Natural and Designed Cyclic Peptides as Potential Antiviral Drugs to Combat Future Coronavirus Outbreaks
Abstract
1. Introduction
2. Health, Social, and Economic Consequences of the COVID-19 Pandemic
3. Coronaviruses
3.1. SARS-CoV
3.2. MERS-CoV
3.3. SARS-CoV-2
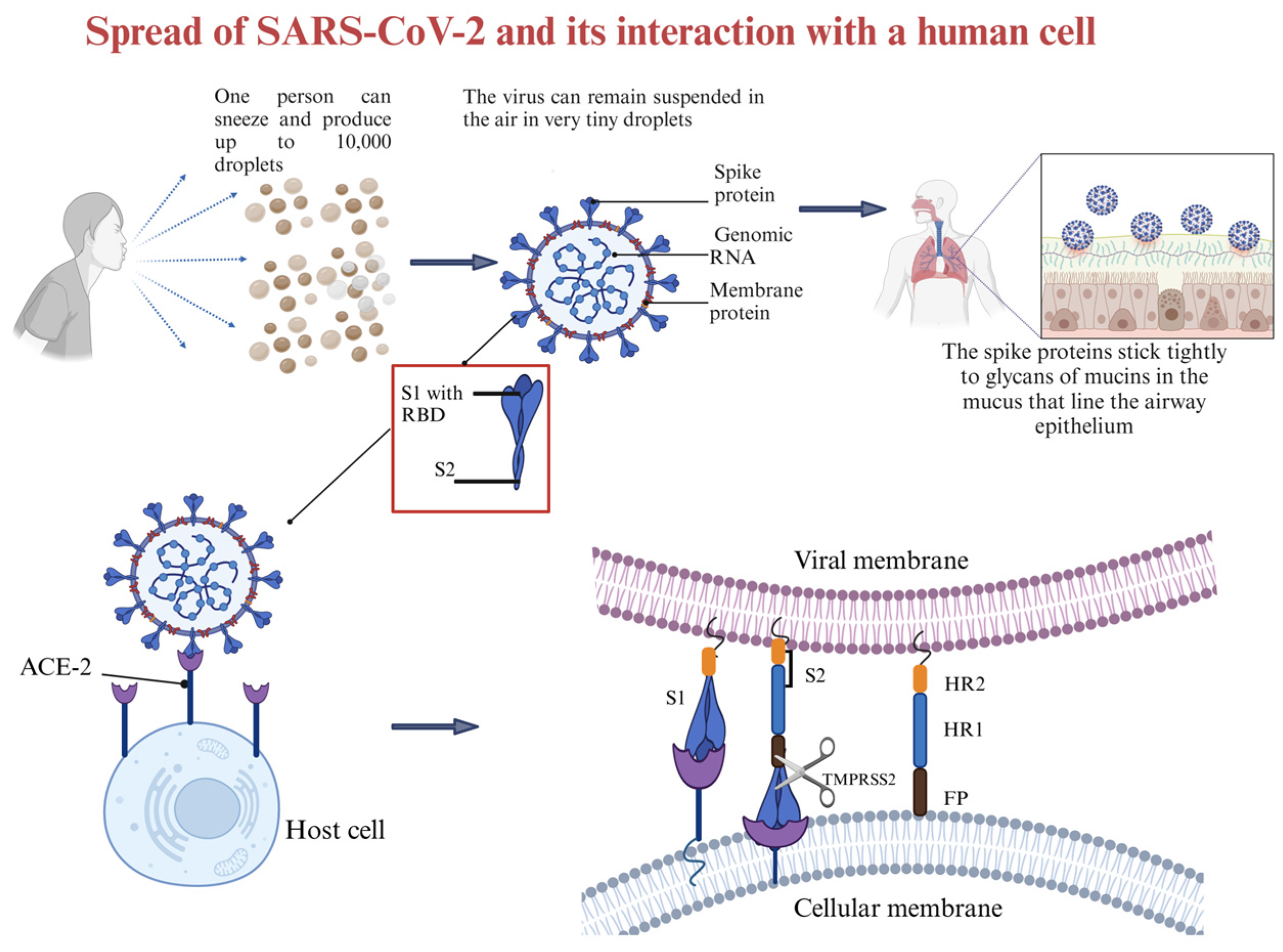
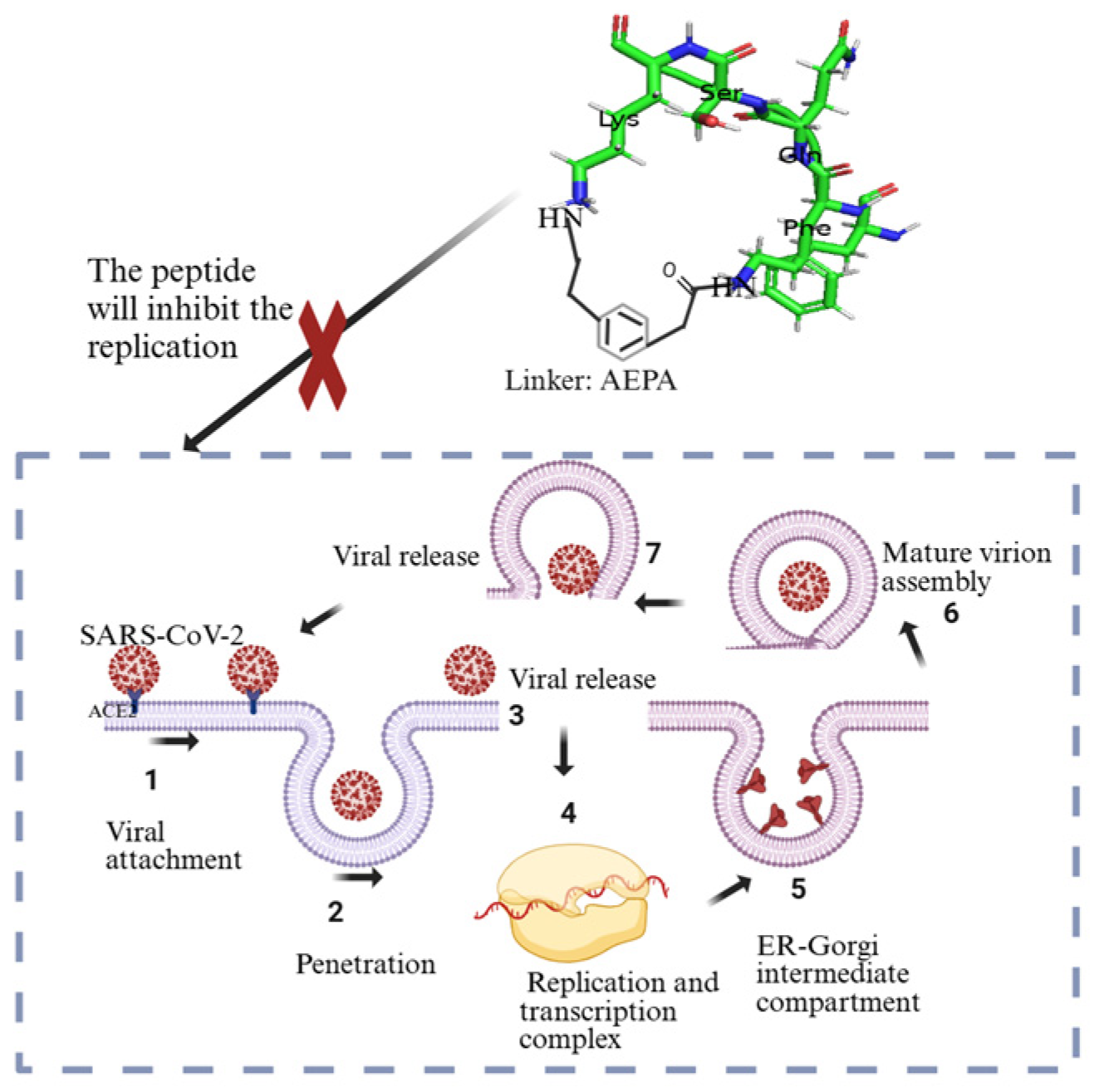
4. Interaction Between SARS-CoV-2 and Host Cells
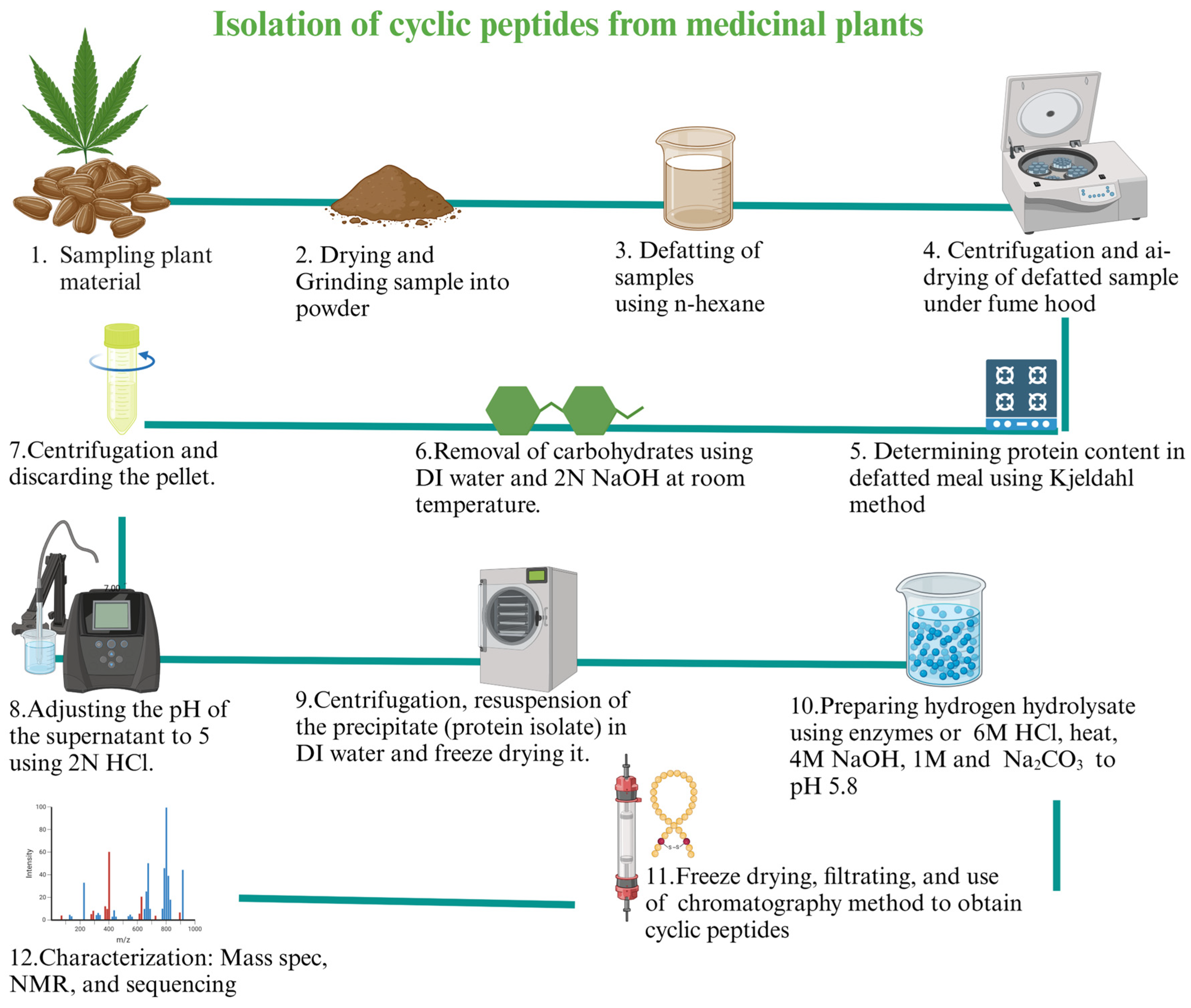
5. Use of Natural and Synthetic Antiviral Cyclic Peptides
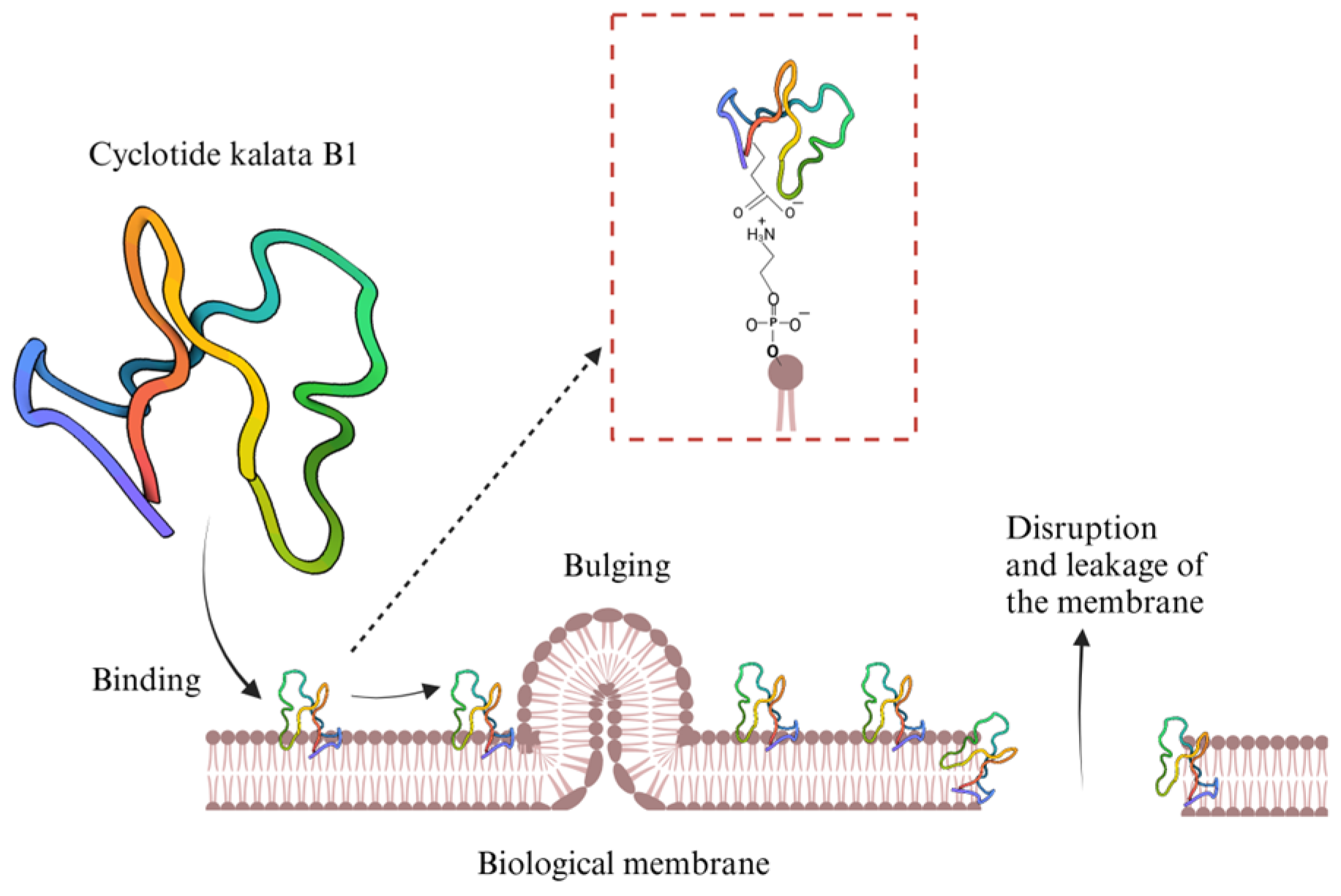
5.1. Natural Cyclic Peptides
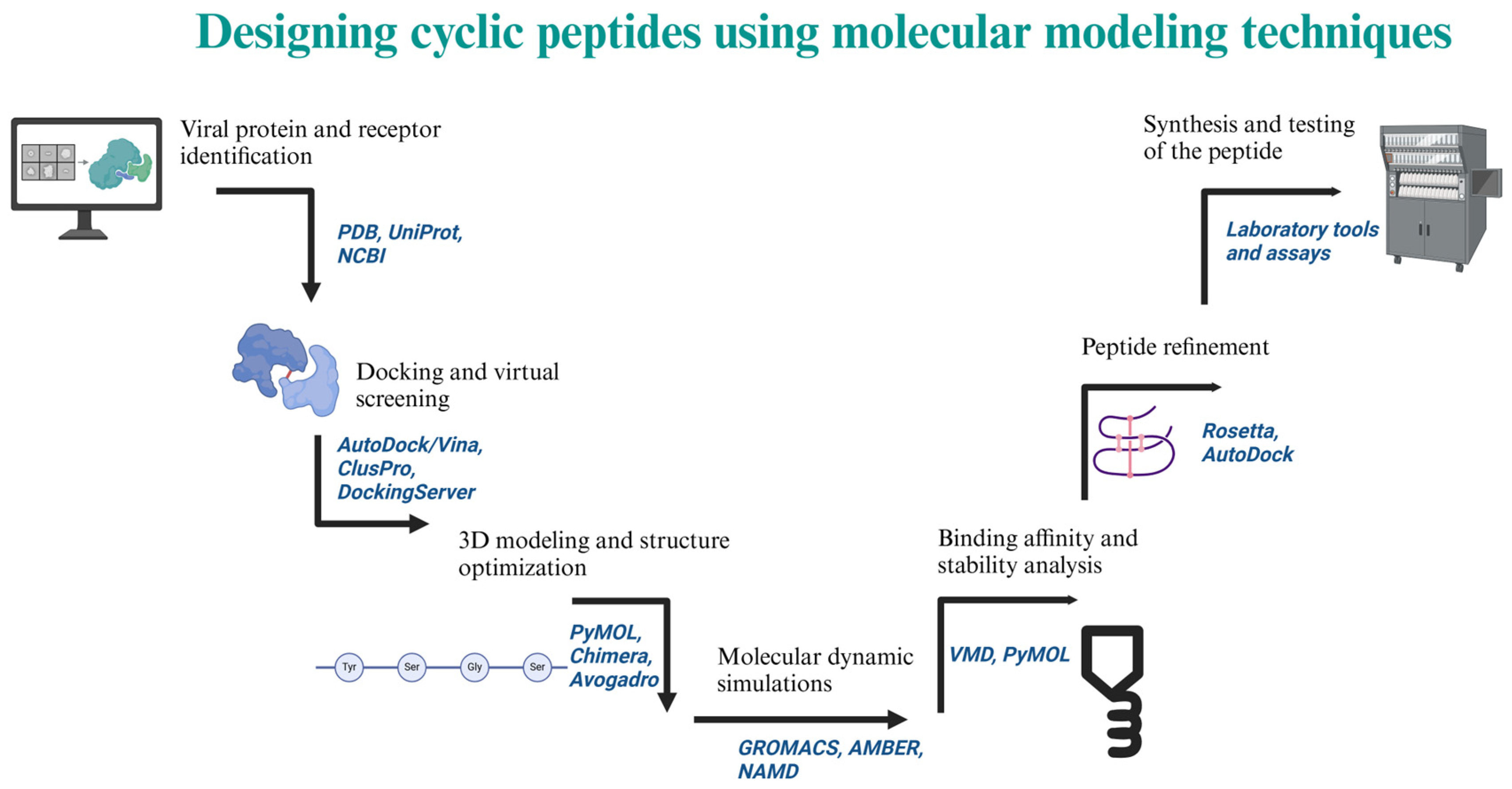
5.2. Designed and Synthetized Cyclic Peptides
6. Future Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global Trends in Emerging Infectious Diseases. Nature 2008, 451, 990–993. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Hernandez, R.; Jácome, R.; Vidal, Y.L.; de León, S.P. Are RNA Viruses Candidate Agents for the next Global Pandemic? A Review. ILAR J. 2017, 58, 343–358. [Google Scholar] [CrossRef]
- Baize, S.; Pannetier, D.; Oestereich, L.; Rieger, T.; Koivogui, L.; Magassouba, N.; Soropogui, B.; Sow, M.S.; Keïta, S.; De Clerck, H.; et al. Emergence of Zaire Ebola Virus Disease in Guinea. N. Engl. J. Med. 2014, 371, 1418–1425. [Google Scholar] [CrossRef]
- Bray, M. Highly Pathogenic RNA Viral Infections: Challenges for Antiviral Research. Antivir. Res. 2008, 78, 1–8. [Google Scholar]
- Holmes, E.C. Evolution and Emergence of RNA Viruses; Oxford University Press Inc.: New York, NY, USA, 2009; ISBN 978-0-19-921112-8. [Google Scholar]
- Markiewicz, L.; Drazkowska, K.; Sikorski, P.J. Tricks and Threats of RNA Viruses–towards Understanding the Fate of Viral RNA. RNA Biol. 2021, 18, 669–687. [Google Scholar]
- Figlerowicz, M.; Alejska, M.; Kurzyńska-Kokorniak, A.; Figlerowicz, M. Genetic Variability: The Key Problem in the Prevention and Therapy of RNA-Based Virus Infections. Med. Res. Rev. 2003, 23, 488–518. [Google Scholar] [CrossRef] [PubMed]
- Villa, T.G.; Abril, A.G.; Sánchez, S.; de Miguel, T.; Sánchez-Pérez, A. Animal and Human RNA Viruses: Genetic Variability and Ability to Overcome Vaccines. Arch. Microbiol. 2021, 203, 443–464. [Google Scholar] [PubMed]
- World Health Organization (WHO). WHO to Identify Pathogens That Could Cause Future Outbreaks and Pandemics. Available online: https://www.who.int/news/item/21-11-2022-who-to-identify-pathogens-that-could-cause-future-outbreaks-and-pandemics (accessed on 6 December 2024).
- Grubaugh, N.D.; Ladner, J.T.; Lemey, P.; Pybus, O.G.; Rambaut, A.; Holmes, E.C.; Andersen, K.G. Tracking Virus Outbreaks in the Twenty-First Century. Nat. Microbiol. 2019, 4, 10–19. [Google Scholar] [CrossRef]
- Ali, S.I.; Sheikh, W.M.; Rather, M.A.; Venkatesalu, V.; Bashir, S.M.; Nabi, S.U. Medicinal Plants: Treasure for Antiviral Drug Discovery. Phytother. Res. 2021, 35, 3447–3483. [Google Scholar]
- McCloskey, B.; Heymann, D.L. SARS to Novel Coronavirus-Old Lessons and New Lessons. Epidemiol. Infect. 2020, 148, e22. [Google Scholar] [CrossRef]
- Gupta, D.; AK, S.; Bansal, A.; Gupta, N.; Patki, V.; Sood, A.K.; Sachdev, A.; Parekh, B.J. Use of personal protective during COVID-19 in resource limited-the barest minimum. Indian J. Pract. Pediatr. 2020, 22, 195. [Google Scholar]
- Choi, H.; Chatterjee, P.; Lichtfouse, E.; Martel, J.A.; Hwang, M.; Jinadatha, C.; Sharma, V.K. Classical and Alternative Disinfection Strategies to Control the COVID-19 Virus in Healthcare Facilities: A Review. Environ. Chem. Lett. 2021, 19, 1945–1951. [Google Scholar] [PubMed]
- Dhama, K.; Patel, S.K.; Kumar, R.; Masand, R.; Rana, J.; Yatoo, M.I.; Tiwari, R.; Sharun, K.; Mohapatra, R.K.; Natesan, S.; et al. The Role of Disinfectants and Sanitizers during COVID-19 Pandemic: Advantages and Deleterious Effects on Humans and the Environment. Environ. Sci. Pollut. Res. 2021, 28, 34211–34228. [Google Scholar] [CrossRef]
- de Araújo, L.A.; Veloso, C.F.; de Campos Souza, M.; de Azevedo, J.M.C.; Tarro, G. The Potential Impact of the COVID-19 Pandemic on Child Growth and Development: A Systematic Review. J. Pediatr. 2021, 97, 369–377. [Google Scholar]
- Usher, K.; Jackson, D.; Durkin, J.; Gyamfi, N.; Bhullar, N. Pandemic-Related Behaviours and Psychological Outcomes; A Rapid Literature Review to Explain COVID-19 Behaviours. Int. J. Ment. Health Nurs. 2020, 29, 1018–1034. [Google Scholar] [CrossRef]
- Viner, R.M.; Russell, S.J.; Croker, H.; Packer, J.; Ward, J.; Stansfield, C.; Mytton, O.; Bonell, C.; Booy, R. School Closure and Management Practices during Coronavirus Outbreaks Including COVID-19: A Rapid Systematic Review. Lancet Child Adolesc. Health 2020, 4, 397–404. [Google Scholar]
- Altmann, D.M.; Boyton, R.J. COVID-19 Vaccination: The Road Ahead. Science 2022, 375, 1127–1132. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. The CanSino Biologics Ad5-NCoV-S [Recombinant] COVID-19 Vaccine: What You Need to Know. Available online: https://www.who.int/news-room/feature-stories/detail/the--cansino-biologics-ad5-ncov-s--recombinant---covid-19-vaccine--what-you-need-to-know (accessed on 2 February 2025).
- Hotez, P.J.; Bottazzi, M.E. Whole Inactivated Virus and Protein-Based COVID-19 Vaccines. Annu. Rev. Med. 2022, 73, 55–64. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. COVID-19 Vaccines with WHO Emergency Use Listing. Available online: https://extranet.who.int/prequal/vaccines/covid-19-vaccines-who-emergency-use-listing (accessed on 3 February 2025).
- International Vaccine Institute. SK Bioscience’s COVID-19 Vaccine Makes WHO’s Emergency Use Listing. Available online: https://www.ivi.int/sk-biosciences-covid-19-vaccine-makes-whos-emergency-use-listing/ (accessed on 3 February 2025).
- Mann, R.; Sekhon, S.; Sekhon, S. Drug-Induced Liver Injury After COVID-19 Vaccine. Cureus 2021, 13, e16491. [Google Scholar] [CrossRef]
- Gøtzsche, P.C.; Demasi, M. Serious Harms of the COVID-19 Vaccines: A Systematic Review. MedRxiv 2022. [Google Scholar] [CrossRef]
- Kang, C.R.; Choe, Y.J.; Yoon, S.J. COVID-19 Vaccine Injury Compensation Program: Lessons Learned from a Review of 10 Implementing Countries. J. Korean Med. Sci. 2024, 39, e121. [Google Scholar] [PubMed]
- Sridhar, S.; Luedtke, A.; Langevin, E.; Zhu, M.; Bonaparte, M.; Machabert, T.; Savarino, S.; Zambrano, B.; Moureau, A.; Khromava, A.; et al. Effect of Dengue Serostatus on Dengue Vaccine Safety and Efficacy. N. Engl. J. Med. 2018, 379, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Vannice, K.S.; Wilder-Smith, A.; Barrett, A.D.T.; Carrijo, K.; Cavaleri, M.; de Silva, A.; Durbin, A.P.; Endy, T.; Harris, E.; Innis, B.L.; et al. Clinical Development and Regulatory Points for Consideration for Second-Generation Live Attenuated Dengue Vaccines. Vaccine 2018, 36, 3411–3417. [Google Scholar] [CrossRef]
- Springer Nature Limited. Funders, now is the time to invest big in COVID drugs. Nature 2021, 592, 326. [Google Scholar] [CrossRef] [PubMed]
- Whittington, M.D.; Pearson, S.D.; Rind, D.M.; Campbell, J.D. The Cost-Effectiveness of Remdesivir for Hospitalized Patients With COVID-19. Value Health 2022, 25, 744–750. [Google Scholar] [CrossRef]
- Carethers, J.M. Rectifying COVID-19 Disparities with Treatment and Vaccination. JCI Insight 2021, 6, e147800. [Google Scholar] [CrossRef]
- U.S Food & Drug Administration. FDA Approves First Oral Antiviral for Treatment of COVID-19 in Adults. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-oral-antiviral-treatment-covid-19-adults (accessed on 27 January 2025).
- Pfizer If It’s COVID, PAXLOVID. Available online: https://www.paxlovid.com/ (accessed on 27 January 2025).
- Aungst, C. How to Find the COVID-19 Pills Paxlovid and Lagevrio. Available online: https://www.goodrx.com/conditions/covid-19/covid-pill-cost-availability (accessed on 27 January 2025).
- Milisavljevic, N.; Konkolová, E.; Kozák, J.; Hodek, J.; Veselovská, L.; Sýkorová, V.; Čížek, K.; Pohl, R.; Eyer, L.; Svoboda, P.; et al. Antiviral Activity of 7-Substituted 7-Deazapurine Ribonucleosides, Monophosphate Prodrugs, and Triphoshates against Emerging RNA Viruses. ACS Infect. Dis. 2021, 7, 471–478. [Google Scholar] [CrossRef]
- Hassine, I.H.; Ben M’Hadheb, M.; Menéndez-Arias, L. Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity. Viruses 2022, 14, 841. [Google Scholar] [CrossRef]
- Musarra-Pizzo, M.; Pennisi, R.; Ben-Amor, I.; Mandalari, G.; Sciortino, M.T. Antiviral Activity Exerted by Natural Products against Human Viruses. Viruses 2021, 13, 828. [Google Scholar] [CrossRef]
- Mammari, N.; Krier, Y.; Albert, Q.; Devocelle, M.; Varbanov, M. Plant-Derived Antimicrobial Peptides as Potential Antiviral Agents in Systemic Viral Infections. Pharmaceuticals 2021, 14, 774. [Google Scholar] [CrossRef]
- Heydari, H.; Golmohammadi, R.; Mirnejad, R.; Tebyanian, H.; Fasihi-Ramandi, M.; Moghaddam, M.M. Antiviral Peptides against Coronaviridae Family: A Review. Peptides 2021, 139, 170526. [Google Scholar] [PubMed]
- Bera, S.; Mondal, D. Natural Cyclic Peptides as Clinical and Future Therapeutics. Curr. Org. Chem. 2019, 23, 38–75. [Google Scholar] [CrossRef]
- Chia, L.Y.; Kumar, P.V.; Maki, M.A.A.; Ravichandran, G.; Thilagar, S. A Review: The Antiviral Activity of Cyclic Peptides. Int. J. Pept. Res. Ther. 2023, 29, 7. [Google Scholar]
- Ke, J.; Zhang, J.; Li, J.; Liu, J.; Guan, S. Design of Cyclic Peptide-Based Nanospheres and the Delivery of SiRNA. Int. J. Mol. Sci. 2022, 23, 12071. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yang, L.; Cao, S.; Gao, Z.; Yang, B.; Zhang, G.; Zhu, R.; Wu, D. CyclicPepedia: A Knowledge Base of Natural and Synthetic Cyclic Peptides. Brief. Bioinform. 2024, 25, bbae190. [Google Scholar] [CrossRef]
- El Sayed, K.A. Natural Products as Antiviral Agents. Stud. Nat. Prod. Chem. 2000, 24, 473–572. [Google Scholar]
- Cardote, T.A.F.; Ciulli, A. Cyclic and Macrocyclic Peptides as Chemical Tools To Recognise Protein Surfaces and Probe Protein-Protein Interactions. ChemMedChem 2015, 11, 787–794. [Google Scholar] [CrossRef]
- World Health Organization (WHO). WHO COVID-19 Dashboard. Available online: https://data.who.int/dashboards/covid19/cases?n=c (accessed on 11 December 2024).
- Du, J.; Lang, H.M.; Ma, Y.; Chen, A.W.; Qin, Y.Y.; Zhang, X.P.; Huang, C.Q. Global Trends in COVID-19 Incidence and Case Fatality Rates (2019–2023): A Retrospective Analysis. Front. Public Health 2024, 12, 1355097. [Google Scholar] [CrossRef]
- Wordometer Europe Population (LIVE). Available online: https://www.worldometers.info/world-population/europe-population/ (accessed on 28 January 2025).
- Worldometer Africa Population (LIVE). Available online: https://www.worldometers.info/world-population/africa-population/ (accessed on 28 January 2025).
- Soares, P.; Rocha, J.V.; Moniz, M.; Gama, A.; Laires, P.A.; Pedro, A.R.; Dias, S.; Leite, A.; Nunes, C. Factors Associated with COVID-19 Vaccine Hesitancy. Vaccines 2021, 9, 300. [Google Scholar] [CrossRef]
- Hornsey, M.J. Reasons Why People May Refuse COVID-19 Vaccination (and What Can Be Done about It). World Psychiatry 2022, 21, 217–218. [Google Scholar] [CrossRef]
- Akindele, A.J.; Sowemimo, A.; Agunbiade, F.O.; Sofidiya, M.O.; Awodele, O.; Ade-Ademilua, O.; Orabueze, I.; Ishola, I.O.; Ayolabi, C.I.; Salu, O.B.; et al. Bioprospecting for Anti-COVID-19 Interventions From African Medicinal Plants: A Review. Nat. Prod. Commun. 2022, 17, 1934578X221096968. [Google Scholar] [CrossRef]
- Vellingiri, B.; Jayaramayya, K.; Iyer, M.; Narayanasamy, A.; Govindasamy, V.; Giridharan, B.; Ganesan, S.; Venugopal, A.; Venkatesan, D.; Ganesan, H.; et al. COVID-19: A Promising Cure for the Global Panic. Sci. Total. Environ. 2020, 725, 138277. [Google Scholar] [PubMed]
- Ayele, Y.; Kim, J.-A.; Park, E.; Kim, Y.-J.; Retta, N.; Dessie, G.; Rhee, S.-K.; Koh, K.; Nam, K.-W.; Kim, H.S. A Methanol Extract of Adansonia Digitata L. Leaves Inhibits Pro-Inflammatory INOS Possibly via the Inhibition of NF-ΚB Activation. Biomol. Ther. 2013, 21, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.; Khan, M.A.; Mashwani, Z.u.R.; Ullah, N.; Nadhman, A. Therapeutic Potential of Medicinal Plants against COVID-19: The Role of Antiviral Medicinal Metabolites. Biocatal. Agric. Biotechnol. 2021, 31, 101890. [Google Scholar]
- Haider, N.; Osman, A.Y.; Gadzekpo, A.; Akipede, G.O.; Asogun, D.; Ansumana, R.; Lessells, R.J.; Khan, P.; Hamid, M.M.A.; Yeboah-Manu, D.; et al. Lockdown Measures in Response to COVID-19 in Nine Sub-Saharan African Countries. BMJ Glob. Health 2020, 5, e003319. [Google Scholar] [CrossRef]
- Mwiinde, A.M.; Siankwilimba, E.; Sakala, M.; Banda, F.; Michelo, C. Climatic and Environmental Factors Influencing COVID-19 Transmission—An African Perspective. Trop. Med. Infect. Dis. 2022, 7, 433. [Google Scholar] [CrossRef]
- Roberts, J.D.; Tehrani, S.O. Environments, Behaviors, and Inequalities: Reflecting on the Impacts of the Influenza and Coronavirus Pandemics in the United States. Int. J. Environ. Res. Public Health 2020, 17, 4484. [Google Scholar] [CrossRef]
- Quinn, S.C.; Kumar, S.; Freimuth, V.S.; Musa, D.; Casteneda-Angarita, N.; Kidwell, K. Racial Disparities in Exposure, Susceptibility, and Access to Health Care in the US H1N1 Influenza Pandemic. Am. J. Public Health 2011, 101, 285–293. [Google Scholar] [CrossRef]
- Reyes, M.V. The Disproportional Impact of COVID-19 on African Americans. Health Hum. Rights 2020, 22, 299–307. [Google Scholar]
- Snowden, L.R.; Graaf, G. COVID-19, Social Determinants Past, Present, and Future, and African Americans’ Health. J. Racial Ethn. Health Disparities 2021, 8, 12–20. [Google Scholar] [CrossRef]
- Carethers, J.M. Insights into Disparities Observed with COVID-19. J. Intern. Med. 2021, 289, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Bunsawat, K.; Grosicki, G.J.; Jeong, S.; Robinson, A.T. Racial and Ethnic Disparities in Cardiometabolic Disease and COVID-19 Outcomes in White, Black/African American, and Latinx Populations: Physiological Underpinnings. Prog. Cardiovasc. Dis. 2022, 71, 11–19. [Google Scholar] [CrossRef]
- Clay, S.L.; Woodson, M.J.; Mazurek, K.; Antonio, B. Racial Disparities and COVID-19: Exploring the Relationship Between Race/Ethnicity, Personal Factors, Health Access/Affordability, and Conditions Associated with an Increased Severity of COVID-19. Race Soc. Probl. 2021, 13, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Price-Haywood, E.G.; Burton, J.; Fort, D.; Seoane, L. Hospitalization and Mortality among Black Patients and White Patients with COVID-19. N. Engl. J. Med. 2020, 382, 2534–2543. [Google Scholar] [CrossRef]
- Sharma, M.; Batra, K.; Batra, R. A Theory-Based Analysis of COVID-19 Vaccine Hesitancy among African Americans in the United States: A Recent Evidence. Healthcare 2021, 9, 1273. [Google Scholar] [CrossRef] [PubMed]
- Majee, W.; Anakwe, A.; Onyeaka, K.; Harvey, I.S. The Past Is so Present: Understanding COVID-19 Vaccine Hesitancy Among African American Adults Using Qualitative Data. J. Racial Ethn. Health Disparities 2023, 10, 462–474. [Google Scholar] [CrossRef]
- Leneva, I.; Kartashova, N.; Poromov, A.; Gracheva, A.; Korchevaya, E.; Glubokova, E.; Borisova, O.; Shtro, A.; Loginova, S.; Shchukina, V.; et al. Antiviral Activity of Umifenovir in Vitro against a Broad Spectrum of Coronaviruses, Including the Novel SARS-CoV-2 Virus. Viruses 2021, 13, 1665. [Google Scholar] [CrossRef]
- Du, R.; Achi, J.G.; Cui, Q.; Rong, L. Paving New Roads toward the Advancement of Broad-Spectrum Antiviral Agents. J. Med. Virol. 2024, 96, e29369. [Google Scholar] [CrossRef]
- Adamson, C.S.; Chibale, K.; Goss, R.J.M.; Jaspars, M.; Newman, D.J.; Dorrington, R.A. Antiviral Drug Discovery: Preparing for the next Pandemic. Chem. Soc. Rev. 2021, 50, 3647–3655. [Google Scholar] [CrossRef]
- Tian, W.J.; Wang, X.J. Broad-Spectrum Antivirals Derived from Natural Products. Viruses 2023, 15, 1100. [Google Scholar] [CrossRef]
- Gardiner, P.; Whelan, J.; White, L.F.; Filippelli, A.C.; Bharmal, N.; Kaptchuk, T.J. A Systematic Review of the Prevalence of Herb Usage Among Racial/Ethnic Minorities in the United States. J. Immigr. Minor. Health 2013, 15, 817–828. [Google Scholar] [PubMed]
- Burrell, C.J.; Howard, C.R.; Murphy, F.A. Coronaviruses. In Fenner and White’s Medical Virology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 437–446. [Google Scholar]
- Kahn, J.S.; McIntosh, K. History and Recent Advances in Coronavirus Discovery. Pediatr. Infect. Dis. J. 2005, 24, S223–S227. [Google Scholar] [CrossRef] [PubMed]
- Rabaan, A.A.; Al-Ahmed, S.H.; Haque, S.; Sah, R.; Tiwari, R.; Malik, Y.S.; Dhama, K.; Yatoo, M.I.; Bonilla-Aldana, D.K.; Rodriguez-Morales, A.J. SARS-CoV-2, SARS-CoV, and MERS-CoV: A Comparative Overview. Infez. Med. 2020, 28, 174–184. [Google Scholar]
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. Product of Natural Evolution (SARS, MERS, and SARS-CoV-2); Deadly Diseases, from SARS to SARS-CoV-2. Hum. Vaccin. Immunother. 2021, 17, 62–83. [Google Scholar] [PubMed]
- Zhou, Y.; Yang, Y.; Huang, J.; Jiang, S.; Du, L. Advances in MERS-CoV Vaccines and Therapeutics Based on the Receptor-Binding Domain. Viruses 2019, 11, 60. [Google Scholar] [CrossRef]
- Zeidler, A.; Karpinski, T.M. SARS-CoV, MERS-COV, SARS-CoV-2 Comparison of Three Emerging Coronaviruses. Jundishapur J. Microbiol. 2020, 13, e103744. [Google Scholar]
- Johns Hopkins BLOOMBERG SCHOOL of PUBLIC HEALTH and Center for Health Security. Coronaviruses: SARS, MERS, and 2019-NCoV. Nat. Med. 2020, 26, 450–452. [Google Scholar]
- Meo, S.A.; Alhowikan, A.M.; Al-Khlaiwi, T.; Meo, I.M.; Halepoto, D.M.; Iqbal, M.; Usmani, A.M.; Hajjar, W.; Ahmed, N. Novel Coronavirus 2019-NCoV: Prevalence, Biological and Clinical Characteristics Comparison with SARS-CoV and MERS-CoV. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 2012–2019. [Google Scholar]
- World Health Organization. WHO-Convened Global Study of Origins of SARS-CoV-2: China Part; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Knight, D. COVID-19 Pandemic Origins: Bioweapons and the History of Laboratory Leaks. South. Med. J. 2021, 114, 465–467. [Google Scholar] [CrossRef]
- Baker, N. The Lab-Leak Hypothesis. N. Y. Mag. 2021, 4, 1–32. [Google Scholar]
- Kormann, C. The Mysterious Case of the COVID-19 Lab-Leak Theory—Did the Virus Spring from Nature or from Human Error? New Yorker 2021, 12, 1–12. [Google Scholar]
- Center for Disease Control and Prevention Symptoms of COVID-19. Available online: https://www.cdc.gov/covid/signs-symptoms/index.html (accessed on 20 January 2025).
- Zhang, Q.; Xiang, R.; Huo, S.; Zhou, Y.; Jiang, S.; Wang, Q.; Yu, F. Molecular Mechanism of Interaction between SARS-CoV-2 and Host Cells and Interventional Therapy. Signal Transduct. Target. Ther. 2021, 6, 233. [Google Scholar] [PubMed]
- Shang, J.; Wan, Y.; Luo, C.; Ye, G.; Geng, Q.; Auerbach, A.; Li, F. Cell Entry Mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 11727–11734. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Sun, P.D. High Affinity Binding of SARS-CoV-2 Spike Protein Enhances ACE2 Carboxypeptidase Activity. J. Biol. Chem. 2020, 295, 18579–18588. [Google Scholar] [CrossRef]
- Engler, M.; Albers, D.; Von Maltitz, P.; Groß, R.; Münch, J.; Cirstea, I.C. ACE2-EGFR-MAPK Signaling Contributes to SARS-CoV-2 Infection. Life Sci. Alliance 2023, 9, e202201880. [Google Scholar] [CrossRef]
- Wang, K.; Chen, W.; Zhang, Z.; Deng, Y.; Lian, J.Q.; Du, P.; Wei, D.; Zhang, Y.; Sun, X.X.; Gong, L.; et al. CD147-Spike Protein Is a Novel Route for SARS-CoV-2 Infection to Host Cells. Signal Transduct. Target. Ther. 2020, 5, 283. [Google Scholar] [CrossRef]
- Ojha, R.; Jiang, A.; Mäntylä, E.; Quirin, T.; Modhira, N.; Witte, R.; Gaudin, A.; De Zanetti, L.; Gormal, R.S.; Vihinen-Ranta, M.; et al. Dynamin Independent Endocytosis Is an Alternative Cell Entry Mechanism for Multiple Animal Viruses. PLoS Pathog. 2024, 20, e1012690. [Google Scholar] [CrossRef]
- Eroshenko, N.; Gill, T.; Keaveney, M.K.; Church, G.M.; Trevejo, J.M.; Rajaniemi, H. Implications of Antibody-Dependent Enhancement of Infection for SARS-CoV-2 Countermeasures. Nat. Biotechnol. 2020, 38, 789–791. [Google Scholar] [CrossRef]
- Iwasaki, A.; Yang, Y. The Potential Danger of Suboptimal Antibody Responses in COVID-19. Nat. Rev. Immunol. 2020, 20, 339–341. [Google Scholar]
- Liu, Y.; Soh, W.T.; Kishikawa, J.-i.; Hirose, M.; Nakayama, E.E.; Li, S.; Sasai, M.; Suzuki, T.; Tada, A.; Arakawa, A.; et al. An Infectivity-Enhancing Site on the SARS-CoV-2 Spike Protein Targeted by Antibodies. Cell 2021, 184, 3452–3466.e18. [Google Scholar] [CrossRef]
- Sivaraman, H.; Er, S.Y.; Choong, Y.K.; Gavor, E.; Sivaraman, J. Structural Basis of SARS-CoV-2-and SARS-CoV-Receptor Binding and Small-Molecule Blockers as Potential Therapeutics. Annu. Rev. Pharmacol. Toxicol. 2021, 61, 465–493. [Google Scholar] [CrossRef] [PubMed]
- Scialo, F.; Daniele, A.; Amato, F.; Pastore, L.; Matera, M.G.; Cazzola, M.; Castaldo, G.; Bianco, A. ACE2: The Major Cell Entry Receptor for SARS-CoV-2. Lung 2020, 198, 867–877. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Luo, W.; Huang, L.; Xiao, J.; Li, F.; Qin, S.; Song, X.; Wu, Y.; Zeng, Q.; et al. A Comprehensive Investigation of the Mrna and Protein Level of Ace2, the Putative Receptor of SARS-CoV-2, in Human Tissues and Blood Cells. Int. J. Med. Sci. 2020, 17, 1522–1531. [Google Scholar] [CrossRef]
- Ling, R.; Dai, Y.; Huang, B.; Huang, W.; Yu, J.; Lu, X.; Jiang, Y. In Silico Design of Antiviral Peptides Targeting the Spike Protein of SARS-CoV-2. Peptides 2020, 130, 170328. [Google Scholar] [CrossRef] [PubMed]
- Totura, A.L.; Bavari, S. Broad-Spectrum Coronavirus Antiviral Drug Discovery. Expert Opin. Drug Discov. 2019, 14, 397–412. [Google Scholar] [CrossRef]
- Karim, M.; Lo, C.W.; Einav, S. Preparing for the next Viral Threat with Broad-Spectrum Antivirals. J. Clin. Investig. 2023, 133, e170236. [Google Scholar] [CrossRef]
- Adalja, A.; Inglesby, T. Broad-Spectrum Antiviral Agents: A Crucial Pandemic Tool. Expert Rev. Anti. Infect. Ther. 2019, 17, 467–470. [Google Scholar] [CrossRef]
- Rosenke, K.; Feldmann, H.; Westover, J.B.; Hanley, P.W.; Martellaro, C.; Feldmann, F.; Saturday, G.; Lovaglio, J.; Scott, D.P.; Furuta, Y.; et al. Use of Favipiravir to Treat Lassa Virus Infection in Macaques. Emerg. Infect. Dis. 2018, 24, 1696–1699. [Google Scholar] [CrossRef]
- Raabe, V.N.; Kann, G.; Ribner, B.S.; Andres, A.M.; Varkey, J.B.; Mehta, A.K.; Lyon, G.M.; Vanairsdale, S.; Faber, K.; Becker, S.; et al. Favipiravir and Ribavirin Treatment of Epidemiologically Linked Cases of Lassa Fever. Clin. Infect. Dis. 2017, 65, 855–859. [Google Scholar] [CrossRef]
- Sissoko, D.; Laouenan, C.; Folkesson, E.; M’Lebing, A.B.; Beavogui, A.H.; Baize, S.; Camara, A.M.; Maes, P.; Shepherd, S.; Danel, C.; et al. Experimental Treatment with Favipiravir for Ebola Virus Disease (the JIKI Trial): A Historically Controlled, Single-Arm Proof-of-Concept Trial in Guinea. PLoS Med. 2016, 13, e1001967. [Google Scholar] [CrossRef]
- Sanches, P.R.S.; Charlie-Silva, I.; Braz, H.L.B.; Bittar, C.; Calmon, M.F.; Rahal, P.; Cilli, E.M. Recent Advances in SARS-CoV-2 Spike Protein and RBD Mutations Comparison between New Variants Alpha (B.1.1.7, United Kingdom), Beta (B.1.351, South Africa), Gamma (P.1, Brazil) and Delta (B.1.617.2, India). J. Virus Erad. 2021, 7, 100054. [Google Scholar] [CrossRef]
- Meng, B.; Kemp, S.A.; Papa, G.; Datir, R.; Ferreira, I.A.T.M.; Marelli, S.; Harvey, W.T.; Lytras, S.; Mohamed, A.; Gallo, G.; et al. Recurrent Emergence of SARS-CoV-2 Spike Deletion H69/V70 and Its Role in the Alpha Variant B.1.1.7. Cell Rep. 2021, 35, 109292. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Hong, W.; Pan, X.; Lu, G.; Wei, X. SARS-CoV-2 Omicron Variant: Characteristics and Prevention. MedComm 2021, 2, 838–845. [Google Scholar] [PubMed]
- CDC; IDSA. COVID-19 Variant Update. Available online: https://www.idsociety.org/covid-19-real-time-learning-network/diagnostics/covid-19-variant-update/#/+/0/publishedDate_na_dt/desc/ (accessed on 22 January 2025).
- Orio, L.P.; Boschin, G.; Recca, T.; Morelli, C.F.; Ragona, L.; Francescato, P.; Arnoldi, A.; Speranza, G. New ACE-Inhibitory Peptides from Hemp Seed (Cannabis sativa L.) Proteins. J. Agric. Food Chem. 2017, 65, 10482–10488. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.; Enan, G.; Al-Mohammadi, A.R.; Abdel-Shafi, S.; Abdel-Hameid, S.; Sitohy, M.Z.; El-Gazzar, N. Antibacterial Peptides Produced by Alcalase from Cowpea Seed Proteins. Antibiotics 2021, 10, 870. [Google Scholar] [CrossRef]
- Weidmann, J.; Craik, D.J. Discovery, Structure, Function, and Applications of Cyclotides: Circular Proteins from Plants. J. Exp. Bot. 2016, 67, 4801–4812. [Google Scholar] [CrossRef]
- Grover, T.; Mishra, R.; Bushra; Gulati, P.; Mohanty, A. An Insight into Biological Activities of Native Cyclotides for Potential Applications in Agriculture and Pharmaceutics. Peptides 2021, 135, 170430. [Google Scholar] [CrossRef]
- Saether, O.; Craik, D.J.; Campbell, I.D.; Sletten, K.; Juul, J.; Norman, D.G. Elucidation of the Primary and Three-Dimensional Structure of the Uterotonic Polypeptide Kalata B1. Biochemistry 1995, 34, 4147–4158. [Google Scholar] [CrossRef]
- RCSB PDB (Protein Data Bank). REFINED STRUCTURE AND DISULFIDE PAIRING OF THE KALATA B1 PEPTIDE. Available online: https://www.rcsb.org/structure/1K48 (accessed on 18 March 2025).
- Panya, A.; Yongpitakwattana, P.; Budchart, P.; Sawasdee, N.; Krobthong, S.; Paemanee, A.; Roytrakul, S.; Rattanabunyong, S.; Choowongkomon, K.; Yenchitsomanus, P. Novel Bioactive Peptides Demonstrating Anti-Dengue Virus Activity Isolated from the Asian Medicinal Plant Acacia Catechu. Chem. Biol. Drug Des. 2019, 93, 100–109. [Google Scholar] [CrossRef]
- Castilla, V.; Barquero, A.A.; Mersich, S.E.; Coto, C.E. In Vitro Anti-Junin Virus Activity of a Peptide Isolated from Melia azedarach L. Leaves. Int. J. Antimicrob. Agents 1998, 10, 67–75. [Google Scholar] [CrossRef]
- Nguyen, P.Q.T.; Ooi, J.S.G.; Nguyen, N.T.K.; Wang, S.; Huang, M.; Liu, D.X.; Tam, J.P. Antiviral Cystine Knot α-Amylase Inhibitors from Alstonia Scholaris. J. Biol. Chem. 2015, 290, 31138–31150. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Nguyen, Q.; Tam, J. 2MM6 | Pdb_00002mm6 Solution Structure of Alpha Amylase Inhibitor Peptide AS1 from Allatide Scholaris. RCSB PDB. Available online: https://www.rcsb.org/structure/2MM6#entity-1 (accessed on 19 March 2025).
- Wang, S.; Nguyen, Q.; Tam, J. 2MM5 | Pdb_00002mm5 Solution Structure of Alpha-Amylase Inhibitor Peptide AS4 from Allatide Scholaris. RCSB PDB. Available online: https://www.rcsb.org/structure/2MM5#entity-1 (accessed on 19 March 2025).
- Singh, S.; Chauhan, P.; Sharma, V.; Rao, A.; Kumbhar, B.V.; Prajapati, V.K. Identification of Multi-Targeting Natural Antiviral Peptides to Impede SARS-CoV-2 Infection. Struct. Chem. 2023, 34, 1743–1758. [Google Scholar] [CrossRef]
- Yang, X.; Feng, P.; Yin, Y.; Bushley, K.; Spatafora, J.W.; Wang, C. Cyclosporine Biosynthesis in Tolypocladium inflatum Benefits Fungal Adaptation to the Environment. mBio 2018, 9, e01211-18. [Google Scholar] [CrossRef]
- Ciesek, S.; Steinmann, E.; Wedemeyer, H.; Manns, M.P.; Neyts, J.; Tautz, N.; Madan, V.; Bartenschlager, R.; Von Hahn, T.; Pietschmann, T. Cyclosporine A Inhibits Hepatitis C Virus Nonstructural Protein 2 through Cyclophilin A. Hepatology 2009, 50, 1638–1645. [Google Scholar] [CrossRef] [PubMed]
- Ianevski, A.; Zusinaite, E.; Kuivanen, S.; Strand, M.; Lysvand, H.; Teppor, M.; Kakkola, L.; Paavilainen, H.; Laajala, M.; Kallio-Kokko, H.; et al. Novel Activities of Safe-in-Human Broad-Spectrum Antiviral Agents. Antivir. Res. 2018, 154, 174–182. [Google Scholar] [CrossRef]
- Glowacka, P.; Rudnicka, L.; Warszawik-Hendzel, O.; Sikora, M.; Goldust, M.; Gajda, P.; Stochmal, A.; Blicharz, L.; Rakowska, A.; Olszewska, M. The Antiviral Properties of Cyclosporine. Focus on Coronavirus, Hepatitis C Virus, Influenza Virus, and Human Immunodeficiency Virus Infections. Biology 2020, 9, 192. [Google Scholar] [CrossRef]
- Rautenbach, M.; Vlok, N.M.; Stander, M.; Hoppe, H.C. Inhibition of Malaria Parasite Blood Stages by Tyrocidines, Membrane-Active Cyclic Peptide Antibiotics from Bacillus brevis. Biochim. Biophys. Acta Biomembr. 2007, 1768, 1488–1497. [Google Scholar] [CrossRef]
- Pärn, K.; Eriste, E.; Langel, Ü. The Antimicrobial and Antiviral Applications of Cell-Penetrating Peptides. In Cell-Penetrating Peptides; Humana Press: New York, NY, USA, 2015; pp. 223–245. [Google Scholar]
- Rautenbach, M.; Vosloo, J.A.; Van Rensburg, W.; Engelbrecht, Y. Natural Antimicrobial Peptides as Green Microbicides in Agriculture: A Proof of Concept Study on the Tyrocidines from Soil Bacteria; Green Economy Research Report; Green Fund, Development Bank of Southern Africa, Midrand: Midrand, South Africa, 2016. [Google Scholar] [CrossRef]
- Wenzel, M.; Rautenbach, M.; Vosloo, J.A.; Siersma, T.; Aisenbrey, C.H.M.; Zaitseva, E.; Laubscher, W.E.; van Rensburg, W.; Behrends, J.C.; Bechinger, B.; et al. The Multifaceted Antibacterial Mechanisms of the Pioneering Peptide Antibiotics Tyrocidine and Gramicidin S. mBio 2018, 9, e00802-18. [Google Scholar] [CrossRef] [PubMed]
- Tally, F.P.; DeBruin, M.F. Development of Daptomycin for Gram-Positive Infections. J. Antimicrob. Chemother. 2000, 46, 523–526. [Google Scholar] [CrossRef]
- Cao, X.; Huang, L.; Tang, M.; Liang, Y.; Liu, X.; Hou, H.; Liang, S. Antibiotics Daptomycin Interacts with S Protein of SARS-CoV-2 to Promote Cell Invasion of Omicron (B1.1.529) Pseudovirus. Virulence 2024, 15, 2339703. [Google Scholar] [CrossRef]
- Norman, A.; Franck, C.; Christie, M.; Hawkins, P.M.E.; Patel, K.; Ashhurst, A.S.; Aggarwal, A.; Low, J.K.K.; Siddiquee, R.; Ashley, C.L.; et al. Discovery of Cyclic Peptide Ligands to the SARS-CoV-2 Spike Protein Using MRNA Display. ACS Cent. Sci. 2021, 7, 1001–1008. [Google Scholar] [CrossRef]
- Kreutzer, A.G.; Krumberger, M.; Diessner, E.M.; Parrocha, C.M.T.; Morris, M.A.; Guaglianone, G.; Butts, C.T.; Nowick, J.S. A Cyclic Peptide Inhibitor of the SARS-CoV-2 Main Protease. Eur. J. Med. Chem. 2021, 221, 113530. [Google Scholar] [CrossRef] [PubMed]
- Johansen-Leete, J.; Ullrich, S.; Fry, S.E.; Frkic, R.; Bedding, M.J.; Aggarwal, A.; Ashhurst, A.S.; Ekanayake, K.B.; Mahawaththa, M.C.; Sasi, V.M.; et al. Antiviral Cyclic Peptides Targeting the Main Protease of SARS-CoV-2. Chem. Sci. 2022, 13, 3826–3836. [Google Scholar] [CrossRef] [PubMed]
- Staufer, O.; Gantner, G.; Platzman, I.; Tanner, K.; Berger, I.; Spatz, J.P. Bottom-up Assembly of Viral Replication Cycles. Nat. Commun. 2022, 13, 6530. [Google Scholar] [CrossRef]
- Johnson, N.; Pattinson, C.; Burgoyne, K.; Hijazi, K.; Houssen, W.E.; Milne, B.F. SARS-CoV-2 Spike Protein-Derived Cyclic Peptides as Modulators of Spike Interaction with GRP78. ChemBioChem 2024, 25, e202300789. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.; Wang, X.; Wang, L.; Xu, W.; Xia, S.; Sun, L.; Wang, S.; Shen, N.; Yang, Z.; Huang, B.; et al. A Novel Cyclic γ-AApeptide-Based Long-Acting Pan-Coronavirus Fusion Inhibitor with Potential Oral Bioavailability by Targeting Two Sites in Spike Protein. Cell Discov. 2022, 8, 88. [Google Scholar] [CrossRef]
- Iannuzzelli, J.A.; Bonn, R.; Hong, A.S.; Anitha, A.S.; Jenkins, J.L.; Wedekind, J.E.; Fasan, R. Cyclic Peptides Targeting the SARS-CoV-2 Programmed Ribosomal Frameshifting RNA from a Multiplexed Phage Display Library. Chem. Sci. 2024, 15, 19520–19533. [Google Scholar] [CrossRef]
- Freitas, E.D.; Bataglioli, R.A.; Oshodi, J.; Beppu, M.M. Antimicrobial Peptides and Their Potential Application in Antiviral Coating Agents. Colloids Surf B Biointerfaces 2022, 217, 112693. [Google Scholar]
- Ellingson, K.D.; Pogreba-Brown, K.; Gerba, C.P.; Elliott, S.P. Impact of a Novel Antimicrobial Surface Coating on Health Care-Associated Infections and Environmental Bioburden at 2 Urban Hospitals. Clin. Infect. Dis. 2020, 71, 1807–1813. [Google Scholar] [CrossRef]
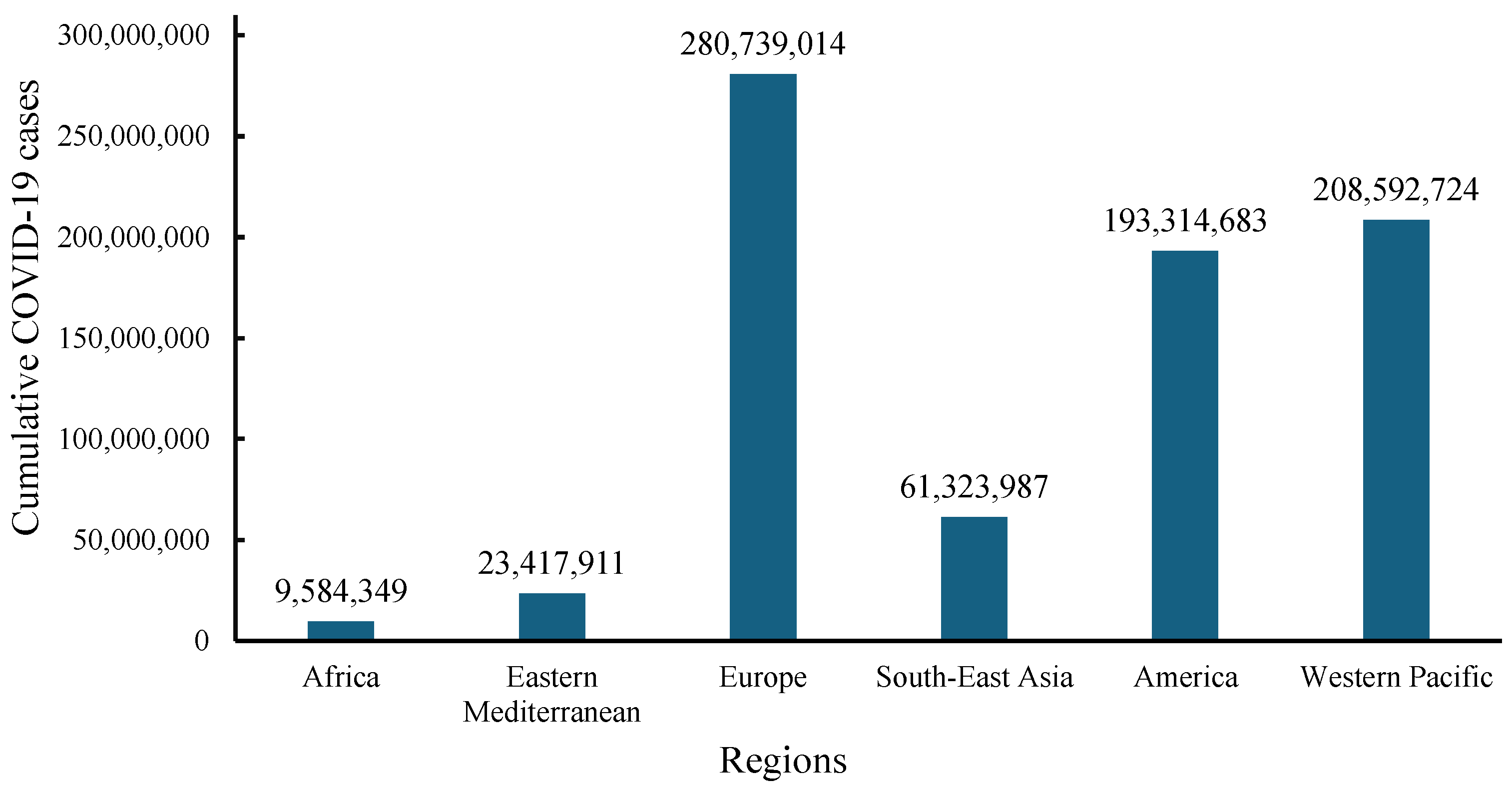



| Region | At Least One Dose (%) | Complete Primary Series (%) | At Least One Booster (%) |
|---|---|---|---|
| Africa | 39 | 33 | 6 |
| Eastern Mediterranean | 60 | 52 | 19 |
| Europe | 69 | 65 | 37 |
| South-East Asia | 77 | 70 | 22 |
| America | 81 | 72 | 42 |
| Western Pacific | 88 | 86 | 55 |
| Virus | Strains/Variants | Country of Origin | Date of Identification | Key Mutations |
|---|---|---|---|---|
| SARS-CoV-2 | Original Wuhan strain (wild type) | China | December 2019 | Wild type |
| Alpha (B1.1.7) | United Kingdom | September 2020 | N501Y, A570D, D614G, P681H, T716I, S982A, D1118H, and two deletions (Δ69–70 and Δ145) [105,106,107] | |
| Beta (B.1.351) | South Africa | May 2020 | D80A, D215G, K417N, E484K, N501Y, D614G, and A701V [105,107] | |
| Gamma (P.1) | Brazil | November 2020 | L18F, T20 N, P26S, D138Y, R190S, K417T, E484K, N501Y, D614G, H655Y, and T1027I [105,107] | |
| Delta (B.1.617.2) | India | October 2020 | T19R, L452R, T478K, D614G, P681R, and D950N [105,107] | |
| Omicron (B1.1.529) | Botswana, South Africa | November 2021 | A67V, T95I, Y145D, L212I, G339D, S371L, S373P, S375F, K417N, N440K, G446S, S477N, T478K, E484A, Q493R, G496S, Q498R, N501Y, Y505H, T547K, D614G, H655Y, N679K, P681H, N764K, D796Y, N856K, Q954H, N969K, L981F, three deletions (H69/V70, G142/V143/Y144, and N211), one insertion (EPE at 214) [107], and deFLiRT [108] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uwamahoro, H.; Collier, W.E.; Nashar, T.O.; Jaynes, J.M.; Mortley, D.G.; Davis, C.G.; Kanyairita, G.G.; Abdelazim, E.F.; Igiramaboko, J.F.R.; Habineza, C.; et al. Natural and Designed Cyclic Peptides as Potential Antiviral Drugs to Combat Future Coronavirus Outbreaks. Molecules 2025, 30, 1651. https://doi.org/10.3390/molecules30081651
Uwamahoro H, Collier WE, Nashar TO, Jaynes JM, Mortley DG, Davis CG, Kanyairita GG, Abdelazim EF, Igiramaboko JFR, Habineza C, et al. Natural and Designed Cyclic Peptides as Potential Antiviral Drugs to Combat Future Coronavirus Outbreaks. Molecules. 2025; 30(8):1651. https://doi.org/10.3390/molecules30081651
Chicago/Turabian StyleUwamahoro, Hilarie, Willard E. Collier, Toufic O. Nashar, Jesse M. Jaynes, Desmond G. Mortley, Cheryl G. Davis, Getrude G. Kanyairita, Eslam F. Abdelazim, Jean Francois Regis Igiramaboko, Concorde Habineza, and et al. 2025. "Natural and Designed Cyclic Peptides as Potential Antiviral Drugs to Combat Future Coronavirus Outbreaks" Molecules 30, no. 8: 1651. https://doi.org/10.3390/molecules30081651
APA StyleUwamahoro, H., Collier, W. E., Nashar, T. O., Jaynes, J. M., Mortley, D. G., Davis, C. G., Kanyairita, G. G., Abdelazim, E. F., Igiramaboko, J. F. R., Habineza, C., Tumushimiyimana, D., Rutayisire, U. N., Davis, Y. A., & Renard, K. L. (2025). Natural and Designed Cyclic Peptides as Potential Antiviral Drugs to Combat Future Coronavirus Outbreaks. Molecules, 30(8), 1651. https://doi.org/10.3390/molecules30081651








