Tin Disulfide Nanosheet as Cathode Materials for Rechargeable Aluminum Ion Batteries: Synthesis, Electrochemical Performance, and Mechanism
Abstract
1. Introduction
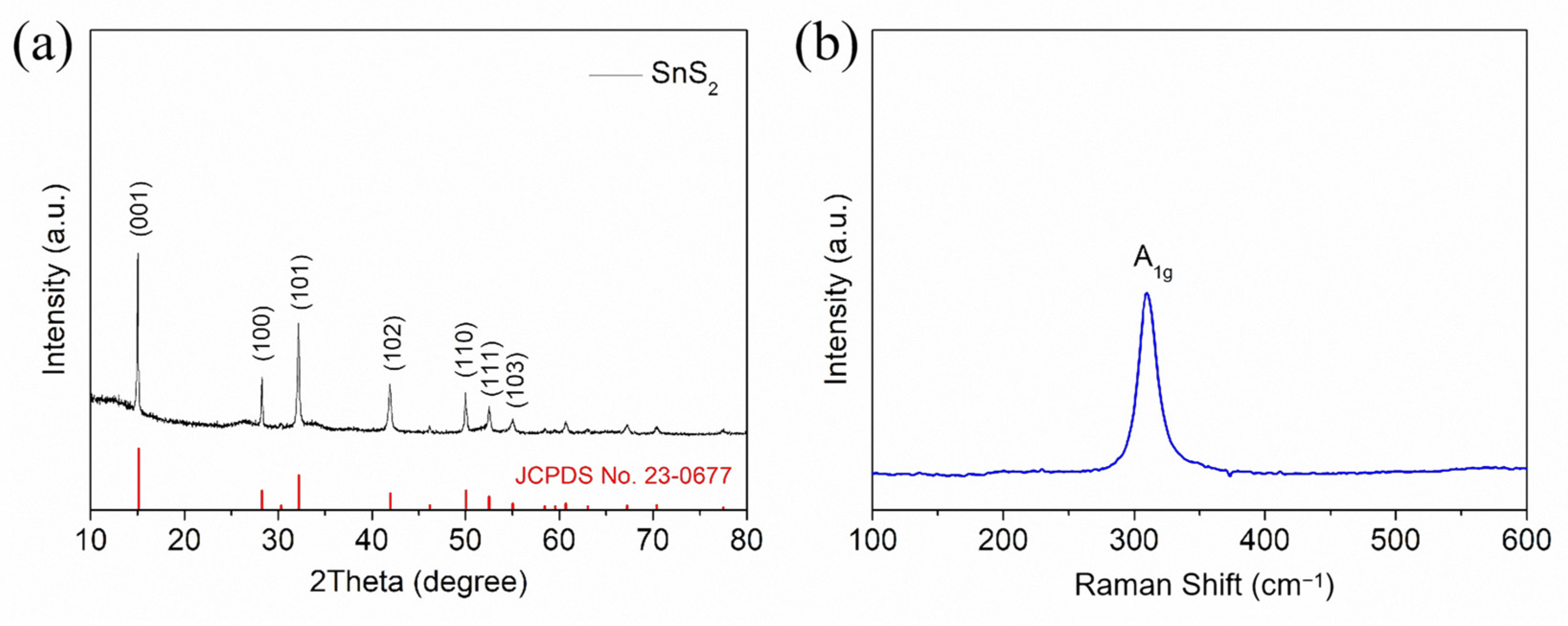
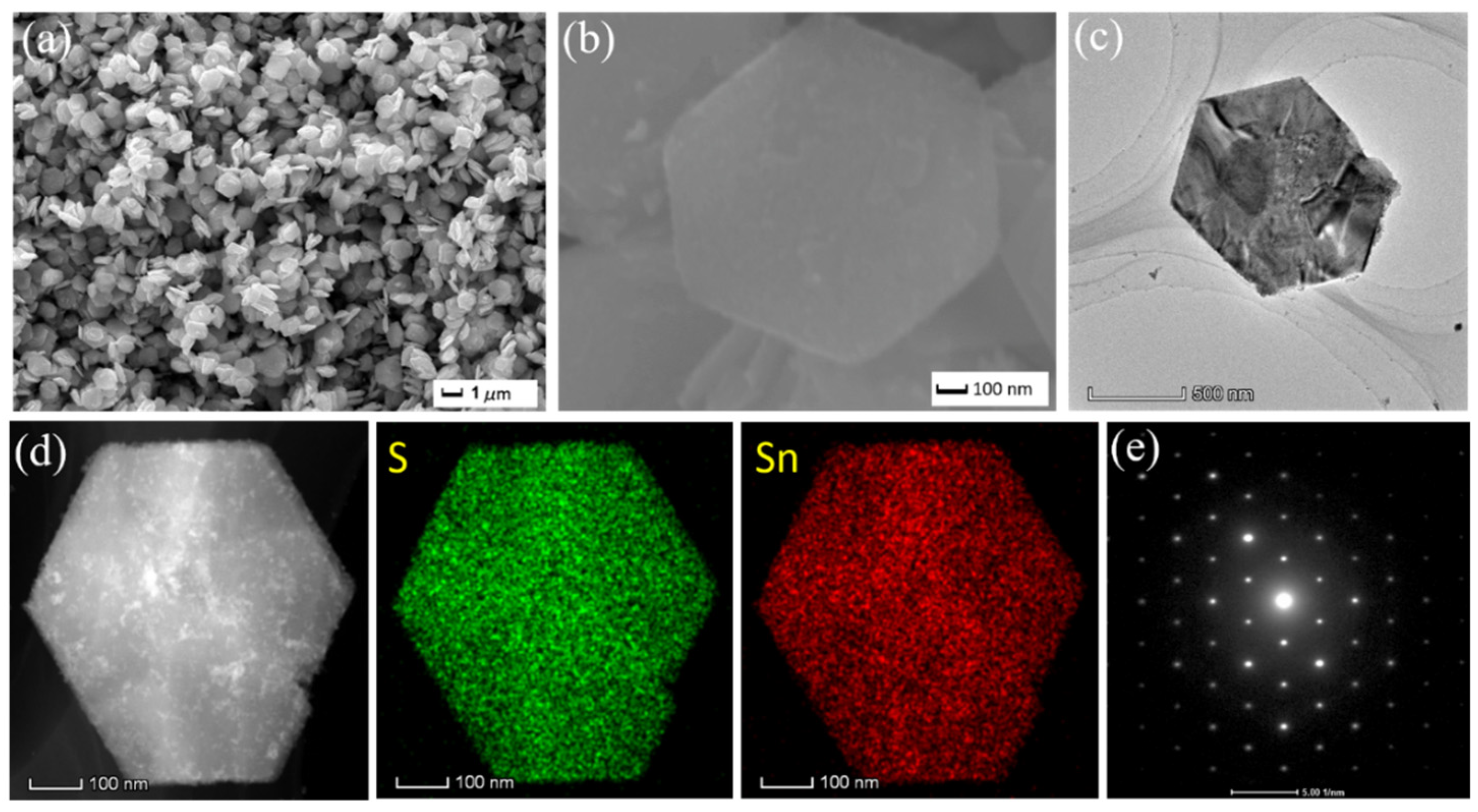
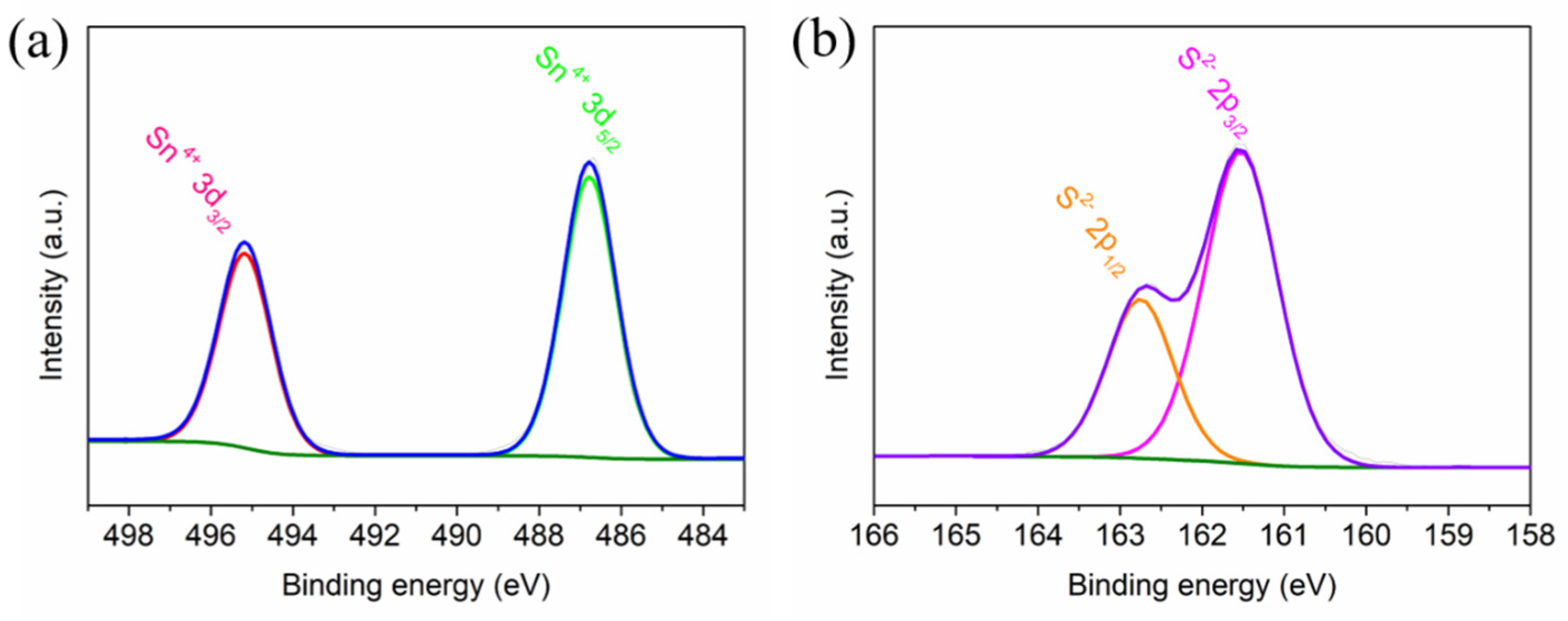
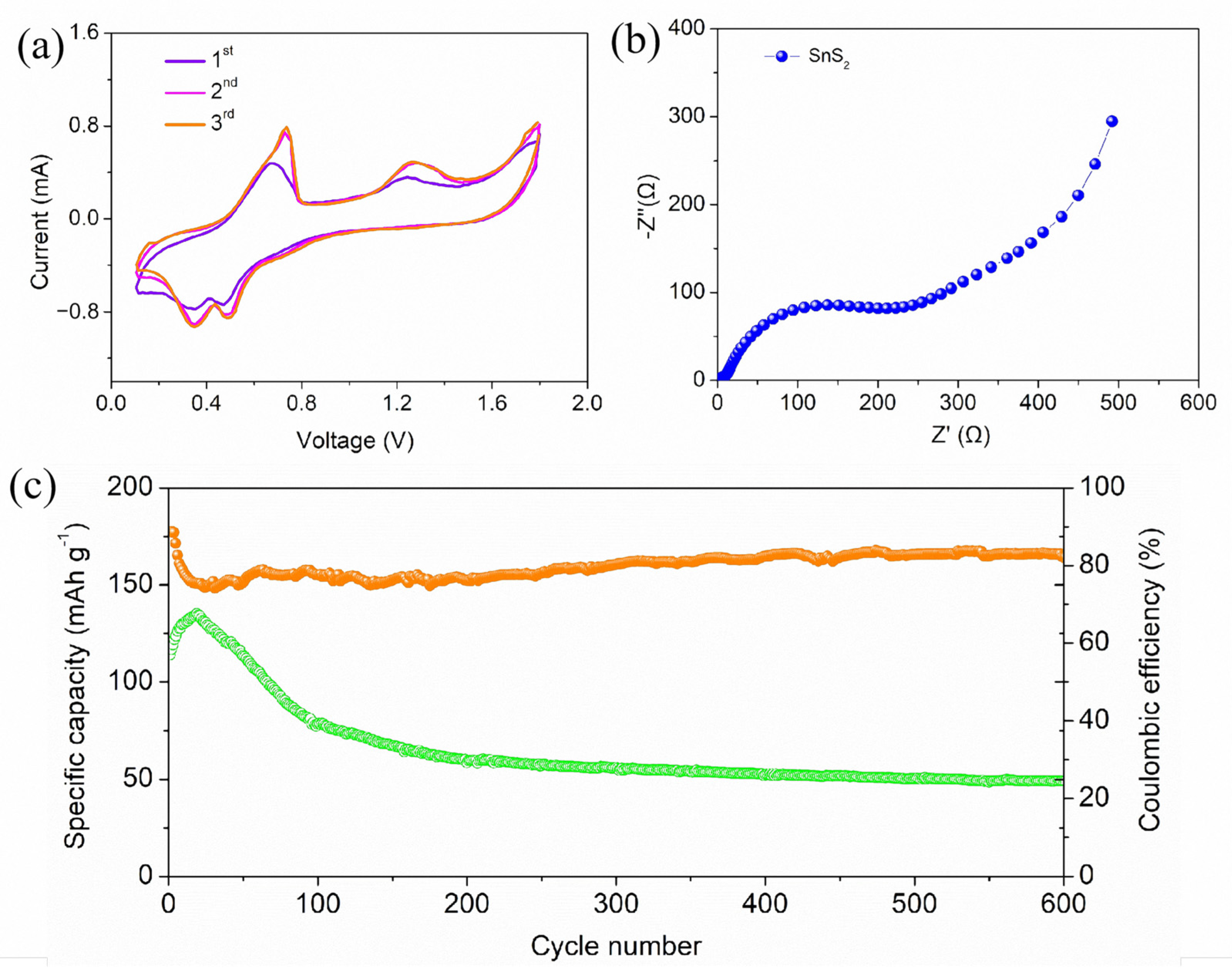
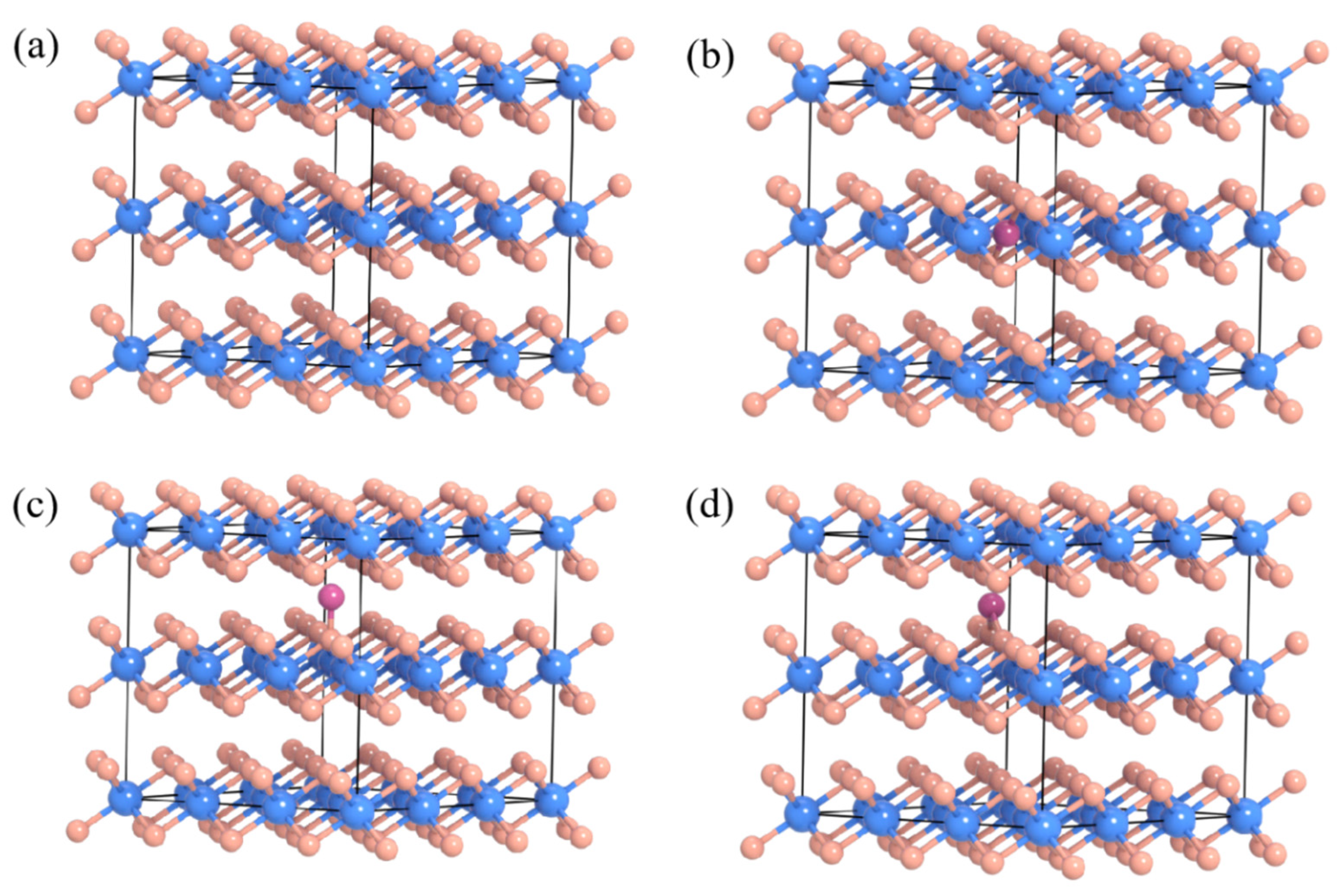
2. Results
3. Materials and Methods
3.1. Synthesis of the SnS2
3.2. Synthesis of the Electrode
3.3. Battery Composition
3.4. Characterization and Measurements
3.5. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gao, Y.; Yu, Q.; Yang, H.; Zhang, J.; Wang, W. The Enormous Potential of Sodium/Potassium-Ion Batteries as the Mainstream Energy Storage Technology for Large-Scale Commercial Applications. Adv. Mater. 2024, 36, 2405989. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, S.; Li, N.; Chen, J.; Chen, Y.; Zhang, Z.; Tan, L.; Niu, X.; Yang, Y.; Zhang, J.; et al. Prospects and Challenges of Practical Nonaqueous Potassium-Ion Batteries. Adv. Funct. Mater. 2024, 34, 2408965. [Google Scholar] [CrossRef]
- Shi, C.M.; Wang, T.Y.; Liao, X.B.; Qie, B.Y.; Yang, P.F.; Chen, M.J.; Wang, X.; Srinivasan, A.; Cheng, Q.; Ye, Q.; et al. Accordion-like stretchable Li-ion batteries with high energy density. Energy Storage Mater. 2019, 17, 136–142. [Google Scholar] [CrossRef]
- Chen, Q.; Yang, Z.; Zhai, P.; Luo, W.; Gong, Y. Lithium Batteries Operating at Wide Temperatures: Opportunities and Challenges. Adv. Funct. Mater. 2024, 35, 2417923. [Google Scholar] [CrossRef]
- Gupta, S.; Vishwakarma, J.; Srivastava, A.; Dhand, C.; Dwivedi, N. Aluminum Batteries: Opportunities and Challenges. Energy Storage Mater. 2024, 70, 103538. [Google Scholar] [CrossRef]
- Yang, C.; Liang, Z.; Dong, B.; Guo, Y.; Xie, W.; Chen, M.; Zhang, K.; Zhou, L. Heterostructure Engineering for Aluminum-Ion Batteries: Mechanism, Challenge, and Perspective. Small 2024, 20, 2405495. [Google Scholar] [CrossRef]
- Hu, E.; Jia, B.; Nong, W.; Zhang, C.; Zhu, B.; Wu, D.; Liu, J.; Wu, C.; Xi, S.; Xia, D.; et al. Boosting Aluminum Adsorption and Deposition on Single-Atom Catalysts in Aqueous Aluminu-Ion Battery. Adv. Energy Mater. 2024, 14, 2401598. [Google Scholar] [CrossRef]
- Yang, X.; Huang, C. Progress in improving the performance of inorganic cathodes for aluminum-ion batteries. J. Energy Storage 2024, 78, 110069. [Google Scholar] [CrossRef]
- Ma, D.; Li, J.; Li, H.; Yuan, D.; Ji, Z.; Manawan, M.; Albarran, C.; Wu, C.; Pan, J. Progress of advanced cathode materials of rechargeable aluminum-ion batteries. Energy Mater. Adv. 2024, 5, 0088. [Google Scholar] [CrossRef]
- Shin, D.; Yang, J.; Mukherjee, S.; Bahrami, A.; Lehmann, S.; Nasiri, N.; Krahl, F.; Pang, C.; Wrzesińska-Lashkova, A.; Vaynzof, Y.; et al. SnS2 Thin Film with In Situ and Controllable Sb Doping via Atomic Layer Deposition for Optoelectronic Applications. Adv. Mater. Technol. 2024, 9, 2302049. [Google Scholar]
- Shan, Y.; Li, Y.; Pang, H. Applications of tin sulfide-based materials in lithium-ion batteries and sodium-ion batteries. Adv. Funct. Mater. 2020, 30, 2001298. [Google Scholar] [CrossRef]
- Hong, S.; Popovitz-Biro, R.; Prior, Y.; Tenne, R. Synthesis of SnS2/SnS fullerene-like nanoparticles: A superlattice with polyhedral shape. J. Am. Chem. Soc. 2003, 125, 10470–10474. [Google Scholar] [CrossRef] [PubMed]
- Setayeshmehr, M.; Haghighi, M.; Mirabbaszadeh, K. A review of tin disulfide (SnS2) composite electrode materials for supercapacitors. Energy Storage 2022, 4, e295. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, H.; Pei, Y.; Chen, Y.; Zeng, Y.; Guo, H. Binder-free ultrathin SnS2 with superior reversibility of conversion reaction for high-rate lithium ion batteries. J. Alloys Compd. 2021, 873, 159623. [Google Scholar] [CrossRef]
- Li, R.; Tong, L.; Jiang, Y.; Wang, Y.; Long, J.; Chen, X.; Wu, J.; Li, X.; Chen, Y. SnS2 nanoparticles embedded in sulfurized polyacrylonitrile composite fibers for high-performance potassium-ion batteries. Interdiscipl. Mater. 2024, 3, 150–159. [Google Scholar] [CrossRef]
- Tai, P.; Chung, R.; Kongvarhodom, C.; Husain, S.; Yougbaré, S.; Chen, H.; Wu, Y.; Lin, L. Structure-directing agent mediated synthesis of SnS2 coupled with UltrapheneTM as highly stable anode material for sodium-ion battery. J. Colloid Interf. Sci. 2025, 679, 691–702. [Google Scholar] [CrossRef]
- Honeker, C.; Thomas, E. Impact of morphological orientation in determining mechanical properties in triblock copolymer systems. Chem. Mater. 1996, 8, 1702–1714. [Google Scholar] [CrossRef]
- Fu, Y.; Zhu, H.; Chen, J.; Hautzinger, M.; Zhu, X.; Jin, S. Metal halide perovskite nanostructures for optoelectronic applications and the study of physical properties. Nat. Rev. Mater. 2019, 4, 169–188. [Google Scholar]
- Roberts, A.; Li, X.; Zhang, H. Porous carbon spheres and monoliths: Morphology control, pore size tuning and their applications as Li-ion battery anode materials. Chem. Soc. Rev. 2014, 43, 4341–4356. [Google Scholar] [CrossRef]
- Li, S.; Wang, K.; Zhang, G.; Li, S.; Xu, Y.; Zhang, X.; Zhang, X.; Zheng, S.; Sun, X.; Ma, Y. Fast charging anode materials for lithium-ion batteries: Current status and perspectives. Adv. Funct. Mater. 2022, 32, 2200796. [Google Scholar] [CrossRef]
- Acerce, M.; Akdoğan, E.; Chhowalla, M. Metallic molybdenum disulfide nanosheet-based electrochemical actuators. Nature 2017, 549, 370–373. [Google Scholar] [PubMed]
- Zhu, X.; Hu, Y.; Wu, G.; Chen, W.; Bao, N. Two-dimensional nanosheets-based soft electro-chemo-mechanical actuators: Recent advances in design, construction, and applications. ACS Nano 2021, 15, 9273–9298. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.; Hoa, N.; Hung, C.; Le, D.; Duy, N.; Hieu, N. A comparative study on the electrochemical properties of nanoporous nickel oxide nanowires and nanosheets prepared by a hydrothermal method. RSC Adv. 2018, 8, 19449–19455. [Google Scholar]
- Gholamvand, Z.; McAteer, D.; Harvey, A.; Backes, C.; Coleman, J. Electrochemical applications of two-dimensional nanosheets: The effect of nanosheet length and thickness. Chem. Mater. 2016, 28, 2641–2651. [Google Scholar] [CrossRef]
- Liang, F.; Dong, H.; Dai, J.; He, H.; Zhang, W.; Chen, S.; Lv, D.; Liu, H.; Kim, I.; Lai, Y.; et al. Fast Energy Storage of SnS2 Anode Nanoconfined in Hollow Porous Carbon Nanofibers for Lithium-Ion Batteries. Adv. Sci. 2024, 11, 2306711. [Google Scholar]
- Dong, C.; Xia, Y.; Su, Z.; Han, Z.; Dong, Y.; Chen, J.; Hao, F.; Yu, Q.; Jiang, Q.; Ye, J. Synergistic effect of carbon nanotube and encapsulated carbon layer enabling high-performance SnS2-based anode for lithium storage. J. Energy Chem. 2024, 97, 700–709. [Google Scholar] [CrossRef]
- Li, H.; Diao, M.; Boukhvalov, D.; Ke, Y.; Humphrey, M.; Zhang, C.; Huang, Z. Prominent nonlinear optical absorption in SnS2-based hybrid inorganic-organic superlattice. Adv. Funct. Mater. 2024, 34, 2400077. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, H.; Du, Z.; Chang, X.; Zhao, L.; Du, X.; Li, Z.; Teng, Y.; Fang, J.; Świerczek, K. (101) plane-oriented SnS2 nanoplates with carbon coating: A high-rate and cycle-stable anode material for lithium ion batteries. ACS Appl. Mater. Interfaces 2017, 9, 35880–35887. [Google Scholar]
- Wu, Y.; Zhao, Y.; Meng, W.; Xie, Y.; Zhang, J.; He, C.; Zhao, D. Nanoplates-assembled SnS2 nanoflowers with carbon coating anchored on reduced graphene oxide for high performance Li-ion batteries. Appl. Surf. Sci. 2021, 539, 148283. [Google Scholar] [CrossRef]
- Kim, G.; Lim, Y.; Shin, J.; Yim, J.; Hur, S.; Song, H.; Baek, S.; Kim, S.; Kim, J.; Kang, C.; et al. Breathable MOFs layer on atomically grown 2D SnS2 for stable and selective surface activation. Adv. Sci. 2023, 10, 2301002. [Google Scholar]
- Jang, J.; Lee, M.; Park, S.; Oh, J.; Park, J.; Paek, S. Long-term cycling stability of a SnS2-based covalent organic nanosheet anode for lithium-ion batteries. J. Mater Chem. A 2023, 11, 13320–13330. [Google Scholar] [CrossRef]
- Zhao, Z.; Sun, G.; Zhang, Y.; Hua, R.; Wang, X.; Wu, N.; Li, J.; Liu, G.; Guo, D.; Cao, A.; et al. Introduction of SnS2 to regulate the ferrous disulfide phase evolution for the construction of triphasic heterostructures enabling kinetically accelerated and durable sodium storage. Adv. Funct. Mater. 2024, 34, 2314679. [Google Scholar] [CrossRef]
- Li, C.; Hou, J.; Zhang, J.; Li, X.; Jiang, S.; Zhang, G.; Yao, Z.; Liu, T.; Shen, S.; Liu, Z.; et al. Heterostructured NiS2@SnS2 hollow spheres as superior high-rate and durable anodes for sodium-ion batteries. Sci. China Chem. 2022, 65, 1420–1432. [Google Scholar]
- He, H.; Cong, H.; Sun, Y.; Zan, L.; Zhang, Y. Spinel-layered integrate structured nanorods with both high capacity and superior high-rate capability as cathode material for lithium-ion batteries. Nano Res. 2017, 10, 556–569. [Google Scholar]
- Li, Y.; Huang, Y.; Wang, X.; Liu, W.; Yu, K.; Liang, C. Simple synthesis of rice husk hollow carbon-coated flower ZnO for the anode in a high performance lithium-ion battery. J. Phys. Chem. Solids 2020, 145, 109540. [Google Scholar] [CrossRef]
- Hu, Y.; Luo, B.; Ye, D.; Zhu, X.; Lyu, M.; Wang, L. An innovative freeze-dried reduced graphene oxide supported SnS2 cathode active material for aluminum-iaon batteries. Adv. Mater. 2017, 29, 1606132. [Google Scholar] [CrossRef]
- Yang, W.; Lu, H.; Cao, Y.; Jing, P. Single-/few-layered ultrasmall WS2 nanoplates embedded in nitrogen-doped carbon nanofibers as a cathode for rechargeable aluminum batteries. J. Power Sources 2019, 441, 227173. [Google Scholar] [CrossRef]
- Wu, L.; Sun, R.M.; Xiong, F.Y.; Pei, G.Y.; Han, K.; Peng, C.; Fan, Y.Q.; Yang, W.; An, Q.Y.; Mai, L.Q. A rechargeable aluminum-ion battery based on a VS2 nanosheet cathode. Phys. Chem. Chem. Phys. 2018, 20, 22563–22568. [Google Scholar]
- Xing, L.L.; Owusu, K.A.; Liu, X.Y.; Meng, J.S.; Wang, K.; An, Q.Y.; Mai, L.Q. Insights into the storage mechanism of VS4 nanowire clusters in aluminum-ion battery. Nano Energy 2021, 79, 105384. [Google Scholar] [CrossRef]
- Wang, S.; Yu, Z.; Tu, J.; Wang, J.; Tian, D.; Liu, Y.; Jiao, S. A novel aluminum-ion battery: Al/AlCl3-[EMIm]Cl/Ni3S2@Graphene. Adv. Energy Mater. 2016, 6, 1600137. [Google Scholar]
- Geng, L.; Lv, G.; Xing, X.; Guo, J. Reversible electrochemical intercalation of aluminum in Mo6S8. Chem. Mater. 2015, 27, 4926–4929. [Google Scholar] [CrossRef]
- Li, H.; Yang, H.; Sun, Z.; Shi, Y.; Cheng, H.-M.; Li, F. A highly reversible Co3S4 microsphere cathode material for aluminum-ion batteries. Nano Energy 2019, 56, 100–108. [Google Scholar] [CrossRef]
- Liang, K.; Ju, L.; Koul, S.; Kushima, A.; Yang, Y. Self-supported Tin sulfide porous films for flexible aluminum-ion batteries. Adv. Energy Mater. 2019, 9, 1802543. [Google Scholar] [CrossRef]
- Geng, L.; Scheifers, J.P.; Fu, C.; Zhang, J.; Fokwa, B.P.T.; Guo, J. Titanium sulfides as intercalation-type cathode materials for rechargeable aluminum batteries. ACS Appl. Mater. Inter. 2017, 9, 21251–21257. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, S.; Tu, J.; Zhang, G.; Li, S.; Tian, D.; Jiao, S. Flower-like vanadium suflide/reduced graphene oxide composite: An energy storage material for aluminum-ion batteries. Chemsuschem 2018, 11, 709–715. [Google Scholar] [CrossRef]
- Li, Z.; Niu, B.; Liu, J.; Li, J.; Kang, F. Rechargeable aluminum-ion battery based on MoS2 microsphere cathode. ACS Appl. Mater. Inter. 2018, 10, 9451–9459. [Google Scholar] [CrossRef]
- Zhang, K.Q.; Lee, T.H.; Cha, J.H.; Jang, H.W.; Shokouhimehr, M.; Choi, J. -W. Properties of CoS2/CNT as a cathode material of rechargeable aluminum-ion batteries. Electron. Mater. Lett. 2019, 15, 727–732. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuang, R.; Tan, X.; Wang, Y.; Wang, J.; Zhan, J.; Yan, J.; Zhang, J.; Wang, L. Tin Disulfide Nanosheet as Cathode Materials for Rechargeable Aluminum Ion Batteries: Synthesis, Electrochemical Performance, and Mechanism. Molecules 2025, 30, 1649. https://doi.org/10.3390/molecules30081649
Zhuang R, Tan X, Wang Y, Wang J, Zhan J, Yan J, Zhang J, Wang L. Tin Disulfide Nanosheet as Cathode Materials for Rechargeable Aluminum Ion Batteries: Synthesis, Electrochemical Performance, and Mechanism. Molecules. 2025; 30(8):1649. https://doi.org/10.3390/molecules30081649
Chicago/Turabian StyleZhuang, Ruiyuan, Xinming Tan, Yuxin Wang, Junhong Wang, Jianfeng Zhan, Jiangnan Yan, Jun Zhang, and Lixiang Wang. 2025. "Tin Disulfide Nanosheet as Cathode Materials for Rechargeable Aluminum Ion Batteries: Synthesis, Electrochemical Performance, and Mechanism" Molecules 30, no. 8: 1649. https://doi.org/10.3390/molecules30081649
APA StyleZhuang, R., Tan, X., Wang, Y., Wang, J., Zhan, J., Yan, J., Zhang, J., & Wang, L. (2025). Tin Disulfide Nanosheet as Cathode Materials for Rechargeable Aluminum Ion Batteries: Synthesis, Electrochemical Performance, and Mechanism. Molecules, 30(8), 1649. https://doi.org/10.3390/molecules30081649





