Optimization of Squalene Production by Pseudozyma sp. P4-22
Abstract
1. Introduction
| Strains | Squalene Titer | Media or Culture Method | Reference |
|---|---|---|---|
| Rhodosporidium sp. DR37 | 619 mg/L | modified YEPD media | [25] |
| Saccharomyces cerevisiae EGY48 | 3129 μg/L | glucose, soy peptone, and yeast extract, under semi-aerobic conditions | [26] |
| Torulaspora delbrueckii | 237.25 μg/gDW | molasses | [27] |
| Pseudozyma sp. JCC207 | 340.52 mg/L | glucose, yeast extract, sea salt | [28] |
| Saccharomyces cerevisiae EGY48 | 20.70 mg/L | glucose, soy peptone, and yeast extract with 0.442 mM terbinafine and 0.044 mM methyl jasmonate | [29] |
| Schizochytrium sp. HX-308 | 439.98 mg/L | glucose and yeast extract; agitation and ventilation rates were set at 250 rpm and 0.6 m3h−1 | [30] |
| Thraustochytrium striatum N5997 | 13 mg/gDW | glucose and yeast extract | [31] |
| Thraustochytrium ATCC 26185 | 72.9 mg/L | glucose and yeast extract with mannitol | [32] |
| Aurantiochytrium sp. TWZ-97 | 240.2 mg/L | glucose, yeast extract, 0.7 g/L α-tocopherol | [33] |
| Aurantiochytrium sp. Yonez 5−1 | 1073.66 mg/L | glucose, tryptone, yeast extract | [34] |
| Aurantiochytrium sp. 18W-13a | 1.29 g/L | glucose, proteose-peptone, yeast extract | [35] |
| Thraustochytrium MST1253 | 65.2 mg/L | glucose, peptone, yeast extract | [36] |
| Thraustochytrium sp. MAN37 FRU | 10.2 mg/gDW | peptone, yeast extract, glucose | [37] |
| Cutaneotrichosporon oleaginosus | 367.89 mg/L | glucose, urea, peptone, yeast extract, 10 mg/L terbinafine | [38] |
| Phormidium autumnale | 0.18 g/kgDW | glucose and slaughterhouse wastewater | [39] |
| Pseudozyma sp. SD 301 | 2.45 g/L | glucose and yeast extract | [23] |
| Pseudozyma sp. P4-22 | 2.06 g/L | glucose and corn steep liquor | This study |
2. Results and Discussion
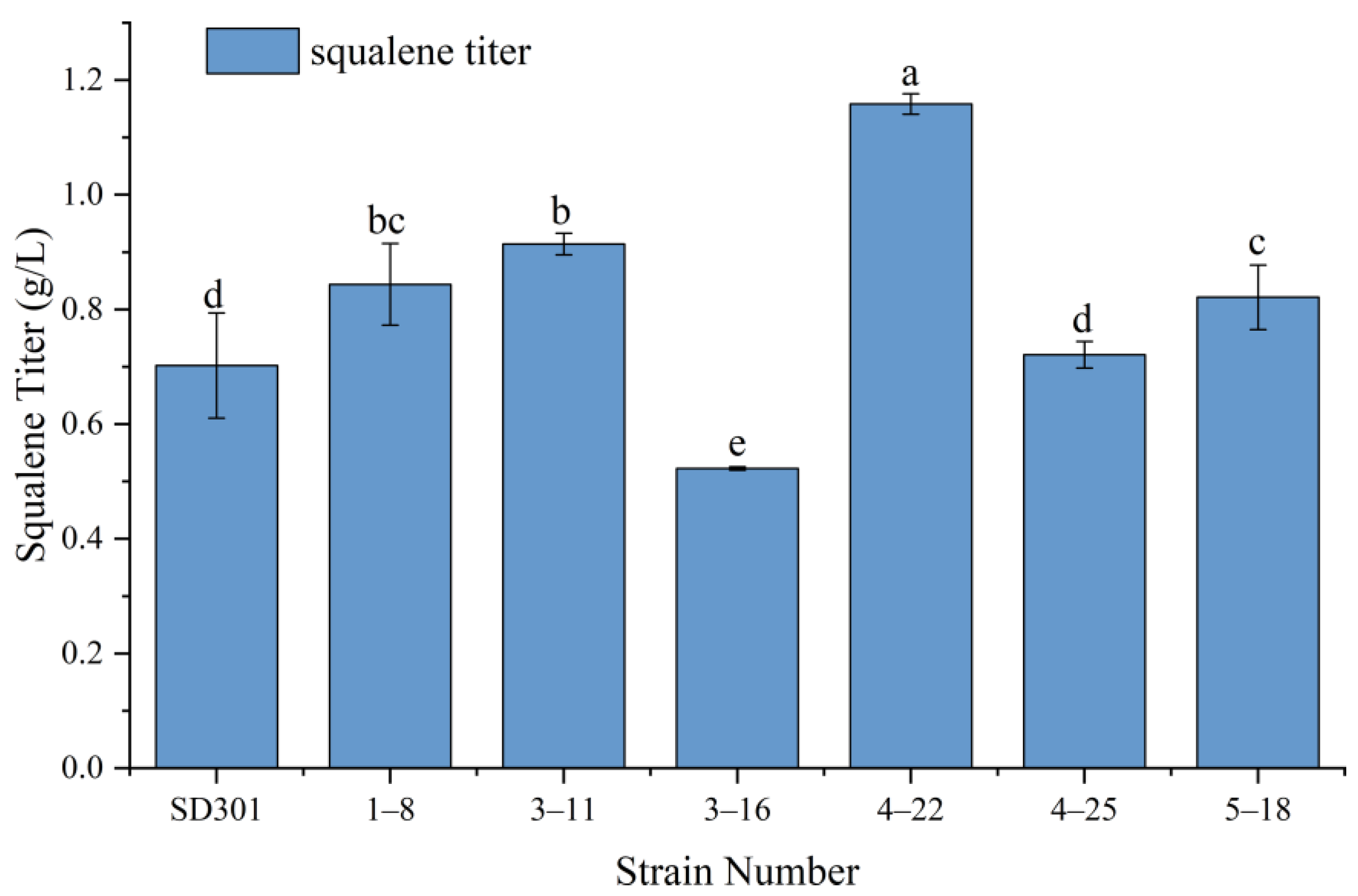
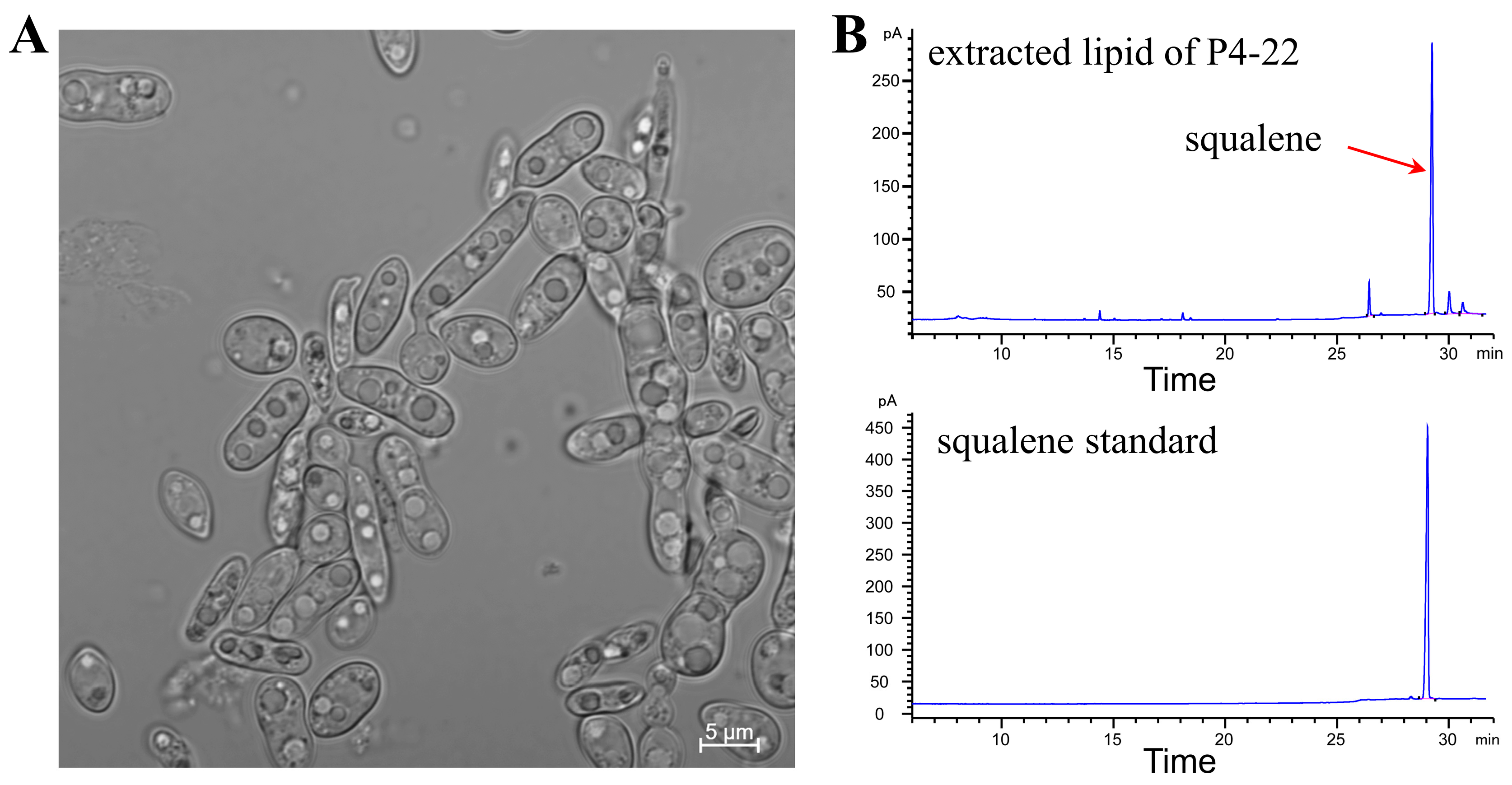
2.1. ARTP Mutagenesis
2.2. Effect of Carbon Source on P4-22 Fermentation Performance
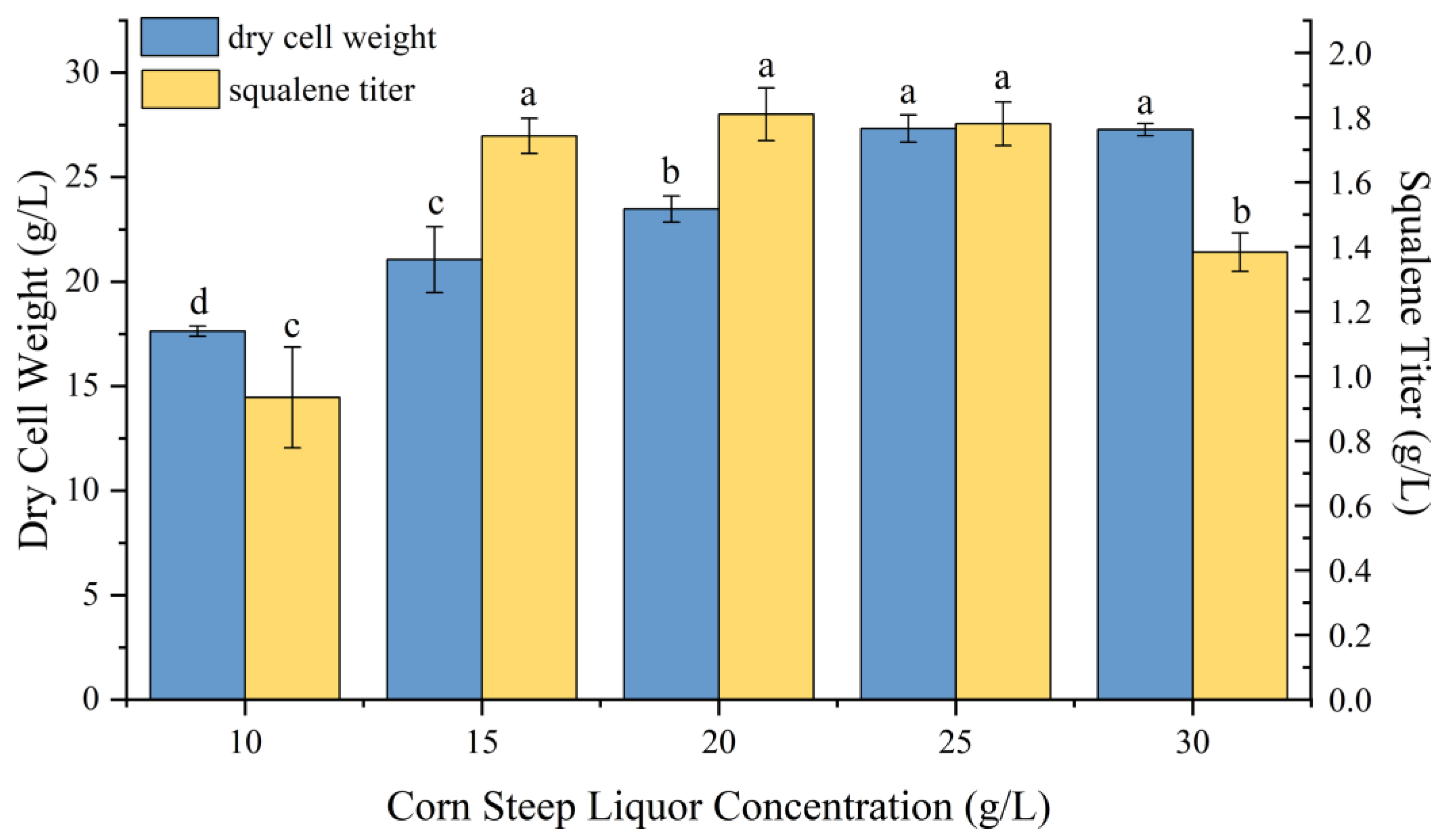
2.3. Effect of Nitrogen Source on P4-22 Fermentation Performance
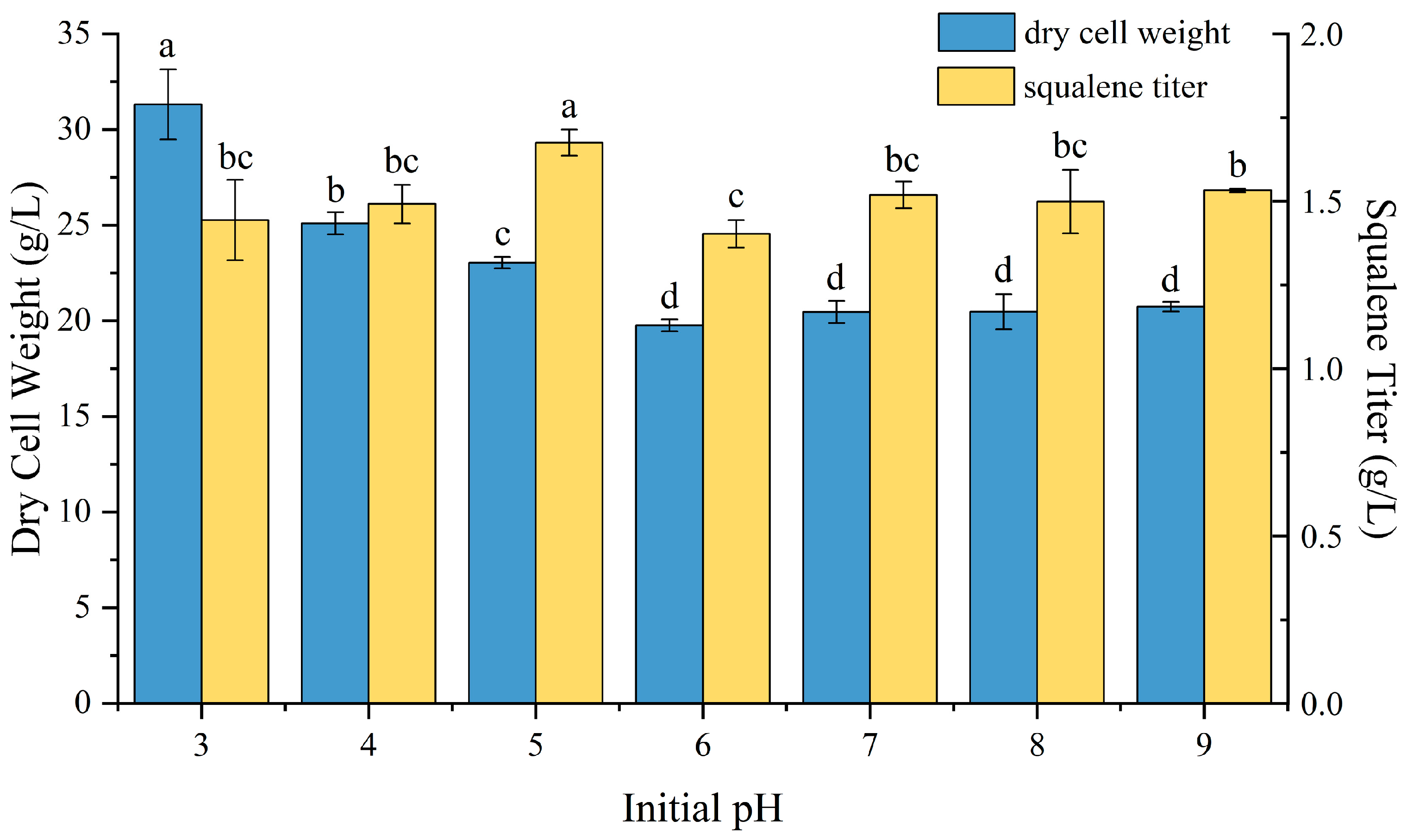
2.4. Effect of pH on P4-22 Fermentation
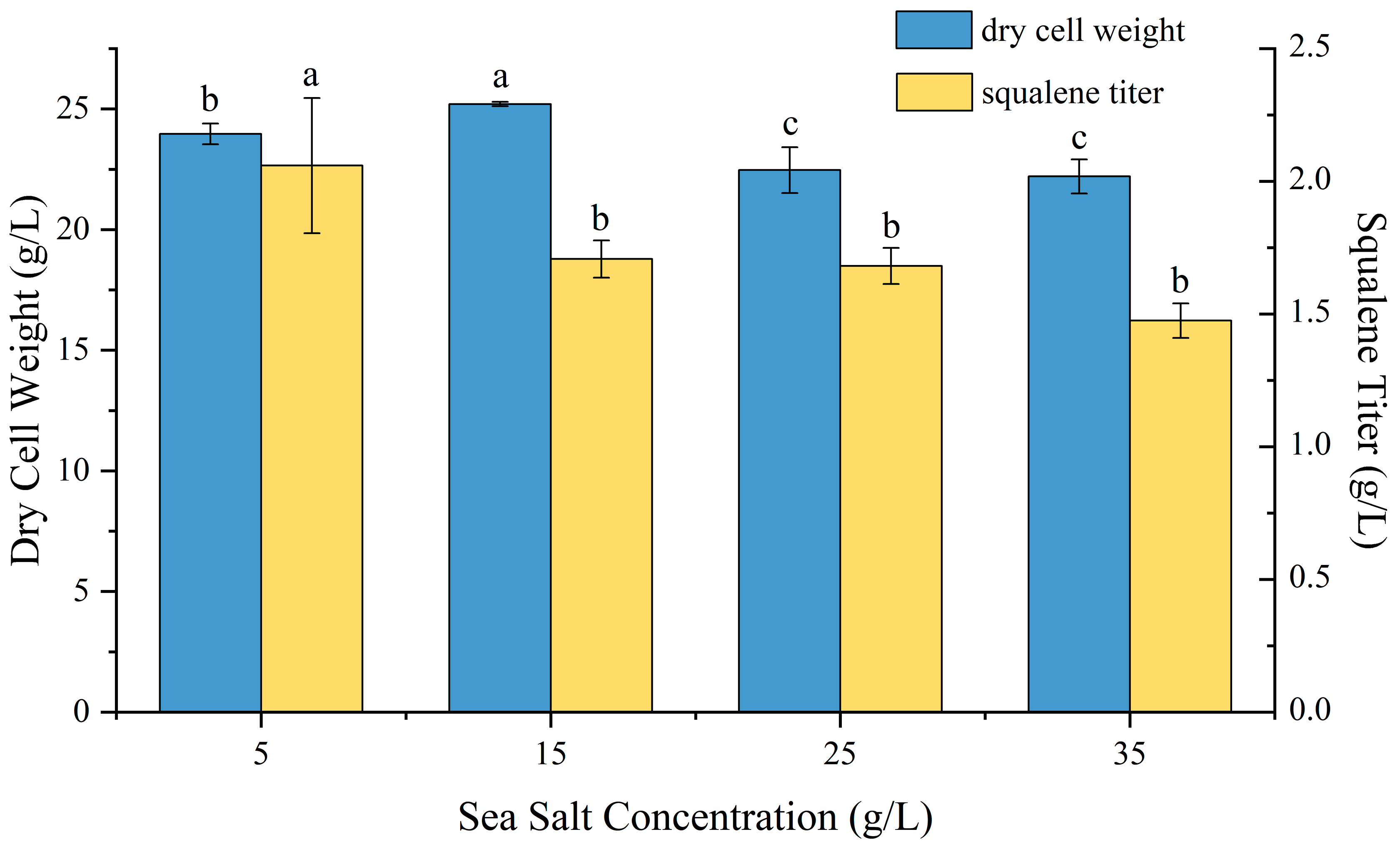
2.5. Effect of Sea Salt Concentration on P4-22 Fermentation
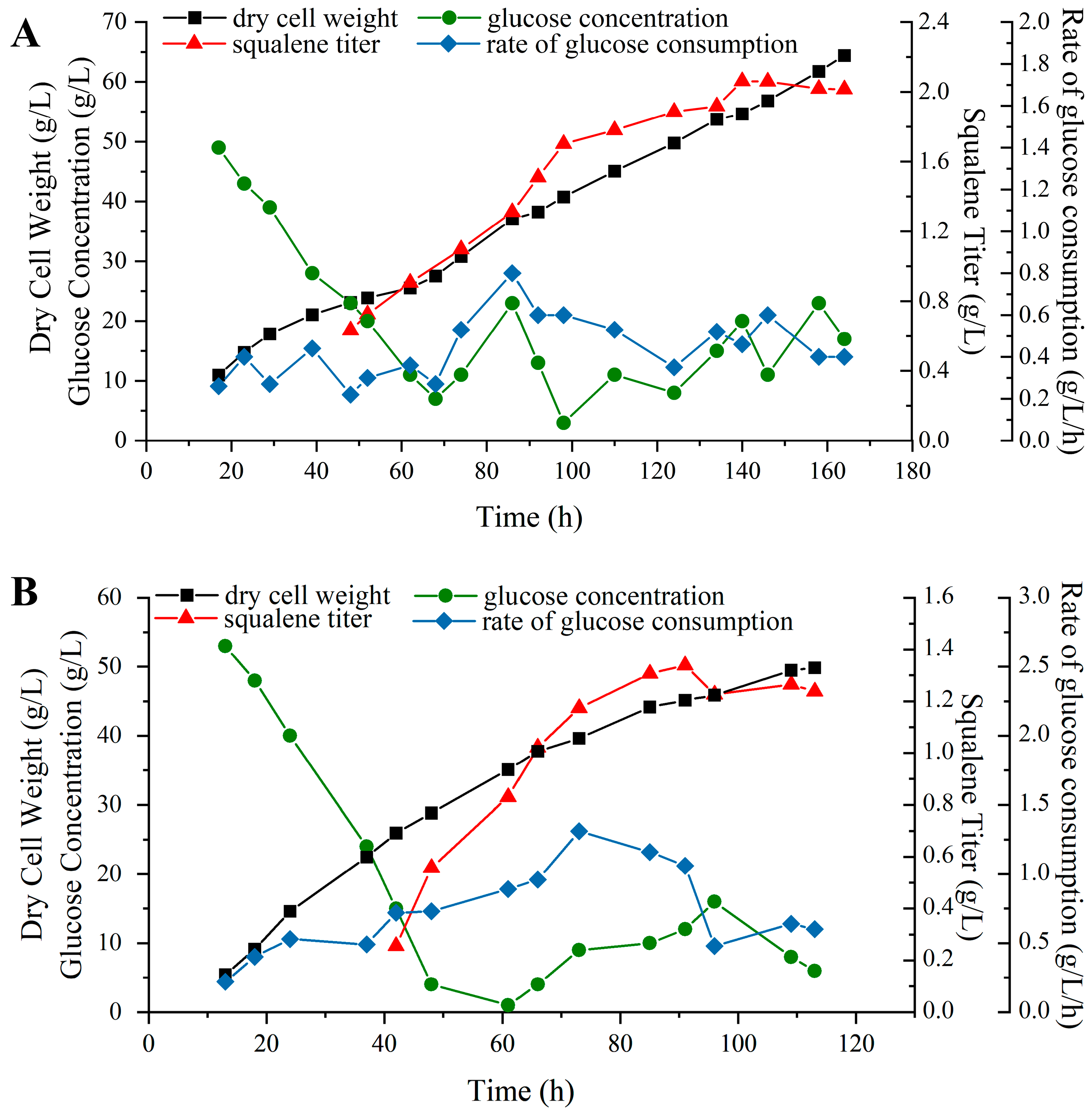
2.6. Fed-Batch Fermentation Experiments for Squalene Production
3. Materials and Methods
3.1. Strain and Culture
3.2. ARTP Mutagenesis
3.3. Optimization of Cultivation Conditions
3.4. Fed-Batch Fermentations in a 5 L Fermenter
3.5. Analysis of Biomass
3.6. Extraction and Detection of Lipids and Squalene
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ARTP | atmospheric and room temperature plasma |
| MEP | 2-methyl-D-erythritol 4-phosphate |
| MVA | mevalonate acid |
| HMGS | 3-hydroxy-3-methylglutaryl-CoA synthase |
| HMGR | 3-hydroxy-3-methylglutaryl-CoA reductase |
| IPP | isopentenyl pyrophosphate |
| DMAPP | dimethylallyl pyrophosphate |
| FPP | farnesyl pyrophosphate |
| SQS | squalene synthase |
References
- Du, X.; Ma, X.; Gao, Y. The physiological function of squalene and its application prospects in animal husbandry. Front. Vet. Sci. 2023, 10, 1284500. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Karadeniz, F. Biological importance and applications of squalene and squalane. Adv. Food Nutr. Res. 2012, 65, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Warleta, F.; Campos, M.; Allouche, Y.; Sánchez-Quesada, C.; Ruiz-Mora, J.; Beltrán, G.; Gaforio, J.J. Squalene protects against oxidative DNA damage in MCF10A human mammary epithelial cells but not in MCF7 and MDA-MB-231 human breast cancer cells. Food Chem. Toxicol. 2010, 48, 1092–1100. [Google Scholar] [CrossRef]
- Patel, A.; Bettiga, M.; Rova, U.; Christakopoulos, P.; Matsakas, L. Microbial genetic engineering approach to replace shark livering for squalene. Trends Biotechnol. 2022, 40, 1261–1273. [Google Scholar] [CrossRef]
- Kedl, J.D.; Kedl, R.M. How squalene GLAdly helps generate antigen-specific T cells via antigen-carrying neutrophils and IL-18. Eur. J. Immunol. 2015, 45, 376–379. [Google Scholar] [CrossRef]
- Nakkala, J.R.; Li, Y.; Akter, L.; Kang, X.; Chen, X. Differential regulation of DC function, adaptive immunity, and MyD88 dependence by MF59 and AS03-like adjuvants. Vaccines 2024, 12, 531. [Google Scholar] [CrossRef]
- Arunachalam, P.S.; Walls, A.C.; Golden, N.; Atyeo, C.; Fischinger, S.; Li, C.; Aye, P.; Navarro, M.J.; Lai, L.; Edara, V.V.; et al. Adjuvanting a subunit COVID-19 vaccine to induce protective immunity. Nature 2021, 594, 253–258. [Google Scholar] [CrossRef]
- Iwata, S.; Pollard, A.J.; Tada, Y.; Omoto, S.; Shibata, R.Y.; Igarashi, K.; Hasegawa, T.; Ariyasu, M.; Sonoyama, T. A phase 3 randomized controlled trial of a COVID-19 recombinant vaccine S-268019-b versus ChAdOx1 nCoV-19 in Japanese adults. Sci. Rep. 2024, 14, 9830. [Google Scholar] [CrossRef]
- Lou-Bonafonte, J.M.; Martínez-Beamonte, R.; Sanclemente, T.; Surra, J.C.; Herrera-Marcos, L.V.; Sanchez-Marco, J.; Arnal, C.; Osada, J. Current insights into the biological action of squalene. Mol. Nutr. Food Res. 2018, 62, e1800136. [Google Scholar] [CrossRef]
- Reddy, L.H.; Couvreur, P. Squalene: A natural triterpene for use in disease management and therapy. Adv. Drug Del. Rev. 2009, 61, 1412–1426. [Google Scholar] [CrossRef]
- Gumus, E.; Erdogan, O.; Demirkan, B.; Cevik, O. Squalene suppresses Jam-A, Claudin 5, and Occludin by accelerating cell death and reducing neuronal interaction in the neuroblastoma cell line SH-SY5Y. J. Res. Pharm. 2022, 26, 1676–1684. [Google Scholar] [CrossRef]
- Lei, X.; Zhao, S.; Chen, W.; Song, F. Comparative study on anti-aging effect of squalene and vitamin E to skin. Sci. Technol. Food Ind. 2013, 34, 91–93. [Google Scholar] [CrossRef]
- Ronco, A.L.; Stéfani, E.D. Squalene: A multi-task link in the crossroads of cancer and aging. Funct. Foods Health Dis. 2013, 3, 462–476. [Google Scholar] [CrossRef]
- Luke, S.S.; Raj, M.N.; Ramesh, S.; Bhatt, N.P. Network pharmacology prediction and molecular docking-based strategy to explore the potential mechanism of squalene against inflammation. In Silico Pharmacol. 2024, 12, 44. [Google Scholar] [CrossRef]
- Cárdeno, A.; Aparicio-Soto, M.; Montserrat-de la Paz, S.; Bermudez, B.; Muriana, F.J.G.; Alarcón-de-la-Lastra, C. Squalene targets pro- and anti-inflammatory mediators and pathways to modulate over-activation of neutrophils, monocytes and macrophages. J. Funct. Foods 2015, 14, 779–790. [Google Scholar] [CrossRef]
- Del Rosso, J.Q.; Kircik, L. The primary role of sebum in the pathophysiology of acne vulgaris and its therapeutic relevance in acne management. J. Dermatolog. Treat. 2024, 35, 2296855. [Google Scholar] [CrossRef]
- Gohil, N.; Bhattacharjee, G.; Khambhati, K.; Braddick, D.; Singh, V. Engineering strategies in microorganisms for the enhanced production of squalene: Advances, challenges and opportunities. Front. Bioeng. Biotechnol. 2019, 7, 50. [Google Scholar] [CrossRef]
- Xu, W.; Ma, X.; Wang, Y. Production of squalene by microbes: An update. World J. Microbiol. Biotechnol. 2016, 32, 195. [Google Scholar] [CrossRef]
- Shalu, S.; Raveendranathan, P.K.; Vaidyanathan, V.K.; Blank, L.M.; Germer, A.; Balakumaran, P.A. Microbial squalene: A sustainable alternative for the cosmetics and pharmaceutical industry—A Review. Eng. Life Sci. 2024, 24, e202400003. [Google Scholar] [CrossRef]
- Chai, L.; Che, J.X.; Qi, Q.S.; Hou, J. Metabolic engineering for squalene production: Advances and perspectives. J. Agric. Food Chem. 2024, 72, 27715–27725. [Google Scholar] [CrossRef]
- Paramasivan, K.; Mutturi, S. Recent advances in the microbial production of squalene. World J. Microbiol. Biotechnol. 2022, 38, 91. [Google Scholar] [CrossRef] [PubMed]
- Valasques Junior, G.L.; Dos Santos, J.D.G.; Chaves, P.F.P.; Cordeiro, L.M.C.; de Jesus, C.L.; de Lima, F.O.; Boffo, E.F.; de Assis, S.A. Antinociceptive and anti-inflammatory activity of α-d-mannan from Pseudozyma sp. 3 Biotech 2021, 11, 73. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Wang, X.; Tan, Y.; Feng, Y.; Li, W.; Cui, Q. High production of squalene using a newly isolated yeast-like strain Pseudozyma sp. SD301. J. Agric. Food Chem. 2015, 63, 8445–8451. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, L.; Wang, S.; Cong, P.; Lu, D.; Feng, Y.; Cui, Q.; Song, X. Mutation and selection of high squalene production yeast Pseudozyma sp. induced by carbon-ions beam irradiation and its electrotransfor-mation. South China Fish. Sci. 2022, 18, 98–104. [Google Scholar] [CrossRef]
- Shakeri, S.; Khoshbasirat, F.; Maleki, M. Rhodosporidium sp. DR37: A novel strain for production of squalene in optimized cultivation conditions. Biotechnol. Biofuels 2021, 14, 95. [Google Scholar] [CrossRef]
- Mantzouridou, F.; Naziri, E.; Tsimidou, M.Z. Squalene versus ergosterol formation using Saccharomyces cerevisiae: Combined effect of oxygen supply, inoculum size, and fermentation time on yield and selectivity of the bioprocess. J. Agric. Food Chem. 2009, 57, 6189–6198. [Google Scholar] [CrossRef]
- Bhattacharjee, P.; Shukla, V.B.; Singhal, R.S.; Kulkarni, P.R. Studies on fermentative production of squalene. World J. Microbiol. Biotechnol. 2001, 17, 811–816. [Google Scholar] [CrossRef]
- Chang, M.H.; Kim, H.J.; Jahng, K.Y.; Hong, S.C. The isolation and characterization of Pseudozyma sp. JCC 207, a novel producer of squalene. Appl. Microbiol. Biotechnol. 2008, 78, 963–972. [Google Scholar] [CrossRef]
- Naziri, E.; Mantzouridou, F.; Tsimidou, M.Z. Enhanced squalene production by wild-type Saccharomyces cerevisiae strains using safe chemical means. J. Agric. Food Chem. 2011, 59, 9980–9989. [Google Scholar] [CrossRef]
- Ren, L.J.; Sun, G.N.; Ji, X.J.; Hu, X.C.; Huang, H. Compositional shift in lipid fractions during lipid accumulation and turnover in Schizochytrium sp. Bioresour. Technol. 2014, 157, 107–113. [Google Scholar] [CrossRef]
- Koopmann, I.K.; Müller, B.A.; Labes, A. Screening of a Thraustochytrid strain collection for carotenoid and squalene production characterized by cluster analysis, comparison of 18S rRNA gene sequences, growth behavior, and morphology. Mar. Drugs 2023, 21, 204. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.K.; Sen, B.; He, Y.; Bai, M.; Wang, G. Media supplementation with mannitol and biotin enhances squalene production of Thraustochytrium ATCC 26185 through increased glucose uptake and antioxidative mechanisms. Molecules 2022, 27, 2449. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.K.; Liu, X.; Li, J.; Zhu, X.; Sen, B.; Wang, G. Alpha-tocopherol significantly improved squalene production yield of Aurantiochytrium sp. TWZ-97 through lowering ROS levels and up-regulating key genes of central carbon metabolism pathways. Antioxidants 2023, 12, 1034. [Google Scholar] [CrossRef]
- Nakazawa, A.; Kokubun, Y.; Matsuura, H.; Yonezawa, N.; Kose, R.; Yoshida, M.; Tanabe, Y.; Kusuda, E.; Van Thang, D.; Ueda, M.; et al. TLC screening of thraustochytrid strains for squalene production. J. Appl. Phycol. 2014, 26, 29–41. [Google Scholar] [CrossRef]
- Kaya, K.; Nakazawa, A.; Matsuura, H.; Honda, D.; Inouye, I.; Watanabe, M.M. Thraustochytrid Aurantiochytrium sp. 18W-13a accummulates high amounts of squalene. Biosci. Biotechnol. Biochem. 2011, 75, 2246–2248. [Google Scholar] [CrossRef]
- Otagiri, M.; Khalid, A.; Moriya, S.; Osada, H.; Takahashi, S. Novel squalene-producing thraustochytrids found in mangrove water. Biosci. Biotechnol. Biochem. 2017, 81, 2034–2037. [Google Scholar] [CrossRef][Green Version]
- Miranda, A.F.; Nham Tran, T.L.; Abramov, T.; Jehalee, F.; Miglani, M.; Liu, Z.; Rochfort, S.; Gupta, A.; Cheirsilp, B.; Adhikari, B.; et al. Marine protists and Rhodotorula yeast as bio-convertors of marine waste into nutrient-rich deposits for mangrove ecosystems. Protist 2020, 171, 125738. [Google Scholar] [CrossRef]
- Stellner, N.I.; Rerop, Z.S.; Kyselka, J.; Alishevich, K.; Beneš, R.; Filip, V.; Celik, G.; Haack, M.; Ringel, M.; Masri, M.; et al. Value-added squalene in single-cell oil produced with Cutaneotrichosporon oleaginosus for food applications. J. Agric. Food Chem. 2023, 71, 8540–8550. [Google Scholar] [CrossRef]
- Fagundes, M.B.; Vendruscolo, R.G.; Maroneze, M.M.; Barin, J.S.; de Menezes, C.R.; Zepka, L.Q.; Jacob-Lopes, E.; Wagner, R. Towards a sustainable route for the production of squalene using cyanobacteria. Waste Biomass Valorization 2019, 10, 1295–1302. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, L.Y.; Ma, K.; Li, G.; Zhang, C.; Zhao, H.X.; Lai, Q.H.; Li, H.P.; Xing, X.H. Characteristics of hydrogen production of an Enterobacter aerogenes mutant generated by a new atmospheric and room temperature plasma (ARTP). Biochem. Eng. J. 2011, 55, 17–22. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.-F.; Li, H.-P.; Wang, L.-Y.; Zhang, C.; Xing, X.-H.; Bao, C.-Y. Atmospheric and room temperature plasma (ARTP) as a new powerful mutagenesis tool. Appl. Microbiol. Biotechnol. 2014, 98, 5387–5396. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Shen, J.; Ding, Q.; Wu, J.; Chen, X. Recent progress of atmospheric and room-temperature plasma as a new and promising mutagenesis technology. Cell Biochem. Funct. 2024, 42, e3991. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-J.; Li, B.; Feng, Y.; Cui, Q. Consolidated bio-saccharification: Leading lignocellulose bioconversion into the real world. Biotechnol. Adv. 2020, 40, 107535. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Cui, Q.; Feng, Y.; Xuan, J. Composition of lignocellulose hydrolysate in different biorefinery strategies: Nutrients and inhibitors. Molecules 2024, 29, 2275. [Google Scholar] [CrossRef]
- Faria, N.T.; Santos, M.V.; Fernandes, P.; Fonseca, L.L.; Fonseca, C.; Ferreira, F.C. Production of glycolipid biosurfactants, mannosylerythritol lipids, from pentoses and d-glucose/d-xylose mixtures by Pseudozyma yeast strains. Process Biochem. 2014, 49, 1790–1799. [Google Scholar] [CrossRef][Green Version]
- Mierke, F.; Brink, D.P.; Norbeck, J.; Siewers, V.; Andlid, T. Functional genome annotation and transcriptome analysis of Pseudozyma hubeiensis BOT-O, an oleaginous yeast that utilizes glucose and xylose at equal rates. Fungal Genet. Biol. 2023, 166, 103783. [Google Scholar] [CrossRef]
- Kaya, K.; Kazama, Y.; Abe, T.; Shiraishi, F. Influence of medium components and pH on the production of odd-carbon fatty acids by Aurantiochytrium sp. SA-96. J. Appl. Phycol. 2020, 32, 1597–1606. [Google Scholar] [CrossRef]
- Zhong, Y.; Gu, J.; Shang, C.; Deng, J.; Liu, Y.; Cui, Z.; Lu, X.; Qi, Q. Sustainable succinic acid production from lignocellulosic hydrolysates by engineered strains of Yarrowia lipolytica at low pH. Bioresour. Technol. 2024, 408, 131166. [Google Scholar] [CrossRef]
- Dias, C.; Silva, C.; Freitas, C.; Reis, A.; da Silva, T.L. Effect of medium pH on Rhodosporidium toruloides NCYC 921 carotenoid and lipid production evaluated by flow cytometry. Appl. Biochem. Biotechnol. 2016, 179, 776–787. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, R.N.; Song, X.P.; Singh, R.K.; Guo, D.J.; Singh, P.; Verma, K.K.; Li, Y.R. Genome analysis of a halophilic Virgibacillus halodenitrificans ASH15 revealed salt adaptation, plant growth promotion, and isoprenoid biosynthetic machinery. Front. Microbiol. 2023, 14, 1229955. [Google Scholar] [CrossRef]
- Zhang, A.; He, Y.; Sen, B.; Wang, W.; Wang, X.; Wang, G. Optimal NaCl Medium enhances squalene accumulation in Thraustochytrium sp. ATCC 26185 and influences the expression levels of key metabolic genes. Front. Microbiol. 2022, 13, 900252. [Google Scholar] [CrossRef] [PubMed]
- Toderich, K.N.; Mamadrahimov, A.A.; Khaitov, B.B.; Karimov, A.A.; Soliev, A.A.; Nanduri, K.R.; Shuyskaya, E.V. Differential impact of salinity stress on seeds minerals, storage proteins, fatty acids, and squalene composition of new quinoa genotype, grown in hyper-arid desert environments. Front. Plant Sci. 2020, 11, 607102. [Google Scholar] [CrossRef] [PubMed]
| Carbon Source | Strain Biomass (g/L) | Squalene Titer (g/L) 1 |
|---|---|---|
| glucose | 21.51 ± 0.18 | 0.99 ± 0.03 |
| sucrose | 19.98 ± 0.32 | 0.82 ± 0.07 |
| maltose | 6.05 ± 0.52 | 0.05 ± 0.01 |
| xylose | 17.79 ± 0.75 | 0.98 ± 0.03 |
| cellobiose | 3.12 ± 0.27 | - |
| lactose | 10.27 ± 1.67 | 0.25 ± 0.17 |
| starch soluble | 5.84 ± 0.22 | - |
| citric acid | 5.36 ± 0.05 | - |
| malic acid | 3.06 ± 1.79 | - |
| succinic acid | 12.83 ± 3.49 | 0.11 ± 0.003 |
| glycerin | 5.77 ± 0.08 | - |
| methanol | 1.02 ± 0.06 | - |
| ethanol | 4.37 ± 0.11 | 0.003 ± 0.001 |
| lactic acid | 3.75 ± 0.03 | - |
| sorbitol | 0.96 ± 0.11 | - |
| Nitrogen Source | Strain Biomass (g/L) | Squalene Titer (g/L) 1 |
|---|---|---|
| yeast extract | 23.07 ± 1.44 | 1.29 ± 0.11 |
| tryptone | 12.14 ± 0.16 | 0.04 ± 0.02 |
| corn steep liquor | 23.28 ± 0.45 | 1.74 ± 0.003 |
| urea | 1.03 ± 0.25 | - |
| NH4Cl | 1.26 ± 0.14 | - |
| (NH4)2SO4 | 1.54 ± 0.19 | - |
| NaNO3 | 2.1 ± 0.45 | - |
| KNO3 | 1.69 ± 0.09 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.; Song, X.; Li, J.; Cui, Q.; Gu, P.; Feng, Y. Optimization of Squalene Production by Pseudozyma sp. P4-22. Molecules 2025, 30, 1646. https://doi.org/10.3390/molecules30071646
Huang C, Song X, Li J, Cui Q, Gu P, Feng Y. Optimization of Squalene Production by Pseudozyma sp. P4-22. Molecules. 2025; 30(7):1646. https://doi.org/10.3390/molecules30071646
Chicago/Turabian StyleHuang, Chen, Xiaojin Song, Jingyi Li, Qiu Cui, Pengfei Gu, and Yingang Feng. 2025. "Optimization of Squalene Production by Pseudozyma sp. P4-22" Molecules 30, no. 7: 1646. https://doi.org/10.3390/molecules30071646
APA StyleHuang, C., Song, X., Li, J., Cui, Q., Gu, P., & Feng, Y. (2025). Optimization of Squalene Production by Pseudozyma sp. P4-22. Molecules, 30(7), 1646. https://doi.org/10.3390/molecules30071646








