Using Green Solvents for Phase Inversion of PVDF/TiO2 Hybrid Coatings for Gas Phase Photocatalysis
Abstract
1. Introduction
2. Results and Discussion
2.1. Structural Analysis
2.2. Diffuse Reflectance
2.3. Photocatalytic Degradation of Ethanol
2.4. PVDF UV and Photocatalysis Stability
3. Materials and Methods
3.1. Materials
3.2. Synthesis
3.3. Experimental Setup
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DI | Deionized |
| EDX | Energy dispersive X-ray spectroscopy |
| LED | Light emitting diode |
| NMP | N-methyl-2-pyrrolidone |
| PC | Propylene carbonate |
| PLA | Polylactic acid |
| PVDF | Poly(vinylidene fluoride-co-hexa-fluoropropylene) |
| QMS | Quadrupole mass spectrometer |
| SEM | Scanning electron microscopy |
| TEP | Triethyl phosphate |
| UV | Ultraviolet |
| VOC | Volatile organic compound |
| XPS | X-ray photoelectron spectroscopy |
References
- Guo, H.; Lee, S.; Chan, L.; Li, W. Risk assessment of exposure to volatile organic compounds in different indoor environments. Environ. Res. 2004, 94, 57–66. [Google Scholar] [CrossRef]
- Alford, K.L.; Kumar, N. Pulmonary Health Effects of Indoor Volatile Organic Compounds-A Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 1578. [Google Scholar] [CrossRef]
- Rodriguez, C.; Linge, K.; Blair, P.; Busetti, F.; Devine, B.; Van Buynder, P.; Weinstein, P.; Cook, A. Recycled water: Potential health risks from volatile organic compounds and use of 1,4-dichlorobenzene as treatment performance indicator. Water Res. 2012, 46, 93–106. [Google Scholar] [CrossRef]
- Mangotra, A.; Singh, S.K. Volatile organic compounds: A threat to the environment and health hazards to living organisms—A review. J. Biotechnol. 2024, 382, 51–69. [Google Scholar] [CrossRef]
- Peiris, S.; de Silva, H.B.; Ranasinghe, K.N.; Bandara, S.V.; Perera, I.R. Recent development and future prospects of TiO2 photocatalysis. J. Chin. Chem. Soc. 2021, 68, 738–769. [Google Scholar] [CrossRef]
- Mamaghani, A.H.; Haghighat, F.; Lee, C. Photocatalytic oxidation technology for indoor environment air purification: The state-of-the-art. Appl. Catal. B Environ. 2017, 203, 247–269. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Kumar, B.G.; Rajendran, S.; Qin, J.; Vadivel, S.; Durgalakshmi, D.; Gracia, F.; Soto-Moscoso, M.; Orooji, Y.; Karimi, F. Tuning of metal oxides photocatalytic performance using Ag nanoparticles integration. J. Mol. Liq. 2020, 314, 113588. [Google Scholar] [CrossRef]
- Cheng, C.; He, B.; Fan, J.; Cheng, B.; Cao, S.; Yu, J. An Inorganic/Organic S-Scheme Heterojunction H2-Production Photocatalyst and its Charge Transfer Mechanism. Adv. Mater. 2021, 33, 2100317. [Google Scholar] [CrossRef]
- Arun, J.; Nachiappan, S.; Rangarajan, G.; Alagappan, R.P.; Gopinath, K.P.; Lichtfouse, E. Synthesis and application of titanium dioxide photocatalysis for energy, decontamination and viral disinfection: A review. Environ. Chem. Lett. 2023, 21, 339–362. [Google Scholar] [CrossRef]
- Bono, N.; Ponti, F.; Punta, C.; Candiani, G. Effect of UV Irradiation and TiO2-Photocatalysis on Airborne Bacteria and Viruses: An Overview. Materials 2021, 14, 1075. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Zhang, W.; Shi, Z.; Zhang, F.; Liu, X.; Jin, J.; Jiang, L. Superhydrophobic and Superoleophilic PVDF Membranes for Effective Separation of Water-in-Oil Emulsions with High Flux. Adv. Mater. 2013, 25, 2071–2076. [Google Scholar] [CrossRef]
- Mortazavi Milani, H.; Sabbagh Alvani, A.A.; Salimi, R.; Sameie, H.; Poelman, D. Ag-functionalized Bi2W(Mo)O6/PVDF membrane for photocatalytic water treatment. J. Mater. Sci. 2021, 56, 16339–16350. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Magnone, E.; Lee, J.I.; Zhuang, X.; Shin, M.C.; Park, J.H. S- and N-Co-Doped TiO2-Coated Al2O3 Hollow Fiber Membrane for Photocatalytic Degradation of Gaseous Ammonia. Membranes 2022, 12, 1101. [Google Scholar] [CrossRef]
- Magnone, E.; Hwang, J.Y.; Shin, M.C.; Zhuang, X.; Lee, J.I.; Park, J.H. Al2O3-Based Hollow Fiber Membranes Functionalized by Nitrogen-Doped Titanium Dioxide for Photocatalytic Degradation of Ammonia Gas. Membranes 2022, 12, 693. [Google Scholar] [CrossRef]
- Tarrass, F.; Benjelloun, M. Health and environmental effects of the use of N-methyl-2-pyrrolidone as a solvent in the manufacture of hemodialysis membranes: A sustainable reflexion. Nefrología 2022, 42, 122–124. [Google Scholar] [CrossRef]
- Drouin, D.; Couture, A.R.; Joly, D.; Tastet, X.; Aimez, V.; Gauvin, R. CASINO V2.42—A Fast and Easy-to-use Modeling Tool for Scanning Electron Microscopy and Microanalysis Users. Scanning 2007, 29, 92–101. [Google Scholar] [CrossRef]
- Cosaert, E.; Wolfs, C.; Lambert, S.D.; Heynderickx, G.J.; Poelman, D. Deposition of Hybrid Photocatalytic Layers for Air Purification Using Commercial TiO2 Powders. Molecules 2021, 26, 6584. [Google Scholar] [CrossRef]
- Chin, S.; Chiang, K.; Fane, A. The stability of polymeric membranes in a TiO2 photocatalysis process. J. Membr. Sci. 2006, 275, 202–211. [Google Scholar] [CrossRef]
- Landry, V.; Blanchet, P. Weathering resistance of opaque PVDF-acrylic coatings applied on wood substrates. Prog. Org. Coatings 2012, 75, 494–501. [Google Scholar] [CrossRef]
- Botelho, G.; Silva, M.M.; Goncalves, A.M.; Sencadas, V.; Serrado-Nunes, J.; Lanceros-Mendez, S. Performance of electroactive poly(vinylidene fluoride) against UV radiation. Polym. Test. 2008, 27, 818–822. [Google Scholar] [CrossRef]
- Mortazavi Milani, H.; Van Neste, B.; Cosaert, E.; Poelman, D. Assessing the Stability and Photocatalytic Efficiency of a Biodegradable PLA-TiO2 Membrane for Air Purification. Adv. Sustain. Syst. 2025, 9, 2400594. [Google Scholar] [CrossRef]
- Dallaev, R.; Pisarenko, T.; Sobola, D.; Orudzhev, F.; Ramazanov, S.; Trčka, T. Brief Review of PVDF Properties and Applications Potential. Polymers 2022, 14, 4793. [Google Scholar] [CrossRef]
- Fairley, N.; Fernandez, V.; Richard-Plouet, M.; Guillot-Deudon, C.; Walton, J.; Smith, E.; Flahaut, D.; Greiner, M.; Biesinger, M.; Tougaard, S.; et al. Systematic and collaborative approach to problem solving using X-ray photoelectron spectroscopy. Appl. Surf. Sci. Adv. 2021, 5, 100112. [Google Scholar] [CrossRef]
- Eufinger, K.; De Gryse, R.; Poelman, D. Effect of Deposition Conditions and Doping on the Structure, Optical Properties and Photocatalytic Activity of D.C. Magnetron Sputtered TiO2 Thin Films. Ph.D. Thesis, Ghent University, Faculty of Sciences, Ghent, Belgium, 2007. [Google Scholar]
- Wallace, W.E. Mass Spectrum (Electron Ionization)—Ethanol; Technical report; NIST Mass Spectrometry Data Center: Gaithersburg, MD, USA, 2014. [Google Scholar]
- Wallace, W.E. Mass Spectrum (Electron Ionization)—Carbon Dioxide; Technical report; NIST Mass Spectrometry Data Center: Gaithersburg, MD, USA, 2014. [Google Scholar]
- ISO 9845-1:2022; Solar Energy—Reference Solar Spectral Irradiance at the Ground at Different Receiving Conditions—Part 1: Direct Normal and Hemispherical Solar Irradiance for Air Mass 1,5. Technical report; International Organization for Standardization: Geneva, Switzerland, 2022.

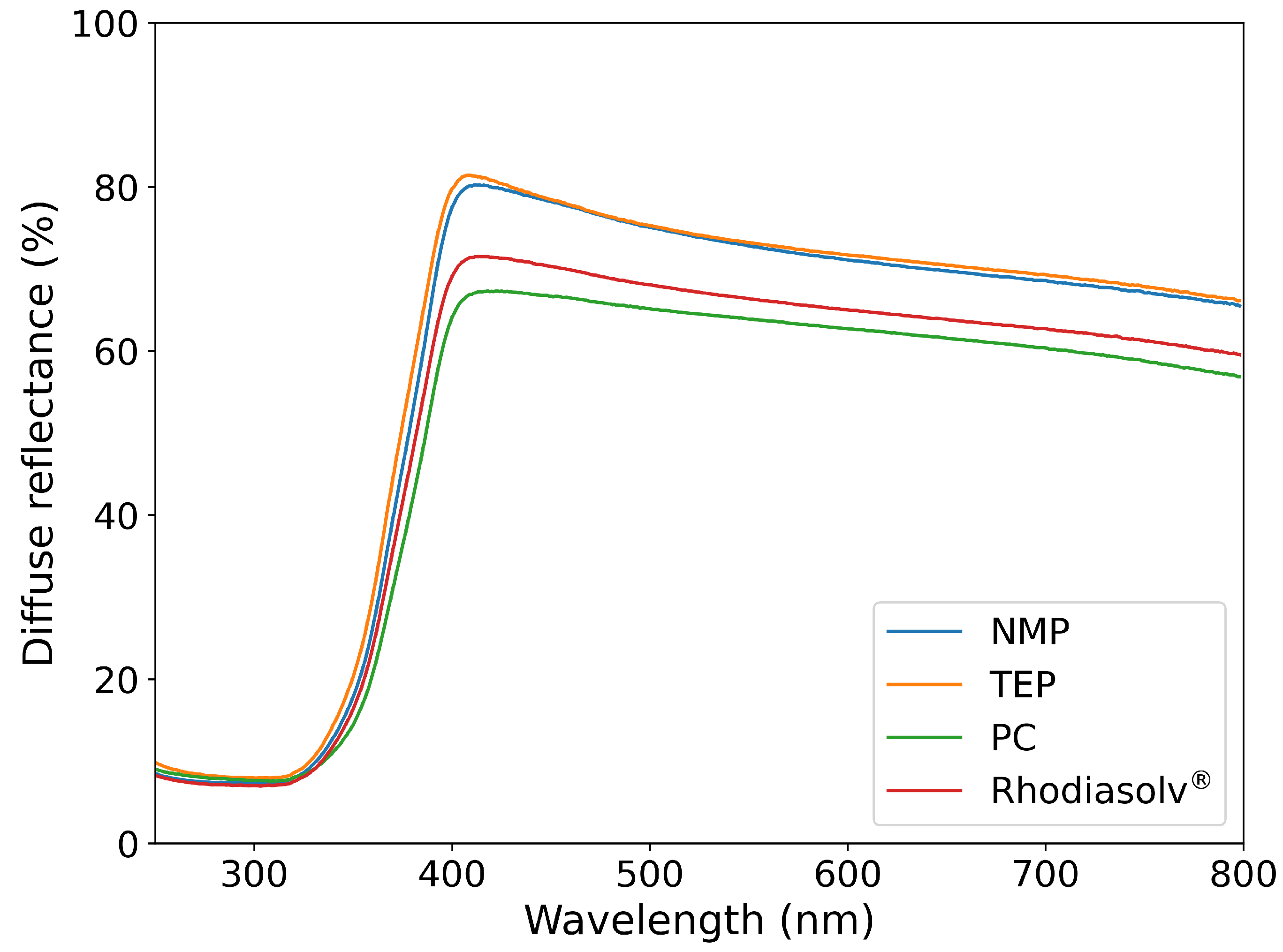


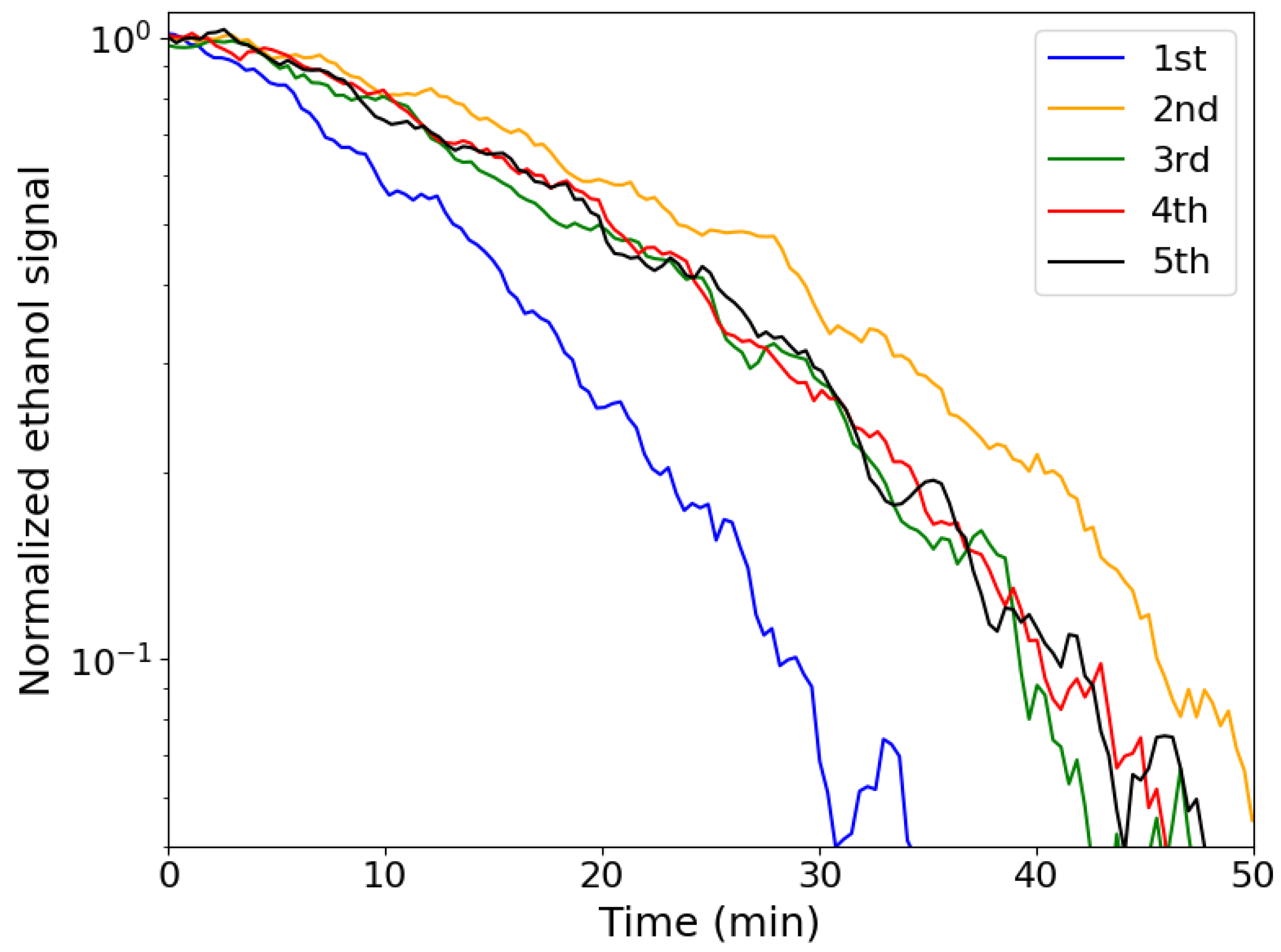
| NMP | TEP | Rhodiasolv® | PC | |
|---|---|---|---|---|
| EDX (7 kV) | 0.30 | 0.31 | 0.32 | 0.27 |
| EDX (1.5 kV) | 0.25 | 0.20 | 0.20 | 0.21 |
| XPS | 0.06 | 0.03 | 0.04 | 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cosaert, E.; Mortazavi Milani, H.; Heynderickx, G.J.; Poelman, D. Using Green Solvents for Phase Inversion of PVDF/TiO2 Hybrid Coatings for Gas Phase Photocatalysis. Molecules 2025, 30, 1700. https://doi.org/10.3390/molecules30081700
Cosaert E, Mortazavi Milani H, Heynderickx GJ, Poelman D. Using Green Solvents for Phase Inversion of PVDF/TiO2 Hybrid Coatings for Gas Phase Photocatalysis. Molecules. 2025; 30(8):1700. https://doi.org/10.3390/molecules30081700
Chicago/Turabian StyleCosaert, Ewoud, Hadis Mortazavi Milani, Geraldine J. Heynderickx, and Dirk Poelman. 2025. "Using Green Solvents for Phase Inversion of PVDF/TiO2 Hybrid Coatings for Gas Phase Photocatalysis" Molecules 30, no. 8: 1700. https://doi.org/10.3390/molecules30081700
APA StyleCosaert, E., Mortazavi Milani, H., Heynderickx, G. J., & Poelman, D. (2025). Using Green Solvents for Phase Inversion of PVDF/TiO2 Hybrid Coatings for Gas Phase Photocatalysis. Molecules, 30(8), 1700. https://doi.org/10.3390/molecules30081700







