Benzoxanthenone Lignans Related to Carpanone, Polemanone, and Sauchinone: Natural Origin, Chemical Syntheses, and Pharmacological Properties
Abstract
1. Introduction
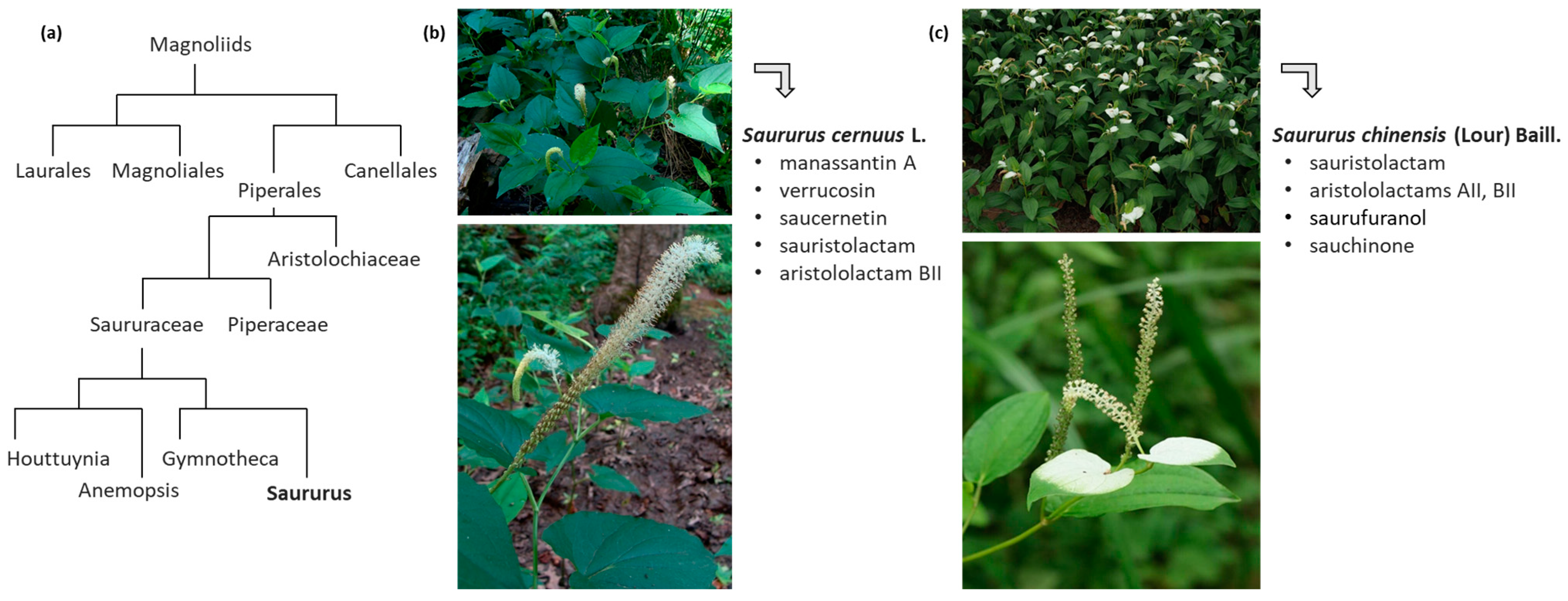
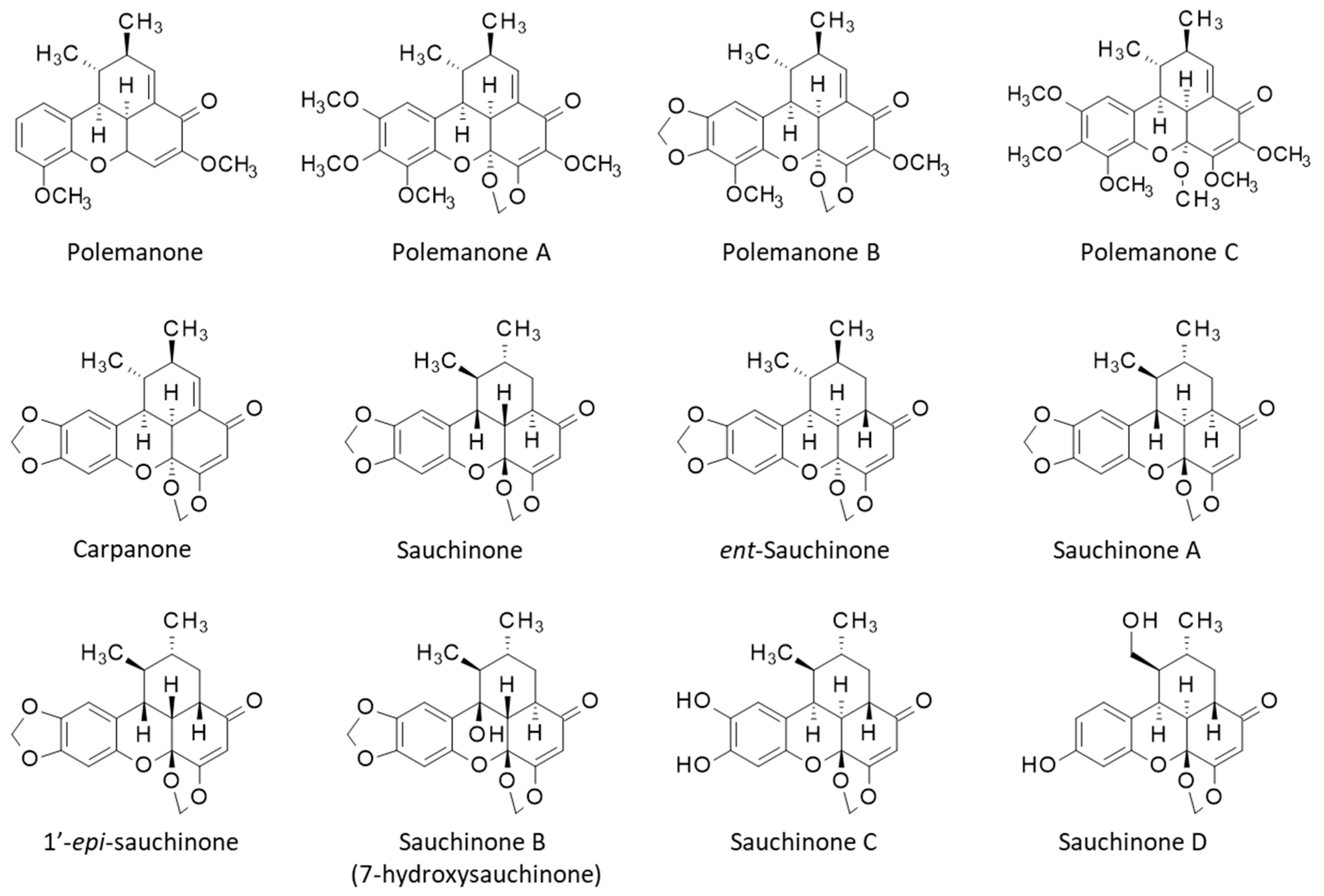
2. Isolation and Biosynthesis of Sauchinone and Related Lignans
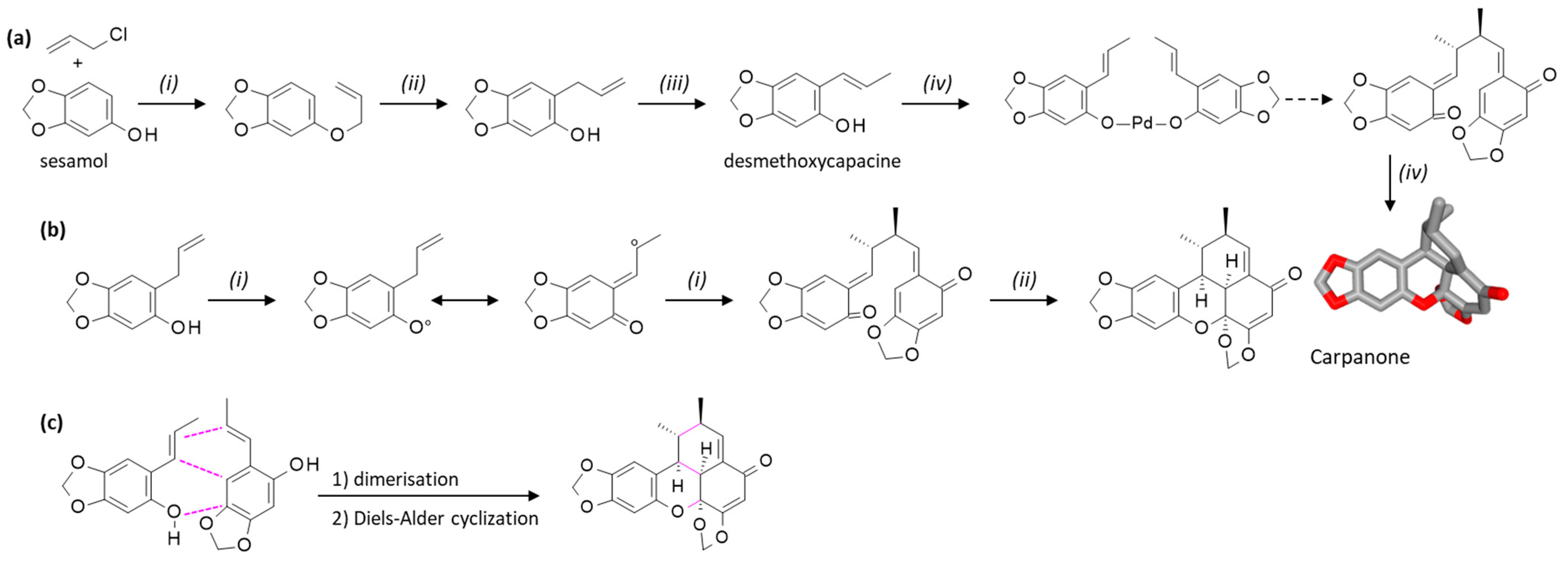
3. Chemical Syntheses of Carpanone, Polemannone, and Sauchinone
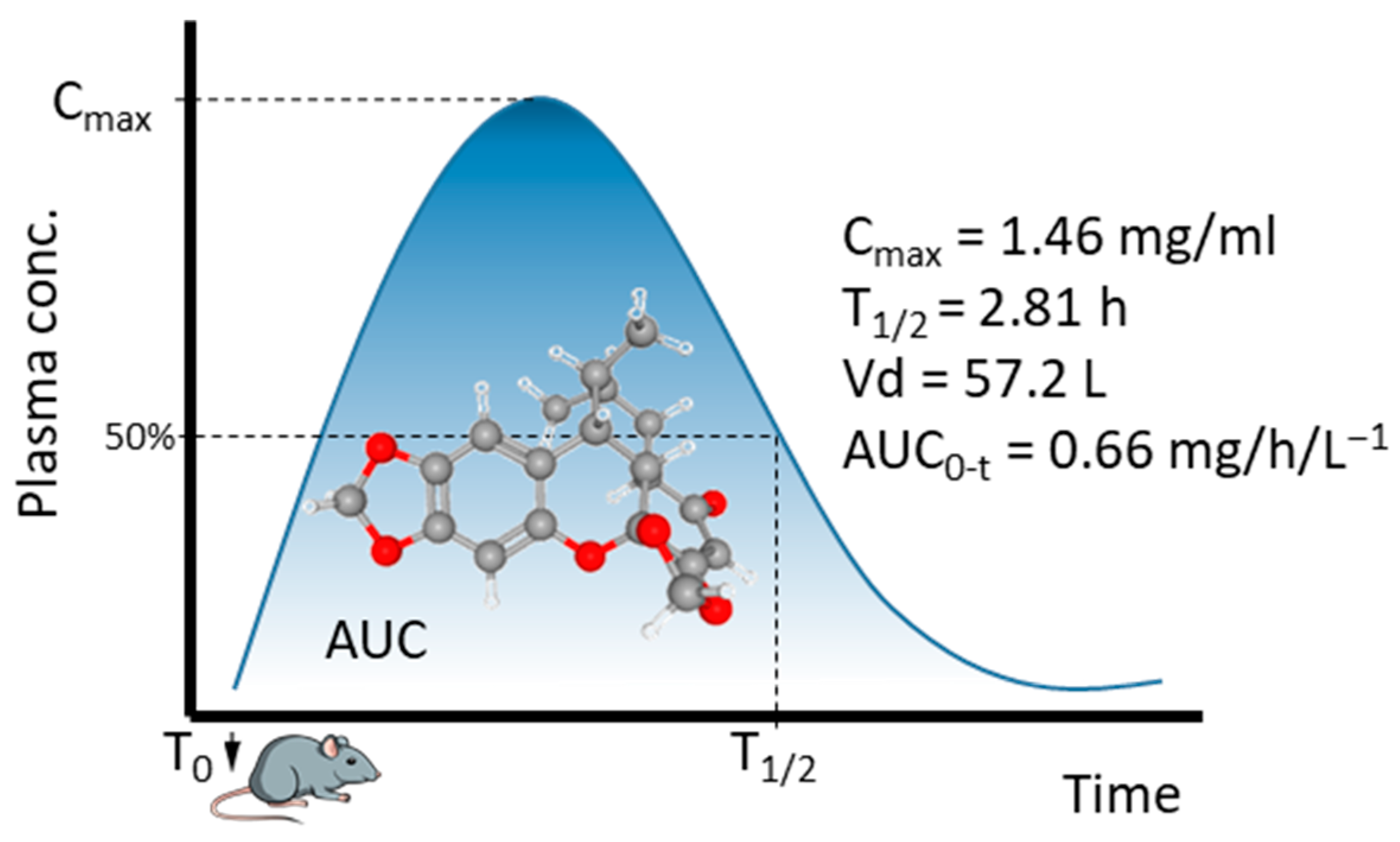
4. Metabolism of Sauchinone
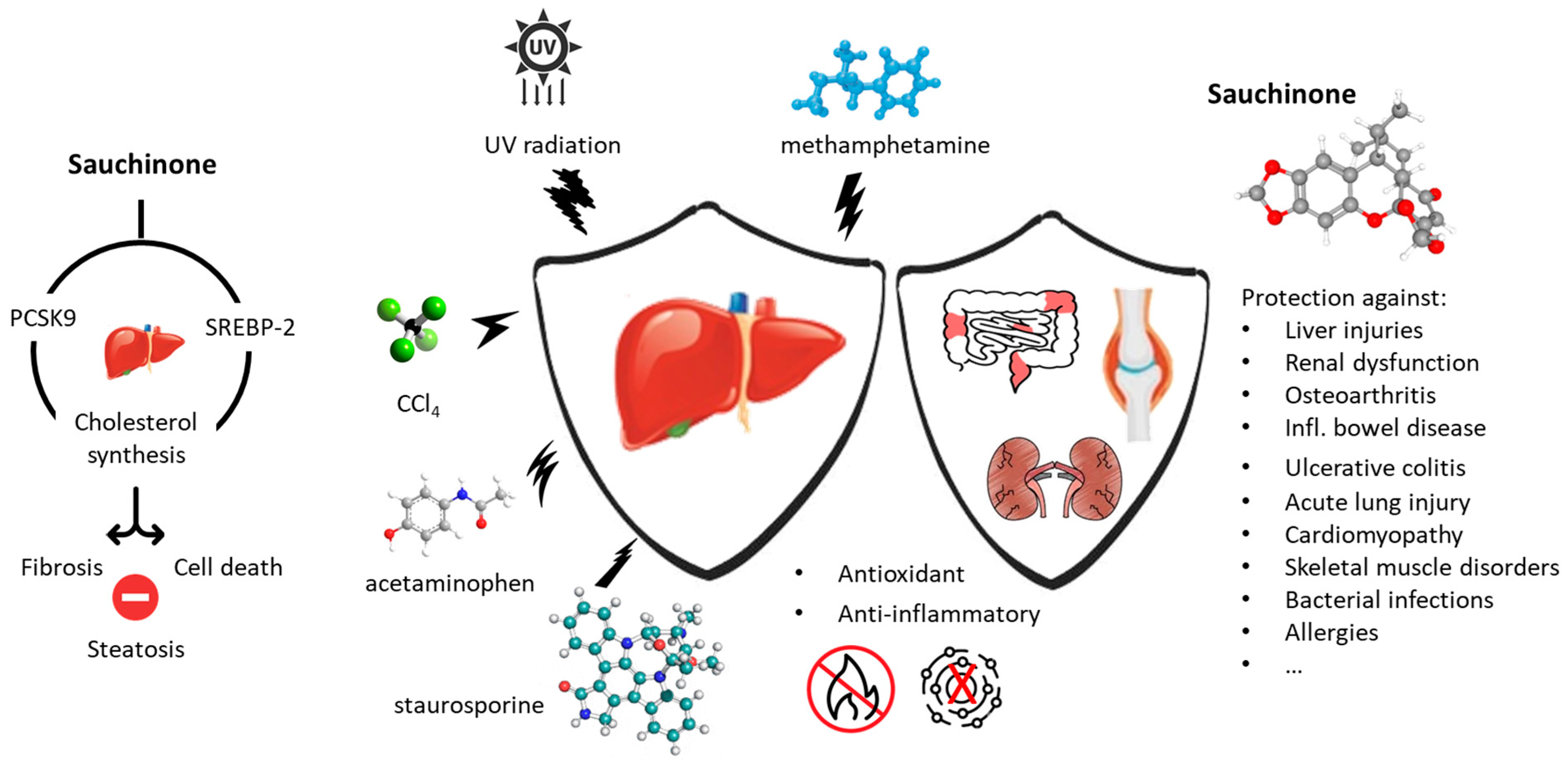
5. Pharmacological Properties of Sauchinone and Congeners
5.1. Potential Antiviral Properties
5.2. Protection from Organ Damages
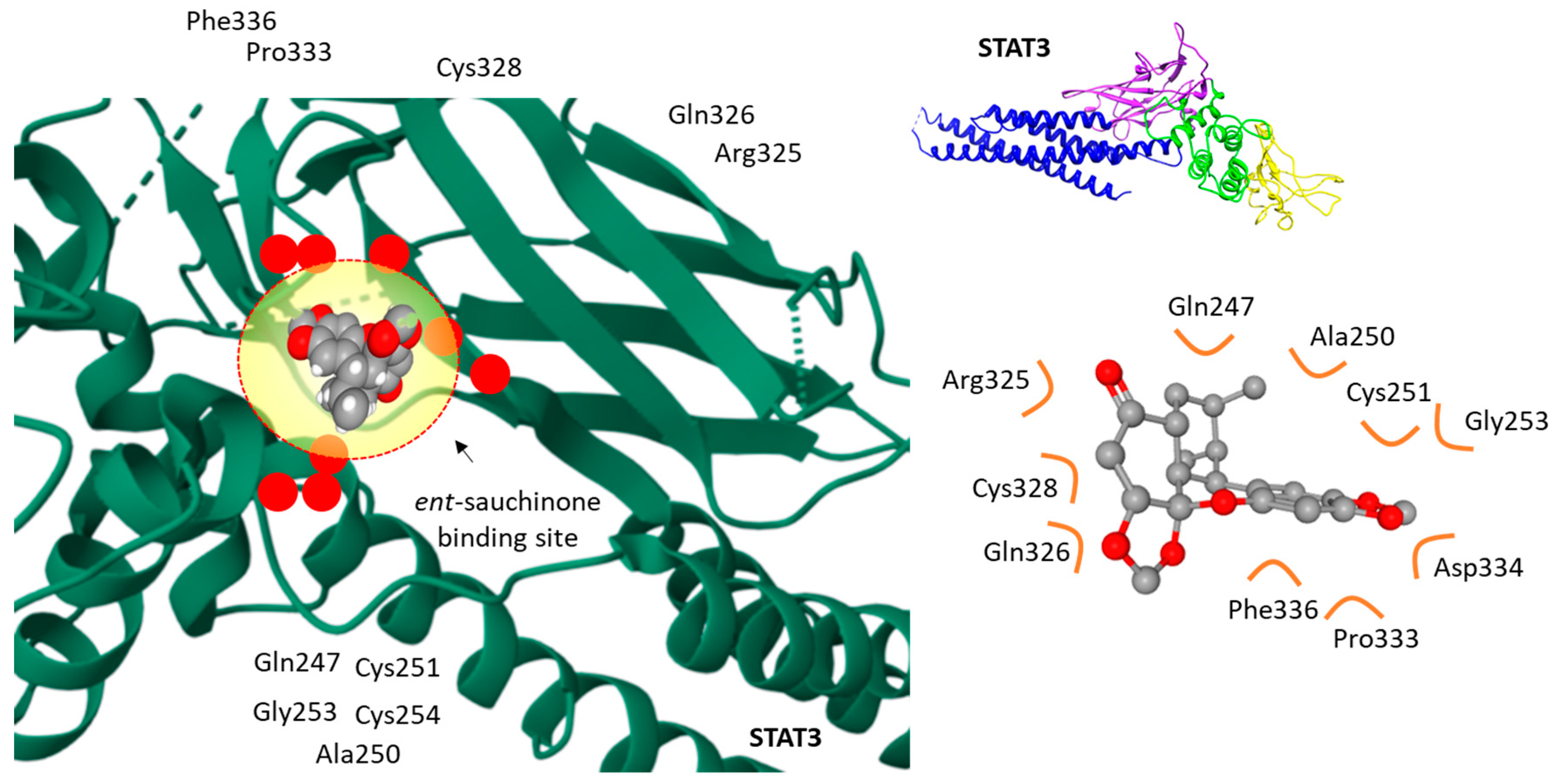
5.3. Anticancer Activities
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EIF4EBP1 | eukaryotic translation initiation factor 4E-binding protein 1 |
| EMT | epithelial-mesenchymal transition |
| GSK3β | glycogen synthase kinase-3β |
| HO-1 | heme oxygenase-1 |
| HUVEC | human umbilical vein endothelial cells |
| IBD | inflammatory bowel disease |
| IκB-α | inhibitor of kappaB alpha |
| MMP | matrix metalloproteinase |
| NF-κB | nuclear factor kappa B |
| Nrf2 | nuclear factor E2-related factor 2 |
| PCSK9 | proprotein convertase subtilisin/kexin type 9 |
| PD-L1 | programmed death-ligand 1 |
| PKCδ | protein kinase C-δ |
| rt | room temperature |
| SREBP-2 | sterol regulatory element binding protein-2 |
| STAT3 | signal transducer and activator of transcription 3 |
References
- Liu, G.; Zhao, Z.; Shen, M.; Zhao, X.; Xie, J.; He, X.; Li, C. A review of traditional uses, phytochemistry, and pharmacological properties of the genus Saururus. Am. J. Chin. Med. 2020, 48, 47–76. [Google Scholar] [CrossRef] [PubMed]
- Grímsson, F.; Grimm, G.W.; Zetter, R. Tiny pollen grains: First evidence of Saururaceae from the late Cretaceous of western North America. PeerJ 2017, 5, e3434. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.Y.; Li, Z.; Hu, S.Y.; Kao, S.M.; Zhao, T.; Wang, J.Y.; Wang, Y.; Chen, M.; Qiu, Y.; Fan, H.Y.; et al. The Saururus chinensis genome provides insights into the evolution of pollination strategies and herbaceousness in magnoliids. Plant J. 2023, 113, 1021–1034. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.H.; Zhang, X.M.; Li, D.Z. Development of the petaloid bracts of a paleoherb species, Saururus chinensis. PLoS ONE 2021, 16, e0255679. [Google Scholar] [CrossRef]
- Phares, D.L. Saururus cernuus. Atlanta Med. Surg. J. 1867, 8, 202–203. [Google Scholar]
- Badisa, R.B.; Badisa, V.L.; Walker, E.H.; Latinwo, L.M. Potent cytotoxic activity of Saururus cernuus extract on human colon and breast carcinoma cultures under normoxic conditions. Anticancer Res. 2007, 27, 189–193. [Google Scholar]
- Byun, J.K.; Lee, S.H.; Moon, E.J.; Park, M.H.; Jang, H.; Weitzel, D.H.; Kim, H.H.; Basnet, N.; Kwon, D.Y.; Lee, C.T.; et al. Manassantin A inhibits tumour growth under hypoxia through the activation of chaperone-mediated autophagy by modulating Hsp90 activity. Br. J. Cancer 2023, 128, 1491–1502. [Google Scholar] [CrossRef]
- Ma, Y.; Min, H.K.; Oh, U.; Hawkridge, A.M.; Wang, W.; Mohsin, A.A.; Chen, Q.; Sanyal, A.; Lesnefsky, E.J.; Fang, X. The lignan manassantin is a potent and specific inhibitor of mitochondrial complex I and bioenergetic activity in mammals. J. Biol. Chem. 2017, 292, 20989–20997. [Google Scholar] [CrossRef]
- Geer Wallace, M.A.; Kwon, D.Y.; Weitzel, D.H.; Lee, C.T.; Stephenson, T.N.; Chi, J.T.; Mook, R.A.; Dewhirst, M.W.; Hong, J.; Fitzgerald, M.C. Discovery of manassantin A protein targets using large-scale protein folding and stability measurements. J. Proteome Res. 2016, 15, 2688–2696. [Google Scholar] [CrossRef]
- Toneto, N.P.A.; de Brito, J.R.; Sartorelli, P.; Uemi, M.; Gonçalves, M.M.; de Rubio, I.G.S.; Lago, J.H.G.; Tamura, R.E. Cytotoxic effects of neolignans from Saururus cernuus (Saururaceae) against prostate cancer cells. Chem. Biol. Drug Des. 2023, 101, 1299–1306. [Google Scholar] [CrossRef]
- Brito, J.R.; da Costa-Silva, T.A.; Tempone, A.G.; Ferreira, E.A.; Lago, J.H.G. Dibenzylbutane neolignans from Saururus cernuus L. (Saururaceae) displayed anti-Trypanosoma cruzi activity via alterations in the mitochondrial membrane potential. Fitoterapia 2019, 137, 104251. [Google Scholar] [CrossRef] [PubMed]
- Brito, J.R.; Wilairatana, P.; Roquini, D.B.; Parra, B.C.; Gonçalves, M.M.; Souza, D.C.S.; Ferreira, E.A.; Salvadori, M.C.; Teixeira, F.S.; Lago, J.H.G.; et al. Neolignans isolated from Saururus cernuus L. (Saururaceae) exhibit efficacy against Schistosoma mansoni. Sci. Rep. 2022, 12, 19320. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.V.; Reddy, G.C. Chemistry of Saururus cernuus, V. sauristolactam and other nitrogenous constituents. J. Nat. Prod. 1990, 53, 309–312. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Sung, S.H.; Kang, S.Y.; Koo, K.A.; Kim, S.H.; Ma, C.J.; Lee, H.S.; Park, M.J.; Kim, Y.C. Aristolactam BII of Saururus chinensis attenuates glutamate-induced neurotoxicity in rat cortical cultures probably by inhibiting nitric oxide production. Planta Med. 2004, 70, 391–396. [Google Scholar]
- Dong, Y.K.; Huh, H.; Choong, K.Y.; Kim, J. Two new nitroalkyl indole alkaloids from Saururus chinensis. In Proceedings of the PSK Conference, Washington, DC, USA, 29 June 2001; p. 260. [Google Scholar]
- Li, R.; Ren, L.; Chen, Y. Chemical constituents of Saururus chinensis (Lour.) Bail. Zhongguo Zhong Yao Za Zhi 1999, 24, 479–481. [Google Scholar]
- Nguyen, T.T.O.; Nguyen, T.M.H.; Tao Vu, M.; Seo, Y.; Park, S.; Pham, V.C.; Nguyen, V.H.; Nguyen, X.N. A new furanoditerpene from Saururus chinensis aerial parts. Phytochem. Lett. 2025, 65, 24–27. [Google Scholar] [CrossRef]
- Wang, E.C.; Shih, M.H.; Liu, M.C.; Chen, M.T.; Lee, G.H. Studies on constituents of Saururus chinensis. Heterocycles 1996, 43, 969–972. [Google Scholar] [CrossRef]
- Sung, S.H.; Kim, Y.C. Hepatoprotective diastereomeric lignans from Saururus chinensis herbs. J. Nat. Prod. 2000, 63, 1019–1021. [Google Scholar] [CrossRef]
- Gao, X.; He, J.; Wu, X.D.; Peng, L.Y.; Dong, L.B.; Deng, X.; Li, Y.; Cheng, X.; Zhao, Q.S. Further lignans from Saururus chinensis. Planta Med. 2013, 79, 1720–1723. [Google Scholar]
- Seo, C.S.; Lee, Y.K.; Kim, Y.J.; Jung, J.S.; Jahng, Y.; Chang, H.W.; Song, D.K.; Son, J.K. Protective effect of lignans against sepsis from the roots of Saururus chinensis. Biol. Pharm. Bull. 2008, 31, 523–526. [Google Scholar] [CrossRef][Green Version]
- Zhuang, T.; Liang, J.Y.; Sun, J.B.; Wu, Y.; Huang, L.R.; Qu, W. Secondary metabolites from Saururus chinensis and their chemotaxonomic significance. Biochem. Syst. Ecol. 2014, 56, 95–98. [Google Scholar] [CrossRef]
- Wang, C.; Qin, X.; Wang, Y.; Wang, W.; Sun, S.; Wang, H.; Li, S.; Dai, X.; Zhu, X.; Gao, X.; et al. Noval selective ligand-extraction of bioactive components in complex natural products applying immobilized multi-target magnetic beads. Biomed. Chromatogr. 2025, 39, e6060. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Huang, X.; Wang, H.B.; Li, Z.G. Determination in the content of sauchinone from different medicament portions in Saururus chinensis (Lour.) Baill. by HPLC. Chin. J. Pharm. Anal. 2015, 35, 1505–1508. [Google Scholar]
- Chen, H.J.; Li, X.; Chen, J.W.; Guo, S.; Cai, B.C. Simultaneous determination of eleven bioactive compounds in Saururus chinensis from different harvesting seasons by HPLC-DAD. J. Pharm. Biomed. Anal. 2010, 51, 1142–1146. [Google Scholar] [CrossRef]
- Kim, S.H.; Choi, E.J.; Lee, M.K.; Lee, K.Y.; Park, J.H.; Kim, Y.C.; Sung, S.H. Simultaneous determination of five active constituents in the aerial parts of Saururus chinensis by HPLC-DAD. J. Liq. Chromatogr. Relat. Technol. 2009, 32, 2943–2953. [Google Scholar] [CrossRef]
- Lee, K.Y.; Park, J.H.; Cho, E.J.; Kim, S.H.; Kim, Y.C.; Sung, S.H. Simultaneous determination of lignans, aristolactam alkaloids and flavonoids in the Saururus chinensis by validated HPLC-DAD-MS/MS. Planta Med. 2008, 74, PC80. [Google Scholar] [CrossRef]
- Meng, X.; Kim, I.; Jeong, Y.J.; Cho, Y.M.; Kang, S.C. Anti-inflammatory effects of Saururus chinensis aerial parts in murine macrophages via induction of heme oxygenase-1. Exp. Biol. Med. 2016, 241, 396–408. [Google Scholar] [CrossRef]
- Jeong, G.S.; Li, B.; Lee, D.S.; Kwon, J.W.; Lee, H.S.; Kwon, T.O.; Kim, Y.C. Hepatoprotective Constituents of Saururus chinensis roots against tacrine-induced cytotoxicity in human liver-derived Hep G2 cells. Korean J. Pharmacogn. 2007, 38, 176–180. [Google Scholar]
- Li, N.; Tuo, Z.D.; Qi, S.Z.; Xing, S.S.; Lee, H.S.; Chen, J.G.; Cui, L. Two new lignans from Saururus chinensis and their DGAT inhibitory activity. Fitoterapia 2015, 101, 46–50. [Google Scholar] [CrossRef]
- Liang, X.L.; Zhang, C.M.; Liang, Q.P.; Li, K.P. Tissue-specific comparative transcriptome analysis reveals candidate genes involved in lignans biosynthesis in Saururus chinensis. J. Holist. Integr. Pharm. 2023, 4, 14–28. [Google Scholar] [CrossRef]
- Jin, Q.; Lee, J.W.; Kim, J.G.; Lee, D.; Hong, J.T.; Kim, Y.; Lee, M.K.; Hwang, B.Y. Lignans from Saururus chinensis with inhibitory effects on nitric oxide production. J. Nat. Prod. 2019, 82, 3002–3009. [Google Scholar] [CrossRef] [PubMed]
- Jakupovic, J.; Eid, F. Benzoxanthenone derivatives from Polemannia montana. Phytochemistry 1987, 26, 2427–2429. [Google Scholar] [CrossRef]
- Brophy, G.C.; Mohandas, J.; Slaytor, M.; Sternhell, S.; Watson, T.R.; Wilson, L.A. Novel lignans from a Cinnamomum sp. from Bougainville. Tetrahedron Lett. 1969, 59, 5159–5162. [Google Scholar] [CrossRef] [PubMed]
- Chapman, O.L.; Engel, M.R.; Springer, J.P.; Clardy, J.C. Total synthesis of carpanone. J. Am. Chem. Soc. 1971, 93, 6696–6698. [Google Scholar] [CrossRef]
- Carreira, E.M.; Hönig, M. Concise four-step total synthesis of (±)-carpanone. Synfacts 2019, 15, 221. [Google Scholar]
- Matsumoto, M.; Kuroda, K. Transition metal(ii) schiff’s base complexes catalyzed oxidation of trans-2-(1-propenyl)-4,5-methylenedioxyphenol to carpanone by molecular oxygen. Tetrahedron Lett. 1981, 22, 4437–4440. [Google Scholar] [CrossRef]
- Neuhaus, W.C.; Kozlowski, M.C. Diastereoselective synthesis of benzoxanthenones. Chem. Asian J. 2020, 15, 1039–1043. [Google Scholar] [CrossRef]
- Cortés-Borda, D.; Wimmer, E.; Gouilleux, B.; Barré, E.; Oger, N.; Goulamaly, L.; Peault, L.; Charrier, B.; Truchet, C.; Giraudeau, P.; et al. An autonomous self-optimizing flow reactor for the synthesis of natural product carpanone. J. Org. Chem. 2018, 83, 14286–14299. [Google Scholar] [CrossRef]
- Aka, E.C.; Wimmer, E.; Barré, E.; Vasudevan, N.; Cortés-Borda, D.; Ekou, T.; Ekou, L.; Rodriguez-Zubiri, M.; Felpin, F.X. Reconfigurable flow platform for automated reagent screening and autonomous optimization for bioinspired lignans synthesis. J. Org. Chem. 2019, 84, 14101–14112. [Google Scholar] [CrossRef]
- Liron, F.; Fontana, F.; Zirimwabagabo, J.O.; Prestat, G.; Rajabi, J.; La Rosa, C.; Poli, G. A new cross-coupling-based synthesis of carpanone. Org. Lett. 2009, 11, 4378–4381. [Google Scholar] [CrossRef]
- Daniels, R.N.; Fadeyi, O.O.; Lindsley, C.W. A new catalytic Cu(II)/sparteine oxidant system for β,β-phenolic couplings of styrenyl phenols: Synthesis of carpanone and unnatural analogs. Org. Lett. 2008, 10, 4097–4100. [Google Scholar] [CrossRef] [PubMed]
- Sloop, J.C. Microscale synthesis of the natural products carpanone and piperine. J. Chem. Educ. 1995, 72, A25. [Google Scholar] [CrossRef]
- Constantin, M.A.; Conrad, J.; Merişor, E.; Koschorreck, K.; Urlacher, V.B.; Beifuss, U. Oxidative dimerization of (E)- and (Z)-2-propenylsesamol with O2 in the presence and absence of laccases and other catalysts: Selective formation of carpanones and benzopyrans under different reaction conditions. J. Org. Chem. 2012, 77, 4528–4543. [Google Scholar] [CrossRef] [PubMed]
- Cardullo, N.; Muccilli, V.; Tringali, C. Laccase-mediated synthesis of bioactive natural products and their analogues. RSC Chem. Biol. 2022, 3, 614–647. [Google Scholar] [CrossRef]
- Rashid, S.; Lone, W.I.; Rashid, A.; Bhat, B.A. Inverse electron demand Diels-Alder reaction in total synthesis of bioactive natural products. Tetrahedron Chem. 2024, 9, 100066. [Google Scholar] [CrossRef]
- Fadeyi, O.O.; Daniels, R.N.; DeGuire, S.M.; Lindsley, C.W. Total synthesis of polemannones B and C. Tetrahedron Lett. 2009, 50, 3084–3087. [Google Scholar] [CrossRef]
- Baxendale, I.R.; Lee, A.L.; Ley, S.V. A concise synthesis of carpanone using solid-supported reagents and scavengers. J. Chem. Soc. Perkin Trans. 2002, 1, 1850–1857. [Google Scholar] [CrossRef]
- Lindsley, C.W.; Chan, L.K.; Goess, B.C.; Joseph, R.; Shair, M.D. Solid-phase biomimetic synthesis of carpanone-like molecules. J. Am. Chem. Soc. 2000, 122, 422–423. [Google Scholar] [CrossRef]
- Goess, B.C.; Hannoush, R.N.; Chan, L.K.; Kirchhausen, T.; Shair, M.D. Synthesis of a 10,000-membered library of molecules resembling carpanone and discovery of vesicular traffic inhibitors. J. Am. Chem. Soc. 2006, 128, 5391–5403. [Google Scholar] [CrossRef]
- Uozumi, Y.; Yamada, Y.M.A. Diversity-oriented synthesis of a 10,102-membered library of carpanones. Synfacts 2006, 8, 849. [Google Scholar]
- He, Y.J.; Xiao, D.; Gong, Q.Z.; Zhu, J.X.; Jiang, X.Y.; Dai, W.Q.; Wen, X.D.; Feng, F.; Qu, W. Synthesis, biological evaluation, and molecular docking of ent-sauchinone-based amide derivatives with potential anti-invasion and anti-migration activities. Nat. Prod. Res. 2025. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Joshi, T.; Joshi, T.; Pundir, H.; Sharma, P.; Mathpal, S.; Chandra, S. Predictive modeling by deep learning, virtual screening and molecular dynamics study of natural compounds against SARS-CoV-2 main protease. J. Biomol. Struct. Dyn. 2021, 39, 6728–6746. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.L.; Chen, J.W.; Ju, W.Z.; Liu, S.J.; Chen, Y.; Chen, Z.P.; Xue, P.; Chen, H.J.; Li, X. Quantitative determination of sauchinone in rat plasma by liquid chromatography-mass spectrometry. Biomed. Chromatogr. 2012, 26, 1210–1214. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Han, S.Y.; Seo, J.S.; Chin, Y.W.; Choi, Y.H. Pharmacokinetics, tissue distribution, and tentative metabolite identification of sauchinone in mice by microsampling and HPLC-MS/MS methods. Biol. Pharm. Bull. 2015, 38, 218–227. [Google Scholar] [CrossRef]
- Abdelkawy, K.S.; Lack, K.; Elbarbry, F. Pharmacokinetics and pharmacodynamics of promising arginase inhibitors. Eur. J. Drug Metab. Pharmacokinet. 2017, 42, 355–370. [Google Scholar] [CrossRef]
- You, B.H.; Gong, E.C.; Choi, Y.H. Inhibitory effect of sauchinone on UDP-glucuronosyltransferase (UGT) 2B7 activity. Molecules 2018, 23, 366. [Google Scholar] [CrossRef]
- Gong, E.C.; Chea, S.; Balupuri, A.; Kang, N.S.; Chin, Y.W.; Choi, Y.H. Enzyme kinetics and molecular docking studies on cytochrome 2B6, 2C19, 2E1, and 3A4 activities by sauchinone. Molecules 2018, 23, 555. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, T.; Xi, H.; Juhas, M.; Li, J. Deep learning driven drug discovery: Tackling severe acute respiratory syndrome coronavirus 2. Front. Microbiol. 2021, 12, 739684. [Google Scholar] [CrossRef]
- Romeo, A.; Iacovelli, F.; Falconi, M. Fighting SARS-CoV-2 using natural compounds: A virtual screening analysis. In High Performance Computing on CRESCO Infrastructure: Research Activity and Results 2020; De Chiara, D., Giusepponi, S., Eds.; The CRESCO infrastructure Scientific: Portici, Italy, 2020; pp. 159–166. ISBN 978-88-8286-429-3. [Google Scholar]
- Saadh, M.J.; Almaaytah, A.M.; Alaraj, M. Sauchinone with zinc sulfate significantly inhibits the activity of the SARS-CoV-2 3CL-protease. Pharmacologyonline 2021, 2, 242–248. [Google Scholar]
- Song, J.H.; Mun, S.H.; Yang, H.; Kwon, Y.S.; Kim, S.R.; Song, M.Y.; Ham, Y.; Choi, H.J.; Baek, W.J.; Cho, S.; et al. Antiviral mechanisms of saucerneol from Saururus chinensis against enterovirus A71, coxsackievirus A16, and coxsackievirus B3: Role of mitochondrial ROS and the STING/TKB-1/IRF3 pathway. Viruses 2023, 16, 16. [Google Scholar] [CrossRef]
- Cui, H.; Xu, B.; Wu, T.; Xu, J.; Yuan, Y.; Gu, Q. Potential antiviral lignans from the roots of Saururus chinensis with activity against Epstein-Barr virus lytic replication. J. Nat. Prod. 2014, 77, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Li, X.; Xu, Y.; Li, E. Effect and mechanism of Saururus chinensis against herpes dimplex virus. Zhongguo Zhong Yao Za Zhi 2012, 37, 1642–1645. [Google Scholar] [PubMed]
- Lee, J.; Huh, M.S.; Kim, Y.C.; Hattori, M.; Otake, T. Lignan, sesquilignans and dilignans, novel HIV-1 protease and cytopathic effect inhibitors purified from the rhizomes of Saururus chinensis. Antivir. Res. 2010, 85, 425–428. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.M.; Choi, J.I.; Lee, S.H.; Lee, H.J.; Son, J.K.; Seo, C.S.; Song, S.W.; Kwak, S.H.; Bae, H.B. Effect of sauchinone, a lignan from Saururus chinensis, on bacterial phagocytosis by macrophages. Eur. J. Pharmacol. 2014, 728, 176–182. [Google Scholar] [CrossRef]
- Li, B.; Lee, Y.J.; Kim, Y.C.; Yoon, J.J.; Lee, S.M.; Lee, Y.P.; Kang, D.G.; Lee, H.S. Sauchinone from Saururus chinensis protects vascular inflammation by heme oxygenase-1 induction in human umbilical vein endothelial cells. Phytomedicine 2014, 21, 101–108. [Google Scholar] [CrossRef]
- Li, B.; Lee, D.S.; Choi, H.G.; Kim, K.S.; Kang, D.G.; Lee, H.S.; Jeong, G.S.; Kim, Y.C. Sauchinone suppresses pro-inflammatory mediators by inducing heme oxygenase-1 in RAW264.7 macrophages. Biol. Pharm. Bull. 2011, 34, 1566–1571. [Google Scholar] [CrossRef]
- Jeong, G.S.; Lee, D.S.; Li, B.; Byun, E.; Kwon, D.Y.; Park, H.; Kim, Y.C. Protective effect of sauchinone by upregulating heme oxygenase-1 via the P38 MAPK and Nrf2/ARE pathways in HepG2 cells. Planta Med. 2010, 76, 41–47. [Google Scholar] [CrossRef]
- Kay, H.Y.; Kim, Y.W.; Ryu, D.H.; Sung, S.H.; Hwang, S.J.; Kim, S.G. Nrf2-mediated liver protection by sauchinone, an antioxidant lignan, from acetaminophen toxicity through the PKCδ-GSK3β pathway. Br. J. Pharmacol. 2011, 163, 1653–1665. [Google Scholar] [CrossRef]
- Hassanein, E.H.M.; Althagafy, H.S.; Baraka, M.A.; Abd-Alhameed, E.K.; Ibrahim, I.M.; Abd El-Maksoud, M.S.; Mohamed, N.M.; Ross, S.A. The promising antioxidant effects of lignans: Nrf2 activation comes into view. Naunyn Schmiedebergs Arch. Pharmacol. 2024, 397, 6439–6458. [Google Scholar] [CrossRef]
- Jang, E.Y.; Park, K.A.; Lee, J.R.; Yang, C.H.; Hwang, M. Protective effect of sauchinone on methamphetamine-induced neurotoxicity in mice. J. Pharmacol. Sci. 2012, 118, 531–536. [Google Scholar] [CrossRef]
- Song, H.; Kim, Y.C.; Moon, A. Sauchinone, a lignan from Saururus chinensis, inhibits staurosporine-induced apoptosis in C6 rat glioma cells. Biol. Pharm. Bull. 2003, 26, 1428–1430. [Google Scholar] [CrossRef] [PubMed]
- Sung, S.H.; Lee, E.J.; Cho, J.H.; Kim, H.S.; Kim, Y.C. Sauchinone, a lignan from Saururus chinensis, attenuates CCl4-induced toxicity in primary cultures of rat hepatocytes. Biol. Pharm. Bull. 2000, 23, 666–668. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Park, G.; Kim, H.G.; Sim, Y.; Sung, S.H.; Oh, M.S. Sauchinone, a lignan from Saururus chinensis, protects human skin keratinocytes against ultraviolet B-induced photoaging by regulating the oxidative defense system. Biol. Pharm. Bull. 2013, 36, 1134–1139. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jung, M.H.; Song, M.C.; Bae, K.; Kim, H.S.; Kim, S.H.; Sung, S.H.; Ye, S.K.; Lee, K.H.; Yun, Y.P.; Kim, T.J. Sauchinone attenuates oxidative stress-induced skeletal muscle myoblast damage through the down-regulation of ceramide. Biol. Pharm. Bull. 2011, 34, 575–579. [Google Scholar] [CrossRef]
- Kim, Y.W.; Kim, Y.M.; Yang, Y.M.; Kim, T.H.; Hwang, S.J.; Lee, J.R.; Kim, S.C.; Kim, S.G. Inhibition of SREBP-1c-mediated hepatic steatosis and oxidative stress by sauchinone, an AMPK-activating lignan in Saururus chinensis. Free Radic. Biol. Med. 2010, 48, 567–578. [Google Scholar] [CrossRef]
- Choi, I.Y.; Yan, H.; Park, Y.K.; Kim, W.K. Sauchinone reduces oxygen-glucose deprivation-evoked neuronal cell death via suppression of intracellular radical production. Arch. Pharm. Res. 2009, 32, 1599–1606. [Google Scholar] [CrossRef]
- Chae, H.S.; You, B.H.; Kim, D.Y.; Lee, H.; Ko, H.W.; Ko, H.J.; Choi, Y.H.; Choi, S.S.; Chin, Y.W. Sauchinone controls hepatic cholesterol homeostasis by the negative regulation of PCSK9 transcriptional network. Sci. Rep. 2018, 8, 6737. [Google Scholar] [CrossRef]
- Han, L.; Wu, L.; Yin, Q.; Li, L.; Zheng, X.; Du, S.; Huang, X.; Bai, L.; Wang, Y.; Bian, Y. A promising therapy for fatty liver disease: PCSK9 inhibitors. Phytomedicine 2024, 128, 155505. [Google Scholar] [CrossRef]
- Lee, J.H.; Jang, E.J.; Seo, H.L.; Ku, S.K.; Lee, J.R.; Shin, S.S.; Park, S.D.; Kim, S.C.; Kim, Y.W. Sauchinone attenuates liver fibrosis and hepatic stellate cell activation through TGF-beta/Smad signaling pathway. Chem. Biol. Interact. 2014, 224, 58–67. [Google Scholar] [CrossRef]
- Kim, Y.W.; Lee, S.M.; Shin, S.M.; Hwang, S.J.; Brooks, J.S.; Kang, H.E.; Lee, M.G.; Kim, S.C.; Kim, S.G. Efficacy of sauchinone as a novel AMPK-activating lignan for preventing iron-induced oxidative stress and liver injury. Free Radic. Biol. Med. 2009, 47, 1082–1092. [Google Scholar] [CrossRef]
- Yoon, J.J.; Lee, H.K.; Kim, H.Y.; Han, B.H.; Lee, H.S.; Lee, Y.J.; Kang, D.G. Sauchinone protects renal mesangial cell dysfunction against angiotensin II by improving renal fibrosis and inflammation. Int. J. Mol. Sci. 2020, 21, 7003. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, X.; Huang, X.; Ma, S.; Xing, Y.; Geng, X.; He, X. Sauchinone inhibits angiotensin II-induced proliferation and migration of vascular smooth muscle cells. Clin. Exp. Pharmacol. Physiol. 2020, 47, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhao, H.; Li, Y. Sauchinone prevents IL-1beta-induced inflammatory response in human chondrocytes. J. Biochem. Mol. Toxicol. 2018, 32, e22033. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Jin, S.; Lin, Z.; Chen, R.; Pan, T.; Kang, X.; Huang, H.; Lin, C.; Pan, J. Sauchinone inhibits IL-1beta induced catabolism and hypertrophy in mouse chondrocytes to attenuate osteoarthritis via Nrf2/HO-1 and NF-kappaB pathways. Int. Immunopharmacol. 2018, 62, 181–190. [Google Scholar] [CrossRef]
- Han, K.Y.; Yang, D.; Chang, E.J.; Lee, Y.; Huang, H.; Sung, S.H.; Lee, Z.H.; Kim, Y.C.; Kim, H.H. Inhibition of osteoclast differentiation and bone resorption by sauchinone. Biochem. Pharmacol. 2007, 74, 911–923. [Google Scholar] [CrossRef]
- Chen, Q.; He, Q.; Xiu, W.; Chen, Y.; Guo, Z. miR-340 affects sauchinone inhibition of Th17 cell differentiation and promotes intestinal inflammation in inflammatory bowel disease. Biochem. Biophys. Res. Commun. 2020, 526, 1157–1163. [Google Scholar] [CrossRef]
- Xiu, W.; Chen, Y.; Chen, Q.; Deng, B.; Su, J.; Guo, Z. Sauchinone attenuates inflammatory responses in dendritic cells via Blimp-1 and ameliorates dextran sulfate sodium (DSS)-induced colitis. Biochem. Biophys. Res. Commun. 2020, 527, 902–908. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, J.; Chen, Y.; Zhou, Z.; Gao, C.; Guo, Z. Sauchinone ameliorates intestinal inflammation and promotes Th17 cell production of IL-10 via Blimp-1. Biochem. Biophys. Res. Commun. 2020, 522, 435–441. [Google Scholar] [CrossRef]
- Wu, K.; Liu, X.; Meng, X.; Cao, L.; Li, H.; Bi, Y.; Wang, M.; Wang, M.; Jiang, Y. Sauchinone alleviates dextran sulfate sodium-induced ulcerative colitis via NAD(P)H dehydrogenase [quinone] 1/NF-kB pathway and gut microbiota. Front. Microbiol. 2023, 13, 1084257. [Google Scholar] [CrossRef]
- Han, H.J.; Li, M.; Son, J.K.; Seo, C.S.; Song, S.W.; Kwak, S.H.; Bae, H.B. Sauchinone, a lignan from Saururus chinensis, attenuates neutrophil pro-inflammatory activity and acute lung injury. Int. Immunopharmacol. 2013, 17, 471–477. [Google Scholar] [CrossRef]
- Bae, H.B.; Li, M.; Son, J.K.; Seo, C.S.; Chung, S.H.; Kim, S.J.; Jeong, C.W.; Lee, H.G.; Kim, W.; Park, H.C.; et al. Sauchinone, a lignan from Saururus chinensis, reduces tumor necrosis factor-alpha production through the inhibition of c-raf/MEK1/2/ERK 1/2 pathway activation. Int. Immunopharmacol. 2010, 10, 1022–1028. [Google Scholar] [CrossRef] [PubMed]
- Thapalia, B.A.; Zhou, Z.; Lin, X. Sauchinone augments cardiomyocyte viability by enhancing autophagy proteins -PI3K, ERK(1/2), AMPK and Beclin-1 during early ischemia-reperfusion injury in vitro. Am. J. Transl. Res. 2016, 8, 3251–3265. [Google Scholar] [PubMed]
- Kim, S.J.; Jeong, C.W.; Bae, H.B.; Kwak, S.H.; Son, J.K.; Seo, C.S.; Lee, H.J.; Lee, J.; Yoo, K.Y. Protective effect of sauchinone against regional myocardial ischemia/reperfusion injury: Inhibition of p38 MAPK and JNK death signaling pathways. J. Korean Med. Sci. 2012, 27, 572–575. [Google Scholar] [CrossRef] [PubMed]
- Min, H.J.; Won, H.Y.; Kim, Y.C.; Sung, S.H.; Byun, M.R.; Hwang, J.H.; Hong, J.H.; Hwang, E.S. Suppression of Th2-driven, allergen-induced airway inflammation by sauchinone. Biochem. Biophys. Res. Commun. 2009, 385, 204–209. [Google Scholar] [CrossRef]
- Quan, Z.; Lee, Y.J.; Yang, J.H.; Lu, Y.; Li, Y.; Lee, Y.K.; Jin, M.; Kim, J.Y.; Choi, J.H.; Son, J.K.; et al. Ethanol extracts of Saururus chinensis suppress ovalbumin-sensitization airway inflammation. J. Ethnopharmacol. 2010, 132, 143–149. [Google Scholar] [CrossRef]
- Jang, E.Y.; Yang, C.H.; Han, M.H.; Choi, Y.H.; Hwang, M. Sauchinone suppresses lipopolysaccharide-induced inflammatory responses through Akt signaling in BV2 cells. Int. Immunopharmacol. 2012, 14, 188–194. [Google Scholar] [CrossRef]
- Lee, C.S.; Won, C.; Yoo, H.; Yi, E.H.; Cho, Y.; Maeng, J.W.; Sung, S.H.; Ye, S.K.; Chung, M.H. Inhibition of double-stranded RNA-induced inducible nitric oxide synthase expression by fraxinellone and sauchinone in murine microglia. Biol. Pharm. Bull. 2009, 32, 1870–1874. [Google Scholar] [CrossRef][Green Version]
- Jiao, Y.; Kim, S.C.; Wang, Y.; Wu, T.; Jin, H.; Lee, C.W.; Park, S.J.; Lee, B.H.; Kim, H.Y.; Yang, C.H.; et al. Sauchinone blocks ethanol withdrawal-induced anxiety but spares locomotor sensitization: Involvement of nitric oxide in the bed nucleus of the stria terminalis. Evid. Based Complement. Altern. Med. 2021, 2021, 6670212. [Google Scholar] [CrossRef]
- Deng, Y.; Jin, F.; Li, X.; Park, S.J.; Chang, J.H.; Kim, D.Y.; Kim, J.A.; Nam, J.W.; Choi, H.; Lee, Y.J.; et al. Sauchinone suppresses FcepsilonRI-mediated mast cell signaling and anaphylaxis through regulation of LKB1/AMPK axis and SHP-1-Syk signaling module. Int. Immunopharmacol. 2019, 74, 105702. [Google Scholar] [CrossRef]
- Song, S.Y.; Jung, Y.Y.; Hwang, C.J.; Lee, H.P.; Sok, C.H.; Kim, J.H.; Lee, S.M.; Seo, H.O.; Hyun, B.K.; Choi, D.Y.; et al. Inhibitory effect of ent-Sauchinone on amyloidogenesis via inhibition of STAT3-mediated NF-κB activation in cultured astrocytes and microglial BV-2 cells. J. Neuroinflamm. 2014, 11, 118. [Google Scholar] [CrossRef]
- Gong, Q.Z.; Xiao, D.; Feng, F.; Wen, X.D.; Qu, W. ent-Sauchinone as potential anticancer agent inhibiting migration and invasion of human liver cancer cells via suppressing the STAT3 signaling pathway. Chem. Biodivers. 2018, 15, e1800024. [Google Scholar] [CrossRef] [PubMed]
- Jeong, G.S.; Lee, D.S.; Park, B.H.; Kwon, K.B.; Kim, Y.C. Sauchinone protects pancreatic beta cells against cytokine-mediated toxicity. Toxicol. Vitr. 2011, 25, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.H.; Sung, N.J.; Shin, S.; Ryu, D.S.; Youn, H.S.; Park, S.A. Sauchinone inhibits the proliferation, migration and invasion of breast cancer cells by suppressing Akt-CREB-MMP13 signaling pathway. Biosci. Rep. 2021, 41, BSR20211067. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.K.; Sung, S.H.; Kim, Y.C.; Kim, S.G. Inhibition of lipopolysaccharide-inducible nitric oxide synthase, TNF-alpha and COX-2 expression by sauchinone effects on I-kappaBalpha phosphorylation, C/EBP and AP-1 activation. Br. J. Pharmacol. 2003, 139, 11–20. [Google Scholar] [CrossRef]
- Kim, H.Y.; Choi, T.W.; Kim, H.J.; Kim, S.M.; Park, K.R.; Jang, H.J.; Lee, E.H.; Kim, C.Y.; Jung, S.H.; Shim, B.S.; et al. A methylene chloride fraction of Saururus chinensis induces apoptosis through the activation of caspase-3 in prostate and breast cancer cells. Phytomedicine 2011, 18, 567–574. [Google Scholar] [CrossRef]
- Kim, Y.W.; Jang, E.J.; Kim, C.H.; Lee, J.H. Sauchinone exerts anticancer effects by targeting AMPK signaling in hepatocellular carcinoma cells. Chem. Biol. Interact. 2017, 261, 108–117. [Google Scholar] [CrossRef]
- He, Z.; Dong, W.; Li, Q.; Qin, C.; Li, Y. Sauchinone prevents TGF-beta-induced EMT and metastasis in gastric cancer cells. Biomed. Pharmacother. 2018, 101, 355–361. [Google Scholar] [CrossRef]
- Li, S.Q.; Feng, J.; Yang, M.; Ai, X.P.; He, M.; Liu, F. Sauchinone: A prospective therapeutic agent-mediated EIF4EBP1 down-regulation suppresses proliferation, invasion and migration of lung adenocarcinoma cells. J. Nat. Med. 2020, 74, 777–787. [Google Scholar] [CrossRef]
- Qiao, Y.; Yan, L.J.; Yan, C. Sauchinone inhibits hypoxia-induced epithelial-mesenchymal transition in pancreatic ductal adenocarcinoma cells through the Wnt/beta-catenin pathway. Anticancer Drugs 2020, 31, 918–924. [Google Scholar] [CrossRef]
- Zhou, D.; He, L. Sauchinone inhibits hypoxia-induced invasion and epithelial-mesenchymal transition in osteosarcoma cells via inactivation of the sonic hedgehog pathway. J. Recept. Signal Transduct. Res. 2022, 42, 173–179. [Google Scholar] [CrossRef]
- Hu, X.; Wang, J.; Shang, P.; Wang, S.; Chen, L.; Ye, C.; Yao, G. Sauchinone inhibits breast cancer cell proliferation through regulating microRNA-148a-3p/HER-2 axis. Thorac. Cancer 2023, 14, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yu, M.; Ye, J.; Liang, Y.; Gao, J.; Ji, Z.; Wang, J. Sauchinone inhibits the proliferation and immune invasion capacity of colorectal cancer cells through the suppression of PD-L1 and MMP2/MM9. Anticancer Agents Med. Chem. 2023, 23, 1406–1414. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Cui, Y.; Zhen, W.; Li, P.; Wei, H. Study on the apoptosis of PC3 cells induced by sauchinone and its mechanism. Anti-Tumor Pharm. 2018, 8, 537–540. [Google Scholar]
- Xin, W.; Yang, H.; Heng, X.; Xu, T.; Zhang, K.; Zhao, Y.; Liu, Y.; Han, D.; Wu, Y.; Zhang, W.; et al. Sauchinone preserves cardiac function in doxorubicin-induced cardiomyopathy by inhibiting the NLRP3 inflammasome. Phytomedicine 2025, 140, 156624. [Google Scholar] [CrossRef]
- Barbera, M.; Kettunen, M.I.; Caputo, A.; Hu, D.E.; Gobbi, S.; Brindle, K.M.; Carrara, M. Immune-modulating and anti-vascular activities of two xanthenone acetic acid analogues: A comparative study to DMXAA. Int. J. Oncol. 2009, 34, 273–279. [Google Scholar] [CrossRef]
- Baguley, B.C.; Ching, L.M. Immunomodulatory actions of xanthenone anticancer agents. BioDrugs 1997, 8, 119–127. [Google Scholar] [CrossRef]
- Patel, K.; Gupta, V.; Patel, D.K. Sauchinone, an active phytochemical of Saururus chinensis, and its use as therapeutic. Pharmacol. Res.–Mod. Chin. Med. 2024, 12, 100486. [Google Scholar] [CrossRef]
- Kuk, M.U.; Lee, Y.H.; Kim, D.; Lee, K.S.; Park, J.H.; Yoon, J.H.; Lee, Y.J.; So, B.; Kim, M.; Kwon, H.W.; et al. Sauchinone ameliorates senescence through reducing mitochondrial ROS production. Antioxidants 2025, 14, 259. [Google Scholar] [CrossRef]


| Patent Numbers | Titles | Publication Dates (Owner) |
|---|---|---|
| CN119235730A | Composition with red fading effect and application thereof. Filed by Shanghai Jiakai Biological Co., Ltd. Published 2025-01-03. | 2025-01-03 (Shanghai Jiakai Biological Co., Ltd.) |
| CN118845919A | A bacteriostatic, whitening, and tender composition containing Impatiens balsamina extract and its application. | 2024-10-29 (Zhengzhou Yipin Pharmaceutical Technology Co., Ltd.) |
| CN118512378A | Whitening composition. | 2024-08-20 (Plant Doctor Guangdong Biotechnology Co., Ltd.) |
| CN109828055A | A kind of method for building up of the HPLC fingerprint of Saururus chinensis medicinal material. | 2022-03-22 (Guangdong Second Traditional Chinese Medicine Hospital. Sun Yat Sen University) |
| KR102138073B1 | Composition comprising sauchinone for preventing or treating chronic pulmonary disease. | 2020-07-27 (*) |
| CN106749311A | Reflect sauchinone analog derivative and its preparation method and application. | 2019-03-29 (China Pharmaceutical University) |
| KR101887631B1 | Composition for delaying senescence, lifespan extension, or preventing, treating, or improving diseases caused by lipofuscin accumulation comprising sauchinone as an active ingredient of Saururus chinensis. | 2018-08-13 (*) |
| CN106810565B | Reflect sauchinone analog derivative and its application in antitumor drug is prepared. | 2018-04-17 (China Pharmaceutical University) |
| CN103550370B | A kind of Chinese medicine compound for the treatment of gynecological inflammation. | 2015-09-16 (Hunan Agricultural University) |
| KR101269208B1 | Composition comprising sauchinone as an active ingredient for preventing or treating insulin resistance. | 2013-05-30 (*) |
| CN103073560A | Sauchinone derivative and preparing method and application thereof. | 2013-05-01 (Nanjing University of Chinese Medicine) |
| KR20130005118A | Pharmaceutical composition for preventing or treating muscle disease and disorders containing sauchinone. | 2013-01-15 (*) |
| KR20090095309A | Composition for alleviation or treatment of atopic skin disease comprising sauchinone or Saururus chinensis (lour.) Baill extract having sauchinone. | 2010-07-29 (*) |
| CN101372491A | A kind of enantiomer of lignan tribagudone 1 and its preparation method and liver protection application. | 2009-02-25 (South China Sea Institute of Oceanology of CAS) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bailly, C. Benzoxanthenone Lignans Related to Carpanone, Polemanone, and Sauchinone: Natural Origin, Chemical Syntheses, and Pharmacological Properties. Molecules 2025, 30, 1696. https://doi.org/10.3390/molecules30081696
Bailly C. Benzoxanthenone Lignans Related to Carpanone, Polemanone, and Sauchinone: Natural Origin, Chemical Syntheses, and Pharmacological Properties. Molecules. 2025; 30(8):1696. https://doi.org/10.3390/molecules30081696
Chicago/Turabian StyleBailly, Christian. 2025. "Benzoxanthenone Lignans Related to Carpanone, Polemanone, and Sauchinone: Natural Origin, Chemical Syntheses, and Pharmacological Properties" Molecules 30, no. 8: 1696. https://doi.org/10.3390/molecules30081696
APA StyleBailly, C. (2025). Benzoxanthenone Lignans Related to Carpanone, Polemanone, and Sauchinone: Natural Origin, Chemical Syntheses, and Pharmacological Properties. Molecules, 30(8), 1696. https://doi.org/10.3390/molecules30081696








