Static and Dynamic Assessments of a Sulfur-Triglyceride Composite for Antimicrobial Surface Applications
Abstract
1. Introduction
2. Results and Discussion
2.1. Microbe Selection and Antimicrobial Properties of Static Hard Surfaces (ASTM E 2180-18)
2.1.1. Preparation of Tile Test Samples and Test Organism Rationale
2.1.2. Efficacy Against Gram-Positive Bacteria
2.1.3. Variable Activity Against Gram-Negative Bacteria
2.1.4. Efficacy Against Fungi
2.2. Microbe Selection and Antimicrobial Properties of SunBG90-Infused Fabric Under Dynamic Conditions (ASTM E 2149-20)
2.2.1. Preparation of SunBG90-Infused Fabric Squares
2.2.2. Activity Against Gram-Positive Bacteria
2.2.3. Activity Against Gram-Negative Bacteria
2.2.4. Activity Against Yeast
2.3. Summary of Antimicrobial Properties
3. Materials and Methods
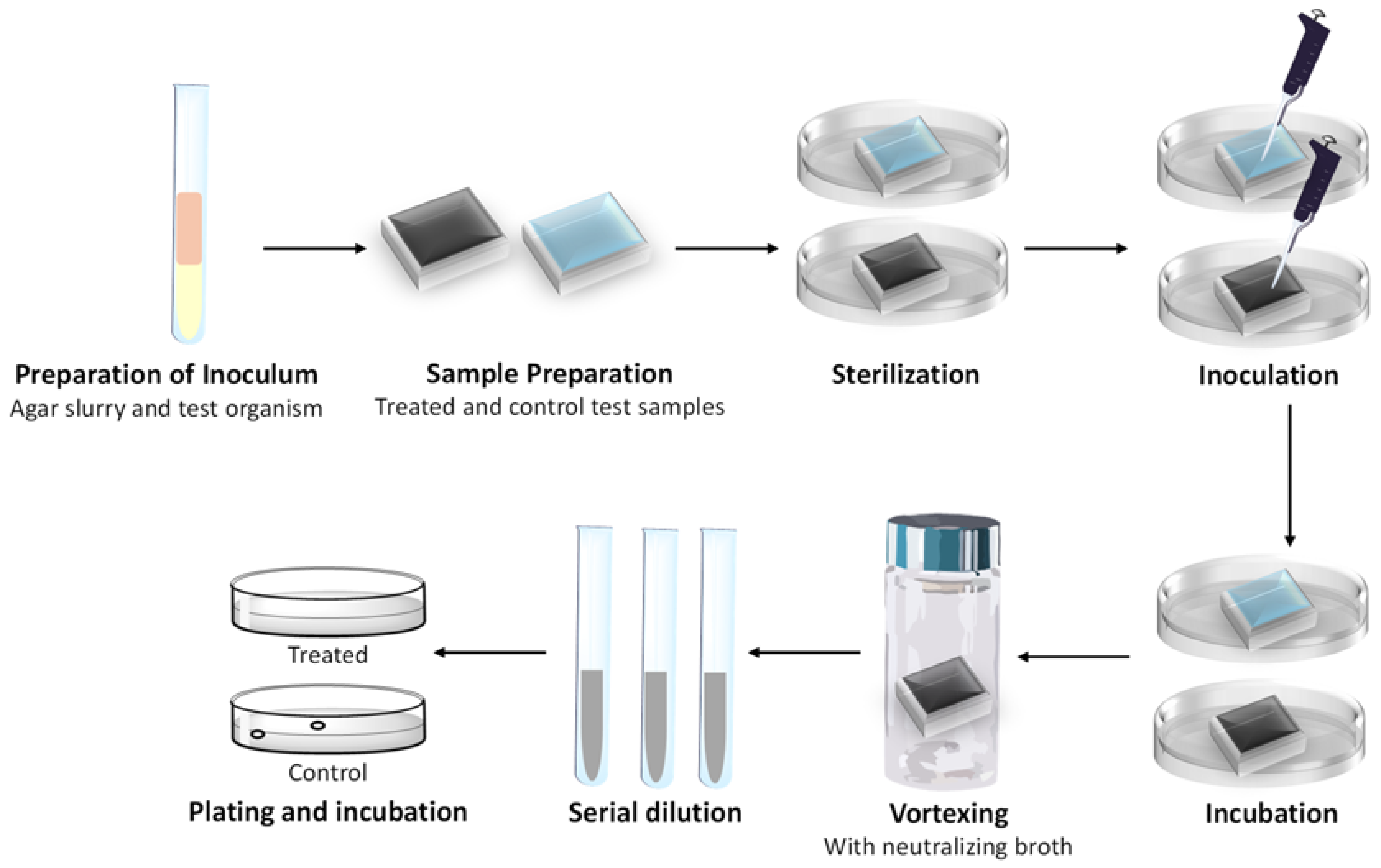
3.1. Testing of Tiles According to ASTM E 2180-18
3.1.1. Sample Preparation
3.1.2. Microorganisms and Growth Conditions
- Campylobacter jejuni (ATCC 33291, ATCC 29428);
- Aspergillus fumigatus (ATCC 204305);
- Salmonella typhi (ATCC 14028);
- Salmonella enteritidis (ATCC 13076);
- Clostridium perfringens (ATCC 13124);
- Listeria monocytogenes (ATCC 19115);
- Candida auris (ATCC CDC B11903);
- Staphylococcus aureus (ATCC 6538);
- Escherichia coli (ATCC 8739).
- Aerobic incubation (30–35 °C) for most bacteria;
- Anaerobic incubation (30–35 °C) for C. perfringens;
- Microaerophilic incubation (37 ± 2 °C) for C. jejuni;
- Fungal incubation (20–25 °C) for A. fumigatus and C. Auris.
3.1.3. Preparation of Inoculated Agar Slurry
3.1.4. Experimental Procedure
Surface Preparation
Inoculation and Incubation
Microbial Recovery and Enumeration
3.1.5. Controls and Quality Assurance:
3.1.6. Data Analysis
3.1.7. Interpretation of Results
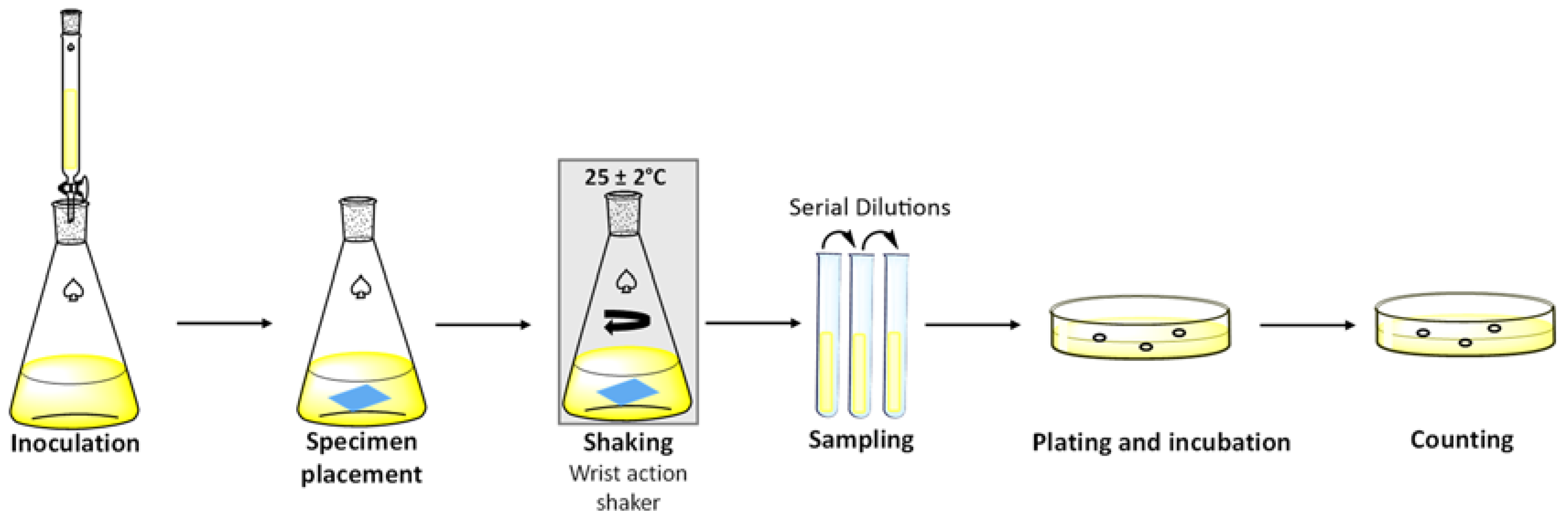
3.2. Testing of Fabric Squares According to ASTM E 2149-20
3.2.1. Sample Preparation
3.2.2. Bacterial Culture Preparation
3.2.3. Experimental Procedure
3.2.4. Control and Quality Assurance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HSM | High Sulfur Content Material |
| SunBG90 | Composite made from 90 wt. % sulfur, 5 wt. % brown grease, and 5 wt. % sunflower oil |
| DIB | 1,3-Diisopropenylbenzene |
| SMS | Spunbond–Meltblown–Spunbond |
| ASTM | American Society for Testing and Materials |
References
- Pople, J.M.M.; Nicholls, T.P.; Pham, L.N.; Bloch, W.M.; Lisboa, L.S.; Perkins, M.V.; Gibson, C.T.; Coote, M.L.; Jia, Z.; Chalker, J.M. Electrochemical Synthesis of Poly(trisulfides). J. Am. Chem. Soc. 2023, 145, 11798–11810. [Google Scholar] [CrossRef] [PubMed]
- Newman, B.; Doeven, E.H.; Francis, P.S.; Stojcevski, F.; Hayne, D.J.; Chalker, J.M.; Henderson, L.C. A high value application of reclaimed carbon fibers: Environmental remediation and redeployment in structural composites. Sustain. Mater. Technol. 2023, 35, e00546. [Google Scholar] [CrossRef]
- Mueller, F.G.; Lisboa, L.S.; Chalker, J.M. Inverse Vulcanized Polymers for Sustainable Metal Remediation. Adv. Sustain. Syst. 2023, 7, 2300010. [Google Scholar] [CrossRef]
- Yan, P.; Zhao, W.; Tonkin, S.J.; Chalker, J.M.; Schiller, T.L.; Hasell, T. Stretchable and Durable Inverse Vulcanized Polymers with Chemical and Thermal Recycling. Chem. Mater. 2022, 34, 1167–1178. [Google Scholar] [CrossRef]
- Worthington, M.J.H.; Mann, M.; Muhti, I.Y.; Tikoalu, A.D.; Gibson, C.T.; Jia, Z.; Miller, A.D.; Chalker, J.M. Modelling mercury sorption of a polysulfide coating made from sulfur and limonene. Phys. Chem. Chem. Phys. 2022, 24, 12363–12373. [Google Scholar] [CrossRef]
- Stojcevski, F.; Stanfield, M.K.; Hayne, D.J.; Mann, M.; Lundquist, N.A.; Chalker, J.M.; Henderson, L.C. Inverse Vulcanisation of canola oil as a route to recyclable chopped carbon fibre composites. Sustain. Mater. Technol. 2022, 32, e00400. [Google Scholar] [CrossRef]
- Mann, M.; Pauling, P.J.; Tonkin, S.J.; Campbell, J.A.; Chalker, J.M. Chemically Activated S-S Metathesis for Adhesive-Free Bonding of Polysulfide Surfaces. Macromol. Chem. Phys. 2022, 223, 2100333. [Google Scholar] [CrossRef]
- Gupta, A.; Worthington, M.J.H.; Patel, H.D.; Johnston, M.R.; Puri, M.; Chalker, J.M. Reaction of sulfur and sustainable algae oil for polymer synthesis and enrichment of saturated triglycerides. ACS Sust. Chem. Eng. 2022, 10, 9022–9028. [Google Scholar] [CrossRef]
- Chalker, J.M.; Mann, M.; Worthington, M.J.H.; Esdaile, L.J. Polymers Made by Inverse Vulcanization for Use as Mercury Sorbents. Org. Mater. 2021, 3, 362–373. [Google Scholar] [CrossRef]
- Bu Najmah, I.; Lundquist, N.A.; Stanfield, M.K.; Stojcevski, F.; Campbell, J.A.; Esdaile, L.J.; Gibson, C.T.; Lewis, D.A.; Henderson, L.C.; Hasell, T.; et al. Insulating Composites Made from Sulfur, Canola Oil, and Wool. ChemSusChem 2021, 14, 2352–2359. [Google Scholar] [CrossRef]
- Molineux, J.; Lee, T.; Kim, K.J.; Kang, K.-S.; Lyons, N.P.; Nishant, A.; Kleine, T.S.; Durfee, S.W.; Pyun, J.; Norwood, R.A. Fabrication of Plastic Optics from Chalcogenide Hybrid Inorganic/Organic Polymers for Infrared Thermal Imaging. Adv. Opt. Mater. 2024, 12, 2301971. [Google Scholar] [CrossRef]
- Qureshi, M.H.; Bao, J.; Kleine, T.S.; Kim, K.-J.; Carothers, K.J.; Molineux, J.; Cho, E.; Kang, K.-S.; Godman, N.P.; Coropceanu, V.; et al. Synthesis of Deuterated and Sulfurated Polymers by Inverse Vulcanization: Engineering Infrared Transparency via Deuteration. J. Am. Chem. Soc. 2023, 145, 27821–27829. [Google Scholar] [CrossRef]
- Bischoff, D.J.; Lee, T.; Kang, K.-S.; Molineux, J.; O’Neil Parker, W., Jr.; Pyun, J.; Mackay, M.E. Unraveling the rheology of inverse vulcanized polymers. Nat. Commun. 2023, 14, 7553. [Google Scholar] [CrossRef]
- Bao, J.; Martin, K.P.; Cho, E.; Kang, K.-S.; Glass, R.S.; Coropceanu, V.; Bredas, J.-L.; Parker, W.O.N., Jr.; Njardarson, J.T.; Pyun, J. On the Mechanism of the Inverse Vulcanization of Elemental Sulfur: Structural Characterization of Poly(sulfur-random-(1,3-diisopropenylbenzene)). J. Am. Chem. Soc. 2023, 145, 12386–12397. [Google Scholar] [CrossRef] [PubMed]
- Pyun, J.; Carrozza, C.F.; Silvano, S.; Boggioni, L.; Losio, S.; de Angelis, A.R.; O’Neil Parker, W., Jr. Nuclear magnetic resonance structural characterization of sulfur-derived copolymers from inverse vulcanization. Part 1: Styrene. J. Polym. Sci. 2022, 60, 3471–3477. [Google Scholar] [CrossRef]
- Nishant, A.; Kim, K.-J.; Showghi, S.A.; Himmelhuber, R.; Kleine, T.S.; Lee, T.; Pyun, J.; Norwood, R.A. High Refractive Index Chalcogenide Hybrid Inorganic/Organic Polymers for Integrated Photonics. Adv. Opt. Mater. 2022, 10, 2200176. [Google Scholar] [CrossRef]
- Lee, T.; Dirlam, P.T.; Njardarson, J.T.; Glass, R.S.; Pyun, J. Polymerizations with Elemental Sulfur: From Petroleum Refining to Polymeric Materials. J. Am. Chem. Soc. 2022, 144, 5–22. [Google Scholar] [CrossRef]
- Kang, K.-S.; Olikagu, C.; Lee, T.; Bao, J.; Molineux, J.; Holmen, L.N.; Martin, K.P.; Kim, K.-J.; Kim, K.H.; Bang, J.; et al. Sulfenyl Chlorides: An Alternative Monomer Feedstock from Elemental Sulfur for Polymer Synthesis. J. Am. Chem. Soc. 2022, 144, 23044–23052. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.-S.; Iyer, K.A.; Pyun, J. On the Fundamental Polymer Chemistry of Inverse Vulcanization for Statistical and Segmented Copolymers from Elemental Sulfur. Chem. Eur. J. 2022, 28, e202200115. [Google Scholar] [CrossRef]
- Zhang, Y.; Konopka, K.M.; Glass, R.S.; Char, K.; Pyun, J. Chalcogenide hybrid inorganic/organic polymers (CHIPs) via inverse vulcanization and dynamic covalent polymerizations. Polym. Chem. 2017, 8, 5167–5173. [Google Scholar] [CrossRef]
- Kang, K.-S.; Phan, A.; Olikagu, C.; Lee, T.; Loy, D.A.; Kwon, M.; Paik, H.-j.; Hong, S.J.; Bang, J.; Parker, W.O., Jr.; et al. Segmented Polyurethanes and Thermoplastic Elastomers from Elemental Sulfur with Enhanced Thermomechanical Properties and Flame Retardancy. Angew. Chem. Int. Ed. 2021, 60, 22900–22907. [Google Scholar] [CrossRef]
- Kwon, M.; Lee, H.; Lee, S.-H.; Jeon, H.B.; Oh, M.-C.; Pyun, J.; Paik, H.-j. Dynamic Covalent Polymerization of Chalcogenide Hybrid Inorganic/Organic Polymer Resins with Norbornenyl Comonomers. Macromol. Res. 2020, 28, 1003–1009. [Google Scholar] [CrossRef]
- Gomez, I.; Mantione, D.; Leonet, O.; Blazquez, J.A.; Mecerreyes, D. Hybrid Sulfur-Selenium Co-polymers as Cathodic Materials for Lithium Batteries. ChemElectroChem 2018, 5, 260–265. [Google Scholar] [CrossRef]
- Gomez, I.; Mecerreyes, D.; Blazquez, J.A.; Leonet, O.; Ben Youcef, H.; Li, C.; Gomez-Camer, J.L.; Bundarchuk, O.; Rodriguez-Martinez, L. Inverse vulcanization of sulfur with divinylbenzene: Stable and easy processable cathode material for lithium-sulfur batteries. J. Power Sources 2016, 329, 72–78. [Google Scholar] [CrossRef]
- Gomez, I.; Leonet, O.; Blazquez, J.A.; Mecerreyes, D. Inverse Vulcanization of Sulfur using Natural Dienes as Sustainable Materials for Lithium-Sulfur Batteries. ChemSusChem 2016, 9, 3419–3425. [Google Scholar] [CrossRef]
- Grimm, A.P.; Scheiger, J.M.; Roesky, P.W.; Theato, P. Inverse vulcanization of trimethoxyvinylsilane particles. Polym. Chem. 2022, 13, 5852–5860. [Google Scholar] [CrossRef]
- Scheiger, J.M.; Direksilp, C.; Falkenstein, P.; Welle, A.; Koenig, M.; Heissler, S.; Matysik, J.; Levkin, P.A.; Theato, P. Inverse Vulcanization of Styrylethyltrimethoxysilane-Coated Surfaces, Particles, and Crosslinked Materials. Angew. Chem. Int. Ed. 2020, 59, 18639–18645. [Google Scholar] [CrossRef]
- Duarte, M.E.; Huber, B.; Theato, P.; Mutlu, H. The unrevealed potential of elemental sulfur for the synthesis of high sulfur content bio-based aliphatic polyesters. Polym. Chem. 2020, 11, 241–248. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Hoefling, A.; Yee, M.; Nguyen, G.T.H.; Theato, P.; Lee, Y.J.; Song, S.-W. Enabling High-Rate and Safe Lithium Ion-Sulfur Batteries by Effective Combination of Sulfur-Copolymer Cathode and Hard-Carbon Anode. ChemSusChem 2019, 12, 480–486. [Google Scholar] [CrossRef]
- Mutlu, H.; Theato, P.; Ceper Ezgi, B.; Ozmen Mehmet, M.; Li, X.; Yang, J.; Dong, W.; Theato, P.; Yang, J. Sulfur Chemistry in Polymer and Materials Science. Macromol. Rapid Commun. 2019, 40, e1800650. [Google Scholar]
- Jia, J.; Yan, P.; Cai, S.D.; Cui, Y.; Xun, X.; Liu, J.; Wang, H.; Dodd, L.; Hu, X.; Lester, D.; et al. Solvated Inverse vulcanisation by photopolymerisation. Eur. Polym. J. 2024, 207, 112815. [Google Scholar] [CrossRef]
- Hanna, V.; Graysmark, M.; Willcock, H.; Hasell, T. Liquid polybutadiene reinforced inverse vulcanised polymers. J. Mater. Chem. A 2024, 12, 1211–1217. [Google Scholar] [CrossRef]
- Dale, J.J.; Stanley, J.; Dop, R.A.; Chronowska-Bojczuk, G.; Fielding, A.J.; Neill, D.R.; Hasell, T. Exploring Inverse Vulcanisation Mechanisms from the Perspective of Dark Sulfur. Eur. Polym. J. 2023, 195, 112198. [Google Scholar] [CrossRef]
- Dale, J.J.; Hanna, V.; Hasell, T. Manipulating Inverse Vulcanization Comonomers to Generate High-Tensile-Strain Polymers. ACS Appl. Polym. Mater. 2023, 5, 6761–6765. [Google Scholar] [CrossRef]
- Zhang, B.; Petcher, S.; Dop, R.A.; Yan, P.; Zhao, W.; Wang, H.; Dodd, L.J.; McDonald, T.O.; Hasell, T. Inverse vulcanised sulfur polymer nanoparticles prepared by antisolvent precipitation. J. Mater. Chem. A 2022, 10, 13704–13710. [Google Scholar] [CrossRef]
- Jia, J.; Liu, J.; Wang, Z.-Q.; Liu, T.; Yan, P.; Gong, X.-Q.; Zhao, C.; Chen, L.; Miao, C.; Zhao, W.; et al. Photoinduced inverse vulcanization. Nat. Chem. 2022, 14, 1249–1257. [Google Scholar] [CrossRef]
- Hanna, V.; Yan, P.; Petcher, S.; Hasell, T. Incorporation of fillers to modify the mechanical performance of inverse vulcanised polymers. Polym. Chem. 2022, 13, 3930–3937. [Google Scholar] [CrossRef]
- Zhang, B.; Dodd, L.J.; Yan, P.; Hasell, T. Mercury capture with an inverse vulcanized polymer formed from garlic oil, a bioderived comonomer. React. Funct. Polym. 2021, 161, 104865. [Google Scholar] [CrossRef]
- Wijeyatunga, S.K.; Tennyson, A.G.; Smith, R.C. High-Sulfur-Content Materials Derived from Postconsumer Polystyrene Wastes: Thermomechanical Properties, Environmental Impacts, and Microstructural Insights. ACS Sustain. Resour. Manag. 2024, 1, 2173–2183. [Google Scholar] [CrossRef]
- Lopez, C.V.; Smith, R.C. Composites produced from Waste Plastic with Agricultural and Energy Sector By-Products. J. Appl. Polym. Sci. 2023, 141, e54828. [Google Scholar] [CrossRef]
- Lopez, C.V.; Smith, R.C. Chemical Recycling of Poly(ethylene terephthalate) via Sequential Glycolysis, Oleoyl Chloride Esterification and Vulcanization to yield Durable Composites. Mater. Adv. 2023, 4, 2785–2793. [Google Scholar] [CrossRef]
- Wijeyatunga, S.K.; Derr, K.M.; Maladeniya, C.P.; Sauceda-Oloño, P.Y.; Tennyson, A.G.; Smith, R.C. Upcycling waste PMMA to durable composites via a transesterification-inverse vulcanization process. J. Polym. Sci. 2024, 62, 554–563. [Google Scholar] [CrossRef]
- Derr, K.M.; Smith, R.C. Thiocracking of Multi-Materials: High-Strength Composites from Post-Consumer Food Packaging Jars. Sustainability 2024, 16, 7023. [Google Scholar] [CrossRef]
- Derr, K.M.; Smith, R.C. One-Pot Method for Upcycling Polycarbonate Waste to Yield High-Strength, BPA-Free Composites. J. Polym. Sci. 2023, 62, 1115–1122. [Google Scholar] [CrossRef]
- Derr, K.M.; Lopez, C.V.; Maladeniya, C.P.; Tennyson, A.G.; Smith, R.C. Transesterification-vulcanization route to durable composites from post-consumer poly(ethylene terephthalate), terpenoids, and industrial waste sulfur. J. Polym. Sci. 2023, 61, 3075–3086. [Google Scholar] [CrossRef]
- Davis, A.E.; Sayer, K.B.; Jenkins, C.L. A comparison of adhesive polysulfides initiated by garlic essential oil and elemental sulfur to create recyclable adhesives. Polym. Chem. 2022, 13, 4634–4640. [Google Scholar] [CrossRef]
- Herrera, C.; Ysinga, K.J.; Jenkins, C.L. Polysulfides Synthesized from Renewable Garlic Components and Repurposed Sulfur Form Environmentally Friendly Adhesives. ACS Appl. Mater. Interfaces 2019, 11, 35312–35318. [Google Scholar] [CrossRef]
- Lopez, C.V.; Karunarathna, M.S.; Lauer, M.K.; Maladeniya, C.P.; Thiounn, T.; Ackley, E.D.; Smith, R.C. High Strength, Acid-Resistant Composites from Canola, Sunflower, or Linseed Oils: Influence of Triglyceride Unsaturation on Material Properties. J. Poly. Sci. 2020, 58, 2259–2266. [Google Scholar] [CrossRef]
- Tikoalu, A.D.; Lundquist, N.A.; Chalker, J.M. Mercury Sorbents Made By Inverse Vulcanization of Sustainable Triglycerides: The Plant Oil Structure Influences the Rate of Mercury Removal from Water. Adv. Sustain. Syst. 2020, 4, 1900111. [Google Scholar] [CrossRef]
- Lopez, C.V.; Maladeniya, C.P.; Smith, R.C. Lithium-Sulfur Batteries: Advances and Trends. Electrochem. 2020, 1, 226–259. [Google Scholar] [CrossRef]
- Guinati, B.G.S.; Sauceda Oloño, P.Y.; Kapuge Dona, N.L.; Derr, K.M.; Wijeyatunga, S.K.; Tennyson, A.G.; Smith, R.C. Upcycling mixed-material waste with elemental sulfur: Applications to plant oil, unseparated biomass, and raw post-consumer food waste. RSC Sustain. 2024, 2, 1819–1827. [Google Scholar] [CrossRef]
- Lopez, C.V.; Smith, A.D.; Smith, R.C. Evaluation of Animal Fats and Vegetable Oils as Comonomers in Polymer Composite Synthesis: Effects of Plant/Animal Sources and Comonomer Composition on Composite Properties. Macromol. Chem. Phys. 2023, 9, 2300233. [Google Scholar] [CrossRef]
- Lopez, C.V.; Derr, K.M.; Smith, A.D.; Tennyson, A.G.; Smith, R.C. Chemical, Thermal, and Mechanical Properties of Sulfur Polymer Composites Comprising Low-Value Fats and Pozzolan Additives. Chemistry 2023, 5, 2166–2181. [Google Scholar] [CrossRef]
- Lopez, C.V.; Smith, A.D.; Smith, R.C. High strength composites from low-value animal coproducts and industrial waste sulfur. RSC Adv. 2022, 12, 1535–1542. [Google Scholar] [CrossRef] [PubMed]
- Tisdale, K.A.; Wijeyatunga, S.K.; Graham, M.J.; Sauceda-Oloño, P.Y.; Tennyson, A.G.; Smith, A.D.; Smith, R.C. High Strength Composites from Wastewater Sludge, Plant Oils, and Fossil Fuel By-Product Elemental Sulfur. J. Polym. Environ. 2025, 33, 1972–1983. [Google Scholar] [CrossRef]
- Tisdale, K.A.; Maladeniya, C.P.; Lopez, C.V.; Tennyson, A.G.; Smith, R.C. Sustainable Composites from Waste Sulfur, Terpenoids, and Pozzolan Cements. J. Compos. Sci. 2023, 7, 35. [Google Scholar] [CrossRef]
- Lauer, M.K.; Tennyson, A.G.; Smith, R.C. Thermomorphological and mechanical properties of vulcanized octenyl succinate/terpenoid-derivatized corn starch composites. Mater. Adv. 2022, 3, 4186–4193. [Google Scholar] [CrossRef]
- Maladeniya, C.P.; Smith, R.C. Influence of Component Ratio on Thermal and Mechanical Properties of Terpenoid-Sulfur Composites. J. Compos. Sci. 2021, 5, 257. [Google Scholar] [CrossRef]
- Maladeniya, C.P.; Karunarathna, M.S.; Lauer, M.K.; Lopez, C.V.; Thiounn, T.; Smith, R.C. A Role for Terpenoid Cyclization in the Atom Economical Polymerization of Terpenoids with Sulfur to Yield Durable Composites. Mater. Adv. 2020, 1, 1665–1674. [Google Scholar] [CrossRef]
- Lauer, M.K.; Tennyson, A.G.; Smith, R.C. Green Synthesis of Thermoplastic Composites from a Terpenoid-Cellulose Ester. ACS Appl. Polym. Mater. 2020, 2, 3761–3765. [Google Scholar] [CrossRef]
- Lauer, M.K.; Estrada-Mendoza, T.A.; McMillen, C.D.; Chumanov, G.; Tennyson, A.G.; Smith, R.C. Durable Cellulose–Sulfur Composites Derived from Agricultural and Petrochemical Waste. Adv. Sust. Syst. 2019, 3, 1900062. [Google Scholar] [CrossRef]
- Lauer, M.K.; Karunarathna, M.S.; Tennyson, A.G.; Smith, R.C. Robust, remeltable and remarkably simple to prepare biomass-sulfur composites. Mater. Adv. 2020, 1, 2271–2278. [Google Scholar] [CrossRef]
- Lauer, M.K.; Sanders, Z.E.; Smith, A.D.; Smith, R.C. Morphological and mechanical characterization of high-strength sulfur composites prepared with variably-sized lignocellulose particles. Mater. Adv. 2021, 2, 7413–7422. [Google Scholar] [CrossRef]
- Lauer, M.K.; Tennyson, A.G.; Smith, R.C. Inverse vulcanization of octenyl succinate-modified corn starch as a route to biopolymer-sulfur composites. Mater. Adv. 2021, 2, 2391–2397. [Google Scholar] [CrossRef]
- Karunarathna, M.S.; Lauer, M.K.; Smith, R.C. Facile route to an organosulfur composite from biomass-derived guaiacol and waste sulfur. J. Mater. Chem. A 2020, 8, 20318–20322. [Google Scholar] [CrossRef]
- Karunarathna, M.S.; Lauer, M.K.; Tennyson, A.G.; Smith, R.C. Copolymerization of an aryl halide and elemental sulfur as a route to high sulfur content materials. Polym. Chem. 2020, 11, 1621–1628. [Google Scholar] [CrossRef]
- Karunarathna, M.S.; Lauer, M.K.; Thiounn, T.; Smith, R.C.; Tennyson, A.G. Valorization of waste to yield recyclable composites of elemental sulfur and lignin. J. Mater. Chem. A 2019, 7, 15683–15690. [Google Scholar] [CrossRef]
- Karunarathna, M.S.; Maladeniya, C.P.; Lauer, M.K.; Tennyson, A.G.; Smith, R.C. Durable Composites by Vulcanization of Oleyl-Esterified Lignin. RSC Adv. 2023, 13, 3234–3240. [Google Scholar] [CrossRef]
- Karunarathna, M.S.; Smith, R.C. Valorization of Lignin as a Sustainable Component of Structural Materials and Composites: Advances from 2011 to 2019. Sustainability 2020, 12, 734. [Google Scholar] [CrossRef]
- Karunarathna, M.S.; Tennyson, A.G.; Smith, R.C. Facile new approach to high sulfur-content materials and preparation of sulfur-lignin copolymers. J. Mater. Chem. A 2020, 8, 548–553. [Google Scholar] [CrossRef]
- Sahadevan, S.A.; Xiao, X.; Ma, Y.; Forsberg, K.; Olsson, R.T.; Gardner, J.M. Sulfur-oleylamine copolymer synthesized via inverse vulcanization for the selective recovery of copper from lithium-ion battery E-waste. Mater. Chem. Front. 2023, 7, 1374–1384. [Google Scholar] [CrossRef]
- Jeong, J.; Kyu, T. Development of Multifunctional Cathode Binder via Inverse Vulcanization for Lithium-Sulfur Battery with Enhanced Capacity Retention. J. Phys. Chem. C 2023, 127, 11836–11844. [Google Scholar] [CrossRef]
- Zhao, F.; Li, Y.; Feng, W. Recent Advances in Applying Vulcanization/Inverse Vulcanization Methods to Achieve High-Performance Sulfur-Containing Polymer Cathode Materials for Li-S Batteries. Small Methods 2018, 2, 1–34. [Google Scholar] [CrossRef]
- Zhang, Y.; Griebel, J.J.; Dirlam, P.T.; Nguyen, N.A.; Glass, R.S.; MacKay, M.E.; Char, K.; Pyun, J. Inverse vulcanization of elemental sulfur and styrene for polymeric cathodes in Li-S batteries. J. Polym. Sci. Part. A Polym. Chem. 2017, 55, 107–116. [Google Scholar] [CrossRef]
- Arslan, M.; Kiskan, B.; Cengiz, E.C.; Demir-Cakan, R.; Yagci, Y. Inverse vulcanization of bismaleimide and divinylbenzene by elemental sulfur for lithium sulfur batteries. Eur. Polym. J. 2016, 80, 70–77. [Google Scholar] [CrossRef]
- Dirlam, P.T.; Simmonds, A.G.; Kleine, T.S.; Nguyen, N.A.; Anderson, L.E.; Klever, A.O.; Florian, A.; Costanzo, P.J.; Theato, P.; Mackay, M.E.; et al. Inverse vulcanization of elemental sulfur with 1,4-diphenylbutadiyne for cathode materials in Li-S batteries. RSC Adv. 2015, 5, 24718–24722. [Google Scholar] [CrossRef]
- Simmonds, A.G.; Griebel, J.J.; Park, J.; Kim, K.R.; Chung, W.J.; Oleshko, V.P.; Kim, J.; Kim, E.T.; Glass, R.S.; Soles, C.L.; et al. Inverse Vulcanization of Elemental Sulfur to Prepare Polymeric Electrode Materials for Li-S Batteries. ACS Macro Lett. 2014, 3, 229–232. [Google Scholar] [CrossRef]
- Sayer, K.B.; Miller, V.L.; Merrill, Z.; Davis, A.E.; Jenkins, C.L. Allyl sulfides in garlic oil initiate the formation of renewable adhesives. Polym. Chem. 2023, 14, 3091–3098. [Google Scholar] [CrossRef]
- Lundquist, N.A.; Chalker, J.M. Confining a spent lead sorbent in a polymer made by inverse vulcanization prevents leaching. Sustain. Mater. Technol. 2020, 26, e00222. [Google Scholar] [CrossRef]
- Lundquist, N.A.; Worthington, M.J.H.; Adamson, N.; Gibson, C.T.; Johnston, M.R.; Ellis, A.V.; Chalker, J.M. Polysulfides made from re-purposed waste are sustainable materials for removing iron from water. RSC Adv. 2018, 8, 1232–1236. [Google Scholar] [CrossRef]
- Esdaile, L.J.; Chalker, J.M. The Mercury Problem in Artisanal and Small-Scale Gold Mining. Chem. Eur. J. 2018, 24, 6905–6916. [Google Scholar] [CrossRef] [PubMed]
- Crockett, M.P.; Evans, A.M.; Worthington, M.J.H.; Albuquerque, I.S.; Slattery, A.D.; Gibson, C.T.; Campbell, J.A.; Lewis, D.A.; Bernardes, G.J.L.; Chalker, J.M. Sulfur-Limonene Polysulfide: A Material Synthesized Entirely from Industrial By-Products and Its Use in Removing Toxic Metals from Water and Soil. Angew. Chem. Int. Ed. 2016, 55, 1714–1718. [Google Scholar] [CrossRef] [PubMed]
- Petcher, S.; Parker, D.J.; Hasell, T. Macroporous sulfur polymers from a sodium chloride porogen-a low cost, versatile remediation material. Environ. Sci. Water Res. Technol. 2019, 5, 2142–2149. [Google Scholar] [CrossRef]
- Parker, D.J.; Jones, H.A.; Petcher, S.; Cervini, L.; Griffin, J.M.; Akhtar, R.; Hasell, T. Low cost and renewable sulfur-polymers by inverse vulcanization, and their potential for mercury capture. J. Mater. Chem. A 2017, 5, 11682–11692. [Google Scholar] [CrossRef]
- Hasell, T.; Parker, D.J.; Jones, H.A.; McAllister, T.; Howdle, S.M. Porous inverse vulcanized polymers for mercury capture. Chem. Commun. 2016, 52, 5383–5386. [Google Scholar] [CrossRef] [PubMed]
- Miao, C.; Xun, X.; Dodd, L.J.; Niu, S.; Wang, H.; Yan, P.; Wang, X.-C.; Li, J.; Wu, X.; Hasell, T.; et al. Inverse Vulcanization with SiO2-Embedded Elemental Sulfur for Superhydrophobic, Anticorrosion, and Antibacterial Coatings. ACS Appl. Polym. Mater. 2022, 4, 4901–4911. [Google Scholar] [CrossRef]
- Smith, J.A.; Mulhall, R.; Goodman, S.; Fleming, G.; Allison, H.; Raval, R.; Hasell, T. Investigating the Antibacterial Properties of Inverse Vulcanized Sulfur Polymers. ACS Omega 2020, 5, 5229–5234. [Google Scholar] [CrossRef]
- Dop, R.A.; Neill, D.R.; Hasell, T. Antibacterial Activity of Inverse Vulcanized Polymers. Biomacromolecules 2021, 22, 5223–5233. [Google Scholar] [CrossRef]
- Dop, R.A.; Neill, D.R.; Hasell, T. Sulfur-Polymer Nanoparticles: Preparation and Antibacterial Activity. ACS Appl. Mater. Interfaces 2023, 15, 20822–20832. [Google Scholar] [CrossRef]
- Wadi, V.S.; Jena, K.K.; Halique, K.; Alhassan, S.M. Linear Sulfur–Nylon Composites: Structure, Morphology, and Antibacterial Activity. ACS Appl. Polym. Mater. 2020, 2, 198–208. [Google Scholar] [CrossRef]
- Farioli, A.S.; Martinez, M.V.; Barbero, C.A.; Acevedo, D.F.; Yslas, E.I. Antimicrobial Activity of Gentamicin-Loaded Biocomposites Synthesized through Inverse Vulcanization from Soybean and Sunflower Oils. Sustain. Chem. 2024, 5, 229–243. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, R.; Mao, Y.; Zhong, L.; Lin, P.; Deng, Q.; Zheng, B.; Shen, H.; Feng, Z.; Zhang, H. Development of a toughened and antibacterial Poly(lactide acid) (PLA) with preserved strength by elemental sulfur-based bio-renewable dynamically crosslinked elastomers. Chem. Eng. J. 2023, 467, 143419. [Google Scholar] [CrossRef]
- Shen, H.; Qiao, H.; Zhang, H. Sulfur-urushiol copolymer: A material synthesized through inverse vulcanization from renewable resources and its latent application as self-repairable and antimicrobial adhesive. Chem. Eng. J. 2022, 450, 137905. [Google Scholar] [CrossRef]
- Shen, H.; Chen, X.; Zheng, B.; Zhang, H. Sorbic Acid–Tung Oil–Sulfur Terpolymer Prepared via Inverse Vulcanization and Its Application as Antibacterial and Toughness Modifier for Polylactide. ACS Appl. Polym. Mater. 2024, 6, 14351–14364. [Google Scholar] [CrossRef]
- Sauceda-Oloño, P.Y.; Lopez, C.V.; Patel, B.K.; Smith, A.D.; Smith, R.C. Influence of Thermal and Chemical Stresses on Thermal Properties, Crystal Morphology, and Mechanical Strength Development of a Sulfur Polymer Composite. Macromol 2024, 4, 240–252. [Google Scholar] [CrossRef]
- Sauceda-Oloño, P.Y.; Guinati, B.G.S.; Smith, A.D.; Smith, R.C. Influence of Additives on Flame-Retardant, Thermal, and Mechanical Properties of a Sulfur–Triglyceride Polymer Composite. J. Compos. Sci. 2024, 8, 304. [Google Scholar] [CrossRef]
- Sauceda-Olono, P.Y.; Borbon-Almada, A.C.; Gaxiola, M.; Smith, A.D.; Tennyson, A.G.; Smith, R.C. Thermal and Mechanical Properties of Recyclable Composites Prepared from Bio-Olefins and Industrial Waste. J. Compos. Sci. 2023, 7, 248. [Google Scholar] [CrossRef]
- van Hoogstraten, S.W.G.; Fechter, J.; Bargon, R.; van Agtmaal, J.L.; Peeters, L.C.W.; Geurts, J.; Arts, J.J.C. The Antibacterial Properties of a Silver Multilayer Coating for the Prevention of Bacterial Biofilm Formation on Orthopedic Implants-An In Vitro Study. Coatings 2024, 14, 216. [Google Scholar] [CrossRef]
- Cui, J.; Yeasmin, R.; Shao, Y.; Zhang, H.; Zhang, H.; Zhu, J. Fabrication of Ag+, Cu2+, and Zn2+ Ternary Ion-Exchanged Zeolite as an Antimicrobial Agent in Powder Coating. Ind. Eng. Chem. Res. 2020, 59, 751–762. [Google Scholar] [CrossRef]
- Tabriz, K.R.; Katbab, A.A. Preparation of modified-TiO2/PLA nanocomposite films: Micromorphology, photo-degradability and antibacterial studies. AIP Conf. Proc. 2017, 1914, 070009/1. [Google Scholar] [CrossRef]
- Tinteri, C.; Potenza, M.; Rizzetto, R. Antimicrobial efficacy and longevity of silver+zeolite incorporating preinsulated ducts installed in real healthcare settings. J. Prev. Med. Hyg. 2012, 53, 177–180. [Google Scholar]
- Wynne, K.J.; Zolotarskaya, O.; Jarrell, R.; Wang, C.; Amin, Y.; Brunson, K. Facile Modification of Medical-Grade Silicone for Antimicrobial Effectiveness and Biocompatibility: A Potential Therapeutic Strategy against Bacterial Biofilms. ACS Appl. Mater. Interfaces 2023, 15, 46626–46638. [Google Scholar] [CrossRef] [PubMed]
- Roman, L.E.; Villalva, C.; Uribe, C.; Paraguay-Delgado, F.; Sousa, J.; Vigo, J.; Vera, C.M.; Gomez, M.M.; Solis, J.L. Textiles Functionalized with Copper Oxides: A Sustainable Option for Prevention of COVID-19. Polymers 2022, 14, 3066. [Google Scholar] [CrossRef] [PubMed]
- Lopez-R, M.; Barrios, Y.; Perez, L.D.; Soto, C.Y.; Sierra, C. Metal-Organic Framework (MOFs) tethered to cotton fibers display antimicrobial activity against relevant nosocomial bacteria. Inorg. Chim. Acta 2022, 537, 120955. [Google Scholar] [CrossRef]
- Huang, C.; Hu, C.; Sun, G.; Ji, B.; Yan, K. Antimicrobial finish of cotton fabrics treated by sophorolipids combined with 1,2,3,4-butanetetracarboxyic acid. Cellulose 2020, 27, 2859–2872. [Google Scholar] [CrossRef]
- Pulingam, T.; Parumasivam, T.; Gazzali, A.M.; Sulaiman, A.M.; Chee, J.Y.; Lakshmanan, M.; Chin, C.F.; Sudesh, K. Antimicrobial resistance: Prevalence, economic burden, mechanisms of resistance and strategies to overcome. Eur. J. Pharm. Sci. 2022, 170, 106103. [Google Scholar] [CrossRef]
- Chang, R.Y.K.; Nang, S.C.; Chan, H.-K.; Li, J. Novel antimicrobial agents for combating antibiotic-resistant bacteria. Adv. Drug Deliv. Rev. 2022, 187, 114378. [Google Scholar] [CrossRef]
- Bai, A.D.; Lo, C.K.L.; Komorowski, A.S.; Suresh, M.; Guo, K.; Garg, A.; Tandon, P.; Senecal, J.; Del Corpo, O.; Stefanova, I.; et al. Staphylococcus aureus bacteremia mortality: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2022, 28, 1076–1084. [Google Scholar] [CrossRef]
- Friedman, M. Chemistry, Antimicrobial Mechanisms, and Antibiotic Activities of Cinnamaldehyde against Pathogenic Bacteria in Animal Feeds and Human Foods. J. Agric. Food Chem. 2017, 65, 10406–10423. [Google Scholar] [CrossRef]
- Allen, K.J.; Walecka-Zacharska, E.; Chen, J.C.; Katarzyna, K.-P.; Devlieghere, F.; Van Meervenne, E.; Osek, J.; Wieczorek, K.; Bania, J. Listeria monocytogenes—An examination of food chain factors potentially contributing to antimicrobial resistance. Food Microbiol. 2016, 54, 178–189. [Google Scholar] [CrossRef]
- Beceiro, A.; Tomas, M.; Bou, G. Antimicrobial resistance and virulence: A successful or deleterious association in the bacterial world? Clin. Microbiol. Rev. 2013, 26, 185–230. [Google Scholar] [CrossRef] [PubMed]
- Temple, M.E.; Nahata, M.C. Treatment of listeriosis. Ann. Pharmacother. 2000, 34, 656–661. [Google Scholar] [CrossRef]
- Venhorst, J.; van der Vossen, J.M.B.M.; Agamennone, V. Battling Enteropathogenic Clostridia: Phage Therapy for Clostridioides difficile and Clostridium perfringens. Front. Microbiol. 2022, 13, 891790. [Google Scholar] [CrossRef] [PubMed]
- Sharan, M.; Vijay, D.; Dhaka, P.; Bedi, J.S.; Gill, J.P.S. Biofilms as a microbial hazard in the food industry: A scoping review. J. Appl. Microbiol. 2022, 133, 2210–2234. [Google Scholar] [CrossRef]
- Mak, P.H.W.; Rehman, M.A.; Kiarie, E.G.; Topp, E.; Diarra, M.S. Production systems and important antimicrobial resistant-pathogenic bacteria in poultry: A review. J. Anim. Sci. Biotechnol. 2022, 13, 148. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Elbestawy, A.R.; El-Shall, N.A.; Saad, A.M.; Salem, H.M.; El-Tahan, A.M.; Khafaga, A.F.; Taha, A.E.; AbuQamar, S.F.; et al. Necrotic enteritis in broiler chickens: Disease characteristics and prevention using organic antibiotic alternatives—A comprehensive review. Poult. Sci. 2022, 101, 101590. [Google Scholar] [CrossRef] [PubMed]
- Castro-Vargas, R.E.; Herrera-Sanchez, M.P.; Rodriguez-Hernandez, R.; Rondon-Barragan, I.S. Antibiotic resistance in Salmonella spp. isolated from poultry: A global overview. Vet. World 2020, 13, 2070–2084. [Google Scholar] [CrossRef]
- Lamas, A.; Miranda, J.M.; Regal, P.; Vazquez, B.; Franco, C.M.; Cepeda, A. A comprehensive review of non-enterica subspecies of Salmonella enterica. Microbiol. Res. 2018, 206, 60–73. [Google Scholar] [CrossRef]
- Shah, D.H.; Paul, N.C.; Sischo, W.C.; Crespo, R.; Guard, J. Population dynamics and antimicrobial resistance of the most prevalent poultry-associated Salmonella serotypes. Poult. Sci. 2017, 96, 687–702. [Google Scholar] [CrossRef]
- Gantois, I.; Ducatelle, R.; Pasmans, F.; Haesebrouck, F.; Gast, R.; Humphrey, T.J.; Van Immerseel, F. Mechanisms of egg contamination by Salmonella enteritidis. FEMS Microbiol. Rev. 2009, 33, 718–738. [Google Scholar] [CrossRef]
- Klancnik, A.; Simunovic, K.; Sternisa, M.; Ramic, D.; Smole Mozina, S.; Bucar, F. Anti-adhesion activity of phytochemicals to prevent Campylobacter jejuni biofilm formation on abiotic surfaces. Phytochem. Rev. 2021, 20, 55–84. [Google Scholar] [CrossRef]
- Elgamoudi, B.A.; Korolik, V. Campylobacter Biofilms: Potential of Natural Compounds to Disrupt Campylobacter jejuni Transmission. Int. J. Mol. Sci. 2021, 22, 12159. [Google Scholar] [CrossRef] [PubMed]
- Bastidas-Caldes, C.; de Waard, J.H.; Salgado, M.S.; Villacís, M.J.; Coral-Almeida, M.; Yamamoto, Y.; Calvopiña, M. Worldwide Prevalence of mcr-mediated Colistin-Resistance Escherichia coli in Isolates of Clinical Samples, Healthy Humans, and Livestock—A Systematic Review and Meta-Analysis. Pathogens 2022, 11, 659. [Google Scholar] [CrossRef] [PubMed]
- Laird, T.J.; Abraham, S.; Jordan, D.; Pluske, J.R.; Hampson, D.J.; Trott, D.J.; O’Dea, M. Porcine enterotoxigenic Escherichia coli: Antimicrobial resistance and development of microbial-based alternative control strategies. Vet. Microbiol. 2021, 258, 109117. [Google Scholar] [CrossRef]
- Loayza, F.; Graham, J.P.; Trueba, G. Factors obscuring the role of E. coli from domestic animals in the global antimicrobial resistance crisis: An evidence-based review. Int. J. Environ. Res. Public. Health 2020, 17, 3061. [Google Scholar] [CrossRef]
- Cho, S.; Jackson, C.R.; Frye, J.G. The prevalence and antimicrobial resistance phenotypes of Salmonella, Escherichia coli and Enterococcus sp. in surface water. Lett. Appl. Microbiol. 2020, 71, 3–25. [Google Scholar] [CrossRef]
- Shahid, S.; Mahesar, M.; Ghouri, N.; Noreen, S. A review of clinical profile, complications and antibiotic susceptibility pattern of extensively drug-resistant (XDR) Salmonella Typhi isolates in children in Karachi. BMC Infect. Dis. 2021, 21, 900. [Google Scholar] [CrossRef]
- Marchello, C.S.; Carr, S.D.; Crump, J.A. A systematic review on antimicrobial resistance among Salmonella Typhi worldwide. Am. J. Trop. Med. Hyg. 2020, 103, 2518–2527. [Google Scholar] [CrossRef]
- Karkey, A.; Thwaites, G.E.; Baker, S. The evolution of antimicrobial resistance in Salmonella Typhi. Curr. Opin. Gastroenterol. 2018, 34, 25–30. [Google Scholar] [CrossRef]
- Dougan, G.; Baker, S. Salmonella enterica serovar Typhi and the pathogenesis of typhoid fever. Annu. Rev. Microbiol. 2014, 68, 317–336. [Google Scholar] [CrossRef]
- Du, H.; Bing, J.; Hu, T.; Ennis, C.L.; Nobile, C.J.; Huang, G. Candida auris: Epidemiology, biology, antifungal resistance, and virulence. PLoS Pathog. 2020, 16, e1008921. [Google Scholar] [CrossRef] [PubMed]
- Forsberg, K.; Woodworth, K.; Walters, M.; Berkow, E.L.; Jackson, B.; Chiller, T.; Vallabhaneni, S. Candida auris: The recent emergence of a multidrug-resistant fungal pathogen. Med. Mycol. 2019, 57, 1–12. [Google Scholar] [CrossRef]
- Perlin, D.S.; Rautemaa-Richardson, R.; Alastruey-Izquierdo, A. The global problem of antifungal resistance: Prevalence, mechanisms, and management. Lancet Infect. Dis. 2017, 17, e383–e392. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Patterson, T.F. Multidrug-resistant Candida: Epidemiology, molecular mechanisms, and treatment. J. Infect. Dis. 2017, 216, S445. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.; Valerio, M.; Alvarez-Uria, A.; Olmedo, M.; Veintimilla, C.; Padilla, B.; De la Villa, S.; Guinea, J.; Escribano, P.; Ruiz-Serrano, M.J.; et al. Invasive pulmonary aspergillosis in the COVID-19 era: An expected new entity. Mycoses 2021, 64, 132–143. [Google Scholar] [CrossRef]
- Lai, C.-C.; Yu, W.-L. COVID-19 associated with pulmonary aspergillosis: A literature review. J. Microbiol. Immunol. Infect. 2021, 54, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Arastehfar, A.; Carvalho, A.; Houbraken, J.; Lombardi, L.; Garcia-Rubio, R.; Jenks, J.D.; Rivero-Menendez, O.; Aljohani, R.; Jacobsen, I.D.; Berman, J.; et al. Aspergillus fumigatus and aspergillosis: From basics to clinics. Stud. Mycol. 2021, 100, 100115. [Google Scholar] [CrossRef]
- Latge, J.-P.; Chamilos, G. Aspergillus fumigatus and aspergillosis in 2019. Clin. Microbiol. Rev. 2020, 33, e00140. [Google Scholar] [CrossRef]


| Organism | ATCC # or CDC# | Classification | Time (h) | Reduction (%) |
|---|---|---|---|---|
| L. monocytogenes | 19115 | Gram-positive Bacteria | 24 h | 91.52 |
| S. aureus | 6538 | Gram-positive Bacteria | 24 h | 96.84 |
| C. perfringens | 13124 | Gram-positive Bacteria | 24 h | 0 |
| S. enteritidis | 13076 | Gram-negative Bacteria | 24 h | 62.15 |
| E. coli | 8739 | Gram-negative Bacteria | 24 h | 61.10 |
| C. jejuni | 29428 | Gram-negative Bacteria | 24 h | 37.70 |
| C. jejuni | 33291 | Gram-negative Bacteria | 24 h | 37.53 |
| S. typhi | 14028 | Gram-negative Bacteria | 24 h | 32.87 |
| C. auris | CDC B11903 | Yeast | 24 h | 96.20 |
| A. fumigatus | 204305 | Mold | 24 h | 83.77 |
| Organism | ATCC # or CDC # | Classification | Time (h) | Reduction (%) |
|---|---|---|---|---|
| L. monocytogenes | 19115 | Gram-positive Bacteria | 1 h | 13.35 |
| L. monocytogenes | 19115 | Gram-positive Bacteria | 8 h | 99.91 |
| S. aureus | 6538 | Gram-positive Bacteria | 8 h | 0 |
| E. coli | 8739 | Gram-negative Bacteria | 8 h | 98.49 |
| S. enteritidis | 13076 | Gram-negative Bacteria | 8 h | 0 |
| C. auris | CDC B11903 | Yeast | 8 h | 96.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wijeyatunga, S.K.; Sauceda-Oloño, P.Y.; Kapuge Dona, N.L.; Guinati, B.G.S.; Derr, K.M.; Tisdale, K.A.; Smith, A.D.; Tennyson, A.G.; Smith, R.C. Static and Dynamic Assessments of a Sulfur-Triglyceride Composite for Antimicrobial Surface Applications. Molecules 2025, 30, 1614. https://doi.org/10.3390/molecules30071614
Wijeyatunga SK, Sauceda-Oloño PY, Kapuge Dona NL, Guinati BGS, Derr KM, Tisdale KA, Smith AD, Tennyson AG, Smith RC. Static and Dynamic Assessments of a Sulfur-Triglyceride Composite for Antimicrobial Surface Applications. Molecules. 2025; 30(7):1614. https://doi.org/10.3390/molecules30071614
Chicago/Turabian StyleWijeyatunga, Shalini K., Perla Y. Sauceda-Oloño, Nawoda L. Kapuge Dona, Bárbara G. S. Guinati, Katelyn M. Derr, Katelyn A. Tisdale, Ashlyn D. Smith, Andrew G. Tennyson, and Rhett C. Smith. 2025. "Static and Dynamic Assessments of a Sulfur-Triglyceride Composite for Antimicrobial Surface Applications" Molecules 30, no. 7: 1614. https://doi.org/10.3390/molecules30071614
APA StyleWijeyatunga, S. K., Sauceda-Oloño, P. Y., Kapuge Dona, N. L., Guinati, B. G. S., Derr, K. M., Tisdale, K. A., Smith, A. D., Tennyson, A. G., & Smith, R. C. (2025). Static and Dynamic Assessments of a Sulfur-Triglyceride Composite for Antimicrobial Surface Applications. Molecules, 30(7), 1614. https://doi.org/10.3390/molecules30071614









