sun, L.J.; Yuan, H.B.; Ma, H.Q.; Wang, Y.N. Effects of Cordyceps cicadae polysaccharide on gut microbiota, the intestinal mucosal barrier, and inflammation in diabetic mice. Metabolites 2025, 15, 8. [Google Scholar] [CrossRef] [PubMed]
- Teyani, R.; Moniri, N.H. Gut feelings in the islets: The role of the gut microbiome and the FFA2 and FFA3 receptors for short chain fatty acids on β-cell function and metabolic regulation. Br. J. Pharmacol. 2023, 180, 3113–3129. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.Y.; Shan, Z.Y.; Shi, L.; Duan, Y.H.; An, Y.C.; He, C.H.; Lyu, Y.; Zhao, Y.G.; Wang, M.L.; Du, Y.H.; et al. Mulberry leaf polysaccharides ameliorate glucose and lipid metabolism disorders via the gut microbiota-bile acids metabolic pathway. Int. J. Biol. Macromol. 2024, 282, 136876. [Google Scholar] [CrossRef]
- Ye, D.; Pei, D.; Ding, F.F.; Zhao, Q.Q.; Huang, D.F. Polypeptides from sea cucumber Acaudina molpadioides ameliorate glucolipid metabolism disorder and reverse gut microbiota dysbiosis in type 2 diabetic rats. J. Aquat. Food Prod. Technol. 2024, 33, 714–733. [Google Scholar] [CrossRef]
- Liu, H.; Xing, Y.; Wang, Y.; Ren, X.; Zhang, D.; Dai, J.; Xiu, Z.; Yu, S.; Dong, Y. Dendrobium officinale polysaccharide prevents diabetes via the regulation of gut microbiota in prediabetic mice. Foods 2023, 12, 2310. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, X.X.; Ke, J.X.; Hou, X.Y.; Shen, G.H.; Li, S.S.; Chen, H.; Cui, Q.; Yu, J.; Luo, Q.Y.; et al. Chayote pectin regulates blood glucose through the gut-liver axis: Gut microbes/SCFAs/FoxO1 signaling pathways. Food Res. Int. 2025, 202, 115706. [Google Scholar] [CrossRef]
- Bizerra, P.; Gilglioni, E.H.; Li, H.L.; Go, S.; Elferink, R.; Verhoeven, A.J.; Chang, J.C. Opposite regulation of glycogen metabolism by camp produced in the cytosol and at the plasma membrane. Biochim. Biophys. Acta-Mol. Cell Res. 2024, 1871, 119585. [Google Scholar] [CrossRef]
- Tang, C.; Zhou, R.Z.; Cao, K.X.; Liu, J.; Kan, J.; Qian, C.L.; Jin, C.H. Current progress in the hypoglycemic mechanisms of natural polysaccharides. Food Funct. 2023, 14, 4490–4506. [Google Scholar] [CrossRef]
- Zhu, Z.P.; Lin, W.D.; Ma, J.X.; Li, N.; Wang, J.X.; Li, J.M. The physiochemical characteristics of Cyclocarya paliurus polysaccharides and in vitro anti-diabetic effects of their different fractions. Ind. Crop. Prod. 2024, 211, 118188. [Google Scholar] [CrossRef]
- Liu, T.; Chen, S.; Qu, Y.H.; Zheng, L.J.; Yang, X.X.; Men, S.; Wang, Y.N.; Ma, H.R.; Zhou, Y.F.; Fan, Y.Y. Mannogalactoglucan from mushrooms protects pancreatic islets via restoring UPR and promotes insulin secretion in T1DM mice. Food Sci. Hum. Wellness 2024, 13, 1390–1401. [Google Scholar] [CrossRef]
- Xia, T.; He, W.; Luo, Z.Y.; Wang, K.X.; Tan, X.M. Achyranthes bidentata polysaccharide ameliorates type 2 diabetes mellitus by gut microbiota-derived short-chain fatty acids-induced activation of the GLP-1/GLP-1r/cAMP/PKA/CREB/INS pathway. Int. J. Biol. Macromol. 2024, 270, 132256. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.W.; He, Z.W.; Chen, Y.G.; Chao, J.T.; Cheng, X.M.; Mao, J.Y.; Chen, Y.L.; Li, B.; Yu, J.J.; Yan, M.Q.; et al. Cordyceps polysaccharide improves polycystic ovary syndrome by inhibiting gut-derived LPS/TLR4 pathway to attenuates insulin resistance. Int. J. Biol. Macromol. 2024, 280, 135844. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.W.; Li, X.X.; Xu, S.G.; Li, J.Y.; Shi, L.; Wang, Z.Y.; Chen, P.Y.; Jia, L.; Zhang, J.J. The acetylation of Ganoderma applanatum polysaccharides on ameliorating T2DM-induced hepatic and colonic injuries by modulating the rf2/keap1-TLR4/NFκB-Bax/Bcl-2 pathways. Int. J. Biol. Macromol. 2025, 294, 140055. [Google Scholar] [CrossRef]
- Ding, Y.Y.; Lan, J.C.; Wang, Y.X.; Pan, Y.X.; Song, T.Y.; Liu, S.Z.; Gu, Z.Y.; Ge, Y.J. Structure characterization of Grifola frondosa polysaccharide and its effect on insulin resistance in HFD-fed mice. NPJ Sci. Food 2025, 9, 1–15. [Google Scholar] [CrossRef]
- Liu, W.; Jin, R.M.; Ma, F.Y.; Zhao, P.; Su, Y.T.; Wang, J.N.; Zhang, Y.; Wang, R.J.; Zhu, J.H.; Liu, X.H. Effects of Dioscorea opposita polysaccharides on insulin resistance and gut microbiota in high-fat-diet induced type 2 diabetic rats. Int. J. Biol. Macromol. 2025, 304, 141004. [Google Scholar] [CrossRef]
- Qiao, Z.J.; Du, X.X.; Zhuang, W.Y.; Yang, S.; Li, H.; Sun, J.H.; Chen, J.G.; Wang, C.M. Schisandra chinensis acidic polysaccharide improves the insulin resistance in type 2 diabetic rats by inhibiting inflammation. J. Med. Food 2020, 23, 358–366. [Google Scholar] [CrossRef]
- Bo, S.; Dan, M.; Li, W.; Chen, C. The regulatory mechanism of natural polysaccharides in type 2 diabetes mellitus treatment. Drug Discov. Today 2024, 29, 104182. [Google Scholar] [CrossRef]
- Chen, X.; Wu, J.; Fu, X.; Wang, P.; Chen, C. Fructus mori polysaccharide alleviates diabetic symptoms by regulating intestinal microbiota and intestinal barrier against TLR4/NF-κB pathway. Int. J. Biol. Macromol. 2023, 249, 126038. [Google Scholar] [CrossRef]
- Siddiqui, N.Z.; Rehman, A.U.; Yousuf, W.; Khan, A.I.; Farooqui, N.A.; Zang, S.Z.; Xin, Y.; Wang, L. Effect of crude polysaccharide from seaweed, Dictyopteris divaricata (CDDP) on gut microbiota restoration and anti-diabetic activity in streptozotocin (STZ)-induced T1DM mice. Gut Pathog. 2022, 14, 1–26. [Google Scholar] [CrossRef]
- Wang, J.Y.; Wang, X.; Xiu, W.Y.; Zhou, Z.; Yu, S.Y.; Yang, M.Y.; Zhou, K.C.; Ma, Y.Q. The sweet corn cob selenium polysaccharide alleviates type 2 diabetes via modulation of LPS/IκBα/NFκB and the intestinal microbiota. Food Biosci. 2024, 58, 103742. [Google Scholar] [CrossRef]
- Song, Q.; Cheng, S.W.; Li, D.; Cheng, H.; Lai, Y.S.; Han, Q.; Wu, H.Y.; Shaw, P.C.; Zuo, Z. Gut microbiota mediated hypoglycemic effect of Astragalus membranaceus polysaccharides in db/db mice. Front. Pharmacol. 2022, 13, 1043527. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.C.; Yao, Q.G.; Lv, J.H.; Li, Z.; Wang, L.A.; Zhang, J.X. Anti-hyperglycemic effect of the brown slime cap mushroom Chroogomphus rutilus (agaricomycetes) crude polysaccharide in vitro and in vivo. Int. J. Med. Mushrooms 2024, 26, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.B.; Gao, S.; Huang, Z.R.; Li, Z.R.; Wang, H.Z.; Wu, J.; Zhou, C.X.; Zhao, M.M. The potential auxiliary effects of Sargassum fusiform polysaccharides on sitagliptin in the treatment of diabetes mellitus. Int. J. Biol. Macromol. 2024, 281, 136154. [Google Scholar] [CrossRef]
- Wang, X.; Yang, M.Y.; Shen, Y.; Zhang, Y.P.; Xiu, W.Y.; Yu, S.Y.; Ma, Y.Q. Structural characterization and hypoglycemic effect of polysaccharides of Polygonatum sibiricum. J. Food Sci. 2024, 89, 4771–4790. [Google Scholar] [CrossRef]
- Li, R.Y.; Hu, R.; Huang, Y.; Li, D.; Ma, X.L.; Yang, Y. Astragalus polysaccharide alleviates polycystic ovary syndrome by reducing insulin resistance and oxidative stress and increasing the diversity of gut microbiota. Endocrine 2024, 83, 783–797. [Google Scholar] [CrossRef]
- Yu, F.Z.; Wang, Y.X.; Teng, Y.L.; Yang, S.T.; He, Y.M.; Zhang, Z.; Yang, H.J.; Ding, C.F.; Zhou, P. Interaction and inhibition of a ganoderma lucidum proteoglycan on PTP1B activity for anti-diabetes. ACS Omega 2021, 6, 29804–29813. [Google Scholar] [CrossRef]
- Wang, Y.N.; Zeng, T.T.; Li, H.; Wang, Y.D.; Wang, J.H.; Yuan, H.B. Structural characterization and hypoglycemic function of polysaccharides from Cordyceps cicadae. Molecules 2023, 28, 526. [Google Scholar] [CrossRef]
- He, Y.X.; Wang, Y.Y.; Li, X.; Qi, Y.Q.; Qu, Z.W.; Hu, Y.L. Lycium barbarum polysaccharides improves cognitive functions in ICV-STZ-induced Alzheimer’s disease mice model by improving the synaptic structural plasticity and regulating IRS1/PI3K/AKT signaling pathway. Neuromol. Med. 2024, 26, 1–18. [Google Scholar] [CrossRef]
- Chen, Y.; Dong, J.D.; Liu, W.T.; Xia, Q.L.; Liu, T.; Liu, S.H.; Song, Z.Y.; Li, S.J. Polysaccharides from Ostrea rivularis alleviate type ii diabetes induced-retinopathy and vgef165-induced angiogenesis via PI3K/AKT signaling pathway. Int. J. Biol. Macromol. 2024, 279, 135547. [Google Scholar] [CrossRef]
- Wang, Y.T.; Wu, H.; Wu, J.J.; Yu, Y.S.; Wen, J.; Zou, B.; Li, L.; Peng, J.; Cheng, L.N.; Bu, Z.B.; et al. The hypoglycemic effect of mulberry (Morus atropurpurea) fruit lacking fructose and glucose by regulation of the gut microbiota. Food Funct. 2025, 16, 2444–2460. [Google Scholar] [CrossRef]
- Jiang, Q.H.; Chen, L.; Wang, R.; Chen, Y.; Deng, S.G.; Shen, G.X.; Liu, S.L.; Xiang, X.W. Hypoglycemic mechanism of Tegillarca granosa polysaccharides on type 2 diabetic mice by altering gut microbiota and regulating the PI3K-akt signaling pathwaye. Food Sci. Hum. Wellness 2024, 13, 842–855. [Google Scholar] [CrossRef]
- Wang, R.; Yang, X.W.; Jiang, Q.H.; Chen, L.; Gu, S.Q.; Shen, G.X.; Liu, S.L.; Xiang, X.W. Effect of mussel polysaccharide on glucolipid metabolism and intestinal flora in type 2 diabetic mice. J. Sci. Food. Agric. 2023, 103, 3353–3366. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.B.; Deng, X.M.; Chen, R.H.; Li, Q.H.; Sun, L.L.; Cao, J.X.; Lai, Z.X.; Lai, X.F.; Wang, Z.H.; Sun, S.L.; et al. Effect of black tea polysaccharides on alleviating type 2 diabetes mellitus by regulating PI3K/Akt/GLUT2 pathway. Foods 2024, 13, 1908. [Google Scholar] [CrossRef]
- Zhang, J.W.; Chen, L.G.; Zhao, C.Y.; Chen, Z.; Xiao, S.Q.; Yin, X.M.; Wu, N.; Yang, L.; Xu, J.D.; Zhou, H.C.; et al. Polysaccharides from Cynanchum auriculatum royle ex wight ameliorate symptoms of hyperglycemia by regulating gut microbiota in type 2 diabetes mellitus mice. Int. J. Biol. Macromol. 2025, 299, 139878. [Google Scholar] [CrossRef]
- Guo, W.H.; Wang, X.R.; Wang, B.; Zhang, Y.J.; Zhao, F.Y.; Qu, Y.L.; Yao, L.; Yun, J.M. In vitro digestion and fecal fermentation behaviors of exopolysaccharide from Morchella esculenta and its impacts on hypoglycemic activity via PI3K/Akt signaling and gut microbiota modulation. Food Chem. X 2024, 24, 101870. [Google Scholar] [CrossRef]
- Su, J.-P.; Fang, J.-Q.; Liu, C.; Liu, S.-P.; Chen, C.; Tan, C.-P.; Wang, P.-P.; Peng, Y.-P.; Fu, X. Advances in structure-hypoglycemic activity relationship and mechanisms of berry polysaccharides. Food Biosci. 2024, 62, 105472. [Google Scholar] [CrossRef]
- Sun, W.J.; Kou, X.H.; Wu, C.E.; Fan, G.J.; Li, T.T.; Cheng, X.; Xu, K.Q.; Suo, A.D.; Tao, Z. Low-temperature plasma modification, structural characterization and anti-diabetic activity of an apricot pectic polysaccharide. Int. J. Biol. Macromol. 2023, 240, 124301. [Google Scholar] [CrossRef]
- Wu, G.Z.; Liu, S.; Wang, Z.Q.; Wang, X. Structural characteristics of neutral polysaccharides purified from coix seed and its anti-insulin resistance effects on HepG2 cells. Food Sci. Nutr. 2024, 12, 8419–8431. [Google Scholar] [CrossRef]
- El-Nashar, H.; Taleb, M.; El-Shazly, M.; Zhao, C.; Farag, M.A. Polysaccharides (pectin, mucilage, and fructan inulin) and their fermented products: A critical analysis of their biochemical, gut interactions, and biological functions as antidiabetic agents. Phytother. Res. 2024, 38, 662–693. [Google Scholar] [CrossRef]
- Jiang, W.; Tan, J.; Zhang, J.C.; Deng, X.; He, X.Y.; Zhang, J.; Liu, T.; Sun, R.; Sun, M.X.; Chen, K.; et al. Polysaccharides from Dendrobium officinale improve obesity-induced insulin resistance through the gut microbiota and the SOCS3-mediated insulin receptor substrate-1 signaling pathway. J. Sci. Food. Agric. 2024, 104, 3437–3447. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Mao, G.X.; Zuo, J.H.; Li, S.J.; Yang, Y.; Thring, R.W.; Wu, M.J.; Tong, H.B. Sargassum fusiforme fucoidan alleviates diet-induced insulin resistance by inhibiting colon-derived ceramide biosynthesis. Food Funct. 2021, 12, 8440–8453. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Hou, X.Y.; Cui, Q.; Li, S.S.; Shen, G.H.; Luo, Q.Y.; Wu, H.J.; Chen, H.; Liu, Y.T.; Chen, A.J.; et al. Pectin mediates the mechanism of host blood glucose regulation through intestinal flora. Crit. Rev. Food. Sci. Nutr. 2024, 64, 6714–6736. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.Y.; Lin, C.Q.; Zhang, X.T.; Yan, X.P. Angelica polysaccharide alleviates TNF-α-induced MIN6 cell damage a through theup-reegulation. Biofactors 2023, 49, 200. [Google Scholar] [CrossRef]
- Pan, Y.N.; Yuan, S.L.; Teng, Y.L.; Zhang, Z.; He, Y.M.; Zhang, Y.; Liang, H.H.; Wu, X.; Li, J.Q.; Yang, H.J.; et al. Antioxidation of a proteoglycan from Ganoderma lucidum protects pancreatic β-cells against oxidative stress-induced apoptosis in vitro and in vivo. Int. J. Biol. Macromol. 2022, 200, 470–486. [Google Scholar] [CrossRef]
- Tannous, S.; Stellbrinck, T.; Hoter, A.; Naim, H.Y. Interaction between the α-glucosidases, sucrase-isomaltase and maltase-glucoamylase, in human intestinal brush border membranes and its potential impact on disaccharide digestion. Front. Mol. Biosci. 2023, 10, 1160860. [Google Scholar] [CrossRef]
- Zhang, X.L.; Shi, C.Y.; Wang, Z.L.; Dai, J.H.; Guan, C.H.; Sheng, J.; Tao, L.; Tian, Y. Separation, purification, structural characterization, and in vitro hypoglycemic activity of polysaccharides from Panax notoginseng leaves. Molecules 2025, 30, 830. [Google Scholar] [CrossRef]
- Ni, Z.Z.; Li, J.T.; Qian, X.Y.; Yong, Y.D.; Zhang, P.; Geng, Y.; Wang, Y.A.; Chen, A.H.; Shao, Y. Optimization of the conditions for microwave-assisted hot-water extraction of polysaccharides from Cordyceps militaris and analysis of their hypoglycemic activity. J. Food Meas. Charact. 2024, 18, 5766–5778. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, M.H.; Li, P.; Lv, G.; Yao, J.; Zhao, L. Hypoglycemic effect of polysaccharides from Physalis alkekengi l. In type 2 diabetes mellitus mice. Biology 2024, 13, 496. [Google Scholar] [CrossRef]
- Yu, J.Q.; Zhao, L.; Wang, Z.Q.; Yue, T.; Wang, X.; Liu, W. Correlations between the structure and anti-diabetic activity of novel polysaccharides from raw and “nine steaming nine sun-drying” polygonti rhizome. Int. J. Biol. Macromol. 2024, 260, 129171. [Google Scholar] [CrossRef]
- Liu, J.K.; Liu, W.L.; Zhang, Y.Y.; Tian, Q.D.; Xia, M.S.; Zhao, Q.Y.; Zhang, D.W.; He, J.J.; Wang, D.C.; Zhu, X.Q.; et al. Preparation and hypoglycemic effect of Magnolia officinalis polysaccharide oral liquid. Plant Food Hum. Nutr. 2025, 80, 1–10. [Google Scholar] [CrossRef]
- Sun, J.; Wu, K.Y.; Wang, P.; Wang, Y.B.; Wang, D.; Zhao, W.T.; Zhao, Y.Y.; Zhang, C.H.; Zhao, X.Y. Dietary tomato pectin attenuates hepatic insulin resistance and inflammation in high-fat-diet mice by regulating the PI3K/AKT pathway. Foods 2024, 13, 444. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Q.; Huang, Y.X.; Gan, Q.R.; Zhang, W.H.; Sun, H.; Zhu, L.L.; Wang, W.X. Characterization of tea polysaccharides from Tieguanyin oolong tea and their hepatoprotective effects via amp-activated protein kinase-mediated signaling pathways. J. Food Sci. 2024, 89, 10064–10078. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xiu, W.Y.; Han, Y.; Wang, Z.L.; Luo, Y.; Ma, Y.Q. Hypoglycemic effect and the mechanism of action of a polysaccharide from sweet corncob in a high-fat diet and streptozotocin-induced diabetic mice. Food Sci. Hum. Wellness 2024, 13, 1543–1555. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, L.; Chai, T.; Xu, H.; Du, H.Y.; Jiang, Y. Mulberry leaf multi-components exert hypoglycemic effects through regulation of the PI-3K/AKT insulin signaling pathway in type 2 diabetic rats. J. Ethnopharmacol. 2024, 319, 117307. [Google Scholar] [CrossRef]
- Ma, D.; Sheng, Q.; Liang, W.; Zhang, J.; Wang, Y.N.; Chen, H.M. A neutral polysaccharide from Medicago sativa L.: Structural properties and hypoglycemic activity in vitro and in vivo. Chem. Biodivers. 2024, 21, e202401162. [Google Scholar] [CrossRef]
- Xu, Y.M.; Xu, C.; Huang, J.; Xu, C.W.; Xiong, Y. Astragalus polysaccharide attenuates diabetic nephropathy by reducing apoptosis and enhancing autophagy through activation of Sirt1/FoxO1 pathway. Int. Urol. Nephrol. 2024, 56, 3067–3078. [Google Scholar] [CrossRef]
- Luo, D.; Dong, X.K.; Huang, J.; Huang, C.C.; Fang, G.W.; Huang, Y.Q. Pueraria lobata root polysaccharide alleviates glucose and lipid metabolic dysfunction in diabetic db/db mice. Pharm. Biol. 2021, 59, 382–390. [Google Scholar] [CrossRef]
- Song, Q.Y.; Zhang, K.P.; Li, S.Y.; Weng, S.T. Trichosanthes kirilowii maxim. Polysaccharide attenuates diabetes through the synergistic impact of lipid metabolism and modulating gut microbiota. Curr. Res. Food Sci. 2025, 10, 100977. [Google Scholar] [CrossRef]
- Zhu, Y.Z.; Wang, D.; Zhou, S.B.; Zhou, T. Hypoglycemic effects of Gynura divaricata (L.) Dc polysaccharide and action mechanisms via modulation of gut microbiota in diabetic mice. J. Agric. Food. Chem. 2024, 72, 9893–9905. [Google Scholar] [CrossRef]
- Zou, P.; Li, X.Y.; Wang, L.P.; She, Y.; Xiao, C.Y.; Peng, Y.; Qian, X.; Luo, P.; Wei, S.F. Grifola frondosa polysaccharide ameliorates inflammation by regulating macrophage polarization of liver in type 2 diabetes mellitus rats. Mol. Nutr. Food Res. 2024, 68, e2400392. [Google Scholar] [CrossRef]
- Li, H.M.; Tao, W.H.; Xu, X.C.; Chen, G.L.; Ma, W.P.; Jia, S.Q. Lycium barbarum polysaccharides alleviate pancreatic β-cells apoptosis through the inhibition of IFNγ pathway. J. Funct. Foods 2023, 107, 105706. [Google Scholar] [CrossRef]
- Dong, Y.T.; Wang, T.; Gan, B.C.; Wasser, S.P.; Zhang, Z.Y.; Zhao, J.; Duan, X.L.; Cao, L.P.; Feng, R.C.; Miao, R.Y.; et al. Antioxidant activity of Phellinus igniarius fermentation mycelia contributions of different solvent extractions and their inhibitory effect on α-amylase. Heliyon 2024, 10, e23370. [Google Scholar] [CrossRef] [PubMed]
- Oumeddour, D.Z.; Lin, W.; Lian, C.; Zhao, L.; Wang, X.; Zhao, L.; Guo, L. The anti-diabetic effect of non-starch polysaccharides extracted from wheat beer on diet/STZ-induced diabetic mice. Foods 2024, 13, 2692. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, B.J.; Sun, W.; Sun, X.; Zhao, J.; Li, Q.H. Structural characterization of squash polysaccharide and its effect on stz-induced diabetes mellitus model in min6 cells. Int. J. Biol. Macromol. 2024, 270, 132226. [Google Scholar] [CrossRef]
- Zhao, F.Q.; Liu, Q.B.; Cao, J.; Xu, Y.S.; Pei, Z.S.; Fan, H.F.; Yuan, Y.Q.; Shen, X.R.; Li, C. A sea cucumber (Holothuria leucospilota) polysaccharide improves the gut microbiome to alleviate the symptoms of type 2 diabetes mellitus in goto-kakizaki rats. Food. Chem. Toxicol. 2020, 135, 110886. [Google Scholar] [CrossRef]
- Li, W.L.; Lin, K.; Zhou, M.; Xiong, Q.; Li, C.Y.; Ru, Q. Polysaccharides from Opuntia milpa alta alleviate alloxan-induced INS-1 cells apoptosis via reducing oxidative stress and upregulating Nrf2 expression. Nutr. Res. 2020, 77, 108–118. [Google Scholar] [CrossRef]
- Li, X.Z.; Gong, H.Q.; Yang, S.W.; Yang, L.L.; Fan, Y.Y.; Zhou, Y.F. Pectic bee pollen polysaccharide from Rosa rugosa alleviates diet-induced hepatic steatosis and insulin resistance via induction of AMPK/mTOR-mediated autophagy. Molecules 2017, 22, 699. [Google Scholar] [CrossRef]
- Ren, X.D.; Dai, Y.; Shan, M.Y.; Zheng, J.; Zhang, Z.Y.; Shen, T. Astragalus polysaccharide restores insulin secretion impaired by lipopolysaccharides through the protein kinase B/mammalian target of rapamycin/glucose transporter 2 pathway. BMC Complement. Med. Ther. 2023, 23, 358. [Google Scholar] [CrossRef]
- Chang, M.W.; Liu, K.L.; Zhu, G.S.; Gul, P.; Khan, J. Structural characterization and hypoglycaemic effects on type 2 diabetic mice of spirulina platensis polysaccharides and se-modified polysaccharides. Food Biosci. 2025, 64, 105826. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, S.D.; Sun, W.X.; Yang, Y.C.; Xu, Y.X.; Tang, Y.X.; Jiang, W.; Li, J.; Zhang, Y.J. Study on the mechanisms by which pumpkin polysaccharides regulate abnormal glucose and lipid metabolism in diabetic mice under oxidative stress. Int. J. Biol. Macromol. 2024, 270, 132249. [Google Scholar] [CrossRef]
- Gu, F.; Tao, L.; Chen, R.; Zhang, J.; Wu, X.; Yang, M.; Sheng, J.; Tian, Y. Ultrasonic-cellulase synergistic extraction of crude polysaccharides from Moringa oleifera leaves and alleviation of insulin resistance in HepG2 cells. Int. J. Mol. Sci. 2022, 23, 12405. [Google Scholar] [CrossRef]
- Zhou, S.Y.; Huang, G.L.; Ren, F.M. Ultrasonic-assisted extraction and the antioxidative mechanism of eggplant root polysaccharide. J. Funct. Foods 2024, 123, 106590. [Google Scholar] [CrossRef]
- Zhu, R.Y.; Ouyang, Y.Z.; Chen, Y.H.; Zhang, L.Z.; Nie, J.P.; Farag, M.A.; Capanoglu, E.; Zhao, C. The therapeutic potential for senescence-associated diabetes of green alga Enteromorpha prolifera polysaccharide. Int. J. Biol. Macromol. 2023, 232, 123465. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.J.; Bai, Z.Y.; Wan, Y.J.; Shi, H.F.; Huang, X.J.; Nie, S.P. Antidiabetic effects of polysaccharide from azuki bean (Vigna angularis) in type 2 diabetic rats via insulin/PI3K/AKT signaling pathway. Food Hydrocoll. 2020, 101, 105456. [Google Scholar] [CrossRef]
- Sun, J.; Wei, N.; Yu, C.X.; Li, C.; Li, W.; Sun, X.Y.; Zhang, Y.Q.; Li, Y.X.; Xie, J.B. Natural polysaccharides: The potential biomacromolecules for treating diabetes and its complications via ages-rage-oxidative stress axis. Int. Immunopharmacol. 2024, 143, 113426. [Google Scholar] [CrossRef]
- Liu, D.; Mei, X.Y.; Mao, Y.T.; Li, Y.J.; Wang, L.; Cao, X.Y. Lentinus edodes mycelium polysaccharide inhibits ages-induced HUVECs pyroptosis by regulating LncRNA MALAT1/miR-199b/mTOR axis and NLRP3/Caspase-1/GSDMD pathway. Int. J. Biol. Macromol. 2024, 267, 131387. [Google Scholar] [CrossRef]
- Wang, M.J.; Li, Y.D.; Yang, S.Y.; Wei, P. The primary mechanisms of drug-food homologous traditional Chinese medicine polysaccharides in the prevention and treatment of diabetes. J. Med. Food 2024, 27, 693–703. [Google Scholar] [CrossRef]
- Liu, S.Y.; Wang, L.Y.; Zhang, Z.H.; Leng, Y.L.; Yang, Y.; Fu, X.X.; Xie, H.Y.; Gao, H.; Xie, C.G. The potential of astragalus polysaccharide for treating diabetes and its action mechanism. Front. Pharmacol. 2024, 15, 1339406. [Google Scholar] [CrossRef]
- Wang, L.F.; Wu, R.T.; Yao, Y.F.; Fu, W.W.; Wan, M.; Sang, T.; Li, W.J. Cardioprotective effects of Ganoderma atrum polysaccharide in a type 2 diabetes mellitus involvement with gut-derived metabolites and NLRP3 inflammasome. J. Funct. Foods 2024, 112, 105991. [Google Scholar] [CrossRef]
- Ruan, Q.L.; Chen, Y.H.; Wen, J.H.; Qiu, Y.H.; Huang, Y.J.; Zhang, Y.; Farag, M.A.; Zhao, C. Regulatory mechanisms of the edible alga Ulva lactuca polysaccharide via modulation of gut microbiota in diabetic mice. Food Chem. 2023, 409, 135287. [Google Scholar] [CrossRef]
- Liu, W.J.; Li, Z.Z.; Feng, C.X.; Hu, S.W.; Yang, X.; Xiao, K.M.; Nong, Q.N.; Xiao, Q.H.; Wu, K.H.; Li, X.Q.; et al. The structures of two polysaccharides from Angelica sinensis and their effects on hepatic insulin resistance through blocking rage. Carbohydr. Polym. 2022, 280, 119001. [Google Scholar] [CrossRef] [PubMed]
- Zhong, R.F.; Liu, C.J.; Hao, K.X.; Fan, X.D.; Jiang, J.G. Polysaccharides from Flos Sophorae immaturus ameliorates insulin resistance in IR-HepG2 cells by co-regulating signaling pathways of AMPK and IRS-1/PI3K/AKT. Int. J. Biol. Macromol. 2024, 280, 136088. [Google Scholar] [CrossRef]
- Chuang, A.; Chen, Y.L.; Chiu, H.J.; Nguyen, H.T.; Liu, C.H. Nasal administration of polysaccharides-based nanocarrier combining hemoglobin and diferuloylmethane for managing diabetic kidney disease. Int. J. Biol. Macromol. 2024, 282, 136534. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.H.; Zhang, X.; Zhao, Y.; Zhi, J.L.; Xu, W.Y.; Yang, Y.Q.; Xu, Y.; Luo, K.; Wang, D.F. Mn(ii)-mediated self-assembly of tea polysaccharide nanoparticles and their functional role in mice with type 2 diabetes. ACS Appl. Mater. Interfaces 2022, 14, 30607–30617. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.J.; Cao, M.; Diao, N.N.; Wang, W.X.; Geng, H.X.; Su, Y.G.; Sun, T.Y.; Lu, X.Y.; Kong, M.; Chen, D.Q. Novel pH-responsive e-selectin targeting natural polysaccharides hybrid micelles for diabetic nephropathy. Nanomed.-Nanotechnol. Biol. Med. 2023, 52, 102696. [Google Scholar] [CrossRef]
- Li, X.M.; Bai, L.M.; Zhang, X.W.; Fang, Q.W.; Chen, G.; Xu, G. Application of Bletilla striata polysaccharide hydrogel for wound healing among in diabetes. Colloid Surf. B Biointerfaces 2024, 241, 114033. [Google Scholar] [CrossRef]
- Tang, L.T.; Xie, S.Y.; Wang, D.Y.; Wei, Y.Y.; Ji, X.P.; Wang, Y.C.; Zhao, N.A.; Mou, Z.L.; Li, B.P.; Sun, W.R.; et al. Astragalus polysaccharide/carboxymethyl chitosan/sodium alginate based electroconductive hydrogels for diabetic wound healing and muscle function assessment. Carbohydr. Polym. 2025, 350, 123058. [Google Scholar] [CrossRef]
- Wu, J.; Jia, R.B.; Luo, D.H.; Li, Z.R.; Lin, L.Z.; Zheng, Q.W.; Zhao, M.M. Sargassum fusiforme polysaccharide is a potential auxiliary substance for metformin in the management of diabetes. Food Funct. 2022, 13, 3023–3035. [Google Scholar] [CrossRef]
- Sun, L.; Jiang, J.; Jing, T.; Hu, D.J.; Zhu, J.; Zeng, Y.; Pang, Y.L.; Huang, D.C.; Cheng, S.J.; Cao, C.J. A polysaccharide NAP-3 from Naematelia aurantialba: Structural characterization and adjunctive hypoglycemic activity. Carbohydr. Polym. 2023, 318, 121124. [Google Scholar] [CrossRef]
- Long, J.L.; Li, M.; Yao, C.C.; Ma, W.J.; Liu, H.T.; Yan, D. Structural characterization of astragalus polysaccharide-D1 and its improvement of low-dose metformin effect by enriching Staphylococcus lentus. Int. J. Biol. Macromol. 2024, 272, 132860. [Google Scholar] [CrossRef]
- Tian, H.C.; Wen, Z.Y.; Chen, J.Y.; Zhao, C.; Yang, C.; Guo, Y.Q.; Sun, B.L. Alleviation of DSS-induced ulcerative colitis by pomelo peel polysaccharides: Exploration for the potential mechanism from comprehensive analysis of multi-omics. Food Biosci. 2025, 65, 105955. [Google Scholar] [CrossRef]
- Khursheed, R.; Singh, S.K.; Wadhwa, S.; Gulati, M.; Kapoor, B.; Jain, S.K.; Gowthamarajan, K.; Zacconi, F.; Chellappan, D.K.; Gupta, G.; et al. Development of mushroom polysaccharide and probiotics based solid self-nanoemulsifying drug delivery system loaded with curcumin and quercetin to improve their dissolution rate and permeability: State of the art. Int. J. Biol. Macromol. 2021, 189, 744–757. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Han, L.; Raza, S.H.A.; Yue, Q.; Sun, S.; Zhao, Y.; Lv, L.; Deng, Y.; Yuan, Z.; Alsharif, I.; et al. Polysaccharides in berberis dasystachya improve intestinal flora depending on the molecular weight and ameliorate type 2 diabetes in rats. J. Funct. Foods 2023, 100, 105381. [Google Scholar] [CrossRef]
- Zhai, J.; Fan, F.; Wang, C.; Zhang, Z.; Zhang, S.; Zhao, Y. Molecular dynamics study on the binding characteristics and transport mechanism of polysaccharides with different molecular weights in Camellia oleifera abel. J. Comput. Biophys. Chem. 2024, 23, 367–377. [Google Scholar] [CrossRef]
- Yang, L.; Huang, J.; Qin, S.; Shao, H.; Li, Y.; Zhou, Y.; Zi, C.; Hu, J. “Md” method for the precise analysis of the o-acetyl-mannan structure and disclosure of the role in the conformational stability of insulin. Int. J. Biol. Macromol. 2024, 263, 129944. [Google Scholar] [CrossRef]
- Yu, X.M.; Liu, C.K.; Kuang, Z.; Song, S.Y.; Tian, L.M.; Wang, Y. Islet organoids: A new hope for islet transplantation in diabetes. Front. Immunol. 2025, 15, 1540209. [Google Scholar] [CrossRef]
- Ding, Q.Q.; Zu, X.P.; Chen, W.; Xin, J.Y.; Xu, X.K.; Lv, Y.H.; Wei, X.T.; Wang, J.; Wei, Y.P.; Li, Z.H.; et al. Astragalus polysaccharide promotes the regeneration of intestinal stem cells through HIF-1 signalling pathway. J. Cell. Mol. Med. 2024, 28, 18058. [Google Scholar] [CrossRef]
- Wang, Q.; Li, G.; Qin, W.J.; Cai, J.; Wang, N.F. Evaluation of in vitro simulated digestion and fermentation characteristics of the crude exopolysaccharide from Levilactobacillus brevis M-14. J. Food Sci. 2024, 89, 9860–9878. [Google Scholar] [CrossRef]
- Song, Q.; Zou, J.; Li, D.; Cheng, S.W.; Li, K.L.S.; Yang, X.; Shaw, P.C.; Zuo, Z. Gastrointestinal metabolism of Astragalus membranaceus polysaccharides and its related hypoglycemic mechanism based on gut microbial transformation. Int. J. Biol. Macromol. 2024, 280, 135847. [Google Scholar] [CrossRef]
| Polysaccharide Type | Molecular Weight (kDa) | Experimental Model | Core Mechanisms | Reference |
|---|---|---|---|---|
| Fructus corni polysaccharides | 59 | IR-HepG2 cells, T2D mice | GLUT2 ↑, GK ↑ | [22] |
| Pumpkin polysaccharides | 33.72 | Acute diabetic mice | SOD ↑, CAT ↑, GSH ↑, PK ↑, PEPCK ↓ | [23] |
| Euglena gracilis polysaccharides | 130.8 | IR-HeoG2 cells | PI3K ↑, Akt ↑, GLUT4 ↑ | [24] |
| Abelmoschus esculentus L. Moench (okra) polysaccharide | 3.02 × 103 | T2D mice | GSK-3β ↑, GS ↑, PI3K ↑, Akt ↑ | [25] |
| Panax notoginseng polysaccharide | 8.27 | High-fat diet mice | GLUT2 ↓, SGLT-1 ↓. p-IRS ↑, p-AMPK ↑ | [26] |
| Enzyme | Effect | Mechanism | Direction of NPs Regulation |
|---|---|---|---|
| GK | Catalyzes the phosphorylation of glucose to G6P | INS activates GK through the PI3K/Akt pathway | Activate |
| G6Pase | Catalyzes the hydrolysis of G6P to glucose | FoxO1 transcriptionally activates G6Pase | Inhibit |
| GS | Promotes glycogen synthesis | Inhibition of GSK-3β by INS activation of the PI3K/Akt pathway deregulates the inhibition of GS phosphorylation | Activate |
| GP | Catalyzes the breakdown of glycogen to glucose-1-phosphate | Activation of PKA pathway by glucagon | Inhibit |
| HK | Catalyzes the phosphorylation of glucose to G6P | Insulin signaling pathway | Activate |
| PK | Catalyzes the formation of pyruvate from phosphoenolpyruvate (PEP) | Activated by FBP-1 (fructose-1,6-bisphosphate) denaturation | Activate |
| GSK-3β | Phosphorylation inhibits GS and blocks glycogen synthesis | INS phosphorylates GSK-3β through the PI3K/Akt pathway and inactivates it | Inhibit |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, L.; Li, J.; Ding, C.; Zhou, Y.; Xiao, Z. Mechanistic Advances in Hypoglycemic Effects of Natural Polysaccharides: Multi-Target Regulation of Glycometabolism and Gut Microbiota Crosstalk. Molecules 2025, 30, 1980. https://doi.org/10.3390/molecules30091980
Zhou L, Li J, Ding C, Zhou Y, Xiao Z. Mechanistic Advances in Hypoglycemic Effects of Natural Polysaccharides: Multi-Target Regulation of Glycometabolism and Gut Microbiota Crosstalk. Molecules. 2025; 30(9):1980. https://doi.org/10.3390/molecules30091980
Chicago/Turabian StyleZhou, Liquan, Jiani Li, Chen Ding, Yimiao Zhou, and Zuowei Xiao. 2025. "Mechanistic Advances in Hypoglycemic Effects of Natural Polysaccharides: Multi-Target Regulation of Glycometabolism and Gut Microbiota Crosstalk" Molecules 30, no. 9: 1980. https://doi.org/10.3390/molecules30091980
APA StyleZhou, L., Li, J., Ding, C., Zhou, Y., & Xiao, Z. (2025). Mechanistic Advances in Hypoglycemic Effects of Natural Polysaccharides: Multi-Target Regulation of Glycometabolism and Gut Microbiota Crosstalk. Molecules, 30(9), 1980. https://doi.org/10.3390/molecules30091980






