Fermentation of Pediococcus pentosaceus JC30 Improves Phytochemical, Flavor Characteristics and Antioxidant Activity of Mulberry Leaves
Abstract
1. Introduction
2. Results and Discussion
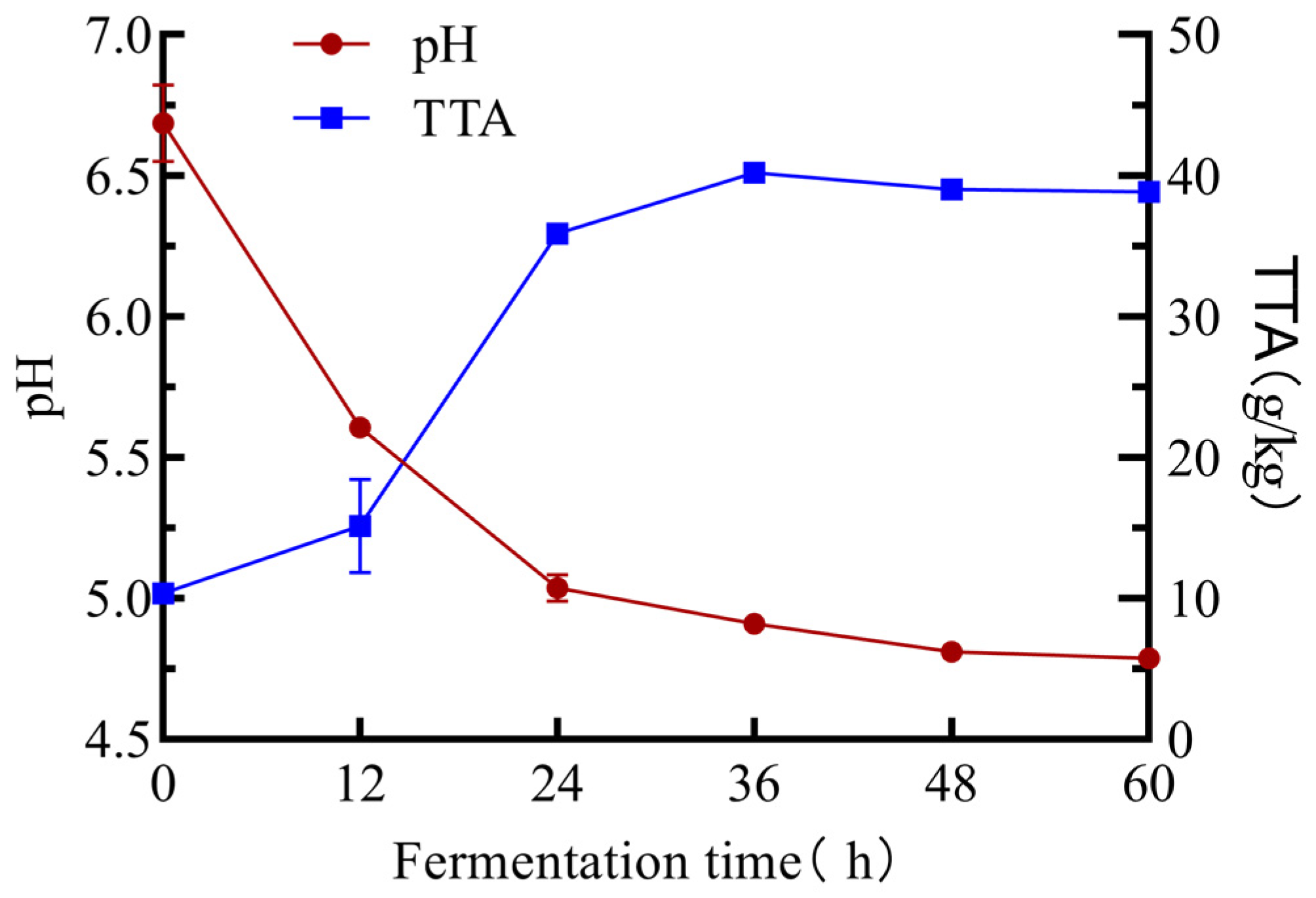
2.1. Analysis of pH, Total Titratable Acidity, and Organic Acids
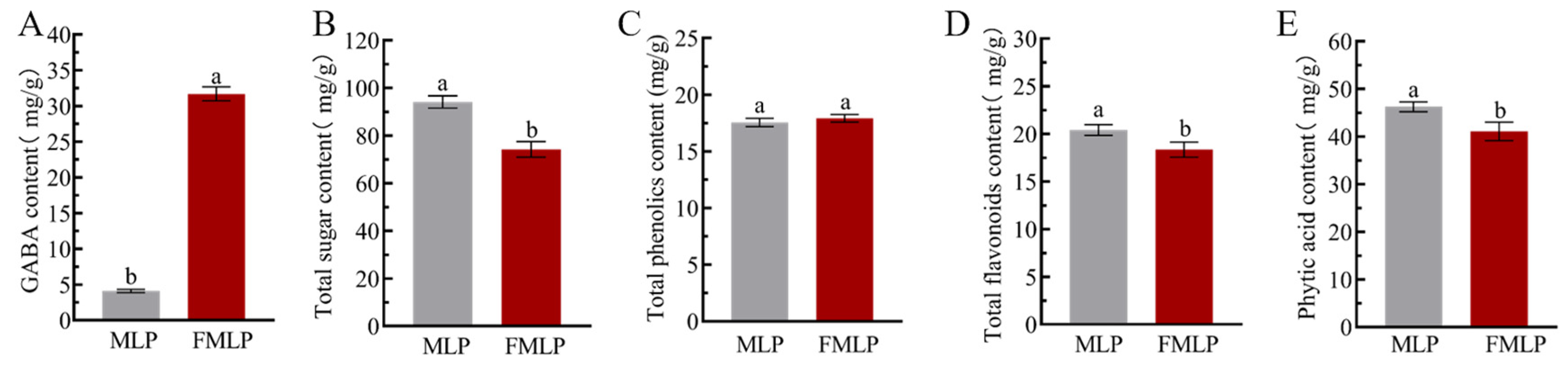
2.2. Changes in Chemical Compositions Contents
2.3. Qualitative Analysis of Free and Bound Phenolic Compounds from Mulberry Leaf Powder
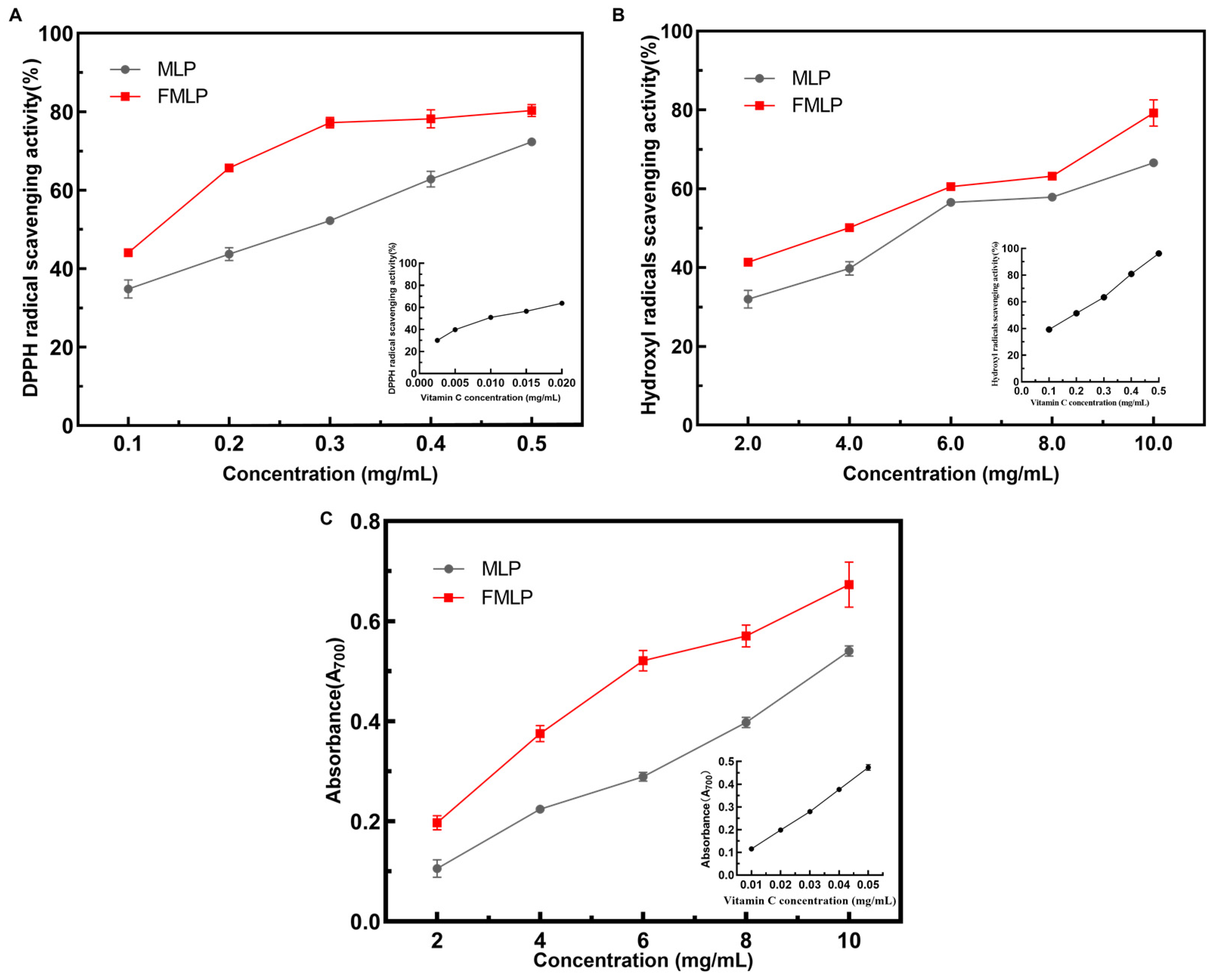
2.4. Effects of Solid-State Fermentation (SSF) on the Antioxidant Activities of Mulberry Leaves
2.5. Changes in Free Amino Acid (FAA) Content
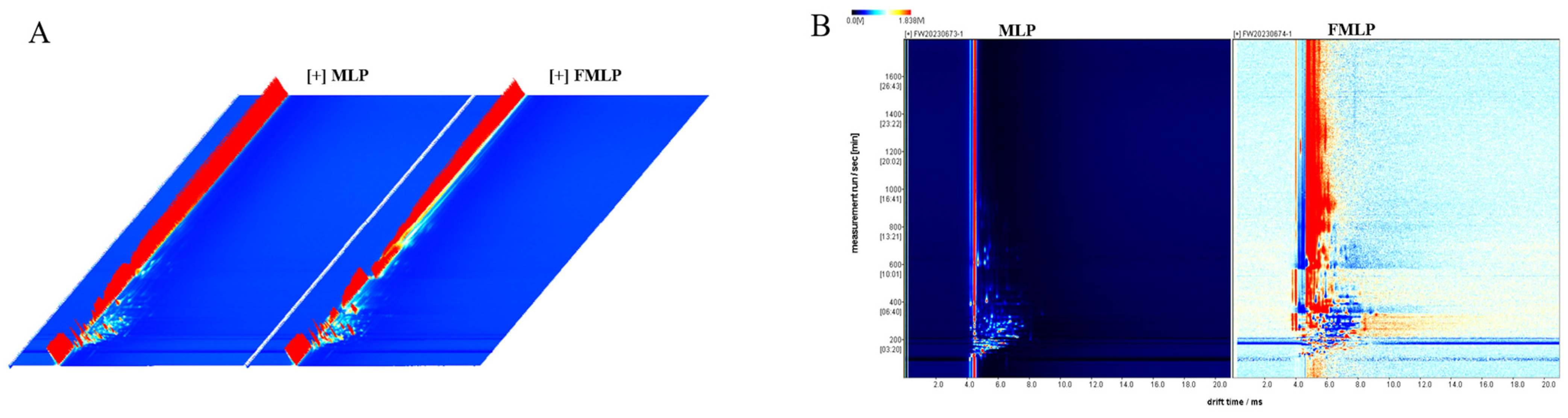
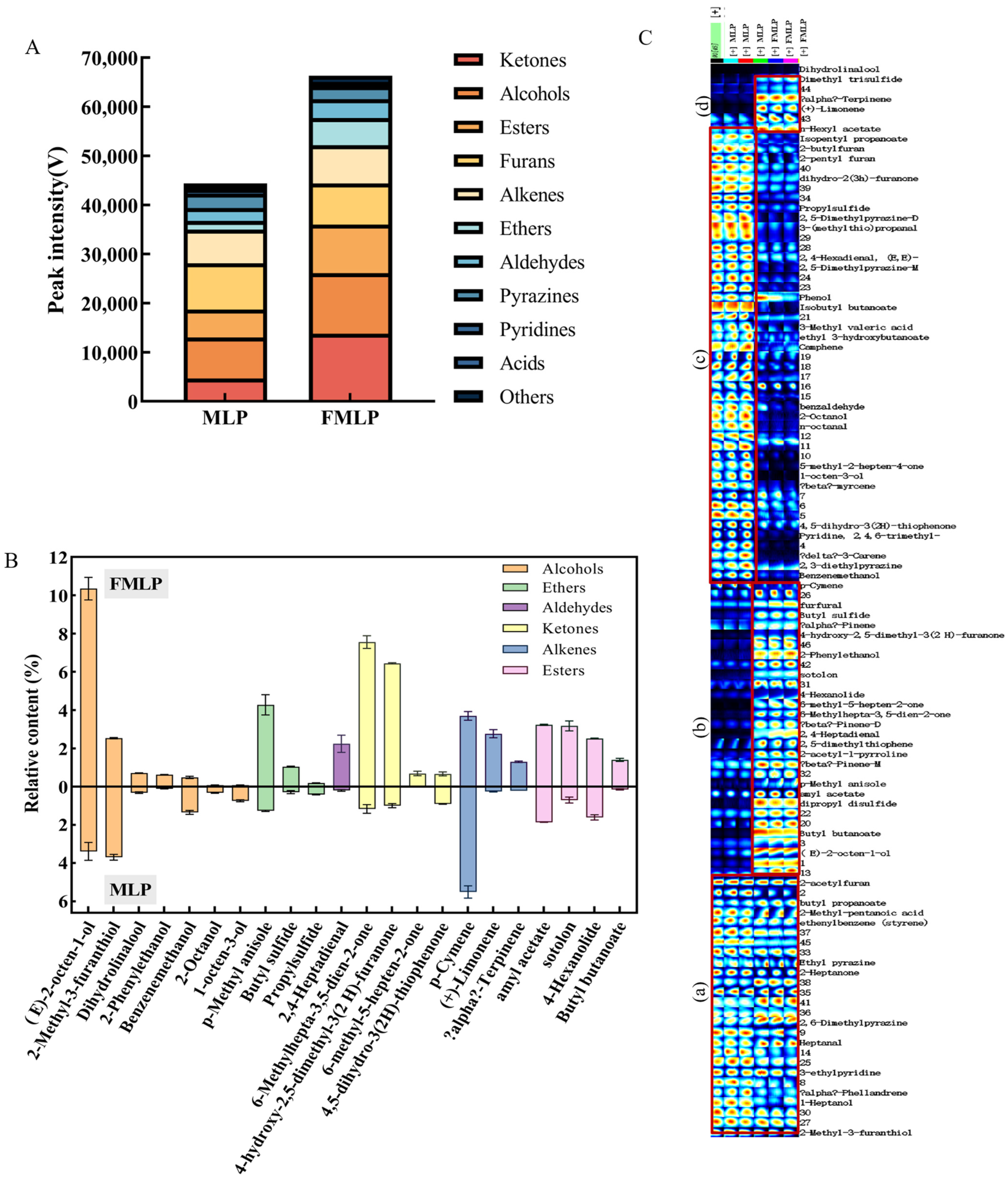
2.6. Changes in Volatile Compounds
3. Materials and Methods
3.1. Chemicals and Materials
3.2. SSF of Mulberry Leaf Powder
3.3. Determination of pH and Total Titratable Acidity
3.4. Determination of Organic Acids
3.5. Determination of GABA, Total Sugar, Total Phenolic, and Total Flavonoid Contents
3.6. Determination of Phytic Acid Contents
3.7. Analysis of Phenolic Compounds of Mulberry Leaf Powder
3.7.1. Extraction of Free Phenolic Compounds from Mulberry Leaf Powder
3.7.2. Extraction of Bound Phenolic Compounds from Mulberry Leaf Powder
3.7.3. UPLC-Q-TOF-MS Analysis
3.8. Evaluation of Antioxidant Activities
3.8.1. DPPH Radical Scavenging Assay
3.8.2. Hydroxyl Radical Scavenging Assay
3.8.3. Total Reducing Power Assay
3.9. Determination of FAAs and Evaluation of Nutritional Value
3.9.1. Determination of FAAs
3.9.2. Evaluation of Nutritional Value of Amino Acids
3.10. Flavor Analysis of Mulberry Leaf Powder
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GABA | γ-aminobutyric acid |
| LAB | Lactic acid bacteria |
| P. pentosaceus | Pediococcus pentosaceus |
| SSF | Solid-state fermentation |
| FMLP | Fermented mulberry leaf powder |
| MLP | Non-fermented mulberry leaf powder |
| TTA | Total titratable acidity |
| TPC | Total phenolic content |
| TFC | Total flavonoid content |
| DPPH | 1,1-Diphenyl-2-picrylhydrazyl free radical |
| •OH | Hydroxyl radical |
| TRP | Total reducing power |
| BPs | Bound phenolics |
| FPs | Free phenolics |
| FAAs | Free amino acids |
| AAS | Amino acid score |
| CS | Chemical score |
| EAAI | Essential amino acid index |
| TAAs | Total amino acids |
| EAAs | Essential l amino acids |
| NEAAs | Non-essential amino acids |
References
- Li, R.; Wang, C.; Chen, Y.; Li, N.; Wang, Q.; Zhang, M.; He, C.; Chen, H. A combined network pharmacology and molecular biology approach to investigate the active ingredients and potential mechanisms of mulberry (Morus alba L.) leaf on obesity. Phytomedicine 2021, 92, 153714. [Google Scholar] [CrossRef]
- Maqsood, M.; Anam Saeed, R.; Sahar, A.; Khan, M.I. Mulberry plant as a source of functional food with therapeutic and nutritional applications: A review. J. Food Biochem. 2022, 46, e14263. [Google Scholar] [CrossRef]
- Gao, T.Q.; Chen, J.L.; Xu, F.; Wang, Y.L.; Zhao, P.P.; Ding, Y.F.; Han, Y.B.; Yang, J.; Tao, Y. Mixed mulberry fruit and mulberry leaf fermented alcoholic beverages: Assessment of chemical composition, antioxidant capacity in vitro and sensory evaluation. Foods 2022, 11, 3125. [Google Scholar] [CrossRef]
- Wang, N.W.; Xiong, Y.; Wang, X.K.; Guo, L.N.; Lin, Y.L.; Ni, K.K.; Yang, F.Y. Effects of Lactobacillus plantarum on fermentation quality and anti-nutritional factors of paper mulberry silage. Fermentation 2022, 8, 144. [Google Scholar] [CrossRef]
- Chuah, H.Q.; Tang, P.L.; Ang, N.J.; Tan, H.Y. Submerged fermentation improves bioactivity of mulberry fruits and leaves. Chin. Herb. Med. 2021, 13, 565–572. [Google Scholar] [CrossRef]
- Guan, X.F.; Zhao, D.Z.; Yu, T.; Liu, S.Q.; Chen, S.Y.; Huang, J.Y.; Lai, G.T.; Lin, B.; Huang, J.Q.; Lai, C.C.; et al. Phytochemical and flavor characteristics of mulberry juice fermented with Lactiplantibacillus plantarum BXM2. Foods 2024, 13, 2648. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, X.; Wang, C.; He, L.; Zhou, W.; Yang, F.; Zhang, Q. The bacterial community and fermentation quality of mulberry (Morus alba) leaf silage with or without Lactobacillus casei and sucrose. Bioresour. Technol. 2019, 293, 122059. [Google Scholar] [CrossRef]
- Tang, S.X.; Cheng, Y.X.; Xu, T.T.; Wu, T.; Pan, S.Y.; Xu, X.Y. Hypoglycemic effect of Lactobacillus plantarum-fermented mulberry pomace extract in vitro and in Caenorhabditis elegans. Food Funct. 2023, 14, 9253–9264. [Google Scholar] [CrossRef]
- Xiao, Y.Y.; Liu, Z.Q.; Li, P.C.; Wang, Y.B.; Wang, X.J.; Piao, C.H.; Yuan, L.H. Lipid-lowering capacity of GABA-rich supernatant from fermented okara in OA-induced HepG2 cells. Food Biosci. 2024, 58, 103659. [Google Scholar] [CrossRef]
- Chen, W.; Xie, C.; He, Q.; Sun, J.; Bai, W. Improvement in color expression and antioxidant activity of strawberry juice fermented with lactic acid bacteria: A phenolic-based research. Food Chem. X 2023, 17, 100535. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Cai, L.; Lv, L.; Li, L. Pediococcus pentosaceus, a future additive or probiotic candidate. Microb. Cell Factories 2021, 20, 45. [Google Scholar] [CrossRef]
- Kuppusamy, P.; Kim, D.; Soundharrajan, I.; Park, H.S.; Jung, J.S.; Yang, S.H.; Choi, K.C. Low-Carbohydrate Tolerant LAB Strains Identified from Rumen Fluid: Investigation of Probiotic Activity and Legume Silage Fermentation. Microorganisms 2020, 8, 1044. [Google Scholar] [CrossRef]
- Jin, D.X.; Jin, Y.X.; Zhang, W.; Cao, W.; Liu, R.; Wu, M.G.; Ge, Q.F.; Yu, H. Exploring Jiangshui-originated probiotic lactic acid bacteria as starter cultures: Functional properties and fermentation performances in pear juice. Food Biosci. 2024, 61, 104982. [Google Scholar] [CrossRef]
- Xuan, J.; Han, X.; Che, J.; Zhuo, J.; Xu, J.; Lu, J.; Mu, H.; Wang, J.; Tu, J.; Liu, G. Production of γ–aminobutyric acid–enriched sourdough bread using an isolated Pediococcus pentosaceus strain JC30. Heliyon 2024, 10, e31236. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Y.; Zhang, H.X.; Lei, H.J. Phenolics profile, antioxidant activity and flavor volatiles of pear juice: Influence of lactic acid fermentation using three Lactobacillus Strains in monoculture and binary mixture. Foods 2021, 11, 11. [Google Scholar] [CrossRef]
- Markkinen, N.; Laaksonen, O.; Nahku, R.; Kuldjärv, R.; Yang, B. Impact of lactic acid fermentation on acids, sugars, and phenolic compounds in black chokeberry and sea buckthorn juices. Food Chem. 2019, 286, 204–215. [Google Scholar] [CrossRef]
- Zhou, M.; Wu, J.; Wu, L.; Sun, X.; Chen, C.; Huang, L. The utilization of N-acetylgalactosamine and its effect on the metabolism of amino acids in Erysipelotrichaceae strain. BMC Microbiol. 2024, 24, 397. [Google Scholar] [CrossRef]
- Ngo, D.-H.; Vo, T.S. An Updated Review on Pharmaceutical Properties of Gamma-Aminobutyric Acid. Molecules 2019, 24, 2678. [Google Scholar] [CrossRef]
- Zhong, Y.; Wu, S.; Chen, F.; He, M.; Lin, J. Isolation of high γ-aminobutyric acid-producing lactic acid bacteria and fermentation in mulberry leaf powders. Exp. Ther. Med. 2019, 18, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.K.; Xi, M.M.; Gao, F.; Li, M.; Dong, T.W.; Geng, Z.X.; Liu, C.Y.; Huang, F.Y.; Wang, J.; Li, X.Y.; et al. Evaluation of mulberry leaves’ hypoglycemic properties and hypoglycemic mechanisms. Front. Pharmacol. 2023, 14, 1045309. [Google Scholar] [CrossRef]
- Adebo, O.A.; Gabriela Medina-Meza, I. Impact of Fermentation on the Phenolic Compounds and Antioxidant Activity of Whole Cereal Grains: A Mini Review. Molecules 2020, 25, 927. [Google Scholar] [CrossRef] [PubMed]
- Li, M.Y.; Xu, X.X.; Jia, Y.F.; Yuan, Y.; Na, G.; Zhu, L.; Xiao, X.W.; Zhang, Y.M.; Ye, H.Q. Transformation of mulberry polyphenols by Lactobacillus plantarum SC-5: Increasing phenolic acids and enhancement of anti-aging effect. Food Res. Int. 2024, 192, 114778. [Google Scholar] [CrossRef]
- Leonard, W.; Zhang, P.Z.; Ying, D.Y.; Adhikari, B.; Fang, Z.X. Fermentation transforms the phenolic profiles and bioactivities of plant-based foods. Biotechnol. Adv. 2021, 49, 107763. [Google Scholar] [CrossRef]
- Du, J.; Li, X.; Liu, N.; Wang, Y.; Li, Y.; Jia, Y.; An, X.; Qi, J. Improving the quality of Glycyrrhiza stems and leaves through solid-state fermentation: Flavonoid content, antioxidant activity, metabolic profile, and release mechanism. Chem. Biol. Technol. Agric. 2024, 11, 1–14. [Google Scholar] [CrossRef]
- Fang, L.P.; Wang, W.J.; Dou, Z.X.; Chen, J.; Meng, Y.C.; Cai, L.Q.; Li, Y.H. Effects of mixed fermentation of different lactic acid bacteria and yeast on phytic acid degradation and flavor compounds in sourdough. Lwt 2023, 174, 114438. [Google Scholar] [CrossRef]
- Augustine, C. Unravelling the Competence of Leucocyanidin in Free Radical Scavenging: A Theoretical Approach Based on Electronic Structure Calculations. J. Struct. Chem. 2019, 60, 198–209. [Google Scholar] [CrossRef]
- Ospina-Posada, A.C.; Porras, O.; Rincón-Cervera, M.A.; Frias, J.; Zielinski, A.A.F.; Bridi, R.; Arias-Santé, M.F.; de Camargo, A.C. Antioxidant properties of phenolic extracts of murtilla pomace: First report on the importance of soluble and insoluble-bound compounds. Food Res. Int. 2024, 196, 115114. [Google Scholar] [CrossRef]
- Zhao, J.R.; Wang, X.H.; Wang, Y.C.; Lv, G.F.; Lin, H.; Lin, Z. UPLC-MS/MS profiling, antioxidant and anti-inflammatory activities, and potential health benefits prediction of phenolic compounds in hazel leaf. Front. Nutr. 2023, 10, 1092071. [Google Scholar] [CrossRef]
- Degrain, A.; Manhivi, V.; Remize, F.; Garcia, C.; Sivakumar, D. Effect of Lactic Acid Fermentation on Color, Phenolic Compounds and Antioxidant Activity in African Nightshade. Microorganisms 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Zhu, Y.W.; Jiang, Y.W.; Li, H.K.; Liu, Z.M.; Wang, W.; Shan, C.H.; Fu, Y.J. Improvement of flavonoid aglycone and biological activity of mulberry leaves by solid-state fermentation. Ind. Crops Prod. 2020, 148, 112287. [Google Scholar] [CrossRef]
- Akhter, S.; Arman, M.S.I.; Tayab, M.A.; Islam, M.N.; Xiao, J. Recent advances in the biosynthesis, bioavailability, toxicology, pharmacology, and controlled release of citrus neohesperidin. Crit. Rev. Food Sci. Nutr. 2022, 64, 5073–5092. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Shen, L.; Li, Y.; Wu, S.; Zhang, X.; Yang, X. Non-targeted metabolomic analysis reveals the mechanism of quality formation of citrus flower-green tea. J. Sci. Food Agric. 2024, 104, 5807–5815. [Google Scholar] [CrossRef]
- Silva, F.; Veiga, F.; Cardoso, C.; Dias, F.; Cerqueira, F.; Medeiros, R.; Cláudia Paiva-Santos, A. A rapid and simplified DPPH assay for analysis of antioxidant interactions in binary combinations. Microchem. J. 2024, 202, 110801. [Google Scholar] [CrossRef]
- Lipinski, B. Hydroxyl Radical and Its Scavengers in Health and Disease. Oxidative Med. Cell. Longev. 2011, 2011, 1–9. [Google Scholar] [CrossRef]
- Tan, J.; Hua, X.; Liu, J.; Wang, M.; Liu, Y.; Yang, R.; Cao, Y. Extraction of sunflower head pectin with superfine grinding pretreatment. Food Chem. 2020, 320, 126631. [Google Scholar] [CrossRef]
- Deekshith, C.; Jois, M.; Radcliffe, J.; Thomas, J. Effects of culinary herbs and spices on obesity: A systematic literature review of clinical trials. J. Funct. Foods 2021, 81, 104449. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Zhao, Q.N.; Yan, X.H.; Yue, Y.; Yue, T.L.; Yuan, Y.H. Improved flavonoid content in mulberry leaves by solid-state fermentation: Metabolic profile, activity, and mechanism. Innov. Food Sci. Emerg. Technol. 2023, 84, 103308. [Google Scholar] [CrossRef]
- Yang, X.H.; Hong, J.Q.; Wang, L.H.; Cai, C.Y.; Mo, H.P.; Wang, J.; Fang, X.; Liao, Z.L. Effect of lactic acid bacteria fermentation on pant-based products. Fermentation 2024, 10, 48. [Google Scholar] [CrossRef]
- Rehman, S.U.; Ali, R.; Zhang, H.; Zafar, M.H.; Wang, M. Research progress in the role and mechanism of Leucine in regulating animal growth and development. Front. Physiol. 2023, 14, 1252089. [Google Scholar] [CrossRef]
- Liao, S.F.; Wang, T.; Regmi, N. Lysine nutrition in swine and the related monogastric animals: Muscle protein biosynthesis and beyond. SpringerPlus 2015, 4, 147. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.-Y.; Guo, Y.; Hu, X.-Y.; Liu, W.-H.; Liu, B.-Q.; Yang, J.; Tu, Z.-C.; Huang, Y.-H. Flavor improvement of fermented soybean foods by co-fermentation with Bacillus velezensis and Lactiplantibacillus plantarum. Lwt 2023, 186, 115257. [Google Scholar] [CrossRef]
- Ding, Y.; Cai, M.; Niu, P.; Zhang, H.; Zhang, S.-Q.; Sun, Y. Ultrashort peptides induce biomineralization. Compos. Part B Eng. 2022, 244, 110196. [Google Scholar] [CrossRef]
- FAO/WHO. Energy and Protein Requirements: Report of a Joint FAO/WHO ad Hoc Expert Committee; FAO/WHO, Ed.; World Health Organization: Geneva, Switzerland, 1973. [Google Scholar]
- Ochiai, M.; Suzuki, Y.; Suzuki, R.; Iwata, K.; Murayama, M. Low protein digestibility-corrected amino acid score and net nitrogen-to-protein conversion factor value of edible insects. Food Chem. 2024, 454, 139781. [Google Scholar] [CrossRef]
- Li, C.; Al-Dalali, S.; Wang, Z.; Xu, B.; Zhou, H. Investigation of volatile flavor compounds and characterization of aroma-active compounds of water-boiled salted duck using GC–MS–O, GC–IMS, and E-nose. Food Chem. 2022, 386, 132728. [Google Scholar] [CrossRef]
- Yaqoob, S.; Imtiaz, A.; Awan, K.A.; Murtaza, M.S.; Mubeen, B.; Yinka, A.A.; Boasiako, T.A.; Alsulami, T.; Rehman, A.; Khalifa, I.; et al. Impact of fermentation through synergistic effect of different lactic acid bacteria (mono and co-cultures) on metabolic and sensorial profile of mulberry juice. J. Food Meas. Charact. 2024, 18, 9364–9384. [Google Scholar] [CrossRef]
- Zhuo, J.; Xuan, J.; Chen, Y.; Tu, J.; Mu, H.R.; Wang, J.; Liu, G.H. Increase of γ-aminobutyric acid content and improvement of physicochemical characteristics of mulberry leaf powder by fermentation with a selected lactic acid bacteria strain. Lwt 2023, 187, 115250. [Google Scholar] [CrossRef]
- GB 12456-2021; Food Safety National Standard—Determination of Total Acids in Foods. China State Administration for Market Regulation and National Health Commission: Beijing, China, 2021.
- Jiang, J.; Yin, R.; Xie, Y.; Ma, X.; Cui, M.; Chen, Y.; Li, Y.; Hu, Y.; Niu, J.; Cheng, W.; et al. Effects of cofermentation of Saccharomyces cerevisiae and different lactic acid bacteria on the organic acid content, soluble sugar content, biogenic amines, phenol content, antioxidant activity and aroma of prune wine. Food Chem. X 2024, 22, 101502. [Google Scholar] [CrossRef]
- Jin, Y.C.; Tu, J.; Han, X.Y.; Zhuo, J.; Liu, G.H.; Han, Y.H.; Du, H.J.; Wang, J.; Xiao, H. Characteristics of mulberry leaf powder enriched with γ-aminobutyric acid and its antioxidant capacity as a potential functional food ingredient. Front. Nutr. 2022, 18, 900718. [Google Scholar] [CrossRef]
- Eom, T.; Ko, G.; Kim, K.C.; Kim, J.-S.; Unno, T. Dendropanax morbifera Leaf Extracts Improved Alcohol Liver Injury in Association with Changes in the Gut Microbiota of Rats. Antioxidants 2020, 9, 911. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Liu, G.; Jin, Y.; Tang, C.; Yao, T.; Zhuo, J.; Li, Q.; Liu, L.; Wang, J. Enrichment of γ-aminobutyric acid in mulberry leaves and the inhibitory effects of the water extract on ACE and α-glucosidase activity. Ind. Crops Prod. 2022, 177, 114485. [Google Scholar] [CrossRef]
- Kahrıman, F.; Songur, U.; Şerment, M.; Akbulut, Ş.; Egesel, C.Ö. Comparison of colorimetric methods for determination of phytic acid content in raw and oil extracted flour samples of maize. J. Food Compos. Anal. 2020, 86, 103380. [Google Scholar] [CrossRef]
- Wang, Z.; Tang, C.; Dai, F.; Xiao, G.; Luo, G. HPLC determination of phenolic compounds in different solvent extracts of mulberry leaves and antioxidant capacity of extracts. Int. J. Food Prop. 2021, 24, 544–552. [Google Scholar] [CrossRef]
- Xiao, Y.; Rui, X.; Xing, G.; Wu, H.; Li, W.; Chen, X.; Jiang, M.; Dong, M. Solid state fermentation with Cordyceps militaris SN-18 enhanced antioxidant capacity and DNA damage protective effect of oats (Avena sativa L.). J. Funct. Foods 2015, 16, 58–73. [Google Scholar] [CrossRef]
- GB/T 30987-2020; Determination of free amino acids in plant. Standard Press of China: Beijing, China, 2020.
- Oser, B.L. CHAPTER 10—An Integrated Essential Amino Acid Index for Predicting the Biological Value of Proteins. In Protein and Amino Acid Nutrition; Oser, B.L., Ed.; Academic Press: Cambridge, MA, USA, 1959; pp. 281–295. [Google Scholar] [CrossRef]

| Organic Acids | MLP | FMLP |
|---|---|---|
| Lactic acid | ND | 15.79 ± 0.40 a |
| Acetic acid | 0.68 ± 0.04 b | 4.02 ± 0.22 a |
| Citric acid | 0.19 ± 0.00 b | 2.44 ± 0.09 a |
| Malic acid | 2.86± 0.31 a | 0.13 ± 0.05 b |
| Tartaric acid | 0.11 ± 0.00 b | 0.98 ± 0.12 a |
| Pyruvic acid | 0.01 ± 0.00 b | 0.22 ± 0.10 a |
| Oxalic acid | 5.38 ± 0.17 a | 5.60 ± 0.33 a |
| Pyroglutamic acid | 3.33 ± 0.23 a | 0.23 ± 0.03 b |
| Succinic acid | 3.88 ± 0.44 a | 0.79 ± 0.13 b |
| Name | Relative Content (%) | |||
|---|---|---|---|---|
| BPs (MLP) | FPs (MLP) | BPs (FMLP) | FPs (FMLP) | |
| 3,4-Dihydroxycinnamic acid | 2.10 ± 0.57 | 2.17 ± 0.16 | 0.53 ± 0.03 | 2.88 ± 0.12 |
| Arundinin | 0.15 ± 0.01 | 0.31 ± 0.04 | ND | 0.09 ± 0.00 |
| Chlorogenic acid | ND | 0.22 ± 0.02 | ND | 0.19 ± 0.02 |
| Dihydrocoumarin | 0.02 ± 0.00 | 0.76 ± 0.05 | 0.01 ± 0.00 | 0.32 ± 0.02 |
| Ellagic acid_1 | 0.07 ± 0.01 | 0.09 ± 0.01 | ND | 3.76 ± 0.00 |
| Gallic acid | 0.23 ± 0.03 | 0.47 ± 0.02 | 0.10 ± 0.02 | 0.21 ± 0.01 |
| Hesperidin | 0.06 ± 0.01 | 0.29 ± 0.01 | 0.08 ± 0.02 | 0.03 ± 0.00 |
| Leucocyanidin | 1.69 ± 0.02 | 0.03 ± 0.01 | 0.37 ± 0.01 | 3.71 ± 0.01 |
| Myricetin | 0.26 ± 0.02 | 0.36 ± 0.03 | 0.01 ± 0.00 | 10.44 ± 0.02 |
| Naringenin | 0.05 ± 0.02 | 0.62 ± 0.02 | ND | 0.03 ± 0.01 |
| Neohesperidin | 6.70 ± 0.03 | 3.10 ± 0.82 | ND | 5.65 ± 0.01 |
| Procyanidin B6 | ND | 0.07 ± 0.01 | ND | 0.30 ± 0.01 |
| Protocatechuic acid | 16.03 ± 0.01 | 0.11 ± 0.00 | 0.14 ± 0.01 | 0.81 ± 0.01 |
| Quercetin | 12.60 ± 0.03 | 3.19 ± 0.03 | ND | 40.55 ± 0.01 |
| Rosavin | 0.29 ± 0.01 | 0.05 ± 0.01 | 0.02 ± 0.00 | 0.51 ± 0.01 |
| Parameter (mg/100 g) | MLP | FMLP |
|---|---|---|
| Lysine (Lys) | 17.32 ± 0.07 b | 77.76 ± 0.08 a |
| Phenylalanine (Phe) | 12.90 ± 0.16 b | 58.00 ± 0.61 a |
| Methionine (Met) | ND | 18.73 ± 0.10 a |
| Threonine (Thr) | 36.58 ± 0.93 b | 51.23 ± 0.28 a |
| Leucine (Leu) | 9.97 ± 0.10 b | 83.81 ± 0.18 a |
| Isoleucine (Ile) | 10.55 ± 0.11 b | 44.60 ± 0.07 a |
| Valine (Val) | 29.79 ± 0.03 b | 90.90 ± 0.10 a |
| Histidine (His) | 12.48 ± 0.05 b | 16.42 ± 0.83 a |
| Arginine (Arg) | 26.02 ± 0.40 a | 4.12 ± 0.00 b |
| Glycine (Gly) | 11.05 ± 0.01 b | 97.56 ± 0.12 a |
| Alanine (Ala) | 33.42 ± 0.15 b | 157.30 ± 0.45 a |
| Proline (Pro) | 119.63 ± 0.97 b | 106.94 ± 0.80 a |
| Tyrosine (Tyr) | ND | 22.94 ± 0.50 a |
| Serine (Ser) | 11.21 ± 0.02 b | 17.52 ± 0.18 a |
| Aspartic acid (Asp) | 54.59 ± 0.04 b | 143.84 ± 0.13 a |
| Cystine (Cys) | 21.87 ± 0.06 a | 22.44 ± 0.01 a |
| TAAs | 407.38 ± 3.10 b | 1014.61 ± 4.44 a |
| EAAs | 117.11 ± 1.40 b | 425.03 ± 1.42 a |
| NEAAs | 290.27 ±1.66 b | 589.58 ± 3.00 a |
| EAAs/TAAs (%) | 28.75 ± 0.35 b | 41.89 ± 0.14 a |
| EAAs/NEAAs (%) | 40.35 ± 0.48 b | 72.09 ± 0.24 a |
| FAO/WHO Recommended Pattern (%) | Whole Egg Protein Pattern (%) | % of Total Amino Acids | AAS (%) | CS (%) | ||||
|---|---|---|---|---|---|---|---|---|
| MLP | FMLP | MLP | FMLP | MLP | FMLP | |||
| Ile | 4.00 | 5.30 | 2.59 ± 0.03 | 4.40 ± 0.01 | 64.74 ± 0.68 | 109.89 ± 0.17 | 48.86 ± 0.51 | 82.94 ± 0.13 |
| Leu | 7.00 | 8.60 | 2.45 ± 0.02 | 8.26 ± 0.02 | 34.96 ± 0.35 | 118.00 ± 0.25 | 28.46 ± 0.29 | 96.05 ± 0.21 |
| Lys | 5.50 | 7.00 | 4.25 ± 0.02 | 7.66 ± 0.01 | 77.30 ± 0.31 | 139.35 ± 0.14 | 60.74 ± 0.25 | 109.49 ± 0.11 |
| Met+Cys | 3.50 | 6.17 | 5.37 ± 0.01 | 4.06 ± 0.01 | 153.38 ± 0.42 | 115.93 ± 0.31 | 87.01 ± 0.24 | 65.77 ± 0.18 |
| Phe+Tyr | 6.00 | 9.04 | 3.17 ± 0.13 | 7.93 ± 0.06 | 52.78 ± 0.65 | 132.11 ± 0.92 | 35.03 ± 0.43 | 87.68 ± 0.61 |
| Thr | 4.00 | 4.70 | 8.98 ± 0.13 | 5.05 ± 0.03 | 224.48 ± 3.21 | 126.23 ± 0.69 | 191.05 ± 2.73 | 107.43 ± 0.59 |
| Val | 5.00 | 6.60 | 7.31 ± 0.01 | 8.96 ± 0.01 | 146.25 ± 0.15 | 179.18 ± 0.20 | 110.80 ± 0.11 | 135.74 ± 0.15 |
| EAAI (%) | / | / | 65.99 ± 0.19 | 95.67 ± 0.26 | / | / | / | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, C.; Xie, J.; Chen, J.; Xuan, J.; Zeng, Z.; Lai, M.; Kang, X.; Li, J.; Liu, G.; Tu, J.; et al. Fermentation of Pediococcus pentosaceus JC30 Improves Phytochemical, Flavor Characteristics and Antioxidant Activity of Mulberry Leaves. Molecules 2025, 30, 1703. https://doi.org/10.3390/molecules30081703
Meng C, Xie J, Chen J, Xuan J, Zeng Z, Lai M, Kang X, Li J, Liu G, Tu J, et al. Fermentation of Pediococcus pentosaceus JC30 Improves Phytochemical, Flavor Characteristics and Antioxidant Activity of Mulberry Leaves. Molecules. 2025; 30(8):1703. https://doi.org/10.3390/molecules30081703
Chicago/Turabian StyleMeng, Caiyan, Jiawen Xie, Jiaqi Chen, Jiajia Xuan, Zhuoying Zeng, Minghua Lai, Xuerui Kang, Jiayun Li, Guanhui Liu, Jie Tu, and et al. 2025. "Fermentation of Pediococcus pentosaceus JC30 Improves Phytochemical, Flavor Characteristics and Antioxidant Activity of Mulberry Leaves" Molecules 30, no. 8: 1703. https://doi.org/10.3390/molecules30081703
APA StyleMeng, C., Xie, J., Chen, J., Xuan, J., Zeng, Z., Lai, M., Kang, X., Li, J., Liu, G., Tu, J., & Tao, H. (2025). Fermentation of Pediococcus pentosaceus JC30 Improves Phytochemical, Flavor Characteristics and Antioxidant Activity of Mulberry Leaves. Molecules, 30(8), 1703. https://doi.org/10.3390/molecules30081703







