Synergistic Adsorption and Fluorescence in Porous Aromatic Frameworks for Highly Sensitive Detection of Radioactive Uranium
Abstract
:1. Introduction
2. Results and Discussion
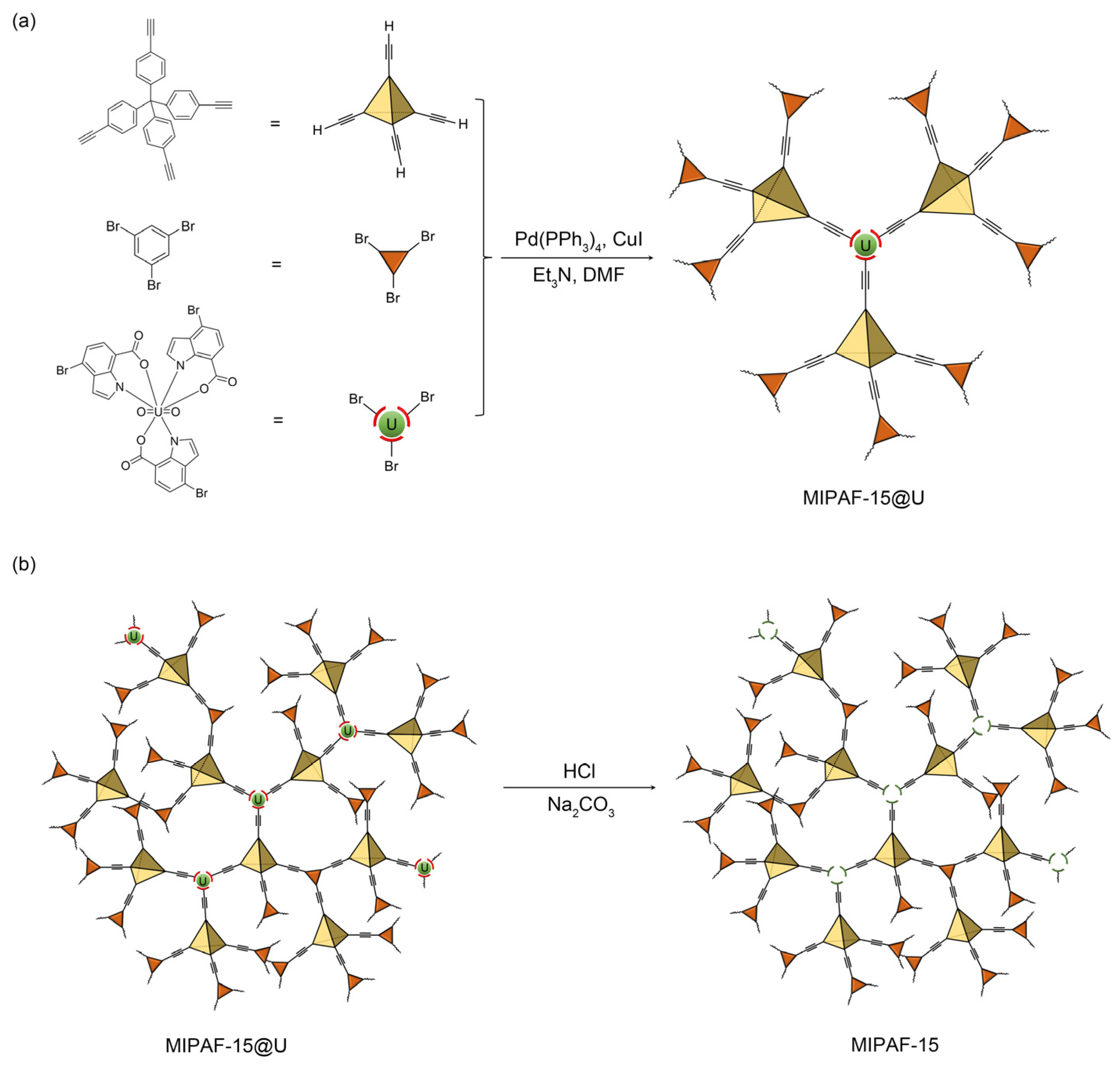
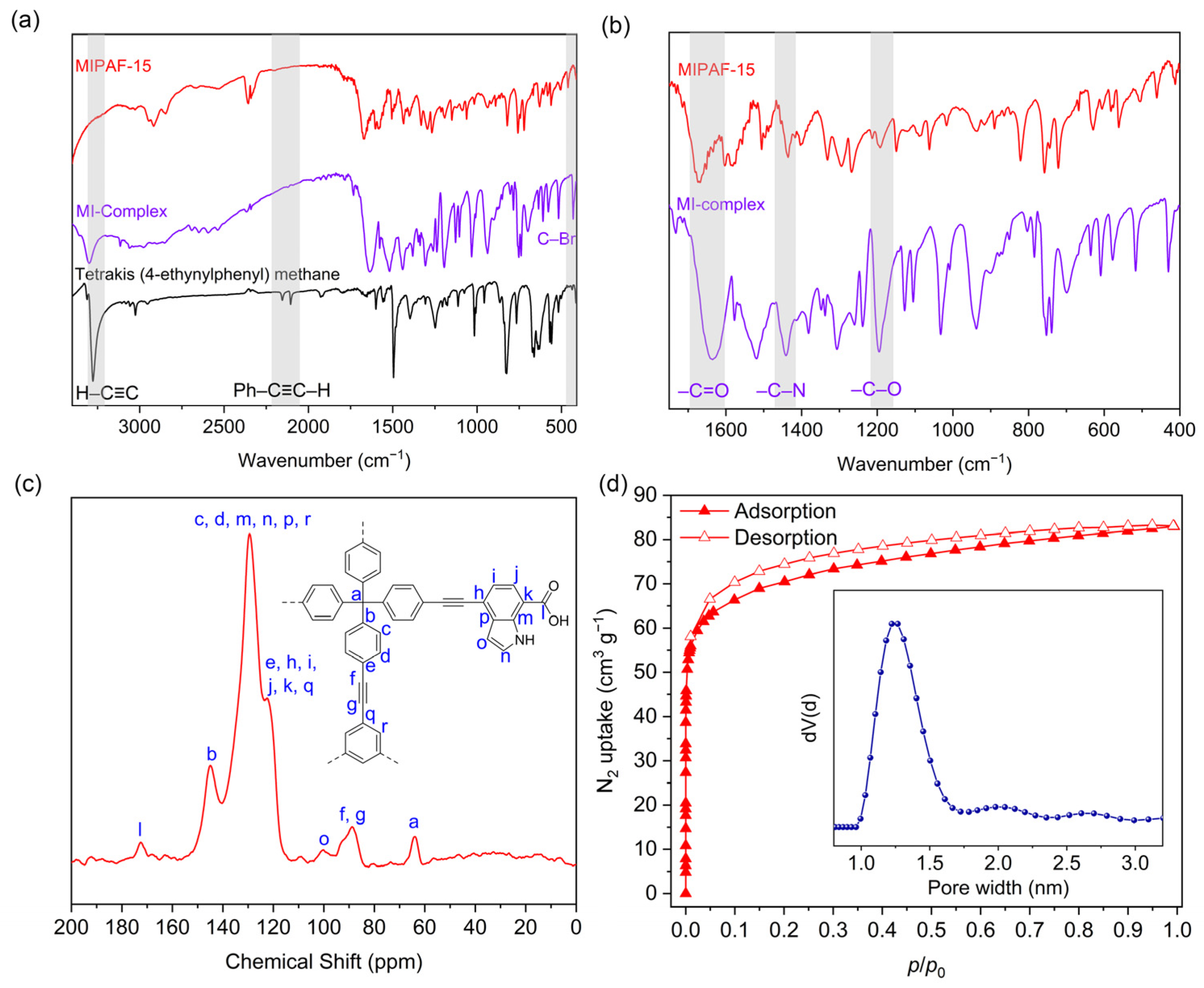
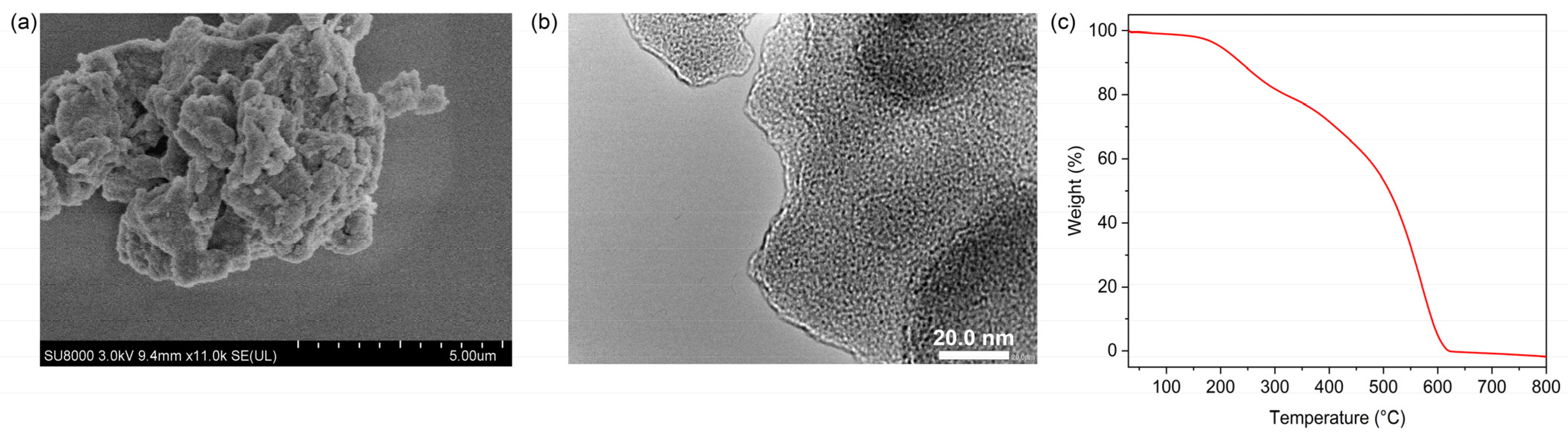
2.1. Structural Characterization of MIPAF-15
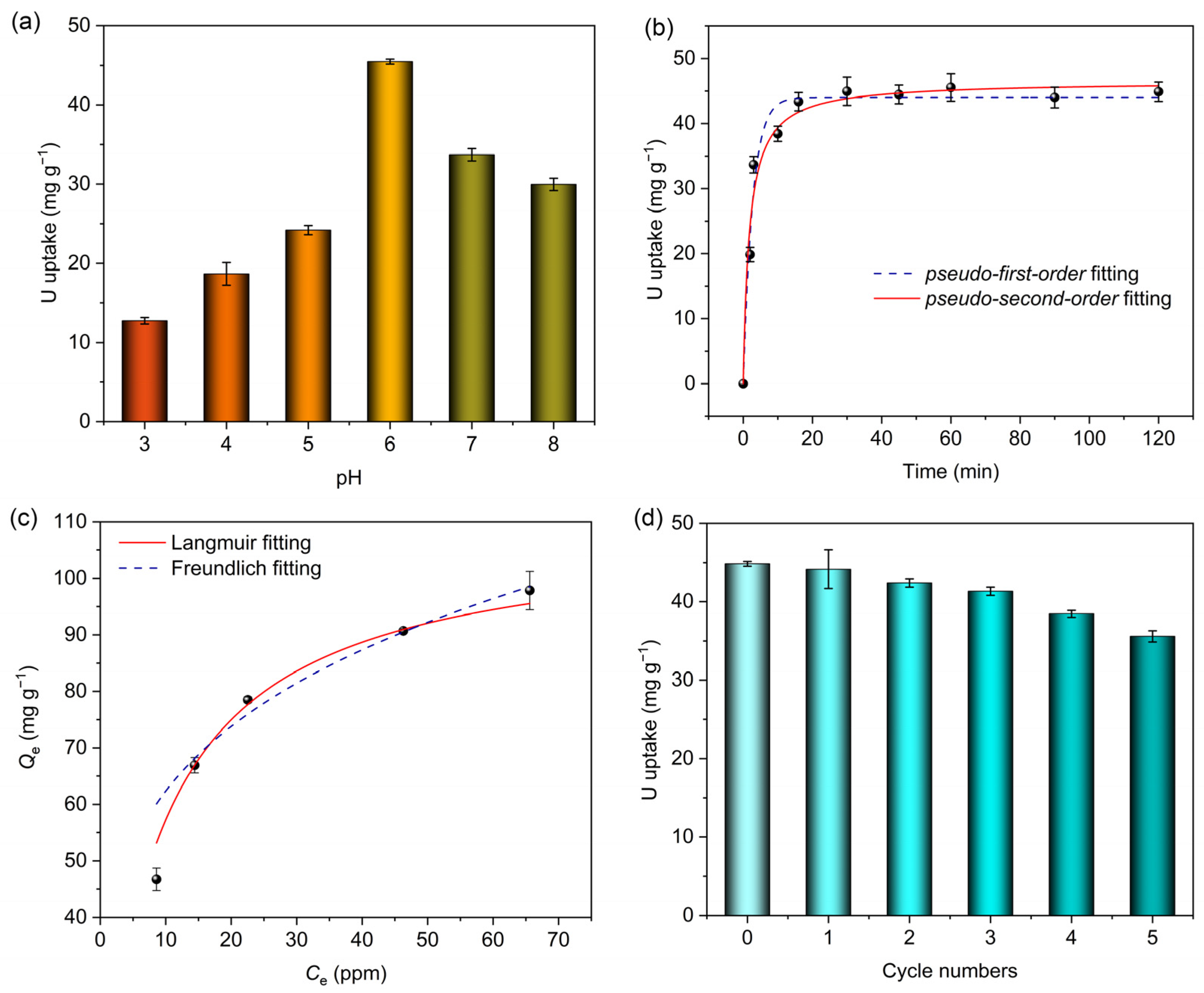
2.2. Uranyl Ion Adsorption Performance of MIPAF-15
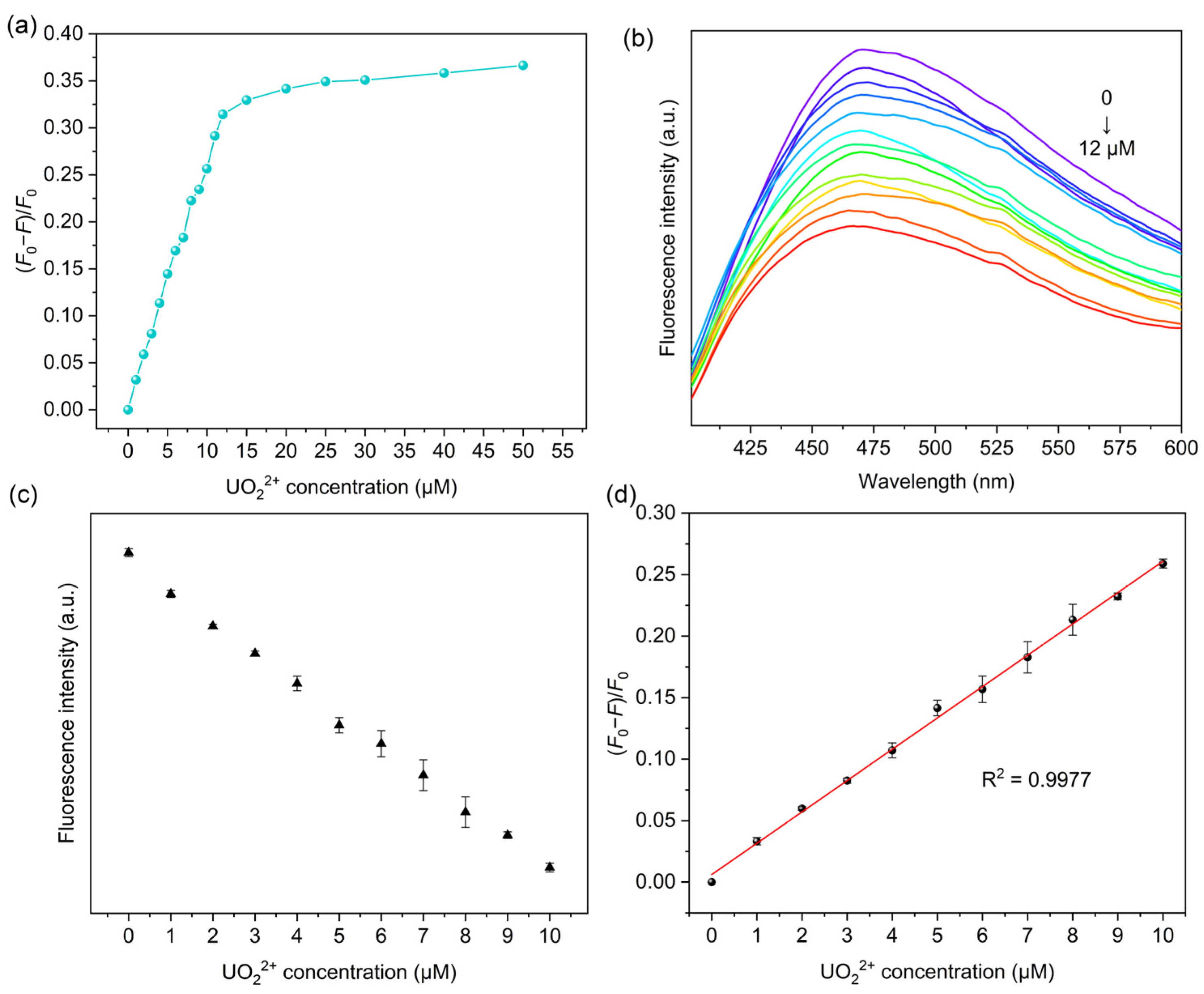
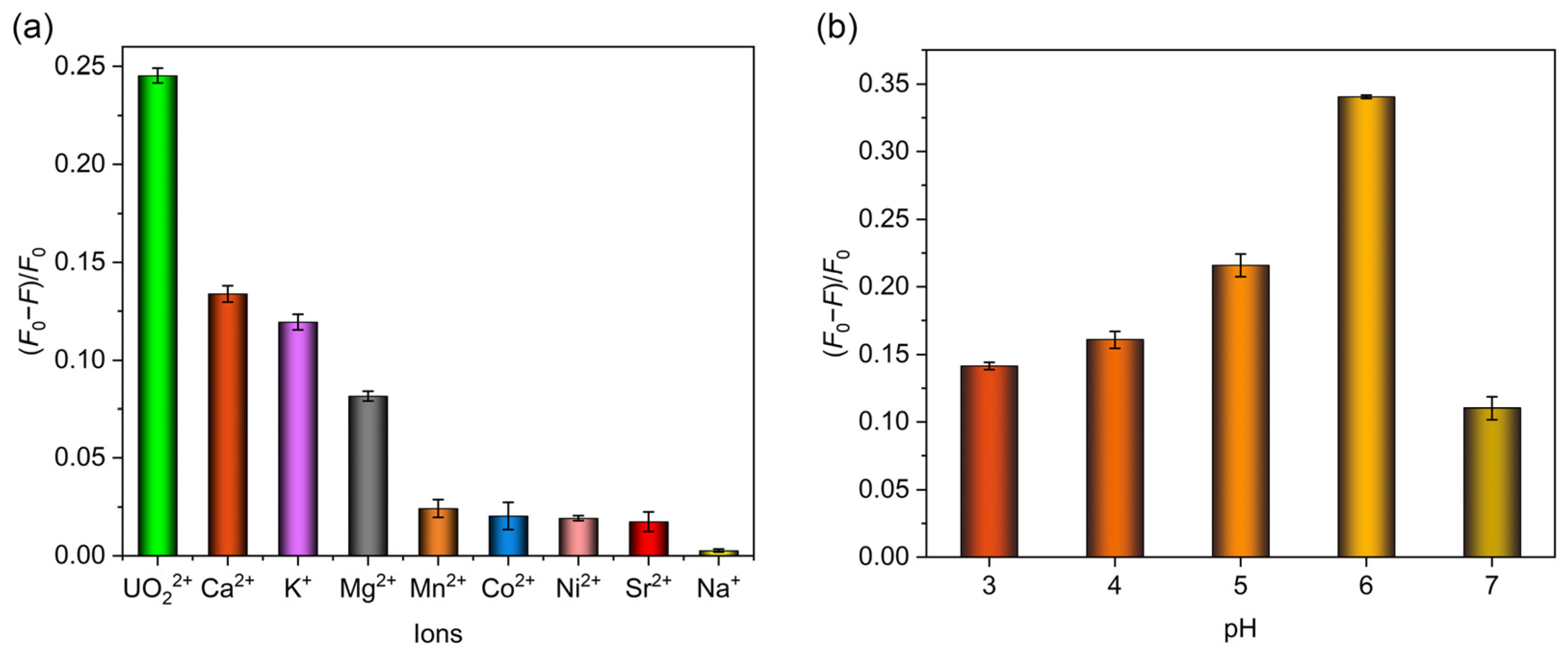
2.3. Fluorescence Sensing Performance of MIPAF-15
3. Materials and Methods
3.1. Materials
3.2. Instrumentation and Physical Measurements
3.3. Synthesis
3.3.1. Synthesis of Molecularly Imprinted Complex
3.3.2. Synthesis of Uranyl Ion Imprinted Porous Aromatic Framework
3.4. Uranyl Ion Adsorption Experiment
3.5. Fluorescence Detection of Uranyl Ion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef]
- Sholl, D.S.; Lively, R.P. Seven chemical separations to change the world. Nature 2016, 532, 435–437. [Google Scholar] [CrossRef]
- Yuan, Y.; Cao, D.D.; Cui, F.C.; Yang, Y.J.; Zhang, C.; Song, Y.B.; Zheng, Y.; Cao, J.R.; Chen, S.S.; Song, Y.; et al. High-capacity uranium extraction from seawater through constructing synergistic multiple dynamic bonds. Nat. Water 2025, 3, 89–98. [Google Scholar] [CrossRef]
- Yin, X.J.; Bai, J.; Fan, F.L.; Cheng, W.W.; Tian, W.; Wang, Y.; Qin, Z. Amidoximed silica for uranium (VI) sorption from aqueous solution. J. Radioanal. Nucl. Chem. 2015, 303, 2135–2142. [Google Scholar] [CrossRef]
- Bleise, A.; Danesi, P.R.; Burkart, W. Properties, use and health effects of depleted uranium (DU): A general overview. J. Environ. Radioact. 2003, 64, 93–112. [Google Scholar] [CrossRef]
- Agius, R.; Batistatou, E.; Gittins, M.; Jones, S.; McNamee, R.; Liu, H.; Rashid, A.; van Tongeren, M.; von Oertzen, G.; Wakeford, R. An epidemiological study of lung cancer and selected other cancers among Namibian uranium workers. Radiat. Res. 2023, 200, 340–348. [Google Scholar] [CrossRef]
- Ahmed, R.S.; Mohammed, R.S.; Mahdi, K.H.; Mahdi, Q.A.; Mostafa, M.Y.; Khalaf, H.N. Evaluation of uranium concentration in the blood breast cancer women with CR-39 detector. Appl. Radiat. Isot. 2022, 182, 110120. [Google Scholar] [CrossRef]
- Abdulrudha, N.H.; Kadhim, S.A. The relationship of cadmium, lead, and uranium with the geographical location of non-smoking thalassemia individuals: A comparative study. Appl. Radiat. Isot. 2025, 220, 111779. [Google Scholar] [CrossRef]
- Berradi, H.; Bertho, J.; Dudoignon, N.; Mazur, A.; Grandcolas, L.; Baudelin, C.; Grison, S.; Voisin, P.; Gourmelon, P.; Dublineau, I. Renal anemia induced by chronic ingestion of depleted uranium in rats. Toxicol. Sci. 2008, 103, 397–408. [Google Scholar] [CrossRef]
- Homma-Takeda, S.; Fujishiro, H.; Tanaka, I.; Yakumaru, H.; Ayama, K.; Uehara, A.; Oikawa, M.; Himeno, S.; Ishihara, H. Single-cell imaging for studies of renal uranium transport and intracellular behavior. Minerals 2021, 11, 191. [Google Scholar] [CrossRef]
- Xiao, F.B.; Sun, Y.F.; Du, W.F.; Shi, W.H.; Wu, Y.; Liao, S.Z.; Wu, Z.Y.; Yu, R.Q. Smart photonic crystal hydrogel material for uranyl ion monitoring and removal in water. Adv. Funct. Mater. 2017, 27, 1702147. [Google Scholar] [CrossRef]
- Cooper, K.L.; Dashner, E.J.; Tsosie, R.; Cho, Y.M.; Lewis, J.; Hudson, L.G. Inhibition of poly (ADP-ribose) polymerase-1 and DNA repair by uranium. Toxicol. Appl. Pharmacol. 2016, 291, 13–20. [Google Scholar] [CrossRef]
- Zhu, J.H.; Wang, J.; Liu, Q.; Yu, J.; Liu, J.Y.; Chen, R.R.; Song, D.L.; Li, R.M.; Wang, J. Amidoxime-functionalized MXene/graphene oxide aerogel for sunlight enhanced uranium adsorption. J. Environ. Chem. Eng. 2025, 13, 116254. [Google Scholar] [CrossRef]
- Burns, H.S.; Biegalski, S.R. Forensic signatures from laser isotope separation. J. Radioanal. Nucl. Chem. 2022, 331, 4947–4952. [Google Scholar] [CrossRef]
- Chen, D.Y.; Sun, M.F.; Zhao, X.Y.; Shi, M.S.; Fu, X.Y.; Hu, W.; Zhao, R. High-efficiency and economical uranium extraction from seawater with easily prepared supramolecular complexes. J. Colloid Interface. Sci. 2024, 668, 343–351. [Google Scholar] [CrossRef]
- Yang, Y.L.; Guo, K.K.; Zhu, M.C.; Zhang, A.G.; Xing, M.; Lu, Y.; Bai, X.; Ji, X.Y.; Hu, Y.J.; Liu, S.X. Exploring electron transfer mechanism in synergistic interactional reduced polyoxometalate-based Cu(I)–organic framework for photocatalytic removal of U(VI). Inorg. Chem. 2024, 63, 7876–7885. [Google Scholar] [CrossRef]
- Ali, T.A.; Akl, Z.F. Ionic liquid-multi-walled carbon nanotubes modified screen-printed electrodes for sensitive electrochemical sensing of uranium. J. Radioanal. Nucl. Chem. 2021, 328, 267–276. [Google Scholar] [CrossRef]
- Korolkov, I.V.; Yeszhanov, A.B.; Shakayeva, A.K.; Shlimas, D.I.; Zhumazhanova, A.; Zdorovets, M.V. Photo-induced graft (co)polymerization of glycidyl methacrylate and acrylonitrile on PET ion-track membranes for electrochemical detection of uranyl ions. Colloids Surf. A 2022, 648, 129086. [Google Scholar] [CrossRef]
- Kersten, B.; Akolkar, R.; Duval, C.E. An electrochemical technique for sensing uranium adsorption and desorption. Anal. Chim. Acta 2023, 1284, 342003. [Google Scholar] [CrossRef]
- Khaing, H.; Lemons, B.G.; Thakur, P. Concentration of uranium in the drinking and surface water around the WIPP site. J. Radioanal. Nucl. Chem. 2016, 310, 523–531. [Google Scholar] [CrossRef]
- Dodd, B.; Cartwright, M.; Goddard, B.; Tepper, G. Investigation of uranium (VI) sorption in mesoporous silica gel using gamma spectroscopy. J. Radioanal. Nucl. Chem. 2018, 318, 1077–1083. [Google Scholar] [CrossRef]
- Jiang, J.L.; Du, Y.F.; Deng, H.; Zhang, Z.J.; Wang, S.F.; Wu, H.X.; Tang, H.; Yun, W.; Zhang, J.; He, W.B.; et al. Surface-enhanced Raman spectroscopy detection of uranium oxides assisted by Ag2O. Appl. Surf. Sci. 2022, 577, 151968. [Google Scholar] [CrossRef]
- Pointurier, F.; Marie, O. An optimized methodology for the determination of the uranium chemical phases in micro-particles by Raman spectrometry within a scanning electron microscope. J. Radioanal. Nucl. Chem. 2023, 332, 2841–2850. [Google Scholar] [CrossRef]
- Wang, N.; Du, J.J.; Li, X.; Ji, X.L.; Wu, Y.L.; Sun, Z.L. Magnetic MOF substrates for the rapid and sensitive surface-enhanced Raman scattering detection of uranyl. Anal. Chem. 2023, 95, 12956–12963. [Google Scholar] [CrossRef]
- Cao, X.Y.; Lv, N.; Lv, J.X.; Guo, H.P. A liquid scintillation analysis method for low-level radioactive wastewater. J. Radiol. Prot. 2021, 41, 337–348. [Google Scholar] [CrossRef]
- Pulhani, V.; Reddy, P.J.; Chaudhury, M.; Tripathi, R.M. Sequential analysis methodology for 210Po and uranium analysis by extractive liquid scintillation spectrometry. J. Radioanal. Nucl. Chem. 2019, 322, 29–36. [Google Scholar] [CrossRef]
- Déjeant, A.; Bourva, L.; Sia, R.; Galoisy, L.; Calas, G.; Phrommavanh, V.; Descostes, M. Field analyses of 238U and 226Ra in two uranium mill tailings piles from Niger using portable HPGe detector. J. Environ. Radioact. 2014, 137, 105–112. [Google Scholar] [CrossRef]
- Zavadilová, A.; Drtinová, B. The matrix influence on the determination of low uranium concentrations by laser induced fluorescence method. J. Radioanal. Nucl. Chem. 2015, 304, 115–122. [Google Scholar] [CrossRef]
- Oba, M.; Akaoka, K.; Miyabe, M.; Wakaida, I. Zeeman effect of atomic uranium in the high lying odd levels measured by laser induced fluorescence spectroscopy. Eur. Phys. J. D 2000, 10, 349–352. [Google Scholar] [CrossRef]
- Fang, Y.T.; Zhang, Z.Q.; Hou, L.D.; Wang, Z.I.; Zhao, Y.P.; Liu, Q.W.; Liu, S.W.; Chang, X.L.; Qin, Y.Q.; Ma, J. Determination of trace uranium concentrations in spent-fuel reprocessing using online graphite crystal pre-diffraction energy-dispersive X-ray fluorescence. X-Ray Spectrom. 2025, 54, 274–283. [Google Scholar] [CrossRef]
- Takahashi, H.; Izumoto, Y.; Matsuyama, T.; Yoshii, H. Trace determination of uranium preconcentrated using graphene oxide by total reflection X-ray fluorescence spectrometry. X-Ray Spectrom. 2019, 48, 366–374. [Google Scholar] [CrossRef]
- Devore, M.A., II; Kerns, S.A.; Gorden, A.E.V. Characterization of quinoxolinol salen ligands as selective ligands for chemosensors for uranium. Eur. J. Inorg. Chem. 2015, 2015, 5708–5714. [Google Scholar] [CrossRef]
- Feng, T.T.; Zhao, S.L.; Cao, M.; Du, X.F.; Wang, H.; Cao, X.W.; Feng, L.J.; Yuan, Y.H.; Wang, N. Highly sensitive and specific uranyl ion detection by a fluorescent sensor containing uranyl-specific recognition sites. Sci. Bull. 2025, 70, 70–77. [Google Scholar] [CrossRef]
- Xiong, L.H.; Tong, Y.Q.; Song, J.Y.; Chen, S.H.; Liu, Y.; Liu, J.Q.; Li, L.; Zhen, D.S. Smartphone-assisted fluorescence/colorimetric dual-mode sensing strategy for uranium ion detection using cerium-sulfonyl calix [4] arene. Microchim. Acta 2025, 192, 158. [Google Scholar] [CrossRef]
- Xia, Z.; Shi, Y.; Xiao, T.; Zheng, X. Multi-stimuli responsive behavior of two Zn (II) complexes based on a Schiff base with high contrast. CrystEngComm 2024, 26, 1015–1021. [Google Scholar] [CrossRef]
- Xiao, T.; Yang, D.D.; Shi, Y.S.; Zheng, H.W.; Xia, Z.G.; Zheng, X.J. Hydrazone-based europium (III) complexes: Mechanochromic luminescence and turn-on fluorescence detection of quinolone antibiotics in human urine. Cryst. Growth Des. 2023, 23, 5957–5964. [Google Scholar] [CrossRef]
- Liu, H.J.; Wang, X.L.; Abeywickrama, T.; Jahanbazi, F.; Min, Z.F.; Lee, Z.R.; Terry, J.; Mao, Y.B. Biomimetically synthesized luminescent Tb3+ -doped fluorapatite/agar nanocomposite for detecting UO22+, Cu2+, and Cr3+ ions. Environ. Sci. Nano 2021, 8, 3711–3721. [Google Scholar] [CrossRef]
- Liu, H.J.; Wang, X.L.; Xiong, W.J.; Mao, Y.B. Luminescent Tb-doped Ca-deficient hydroxyapatite/agar for selective adsorption and detection of UO22+ ion. Mater. Res. Bull. 2022, 152, 111850. [Google Scholar] [CrossRef]
- Su, Z.; Zhang, L.X.; Zhang, H.Q.; Li, Y.S.; Guan, Q.Q. Biplane ion-pairing induced supramolecular assembly for high-performance uranium detection. Adv. Mater. 2025, 37, 2418952. [Google Scholar] [CrossRef]
- Zhou, X.Y.; Wang, Y.; Xiong, L.H.; Song, J.Y.; Zhou, H.; Li, L.; Zhen, D.S. Development of rare earth europium composites for highly sensitive fluorescence enhancement for detection of uranyl ions in water and cells. J. Radioanal. Nucl. Chem. 2025, 334, 1931–1939. [Google Scholar] [CrossRef]
- Harvey, P.; Nonat, A.; Platas Iglesias, C.; Natrajan, L.S.; Charbonnière, L.J. Sensing uranyl (VI) ions by coordination and energy transfer to a luminescent europium (III) complex. Angew. Chem. Int. Ed. 2018, 130, 10069–10072. [Google Scholar] [CrossRef]
- Jiang, M.; Xiao, X.; He, B.; Liu, Y.; Hu, N.; Su, C.L.; Li, Z.Y.; Liao, L.F. A europium (III) complex-based surface fluorescence sensor for the determination of uranium (VI). J. Radioanal. Nucl. Chem. 2019, 321, 161–167. [Google Scholar] [CrossRef]
- Chen, W.M.; Meng, X.L.; Zhuang, G.L.; Wang, Z.; Kurmoo, M.; Zhao, Q.Q.; Wang, X.P.; Shan, B.; Tung, C.H.; Sun, D. A superior fluorescent sensor for Al3+ and UO2 2+ based on a Co (II) metal–organic framework with exposed pyrimidyl Lewis base sites. J. Mater. Chem. A 2017, 5, 13079–13085. [Google Scholar] [CrossRef]
- Rapti, S.; Sarma, D.; Diamantis, S.A.; Skliri, E.; Armatas, G.S.; Tsipis, A.C.; Hassan, Y.S.; Alkordi, M.; Malliakas, C.D.; Kanatzidis, M.G.; et al. All in one porous material: Exceptional sorption and selective sensing of hexavalent chromium by using a Zr4+ MOF. J. Mater. Chem. A 2017, 5, 14707–14719. [Google Scholar] [CrossRef]
- Liu, W.; Dai, X.; Bai, Z.L.; Wang, Y.L.; Yang, Z.X.; Zhang, L.J.; Xu, L.; Chen, L.H.; Li, Y.X.; Gui, D.X.; et al. Highly sensitive and selective uranium detection in natural water systems using a luminescent mesoporous metal–organic framework equipped with abundant Lewis basic sites: A combined batch, X-ray absorption spectroscopy, and first principles simulation investigation. Environ. Sci. Technol. 2017, 51, 3911–3921. [Google Scholar] [CrossRef]
- Li, L.N.; Shen, S.S.; Su, J.; Ai, W.P.; Bai, Y.; Liu, H.W. Facile one-step solvothermal synthesis of a luminescent europium metal-organic framework for rapid and selective sensing of uranyl ions. Anal. Bioanal. Chem. 2019, 411, 4213–4220. [Google Scholar] [CrossRef]
- Chen, N.N.; Wang, J. A serial of 2D Co-Zn isomorphous metal–organic frameworks for photodegradation and luminescent detection properties. Appl. Organomet. Chem. 2020, 34, e5743. [Google Scholar] [CrossRef]
- Qin, X.D.; Yang, W.T.; Yang, Y.H.; Gu, D.X.; Guo, D.Y.; Pan, Q.H. A zinc metal–organic framework for concurrent adsorption and detection of uranium. Inorg. Chem. 2020, 59, 9857–9865. [Google Scholar] [CrossRef]
- Feng, H.; Feng, X.F.; Luo, F. A 1D brick-like coordination polymer containing free-standing sulfonic units for luminescence sensing of uranium in aqueous solution. J. Solid State Chem. 2021, 299, 122153. [Google Scholar] [CrossRef]
- Huang, Z.W.; Li, X.B.; Mei, L.; Han, Y.Z.; Song, Y.T.; Fu, X.; Zhang, Z.H.; Guo, Z.J.; Zeng, J.R.; Bian, F.G.; et al. All-in-one: Photo-responsive lanthanide-organic framework for simultaneous sensing, adsorption, and photocatalytic reduction of uranium. Adv. Funct. Mater. 2024, 34, 2404126. [Google Scholar] [CrossRef]
- Cui, W.R.; Zhang, C.R.; Jiang, W.; Li, F.F.; Liang, R.P.; Liu, J.W.; Qiu, J.D. Regenerable and stable sp2 carbon-conjugated covalent organic frameworks for selective detection and extraction of uranium. Nat. Commun. 2020, 11, 436. [Google Scholar] [CrossRef]
- Xiao, S.J.; Qiu, A.T.; Li, H.H.; Wang, M.P.; Zhang, L.; Guo, K.X.; Guo, J.; Qiu, J. Simultaneous detection and separation of uranium based on a fluorescent amidoxime-functionalized covalent organic polymer. Spectrochim. Acta Part A 2023, 289, 122182. [Google Scholar] [CrossRef]
- Mao, X.L.; Cai, Y.J.; Luo, Q.X.; Liu, X.; Jiang, Q.Q.; Zhang, C.R.; Zhang, L.; Liang, R.P.; Qiu, J.D. Europium (III) functionalized covalent organic framework as sensitive and selective fluorescent switch for detection of uranium. Anal. Chem. 2024, 96, 5037–5045. [Google Scholar] [CrossRef]
- Bhanjana, G.; Toor, I.; Chaudhary, G.R.; Dilbaghi, N.; Kim, K.; Kumar, S. Direct redox sensing of uranium using copper oxide quantum dots. J. Mol. Liq. 2019, 292, 111455. [Google Scholar] [CrossRef]
- Singhal, P.; Pulhani, V. Effect of ligand concentration, dilution, and excitation wavelength on the emission properties of CdSe/CdS core shell quantum dots and their implication on detection of uranium. Chem. Slct. 2019, 4, 4528–4537. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, H.Y.; Yu, D.M.; Qin, W.; Wu, X.H. Ultra-sensitive and stable n-doped carbon dots for selective detection of uranium through electron transfer induced UO2+ (V) sensing mechanism. Carbon 2022, 198, 162–170. [Google Scholar] [CrossRef]
- Yang, M.; Liao, L.F.; Zhang, G.L.; He, B.; Xiao, X.L.; Lin, Y.W.; Nie, C.M. Detection of uranium with a wireless sensing method by using salophen as receptor and magnetic nanoparticles as signal-amplifying tags. J. Radioanal. Nucl. Chem. 2013, 298, 1393–1399. [Google Scholar] [CrossRef]
- Cao, X.H.; Zhang, H.Y.; Ma, R.C.; Yang, Q.; Zhang, Z.B.; Liu, Y.H. Visual colorimetric detection of UO22+ using o-phosphorylethanolamine-functionalized gold nanoparticles. Sens. Actuators B 2015, 218, 67–72. [Google Scholar] [CrossRef]
- Li, W.L.; Mayo, J.T.; Benoit, D.N.; Troyer, L.D.; Lewicka, Z.A.; Lafferty, B.J.; Catalano, J.G.; Lee, S.S.; Colvin, V.L.; Fortner, J.D. Engineered superparamagnetic iron oxide nanoparticles for ultra-enhanced uranium separation and sensing. J. Mater. Chem. A 2016, 4, 15022–15029. [Google Scholar] [CrossRef]
- Wang, X.L.; Zeng, R.; Chu, S.N.; Tang, W.; Lin, N.; Fu, J.; Yang, J.R.; Gao, B. A quencher-free DNAzyme beacon for fluorescently sensing uranyl ions via embedding 2-aminopurine. Biosens. Bioelectron. 2019, 135, 166–172. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhu, G.S. Porous aromatic frameworks as a platform for multifunctional applications. ACS Cent. Sci. 2019, 5, 409–418. [Google Scholar] [CrossRef]
- Yuan, Y.; Meng, Q.H.; Faheem, M.; Yang, Y.J.; Li, Z.; Wang, Z.Y.; Deng, D.; Sun, F.X.; He, H.M.; Huang, Y.H.; et al. A molecular coordination template strategy for designing selective porous aromatic framework materials for uranyl capture. ACS Cent. Sci. 2019, 5, 1432–1439. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, Z.L.; Zhang, P.P.; Rong, H.Z.; Ma, T.T.; Cui, F.C.; Liu, D.T.; Zou, X.Q.; Zhu, G.S. Tailoring the pore chemistry in porous aromatic frameworks for selective separation of acetylene from ethylene. Chem. Sci. 2022, 13, 11126–11131. [Google Scholar] [CrossRef]
- Jang, J.Y.; Kim, Y.K.; Ko, Y.; Son, S.U. Hollow microporous organic polymer@hypercrosslinked polymer catalysts bearing N -heterocyclic carbene–iron species for the synthesis of biomass-derived polymer platforms. ACS Sustain. Chem. Eng. 2024, 12, 8880–8889. [Google Scholar] [CrossRef]
- Ma, H.P.; Ren, H.; Zou, X.Q.; Sun, F.X.; Yan, Z.J.; Cai, K.; Wang, D.Y.; Zhu, G.S. Novel lithium-loaded porous aromatic framework for efficient CO2 and H2 uptake. J. Mater. Chem. A 2013, 1, 752–758. [Google Scholar] [CrossRef]
- Li, X.; Cui, Y.; Yang, C.; Yan, X. Synthesis of carboxyl functionalized microporous organic network for solid phase extraction coupled with high-performance liquid chromatography for the determination of phenols in water samples. Talanta 2020, 208, 120434. [Google Scholar] [CrossRef]
- Kumar, K.; Zapf, A.; Michalik, D.; Tillack, A.; Heinrich, T.; Böttcher, H.; Arlt, M.; Beller, M. Palladium-catalyzed carbonylation of haloindoles: No need for protecting groups. Org. Lett. 2004, 6, 7–10. [Google Scholar] [CrossRef]
- Mistry, S.N.; Shonberg, J.; Draper-Joyce, C.J.; Klein Herenbrink, C.; Michino, M.; Shi, L.; Christopoulos, A.; Capuano, B.; Scammells, P.J.; Lane, J.R. Discovery of a novel class of negative allosteric modulator of the dopamine d2 receptor through fragmentation of a bitopic ligand. J. Med. Chem. 2015, 58, 6819–6843. [Google Scholar] [CrossRef]
- Park, S.I.; Kang, C.W.; Cho, S.Y.; Lee, S.M.; Kim, H.J.; Ko, Y.; Choi, J.; Son, S.U. Fabrication of poly (ethylene terephthalate) fiber @ microporous organic polymer with amino groups @ Cu films for flexible and metal-economical electromagnetic interference shielding materials. Langmuir 2020, 36, 8745–8752. [Google Scholar] [CrossRef]
- Sk, M.; Saifi, S.; Bera, S.; Ghosh, A.; Aijaz, A.; Banerjee, D. Reusable Ni-immobilized MOF catalyst for dehydrogenation of n-heterocycles under milder conditions. Chem. Eur. J. 2025, 31, e202404219. [Google Scholar] [CrossRef]
- Yan, Z.J.; Cui, B.; Zhao, T.; Luo, Y.F.; Zhang, H.C.; Xie, J.L.; Li, N.; Bu, N.S.; Yuan, Y.; Xia, L.X. A carbazole-functionalized porous aromatic framework for enhancing volatile iodine capture via Lewis electron pairing. Molecules 2021, 26, 5263. [Google Scholar] [CrossRef]
- Yan, Z.J.; Qiao, Y.M.; Wang, J.L.; Xie, J.L.; Cui, B.; Fu, Y.; Lu, J.W.; Yang, Y.J.; Bu, N.S.; Yuan, Y.; et al. An azo-group-functionalized porous aromatic framework for achieving highly efficient capture of iodine. Molecules 2022, 27, 6297. [Google Scholar] [CrossRef]
- Yang, Y.J.; Cai, F.L.; Zhang, C.; Gao, N.; Zhang, S.M.; Wang, G.T.; Yuan, Y. Molecularly imprinted porous-organic framework with pH-responsive adsorption sites for the selective adsorption of iron. Chin. J. Chem. 2024, 42, 3324–3330. [Google Scholar] [CrossRef]
- Ben, T.; Ren, H.; Ma, S.Q.; Cao, D.P.; Lan, J.H.; Jing, X.F.; Wang, W.C.; Xu, J.; Deng, F.; Simmons, J.M.; et al. Targeted synthesis of a porous aromatic framework with high stability and exceptionally high surface area. Angew. Chem. Int. Ed. 2009, 48, 9457–9460. [Google Scholar] [CrossRef]
- Yang, Y.J.; Yan, Z.J.; Wang, L.L.; Meng, Q.H.; Yuan, Y.; Zhu, G.S. Constructing synergistic groups in porous aromatic frameworks for the selective removal and recovery of lead (II) ions. J. Mater. Chem. A 2018, 6, 5202–5207. [Google Scholar] [CrossRef]
- Xu, M.Y.; Wang, T.; Gao, P.; Zhao, L.; Zhou, L.; Hua, D.B. Highly fluorescent conjugated microporous polymers for concurrent adsorption and detection of uranium. J. Mater. Chem. A 2019, 7, 11214–11222. [Google Scholar] [CrossRef]
- Zhang, C.R.; Chen, X.J.; Niu, C.P.; Meng, C.; Yi, S.M.; Liu, X.; Qi, J.X.; Luo, Q.X.; Liang, R.P.; Qiu, J.D. Reconstruction of biomimetic ionic channels within covalent organic frameworks for ultrafast and selective uranyl capture. Sci. China Chem. 2024, 67, 3423–3431. [Google Scholar] [CrossRef]
- Li, Z.N.; Meng, Q.H.; Yang, Y.J.; Zou, X.Q.; Yuan, Y.; Zhu, G.S. Constructing amidoxime-modified porous adsorbents with open architecture for cost-effective and efficient uranium extraction. Chem. Sci. 2020, 11, 4747–4752. [Google Scholar] [CrossRef]
- Mollick, S.; Saurabh, S.; More, Y.D.; Fajal, S.; Shirolkar, M.M.; Mandal, W.; Ghosh, S.K. Benchmark uranium extraction from seawater using an ionic macroporous metal–organic framework. Energy Environ. Sci. 2022, 15, 3462–3469. [Google Scholar] [CrossRef]
| Reagent | pH | Usage (per L Water) | Contact Time | LOD (nM) | Cost | Reference |
|---|---|---|---|---|---|---|
| Eu-MOF | 7.0 | 0.20 g | immediately | 900.00 | USD 30.56/g | [46] |
| Au-nanoparticles | 7.0 | 0.50 L | several minutes | 84.00 | USD 406.38/L | [58] |
| Zn-MOF | 3.0 | 0.50 g | immediately | 12.00 | USD 15.32/g | [48] |
| Eu-complex | 7.4 | 0.54 g | immediately | 12,000 | USD 22.35/g | [41] |
| N-doped QD | 7.0 | 0.01 g | 3 h | 20.38 | USD 241.70/g | [56] |
| AO-CMP | 6.0 | 0.20 g | 3 h | 1.70 | USD 831.89/g | [76] |
| MIPAF-15 | 6.0 | 0.20 g | 30 s | 50.40 | USD 150.51/g | This work |
| Cations | Tap Water (mM) | Seawater (mM) |
|---|---|---|
| Na+ | 0.80 | 450.00–470.00 |
| Ca2+ | 2.20 | 50.00 |
| K+ | 0.50 | 10.00 |
| Mg2+ | 1.20 | 9.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Wu, S.; Zhang, C.; Cao, D.; Song, Y.; Zheng, Y.; Cao, J.; Luo, L.; Yang, Y.; Zheng, X.; et al. Synergistic Adsorption and Fluorescence in Porous Aromatic Frameworks for Highly Sensitive Detection of Radioactive Uranium. Molecules 2025, 30, 1920. https://doi.org/10.3390/molecules30091920
Zhang S, Wu S, Zhang C, Cao D, Song Y, Zheng Y, Cao J, Luo L, Yang Y, Zheng X, et al. Synergistic Adsorption and Fluorescence in Porous Aromatic Frameworks for Highly Sensitive Detection of Radioactive Uranium. Molecules. 2025; 30(9):1920. https://doi.org/10.3390/molecules30091920
Chicago/Turabian StyleZhang, Suming, Siyu Wu, Cheng Zhang, Doudou Cao, Yingbo Song, Yue Zheng, Jiarui Cao, Lu Luo, Yajie Yang, Xiangjun Zheng, and et al. 2025. "Synergistic Adsorption and Fluorescence in Porous Aromatic Frameworks for Highly Sensitive Detection of Radioactive Uranium" Molecules 30, no. 9: 1920. https://doi.org/10.3390/molecules30091920
APA StyleZhang, S., Wu, S., Zhang, C., Cao, D., Song, Y., Zheng, Y., Cao, J., Luo, L., Yang, Y., Zheng, X., & Yuan, Y. (2025). Synergistic Adsorption and Fluorescence in Porous Aromatic Frameworks for Highly Sensitive Detection of Radioactive Uranium. Molecules, 30(9), 1920. https://doi.org/10.3390/molecules30091920







