Research Progress on Microwave Synthesis of 3d Transition Metal (Mn, Fe, Co, and Ni) Oxide Nanomaterials for Supercapacitors
Abstract
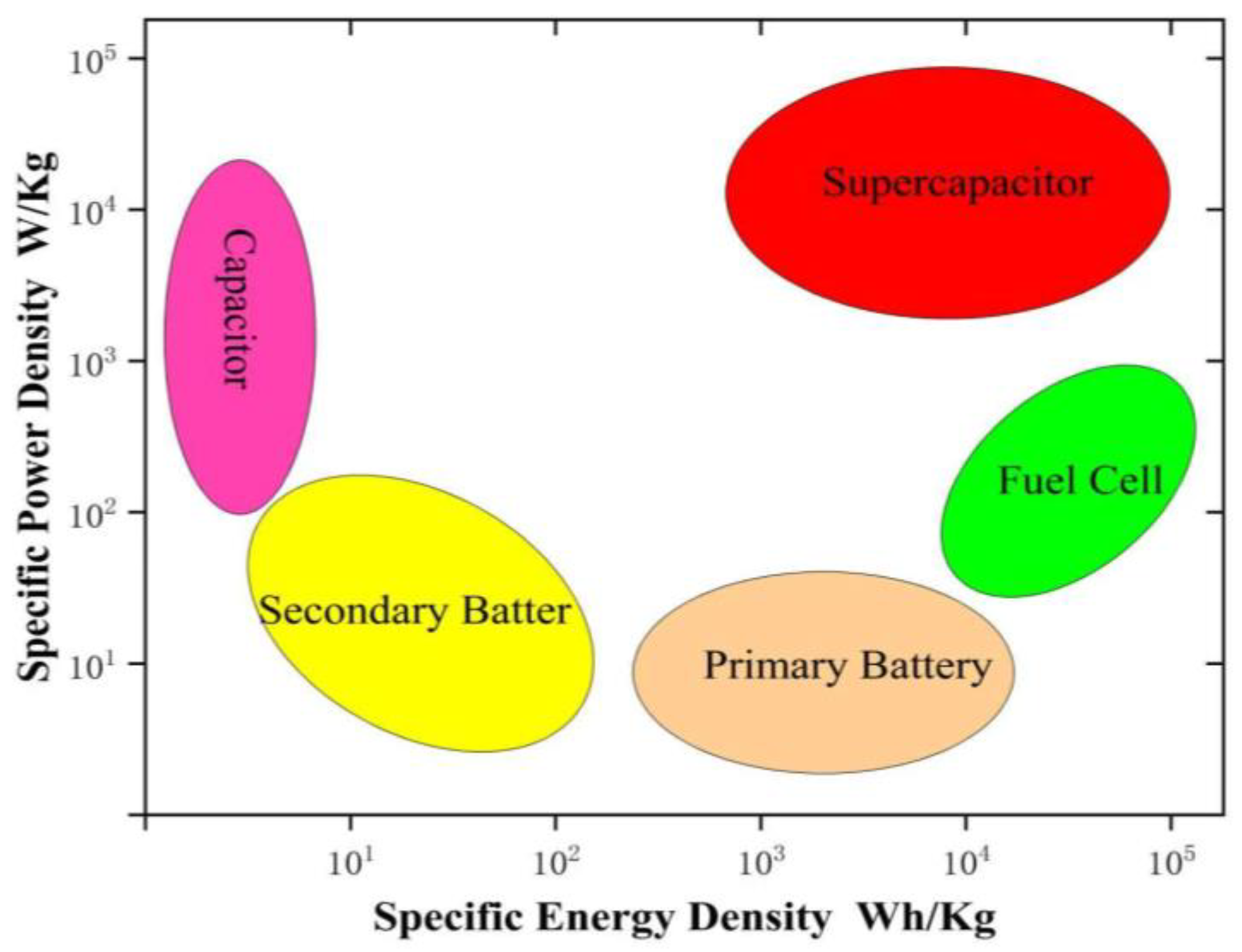
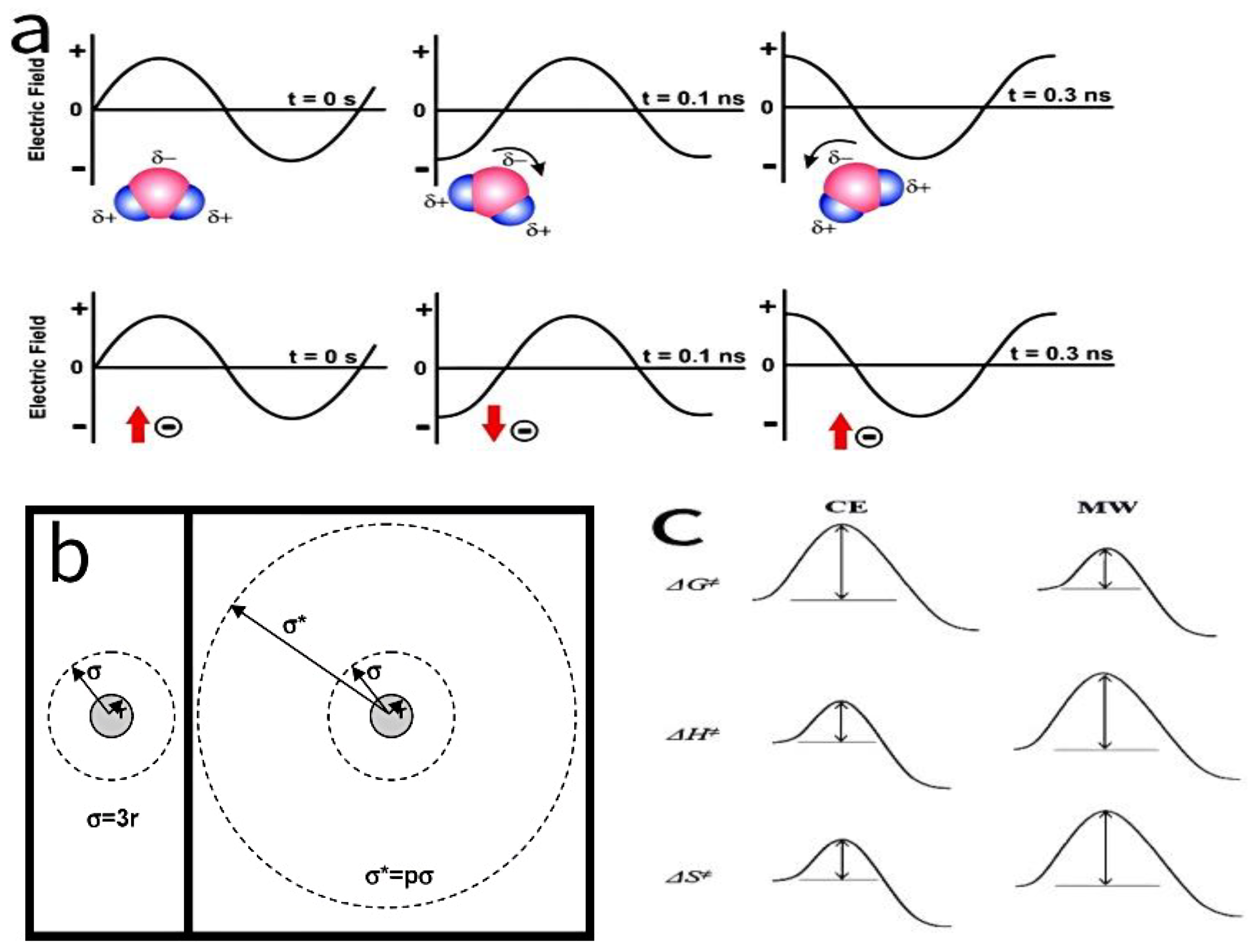
1. Introduction
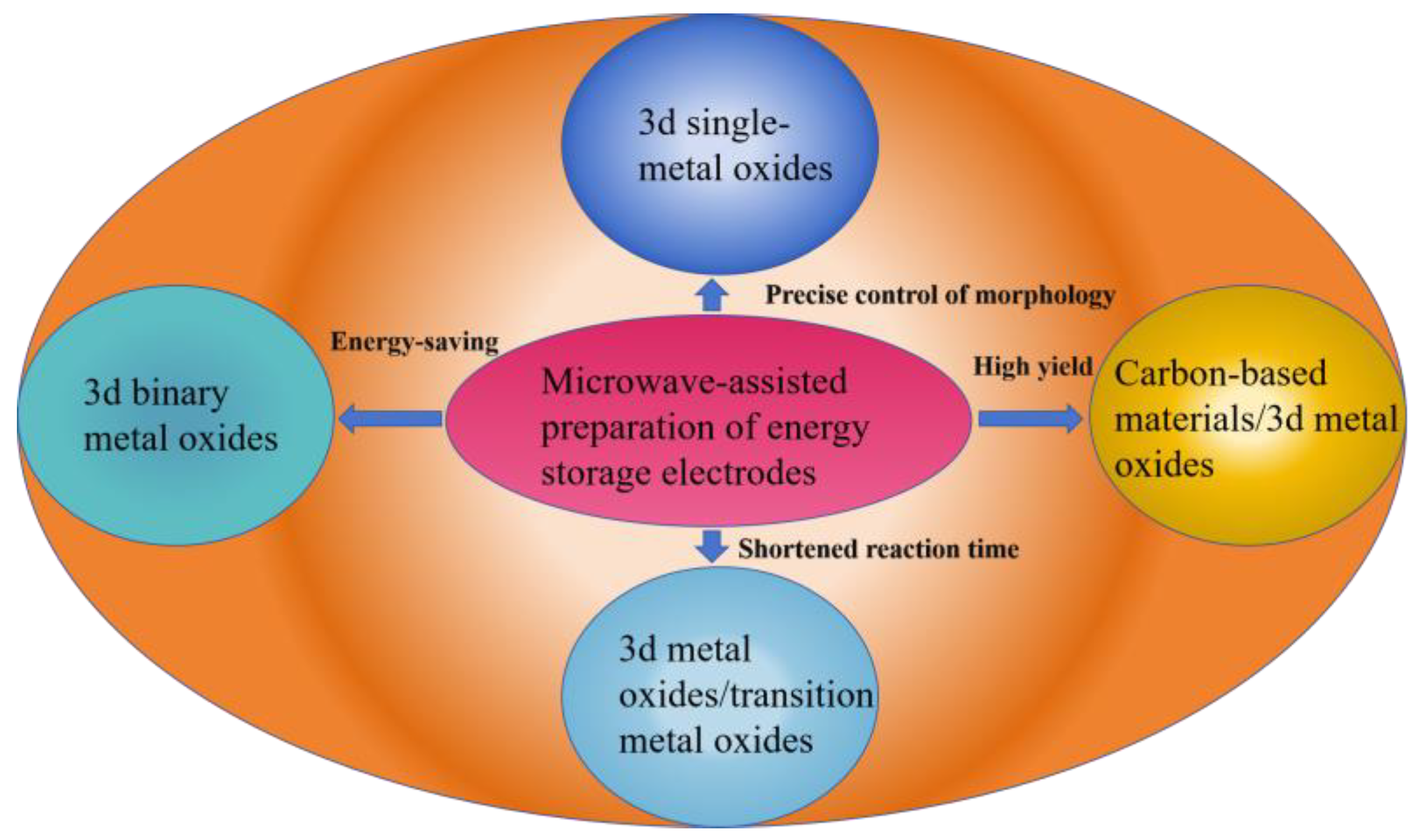
2. Results and Discussion
2.1. 3d Single-Metal Oxides
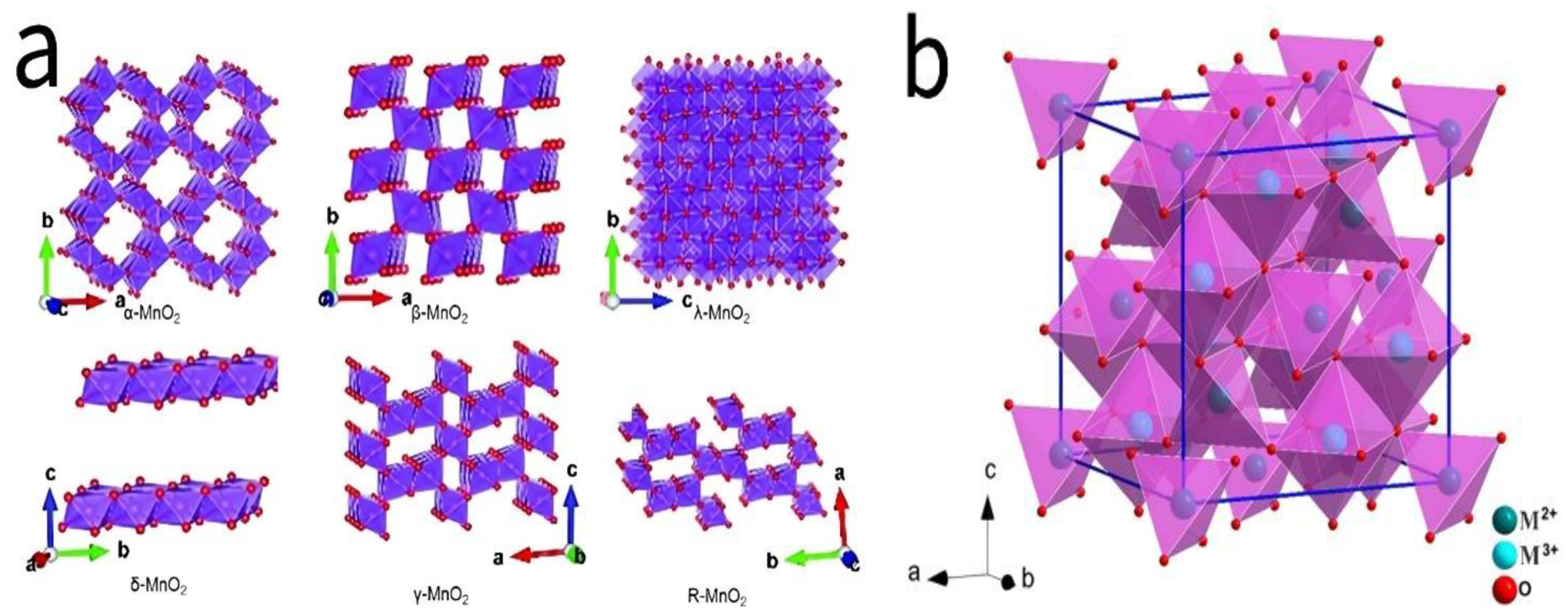
2.1.1. Manganese-Based Oxides (MnO2)
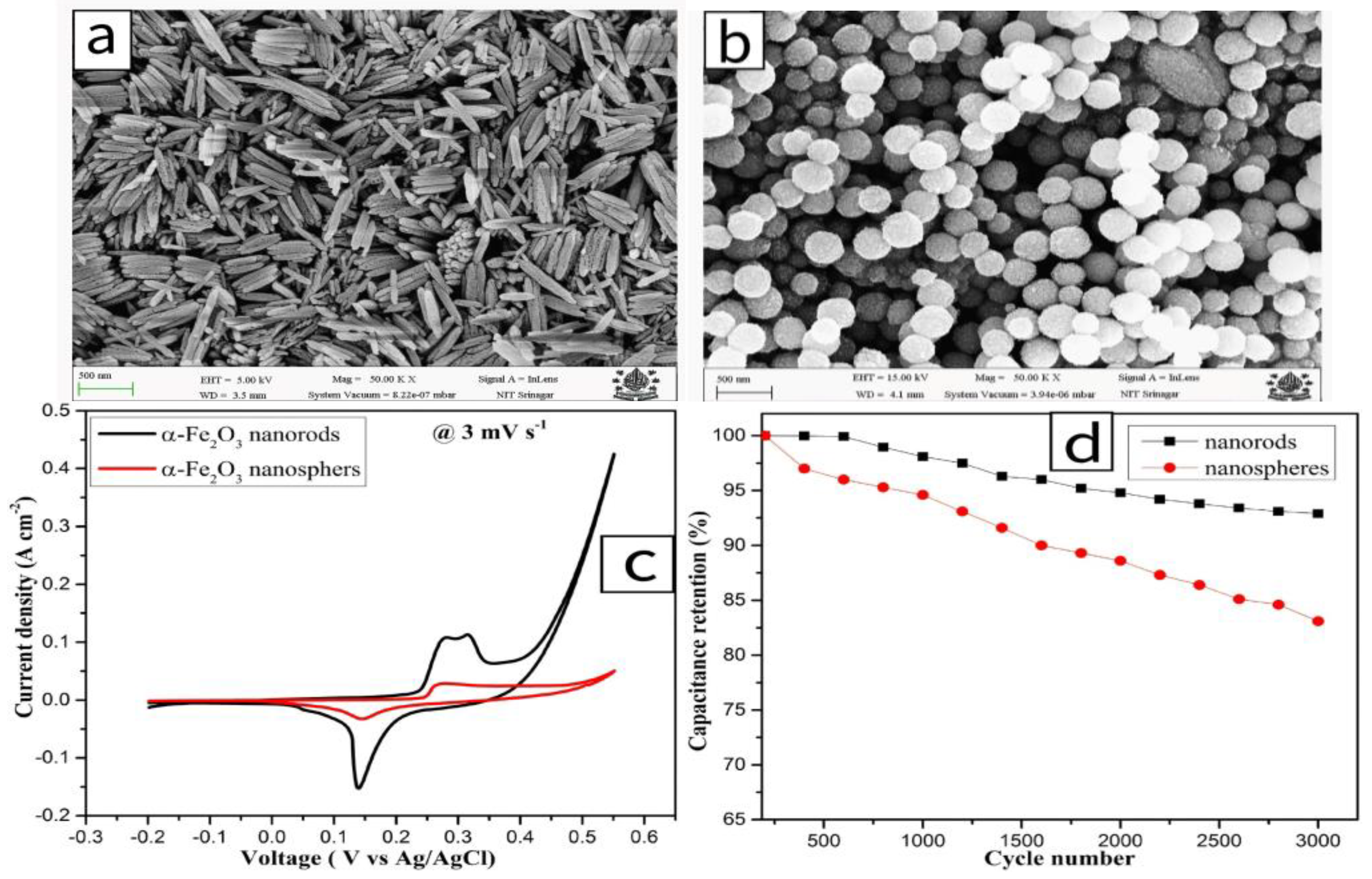
2.1.2. Iron-Based Oxides (α-Fe2O3)
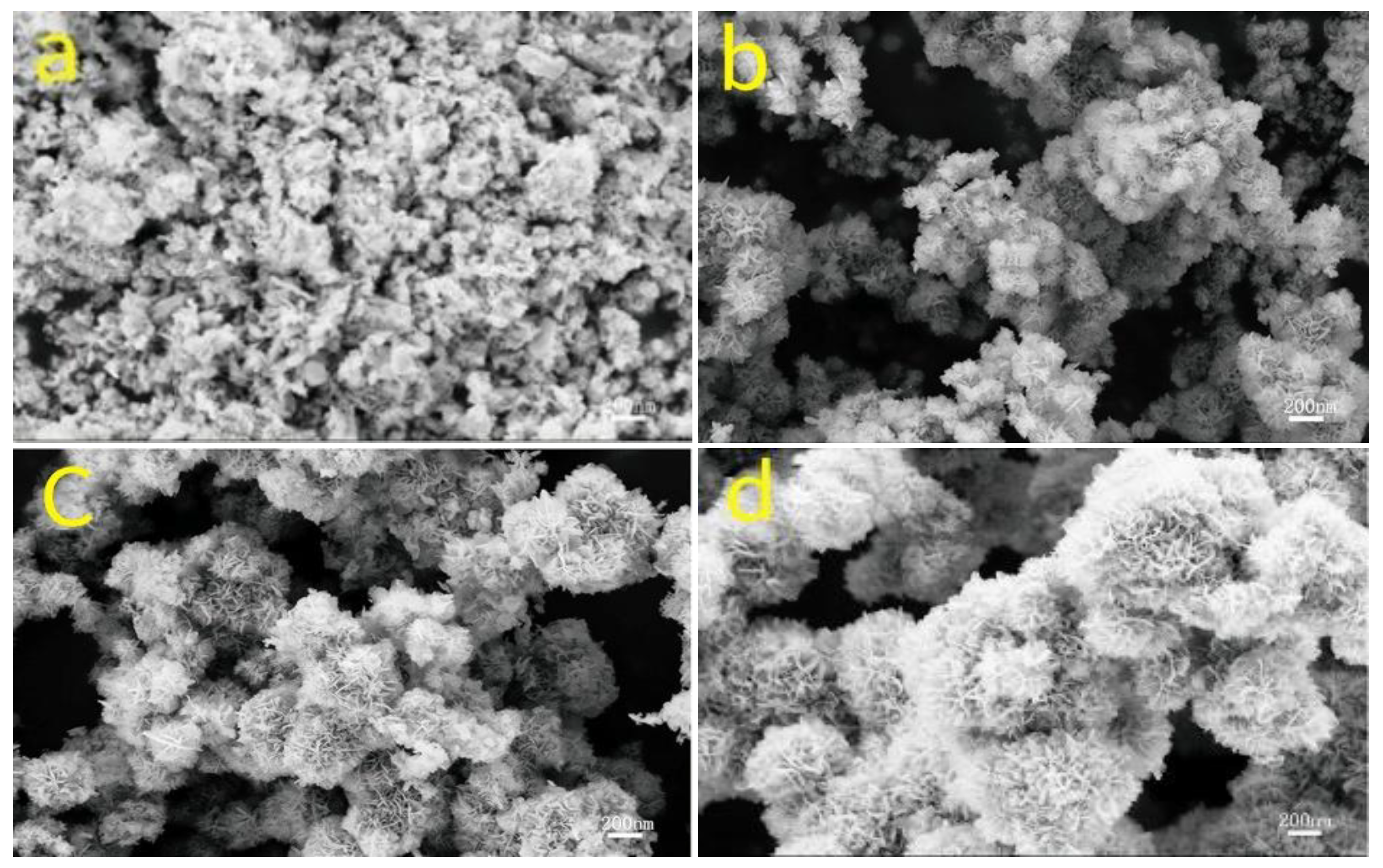
2.1.3. Cobalt-Based Oxides (Co3O4 and CoO)
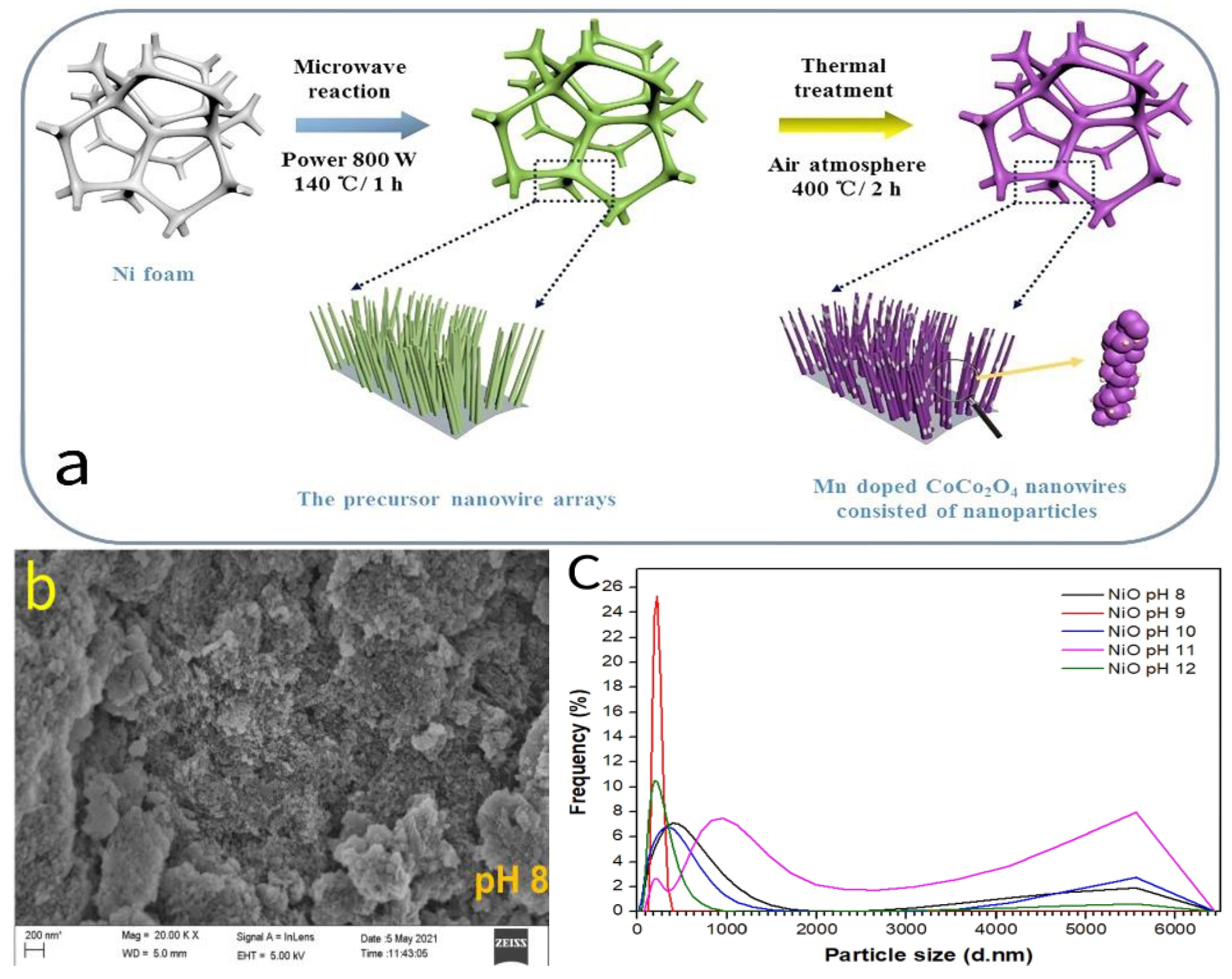
2.1.4. Nickel-Based Oxides (NiO)
2.2. 3d Binary Transition Metal Oxides
2.2.1. Nickel–Manganese Composite Oxides (NiMn2O4 and NiMnO3)
2.2.2. Nickel–Iron Composite Oxides (NiFe2O4)
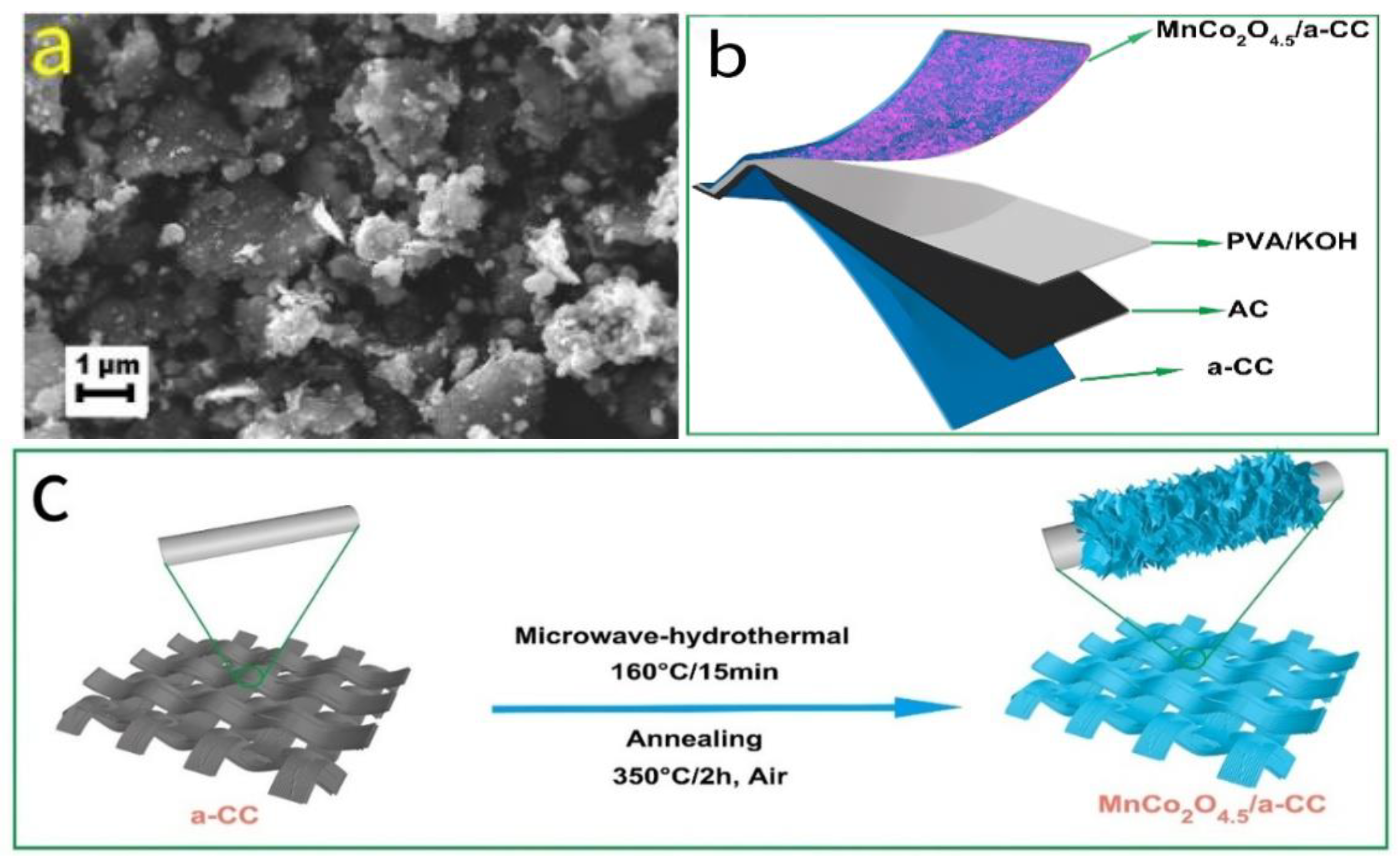
2.2.3. Manganese–Cobalt Composite Oxides (MnCo2O4 and MnCo2O4.5)
2.3. Carbon-Based Materials/3d Transition Metal Oxide Composite Materials
2.3.1. Graphene-Based 3d Transition Metal Oxide Electrode Materials
2.3.2. Carbon Nanotube-Based 3d Transition Metal Oxide Electrode Materials
2.3.3. Multicomponent Carbon-Based 3d Transition Metal Oxide Electrode Materials
2.4. Transition Metal Oxides/3d Transition Metal Oxide Composite Materials
3. Conclusions and Future Perspectives
3.1. Conclusions
3.2. Future Perspectives
- In-depth Investigation of Microwave-Induced Reaction MechanismsCurrently, the regulatory mechanisms of microwave effects in the synthesis process are not yet fully understood. Future studies should focus on elucidating the role of microwave irradiation in the structural evolution and performance modulation of materials, particularly its impact on nucleation, growth, and defect engineering.
- Enhancement of the Practical Specific Capacitance of Electrode MaterialsThe specific capacitance of microwave-synthesized 3d transition metal oxide electrode materials in practical applications remains lower than their theoretical values. Future research should prioritize the development of novel composite structures, such as multi-element doping and multi-dimensional hybrid materials, to further improve specific capacitance, energy density, and power density.
- Advancements in In Situ Characterization TechniquesIn studies on the energy storage mechanisms of microwave-synthesized 3d transition metal oxide electrodes, conventional ex situ techniques such as XRD and XPS fail to precisely capture the dynamic changes occurring on the electrode surface during charge-discharge processes. Future research should focus on integrating in situ characterization techniques to monitor phase transitions, morphological evolution, and interfacial adsorption behavior in real-time. This approach will provide deeper insights into performance evolution mechanisms and guide the rational design of high-performance energy storage materials.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef] [PubMed]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ruan, P.; Wu, X.; Liang, S.; Zhou, J. Crystal Structures, Reaction Mechanisms, and Optimization Strategies of MnO2 Cathode for Aqueous Rechargeable Zinc Batteries. Acta Phys. Chim. Sin. 2021, 38, 2111003. [Google Scholar] [CrossRef]
- Vangari, M.; Pryor, T.; Jiang, L. Supercapacitors: Review of Materials and Fabrication Methods. J. Energy Eng. 2013, 139, 72–79. [Google Scholar] [CrossRef]
- Practical Microwave Synthesis for Organic Chemists: Strategies, Instruments, and Protocols. J. Am. Chem. Soc. 2009, 131, 7204. [CrossRef]
- Godinho, M.; Ribeiro, C.; Longo, E.; Leite, E.R. Influence of Microwave Heating on the Growth of Gadolinium-Doped Cerium Oxide Nanorods. Cryst. Growth Des. 2008, 8, 384–386. [Google Scholar] [CrossRef]
- Haque, E.; Khan, N.A.; Kim, C.M.; Jhung, S.H. Syntheses of Metal–Organic Frameworks and Aluminophosphates under Microwave Heating: Quantitative Analysis of Accelerations. Cryst. Growth Des. 2011, 11, 4413–4421. [Google Scholar] [CrossRef]
- Toupin, M.; Brousse, T.; Bélanger, D. Charge Storage Mechanism of MnO2 Electrode Used in Aqueous Electrochemical Capacitor. Chem. Mater. 2004, 16, 3184–3190. [Google Scholar] [CrossRef]
- Jayachandran, M.; Rose, A.; Maiyalagan, T.; Poongodi, N.; Vijayakumar, T. Effect of various aqueous electrolytes on the electrochemical performance of α-MnO2 nanorods as electrode materials for supercapacitor application. Electrochim. Acta 2021, 366, 137412. [Google Scholar] [CrossRef]
- Wei, W.; Cui, X.; Chen, W.; Ivey, D.G. Manganese oxide-based materials as electrochemical supercapacitor electrodes. Chem. Soc. Rev. 2011, 40, 1697–1721. [Google Scholar] [CrossRef]
- Rios, E.C.; Rosario, A.V.; Mello, R.M.Q.; Micaroni, L. Poly(3-methylthiophene)/MnO2 composite electrodes as electrochemical capacitors. J. Power Sources 2007, 163, 1137–1142. [Google Scholar] [CrossRef]
- Tompsett, G.A.; Conner, W.C.; Yngvesson, K.S. Microwave Synthesis of Nanoporous Materials. ChemPhysChem 2006, 7, 296–319. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.; Cheng, F.; Chen, J. Electrodeposition synthesis and electrochemical properties of nanostructured γ-MnO2 films. J. Power Sources 2006, 162, 727–734. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Huang, W.; Sebastian, P.J.; Gamboa, S. Sol–gel template synthesis of highly ordered MnO2 nanowire arrays. J. Power Sources 2005, 140, 211–215. [Google Scholar] [CrossRef]
- Samal, R.; Kandasamy, M.; Chakraborty, B.; Rout, C.S. Experimental and theoretical realization of an advanced bifunctional 2D δ-MnO2 electrode for supercapacitor and oxygen evolution reaction via defect engineering. Int. J. Hydrogen Energy 2021, 46, 28028–28042. [Google Scholar] [CrossRef]
- Vimuna, V.M.; Athira, A.R.; Dinesh Babu, K.V.; Xavier, T.S. Simultaneous stirring and microwave assisted synthesis of nanoflakes MnO2/rGO composite electrode material for symmetric supercapacitor with enhanced electrochemical performance. Diam. Relat. Mater. 2020, 110, 108129. [Google Scholar] [CrossRef]
- Raskar, N.D.; Dake, D.V.; Mane, V.A.; Sonpir, R.B.; Vasundhara, M.; Asokan, K.; Deshpande, U.; Venkatesh, R.; Mote, V.D.; Dole, B.N. Designing reduced graphene oxide decorated Ni doped δ-MnO2 nanocomposites for supercapacitor applications. Mater. Sci. Semicond. Process. 2024, 178, 108451. [Google Scholar] [CrossRef]
- Seok, J.Y.; Lee, J.; Park, J.H.; Yang, M. Li-Ion Batteries: Ultrafine Copper Nanopalm Tree-Like Framework Decorated with Iron Oxide for Li-Ion Battery Anodes with Exceptional Rate Capability and Cycling Stability (Adv. Energy Mater. 13/2019). Adv. Energy Mater. 2019, 9, 1970039. [Google Scholar] [CrossRef]
- Chen, D.; Zhou, S.; Quan, H.; Zou, R.; Gao, W.; Luo, X.; Guo, L. Tetsubo-like α-Fe2O3/C nanoarrays on carbon cloth as negative electrode for high-performance asymmetric supercapacitors. Chem. Eng. J. 2018, 341, 102–111. [Google Scholar] [CrossRef]
- Lu, X.; Zeng, Y.; Yu, M.; Zhai, T.; Liang, C.; Xie, S.; Balogun, M.; Tong, Y. Oxygen-Deficient Hematite Nanorods as High-Performance and Novel Negative Electrodes for Flexible Asymmetric Supercapacitors. Adv. Mater. 2014, 26, 3148–3155. [Google Scholar] [CrossRef]
- Aalim, M.; Shah, M.A. Role of oxygen vacancies and porosity in enhancing the electrochemical properties of Microwave synthesized hematite (α-Fe2O3) nanostructures for supercapacitor application. Vacuum 2023, 210, 111903. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Ponnalagi, A.K.; Nagamuthu, S.; Muralidharan, G. Microwave assisted synthesis of Co3O4 nanoparticles for high-performance supercapacitors. Electrochim. Acta 2013, 106, 500–505. [Google Scholar] [CrossRef]
- Yan, J.; Wei, T.; Qiao, W.; Shao, B.; Zhao, Q.; Zhang, L.; Fan, Z. Rapid microwave-assisted synthesis of graphene nanosheet/Co3O4 composite for supercapacitors. Electrochim. Acta 2010, 55, 6973–6978. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, Y.; Li, Y.; Liu, J. Construction of High-Capacitance 3D CoO@Polypyrrole Nanowire Array Electrode for Aqueous Asymmetric Supercapacitor. Nano Lett. 2013, 13, 2078–2085. [Google Scholar] [CrossRef]
- Chen, D.; Wang, Q.; Wang, R.; Shen, G. Ternary oxide nanostructured materials for supercapacitors: A review. J. Mater. Chem. A 2015, 3, 10158–10173. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, J.; Liu, S.; Sun, X.; Huang, N. An enhancement on supercapacitor properties of porous CoO nanowire arrays by microwave-assisted regulation of the precursor. Nanotechnology 2021, 32, 195707. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, X.; Zhang, J.; Liu, S.; Guo, L.; Bi, J.; Huang, N. Boosting the electrochemical properties of CoCo2O4 porous nanowire arrays by microwave-assisted synthesis for battery–supercapacitor hybrid devices. Sustain. Energy Fuels 2021, 5, 3918–3928. [Google Scholar] [CrossRef]
- Sun, Y.; Huang, N.; Zhao, D.; Wang, X.; Zhang, J.; Liu, S.; Guo, L.; Sun, X. Microwave-assisted in-situ isomorphism via introduction of Mn into CoCo2O4 for battery-supercapacitor hybrid electrode material. Chem. Eng. J. 2022, 430, 132729. [Google Scholar] [CrossRef]
- Chuminjak, Y.; Daothong, S.; Reanpang, P.; Mensing, J.P.; Phokharatkul, D.; Jakmunee, J.; Wisitsoraat, A.; Tuantranont, A.; Singjai, P. Electrochemical energy-storage performances of nickel oxide films prepared by a sparking method. RSC Adv. 2015, 5, 67795–67802. [Google Scholar] [CrossRef]
- Behm, N.; Brokaw, D.; Overson, C.; Peloquin, D.; Poler, J.C. High-throughput microwave synthesis and characterization of NiO nanoplates for supercapacitor devices. J. Mater. Sci. 2012, 48, 1711–1716. [Google Scholar] [CrossRef]
- Agrawal, S.; Parveen, A.; Azam, A. Microwave assisted synthesis of Co doped NiO nanoparticles and its fluorescence properties. J. Lumin. 2017, 184, 250–255. [Google Scholar] [CrossRef]
- Zhang, J.; Tahmasebi, A.; Omoriyekomwan, J.E.; Yu, J. Microwave-assisted synthesis of biochar-carbon-nanotube-NiO composite as high-performance anode materials for lithium-ion batteries. Fuel Process. Technol. 2021, 213, 106714. [Google Scholar] [CrossRef]
- Rakesh Kumar, T.; Shilpa Chakra, C.H.; Madhuri, S.; Sai Ram, E.; Ravi, K. Microwave-irradiated novel mesoporous nickel oxide carbon nanocomposite electrodes for supercapacitor application. J. Mater. Sci. Mater. Electron. 2021, 32, 20374–20383. [Google Scholar] [CrossRef]
- Wei, X.; Dong, C.; Chen, Z.; Xiao, K.; Li, X. Density functional theory study of SO2-adsorbed Ni(1° 1° 1) and hydroxylated NiO(1° 1° 1) surface. Appl. Surf. Sci. 2015, 355, 429–435. [Google Scholar] [CrossRef]
- Salleh, N.A.; Mohammad, A.H.; Zakaria, Z.; Deghfel, B.; Yaakob, M.K.; Rahiman, W.; Kheawhom, S.; Mohamad, A.A. Microwave assisted synthesis of nickel oxide nanoparticles at different pH via sol gel method: Experimental and first-principles investigations. Inorg. Chem. Commun. 2024, 164, 112415. [Google Scholar] [CrossRef]
- Dubal, D.P.; Chodankar, N.R.; Kim, D.-H.; Gomez-Romero, P. Towards flexible solid-state supercapacitors for smart and wearable electronics. Chem. Soc. Rev. 2018, 47, 2065–2129. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Zhang, Y.; Hu, X. 3D NiS dendritic arrays on nickel foam as binder-free electrodes for supercapacitors. J. Mater. Sci.: Mater. Electron. 2016, 27, 8599–8605. [Google Scholar] [CrossRef]
- Li, X.; Wu, H.; Elshahawy, A.M.; Wang, L.; Pennycook, S.J.; Guan, C.; Wang, J. Cactus-Like NiCoP/NiCo-OH 3D Architecture with tunable composition for high-performance electrochemical capacitors. Adv. Funct. Mater. 2018, 28, 1800036. [Google Scholar] [CrossRef]
- Chen, C.; Yan, D.; Luo, X.; Gao, W.; Huang, G.; Han, Z.; Zeng, Y.; Zhu, Z. Construction of Core–Shell NiMoO4@Ni-Co-S Nanorods as Advanced Electrodes for High-Performance Asymmetric Supercapacitors. ACS Appl. Mater. Interfaces 2018, 10, 4662–4671. [Google Scholar] [CrossRef]
- Chen, C.; Wang, S.; Luo, X.; Gao, W.; Huang, G.; Zeng, Y.; Zhu, Z. Reduced ZnCo2O4@NiMoO4·H2O heterostructure electrodes with modulating oxygen vacancies for enhanced aqueous asymmetric supercapacitors. J. Power Sources 2019, 409, 112–122. [Google Scholar] [CrossRef]
- Qiao, S.; Huang, N.; Gao, Z.; Zhou, S.; Sun, Y. Nickel-Manganese Binary Metal Oxide as Electrode Materials for Supercapacitors. Prog. Chem. 2019, 31, 1177–1186. [Google Scholar]
- Du, X.; Yang, L.; Fu, Y.; Liu, S.; Huang, N.; Wang, S. Microwave-Assisted Synthesis of NiMn2O4 Grown on Nickel Foam as Electrode Material for High-Performance Supercapacitors. ChemistrySelect 2021, 6, 5567–5574. [Google Scholar] [CrossRef]
- Qiao, S.; Huang, N.; Sun, Y.; Zhang, J.; Zhang, Y.; Gao, Z. Microwave-assisted synthesis of novel 3D flower-like NiMnO3 nanoballs as electrode material for high-performance supercapacitors. J. Alloys Compd. 2019, 775, 1109–1116. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, J.; Sun, X.; Huang, N. High-performance spinel NiMn2O4 microspheres self-assembled with nanosheets by microwave-assisted synthesis for supercapacitors. CrystEngComm 2020, 22, 1645–1652. [Google Scholar] [CrossRef]
- Bashir, B.; Shaheen, W.; Asghar, M.; Warsi, M.F.; Khan, M.A.; Haider, S.; Shakir, I.; Shahid, M. Copper doped manganese ferrites nanoparticles anchored on graphene nano-sheets for high performance energy storage applications. J. Alloys Compd. 2017, 695, 881–887. [Google Scholar] [CrossRef]
- Sathiyamurthy, K.; Rajeevgandhi, C.; Guganathan, L.; Bharanidharan, S.; Savithiri, S. Enhancement of magnetic, supercapacitor applications and theoretical approach on cobalt-doped zinc ferrite nanocomposites. J. Mater. Sci. Mater. Electron. 2021, 32, 11593–11606. [Google Scholar] [CrossRef]
- Mayakkannan, M.; Siva, V.; Murugan, A.; Shameem, A.; Thangarasu, S.; Asath Bahadur, S. Microwave-assisted synthesis of ternary transition metal ferrite: Structural, morphological, optical, magnetic and electrochemical properties. Phys. E Low-Dimens. Syst. Nanostruct. 2023, 147, 115573. [Google Scholar] [CrossRef]
- Suresh Kumar, G.; Srinivasan, R.; Karunakaran, G.; Kolesnikov, E.; Kim, M.; Karpenkov, D.Y. Microwave-assisted combustion synthesis of soft ferromagnetic spinel MFe2O4 (M = Ni, Mg, Zn) nanoparticles using Citrus limon fruit extract as a fuel. Appl. Phys. A 2021, 127, 546. [Google Scholar] [CrossRef]
- Gao, Y.; Xia, Y.; Wan, H.; Xu, X.; Jiang, S. Enhanced cycle performance of hierarchical porous sphere MnCo2O4 for asymmetric supercapacitors. Electrochim. Acta 2019, 301, 294–303. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, X.; An, C.; Wang, Y.; Jiao, L.; Yuan, H. Facile synthesis route of porous MnCo2O4 and CoMn2O4 nanowires and their excellent electrochemical properties in supercapacitors. J. Mater. Chem. A 2014, 2, 16480–16488. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, W.; Liang, R.; Tjandra, R.; Yu, A. Graphene quantum dot induced tunable growth of nanostructured MnCo2O4.5 composites for high-performance supercapacitors. Sustain. Energy Fuels 2019, 3, 2499–2508. [Google Scholar] [CrossRef]
- Du, X.; Ren, X.; Xu, C.; Chen, H. Recent advances on the manganese cobalt oxides as electrode materials for supercapacitor applications: A comprehensive review. J. Energy Storage 2023, 68, 107672. [Google Scholar] [CrossRef]
- Li, J.; Xiong, S.; Li, X.; Qian, Y. Spinel Mn1.5Co1.5O4 core–shell microspheres as Li-ion battery anode materials with a long cycle life and high capacity. J. Mater. Chem. 2012, 3, 23254–23259. [Google Scholar] [CrossRef]
- Guo, X.; Li, M.; Liu, Y.; Huang, Y.; Geng, S.; Yang, W.; Yu, Y. Hierarchical core-shell electrode with NiWO4 nanoparticles wrapped MnCo2O4 nanowire arrays on Ni foam for high-performance asymmetric supercapacitors. J. Colloid Interface Sci. 2020, 563, 405–413. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.Q.; Liu, X.Y.; Shi, S.J.; Zhao, X.Y.; Zhang, H.; Ge, X.; Cai, G.F.; Gu, C.D.; Wang, X.L.; et al. One-dimension MnCo2O4 nanowire arrays for electrochemical energy storage. Electrochim. Acta 2014, 116, 467–474. [Google Scholar] [CrossRef]
- Kong, L.-B.; Lu, C.; Liu, M.-C.; Luo, Y.-C.; Kang, L.; Li, X.; Walsh, F.C. The specific capacitance of sol–gel synthesised spinel MnCo2O4 in an alkaline electrolyte. Electrochim. Acta 2014, 115, 22–27. [Google Scholar] [CrossRef]
- Liu, Y.; Du, X.; Li, Y.; Bao, E.; Ren, X.; Chen, H.; Tian, X.; Xu, C. Nanosheet-assembled porous MnCo2O4.5 microflowers as electrode material for hybrid supercapacitors and lithium-ion batteries. J. Colloid Interface Sci. 2022, 627, 815–826. [Google Scholar] [CrossRef]
- Uniform MnCo2O4.5 porous nanowires and quasi-cubes for hybrid supercapacitors with excellent electrochemical performances. Nanoscale Adv. 2021, 3, 4447–4458. [CrossRef]
- Krishnan, S.G.; Harilal, M.; Arshid, N.; Jagadish, P.; Khalid, M.; Li, L.P. Rapid microwave-assisted synthesis of MnCo2O4 nanoflakes as a cathode for battery-supercapacitor hybrid. J. Energy Storage 2021, 44, 103566. [Google Scholar] [CrossRef]
- Sannasi, V.; Subbian, K. High-pseudocapacitance of MnCo2O4 nanostructures prepared by phenolphthalein assisted hydrothermal and microwave methods. Ceram. Int. 2020, 46, 15510–15520. [Google Scholar] [CrossRef]
- Sannasi, V.; Karuppuchamy, S. Preparation of MnCo2O4 by microwave assisted method for supercapacitor applications. In AIP Conference Proceedings; AIP Publishing: Tamil Nadu, India, 2019; p. 020040. [Google Scholar]
- Sun, Y.; Sheng, Y.; Jiang, W.; Sun, C.; Zhao, X.; Deng, B.; Zhang, K.; Gao, Z.; Zhang, S.; Wang, X. Microwave-assistance boosted voltage window in oxygen-deficient MnCo2O4.5 for flexible asymmetric supercapacitors. Vacuum 2024, 225, 113218. [Google Scholar] [CrossRef]
- Lee, H.S.; Gund, G.S.; Park, H.S. Controlled growth and interaction of NiCo2S4 on conductive substrate for enhanced electrochemical performance. J. Power Sources 2020, 451, 227763. [Google Scholar] [CrossRef]
- Eder, D. Carbon Nanotube−Inorganic Hybrids. Chem. Rev. 2010, 110, 1348–1385. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Kim, B.-S.; Chen, S.; Shao-Horn, Y.; Hammond, P.T. Layer-by-Layer Assembly of All Carbon Nanotube Ultrathin Films for Electrochemical Applications. J. Am. Chem. Soc. 2009, 131, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Gopi, C.V.V.M.; Alzahmi, S.; Al-Haik, M.Y.; Kumar, Y.A.; Hamed, F.; Haik, Y.; Obaidat, I.M. Recent advances in pseudocapacitive electrode materials for high energy density aqueous supercapacitors: Combining transition metal oxides with carbon nanomaterials. Mater. Today Sustain. 2024, 28, 100981. [Google Scholar] [CrossRef]
- Geim, A.K. Graphene: Status and Prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef]
- Seabra, A.B.; Paula, A.J.; de Lima, R.; Alves, O.L.; Durán, N. Nanotoxicity of Graphene and Graphene Oxide. Chem. Res. Toxicol. 2014, 27, 159–168. [Google Scholar] [CrossRef]
- Xia, J.; Chen, F.; Li, J.; Tao, N. Measurement of the quantum capacitance of graphene. Nat. Nanotechnol. 2019, 4, 505–509. [Google Scholar] [CrossRef]
- Booth, T.J.; Blake, P.; Nair, R.R.; Jiang, D.; Hill, E.W.; Bangert, U.; Bleloch, A.; Gass, M.; Novoselov, K.S.; Katsnelson, M.I.; et al. Macroscopic Graphene Membranes and Their Extraordinary Stiffness. Nano Lett. 2008, 8, 2442–2446. [Google Scholar] [CrossRef]
- Wu, Z.-S.; Zhou, G.; Yin, L.-C.; Ren, W.; Li, F.; Cheng, H.-M. Graphene/metal oxide composite electrode materials for energy storage. Nano Energy 2012, 1, 107–131. [Google Scholar] [CrossRef]
- Kumar, R.; Joanni, E.; Tan, W.K.; Matsuda, A. Microwave-aided ultra-fast synthesis of Fe3O4 nanoparticles attached reduced graphene oxide edges as electrode materials for supercapacitors. Mater. Today Commun. 2024, 38, 108438. [Google Scholar] [CrossRef]
- Kumar, R.; Youssry, S.M.; Joanni, E.; Sahoo, S.; Kawamura, G.; Matsuda, A. Microwave-assisted synthesis of iron oxide homogeneously dispersed on reduced graphene oxide for high-performance supercapacitor electrodes. J. Energy Storage 2022, 56, 105896. [Google Scholar] [CrossRef]
- Kumar, R.; Sahoo, S.; Joanni, E.; Pandey, R.; Tan, W.K.; Kawamura, G.; Moshkalev, S.A.; Matsuda, A. Microwave-assisted dry synthesis of hybrid electrode materials for supercapacitors: Nitrogen-doped rGO with homogeneously dispersed CoO nanocrystals. J. Energy Storage 2023, 68, 107820. [Google Scholar] [CrossRef]
- Zhai, Z.; Zhang, L.; Du, T.; Ren, B.; Xu, Y.; Wang, S.; Miao, J.; Liu, Z. A review of carbon materials for supercapacitors. Mater. Des. 2022, 221, 111017. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Dresselhaus, G.; Charlier, J.C.; Hernández, E. Electronic, thermal and mechanical properties of carbon nanotubes. Philos. Trans. R. Soc. Lond. Ser. A Math. Phys. Eng. Sci. 2004, 362, 2065–2098. [Google Scholar] [CrossRef]
- Xie, S.; Li, W.; Pan, Z.; Chang, B.; Sun, L. Mechanical and physical properties on carbon nanotube. J. Phys. Chem. Solids 2000, 61, 1153–1158. [Google Scholar] [CrossRef]
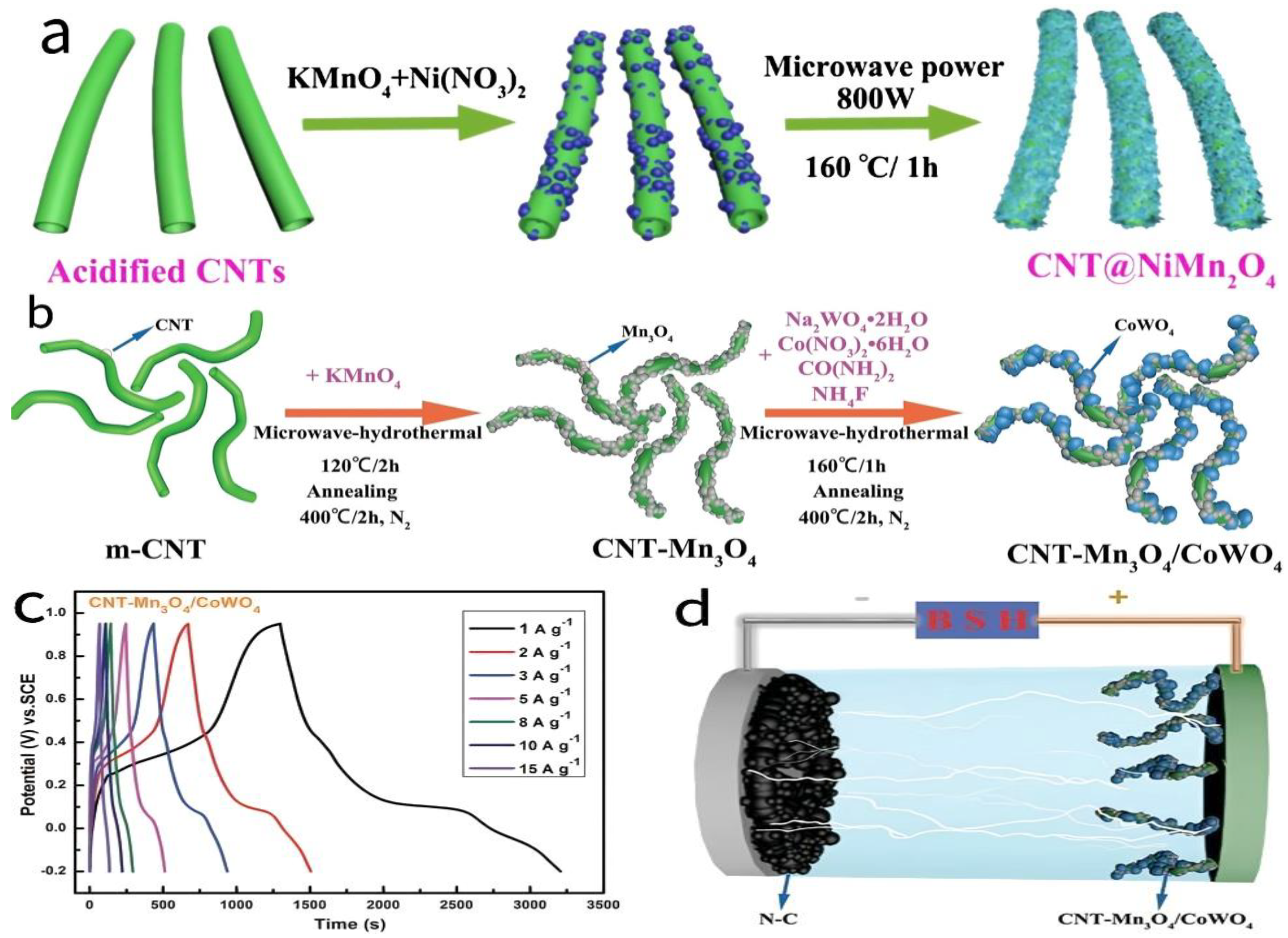
- Sun, Y.; Du, X.; Zhang, J.; Huang, N.; Yang, L.; Sun, X. Microwave-assisted preparation and improvement mechanism of carbon nanotube@NiMn2O4 core-shell nanocomposite for high performance asymmetric supercapacitors. J. Power Sources 2020, 473, 228609. [Google Scholar] [CrossRef]
- Park, S.K.; Sure, J.; Vishnu, D.S.M.; Jo, S.J.; Lee, W.C.; Ahmad, I.A.; Kim, H.-K. Nano-Fe3O4/Carbon Nanotubes Composites by One-Pot Microwave Solvothermal Method for Supercapacitor Applications. Energies 2021, 14, 2908. [Google Scholar] [CrossRef]
- Huang, N.; Sun, Y.; Liu, S.; Wang, X.; Zhang, J.; Guo, L.; Bi, J.; Sun, X. Microwave-Assisted Rational Designed CNT-Mn3O4/CoWO4 Hybrid Nanocomposites for High Performance Battery-Supercapacitor Hybrid Device. Small 2023, 19, 2300696. [Google Scholar] [CrossRef]
- Yetiman, S.; Peçenek, H.; Dokan, F.K.; Onses, M.S.; Yılmaz, E.; Sahmetlioglu, E. Microwave-assisted fabrication of high-performance supercapacitors based on electrodes composed of cobalt oxide decorated with reduced graphene oxide and carbon dots. J. Energy Storage 2022, 49, 104103. [Google Scholar] [CrossRef]
- Jung, M.; Sivakumar, P.; Park, H.S. Carbon nanotube branch-grown nickel nanoparticles/graphene composites for a high-capacitance electrode. J. Phys. Energy 2023, 5, 025005. [Google Scholar] [CrossRef]
- Appiagyei, A.B.; Anang, D.A.; Bonsu, J.O.; Asiedua-Ahenkorah, L.; Mane, S.D.; Kim, H.-S.; Bathula, C. Sucrose-directed porous carbon interfaced α-Fe2O3-rGO for supercapacitors. J. Electroanal. Chem. 2023, 936, 117383. [Google Scholar] [CrossRef]
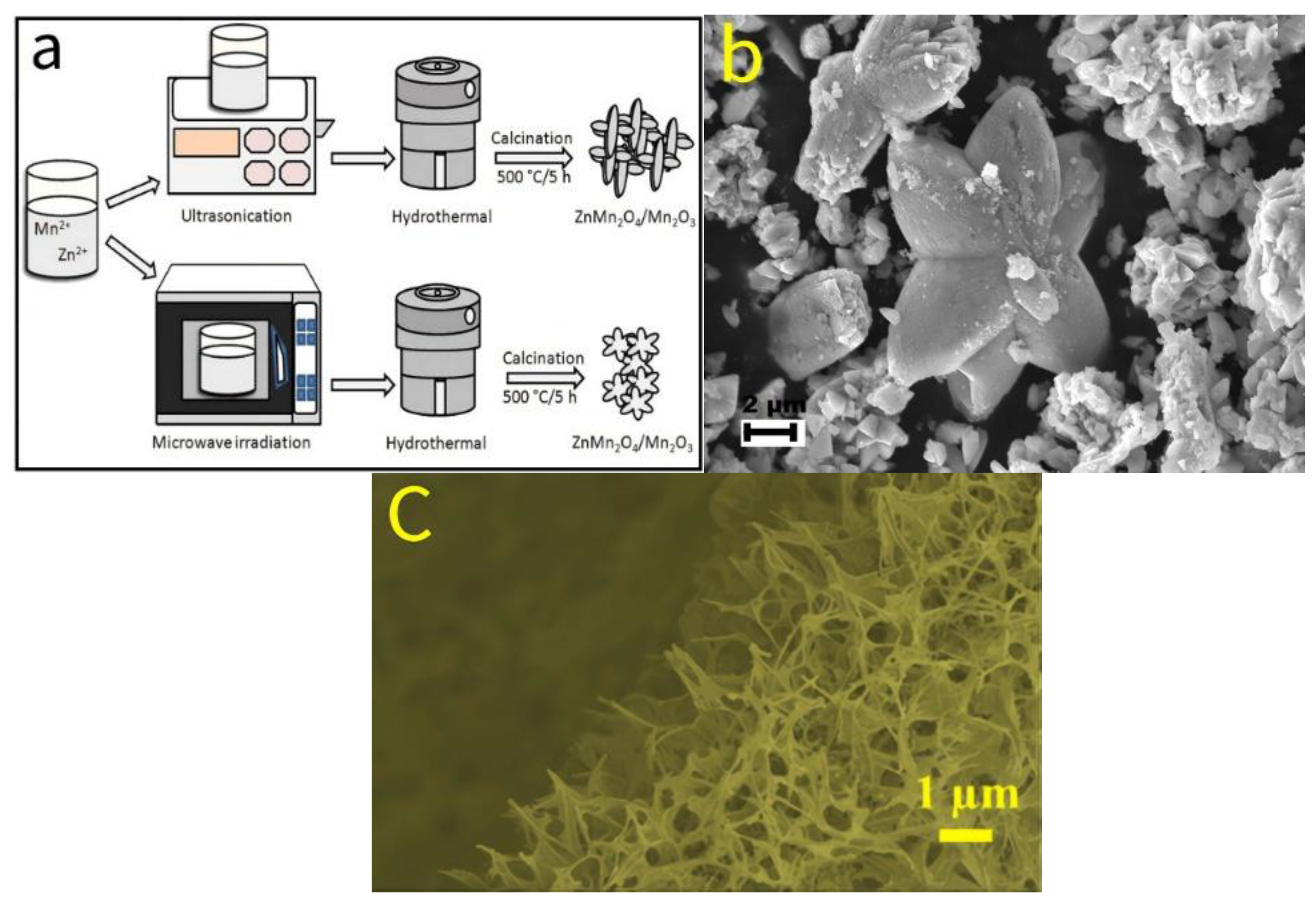
- Sannasi, V.; Subbian, K. A facile synthesis of ZnMn2O4/Mn2O3 composite nanostructures for supercapacitor applications. Ceram. Int. 2021, 47, 12300–12309. [Google Scholar] [CrossRef]
- Keshari, A.S.; Dubey, P. Rapid microwave-assisted vs. hydrothermal synthesis of hierarchical sheet-like NiO/NiMoO4 hybrid nanostructures for high performance extrinsic pseudocapacitor application. J. Energy Storage 2021, 40, 102629. [Google Scholar] [CrossRef]
- Ali, A.; Ammar, M.; Mukhtar, A.; Ahmed, T.; Ali, M.; Waqas, M.; Amin, M.N.; Rasheed, A. 3D NiO nanowires@NiO nanosheets core-shell structures grown on nickel foam for high performance supercapacitor electrode. J. Electroanal. Chem. 2020, 857, 113710. [Google Scholar] [CrossRef]



| Synthesis Methods | Advantages | Disadvantages |
|---|---|---|
| Electrochemical deposition | Large-scale production; low cost; precise control of film thickness, deposition rate, and uniformity | Set process; required current or voltage |
| Chemical vapor deposition (CVD) | Good film uniformity | Expensive equipment, relatively high cost |
| Sol–gel method | Low cost; the prepared products have a uniform particle size distribution, good dispersion, and high purity | Easily influenced by external factors; complex reaction process; difficult to control operation |
| Hydrothermal/solvothermal synthesis | Convenient; adjustable reaction parameters | Low thermal energy efficiency; poor product uniformity; low yield; wall effect |
| Liquid-phase co-precipitation method | Easy to operate; simple process steps; high product purity; reactions are easy to control | The products are prone to aggregation, leading to poor dispersion performance |
| Microwave-assisted method | Economical; simple; safe; controllable; environmentally friendly; energy-saving; rapid, uniform, and selective heating; efficient; short reaction time; high yield; uniform particle size distribution and high purity of the prepared products; good reproducibility | Requires a specialized microwave reactor; most processes require solvents; difficult to commercialize |
| Material | Material Type | Electrolyte | Specific Capacitance | Operating Voltage Window | Retention | Refs. |
|---|---|---|---|---|---|---|
| 3d Single-Metal Oxides | δ-MnO2-UVT | 1 M KOH | - | - | - | [15] |
| MnO2 | 1 M Na2SO4 | 207 F/g @ 10 mV/s | - | - | [16] | |
| δ-MnO2 | 2 M Na2SO4 | 68 F/g @ 10 mV/s | - | - | [17] | |
| α-Fe2O3 | 3 M KOH | 1253 F/g @ 3 mV/s | 0.75 V (−0.2~0.55 V vs. Ag/AgCl) | 93% (3000 cycles @ 3 mV/s) | [21] | |
| CoO | 6 M KOH | 728.8 F/g @ 1 A/g | 0.85 V (−0.3~0.55 V vs. SCE) | 82.3% (5000 cycles @ 15 A/g) | [26] | |
| CoCo2O4 | 6 M KOH | 875 F/g @ 1 A/g | 0.85 V (−0.3~0.55 V vs. SCE) | 89.7% (5000 cycles @ 15 A/g) | [27] | |
| Mn2CoCo2O4 | 6 M KOH | 1389 F/g @ 1 A/g | 1.1 V (−0.3~0.8 V vs. SCE) | 91.5% (5000 cycles @ 15 A/g) | [28] | |
| NiO | 6 M KOH | 270 F/g @ 1 A/g | - | - | [33] | |
| NiO | 1 M KOH | 87.7 F/g @ 10 mV/s | - | 85% (5000 cycles @ 0.5 A/g) | [35] | |
| 3d Binary Transition Metal Oxides | NiMn2O4 | 6 M KOH | 802 F/g @ 1 A/g | 0.8 V (−0.2~0.6 V vs. SCE) | 61% (5000 cycles @ 10 A/g) | [42] |
| NiMnO3 | 6 M KOH | 345.8 F/g @ 1 A/g | 0.8 V (−0.3~0.5 V vs. SCE) | 92% (1000 cycles @ 3 A/g) | [43] | |
| NiMn2O4 | 6 M KOH | 768.9 F/g @ 1 A/g | 0.7 V (−0.2~0.5 V vs. SCE) | 85.8% (6000 cycles @ 5 A/g) | [44] | |
| NiFe2O4 | - | - | - | - | [48] | |
| MnCo2O4 | 3 M KOH | 536 F/g @ 0.5 A/g | 0.5 V (0~0.5 V vs. SCE) | 96% (10,000 cycles @ 5 A/g) | [59] | |
| MnCo2O4 | 2 M KOH | 802 F/g @ 1 A/g | 0.6 V (−0.1~0.5 V vs. SCE) | 70% (1000 cycles @ 5 A/g) | [61] | |
| MnCo2O4 | 2 M KOH | 1053.5 F/g @ 1 A/g | 0.6 V (−0.1~0.5 V vs. SCE) | 90% (2000 cycles @ 8 A/g) | [60] | |
| MnCo2O4.5 | 6 M KOH | 497.8 F/g @ 1 A/g | 1.05 V (0~1.05 V vs. Hg/HgO) | 87.6% (5000 cycles @ 10 A/g) | [62] | |
| Carbon-Based Materials/3d Transition Metal Oxide Composite Materials | rGO@Fe3O4 | 1 M KOH | 771.3 F/g @ 5 mV/s | 1.0 V (−0.2~0.8 V vs. Ag/AgCl) | 95.1% (5000 cycles @ 0.9 A/g) | [73] |
| rGO@Fe3O4 | 1 M KOH | 471 F/g @ 10 mV/s | 0.8 V (−0.2~0.6 V vs. Ag/AgCl) | 96.5% (1000 cycles @ 40 mV/s) | [72] | |
| N-rGO@CoO | 1 M KOH | 744.1 F/g @ 5 mV/s | 0.8 V (−0.2~0.6 V vs. Ag/AgCl) | 91.3% (5000 cycles @ 70 mV/s) | [74] | |
| CNT@NiMn2O4 | 6 M KOH | 915.6 F/g @ 1 A/g | 0.8 V (−0.2~0.6 V vs. SCE) | 93% (5000 cycles @ 5 A/g) | [78] | |
| Fe3O4/CNT | 1 M Na2SO3 | 187.1 F/g @ 1 A/g | 1.0 V (−1.0~0 V vs. SCE) | 80.2% (1000 cycles @ 1 A/g) | [79] | |
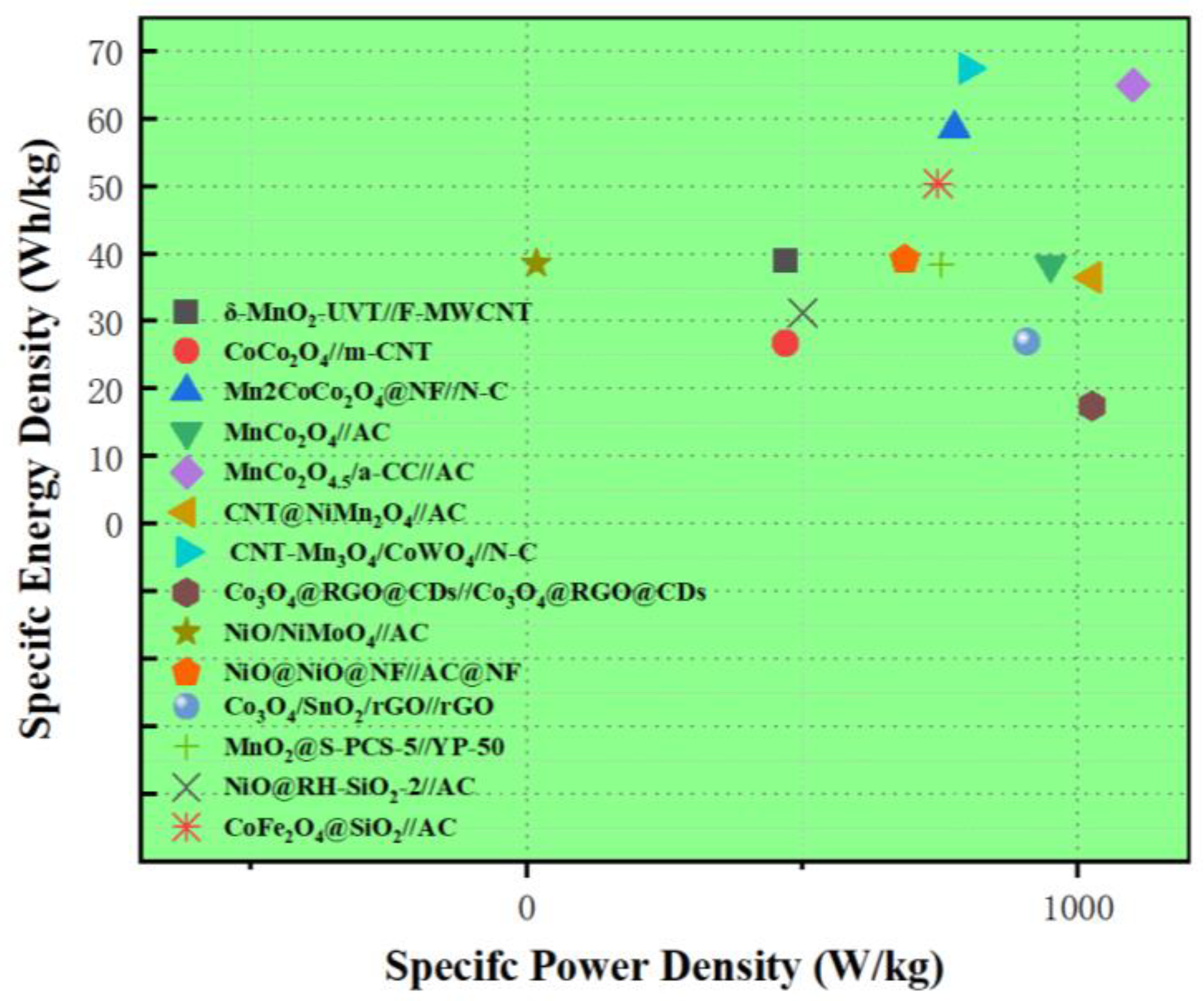
| CNT-Mn3O4/CoWO4 | - | 1658.7 F/g @ 1 A/g | 1.15 V (−0.2~0.95 V vs. SCE) | 117.2% (13,000 cycles @ 5 A/g) | [80] | |
| Co3O4@rGO@CDs | 2 M KOH | 936 F/g @ 0.5 A/g | 0.7 V (−0.1~0.6 V vs. Ag/AgCl) | 88% (10,000 cycles @ 10 A/g) | [81] | |
| NiO@srGO/CNT | 6 M KOH | 1605.81 F/g @ 1 A/g | 0.6 V (−0.1~0.5 V vs. SCE) | 71.56% (10,000 cycles @ 1 A/g) | [82] | |
| rGO/su-GC@Fe2O3 | 2 M Na2SO4 | 1978 F/g @ 1 A/g | 1.0 V (−0.8~0.2 V vs. Ag/AgCl) | 95.2% (8000 cycles @ 10 A/g) | [83] | |
| Transition Metal Oxides/3d Transition Metal Oxide Composite Materials | ZnMn2O4/Mn2O3 | 2 M KOH | 380 F/g @ 0.5 A/g | 0.6 V (0~0.6 V vs. Hg/HgO) | 92% (2000 cycles @ 3 A/g) | [84] |
| NiO/NiMoO4 | 6 M KOH | 1147.5 F/g @ 1 A/g | 0.5 V (0~0.5 V vs. Ag/AgCl) | - | [85] | |
| NiO nanowires@NiO nanosheets | 3 M KOH | 1782 F/g @ 1 A/g | 0.6 V (0~0.6 V vs. RHE) | 70.33% (7000 cycles @ 1 A/g) | [86] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, C.; Ge, M.; Tan, S.; Liu, Y.; Wang, H.; Jiang, W.; Zhang, S.; Sun, Y. Research Progress on Microwave Synthesis of 3d Transition Metal (Mn, Fe, Co, and Ni) Oxide Nanomaterials for Supercapacitors. Molecules 2025, 30, 1843. https://doi.org/10.3390/molecules30081843
Sun C, Ge M, Tan S, Liu Y, Wang H, Jiang W, Zhang S, Sun Y. Research Progress on Microwave Synthesis of 3d Transition Metal (Mn, Fe, Co, and Ni) Oxide Nanomaterials for Supercapacitors. Molecules. 2025; 30(8):1843. https://doi.org/10.3390/molecules30081843
Chicago/Turabian StyleSun, Chengqi, Maosheng Ge, Shuhuang Tan, Yichen Liu, Haowei Wang, Wenhao Jiang, Shoujun Zhang, and Yin Sun. 2025. "Research Progress on Microwave Synthesis of 3d Transition Metal (Mn, Fe, Co, and Ni) Oxide Nanomaterials for Supercapacitors" Molecules 30, no. 8: 1843. https://doi.org/10.3390/molecules30081843
APA StyleSun, C., Ge, M., Tan, S., Liu, Y., Wang, H., Jiang, W., Zhang, S., & Sun, Y. (2025). Research Progress on Microwave Synthesis of 3d Transition Metal (Mn, Fe, Co, and Ni) Oxide Nanomaterials for Supercapacitors. Molecules, 30(8), 1843. https://doi.org/10.3390/molecules30081843




