Phytochemical Characterization and Anti-Biofilm Activity of Primula veris L. Roots
Abstract
1. Introduction
2. Results and Discussion
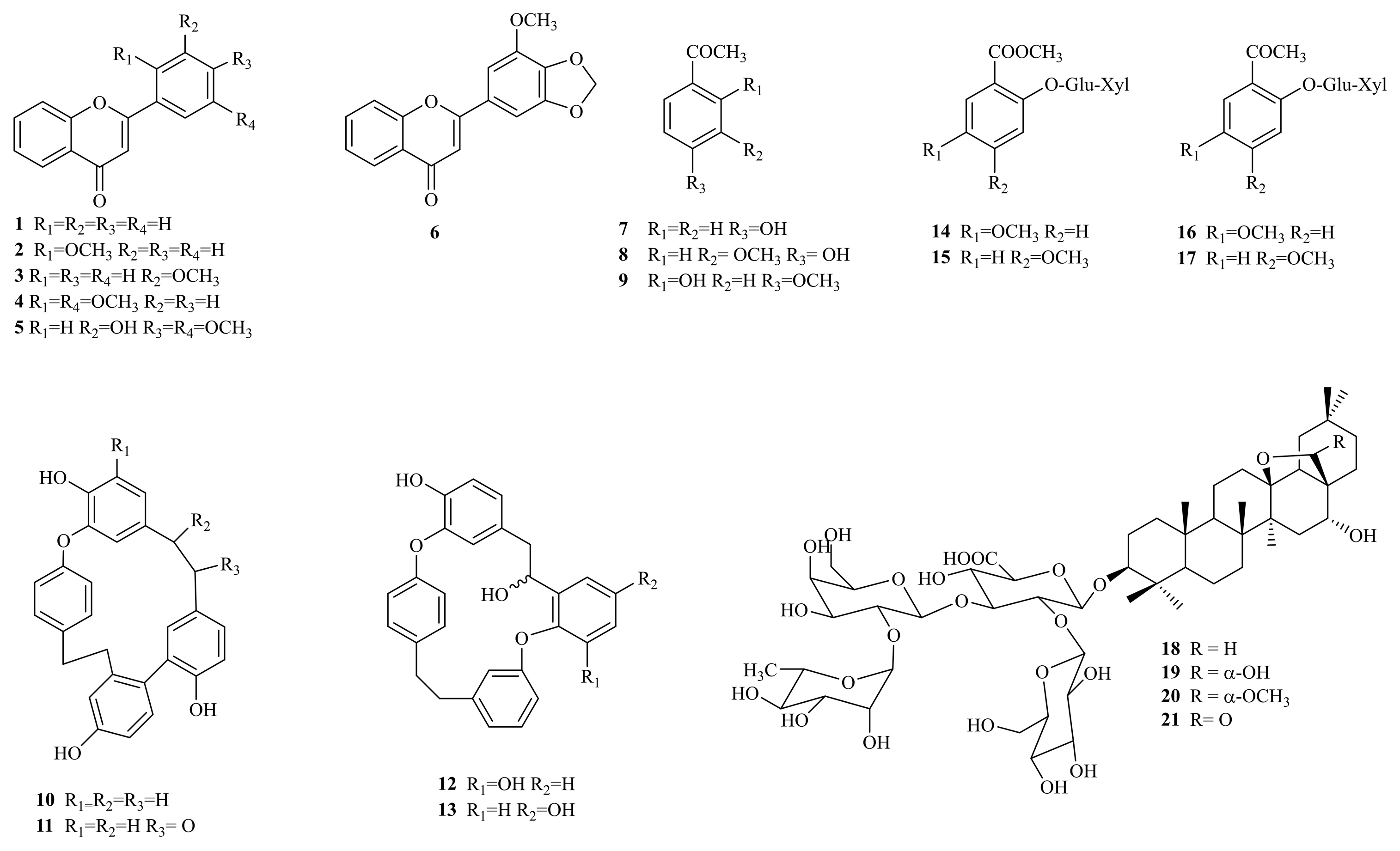
2.1. Phytochemical Characterization of Primula veris
2.2. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of Primula veris Extracts and the Saponin-Enriched Fraction
2.3. Inhibitory Effects of Sub-MICs of Primula veris Extracts and the Saponin-Enriched Fraction on Biofilm Formation
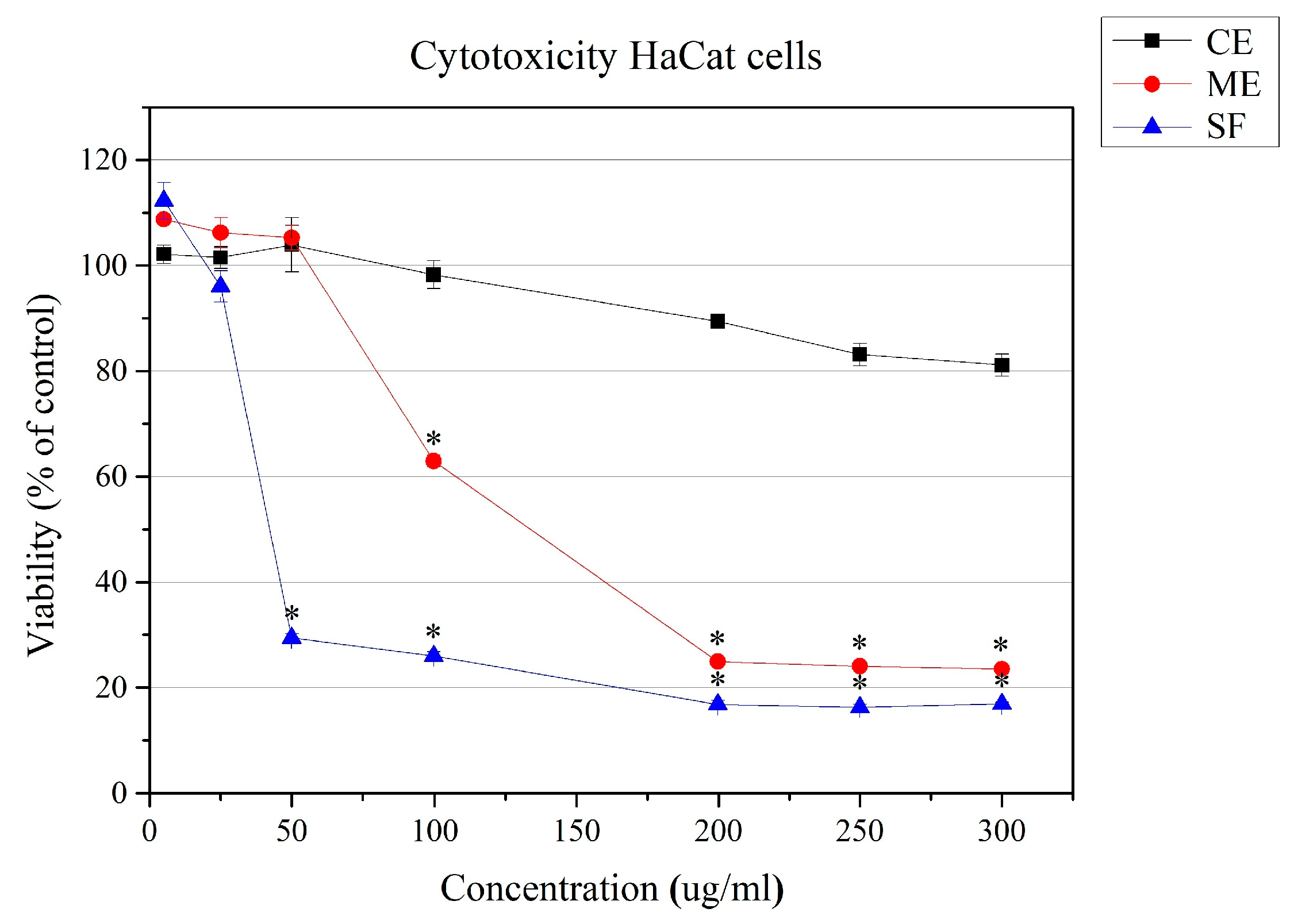
2.4. Cytotoxicity Against Human Keratinocyte Cell Line HaCaT
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation of Individual Compounds
3.4. Hydrolysis of Saponin-Enriched Fraction
3.5. Bacterial Strains, Growth Medium, and Cultural Conditions
3.6. Evaluation of the Minimum Inhibitory Concentration (MIC) and the Minimum Bactericidal Concentration (MBC)
3.7. Biofilm Formation Experiments
3.8. Cytotoxicity Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Colombo, P.S.; Flamini, G.; Rodondi, G.; Giuliani, C.; Santagostini, L.; Fico, G. Phytochemistry of European Primula species. Phytochemistry 2017, 143, 132–144. [Google Scholar] [CrossRef] [PubMed]
- The Plant List. Primula veris L. Available online: http://www.theplantlist.org/tpl1.1/record/kew-2563733 (accessed on 21 February 2025).
- Plants of the World Online Kew Science. Primula veris L. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:702751-1 (accessed on 21 February 2025).
- Kahraman, C.; Sari, S.; Küpeli Akkol, E.; Tatli Cankaya, I. Bioactive saponins of Primula vulgaris Huds. promote wound healing through inhibition of collagenase and elastase enzymes: In vivo, in vitro and in silico evaluations. Chem. Biodivers. 2022, 19, e202200582. [Google Scholar] [CrossRef] [PubMed]
- Alam, F.; Din, K.M.; Sarfraz, M.; Qudoos, A.; Malik, S. Genus Primula and its role in phytomedicine; a systematic review. Phytomedicine Plus 2024, 4, 100510. [Google Scholar] [CrossRef]
- Kemmerich, B. Evaluation of efficacy and tolerability of a fixed combination of dry extracts of Thyme herb and Primrose root in adults suffering from acute bronchitis with productive cough: A prospective, double-blind, placebo-controlled multicentre clinical trial. Arzneim. Forsch. Drug Res. 2007, 57, 607–615. [Google Scholar] [CrossRef]
- Yasar, B.; Kutlu, G.; Tornuk, F. Edible Flowers as Sources of Bioactive Compounds: Determination of phenolic extraction conditions. Int. J. Gastron. Food Sci. 2022, 30, 100618. [Google Scholar] [CrossRef]
- Sarropoulou, V.; Sarrou, E.; Angeli, A.; Martens, S.; Maloupa, E.; Grigoriadou, K. Species-specific secondary metabolites from Primula veris subsp. veris obtained in vitro adventitious root cultures: An alternative for sustainable production. Sustainability 2023, 15, 2452. [Google Scholar] [CrossRef]
- Stefanis, I.; Chatzopoulou, P.; Krigas, N.; Karioti, A. Exploring the chemical content of Primula veris L. subsp. veris wild-growing populations along a climate gradient: An HPLC-PDA-MS quality assessment of flowers, leaves and roots for sustainable exploitation. Horticulturae 2023, 9, 1120. [Google Scholar] [CrossRef]
- Müller, A.; Ganzera, M.; Stuppner, H. Analysis of phenolic glycosides and saponins in Primula elatior and Primula veris (Primula Root) by liquid chromatography, evaporative light scattering detection and mass spectrometry. J. Chromatogr. A 2006, 1112, 218–223. [Google Scholar] [CrossRef]
- Ozkan, M.T.; Aliyazicioglu, R.; Demir, S.; Misir, S.; Turan, I.; Yildirmis, S.; Aliyazicioglu, Y. Phenolic characterisation and antioxidant activity of Primula vulgaris and its antigenotoxic effect on fibroblast cells. Jundishapur. J. Nat. Pharm. Prod. 2017, 12, e40073. [Google Scholar] [CrossRef]
- Latypova, G.M.; Bychenkova, M.A.; Katayev, V.A.; Perfilova, V.N.; Tyurenkov, I.N.; Mokrousov, I.S.; Prokofiev, I.I.; Salikhov, S.M.; Iksanova, G.R. Composition and cardioprotective effects of Primula veris L. solid herbal extract in experimental chronic heart failure. Phytomedicine 2019, 54, 17–26. [Google Scholar] [CrossRef]
- Tünde, J.; Eleonora, M.; Laura, V.; Neagu, O.; Annamaria, P. Bioactive compounds and antioxidant capacity of Primula veris L. flower extracts. Analele Univ. Din Oradea Fasc. Ecotoxicologie Zooteh. De Ind. 2015, XIV B, 235–241. [Google Scholar]
- Tokalov, S.V.; Kind, B.; Wollenweber, E.; Gutzeit, H.O. Biological effects of epicuticular flavonoids from Primula denticulata on human leukemia cells. J. Agric. Food. Chem. 2003, 52, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Bhutia, T.D.; Valant-Vetschera, K.M. Diversification of exudate flavonoid profiles in further Primula spp. Nat. Prod. Commun. 2012, 7, 587–589. [Google Scholar] [CrossRef] [PubMed]
- Valant-Vetschera, K.M.; Bhutia, T.D.; Wollenweber, E. Chemodiversity of exudate flavonoids in Dionysia (Primulaceae): A comparative study. Phytochemistry 2010, 71, 937–947. [Google Scholar] [CrossRef]
- Valant-Vetschera, K.M.; Bhutia, T.D.; Wollenweber, E. Exudate flavonoids of Primula spp: Structural and biogenetic chemodiversity. Nat. Prod. Commun. 2009, 4, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Bhutia, T.D.; Valant-Vetschera, K.M.; Brecker, L. Orphan flavonoids and dihydrochalcones from Primula exudates. Nat. Prod. Commun. 2013, 8, 1081–1084. [Google Scholar] [CrossRef] [PubMed]
- Bhutia, T.D.; Valant-Vetschera, K.M.; Adlassnig, W.; Brecker, L. Flavonoids in selected Primula spp.: Bridging micromorphology with chemodiversity. Nat. Prod. Commun. 2012, 7, 1469–1473. [Google Scholar] [CrossRef]
- Siems, K.; Jaensch, M.; Jakupovic, J. Structures of the two saponins isolated from commercially available root extract of Primula sp. Planta Med. 1998, 64, 272–274. [Google Scholar] [CrossRef]
- Apel, L.; Kammerer, D.R.; Stintzing, F.C.; Spring, O. Comparative metabolite profiling of triterpenoid saponins and flavonoids in flower color mutations of Primula veris L. Int. J. Mol. Sci. 2017, 18, 153. [Google Scholar] [CrossRef]
- Çaliş, İ.; Yürüker, A.; Rüegger, H.; Wright, A.D.; Sticker, O. Triterpene saponins from Primula veris subsp. macrocalyx and Primula elatior subsp. meyeri. J. Nat. Prod. 1992, 55, 1299–1306. [Google Scholar] [CrossRef]
- Włodarczyk, M.; Pasikowski, P.; Osiewała, K.; Frankiewicz, A.; Dryś, A.; Gleńsk, M. In search of high-yielding and single-compound-yielding plants: New sources of pharmaceutically important saponins from the Primulaceae family. Biomolecules 2020, 10, 376. [Google Scholar] [CrossRef] [PubMed]
- Herbal Medicinal Product European Medicines Agency (EMA). Primulae Radix. Available online: https://www.ema.europa.eu/en/medicines/herbal/primulae-radix (accessed on 14 February 2025).
- Kosenkova, Y.S.; Polovinka, M.P.; Komarova, N.I.; Korchagina, D.V.; Kurochkina, N.Y.; Cheremushkina, V.A.; Salakhutdinov, N.F. Riccardin C, a bisbibenzyl compound from Primula Macrocalyx. Chem. Nat. Compd. 2007, 43, 712–713. [Google Scholar] [CrossRef]
- Bukvicki, D.; Kovtonyuk, N.K.; Legin, A.A.; Keppler, B.K.; Brecker, L.; Asakawa, Y.; Valant-Vetschera, K. Hunting for bis-bibenzyls in Primula veris subsp. macrocalyx (Bunge) Lüdi: Organ-specific accumulation and cytotoxic activity. Phytochem. Lett. 2021, 44, 90–97. [Google Scholar] [CrossRef]
- Novakovic, M.; Ilic-Tomic, T.; Djordjevic, I.; Andjelkovic, B.; Tesevic, V.; Milosavljevic, S.; Asakawa, Y. Bisbibenzyls from Serbian Primula veris subsp. columnae (Ten.) Lȕdi and P. acaulis (L.) L. Phytochemistry 2023, 212, 113719. [Google Scholar] [CrossRef]
- Novakovic, M.; Bukvicki, D.; Andjelkovic, B.; Ilic-Tomic, T.; Veljic, M.; Tesevic, V.; Asakawa, Y. Cytotoxic activity of riccardin and perrottetin derivatives from the liverwort Lunularia cruciata. J. Nat. Prod. 2019, 82, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.F.; Qu, J.B.; Wu, X.Z.; Liu, N.; Ji, M.; Lou, H.X. Antifungal macrocyclic bis(bibenzyls) from the Chinese liverwort Ptagiochasm intermedlum L. Nat. Prod. Res. 2010, 24, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Zafer, M.M.; Mohamed, G.A.; Ibrahim, S.R.M.; Ghosh, S.; Bornman, C.; Elfaky, M.A. Biofilm-mediated infections by multidrug-resistant microbes: A comprehensive exploration and forward perspectives. Arch. Microbiol. 2024, 206, 101. [Google Scholar] [CrossRef]
- Olsen, I. Biofilm-specific antibiotic tolerance and resistance. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 877–886. [Google Scholar] [CrossRef]
- Abebe, G.M. The role of bacterial biofilm in antibiotic resistance and food contamination. Int. J. Microbiol. 2020, 2020, 1705814. [Google Scholar] [CrossRef]
- Ciofu, O.; Moser, C.; Jensen, P.Ø.; Høiby, N. Tolerance and resistance of microbial biofilms. Nat. Rev. Microbiol. 2022, 20, 621–635. [Google Scholar] [CrossRef]
- Damyanova, T.; Paunova-Krasteva, T. What we still don’t know about biofilms—current overview and key research information. Microbiol. Res. 2025, 16, 46. [Google Scholar] [CrossRef]
- Oyardi, O.; Hacioglu, M.; Özdemir, E.; Erbay, M.Ş.; Kültür, Ş.; Bozkurt Güzel, C. Screening of antimicrobial, antibiofilm and cytotoxic activities of some medicinal plants from Balıkesir Province, Türkiye: Pointing to the potential effects of Allium paniculatum flower. Turk. J. Pharm. Sci. 2023, 21, 252–258. [Google Scholar] [CrossRef]
- Başbülbül, G.; Özmen, A.; Biyik, H.H.; Şen, Ö. Antimitotic and antibacterial effects of the Primula veris L. flower extracts. Caryologia 2008, 61, 88–91. [Google Scholar] [CrossRef]
- Karapınar, Ç.; Öz, M. Chemical content of volatile oil of Primula veris subsp. columnae, obtaining the methanol extracts and their biological activities. Bioresources 2023, 18, 4475–4491. [Google Scholar] [CrossRef]
- Ivanišová, E.; Horňák, M.; Čech, M.; Harangozo, Ľ.; Kačániová, M.; Grygorieva, O.; Kowalczewski, P.Ł. Polyphenol content, mineral compounds composition, antimicrobial and antioxidant activities of selected medicinal herbs from Slovak Republic. Appl. Sci. 2023, 13, 1918. [Google Scholar] [CrossRef]
- Chilku, E.; Ivic Kolevska, S.; Kadifkova Panovska, T. Antioxidant and antibacterial properties of some commercial plants from Macedonia. World J. Pharm. Pharm. Sci. 2017, 6, 1767–1778. [Google Scholar] [CrossRef][Green Version]
- Tosun, F.; Kizilay, Ç.A.; Şener, B.; Vural, M. The evaluation of plants from Turkey for in vitro antimycobacterial activity. Pharm. Biol. 2005, 43, 58–63. [Google Scholar] [CrossRef]
- Yayli, N.; Tosun, G.; Yayli, B.; Gündoǧan, Z.; Coşkunçelebi, K.; Karaoǧlu, Ş.A. Altitude variation in the composition of essential oils, fatty acid methyl esters, and antimicrobial activities of two subspecies of Primula vulgaris grown in Turkey. Nat. Prod. Commun. 2016, 11, 1505–1510. [Google Scholar] [CrossRef]
- Majid, A.; Hassan, S.; Hussain, W.; Khan, A.; Hassan, A.; Khan, A.; Khan, T.; Ahmad, T.; Rehman, M.U. In vitro approaches of Primula vulgaris leaves and roots extraction against human pathogenic bacterial strains. World Appl. Sci. J. 2014, 30, 575–580. Available online: https://idosi.org/wasj/wasj30(5)14/6.pdf (accessed on 14 February 2025).
- Jaberian, H.; Piri, K.; Nazari, J. Phytochemical composition and in vitro antimicrobial and antioxidant activities of some medicinal plants. Food Chem. 2013, 136, 237–244. [Google Scholar] [CrossRef]
- Khan, S.; Shaheen, H.; Aziz, S.; Nasar, S. Diversity and distribution of genus Primula in Kashmir Region: An indicator genus of the western Himalayan Mountain wetlands and glacial forelands. Biodivers. Conserv. 2021, 30, 1673–1688. [Google Scholar] [CrossRef]
- Najmus-Saqib, Q.; Alam, F.; Ahmad, M. Antimicrobial and cytotoxicity activities of the medicinal plant Primula macrophylla. J. Enzyme. Inhib. Med. Chem. 2009, 24, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Peev, D. Flora of the Republic of Bulgaria; Velchev, V., Ed.; Bulgarian Academy of Sciences: Sofia, Bulgaria, 1982; Volume 8, pp. 324–336. [Google Scholar]
- Assyov, B.; Petrova, A.; Dimitrov, D.; Vassilev, P. Conspectus of the Bulgarian Vascular Flora. Distribution Maps and Floristic Elements, 4th ed.; Assyov, B., Petrova, A., Eds.; Bulgarian Biodiversity Foundation: Sofia, Bulgaria, 2012. [Google Scholar]
- Yankova-Tsvetkova, E.; Yurukova-Grancharova, P.; Aneva, I.; Zhelev, P. On the reproductive potential in Primula veris L. (Primulaceae): Embryological features, pollen and seed viability, genetic diversity. Plants 2021, 10, 2296. [Google Scholar] [CrossRef]
- Yankova-Tsvetkova, E.; Petrova, M.; Grigorova, I.; Traykova, B.; Stanilova, M. The establishment of an ex situ collection of Primula veris in Bulgaria. Plants 2022, 11, 3018. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, M.; Yankova-Tsvetkova, E.; Stefanova, T.; Berkov, S. Exudate flavonoids of Primula veris leaves and their inhibitory activity on Lolium perrene seed germination. Proc. Bulg. Acad. Sci. 2023, 76, 388–393. [Google Scholar] [CrossRef]
- Budzianowski, J.; Morozowska, M.; Wesołowska, M. Lipophilic Flavones of Primula veris L. from field cultivation and in vitro cultures. Phytochemistry 2005, 66, 1033–1039. [Google Scholar] [CrossRef]
- Matsumoto, M. 2′-Hydroxy-4′-Methoxyacetophenone (Paeonol) in Exacum affine Cv. Biosci. Biotechnol. Biochem. 1994, 58, 1892–1893. [Google Scholar] [CrossRef]
- Has, M.; Kucuk, S.; Kurkcuoglu, M. Anatomical and Volatile Components Investigations on Primula vulgaris Huds. subsp. vulgaris (Primulaceae). Ann. Phytomedicine Int. J. 2021, 10, 63–66. [Google Scholar] [CrossRef]
- Qu, G.L.; Zhang, H.M.; Deng, Z.W.; Kong, D.Y.; Geng, Z.F.; Du, S.S. Study on chemical constituents of Primula maximowiczii Regel: Part III. Chin. Pharm. J. 2011, 46, 93–97. [Google Scholar]
- Xu, S.J.; Yang, L.; Zeng, X.; Zhang, M.; Wang, Z.T. Characterization of compounds in the Chinese herbal drug mu-dan-pi by liquid chromatography coupled to electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2006, 20, 3275–3288. [Google Scholar] [CrossRef]
- Baczek, K.; Przybył, J.L.; Mirgos, M.; Kosakowska, O.; Szymborska-Sandhu, I.; Wȩglarz, Z. Phenolics in Primula veris L. and P. elatior (L.) Hill raw materials. Int. J. Anal. Chem. 2017, 2017, 2871579. [Google Scholar] [CrossRef] [PubMed]
- Foubert, K.; Theunis, M.; Apers, S.; Vlietinck, A.J.; Pieters, L. Chemistry, distribution and biological activities of 13,28-epoxy-oleanane saponins from the plant families Myrsinaceae and Primulaceae. Curr. Org. Chem. 2008, 12, 629–642. [Google Scholar] [CrossRef]
- Damyanova, T.; Dimitrova, P.D.; Borisova, D.; Topouzova-Hristova, T.; Haladjova, E.; Paunova-Krasteva, T. An overview of biofilm-associated infections and the role of phytochemicals and nanomaterials in their control and prevention. Pharmaceutics 2024, 16, 162. [Google Scholar] [CrossRef]
- Sarker, S.D.; Nahar, L.; Kumarasamy, Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 2007, 42, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Święciło, A.; Rybczyńska-Tkaczyk, K. Resazurin method for evaluation of bioactive compounds from cranberry extracts using the metabolic activity of a ΔSOD1 mutant of saccharomyces cerevisiae yeast under severe osmotic stress. J. AOAC Int. 2020, 103, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.I.; Anwar, F.; Nigam, P.S.; Sarker, S.D.; Moore, J.E.; Rao, J.R.; Mazumdar, A. Antibacterial activity of some Lamiaceae essential oils using resazurin as an indicator of cell growth. LWT Food Sci. Technol. 2011, 44, 1199–1206. [Google Scholar] [CrossRef]
- Khan, S.; Shaheen, H.; Mehmood, A.; Nasar, S.; Khan, T. Ethnobotanical and antibacterial study of Primula plants traditionally used in the indigenous communities of Western Himalaya, Pakistan. Saudi J. Biol. Sci. 2022, 29, 3244–3254. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, N.R.; Samir, R.; Abdel-Hafez, L.J.M.; Ramadan, M.A. Olive leaf extract modulates quorum sensing genes and biofilm formation in multi-drug resistant Pseudomonas aeruginosa. Antibiotics 2020, 9, 526. [Google Scholar] [CrossRef]
- Paunova-Krasteva, T.; Hemdan, B.A.; Dimitrova, P.D.; Damyanova, T.; El-Feky, A.M.; Elbatanony, M.M.; Stoitsova, S.; El-Liethy, M.A.; El-Taweel, G.E.; El Nahrawy, A.M. Hybrid Chitosan/CaO-based nanocomposites doped with plant extracts from Azadirachta indica and Melia azedarach: Evaluation of antibacterial and antibiofilm activities. Bionanoscience 2023, 13, 88–102. [Google Scholar] [CrossRef]
- Ansari, F.A.; Husain, F.M.; Pichtel, J.; Meena, R.P.; Khan, M.H.; Khan, A.S.; Alam, N. Withania somnifera (L.) Dunal: A medicinally important plant inhibits pathogenic biofilms. Microbe 2025, 6, 100227. [Google Scholar] [CrossRef]
- Laskoski, L.V.; Bandeira, D.M.; Batista, J.M.; da Costa, W.F.; Baeza, L.C.; Kuo, L.H.; Pinto, F.G.d.S. Phytochemical prospection and evaluation of antimicrobial, antioxidant and antibiofilm activities of extracts and essential oil from leaves of Myrsine umbellata Mart. (Primulaceae). Braz. J. Biol. 2022, 82, e263865. [Google Scholar] [CrossRef]
- Ozay, C.; Temel, A.; Turel, S.; Akgul, I. Investigation of anti-inflammatory, antibiofilm, antioxidant and cytotoxic activities of Cyclamen hederifolium (Primulaceae). Farmacia 2023, 71, 1208–1216. [Google Scholar] [CrossRef]
- Hamdan, H.F.; Ross, E.E.R.; Jalil, M.T.M.; Hashim, M.A.; Yahya, M.F.Z.R. Antibiofilm efficacy and mode of action of Etlingera elatior extracts against Staphylococcus aureus. Malays. Appl. Biol. 2024, 53, 27–34. [Google Scholar] [CrossRef]
- Dimitrova, P.D.; Ivanova, V.; Trendafilova, A.; Paunova-Krasteva, T. Anti-biofilm and anti-quorum-sensing activity of Inula extracts: A strategy for modulating Chromobacterium violaceum virulence factors. Pharmaceuticals 2024, 17, 573. [Google Scholar] [CrossRef] [PubMed]
- Dimitrova, P.D.; Damyanova, T.; Paunova-Krasteva, T. Chromobacterium violaceum: A model for evaluating the anti-quorum sensing activities of plant substances. Sci. Pharm. 2023, 91, 33. [Google Scholar] [CrossRef]
- Yu, Y.; Kang, X.; Liu, T.; Wang, Y.; Tang, J.; Peng, W.; Martin, F.M.; Tan, H. Inoculation of the Morchella importuna mycosphere with Pseudomonas chlororaphis alleviated a soil-borne disease caused by Paecilomyces penicillatus. Biol. Fertil. Soils. 2024, 61, 141–161. [Google Scholar] [CrossRef]
- Matilla-Cuenca, L.; Gil, C.; Cuesta, S.; Rapún-Araiz, B.; Žiemytė, M.; Mira, A.; Lasa, I.; Valle, J. Antibiofilm activity of flavonoids on Staphylococcal biofilms through targeting BAP amyloids. Sci. Rep. 2020, 10, 18968. [Google Scholar] [CrossRef]
- Zeng, Y.; Nikitkova, A.; Abdelsalam, H.; Li, J.; Xiao, J. Activity of quercetin and kaemferol against Streptococcus mutans biofilm. Arch. Oral. Biol. 2019, 98, 9–16. [Google Scholar] [CrossRef]
- Pruteanu, M.; Hernández Lobato, J.I.; Stach, T.; Hengge, R. Common plant flavonoids prevent the assembly of amyloid curli fibres and can interfere with bacterial biofilm formation. Environ. Microbiol. 2020, 22, 5280–5299. [Google Scholar] [CrossRef]
- Parai, D.; Islam, E.; Mitra, J.; Mukherjee, S.K. Effect of Bacoside a on growth and biofilm formation by Staphylococcus aureus and Pseudomonas aeruginosa. Can. J. Microbiol. 2016, 63, 169–178. [Google Scholar] [CrossRef]
- Stanković, J.; Godevac, D.; Tešević, V.; Dajić-Stevanović, Z.; Ćirić, A.; Soković, M.; Novaković, M. Antibacterial and antibiofilm activity of flavonoid and saponin derivatives from Atriplex tatarica against Pseudomonas aeruginosa. J. Nat. Prod. 2019, 82, 1487–1495. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, Y.; Li, Y.; Yin, J. Anti-biofilm activity of assamsaponin A, theasaponin E1, and theasaponin E2 against Candida albicans. Int. J. Mol. Sci. 2024, 25, 3599. [Google Scholar] [CrossRef]
- Araújo, N.J.S.; Silva, A.R.P.; Costa, M.S.; Freitas, T.S.; Filho, J.M.B.; Matos, Y.M.L.S.; Morais-Braga, M.F.B.; Pereira Junior, F.N.; Silva, C.A.P.; Souza, E.O.; et al. Chemical characterization UPLC-ESI-QToF-MSE, antibacterial and antibiofilm potential of Sarcomphalus joazeiro (MART.) Hauenschild. Food Biosci. 2022, 50, 102066. [Google Scholar] [CrossRef]
- Choudhary, M.; Verma, V.; Saran, R.; Bhagyawant, S.S.; Srivastava, N. Natural biosurfactant as antimicrobial agent: Strategy to action against fungal and bacterial activities. Cell. Biochem. Biophys. 2022, 80, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Yadav, H.; Mahalvar, A.; Pradhan, M.; Yadav, K.; Kumar Sahu, K.; Yadav, R. Exploring the potential of phytochemicals and nanomaterial: A boon to antimicrobial treatment. Med. Drug Discov. 2023, 17, 100151. [Google Scholar] [CrossRef]
- Silva, E.; Teixeira, J.A.; Pereira, M.O.; Rocha, C.M.R.; Sousa, A.M. Evolving biofilm inhibition and eradication in clinical settings through plant-based antibiofilm agents. Phytomedicine 2023, 119, 154973. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Henning, S.M.; Heber, D. Limitations of MTT and MTS-based assays for measurement of antiproliferative activity of green tea polyphenols. PLoS ONE 2010, 5, e10202. [Google Scholar] [CrossRef]
- Koczurkiewicz, P.; Czyz, J.; Podolak, I.; Wójcik, K.; Galanty, A.; Janeczko, Z.; Michalik, M. Multidirectional effects of triterpene saponins on cancer cells—mini-review of in vitro studies. Acta Biochim. Pol 2015, 62, 383–393. [Google Scholar] [CrossRef]
- Francis, G.; Kerem, Z.; Makkar, H.P.S.; Becker, K. The biological action of saponins in animal systems: A review. Br. J. Nutr. 2002, 88, 587–605. [Google Scholar] [CrossRef]
- Sun, X.H.; Chai, Y.H.; Bai, X.T.; Li, H.X.; Yang, P.P.; Xi, Y.M. Saikosaponin A mediates the anti-acute myeloid leukemia effect via the P-JNK signaling pathway induced by endoplasmic reticulum stress. Drug Des. Devel. Ther. 2025, 19, 1983–2001. [Google Scholar] [CrossRef]
- De Soyza, A.; Hall, A.J.; Mahenthiralingam, E.; Drevinek, P.; Kaca, W.; Drulis-Kawa, Z.; Stoitsova, S.R.; Toth, V.; Coenye, T.; Zlosnik, J.E.A.; et al. Developing an international Pseudomonas aeruginosa reference panel. Microbiologyopen 2013, 2, 1010–1023. [Google Scholar] [CrossRef] [PubMed]
- Trendafilova, A.; Staleva, P.; Petkova, Z.; Ivanova, V.; Evstatieva, Y.; Nikolova, D.; Rasheva, I.; Atanasov, N.; Topouzova-Hristova, T.; Veleva, R.; et al. Phytochemical profile, antioxidant potential, antimicrobial activity, and cytotoxicity of dry extract from Rosa damascena Mill. Molecules 2023, 28, 7666. [Google Scholar] [CrossRef] [PubMed]


| Position | 19 | 20 | 21 | |||
|---|---|---|---|---|---|---|
| δC | δH | δC | δH | δC | δH | |
| 1 | 40.3 | 0.99 m 1 | 40.3 | 0.98 m | 40.2 | 1.02 m |
| 1.74 m | 1.75 m | 1.75 m | ||||
| 2 | 27.1 | 1.75 m | 27.1 | 1.75 m | 27.0 | 1.75 m |
| 2.02 m | 1.95 m | 2.01 m | ||||
| 3 | 92.1 | 3.20 dd (4.0, 11.3) | 92.3 | 3.19 dd (4.0, 11.3) | 91.9 | 3.21 dd (4.0, 11.3) |
| 4 | 40.7 | - | 40.7 | - | 40.7 | - |
| 5 | 56.8 | 0.72 dd (1.8, 11.5) | 56.8 | 0.73 dd (1.8, 11.5) | 56.8 | 0.76 dd (1.8, 11.5) |
| 6 | 18.7 | 1.44 m | 18.7 | 1.43 m | 18.7 | 1.43 m |
| 1.50 m | 1.50 m | 1.55 m | ||||
| 7 | 35.2 | 1.20 m | 35.2 | 1.22 m | 34.9 | 1.24 m |
| 1.56 m | 1.55 m | 1.59 m | ||||
| 8 | 43.3 | - | 43.4 | - | 43.0 | - |
| 9 | 51.4 | 1.22 m | 51.4 | 1.23 m | 50.8 | 1.34 m |
| 10 | 37.8 | - | 37.8 | - | 37.8 | - |
| 11 | 19.9 | 1.45 m | 19.9 | 1.45 m | 19.4 | 1.55 m |
| 1.66 m | 1.65 m | 1.65 m | ||||
| 12 | 33.6 | 1.28 m | 33.7 | 1.28 m | 32.2 | 1.56 m |
| 2.03 m | 2.00 m | 2.15 m | ||||
| 13 | 88.9 | - | 89.0 | - | 94.9 | - |
| 14 | 43.9 | - | 44.0 | - | 43.0 | - |
| 15 | 37.0 | 1.20 m | 37.4 | 1.20 m | 37.4 | 1.20 m |
| 1.99 m | 1.99 m | 1.99 m | ||||
| 16 | 77.3 | 3.78 m | 77.2 | 3.82 m | 76.2 | 3.79 m |
| 17 | 48.7 | - | 48.9 | 49.1 | - | |
| 18 | 47.6 | 1.67 m | 48.0 | 1.68 m | 52.6 | 1.92 m |
| 19 | 39.8 | 1.19 m | 39.8 | 1.17 m | 39.2 | 1.30 m |
| 2.37 dd (12.1, 14.5) | 2.28 dd (12.1, 14.5) | 2.49 dd (12.1, 14.5) | ||||
| 20 | 32.4 | - | 32.4 | - | 32.2 | - |
| 21 | 37.5 | 1.15 m | 37.1 | 1.15 m | 36.7 | 1.21 m |
| 2.06 m | 2.01 m | 2.09 m | ||||
| 22 | 27.2 | 1.42 m | 26.5 | 1.42 m | 28.7 | 1.63 m |
| 1.94 m | 1.61 m | 1.83 m | ||||
| 23 | 28.3 | 1.06 s | 28.2 | 1.06 s | 28.2 | 1.07 s |
| 24 | 16.8 | 0.87 s | 16.8 | 0.87 s | 16.7 | 0.88 s |
| 25 | 16.8 | 0.90 s | 16.8 | 0.91 s | 16.8 | 0.91 s |
| 26 | 18.8 | 1.18 s | 18.9 | 1.18 s | 18.3 | 1.08 s |
| 27 | 19.9 | 1.22 s | 19.8 | 1.21 s | 19.7 | 1.38 s |
| 28 | 99.7 | 4.60 brs | 106.9 | 4.17 brs | 182.0 | - |
| 29 | 33.9 | 0.93 s | 33.9 | 0.92 s | 33.5 | 0.96 |
| 30 | 25.0 | 0.92 s | 24.9 | 0.88 s | 24.9 | 0.92 |
| OCH3 | 55.4 | 3.31 s | ||||
| 1′ | 105.8 | 4.45 d (7.7) | 105.8 | 4.48 d (7.7) | 105.8 | 4.46 d (7.7 |
| 2′ | 79.3 | 3.92 dd (7.7, 8.8) | 79.1 | 3.93 dd (7.7, 8.8) | 79.3 | 3.92 dd (7.7, 8.8) |
| 3′ | 81.2 | 4.06 t (8.8) | 81.0 | 4.05 t (8.8) | 81.2 | 4.07 t (8.8) |
| 4′ | 72.2 | 3.59 m | 72.2 | 3.61 m | 72.3 | 3.59 m |
| 5′ | 75.9 | 3.79 m | 75.9 | 3.78 m | 75.9 | 3.79 m |
| 6′ | 176.6 | - | - | |||
| 1″ | 100.8 | 5.20 d (7.8) | 100.9 | 5.20 d (7.8) | 100.8 | 5.20 d (7.8) |
| 2″ | 76.2 | 3.79 m | 76.1 | 3.79 m | 76.2 | 3.79 m |
| 3″ | 75.9 | 3.72 m | 76.0 | 3.74 m | 76.0 | 3.74 m |
| 4″ | 71.9 | 3.72 m | 71.8 | 3.72 m | 71.8 | 3.72 m |
| 5″ | 76.9 | 3.53 m | 76.9 | 3.53 m | 76.9 | 3.53 m |
| 6″ | 62.7 | 3.65 dd (4.1, 11.5) | 62.8 | 3.65 dd (4.1, 11.5) | 62.7 | 3.67 dd (4.1, 11.5) |
| 3.81 m | 3.81 m | 3.81 m | ||||
| 1‴ | 102.0 | 5.28 d (1.2) | 102.1 | 5.28 d (1.2) | 102.0 | 5.29 d (1.2) |
| 2‴ | 72.6 | 3.97 dd (1.2, 3.5) | 72.6 | 3.96 dd (1.2, 3.5) | 72.6 | 3.96 dd (1.2, 3.5) |
| 3‴ | 72.3 | 3.71 dd (3.5, 9.5) | 72.3 | 3.71 dd (3.5, 9.5) | 72.2 | 3.71 dd (3.5, 9.5) |
| 4‴ | 73.7 | 3.44 t (9.5) | 73.8 | 3.45 t (9.5) | 73.7 | 3.43 t (9.5) |
| 5‴ | 70.3 | 4.10 dq (9.5, 6.5) | 70.2 | 4.11 dq (9.5, 6.5) | 70.2 | 4.11 dq (9.5, 6.5) |
| 6‴ | 17.9 | 1.27 d (6.5) | 17.9 | 1.27 d (6.5) | 17.9 | 1.27 d (6.5) |
| 1⁗ | 102.5 | 4.88 | 102.6 | 4.88 | 102.6 | 4.88 |
| 2⁗ | 76.2 | 3.22 dd (7.2, 9.5) | 76.1 | 3.22 dd (7.2, 9.5) | 76.1 | 3.22 dd (7.2, 9.5) |
| Glu-3 | 77.9 | 3.35 m | 77.9 | 3.35 m | 77.9 | 3.35 m |
| 4⁗ | 72.4 | 3.05 t (9.5) | 72.6 | 3.05 t (9.5) | 72.6 | 3.05 t (9.5) |
| 5⁗ | 78.1 | 3.40 m | 78.1 | 3.40 m | 78.1 | 3.40 m |
| 6⁗ | 63.5 | 3.54 dd (8.0, 12.5) | 63.5 | 3.54 dd (8.0, 12.5) | 63.5 | 3.55 dd (8.0, 12.5) |
| 3.87 dd (2.5, 12.5) | 3.87 dd (2.5, 12.5) | 3.88 dd (2.5, 12.5) | ||||
| Sample 1 | P. aeruginosa | E. coli | S. aureus | S. mutans | ||||
|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| CE | 1.0 | 2.0 | 0.5 | 0.5 | 1.0 | 2.0 | 1.0 | 2.0 |
| ME | 0.5 | 1.0 | 0.5 | 1.0 | 1.0 | 2.0 | 1.0 | 2.0 |
| SF | 0.5 | 1.0 | 0.5 | 1.0 | 0.5 | 2.0 | 0.5 | 2.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trendafilova, A.; Raykova, D.; Ivanova, V.; Novakovic, M.; Nedialkov, P.; Paunova-Krasteva, T.; Veleva, R.; Topouzova-Hristova, T. Phytochemical Characterization and Anti-Biofilm Activity of Primula veris L. Roots. Molecules 2025, 30, 1702. https://doi.org/10.3390/molecules30081702
Trendafilova A, Raykova D, Ivanova V, Novakovic M, Nedialkov P, Paunova-Krasteva T, Veleva R, Topouzova-Hristova T. Phytochemical Characterization and Anti-Biofilm Activity of Primula veris L. Roots. Molecules. 2025; 30(8):1702. https://doi.org/10.3390/molecules30081702
Chicago/Turabian StyleTrendafilova, Antoaneta, Desislava Raykova, Viktoria Ivanova, Miroslav Novakovic, Paraskev Nedialkov, Tsvetelina Paunova-Krasteva, Ralitsa Veleva, and Tanya Topouzova-Hristova. 2025. "Phytochemical Characterization and Anti-Biofilm Activity of Primula veris L. Roots" Molecules 30, no. 8: 1702. https://doi.org/10.3390/molecules30081702
APA StyleTrendafilova, A., Raykova, D., Ivanova, V., Novakovic, M., Nedialkov, P., Paunova-Krasteva, T., Veleva, R., & Topouzova-Hristova, T. (2025). Phytochemical Characterization and Anti-Biofilm Activity of Primula veris L. Roots. Molecules, 30(8), 1702. https://doi.org/10.3390/molecules30081702










