Catalytic Methanation over Natural Clay-Supported Nickel Catalysts
Abstract
1. Introduction
2. Results and Discussion
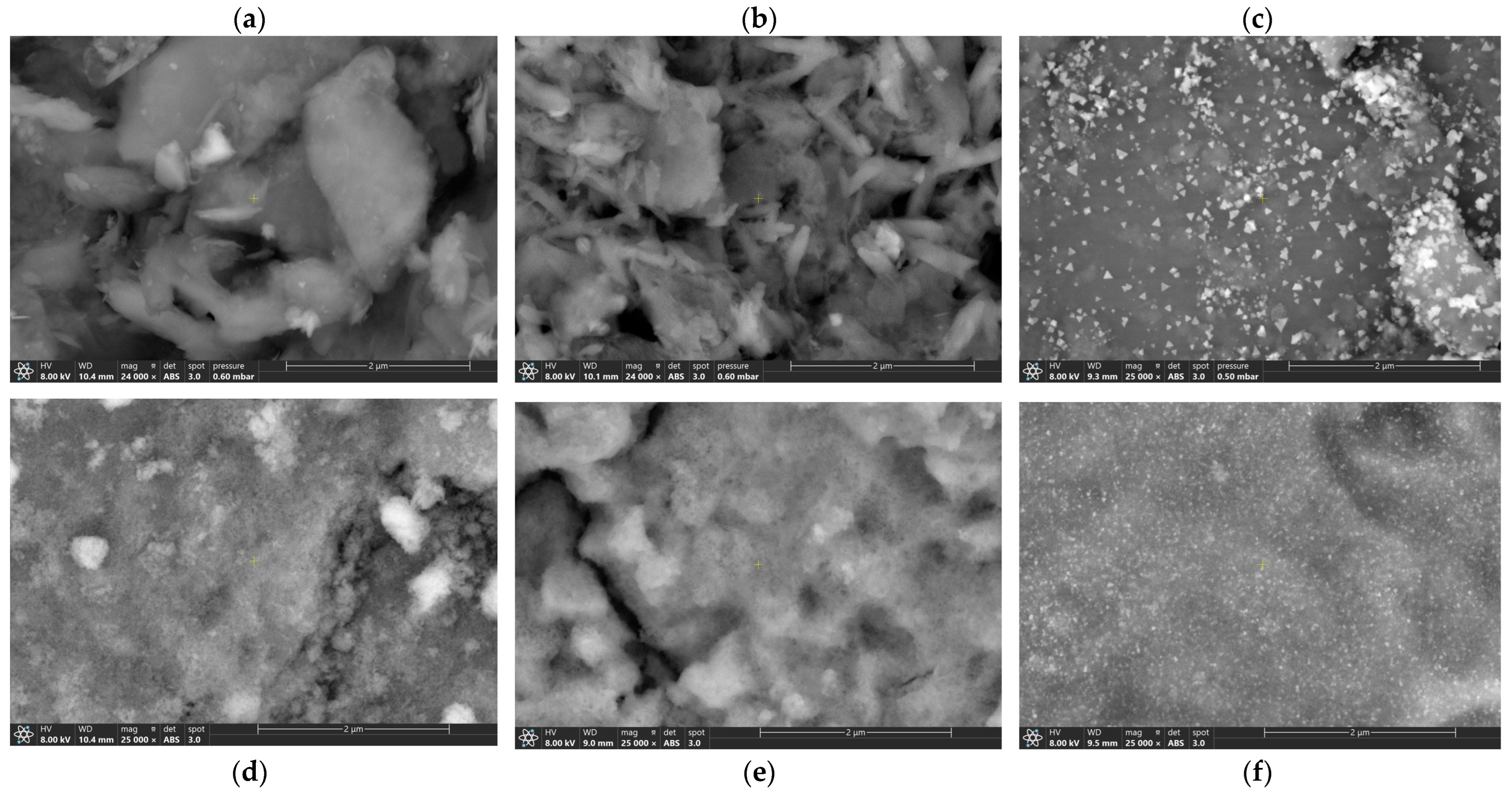
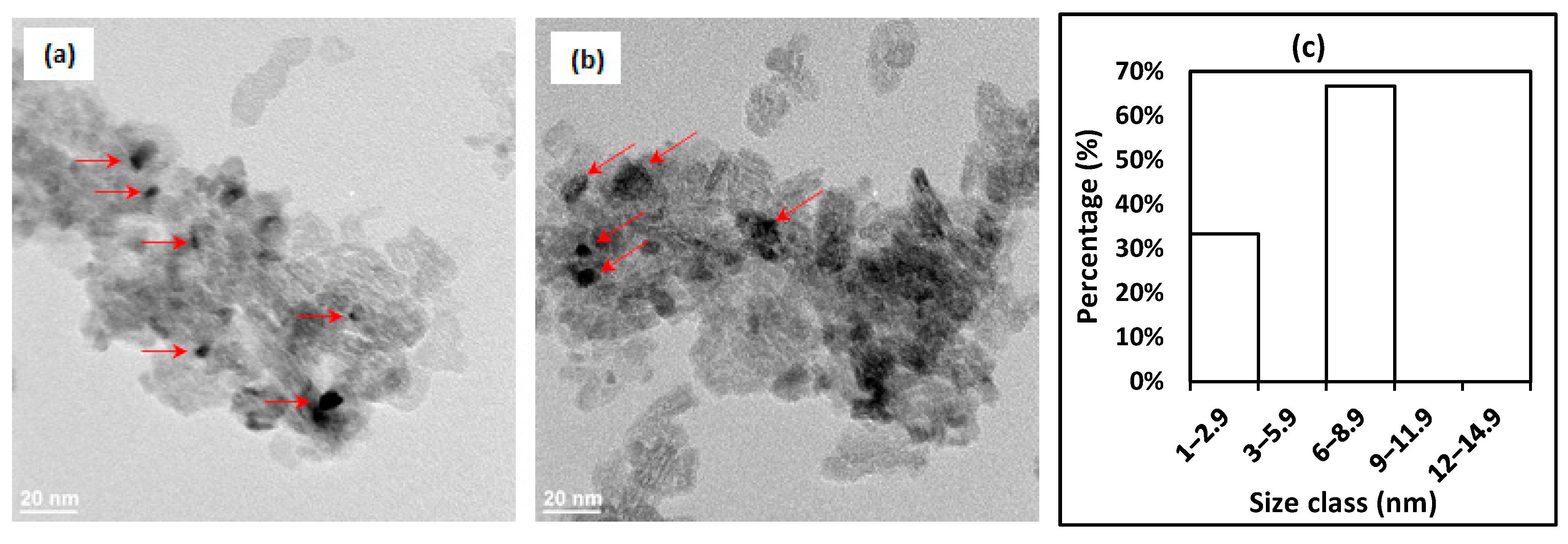
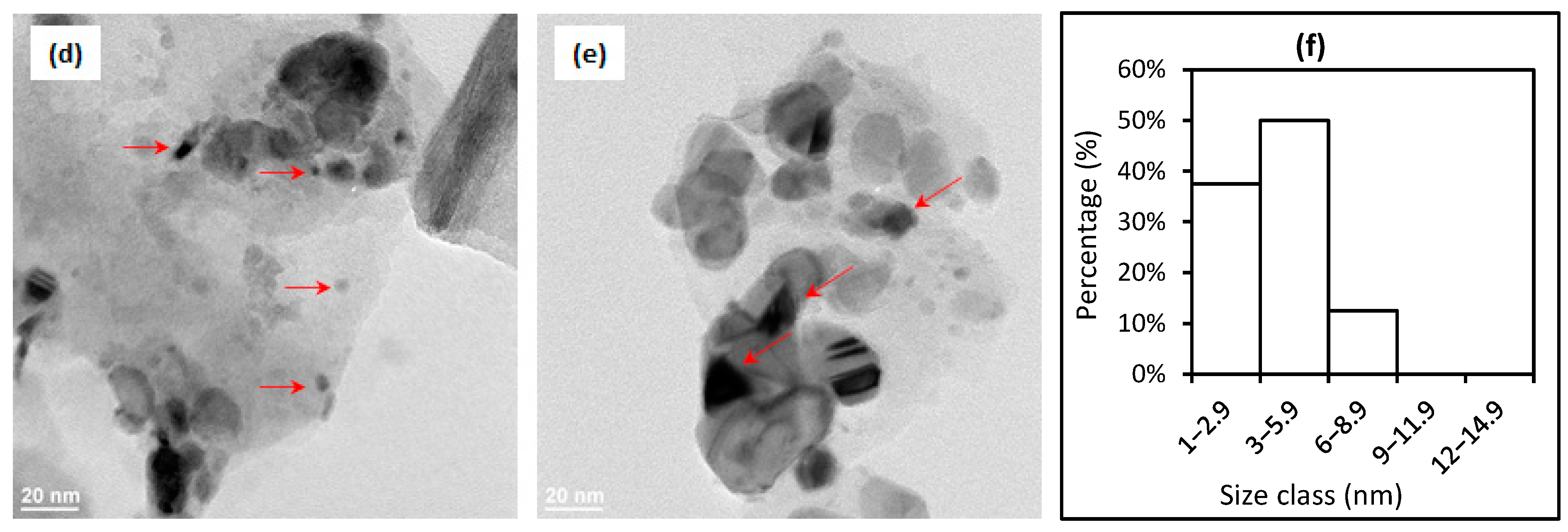
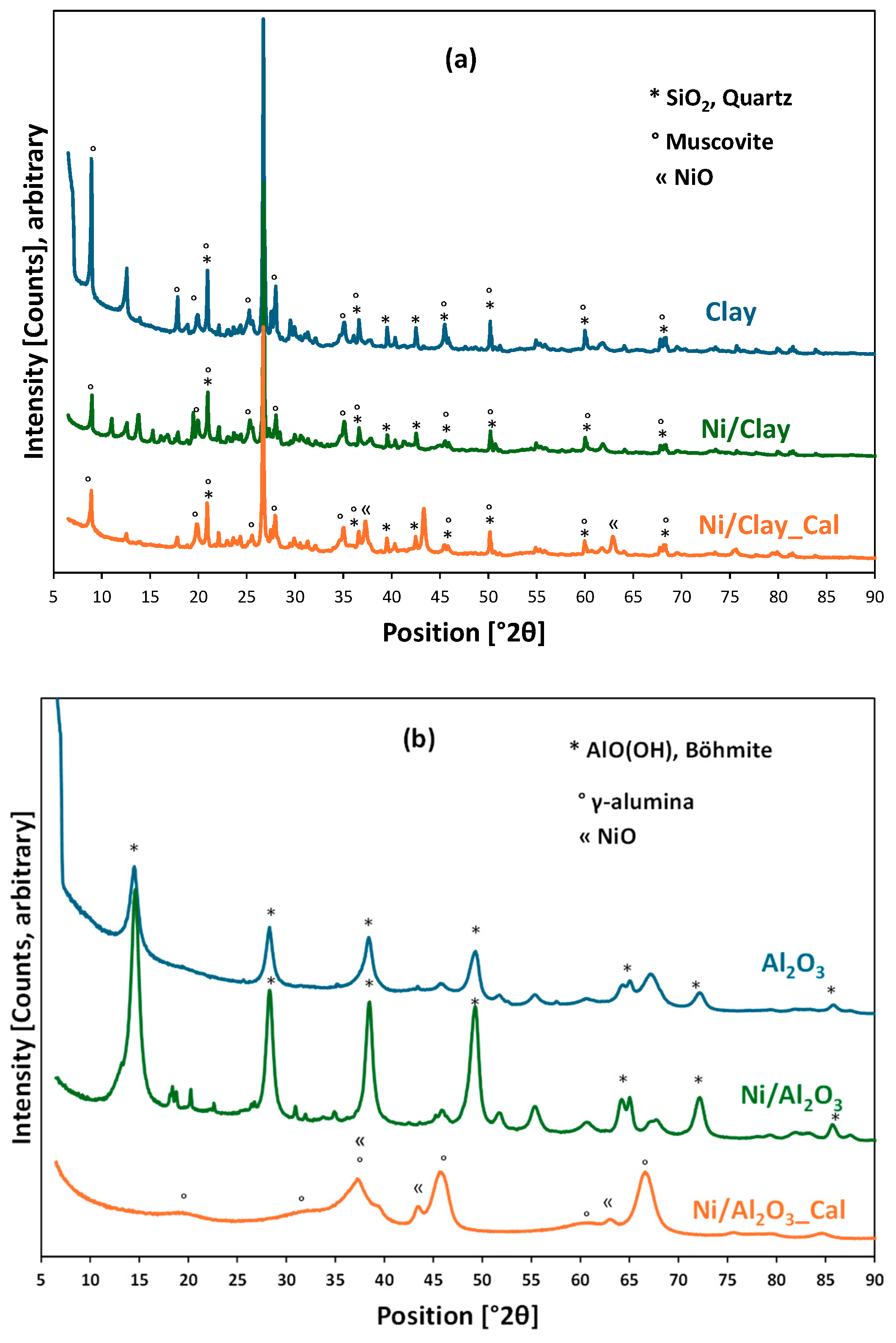
2.1. Catalyst Characterizations
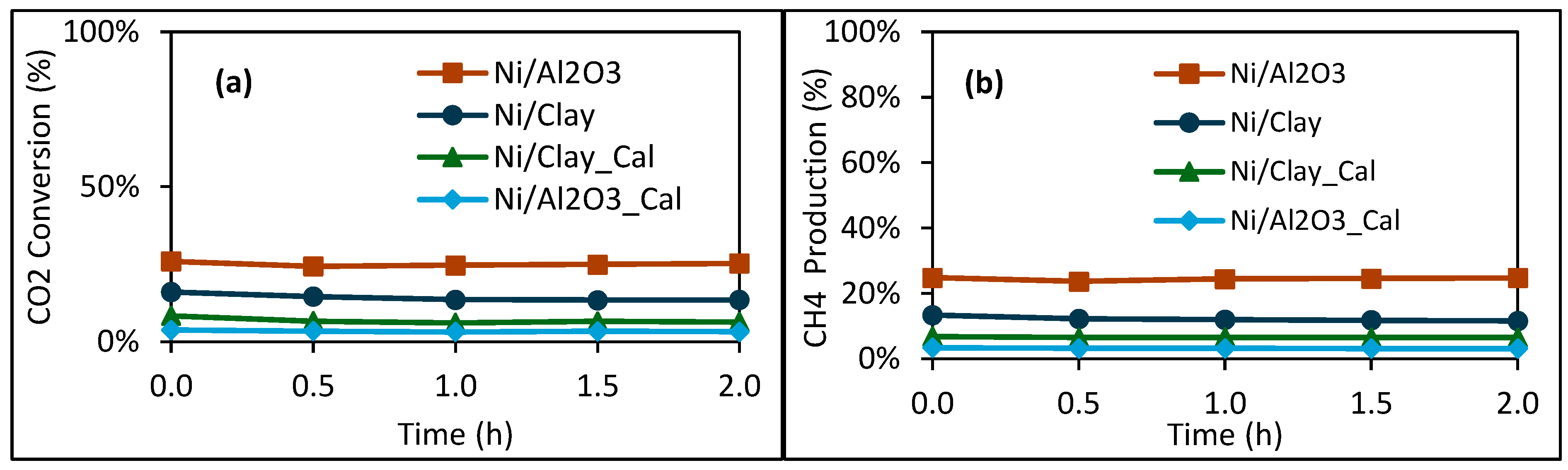
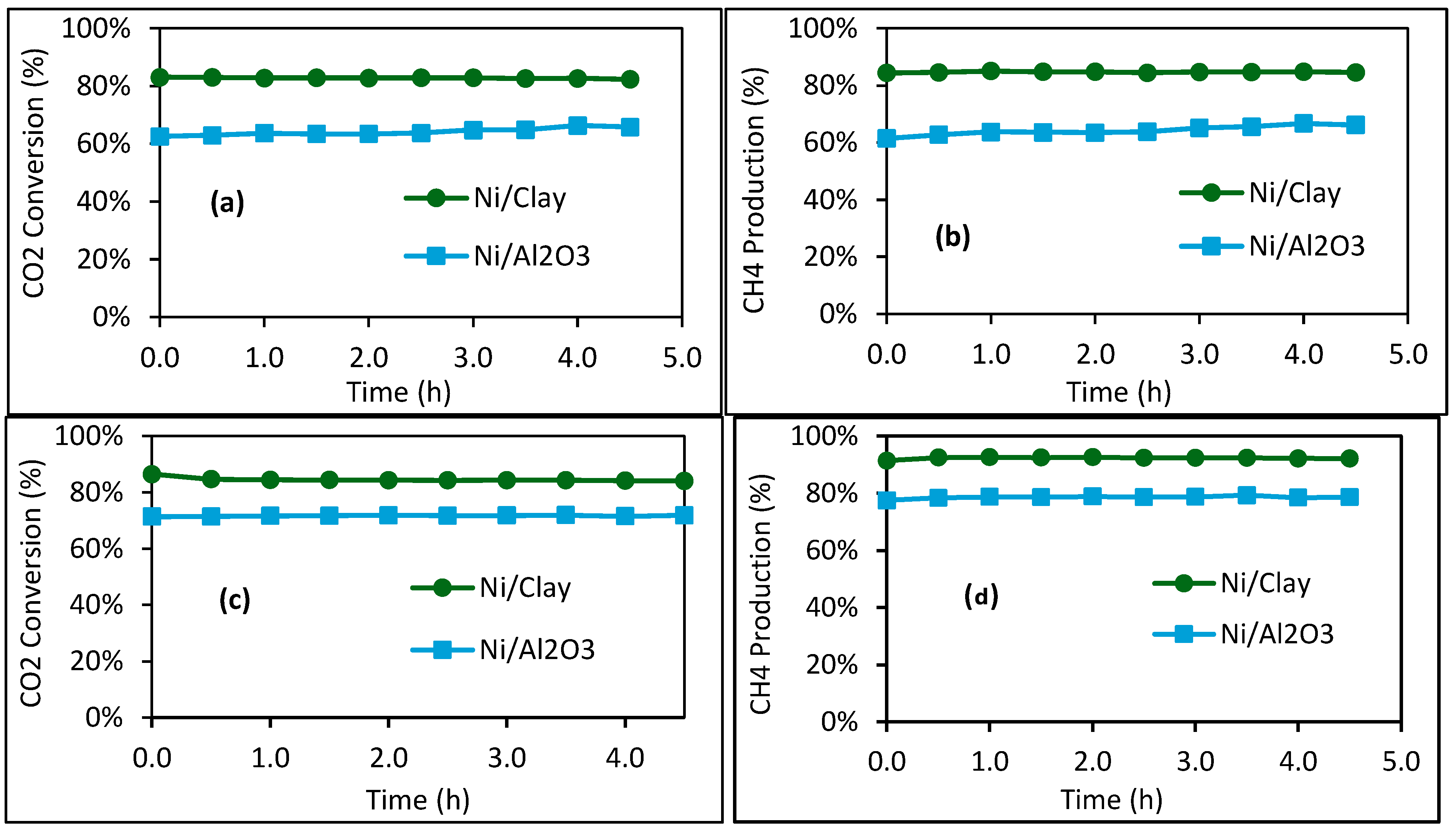
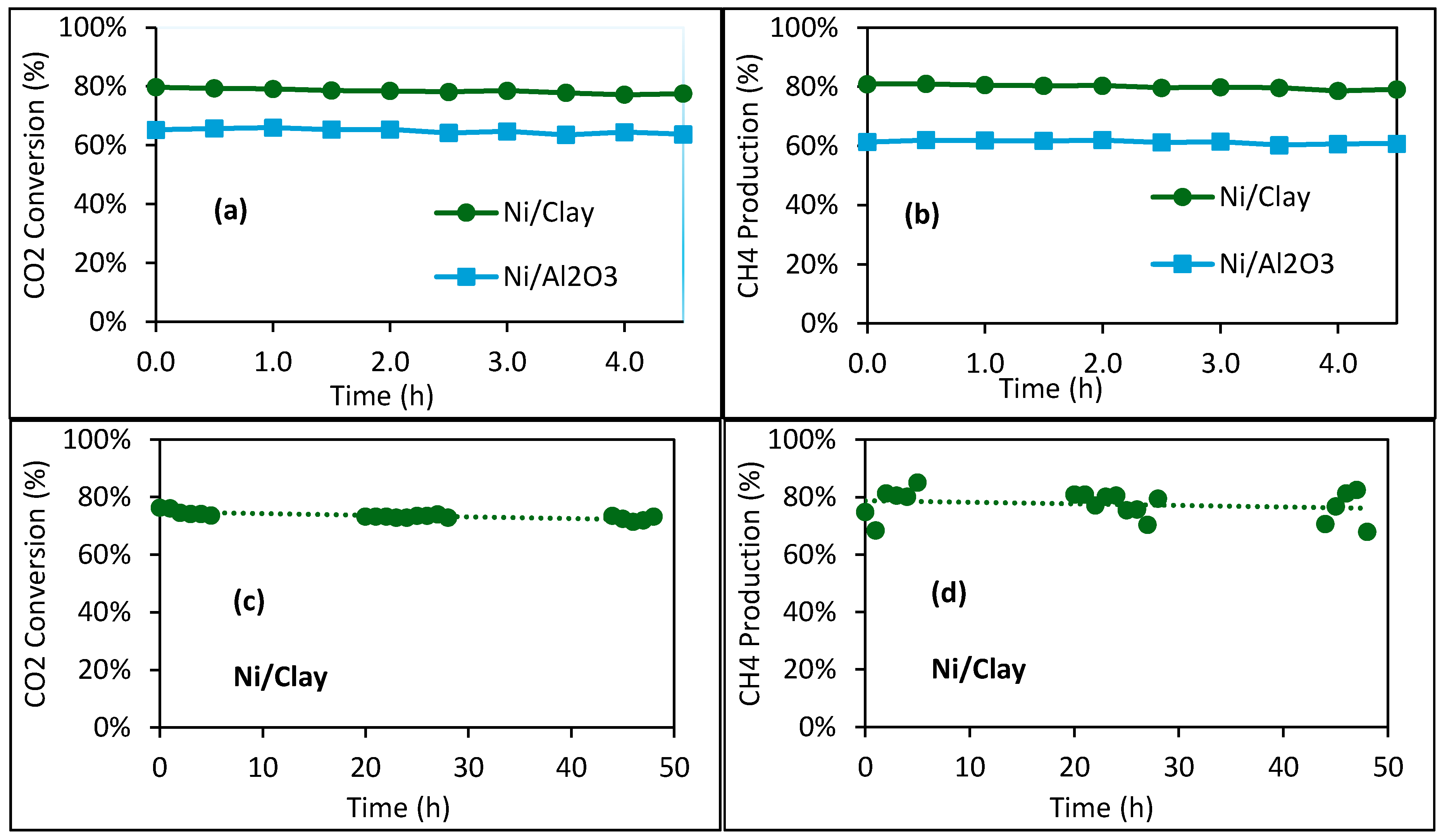
2.2. Catalytic Performance
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Characterization Analysis Techniques
3.3. Catalytic Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IEA. Outlook for Biogas and Biomethane: Prospects for Organic Growth; International Energy Agency: Paris, France, 2020. [Google Scholar]
- IEA. Renewables 2024; International Energy Agency: Paris, France, 2024. [Google Scholar]
- Farghali, M.; Osman, A.I.; Umetsu, K.; Rooney, D.W. Integration of Biogas Systems into a Carbon Zero and Hydrogen Economy: A Review. Environ. Chem. Lett. 2022, 20, 2853–2927. [Google Scholar] [CrossRef]
- Khan, M.U.; Lee, J.T.E.; Bashir, M.A.; Dissanayake, P.D.; Ok, Y.S.; Tong, Y.W.; Shariati, M.A.; Wu, S.; Ahring, B.K. Current Status of Biogas Upgrading for Direct Biomethane Use: A Review. Renew. Sustain. Energy Rev. 2021, 149, 111343. [Google Scholar] [CrossRef]
- GRDF. Le Biométhane en France: Les Sites d’injection Sur le Réseau de GRDF. Available online: https://www.grdf.fr/gaz-vert/reseau-gaz/sites-injection-biomethane-france (accessed on 10 February 2025).
- Alarcón, A.; Guilera, J.; Díaz, J.A.; Andreu, T. Optimization of Nickel and Ceria Catalyst Content for Synthetic Natural Gas Production through CO2 Methanation. Fuel Process. Technol. 2019, 193, 114–122. [Google Scholar] [CrossRef]
- Ghaib, K.; Nitz, K.; Ben-Fares, F. Chemical Methanation of CO2: A Review. ChemBioEng Rev. 2016, 3, 266–275. [Google Scholar] [CrossRef]
- Lee, W.J.; Li, C.; Prajitno, H.; Yoo, J.; Patel, J.; Yang, Y.; Lim, S. Recent Trend in Thermal Catalytic Low Temperature CO2 Methanation: A Critical Review. Catal. Today 2021, 368, 2–19. [Google Scholar] [CrossRef]
- United Nations Transforming Our World: The 2030 Agenda for Sustainable Development|Department of Economic and Social Affairs. Available online: https://sdgs.un.org/2030agenda (accessed on 6 February 2024).
- Gac, W.; Zawadzki, W.; Rotko, M.; Greluk, M.; Słowik, G.; Kolb, G. Effects of Support Composition on the Performance of Nickel Catalysts in CO2 Methanation Reaction. Catal. Today 2020, 357, 468–482. [Google Scholar] [CrossRef]
- Italiano, C.; Llorca, J.; Pino, L.; Ferraro, M.; Antonucci, V.; Vita, A. CO and CO2 Methanation over Ni Catalysts Supported on CeO2, Al2O3 and Y2O3 Oxides. Appl. Catal. B Environ. 2020, 264, 118494. [Google Scholar] [CrossRef]
- Stangeland, K.; Kalai, D.; Li, H.; Yu, Z. CO2 Methanation: The Effect of Catalysts and Reaction Conditions. Energy Procedia 2017, 105, 2022–2027. [Google Scholar] [CrossRef]
- Medina, O.E.; Amell, A.A.; López, D.; Santamaría, A. Comprehensive Review of Nickel-Based Catalysts Advancements for CO2 Methanation. Renew. Sustain. Energy Rev. 2025, 207, 114926. [Google Scholar] [CrossRef]
- Daroughegi, R.; Meshkani, F.; Rezaei, M. Characterization and Evaluation of Mesoporous High Surface Area Promoted Ni- Al2O3 Catalysts in CO2 Methanation. J. Energy Inst. 2020, 93, 482–495. [Google Scholar] [CrossRef]
- Daroughegi, R.; Meshkani, F.; Rezaei, M. Enhanced Low-Temperature Activity of CO2 Methanation over Ceria-Promoted Ni-Al2O3 Nanocatalyst. Chem. Eng. Sci. 2021, 230, 116194. [Google Scholar] [CrossRef]
- Lin, S.; Hao, Z.; Shen, J.; Chang, X.; Huang, S.; Li, M.; Ma, X. Enhancing the CO2 Methanation Activity of Ni/CeO2 via Activation Treatment-Determined Metal-Support Interaction. J. Energy Chem. 2021, 59, 334–342. [Google Scholar] [CrossRef]
- Shen, L.; Xu, J.; Zhu, M.; Han, Y.-F. Essential Role of the Support for Nickel-Based CO2 Methanation Catalysts. ACS Catal. 2020. [Google Scholar] [CrossRef]
- Ma, Y.; Preveniou, A.; Kladis, A.; Pettersen, J.B. Circular Economy and Life Cycle Assessment of Alumina Production: Simulation-Based Comparison of Pedersen and Bayer Processes. J. Clean. Prod. 2022, 366, 132807. [Google Scholar] [CrossRef]
- Sáez-Guinoa, J.; García-Franco, E.; Llera-Sastresa, E.; Romeo, L.M. The Effects of Energy Consumption of Alumina Production in the Environmental Impacts Using Life Cycle Assessment. Int. J. Life Cycle Assess. 2024, 29, 380–393. [Google Scholar] [CrossRef]
- Sayyah, A.; Mahmoudi, E.; Farhoudi, S.; Behmenyar, G.; Turan, A.Z.; Nabavi, S.R.; Niaei, A. Environmental Assessment of Carbon Dioxide Methanation Process Using Mixed Metal Oxide and Zeolite-Supported Catalysts by Life Cycle Assessment Methodology. J. Clean. Prod. 2022, 362, 132529. [Google Scholar] [CrossRef]
- Cañón-Alvarado, M.; Blanco, C.; Daza, C. Exploring Dolomite as a Promising Support for Ni Catalysts in CO2 Methanation. J. Environ. Chem. Eng. 2024, 12, 112224. [Google Scholar] [CrossRef]
- Cortés-Murillo, D.; Blanco-Jiménez, C.; Daza, C.E. Clay Exfoliation Method as a Route to Obtain Mesoporous Catalysts for CO2 Methanation. MethodsX 2023, 10, 101955. [Google Scholar] [CrossRef]
- Han, F.; Liu, Q.; Li, D.; Ouyang, J. An Emerging and High-Performance Sepiolite-Supported Ni Catalyst for Low-Temperature CO2 Methanation: The Critical Role of Hydroxyl Groups. J. Environ. Chem. Eng. 2023, 11, 110331. [Google Scholar] [CrossRef]
- Jiang, Y.; Huang, T.; Dong, L.; Qin, Z.; Ji, H. Ni/Bentonite Catalysts Prepared by Solution Combustion Method for CO2 Methanation. Chin. J. Chem. Eng. 2018, 26, 2361–2367. [Google Scholar] [CrossRef]
- Ye, R.-P.; Gong, W.; Sun, Z.; Sheng, Q.; Shi, X.; Wang, T.; Yao, Y.; Razink, J.J.; Lin, L.; Zhou, Z.; et al. Enhanced Stability of Ni/SiO2 Catalyst for CO2 Methanation: Derived from Nickel Phyllosilicate with Strong Metal-Support Interactions. Energy 2019, 188, 116059. [Google Scholar] [CrossRef]
- Guo, X.; Traitangwong, A.; Hu, M.; Zuo, C.; Meeyoo, V.; Peng, Z.; Li, C. Carbon Dioxide Methanation over Nickel-Based Catalysts Supported on Various Mesoporous Material. Energy Fuels 2018, 32, 3681–3689. [Google Scholar] [CrossRef]
- Kruissink, E.C.; Pelt, H.L.; Ross, J.R.H.; Van Reüen, L.L. The Effect of Sodium on the Methanation Activity of Nickel/Alumina Coprecipitated Catalysts. Appl. Catal. 1981, 1, 23–29. [Google Scholar] [CrossRef]
- Gonzalez, R.D.; Miura, H. Methanation and Fischer-Tropsch Studies on Potassium-Promoted Silica-Supported Ru Catalysts. J. Catal. 1982, 77, 338–347. [Google Scholar] [CrossRef]
- Ayub, N.A.; Bahruji, H.; Mahadi, A.H. Barium Promoted Ni/Sm2O3 Catalysts for Enhanced CO2 Methanation. RSC Adv. 2021, 11, 31807–31816. [Google Scholar] [CrossRef]
- Nguyen-Quang, M.; Azzolina-Jury, F.; Samojeden, B.; Motak, M.; Da Costa, P. Why Is Calcium an Effective Promoter for Plasma CO2 Methanation over Polymer P123-Modified NiMgAlOx-Mixed Oxide Catalysts? Appl. Catal. B Environ. 2023, 336, 122952. [Google Scholar] [CrossRef]
- Yan, P.; Peng, H.; Zhang, X.; Rabiee, H.; Ahmed, M.; Weng, Y.; Rozhkovskaya, A.; Vogrin, J.; Konarova, M.; Zhu, Z. Unlocking the Role of Ni-Fe Species in CO2 Methanation. Fuel 2024, 374, 132373. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Williams, R.T. Physisorption Hysteresis Loops and the Characterization of Nanoporous Materials. Adsorpt. Sci. Technol. 2004, 22, 773–782. [Google Scholar] [CrossRef]
- Keely, W.M.; Maynor, H.W. Thermal Studies of Nickel, Cobalt, Iron and Copper Oxides and Nitrates. J. Chem. Eng. Data 1963, 8, 297–300. [Google Scholar] [CrossRef]
- Guggenheim, S.; Chang, Y.-H.; Koster van Groos, A.F. Muscovite dehydroxylation; high-temperature studies. Am. Mineral. 1987, 72, 537–550. [Google Scholar] [CrossRef]
- Hassanzadeh-Tabrizi, S.A. Precise Calculation of Crystallite Size of Nanomaterials: A Review. J. Alloys Compd. 2023, 968, 171914. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving Surface Chemical States in XPS Analysis of First Row Transition Metals, Oxides and Hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Bagus, P.S.; Nelin, C.J.; Brundle, C.R.; Crist, B.V.; Ilton, E.S.; Lahiri, N.; Rosso, K.M. Main and Satellite Features in the Ni 2p XPS of NiO. Inorg. Chem. 2022, 61, 18077–18094. [Google Scholar] [CrossRef] [PubMed]
- Li, C.P.; Proctor, A.; Hercules, D.M. Curve Fitting Analysis of ESCA Ni 2p Spectra of Nickel-Oxygen Compounds and Ni/Al2 O3 Catalysts. Appl. Spectrosc. 1984, 38, 880–886. [Google Scholar] [CrossRef]
- Quindimil, A.; Bacariza, M.C.; González-Marcos, J.A.; Henriques, C.; González-Velasco, J.R. Enhancing the CO2 Methanation Activity of γ-Al2O3 Supported Mono- and Bi-Metallic Catalysts Prepared by Glycerol Assisted Impregnation. Appl. Catal. B Environ. 2021, 296, 120322. [Google Scholar] [CrossRef]
- Cui, Y.; Chen, B.; Xu, L.; Chen, M.; Wu, C.; Qiu, J.; Cheng, G.; Wang, N.; Xu, J.; Hu, X. CO2 Methanation over the Ni-Based Catalysts Supported on the Hollow ZSM-5 Zeolites: Effects of the Hollow Structure and Alkaline Treatment. Fuel 2023, 334, 126783. [Google Scholar] [CrossRef]


| Element | Na | Mg | Al | Si | P | S | K | Ca | Ti | Mn | Fe | Cu | Zn | Ba | Sr |
| Content (wt.%) | 0.07 | 1.11 | 9.5 | 24.91 | nd. | nd. | 3.78 | 3.38 | 0.84 | 0.18 | 9.77 | nd. | nd. | 0.11 | nd. |
| Entry | Catalyst | mcatalyst (g)/msintered Al2O3 (g) | Total Catalyst Bed Height (cm) | In Situ Reduction Temperature, °C | Reaction Temperature, °C | Inlet Gas Mixture |
|---|---|---|---|---|---|---|
| #1 | Ni/Clay | 1.0/1.0 | 5.5 | 500 | 300 | Mixture 1 |
| #2 | Ni/Al2O3 | 1.0/1.0 | 5.5 | 500 | 300 | Mixture 1 |
| #3 | Ni/Clay_Cal | 1.0/1.0 | 5.5 | 500 | 300 | Mixture 1 |
| #4 | Ni/Al2O3_Cal | 1.0/1.0 | 5.5 | 500 | 300 | Mixture 1 |
| #5 | Ni/Clay | 1.0/1.9 | 5.5 | 400 | 400 | Mixture 1 |
| #6 | Ni/Al2O3 | 1.0/1.9 | 5.5 | 400 | 400 | Mixture 1 |
| #7 | Ni/Clay | 1.0/1.9 | 5.5 | 500 | 500 | Mixture 1 |
| #8 | Ni/Al2O3 | 1.0/1.9 | 5.5 | 500 | 500 | Mixture 1 |
| #9 | Ni/Clay | 0.5/1.0 | 2.75 | 500 | 500 | Mixture 1 |
| #10 | Ni/Al2O3 | 0.5/1.0 | 2.75 | 500 | 500 | Mixture 1 |
| #11 | Ni/Clay | 0.5/1.0 | 2.75 | 500 | 500 | Mixture 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cue Gonzalez, A.; Weiss-Hortala, E.; Pham, Q.N.; Pham Minh, D. Catalytic Methanation over Natural Clay-Supported Nickel Catalysts. Molecules 2025, 30, 2110. https://doi.org/10.3390/molecules30102110
Cue Gonzalez A, Weiss-Hortala E, Pham QN, Pham Minh D. Catalytic Methanation over Natural Clay-Supported Nickel Catalysts. Molecules. 2025; 30(10):2110. https://doi.org/10.3390/molecules30102110
Chicago/Turabian StyleCue Gonzalez, Alejandra, Elsa Weiss-Hortala, Quoc Nghi Pham, and Doan Pham Minh. 2025. "Catalytic Methanation over Natural Clay-Supported Nickel Catalysts" Molecules 30, no. 10: 2110. https://doi.org/10.3390/molecules30102110
APA StyleCue Gonzalez, A., Weiss-Hortala, E., Pham, Q. N., & Pham Minh, D. (2025). Catalytic Methanation over Natural Clay-Supported Nickel Catalysts. Molecules, 30(10), 2110. https://doi.org/10.3390/molecules30102110






