Tyrosinase Inhibitors Among Flora of Lubelskie Region—Application of Bio-Chromatographic Approach and Zebrafish Model in Bioactivity Screening of Plant Material
Abstract
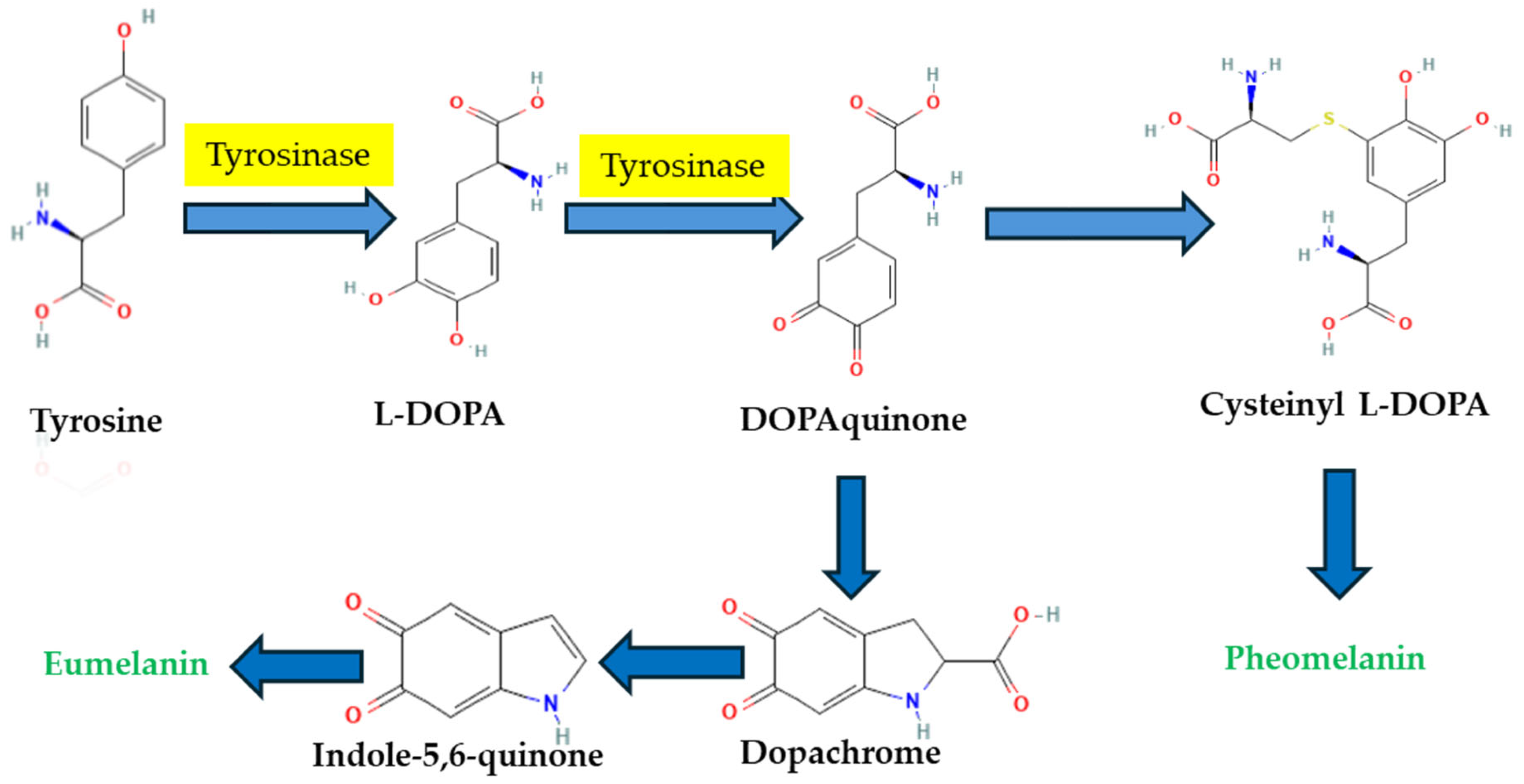
1. Introduction
2. Results
2.1. Extraction and Fingerprinting of the Obtained Extracts
2.2. Qualitative Fingerprinting of Chamomilla recutita Extract by HPLC-ESI-QTOF-MS/MS
2.3. Determination of Whitening Properties of Chamomile In Vivo in Zebrafish Model
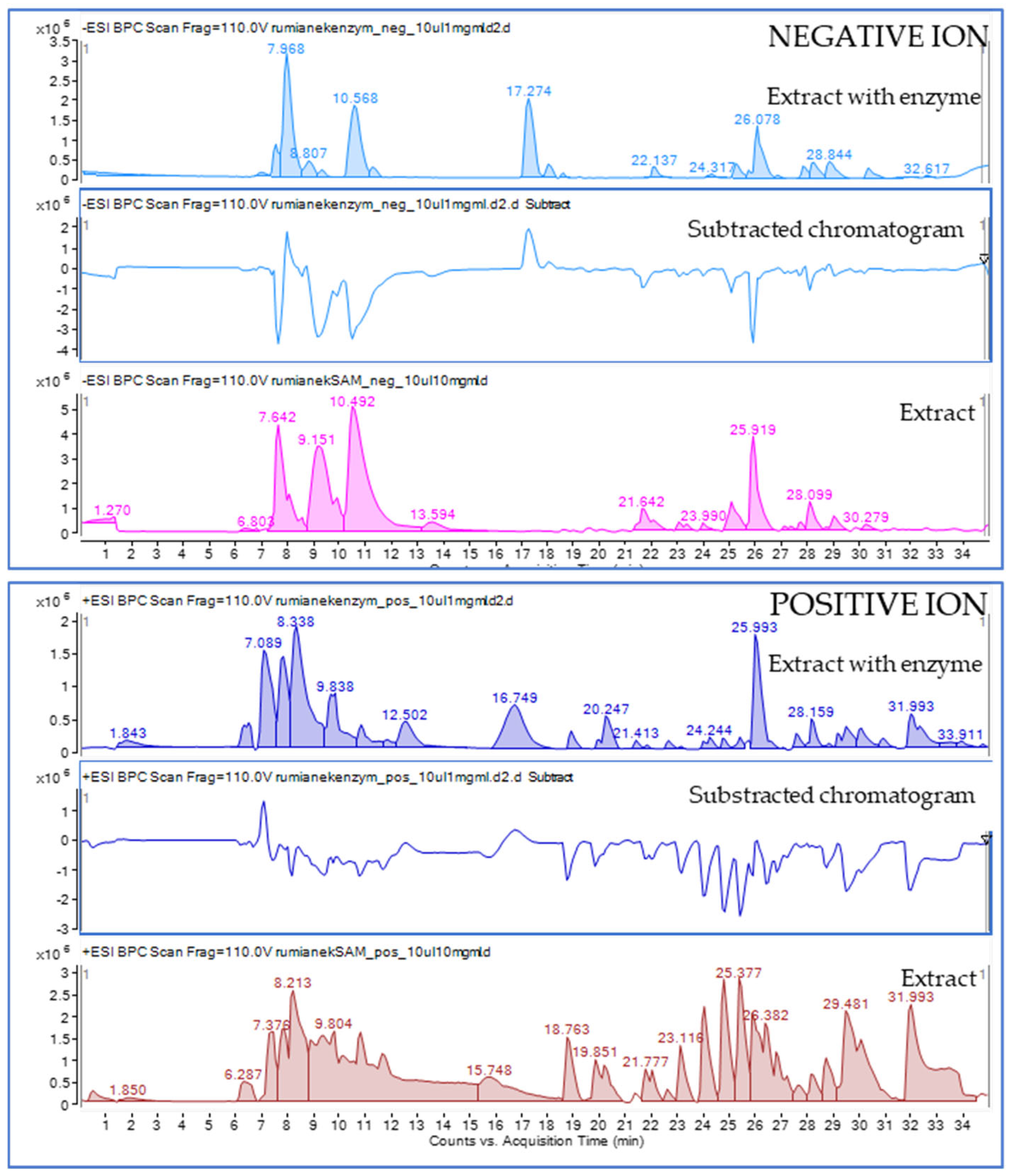
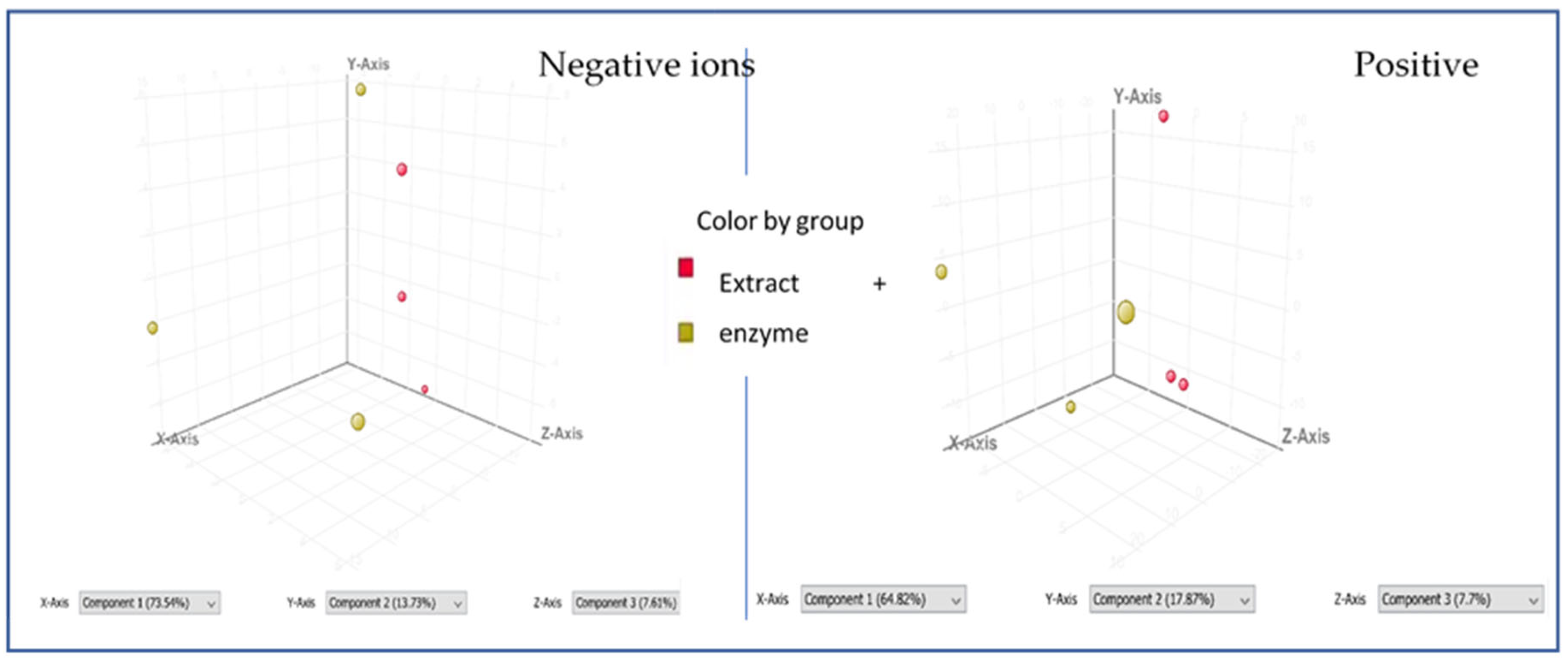
2.4. Bio-Chromatographic Studies Towards the Assignment of Single Molecules Interacting with Mushroom Tyrosinase Enzyme by HPLC-ESI-QTOF-MS/MS
2.5. Evaluation of the Tyrosinase Inhibitory Potential of Single Compounds
3. Materials and Methods
3.1. Plant Material and Extraction
3.2. In Vitro Biological Activity Determination Towards the Tyrosinase Inhibitory Potential
3.3. Compositional Studies of the Most Active Extract by HPLC-ESI-QTOF-MS/MS Approach
3.4. Zebrafish Assay for the Determination of Whitening Properties of Chamomile 50% EtOH Extract
3.4.1. Animals and Ethical Approval
3.4.2. Determination of Whitening Properties of Chamomile In Vivo in Zebrafish Model
3.5. Identification of Individual Components Within the 50% EtOH Extract of Chamomilla recutita Extract Exhibiting Tyrosinase Inhibitory Potential in HPLC-MS Approach
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cosmetics Europe. Market Performance. 2022. Available online: https://www.cosmeticseurope.eu (accessed on 23 February 2025).
- European Commission. CosIng—Cosmetic Ingredients. Available online: https://ec.europa.eu/growth/tools-databases/cosing/ (accessed on 23 February 2025).
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV Radiation and the Skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef]
- Wang, Y.; Hao, M.M.; Sun, Y.; Wang, L.F.; Wang, H.; Zhang, Y.J.; Li, H.Y.; Zhuang, P.W.; Yang, Z. Synergistic Promotion on Tyrosinase Inhibition by Antioxidants. Molecules 2018, 23, 106. [Google Scholar] [CrossRef]
- Prota, G. Progress in the Chemistry of Melanins and Related Metabolites. Med. Res. Rev. 1988, 8, 525–556. [Google Scholar] [CrossRef] [PubMed]
- Bang, E.; Noh, S.G.; Ha, S.; Jung, H.J.; Kim, D.H.; Lee, A.K.; Hyun, M.K.; Kang, D.; Lee, S.; Park, C.; et al. Evaluation of the Novel Synthetic Tyrosinase Inhibitor (Z)-3-(3-Bromo-4-Hydroxybenzylidene)thiochroman-4-one (MHY1498) In Vitro and In Silico. Molecules 2018, 23, 3307. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, P.C.O.; Lessa, R.C.; Ceroullo, M.S.; Wegermann, C.A.; De Moraes, M.C. On-flow enzymatic inhibitor screening: The emerging success of liquid chromatography-based assays. Front. Anal. Sci. 2022, 2, 1004113. [Google Scholar] [CrossRef]
- Peng, M.J.; Shi, S.Y.; Chen, L.; Zhang, S.H.; Cai, P.; Chen, X.Q. Online coupling solid-phase ligand-fishing with high-performance liquid chromatography–diode array detector–tandem mass spectrometry for rapid screening and identification of xanthine oxidase inhibitors in natural products. Anal. Bioanal. Chem. 2016, 408, 6693–6701. [Google Scholar] [CrossRef]
- Fu, Y.; Mo, H.Y.; Gao, W.; Hong, J.Y.; Lu, J.; Li, P.; Chen, C. Affinity selection-based two-dimensional chromatography coupled with high-performance liquid chromatography-mass spectrometry for discovering xanthine oxidase inhibitors from Radix Salviae Miltiorrhizae. Anal. Bioanal. Chem. 2014, 406, 4987–4995. [Google Scholar] [CrossRef]
- Tao, Y.; Chen, Z.; Zhang, Y.; Wang, Y.; Cheng, Y. Immobilized magnetic beads based multi-target affinity selection coupled with high performance liquid chromatography-mass spectrometry for screening anti-diabetic compounds from a Chinese medicine “Tang-Zhi-Qing”. J. Pharm. Biomed. Anal. 2013, 78–79, 190–201. [Google Scholar] [CrossRef]
- Ingkaninan, K.; Phengpa, P.; Yuenyongsawad, S.; Khorana, N. Acetylcholinesterase inhibitors from Stephania venosa tuber. J. Pharm. Pharmacol. 2006, 58, 695–700. [Google Scholar] [CrossRef]
- Lubelskie Voivodeship. Analiza Potencjału Województwa Lubelskiego w Zakresie Gospodarczego Wykorzystania Bioaktywnych Substancji Pochodzenia Naturalnego. Available online: https://www.lubelskie.pl/wp-content/uploads/2023/06/Analiza-potencja%C5%82u-woj.-lubelskiego-w-zakresie-gospodarczego-wykorzystania-bioaktywnych-substancji-pochodzenia-naturalnego.pdf (accessed on 20 January 2025).
- Jo, J.-B.; Kim, M.-U.; Lee, E.-H.; Kim, Y.-J.; Cho, E.-B.; Kang, I.-K.; Cho, Y.-J. Whitening Effect of Extracts from Matricaria chamomilla L. with B16F10 Melanoma Cells. J. Appl. Biol. Chem. 2018, 61, 267–273. [Google Scholar] [CrossRef]
- Dai, Y.L.; Li, Y.; Wang, Q.; Niu, F.J.; Li, K.W.; Wang, Y.Y.; Wang, J.; Zhou, C.Z.; Gao, L.N. Chamomile: A Review of Its Traditional Uses, Chemical Constituents, Pharmacological Activities and Quality Control Studies. Molecules 2022, 28, 133. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.F.; Zhang, D.; Yang, L.X.; Wang, K.; Ma, Y.; Yang, L. Determination of Volatile Components in Different Parts of Chamomile by HS-SPME-GC-MS. Chin. J. Exp. Tradit. Med. Formulae 2018, 24, 69–73. [Google Scholar]
- Li, Q.; Abdulla, R.; Xin, X.; Xue, G.; Kang, X.; Zhao, F.; Asia, H.A. Profiling of Chemical Constituents of Matricaria chamomilla L. by UHPLC-Q-Orbitrap-HRMS and In Vivo Evaluation of Its Anti-Asthmatic Activity. Heliyon 2023, 9, e15470. [Google Scholar] [CrossRef] [PubMed]
- Tabatadze, N.; Tsomaia, I.; Pichette, A.; Mshvildadze, V. HPLC Identification of Caffeoylquinic Acid Derivatives of Cephalaria gigantea. Int. J. Pharm. Sci. Res. 2021, 8, 155–158. [Google Scholar] [CrossRef]
- Ismail, Y.; Fahmy, D.M.; Ghattas, M.H.; Ahmed, M.M.; Zehry, W.; Saleh, S.M.; Abo-Elmatty, D.M. Integrating Experimental Model, LC-MS/MS Chemical Analysis, and Systems Biology Approach to Investigate the Possible Antidiabetic Effect and Mechanisms of Matricaria aurea (Golden Chamomile) in Type 2 Diabetes Mellitus. Front. Pharmacol. 2022, 13, 924478. [Google Scholar] [CrossRef] [PubMed]
- Polcaro, L.M.; Cerulli, A.; Montella, F.; Ciaglia, E.; Masullo, M.; Piacente, S. Chemical Profile and Antioxidant and Tyrosinase Inhibitory Activity of Chamaemelum nobile L. Green Extracts. Cosmetics 2024, 11, 94. [Google Scholar] [CrossRef]
- Choi, T.Y.; Kim, J.H.; Ko, D.H.; Kim, C.H.; Hwang, J.S.; Ahn, S.; Kim, S.Y.; Kim, C.D.; Lee, J.H.; Yoon, T.J. Zebrafish as a New Model for Phenotype-Based Screening of Melanogenic Regulatory Compounds. Pigment Cell Res. 2007, 20, 120–122. [Google Scholar] [CrossRef]
- Logan, D.W.; Burn, S.F.; Jackson, I.J. Regulation of Pigmentation in Zebrafish Melanophores. Pigment Cell Res. 2006, 19, 206–213. [Google Scholar] [CrossRef]
- Rawls, J.F.; Mellgren, E.M.; Johnson, S.L. How the Zebrafish Gets Its Stripes. Dev. Biol. 2001, 240, 301–314. [Google Scholar] [CrossRef]
- Chen, W.-C.; Tseng, T.-S.; Hsiao, N.-W.; Lin, Y.-L.; Wen, Z.-H.; Tsai, C.-C.; Lee, Y.-C.; Lin, H.-H.; Tsai, K.-C. Discovery of Highly Potent Tyrosinase Inhibitor, T1, with Significant Anti-Melanogenesis Ability by Zebrafish In Vivo Assay and Computational Molecular Modeling. Sci. Rep. 2015, 5, 7995. [Google Scholar] [CrossRef]
- Smeriglio, A.; D’Angelo, V.; Denaro, M.; Trombetta, D.; Raimondo, F.M.; Germanò, M.P. Polyphenol Characterization, Antioxidant and Skin Whitening Properties of Alnus cordata Stem Bark. Chem. Biodivers. 2019, 16, e1900314. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, N.; Han, D.; Yan, H. Characterization of Tyrosinase Inhibitors in Dryopteris crassirhizoma Rhizome Using a Combination of High-Speed Counter-Current Chromatography, Affinity-Based Ultrafiltration, and Liquid Chromatography-Tandem Mass Spectrometry. Front. Nutr. 2022, 18, 862773. [Google Scholar] [CrossRef] [PubMed]
- Usuki, A.; Ohashi, A.; Sato, H.; Ochiai, Y.; Ichihashi, M.; Funasaka, Y. The Inhibitory Effect of Glycolic Acid and Lactic Acid on Melanin Synthesis in Melanoma Cells. Exp. Dermatol. 2003, 12, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Kaya, E.D.; Türkhan, A.; Gür, F.; Gür, B. A Novel Method for Explaining the Product Inhibition Mechanisms via Molecular Docking: Inhibition Studies for Tyrosinase from Agaricus bisporus. J. Biomol. Struct. Dyn. 2022, 40, 7926–7939. [Google Scholar] [CrossRef]
- Maruyama, H.; Kawakami, F.; Lwin, T.T.; Imai, M.; Shamsa, F. Biochemical Characterization of Ferulic Acid and Caffeic Acid Which Effectively Inhibit Melanin Synthesis via Different Mechanisms in B16 Melanoma Cells. Biol. Pharm. Bull. 2018, 41, 806–810. [Google Scholar] [CrossRef]
- Liang, C.-P.; Chang, C.-H.; Liang, C.-C.; Hung, K.-Y.; Hsieh, C.-W. In Vitro Antioxidant Activities, Free Radical Scavenging Capacity, and Tyrosinase Inhibitory of Flavonoid Compounds and Ferulic Acid from Spiranthes sinensis (Pers.) Ames. Molecules 2014, 19, 4681–4694. [Google Scholar] [CrossRef]
- Wijayanti, E.D.; Safitri, A.; Siswanto, D.; Fatchiyah, F. Indonesian Purple Rice Ferulic Acid as a Candidate for Anti-Aging through the Inhibition of Collagenase and Tyrosinase Activities. Indones. J. Chem. 2023, 23, 475–488. [Google Scholar] [CrossRef]
- Li, H.-R.; Habasi, M.; Xie, L.-Z.; Aisa, H.A. Effect of Chlorogenic Acid on Melanogenesis of B16 Melanoma Cells. Molecules 2014, 19, 12940–12948. [Google Scholar] [CrossRef]
- De Freitas, M.M.; Fontes, P.R.; Souza, P.M.; Fagg, C.W.; Guerra, E.N.S.; de Medeiros Nóbrega, Y.K.; Silveira, D.; Fonseca-Bazzo, Y.; Simeoni, L.A.; Homem-de-Mello, M.; et al. Extracts of Morus nigra L. Leaves Standardized in Chlorogenic Acid, Rutin and Isoquercitrin: Tyrosinase Inhibition and Cytotoxicity. PLoS ONE 2016, 11, e0163130. [Google Scholar] [CrossRef]
- Nguyen, V.; Taine, E.G.; Meng, D.; Cui, T.; Tan, W. Chlorogenic Acid: A Systematic Review on the Biological Functions, Mechanistic Actions, and Therapeutic Potentials. Nutrients 2024, 16, 924. [Google Scholar] [CrossRef]
- Rodboon, T.; Okada, S.; Suwannalert, P. Germinated Riceberry Rice Enhanced Protocatechuic Acid and Vanillic Acid to Suppress Melanogenesis through Cellular Oxidant-Related Tyrosinase Activity in B16 Cells. Antioxidants 2020, 9, 247. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-D.; Choi, H.; Abekura, F.; Park, J.-Y.; Yang, W.-S.; Yang, S.-H.; Kim, C.-H. Naturally-Occurring Tyrosinase Inhibitors Classified by Enzyme Kinetics and Copper Chelation. Int. J. Mol. Sci. 2023, 24, 8226. [Google Scholar] [CrossRef] [PubMed]
- Ersoy, E.; Ozkan, E.E.; Boga, M.; Yilmaz, M.A.; Mat, A. Anti-Aging Potential and Anti-Tyrosinase Activity of Three Hypericum Species with Focus on Phytochemical Composition by LC–MS/MS. Ind. Crops Prod. 2019, 141, 111735. [Google Scholar] [CrossRef]
- Gou, L.; Lee, J.; Yang, J.M.; Park, Y.D.; Zhou, H.M.; Zhan, Y.; Lü, Z.R. Inhibition of Tyrosinase by Fumaric Acid: Integration of Inhibition Kinetics with Computational Docking Simulations. Int. J. Biol. Macromol. 2017, 105, 1663–1669. [Google Scholar] [CrossRef]
- Jeong, Y.J.; Lee, J.Y.; Park, J.; Park, S.N. An Inhibitory Mechanism of Action of a Novel Syringic-Acid Derivative on α-Melanocyte-Stimulating Hormone (α-MSH)-Induced Melanogenesis. Life Sci. 2017, 191, 52–58. [Google Scholar] [CrossRef]
- Strzępek-Gomółka, M.; Gaweł-Bęben, K.; Angelis, A.; Antosiewicz, B.; Sakipova, Z.; Kozhanova, K.; Głowniak, K.; Kukula-Koch, W. Identification of Mushroom and Murine Tyrosinase Inhibitors from Achillea biebersteinii Afan. Extract. Molecules 2021, 26, 964. [Google Scholar] [CrossRef]
- Oh, K.E.; Shin, H.; Lee, M.K.; Park, B.; Lee, K.Y. Characterization and Optimization of the Tyrosinase Inhibitory Activity of Vitis amurensis Root Using LC-Q-TOF-MS Coupled with a Bioassay and Response Surface Methodology. Molecules 2021, 26, 446. [Google Scholar] [CrossRef]
- Welling, G.W.; Scheffer, A.J.; Welling-Wester, S. Determination of enzyme activity by high-performance liquid chromatography. J. Chromatogr. B Biomed. Sci. Appl. 1994, 659, 209–225. [Google Scholar] [CrossRef]
- Wojtasek, H. Quercetin Is a Substrate, Not an Inhibitor, of Tyrosinase—Comments on “Quercetin as a Tyrosinase Inhibitor: Inhibitory Activity, Conformational Change and Mechanism” Published by Fan et al. (2017). Food Res. Int. 2022, 153, 110944. [Google Scholar] [CrossRef]
- Uchida, R.; Ishikawa, S.; Tomoda, H. Inhibition of Tyrosinase Activity and Melanin Pigmentation by 2-Hydroxytyrosol. Acta Pharm. Sin. B 2013, 4, 141–145. [Google Scholar] [CrossRef]
- Cabanes, J.; Chazarra, S.; Garcia-Carmona, F. Kojic Acid, a Cosmetic Skin Whitening Agent, Is a Slow-Binding Inhibitor of Catecholase Activity of Tyrosinase. J. Pharm. Pharmacol. 1994, 46, 982–985. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-M.; Song, W.-C.; Liu, C.; Yan, S.; Chen, Q.-X.; Zhang, J.; Tang, D.-L.; Chen, S.-M.; Wang, Q. Anti-Melanogenesis of Novel Kojic Acid Derivatives in B16F10 Cells and Zebrafish. Int. J. Biol. Macromol. 2019, 123, 723–731. [Google Scholar] [CrossRef] [PubMed]



| Species Name | Type of Extract | Remaining Tyrosinase Activity [%] | Tyrosinase Inhibition [%] |
|---|---|---|---|
|
Chamomile herb Matricaria chamomilla | 96% EtOH 50% EtOH H2O | 128 ± 4.60 24.2 ± 7.18 60.6 ± 3.95 | 0 75.8 39.4 |
|
Marigold flower Calendula officinalis | 96% EtOH 50% EtOH H2O | 127 ± 26.1 58.4 ± 3.41 56.8 ± 1.43 | 0 41.6 43.2 |
|
Dandelion rhizome Taraxacum officinale | 96% EtOH 50% EtOH H2O | 62.9 ± 15.6 61.5 ± 3.35 72.3 ± 5.14 | 37.1 38.5 27.7 |
|
Rosehip fruit Rosa canina | 96% EtOH 50% EtOH H2O | 111 ± 23.5 184 ± 8.71 167 ± 20.3 | 0 0 0 |
|
Elderberry flower Sambucus nigra | 96% EtOH 50% EtOH H2O | 316 ± 17.4 200 ± 3.60 115 ± 3.03 | 0 0 0 |
|
Hawthorn fruit Crataegus monogyna | 96% EtOH 50% EtOH H2O | 180 ± 27.9 194 ± 9.41 156 ± 10.5 | 0 0 0 |
|
Nettle herb Urtica dioica | 96% EtOH 50% EtOH H2O | 224 ± 28.9 183 ± 1.41 114 ± 20.5 | 0 0 0 |
|
Horsetail herb Equisetum arvense | 96% EtOH 50% EtOH H2O | 259 ± 1.81 151 ± 11.5 276 ± 4.75 | 0 0 0 |
| Kojic acid (1 mg/mL) | H2O | 42.0 | 57.97 |
| No | Ion | Rt [min] | Proposed Compound | Neutral Molecular Formula | Theoretical Mass | Experimental Mass | MS/MS Fragments | Error of Measurement [ppm] | DBE |
|---|---|---|---|---|---|---|---|---|---|
| 1 | [M−H]− | 7.0514 | Gluconic acid | C6H12O7 | 195.0510 | 195.0523 | 177.0404 129.0192 99.0091 75.0097 | −6.5 | 1 |
| 2 | [M−H]− | 8.078 | Quinic acid | C7H12O6 | 191.0561 | 191.0563 | 173.0555 127.0475 103.0099 | −0.98 | 2 |
| 3 | [M−H]− | 9.587 | Malic acid | C4H6O5 | 133.0142 | 133.0148 | 114.9936 89.0169 71.0086 | −4.12 | 2 |
| 4 | [M−H]− | 10.459 | Citric acid | C6H8O7 | 191.0197 | 191.0198 | 173.0064 129.0168 111.0068 87.0074 | −0.38 | 3 |
| 5 | [M−H]− | 17.618 | Baicalin | C21H18O11 | 445.0776 | 445.0788 | - | −2.61 | 13 |
| 6 | [M−H]− | 21.609 | Protocatechuoylglucose | C13H16O9 | 315.0722 | 315.0731 | 203.0012 165.0184 152.0111 108.0214 | −2.99 | 6 |
| 7 | [M−H]− | 21.860 | Glucogallic acid | C13H16O10 | 331.0671 | 331.0673 | 313.0577 168.0061 125.0238 | −0.69 | 6 |
| 8 | [M−H]− | 22.917 | Dihydroferulic acid 4-O-glucuronide; | C16H20O10 | 371.0984 | 371.0988 | 251.0550 209.0314 197.0468 191.0258 167.0335 | −1.15 | 7 |
| 9 | [M−H]− | 23.068 | Chlorogenic acid | C16H18O9 | 353.0872 | 353.0882 | 209.0299 191.0557 179.0349 161.0232 135.0444 | −1.11 | 8 |
| 10 | [M−H]− | 23.4 | Dihydroxybenzoic acid | C7H6O4 | 153.0193 | 153.0195 | 109.0292 81.0350 | −1.09 | 5 |
| 11 | [M−H]− | 23.403 | Protocatechuic acid | C7H6O4 | 153.0212 | 153.0198 | 109.0293 91.0189 81.0352 | −3.4 | 5 |
| 12 | [M−H]− | 24.845 | Isopropylmalic acid | C7H12O5 | 175.0612 | 175.0606 | 157.0456 131.0659 115.0352 | 3.39 | 2 |
| 13 | [M−H]− | 25.164 | Neochlorogenic acid | C16H18O9 | 353.0872 | 353.0856 | 191.0529 179.0312 173.0426 161.0211 | 6.23 | 8 |
| 14 | [M−H]− | 25.248 | Syringetin glucoside | C23H24O13 | 507.1144 | 507.1150 | - | −1.15 | 12 |
| 15 | [M−H]− | 25.751 | Feruloyl hexose | C16H20O9 | 355.1034 | 355.1040 | 193.0499 149.0594 134.0358 | −1.53 | 7 |
| 16 | [M−H]− | 25.919 | Syringin | C17H24O9 | 371.1348 | 371.1353 | 209.0647 191.0683 179.0515 149.0459 | −1.46 | 6 |
| 17 | [M−H]− | 26.003 and 28.434 | Feruloyl-glucose isomers | C16H20O9 | 355.1029 | 355.1042 | 241.1059 193.0494 149.0598 134.0364 | −2.09 | 7 |
| 18 | [M−H]− | 26.103 | Ferulic acid | C10H10O4 | 193.0506 | 193.0513 | 149.0592 134.0363 | −3.44 | 6 |
| 19 | [M−H]− | 26.1870 | (Z)-chlorogenic acid | C16H18O9 | 353.0872 | 353.0875 | 191.0550 161.0235 149.0595 134.0360 119.0337 | −4.78 | 8 |
| 20 | [M−H]− | 26.606 | p-Coumaroyltartaric acid | C13H14O7 | 281.0667 | 281.0647 | - | 7.01 | 7 |
| 21 | [M−H]− | 27.009 | 7,8-Dihydroxycoumarin | C9H6O4 | 177.0193 | 177.0193 | 149.0202 133.0257 105.0311 | 0.18 | 7 |
| 22 | [M−H]− | 27.076 | Esculetin | C9H6O4 | 177.0193 | 177.0202 | 149.0209 133.0251 121.0247 105.0310 | −4.87 | 7 |
| 23 | [M−H]− | 27.260 | Apigenin-7-O-rhamnoglucoside (Rhoifolin) | C27H30O14 | 577.1563 | 577.1568 | 413.0882 293.0461 | −0.9 | 13 |
| 24 | [M−H]− | 27.361 | Caffeic acid | C9H8O4 | 179.035 | 179.0357 | 163.0052 135.0444 93.0340 | −3.99 | 6 |
| 25 | [M−H]− | 27.746 | Luteolin-7-O-rutinoside | C27H30O15 | 593.1506 | 593.1534 | 285.0401 | −3.71 | 13 |
| 26 | [M−H]− | 28.233 | Caffeoylmalic acid | C13H12O8 | 295.0459 | 295.0478 | 179.0343 163.0365 133.0135 115.0026 | −6.28 | 8 |
| 27 | [M + H]+ | 28.308 | 4-Methylumbelliferone | C10H8O3 | 177.0546 | 177.0554 | 149.0590 133.0647 121.0651 | −4.43 | 7 |
| 28 | [M−H]− | 28.669 | Luteolin galactoside | C21H20O11 | 447.0933 | 447.0934 | 327.0514 285.0396 256.0364 151.0020 | −0.26 | 12 |
| 29 | [M−H]− | 28.669 | Kaempferol glucoside | C21H20O11 | 447.0934 | 447.0933 | 285.0396 | 0.26 | 12 |
| 30 | [M−H]− | 28.719 | Patulitrin | C22H22O13 | 493.0990 | 493.0988 | 331.0447 316.0209 287.0193 181.0126 | 0.48 | 12 |
| 31 | [M−H]− | 28.736 | Glucocaffeic acid | C15H18O9 | 341.0881 | 341.0891 | 281.0611 251.0487 179.0297 161.0204 135.0398 | −3.78 | 7 |
| 32 | [M−H]− | 28.736 | Hyperoside | C21H20O12 | 463.0891 | 463.0896 | 300.0274 271.0225 151.0012 | −3.02 | 12 |
| 33 | [M−H]− | 29.189 | Propylglutaric acid | C8H14O4 | 173.0819 | 173.0809 | - | 5.93 | 2 |
| 34 | [M−H]− | 29.574 | Hydroxydecanoic acid | C10H18O5 | 217.1081 | 217.1076 | 199.0939 171.1004 155.1064 137.0957 127.1105 | 2.51 | 2 |
| 35 | [M−H]− | 29.776 | Di-caffeoylquinic acid | C25H24O12 | 515.1195 | 515.1203 | 353.0854 335.0794 308.1009 191.0540 179.0340 173.0435 | −1.55 | 14 |
| 36 | [M−H]− | 30.262 | Isorhamnetin 3-O-glucoside | C22H22O12 | 477.1053 | 477.1038 | 315.0617 299.0130 285.0347 243.0349 161.0189 152.0057 108.0163 | 3.03 | 12 |
| 37 | [M−H]− | 30.396 | Kaempferol 3-(6″-malonylglucoside) | C24H22O14 | 533.0950 | 533.0937 | 489.1002 285.0350 150.9987 | 2.47 | 14 |
| 38 | [M−H]− | 30.447 | Sinapic acid | C11H12O5 | 223.0612 | 223.0604 | 179.0651 163.0362 133.0623 | 3.56 | 6 |
| 39 | [M−H]− | 30.530 | Apigenin 8-C-glucoside | C21H20O10 | 431.0984 | 431.0964 | 268.0321 | 4.56 | 12 |
| 40 | [M−H]− | 30.933 | Di-caffeoyl-quinic acid | C25H24O12 | 515.1205 | 515.1195 | 353.0854 191.0533 179.0325 173.0424 161.0240 135.0435 | 1.94 | 14 |
| 41 | [M−H]− | 31.117 | Di-caffeoylquinic acid | C25H24O12 | 515.1195 | 515.1196 | 353.0880 323.0773 191.0549 179.0337 173.0441 161.0237 | −0.19 | 14 |
| 42 | [M−H]− | 31.537 | Kaempferol-3-O-alpha-L-arabinoside | C20H18O10 | 417.0827 | 417.0849 | - | −5.21 | 12 |
| 43 | [M−H]− | 31.620 | Apigenin | C15H10O5 | 269.0455 | 269.0462 | - | −2.42 | 11 |
| 44 | [M−H]− | 32.62 | Luteolin | C15H10O6 | 285.0405 | 285.0419 | - | −5.03 | 11 |
| Positive Ion Mode | Negative Ion Mode | ||||
|---|---|---|---|---|---|
| m/z of a Neutral Ion | Retention Time | Regulation | m/z of a Neutral Ion | Retention Time | Regulation |
| 78.0148 | 9.2 | up | 176.0655 | 25.094 | up |
| 157.111 | 9.265 | up | 298.0699 | 24.3819 | up |
| 175.0047 | 10.244 | up | 224.0664 | 30.636 | up |
| 522.158 | 10.244 | up | 129.9671 | 7.013 | up |
| 80.011 | 9.185 | up | 296.056 | 28.121 | up |
| 312.0026 | 14.006 | up | 192.062 | 26.241 | up |
| 334.0302 | 10.23 | up | 282.0723 | 26.569 | up |
| 291.9572 | 10.272 | up | 280.056 | 7.798 | up |
| 369.9593 | 10.223 | up | 240.0636 | 25.76 | up |
| 260.1743 | 8.679 | up | 328.0932 | 19.036 | up |
| 541.7257 | 7.159 | up | 297.8905 | 7.027 | up |
| 457.7642 | 7.149 | up | 508.1229 | 25.266 | up |
| 376.0027 | 9.937 | up | 120.0432 | 7.8 | up |
| 335.838 | 6.951 | up | |||
| 179.0642 | 21.53 | up | |||
| 543.7241 | 7.1579 | up | |||
| 109.0024 | 9.8 | down | |||
| 257.2374 | 0.956 | down | |||
| 241.9188 | 6.926 | down | |||
| 259.9874 | 9.33 | down | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kusio-Targońska, K.; Kosheva, N.; Wojtanowski, K.K.; Gaweł-Bęben, K.; Beis, D.; Kukula-Koch, W. Tyrosinase Inhibitors Among Flora of Lubelskie Region—Application of Bio-Chromatographic Approach and Zebrafish Model in Bioactivity Screening of Plant Material. Molecules 2025, 30, 1979. https://doi.org/10.3390/molecules30091979
Kusio-Targońska K, Kosheva N, Wojtanowski KK, Gaweł-Bęben K, Beis D, Kukula-Koch W. Tyrosinase Inhibitors Among Flora of Lubelskie Region—Application of Bio-Chromatographic Approach and Zebrafish Model in Bioactivity Screening of Plant Material. Molecules. 2025; 30(9):1979. https://doi.org/10.3390/molecules30091979
Chicago/Turabian StyleKusio-Targońska, Kamila, Nataliia Kosheva, Krzysztof Kamil Wojtanowski, Katarzyna Gaweł-Bęben, Dimitris Beis, and Wirginia Kukula-Koch. 2025. "Tyrosinase Inhibitors Among Flora of Lubelskie Region—Application of Bio-Chromatographic Approach and Zebrafish Model in Bioactivity Screening of Plant Material" Molecules 30, no. 9: 1979. https://doi.org/10.3390/molecules30091979
APA StyleKusio-Targońska, K., Kosheva, N., Wojtanowski, K. K., Gaweł-Bęben, K., Beis, D., & Kukula-Koch, W. (2025). Tyrosinase Inhibitors Among Flora of Lubelskie Region—Application of Bio-Chromatographic Approach and Zebrafish Model in Bioactivity Screening of Plant Material. Molecules, 30(9), 1979. https://doi.org/10.3390/molecules30091979










