Study of Natural Dyes’ Liposomal Encapsulation in Food Dispersion Model Systems via High-Pressure Homogenization
Abstract
1. Introduction
2. Results and Discussion
2.1. Particle Size of Liposomal Dispersions
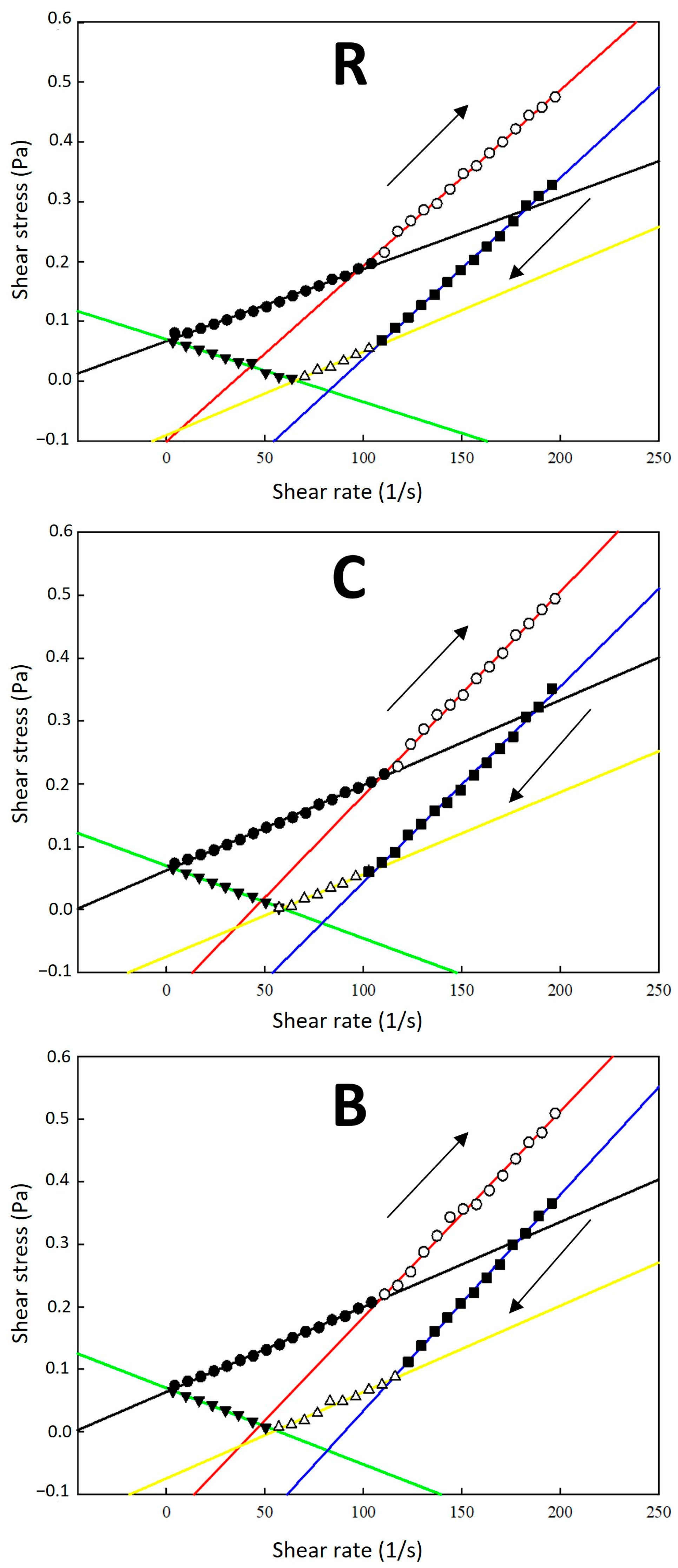
2.2. Rheological Behaviour of Dispersion Systems
2.3. Chromatic Characteristics of Dispersion Systems
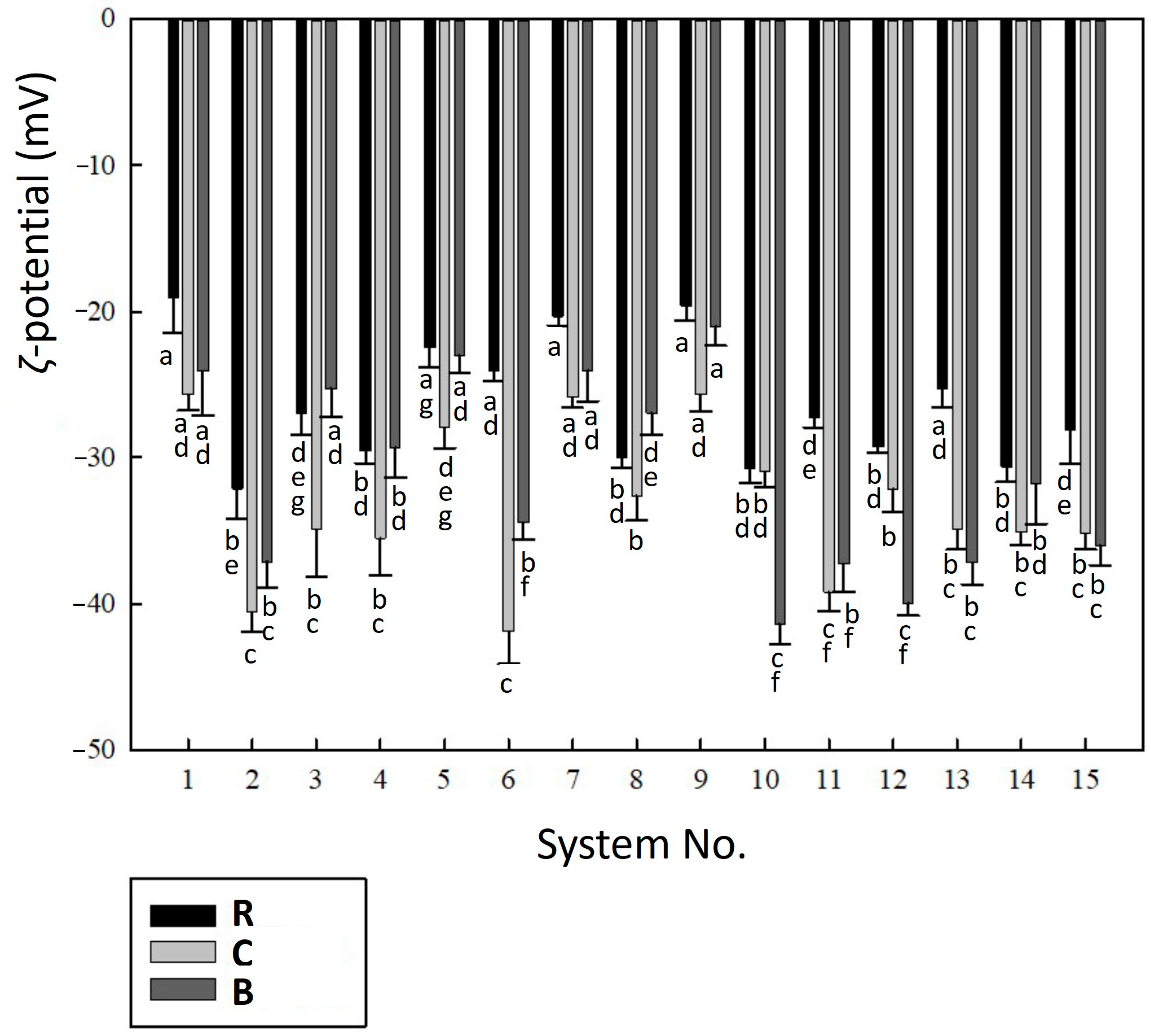
2.4. Electrokinetic Stability of Liposomal Dispersions
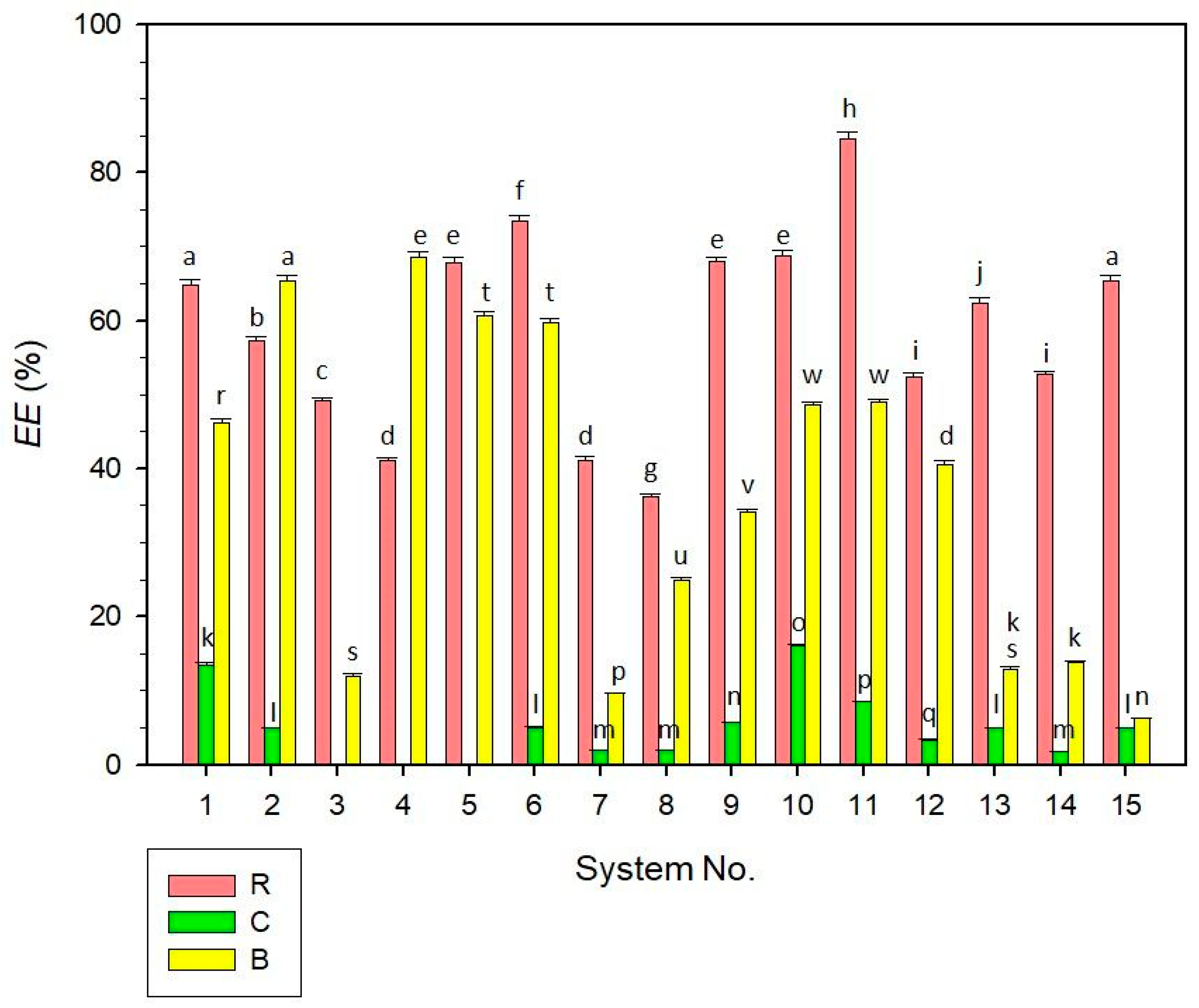
2.5. Encapsulation Efficiency of Liposomal Dispersions
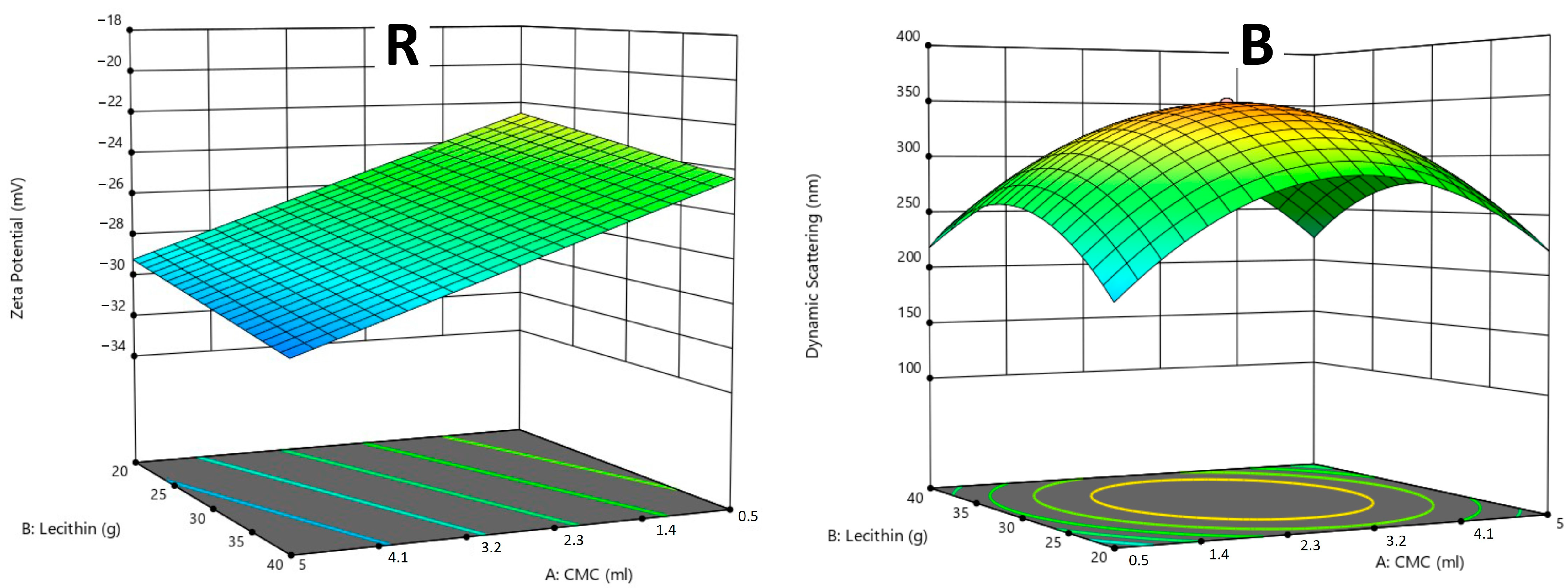
2.6. Design of Optimal Liposomal Formulation Using CCD (Central Composite Design)
3. Materials and Methods
3.1. Materials
3.1.1. Dye Agents
3.1.2. Sunflower Lecithin
3.1.3. Carboxymethylcellulose Sodium Salt (CMC-Na)
3.2. Design of Liposomal Carrier Systems Formulation and Preparation Procedure
3.3. Particle Size Determination by Dynamic Light Scattering (DLS) Measurement
3.4. Rheological Analysis
3.5. CIE L*a*b* Analysis
3.6. ζ-Potential and pH Analyses
3.7. Encapsulation Efficiency Determination
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CCD | Central Composite Design |
| CMC-Na | Carboxymethylcellulose sodium salt |
| EE | Encapsulation efficiency |
| ICP-MS | Inductively coupled plasma-mass spectrometry |
| O/W | Oil-in-water emulsion |
| PDI | Polydispersity index |
| RPM | Rotations per minute |
References
- Demisli, S.; Galani, E.; Goulielmaki, M.; Kyrilis, F.L.; Ilić, T.; Hamdi, F.; Crevar, M.; Kastritis, P.L.; Pletsa, V.; Nallet, F.; et al. Encapsulation of Cannabidiol in Oil-in-Water Nanoemulsions and Nanoemulsion-Filled Hydrogels: A Structure and Biological Assessment Study. J. Colloid Interface Sci. 2023, 634, 300–313. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Nanoemulsions Versus Microemulsions: Terminology, Differences, and Similarities. Soft Matter 2012, 8, 1719–1729. [Google Scholar] [CrossRef]
- Naseema, A.; Kovooru, L.; Behera, A.K.; Kumar, K.P.P.; Srivastava, P. A Critical Review of Synthesis Procedures, Applications and Future Potential of Nanoemulsions. Adv. Colloid Interface Sci. 2021, 287, 102318. [Google Scholar] [CrossRef]
- Oliveira, C.; Ferreira, C.J.O.; Sousa, M.; Paris, J.L.; Gaspar, R.; Silva, B.F.B.; Teixeira, J.A.; Ferreira-Santos, P.; Botelho, C.M. A Versatile Nanocarrier—Cubosomes, Characterization, and Applications. Nanomaterials 2022, 12, 2224. [Google Scholar] [CrossRef]
- Chen, H.; Fan, Y.; Zhang, N.; Trépout, S.; Ptissam, B.; Brûlet, A.; Tang, B.Z.; Li, M. Fluorescent Polymer Cubosomes and Hexosomes with Aggregation-Induced Emission. Chem. Sci. 2021, 12, 5495. [Google Scholar] [CrossRef]
- Huang, Y.; Chang, Z.; Xia, X.; Zhao, Z.; Zhang, X.; Huang, Z.; Wu, C.; Pan, X. Current and Evolving Knowledge Domains of Cubosome Studies in the New Millennium. J. Nanopart. Res. 2023, 25, 176. [Google Scholar] [CrossRef]
- de AV Matos, H.; Ferreira, G.A. Formulating Plant-Based Hexosomes for the Sustained Delivery of Food Proteins. Colloids Surf. B Biointerfaces 2024, 244, 114169. [Google Scholar] [CrossRef]
- Liu, P.; Chen, G.; Zhang, J. A Review of Liposomes as a Drug Delivery System: Current Status of Approved Products, Regulatory Environments, and Future Perspectives. Molecules 2022, 27, 1372. [Google Scholar] [CrossRef]
- Nagle, J.F.; Tristram-Nagle, S. Structure of Lipid Bilayers. Biochim. Biophys. Acta 2000, 1469, 159–195. [Google Scholar] [CrossRef]
- Ullrich, M.; Hanuš, J.; Dohnal, J.; Štěpánek, F. Encapsulation Stability and Temperature-Dependent Release Kinetics from Hydrogel-Immobilised Liposomes. J. Colloid Interface Sci. 2013, 394, 380–385. [Google Scholar] [CrossRef]
- Senjab, R.M.; Alsawaftah, N.; Abuwatfa, W.H.; Husseini, G.A. Advances in Liposomal Nanotechnology: From Concept to Clinics. RSC Pharm. 2024, 1, 928. [Google Scholar] [CrossRef]
- Kumar, A.; Dixit, U.; Singh, K.; Satya, P.G.; Beg, M.S.J. Structure and Properties of Dyes and Pigments. In Dyes and Pigments—Novel Applications and Waste Treatment; Papadakis, R., Ed.; IntechOpen: Rijeka, Croatia, 2021; Volume 8. [Google Scholar] [CrossRef]
- Repon, M.R.; Islam, T.; Islam, T.; Ghorab, A.E.; Rahman, M.M. Cleaner Pathway for Developing Bioactive Textile Materials using Natural Dyes: A Review. Environ. Sci. Pollut. Res. 2023, 30, 48793–48823. [Google Scholar] [CrossRef] [PubMed]
- Safapour, S.; Rather, L.J.; Moradnejad, J.; Mir, S.S. Functional and Colorful Wool Textiles through Ecological Dyeing with Lemon Balm Bio-Dyes and Mordants. Fibers Polym. 2023, 24, 4357–4370. [Google Scholar] [CrossRef]
- Mezgebe, K.; Mulugeta, E. Synthesis and Pharmacological Activities of Azo Dye Derivatives Incorporating Heterocyclic Scaffolds: A Review. RSC Adv. 2022, 12, 25932–25946. [Google Scholar] [CrossRef] [PubMed]
- Schweiggert, R. Handbook on Natural Pigments in Food and Beverages: Industrial Applications for Improving Food Color, 2nd ed.; Woodhead Publishing: Cambridge, UK, 2024; p. 642. [Google Scholar] [CrossRef]
- Ribeiro, J.S.; Veloso, C.M. Microencapsulation of Natural Dyes with Biopolymers for Application in Food: A Review. Food Hydrocoll. 2021, 112, 106374. [Google Scholar] [CrossRef]
- Codex Alimentarius Commission. Discussion Paper on the Use of Colours in Foods. Report of the 32nd Session of the Codex Committee on Food Additives and Contaminants; FAO/WHO: Beijing, China, 2000; Available online: https://openknowledge.fao.org/server/api/core/bitstreams/25486ce9-cb1a-433d-8b10-8ae8104f4e4a/content (accessed on 16 March 2025).
- Rodriguez-Amaya, D.B. Natural Food Pigments and Colorants. Curr. Opin. Food Sci. 2016, 7, 20–26. [Google Scholar] [CrossRef]
- Alouk, I.; Xu, D.; Cao, Y. Encapsulation of Natural Pigments by Double Emulsion: A Review. Food Biosci. 2023, 56, 103411. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. Update on Natural Food Pigments—A Mini-Review on Carotenoids, Anthocyanins, and Betalains. Food Res. Int. 2019, 124, 200–205. [Google Scholar] [CrossRef]
- Jafari, S.M. Nanoencapsulation of Food Bioactive Ingredients: Principles and Applications, 1st ed.; Academic Press: Cambridge, MA, USA, 2017; p. 500. [Google Scholar]
- Chen, J.; Li, F.; Li, Z.; McClements, D.J.; Xiao, H. Encapsulation of Carotenoids in Emulsion-Based Delivery Systems: Enhancement of Β-Carotene Water-Dispersibility and Chemical Stability. Food Hydrocoll. 2017, 69, 49–55. [Google Scholar] [CrossRef]
- Kim, J.; Choi, S.J. Improving the Stability of Lycopene from Chemical Degradation in Model Beverage Emulsions: Impact of Hydrophilic Group Size of Emulsifier and Antioxidant Polarity. Foods 2020, 9, 971. [Google Scholar] [CrossRef]
- Irshad, S.; Sultana, H.; Usman, M.; Ahmad, M.; Al-Hussain, S.A.; Zaki, M.E.A. Enhanced Solubilization and Encapsulation of Direct Dyes through Self-Assembled Single and Mixed Micellar Nanocarriers. Environ. Technol. Innov. 2023, 30, 103061. [Google Scholar] [CrossRef]
- Chatterjee, M.; Hens, A.; Mahato, K.; Jaiswal, N.; Mahato, N.; Nagahanumaiah; Chanda, N. A Novel Approach to Fabricate Dye-Encapsulated Polymeric Micro- and Nanoparticles by Thin Film Dewetting Technique. J. Colloid Interface Sci. 2017, 506, 126–134. [Google Scholar] [CrossRef]
- Andra, V.V.S.N.L.; Pammi, S.V.N.; Bhatraju, L.V.K.P.; Ruddaraju, L.K. A Comprehensive Review on Novel Liposomal Methodologies, Commercial Formulations, Clinical Trials and Patents. Bionanoscience 2022, 12, 274–291. [Google Scholar] [CrossRef] [PubMed]
- Javadi, B.; Farahmand, A.; Soltani-Gorde-Faramarzi, S.; Hesarinejad, M.A. Chitosan-Coated Nanoliposome: An Approach for Simultaneous Encapsulation of Caffeine and Roselle-Anthocyanin in Beverages. Int. J. Biol. Macromol. 2024, 275, 133469. [Google Scholar] [CrossRef]
- Wang, L.; Wang, L.; Wang, X.; Lu, B.; Zhang, J. Preparation of Blueberry Anthocyanin Liposomes and Changes of Vesicle Properties, Physicochemical Properties, in Vitro Release, and Antioxidant Activity before and After Chitosan Modification. Food Sci. Nutr. 2022, 10, 75–87. [Google Scholar] [CrossRef]
- Benchabane, A.; Bekkour, K. Rheological Properties of Carboxymethyl Cellulose (CMC) Solutions. Colloid Polym. Sci. 2008, 286, 1173–1180. [Google Scholar] [CrossRef]
- Liu, G. Novel Protein-Lipid Composite Nanoparticles as Delivery Systems of Vitamin B12. Doctoral Thesis, University of Alberta, Edmonton, AB, Canada, 2019; p. 144. [Google Scholar] [CrossRef]
- da Fonsêca, J.H.L.; d’Ávila, M.A. Rheological Behavior of Carboxymethylcellulose and Cellulose Nanocrystal Aqueous Dispersions. Rheol. Acta 2021, 60, 497–509. [Google Scholar] [CrossRef]
- Azouz, K.B.; Dupuis, D.; Bekkour, K. Rheological Characterizations of Dispersions of Clay Particles in Viscoelastic Polymer Solutions. Appl. Rheol. 2010, 20, 13041. [Google Scholar] [CrossRef]
- Klinger, E.; Salminen, H.; Bause, K.; Weiss, J. Stability of Anthocyanin Extracts from Chokeberry, Grape, Hibiscus, and Purple Sweet Potato in Ω-3-Fatty Acid Rich Oil-in-Water Emulsions. Food Chem. 2024, 459, 140385. [Google Scholar] [CrossRef]
- Nguyen, N.B.; Ozarska, B.; Fergusson, M.; Vinden, P. Dyeing of Eucalyptus Globulus Veneers with Reactive Dye using Sequential Vacuum and Varied Pressures. BioResources 2023, 13, 8690–8708. [Google Scholar] [CrossRef]
- Delgado, A.V.; González-Caballero, F.; Hunter, R.J.; Koopal, L.K.; Lyklema, J. Measurement and Interpretation of Electrokinetic Phenomena. J. Colloid Interface Sci. 2007, 309, 194–224. [Google Scholar] [CrossRef] [PubMed]
- Samimi, S.; Maghsoudnia, N.; Eftekhari, R.B.; Dorkoosh, F. Lipid-Based Nanoparticles for Drug Delivery Systems. In Characterization and Biology of Nanomaterials for Drug Delivery: Nanoscience and Nanotechnology in Drug Delivery, 1st ed.; Mohapatra, S., Ranjan, S., Dasgupta, N., Thomas, S., Mishra, R.K., Eds.; Elsevier: Cambridge, MA, USA, 2019; pp. 47–76. [Google Scholar] [CrossRef]
- Wu, C.; Wang, L.; Harbottle, D.; Masliyah, J.; Xu, Z. Studying Bubble–particle Interactions by Zeta Potential Distribution Analysis. J. Colloid Interface Sci. 2015, 449, 399–408. [Google Scholar] [CrossRef]
- Dukhin, A.S.; Reisel, A. Overlapping Double Layers in Electrokinetics of Concentrated Dispersions. J. Colloid Interface Sci. 2022, 609, 764–774. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R. Impact of Measurement Protocol on ICP-MS Data Quality Objectives: Part II. Spectroscopy 2021, 36, 19–22. [Google Scholar] [CrossRef]
- Scholl, P.F.; Gray, P.J.; Harp, B.P.; Delmonte, P. High Resolution Mass Spectral Data from the Analysis of Copper Chlorophylls and Copper Chlorophyll Degradation Products in Bright Green Table Olives. Data Brief 2020, 30, 105548. [Google Scholar] [CrossRef]
- Hosseini, S.; Gharachorloo, M.; Ghiassi-Tarzi, B.; Ghavami, M. Evaluation the Organic Acids Ability for Extraction of Anthocyanins and Phenolic Compounds from Different Sources and their Degradation Kinetics during Cold Storage. Pol. J. Food Nutr. Sci. 2016, 66, 261–269. [Google Scholar] [CrossRef]
- Hudiyanti, D.; Aminah, S.; Hikmahwati, Y.; Siahaan, P. Cholesterol Implications on Coconut Liposomes Encapsulation of Beta-Carotene and Vitamin C. IOP Conf. Ser. Mater. Sci. Eng. 2019, 509, 012037. [Google Scholar] [CrossRef]
- Deroche, M.E.; Briantais, J.M. Absorption Spectra of Chlorophyll Forms, Β Carotene and Lutein in Freeze Dried Chloroplasts. Photochem. Photobiol. 1974, 19, 233–240. [Google Scholar] [CrossRef]
- Anonymous. Regulation (EC) no 1333/2008 of the European Parliament and of the Council of 16 December 2008 on Food Additives. Off. J. Eur. Union L 2008, 354, 16–33. [Google Scholar]
- Robert, P.; Fredes, C. The Encapsulation of Anthocyanins from Berry-Type Fruits. Trends in Foods. Molecules 2015, 20, 5875–5888. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, O.; Jafarizadeh-Malmiri, H. Green Approach in Food Nanotechnology Based on Subcritical Water: Effects of Thyme Oil and Saponin on Characteristics of the Prepared Oil in Water Nanoemulsions. Food Sci. Biotechnol. 2020, 29, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Nobbmann, U. D90, D50, D10, and Span—For DLS? Malvern Panalytical Ltd.: Malvern, UK, 2017; Available online: https://www.malvernpanalytical.com/en/learn/knowledge-center/insights/d90-d50-d10-and-span-for-dls (accessed on 16 March 2025).
- Lapčíková, B.; Lapčík, L.; Valenta, T.; Chvatíková, M. Plant-Based Emulsions as Dairy Cream Alternatives: Comparison of Viscoelastic Properties and Colloidal Stability of various Model Products. Foods 2024, 13, 1225. [Google Scholar] [CrossRef] [PubMed]
- Chudy, S.; Gierałtowska, U. Influence of the Background Color on the Cheese Color Parameters. Int. J. Dairy Sci. 2020, 15, 108. [Google Scholar] [CrossRef]
- McLellan, M.R.; Lind, L.R.; Kime, R.W. Hue Angle Determinations and Statistical Analysis for Multiquadrant Hunter L, a, B Data. J. Food Qual. 1995, 18, 235–240. [Google Scholar] [CrossRef]
- Schuessler, Z. Delta E 101. 2019. Available online: http://zschuessler.github.io/DeltaE/learn/ (accessed on 8 October 2024).
- Shi, Y.; Wang, W.; Zhu, X.; Wang, B.; Hao, Y.; Wang, L.; Yu, D.; Elfalleh, W. Preparation and Physicochemical Stability of Hemp Seed Oil Liposomes. Ind. Crops Prod. 2021, 162, 113283. [Google Scholar] [CrossRef]
- Chandra Singh, M.; Price, W.E.; Kelso, C.; Arcot, J.; Probst, Y. Measuring the Anthocyanin Content of the Australian Fruit and Vegetables for the Development of a Food Composition Database. J. Food Compos. Anal. 2022, 112, 104697. [Google Scholar] [CrossRef]
- de Freitas Santos, P.D.; Batista, P.S.; Torres, L.C.R.; Thomazini, M.; de Alencar, S.M.; Favaro-Trindade, C.S. Application of Spray Drying, Spray Chilling and the Combination of both Methods to Produce Tucumã Oil Microparticles: Characterization, Stability, and Β-Carotene Bioaccessibility. Food Res. Int. 2023, 172, 113174. [Google Scholar] [CrossRef]
- Harp, B.P.; Scholl, P.F.; Gray, P.J.; Delmonte, P. Quantitation of Copper Chlorophylls in Green Table Olives by Ultra-High-Performance Liquid Chromatography with Inductively Coupled Plasma Isotope Dilution Mass Spectrometry. J. Chromatogr. A 2020, 1620, 461008. [Google Scholar] [CrossRef]

| R Samples | C Samples | B Samples | ||||
|---|---|---|---|---|---|---|
| System No. | 1 DZ50 (nm) | Span (–) | DZ50 (nm) | Span (–) | DZ50 (nm) | Span (–) |
| 1 | 208.4 ± 2.1 a | 0.972 ± 0.032 a | 200.7 ± 3.4 a | 0.648 ± 0.004 ad | 171.6 ± 3.4 a | 1.230 ± 0.011 a |
| 2 | 227.6 ± 3.6 a | 0.690 ± 0.009 b | 232.2 ± 4.0 a | 0.643 ± 0.013 ad | 196.1 ± 2.2 a | 1.095 ± 0.001 bd |
| 3 | 208.4 ± 4.0 a | 0.715 ± 0.008 bc | 198.0 ± 2.5 a | 0.625 ± 0.007 ac | 168.9 ± 3.0 a | 1.039 ± 0.012 bch |
| 4 | 211.1 ± 3.7 a | 0.805 ± 0.002 ce | 221.5 ± 5.0 a | 0.585 ± 0.007 b | 180.2 ± 2.9 a | 1.139 ± 0.014 cdf |
| 5 | 166.1 ± 2.9 a | 1.261 ± 0.049 d | 206.7 ± 3.3 a | 0.594 ± 0.005 bc | 172.8 ± 1.9 a | 1.118 ± 0.023 d |
| 6 | 201.1 ± 5.5 a | 0.972 ± 0.062 a | 229.1 ± 3.9 a | 0.606 ± 0.001 bc | 219.9 ± 5.9 a | 1.090 ± 0.016 bd |
| 7 | 207.2 ± 5.0 a | 0.738 ± 0.013 be | 204.1 ± 3.6 a | 0.626 ± 0.012 ac | 176.1 ± 3.0 a | 0.962 ± 0.009 e |
| 8 | 215.5 ± 3.1 a | 0.833 ± 0.005 e | 222.6 ± 4.1 a | 0.587 ± 0.013 b | 177.5 ± 2.8 a | 1.085 ± 0.002 bd |
| 9 | 213.1 ± 3.3 a | 0.719 ± 0.003 bc | 200.1 ± 3.3 a | 0.665 ± 0.013 de | 165.1 ± 1.8 a | 1.080 ± 0.014 bd |
| 10 | 246.0 ± 2.8 a | 0.681 ± 0.007 b | 219.8 ± 3.6 a | 0.654 ± 0.010 ae | 199.3 ± 3.1 a | 1.102 ± 0.003 d |
| 11 | 249.3 ± 3.0 a | 0.826 ± 0.050 e | 225.2 ± 4.5 a | 0.626 ± 0.016 ac | 204.5 ± 4.0 a | 1.092 ± 0.005 bd |
| 12 | 212.7 ± 4.2 a | 0.701 ± 0.002 bf | 206.6 ± 3.8 a | 0.626 ± 0.002 ac | 312.6 ± 5.5 b | 1.253 ± 0.019 a |
| 13 | 219.7 ± 5.0 a | 0.747 ± 0.012 be | 228.8 ± 4.0 a | 0.674 ± 0.016 de | 316.5 ± 5.9 b | 1.196 ± 0.014 af |
| 14 | 226.1 ± 3.2 a | 0.731 ± 0.002 be | 209.0 ± 2.9 a | 0.641 ± 0.003 ad | 406.4 ± 6.1 c | 0.802 ± 0.037 g |
| 15 | 228.1 ± 2.9 a | 0.778 ± 0.001 cef | 222.9 ± 3.1 a | 0.598 ± 0.004 bc | 382.1 ± 5.2 c | 0.981 ± 0.021 eh |
| R Samples | C Samples | B Samples | |||||||
|---|---|---|---|---|---|---|---|---|---|
| System No. | 1 τ0 (Pa) | 2 K (Pa.sn) | 3 n (–) | τ0 (Pa) | K (Pa.sn) | n (–) | τ0 (Pa) | K (Pa.sn) | n (–) |
| 1 | 0.083 ± 0.002 a | (3.88 ± 0.21) × 10−5 aeg | 1.76 ± 0.07 ab | 0.085 ± 0.002 a | (2.79 ± 0.11) × 10−5 a | 1.82 ± 0.05 a | 0.087 ± 0.001 a | (2.75 ± 0.10) × 10−5 a | 1.82 ± 0.04 ad |
| 2 | 0.099 ± 0.003 bcd | (5.92 ± 0.19) × 10−5 b | 1.69 ± 0.05 ab | 0.087 ± 0.001 af | (5.10 ± 0.27) × 10−4 b | 1.30 ± 0.04 b | 0.076 ± 0.002 b | (1.14 ± 0.08) × 10−3 b | 1.14 ± 0.03 b |
| 3 | 0.082 ± 0.001 a | (3.10 ± 0.17) × 10−5 cf | 1.80 ± 0.06 ab | 0.088 ± 0.003 af | (2.41 ± 0.08) × 10−5 a | 1.85 ± 0.07 a | 0.088 ± 0.003 ac | (2.52 ± 0.13) × 10−5 a | 1.84 ± 0.05 a |
| 4 | 0.100 ± 0.003 bd | (2.77 ± 0.14) × 10−5 c | 1.83 ± 0.04 a | 0.096 ± 0.002 be | (2.61 ± 0.12) × 10−5 a | 1.85 ± 0.05 a | 0.093 ± 0.002 af | (2.36 ± 0.09) × 10−4 cf | 1.43 ± 0.04 cf |
| 5 | 0.079 ± 0.001 a | (3.83 ± 0.16) × 10−5 aeg | 1.75 ± 0.05 ab | 0.084 ± 0.001 ad | (2.80 ± 0.10) × 10−5 a | 1.82 ± 0.06 a | 0.085 ± 0.002 cd | (3.56 ± 0.12) × 10−5 a | 1.77 ± 0.05 ad |
| 6 | 0.093 ± 0.002 ce | (2.93 ± 0.11) × 10−5 c | 1.81 ± 0.06 ac | 0.093 ± 0.003 b | (2.11 ± 0.09) × 10−4 c | 1.45 ± 0.03 c | 0.089 ± 0.003 ac | (3.99 ± 0.15) × 10−4 d | 1.34 ± 0.02 c |
| 7 | 0.080 ± 0.002 a | (3.27 ± 0.19) × 10−5 ceg | 1.78 ± 0.04 ab | 0.083 ± 0.002 ad | (3.61 ± 0.16) × 10−5 a | 1.77 ± 0.06 a | 0.086 ± 0.002 ad | (2.67 ± 0.08) × 10−5 a | 1.83 ± 0.06 a |
| 8 | 0.094 ± 0.001 be | (2.87 ± 0.13) × 10−5 c | 1.82 ± 0.05 ac | 0.102 ± 0.002 ce | (4.06 ± 0.13) × 10−5 a | 1.77 ± 0.04 a | 0.101 ± 0.003 eg | (5.57 ± 0.14) × 10−5 a | 1.71 ± 0.04 d |
| 9 | 0.078 ± 0.002 a | (4.00 ± 0.18) × 10−5 ae | 1.74 ± 0.03 ab | 0.082 ± 0.003 ad | (4.62 ± 0.19) × 10−5 a | 1.74 ± 0.05 a | 0.082 ± 0.002 bcd | (3.70 ± 0.13) × 10−5 a | 1.76 ± 0.03 ade |
| 10 | 0.102 ± 0.003 df | (6.76 ± 0.29) × 10−5 d | 1.68 ± 0.04 bc | 0.078 ± 0.001 d | (9.92 ± 0.31) × 10−4 d | 1.17 ± 0.02 b | 0.077 ± 0.001 b | (1.71 ± 0.07) × 10−3 e | 1.07 ± 0.02 b |
| 11 | 0.102 ± 0.002 df | (4.13 ± 0.10) × 10−5 a | 1.77 ± 0.06 ab | 0.102 ± 0.002 ce | (4.46 ± 0.18) × 10−5 a | 1.75 ± 0.04 a | 0.100 ± 0.003 efg | (8.52 ± 0.28) × 10−5 a | 1.64 ± 0.03 e |
| 12 | 0.100 ± 0.003 bd | (3.50 ± 0.16) × 10−5 ef | 1.79 ± 0.05 ab | 0.095 ± 0.002 b | (2.94 ± 0.11) × 10−5 a | 1.82 ± 0.03 a | 0.099 ± 0.003 efg | (1.54 ± 0.10) × 10−4 c | 1.51 ± 0.02 fg |
| 13 | 0.103 ± 0.004 d | (7.00 ± 0.22) × 10−5 d | 1.66 ± 0.04 b | 0.101 ± 0.003 ce | (4.06 ± 0.15) × 10−5 a | 1.76 ± 0.03 a | 0.095 ± 0.002 ef | (2.43 ± 0.11) × 10−4 f | 1.42 ± 0.04 cg |
| 14 | 0.093 ± 0.001 ce | (3.55 ± 0.15) × 10−5 ef | 1.78 ± 0.03 ab | 0.093 ± 0.002 bf | (2.94 ± 0.09) × 10−5 a | 1.82 ± 0.05 a | 0.100 ± 0.003 efg | (4.48 ± 0.16) × 10−5 a | 1.75 ± 0.04 ade |
| 15 | 0.096 ± 0.003 bcf | (3.44 ± 0.13) × 10−5 fg | 1.79 ± 0.04 ab | 0.098 ± 0.001 bc | (2.88 ± 0.10) × 10−5 a | 1.83 ± 0.04 a | 0.103 ± 0.002 g | (4.39 ± 0.14) × 10−5 a | 1.75 ± 0.06 ade |
| R Samples | C Samples | B Samples | ||||
|---|---|---|---|---|---|---|
| System No. | h* (°) | ΔE* (–) | h* (°) | ΔE* (–) | h* (°) | ΔE* (–) |
| 1 | 67.82 ± 0.24 a | 42.09 ± 0.13 a | 115.87 ± 0.23 a | 54.37 ± 0.20 a | 81.13 ± 0.26 ae | 69.08 ± 0.21 a |
| 2 | 78.50 ± 0.26 b | 34.08 ± 0.17 b | 117.23 ± 0.28 b | 51.31 ± 0.20 b | 81.46 ± 0.35 a | 64.33 ± 0.26 b |
| 3 | 75.19 ± 0.34 c | 48.71 ± 0.16 c | 105.25 ± 0.32 c | 59.58 ± 0.18 c | 79.95 ± 0.24 bfe | 67.39 ± 0.31 c |
| 4 | 77.39 ± 0.30 d | 45.60 ± 0.17 d | 105.66 ± 0.34 c | 56.93 ± 0.21 d | 80.06 ± 0.33 bde | 70.91 ± 0.29 d |
| 5 | 76.15 ± 0.24 e | 30.85 ± 0.12 e | 115.49 ± 0.24 a | 46.31 ± 0.17 e | 82.11 ± 0.30 c | 58.20 ± 0.21 e |
| 6 | 73.86 ± 0.27 f | 31.06 ± 0.12 e | 117.07 ± 0.31 b | 43.67 ± 0.16 f | 82.45 ± 0.35 c | 53.57 ± 0.25 f |
| 7 | 77.22 ± 0.23 d | 40.51 ± 0.15 f | 105.86 ± 0.22 c | 51.11 ± 0.17 b | 81.14 ± 0.29 ae | 62.04 ± 0.21 g |
| 8 | 79.25 ± 0.28 g | 38.60 ± 0.19 g | 106.94 ± 0.29 d | 49.88 ± 0.20 g | 81.14 ± 0.36 ae | 63.48 ± 0.26 h |
| 9 | 69.81 ± 0.25 h | 39.71 ± 0.13 h | 109.49 ± 0.34 e | 53.12 ± 0.26 h | 80.37 ± 0.30 dfe | 66.48 ± 0.33 i |
| 10 | 77.47 ± 0.29 d | 35.21 ± 0.14 i | 110.96 ± 0.27 f | 50.27 ± 0.16 g | 81.25 ± 0.26 ae | 62.45 ± 0.30 g |
| 11 | 71.58 ± 0.35 i | 30.58 ± 0.10 e | 121.60 ± 0.24 g | 45.49 ± 0.18 i | 82.41 ± 0.34 c | 57.31 ± 0.26 j |
| 12 | 77.43 ± 0.38 d | 44.92 ± 0.22 j | 104.29 ± 0.31 h | 55.91 ± 0.20 j | 81.36 ± 0.20 a | 61.74 ± 0.26 g |
| 13 | 71.06 ± 0.29 j | 51.07 ± 0.25 k | 109.65 ± 0.28 e | 60.29 ± 0.23 k | 80.38 ± 0.24 e | 73.92 ± 0.37 k |
| 14 | 77.94 ± 0.32 bd | 33.24 ± 0.13 l | 110.66 ± 0.22 f | 46.02 ± 0.17 ei | 82.38 ± 0.30 c | 57.47 ± 0.20 ej |
| 15 | 75.44 ± 0.33 ce | 39.41 ± 0.14 h | 109.44 ± 0.26 e | 52.88 ± 0.21 h | 81.66 ± 0.29 a | 63.43 ± 0.20 h |
| Carrier System No. | CMC-Na Solution 1 wt.% (mL) | Lecithin Solution 1 wt.% (mL) | Distilled Water (mL) |
|---|---|---|---|
| 1 | 0.5 | 20.0 | 60.0 |
| 2 | 5.0 | 20.0 | 60.0 |
| 3 | 0.5 | 40.0 | 60.0 |
| 4 | 5.0 | 40.0 | 60.0 |
| 5 | 0.5 | 20.0 | 90.0 |
| 6 | 5.0 | 20.0 | 90.0 |
| 7 | 0.5 | 40.0 | 90.0 |
| 8 | 5.0 | 40.0 | 90.0 |
| 9 | 0.0 | 30.0 | 75.0 |
| 10 | 6.5 | 30.0 | 75.0 |
| 11 | 2.75 | 13.18 | 75.0 |
| 12 | 2.75 | 46.81 | 75.0 |
| 13 | 2.75 | 30.0 | 49.77 |
| 14 | 2.75 | 30.0 | 100.22 |
| 15 | 2.75 | 30.0 | 75.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lapčík, L.; Lapčíková, B.; Valenta, T.; Vašina, M.; Dudová, P.; Fišera, M. Study of Natural Dyes’ Liposomal Encapsulation in Food Dispersion Model Systems via High-Pressure Homogenization. Molecules 2025, 30, 1845. https://doi.org/10.3390/molecules30081845
Lapčík L, Lapčíková B, Valenta T, Vašina M, Dudová P, Fišera M. Study of Natural Dyes’ Liposomal Encapsulation in Food Dispersion Model Systems via High-Pressure Homogenization. Molecules. 2025; 30(8):1845. https://doi.org/10.3390/molecules30081845
Chicago/Turabian StyleLapčík, Lubomír, Barbora Lapčíková, Tomáš Valenta, Martin Vašina, Pavlína Dudová, and Miroslav Fišera. 2025. "Study of Natural Dyes’ Liposomal Encapsulation in Food Dispersion Model Systems via High-Pressure Homogenization" Molecules 30, no. 8: 1845. https://doi.org/10.3390/molecules30081845
APA StyleLapčík, L., Lapčíková, B., Valenta, T., Vašina, M., Dudová, P., & Fišera, M. (2025). Study of Natural Dyes’ Liposomal Encapsulation in Food Dispersion Model Systems via High-Pressure Homogenization. Molecules, 30(8), 1845. https://doi.org/10.3390/molecules30081845








