Impact of Winemaking Techniques on the Phenolic Composition and Antioxidant Properties of Touriga Nacional Wines
Abstract
:1. Introduction
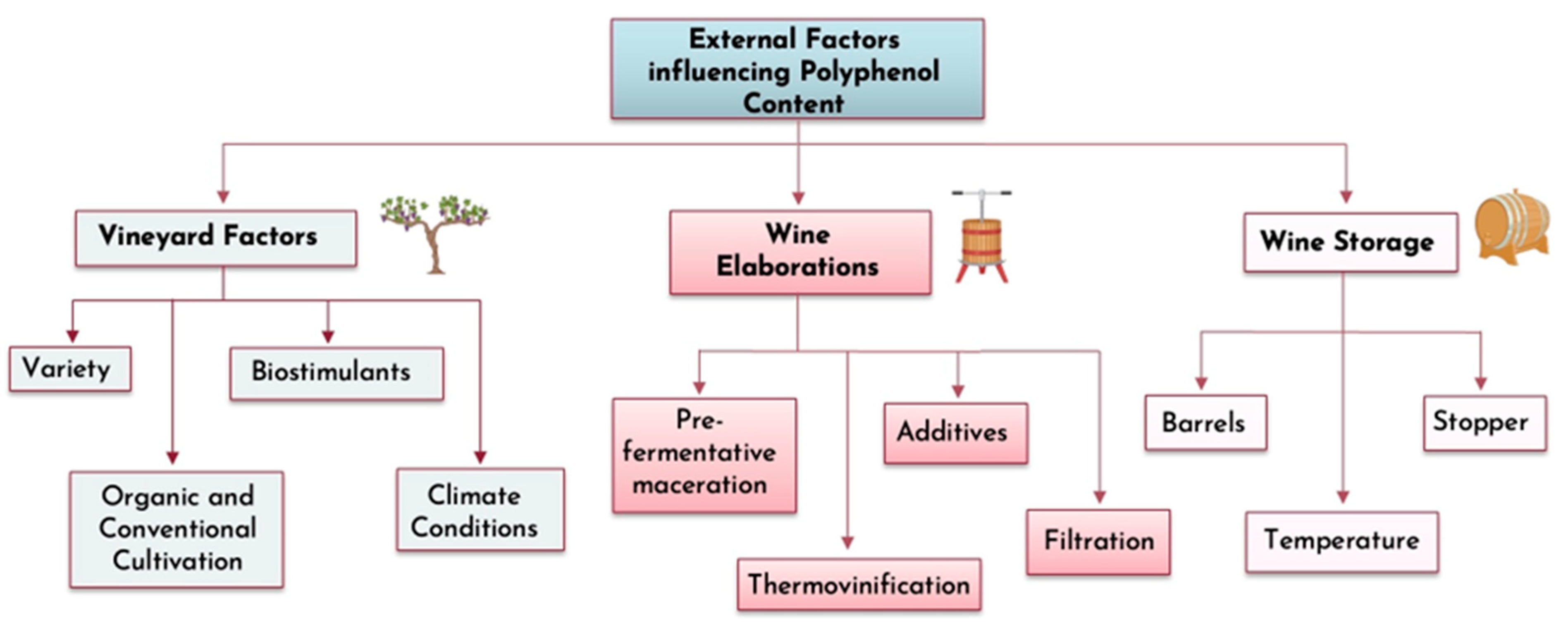
The Role of Polyphenols in Wine: Extraction and the Impact of Winemaking Techniques
2. Results and Discussion
2.1. Phenolic Content
2.2. Anthocyanins and Tannins Content
2.3. Impact of Maceration Temperature on Phenolic Compound Extraction and Wine Quality
2.4. Chromatographic Analysis of Phenolic Compounds
2.4.1. Stilbenes
2.4.2. Hydroxycinnamic Acids
2.4.3. Flavan-3-Ols
2.4.4. Flavonols
2.5. Antioxidant Capacity
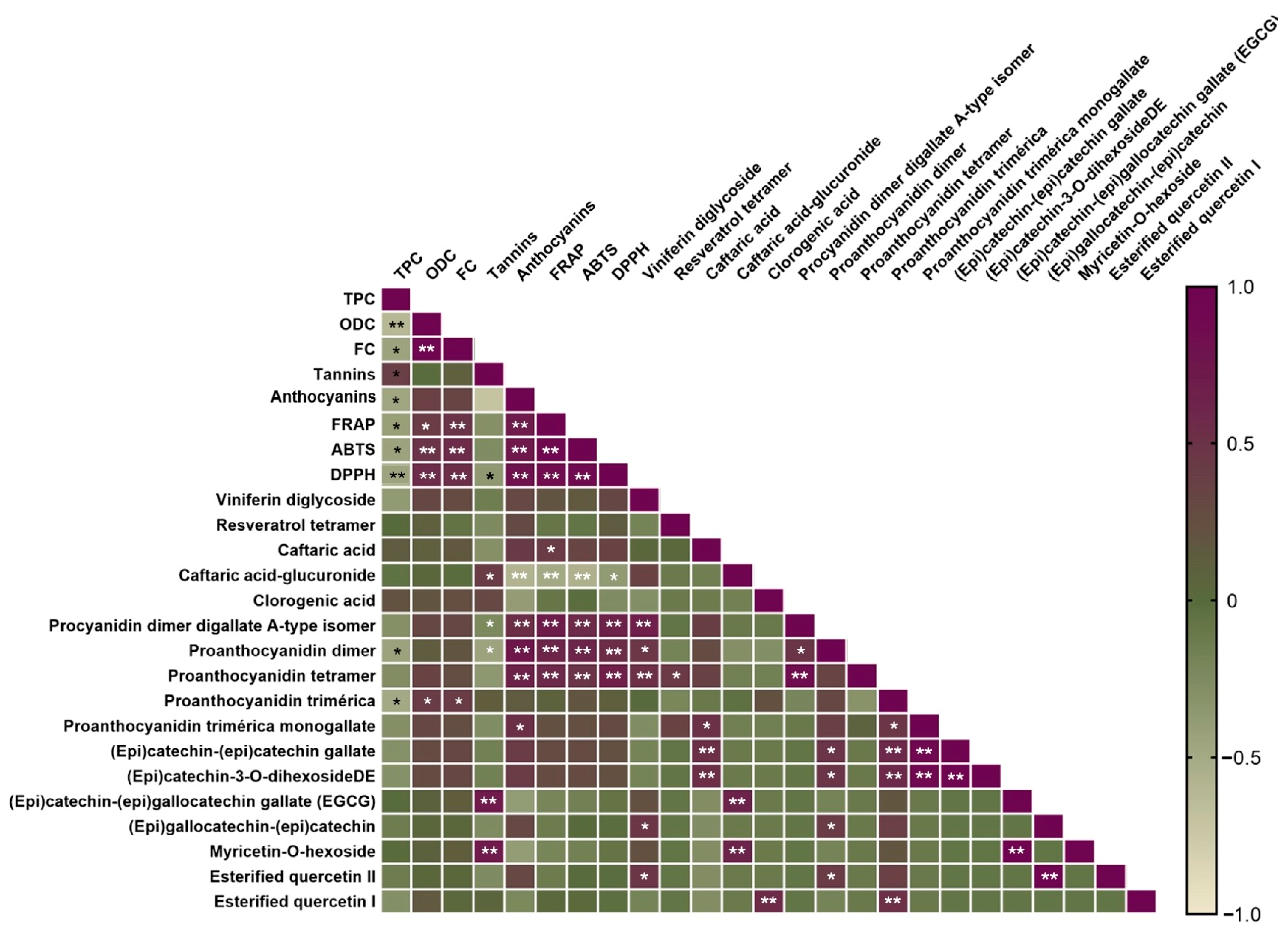
2.6. Pearson Correlation
3. Materials and Methods
3.1. Chemicals
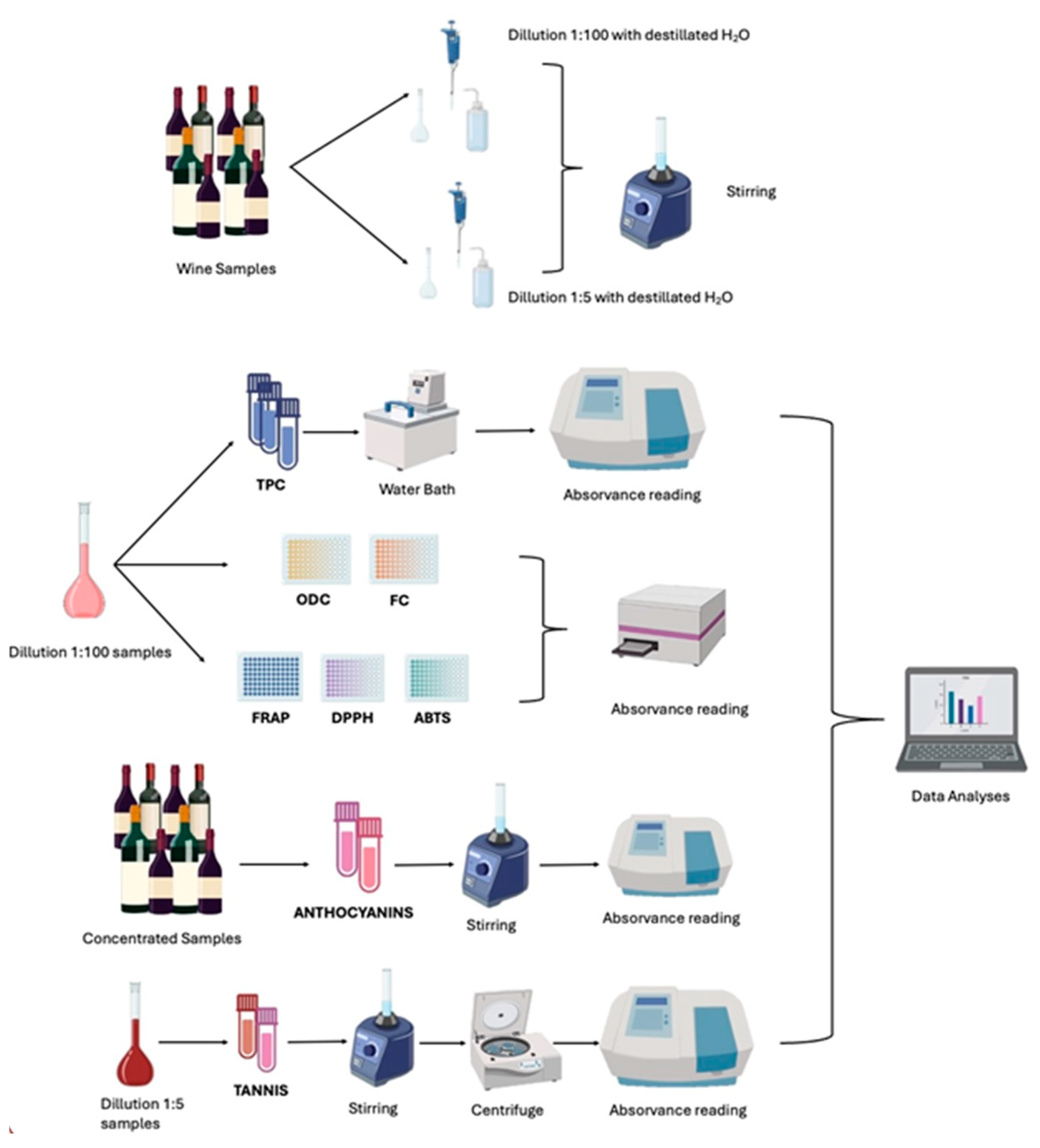
3.2. Sampling
Sampling Preparation
3.3. Determination of Phenolic Content
3.3.1. Total Phenolic Content
3.3.2. Ortho-Diphenols Content
3.3.3. Flavonoid Content
3.4. Determination of Total Anthocyanins
3.5. Determination of Total Tannins
3.6. (Poly) Phenolic Profile
3.7. Determination of Antioxidant Capacity
3.7.1. DPPH Radical Scavenging Assay
3.7.2. ABTS Radical Scavenging Assay
3.7.3. FRAP Assay
3.8. Statictical Assay
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- CE n.º 1493/1999 do Conselho de 22 de outubro.
- Bisson, L.; Waterhouse, A.; Ebeler, S.; Walker, A.; Lapsley, J. The present and future of the international wine industry. Nature 2002, 418, 696–699. [Google Scholar] [CrossRef]
- Sánchez-García, E.; Martínez-Falcó, J.; Alcon-Vila, A.; Marco-Lajara, B. Developing Green Innovations in the Wine Industry: An Applied Analysis. Foods 2023, 12, 1157. [Google Scholar] [CrossRef] [PubMed]
- Peralta, H.C. Conheça Quais os 12 Maiores Países Produtores de Vinho do Mundo. Forbes Portugal, 24 September 2023. Available online: https://www.forbespt.com/conheca-os-12-maiores-produtores-de-vinho-do-mundo (accessed on 3 February 2025).
- Cabo, P.; Verdial, J.; Ribeiro, A. Beirin: Proposta de Marca Internacional Para os Vinhos da Beira Interior. 2019. Available online: https://ubibliorum.ubi.pt/bitstream/10400.6/12107/1/8693_18750.pdf (accessed on 3 February 2025).
- Fortunas, S.; Diniz, F.; Katsioloudes, M. The competitiveness of the Portuguese wine sector. Acta Agric. Scand. Sect. C Food Econ. 2007, 4, 120–138. [Google Scholar] [CrossRef]
- Oliveira, A.; Correia, M. Influence of elevation and slope exposure upon productivity and must quality of touriga nacional (sub-region of douro superior). OENO One 2008, 42, 73–78. [Google Scholar] [CrossRef]
- Magalhães, N. Tratado de Viticultura—A Videira, A Vinha e o “Terroir” [Review of Tratado de Viticultura—A Videira, A Vinha e o “Terroir”]; Esfera Poética: Lisbon, Portugal, 2008. [Google Scholar]
- Pinto-Sintra, A. Establishment of embryogenic cultures and plant regeneration in the Portuguese cultivar ‘Touriga Nacional’ of Vitis vinifera L. Plant Cell Tissue Organ Cult. 2007, 88, 253–265. [Google Scholar] [CrossRef]
- Pinho, P.; Falqué, E.; Castro, M.; Silva, H.; Machado, B.; Ferreira, A. Further insights into the floral character of Touriga Nacional wines. J. Food Sci. 2007, 72, 396–401. [Google Scholar]
- Cabral, I.; Teixeira, A.; Ferrier, M.; Lanoue, A.; Valente, J.; Rogerson, F.; Alves, F.; Carvalho, S.; Gerós, H.; Queiroz, J. Canopy management through crop forcing impacts grapevine cv. ‘Touriga Nacional’ performance, ripening and berry metabolomics profile. OENO One 2023, 57, 7122. [Google Scholar] [CrossRef]
- Falqué, E.; Ferreira, A.; Hogg, T.; Guedes-Pinho, P. Determination of aromatic descriptors of Touriga Nacional wines by sensory descriptive analysis. Flavour Fragr. J. 2004, 19, 298–302. [Google Scholar] [CrossRef]
- Ferreira, A.; Falqué, E.; Castro, M.; Silva, H.; Machado, B.; Pinho, P. Identification of key odorants related with high quality touriga nacional wine. Dev. Food Sci. 2006, 43, 217–220. [Google Scholar] [CrossRef]
- Museu do Douro. Região Demarcada do Douro. Available online: https://www.museudodouro.pt/regiao-demarcada-do-douro (accessed on 3 February 2025).
- Lourenço-Gomes, L.; Pinto, L.; Rebelo, J. Wine and cultural heritage. The experience of the Alto Douro Wine Region. Wine Econ. Policy 2015, 4, 78–87. [Google Scholar] [CrossRef]
- Pina, H.; António; Cardoso, B. A Vinha, A Paisagem e o Património. Available online: https://www.researchgate.net/publication/337316226_A_vinha_a_paisagem_e_o_patrimonio (accessed on 3 February 2025).
- Cheynier, V.; Dueñas-Paton, M.; Salas, E.; Maury, C.; Souquet, J.; Sarni-Manchado, P.; Fulcrand, H. Structure and Properties of Wine Pigments and Tannins. Am. J. Enol. Vitic. 2006, 57, 298–305. [Google Scholar] [CrossRef]
- Ferrières, J. The French paradox: Lessons for other countries. Heart 2004, 90, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A. Wine Phenolics. Ann. N.Y. Acad. Sci. 2002, 957, 21–36. [Google Scholar] [CrossRef]
- Aleixandré-Tudo, J.; Buica, A.; Nieuwoudt, H.; Aleixandre, J.; Toit, W. Spectrophotometric Analysis of Phenolic Compounds in Grapes and Wines. J. Agric. Food Chem. 2017, 65, 4009–4026. [Google Scholar] [CrossRef] [PubMed]
- Vejarano, R.; Luján-Corro, M. Red Wine and Health: Approaches to Improve the Phenolic Content During Winemaking. Front. Nutr. 2022, 9, 890066. [Google Scholar] [CrossRef]
- Alara, O.; Abdurahman, N.; Ukaegbu, C. Extraction of phenolic compounds: A review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef]
- Cheynier, V. Phenolic compounds: From plants to foods. Phytochem. Rev. 2012, 11, 153–177. [Google Scholar] [CrossRef]
- Haminiuk, C.; Maciel, G.; Plata-Oviedo, M.; Peralta, R. Phenolic compounds in fruits—An overview. Int. J. Food Sci. Technol. 2012, 47, 2023–2044. [Google Scholar] [CrossRef]
- Cianciosi, D.; Forbes-Hernández, T.Y.; Afrin, S.; Gasparrini, M.; Reboredo-Rodriguez, P.; Manna, P.P.; Zhang, J.; Bravo Lamas, L.; Martínez Flórez, S.; Agudo Toyos, P.; et al. Phenolic Compounds in Honey and Their Associated Health Benefits: A Review. Molecules 2018, 23, 2322. [Google Scholar] [CrossRef]
- Gutiérrez-Escobar, R.; Aliaño-González, M.J.; Cantos-Villar, E. Wine Polyphenol Content and Its Influence on Wine Quality and Properties: A Review. Molecules 2021, 26, 718. [Google Scholar] [CrossRef]
- FOAM—Organic International; Cravero, M.C. Quality and characteristics of organic and biodynamic wines: A review. Food Chem. 2019, 295, 334–340. Available online: https://www.ifoam.bio (accessed on 3 February 2025). [PubMed]
- Reynolds, A.G. Managing Wine Quality: Viticulture and Wine Quality; Elsevier Inc.: Amsterdam, The Netherlands, 2010; pp. 365–444. [Google Scholar]
- Jara-Palacios, M.J.; Gordillo, B.; González-Miret, M.L.; Hernanz, D.; Escudero-Gilete, M.L.; Heredia, F.J. Comparative study of the oenological potential of different winemaking by-products: Implications for antioxidant activity and color expression of red wine anthocyanins in a model solution. J. Agric. Food Chem. 2014, 62, 6975–6983. [Google Scholar]
- Gambuti, A.; Capuano, R.; Lecce, L.; Fragasso, M.G.; Moio, L. Extraction of phenolic compounds from ‘Aglianico’ and ’Uva di Troia’ grape skins and seeds in model solutions: Influence of ethanol and maceration time. Vitis. J. Grapevine Res. 2009, 48, 193–200. [Google Scholar]
- Casassa, L.F.; Harbertson, J.F. Extraction, Evolution, and Sensory Impact of Phenolic Compounds During Red Wine Maceration. Annu. Rev. Food Sci. Technol. 2014, 5, 83–109. [Google Scholar] [CrossRef]
- Waterhouse, A.L.; Laurie, V.F. Oxidation of Wine Phenolics: A Critical Evaluation and Hypotheses. Am. J. Enol. Vitic. 2006, 57, 306–313. [Google Scholar] [CrossRef]
- McRae, J.M.; Dambergs, R.G.; Kassara, S.; Parker, M.; Jeffery, D.W.; Herderich, M.J.; Smith, P.A. Phenolic compositions of 50- and 30-year-old sequences of Australian red wines: The impact of wine age. J. Agric. Food Chem. 2012, 60, 10093–10102. [Google Scholar]
- Jaffré, J.J.; Valentin, D.; Dacremont, C.; Peyron, D. Burgundy red wines: Representing the potential foraging. Food Qual. Prefer. 2009, 20, 505–513. [Google Scholar] [CrossRef]
- Cadahía, E.; de Simón, B.F.; Jalocha, J. Volatile Compounds in Spanish, French, and American Oak Woods after Natural Seasoning and Toasting. J. Agric. Food Chem. 2003, 51, 5923–5932. [Google Scholar] [CrossRef]
- Sánchez-Rangel, J.; Benavides, J.; Heredia, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D. The Folin–Ciocalteu assay revisited: Improvement of its specificity for total phenolic content determination. Anal. Methods 2013, 5, 5990–5999. [Google Scholar] [CrossRef]
- Lee, E.; Nomura, N.; Patil, B.; Yoo, K. Measurement of total phenolic content in wine using an automatic Folin–Ciocalteu assay method. Int. J. Food Sci. Technol. 2014, 49, 2364–2372. [Google Scholar] [CrossRef]
- Perez, M.; Domínguez-López, I.; Lamuela-Raventós, R. The Chemistry Behind the Folin–Ciocalteu Method for the Estimation of (Poly)phenol Content in Food: Total Phenolic Intake in a Mediterranean Dietary Pattern. J. Agric. Food Chem. 2023, 71, 17543–17553. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lan, Y.; Huang, Y.; Zhao, X.; Duan, C. Targeted metabolomics of anthocyanin derivatives during prolonged wine aging: Evolution, color contribution and aging prediction. Food Chem. 2024, 339, 127795. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, A.M.D.; Carvalheira, J.A.N.; Coimbra, M.A.; Rocha, S.M. Tecnologia dos Vinhos Tintos; Direcção Regional de Agricultura da Beira Litoral: Coimbra, Portugal, 2005. [Google Scholar]
- Vilela, A.; Liquito, Â.; Cosme, F. Caraterização Sensorial E Fenólica De Vinhos Tintos Monovarietais Produzidos Com Castas Tintas Cultivadas Na Região Demarcada Do Douro; CQ-Centro de Química: Vila Real, Portugal, 2016. [Google Scholar] [CrossRef]
- Paixão, N.; Perestrelo, R.; Marques, J.C.; Câmara, J.S. Relationship between antioxidant capacity and total phenolic content of red, rosé and white wines. Food Chem. 2007, 105, 204–214. [Google Scholar] [CrossRef]
- Minussi, R.C.; Rossi, M.; Bologna, L.; Cordi, L.; Rotilio, D.; Pastore, G.M.; Durán, N. Phenolic compounds and total antioxidant potential of commercial wines. Food Chem. 2003, 82, 409–416. [Google Scholar] [CrossRef]
- Lisanti, M.; Capuano, R.; Moio, L.; Gambuti, A. Wood powders of different botanical origin as an alternative to barrel aging for red wine. Eur. Food Res. Technol. 2021, 247, 2309–2320. [Google Scholar] [CrossRef]
- De Rosso, M.; Gardiman, M.; Carraro, R.; Panighel, A.; Fagherazzi, F.; Sansone, L.; Roman, T.; Vettori, L.; Flamini, R. Monoglucoside versus Diglucoside Anthocyanin Evolution of Red Wine Produced Using a Fungus-Resistant Grape Cultivar (Downy Mildew and Powdery Mildew) under Oxidative Conditions. J. Agric. Food Chem. 2024, 72, 7383–7396. [Google Scholar] [CrossRef]
- He, F.; Mu, L.; Yan, G.; Liang, N.; Pan, Q.; Wang, J.; Reeves, M.; Duan, C. Biosynthesis of Anthocyanins and Their Regulation in Colored Grapes. Molecules 2010, 15, 9057–9091. [Google Scholar] [CrossRef]
- Mazza, G.; Francis, F.J. Anthocyanins in grapes and grape products. Crit. Rev. Food Sci. Nutr. 1995, 35, 341–371. [Google Scholar] [CrossRef]
- He, F.; Liang, N.; Mu, L.; Pan, Q.; Wang, J.; Reeves, M.; Duan, C. Anthocyanins and Their Variation in Red Wines I. Monomeric Anthocyanins and Their Color Expression. Molecules 2012, 17, 1571–1601. [Google Scholar] [CrossRef]
- Gambuti, A.; Picariello, L.; Rinaldi, A.; Ugliano, M.; Moio, L. Impact of 5-year bottle aging under controlled oxygen exposure on sulfur dioxide and phenolic composition of tannin-rich red wines. OENO One 2020, 54, 623–636. [Google Scholar] [CrossRef]
- Agazzi, F.; Nelson, J.; Tanabe, C.; Doyle, C.; Boulton, R.; Buscema, F. Aging of Malbec wines from Mendoza and California: Evolution of phenolic and elemental composition. Food Chem. 2018, 269, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Aleixandré-Tudo, J.; Du Toit, W. Cold maceration application in red wine production and its effects on phenolic compounds: A review. LWT Food Sci. Technol. 2018, 95, 200–208. [Google Scholar] [CrossRef]
- Harbertson, J.F.; Hodgins, R.E.; Thurston, L.N.; Schaffer, L.J.; Reid, M.S.; Landon, J.L.; Ross, C.F.; Adams, D.O. Variability of Tannin Concentration in Red Wines. Am. J. Enol. Vitic. 2008, 59, 210–214. [Google Scholar] [CrossRef]
- Watrelot, A.; Schulz, D.; Kennedy, J. Wine polysaccharides influence tannin–protein interactions. Food Hydrocoll. 2017, 63, 571–579. [Google Scholar] [CrossRef]
- Díaz-Plaza, E.; Reyero, J.; Pardo, F.; Alonso, G.; Salinas, M. Influence of oak wood on the aromatic composition and quality of wines with different tannin contents. J. Agric. Food Chem. 2002, 50, 2622–2626. [Google Scholar] [CrossRef] [PubMed]
- González-Centeno, M.; Chira, K.; Teissèdre, P. Comparison between Malolactic Fermentation Container and Barrel Toasting Effects on Phenolic, Volatile, and Sensory Profiles of Red Wines. J. Agric. Food Chem. 2017, 65, 3320–3329. [Google Scholar] [CrossRef]
- Nikolantonaki, M.; Daoud, S.; Noret, L.; Coelho, C.; Badet-Murat, M.-L.; Schmitt-Kopplin, P.; Gougeon, R.D. Impact of oak wood barrel tannin potential and toasting on white wines antioxidant stability. J. Agric. Food Chem. 2019, 67, 8402–8410. [Google Scholar] [CrossRef]
- Olate-Olave, V.; Pino-Ramos, L.; Peña-Martínez, P.; Castro, R.; Muñoz-Vera, M.; Reyes-Manríquez, S.; Casaubon, G.; Laurie, V. Red wine maceration with grapevine-cane residues: Influence of format and toasting level. Heliyon 2025, 11, e42164. [Google Scholar] [CrossRef]
- Leong, S.; Treadwell, M.; Liu, T.; Hochberg, M.; Sack, M.; Mueller, G.; Sigler, J.; Silcock, P.; Oey, I. Influence of Pulsed Electric Fields processing at high-intensity electric field strength on the relationship between anthocyanins composition and colour intensity of Merlot (Vitis vinifera L.) musts during cold maceration. Innov. Food Sci. Emerg. Technol. 2020, 59, 102243. [Google Scholar] [CrossRef]
- Nogales-Bueno, J.; Baca-Bocanegra, B.; Heredia, F.; Hernández-Hierro, J. Phenolic compounds extraction in enzymatic macerations of grape skins identified as low-level extractable total anthocyanin content. J. Food Sci. 2020, 85, 324–331. [Google Scholar] [CrossRef]
- Ruiz-Rodríguez, A.; Palma, M.; Barroso, C. Influence of Temperature during Pre-Fermentative Maceration and Alcoholic Fermentation on the Phenolic Composition of ‘Cabernet Sauvignon’ Wines. Foods 2021, 10, 1053. [Google Scholar] [CrossRef]
- Villamor, R.; Harbertson, J.; Ross, C. Influence of Tannin Concentration, Storage Temperature, and Time on Chemical and Sensory Properties of Cabernet Sauvignon and Merlot Wines. Am. J. Enol. Vitic. 2009, 60, 442–449. [Google Scholar] [CrossRef]
- Del Pino-García, R.; González-Sanjosé, M.; Rivero-Pérez, M.; García-Lomillo, J.; Muñiz, P. The effects of heat treatment on the phenolic composition and antioxidant capacity of red wine pomace seasonings. Food Chem. 2017, 221, 1723–1732. [Google Scholar] [CrossRef]
- Ifie, I.; Abrankó, L.; Villa-Rodriguez, J.; Papp, N.; Ho, P.; Williamson, G.; Marshall, L. The effect of aging temperature on the physicochemical properties, phytochemical profile and α-glucosidase inhibition of Hibiscus sabdariffa (roselle) wine. Food Chem. 2017, 267, 263–270. [Google Scholar] [CrossRef]
- Mafata, M.; Buica, A.; Toit, W.; Jaarsveld, F. The Effect of Grape Temperature at Pressing on Phenolic Extraction and Evolution in Méthode Cap Classique Wines Throughout Winemaking. S. Afr. J. Enol. Vitic. 2018, 39, 141–148. [Google Scholar] [CrossRef]
- Miller, K.; Noguera, R.; Beaver, J.; Medina-Plaza, C.; Oberholster, A.; Block, D. A Mechanistic Model for the Extraction of Phenolics from Grapes During Red Wine Fermentation. Molecules 2019, 24, 1275. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo-Llopart, A.; Saurina, J. Liquid Chromatographic Approach for the Discrimination and Classification of Cava Samples Based on the Phenolic Composition Using Chemometric Methods. Beverages 2020, 6, 54. [Google Scholar] [CrossRef]
- Wolfender, J. HPLC in Natural Product Analysis: The Detection Issue. Planta Med. 2009, 75, 719–734. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, H.; Xie, L.; Hu, Y.; Fang, H.; Sun, X.; Wang, T.; Xiao, R.; Yu, R. Direct and interference-free determination of thirteen phenolic compounds in red wines using a chemometrics-assisted HPLC-DAD strategy for authentication of vintage year. Anal. Methods 2017, 9, 3361–3374. [Google Scholar] [CrossRef]
- Del Alamo-Sanza, M.; Nevares, I. Recent advances in the evaluation of the oxygen transfer rate in oak barrels. Food Chem. 2014, 156, 33–37. [Google Scholar] [CrossRef]
- Burns, J.; Gardner, P.T.; O’Neil, J.; Crawford, S.; McLearie, L.; Lean, M.E.J.; Crozier, A.; Duthie, G.G. Relationship among Antioxidant Activity, Vasodilation Capacity, and Phenolic Content of Red Wines. J. Agric. Food Chem. 2000, 48, 220–230. [Google Scholar]
- Jackson, R.S. Wine Science: Principles and Applications; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Monagas, M.; Bartolomé, B.; Gómez-Cordovés, C. Chemical characterization of commercial dietary ingredients from Vitis vinifera L. Analyses of proanthocyanidins and anthocyanins. J. Agric. Food Chem. 2006, 54, 4319–4325. [Google Scholar]
- Fournand, D.; Vicens, A.; Sidhoum, L.; Souquet, J.-M.; Moutounet, M.; Cheynier, V. Accumulation and extractability of grape skin tannins and anthocyanins at different advanced physiological stages. J. Agric. Food Chem. 2006, 54, 7331–7338. [Google Scholar] [PubMed]
- Cristino, R.; Costa, E.; Cosme, F.; Jordão, A.M. General phenolic characterisation, individual anthocyanin and antioxidant capacityof matured red wines from two Portuguese Appellations of Origins. J. Sci. Food Agric. 2013, 93, 2486–2493. [Google Scholar] [CrossRef]
- Leal, C.; Barros, J.G.L.; Fernandes, R.; Abraão, A.; Costa, R.D.; Aires, A.; Gouvinhas, I.; Granato, D.; Barros, A.N. Characterization of Azorean Plant Leaves for Sustainable Valorization and Future Advanced Applications in the Food, Cosmetic, and Pharmaceutical Industries. Antioxidants 2024, 13, 325. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.D.; Domínguez-Perles, R.; Abraão, A.; Gomes, V.; Gouvinhas, I.; Barros, A.N. Exploring the Antioxidant Potential of Phenolic Compounds from Winery By-Products by Hydroethanolic Extraction. Molecules 2023, 28, 6660. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar]
- Mercurio, M.D.; Dambergs, R.G.; Herderich, M.J.; Smith, P.A. High throughput analysis of red wine and grape phenolics—Adaptation and validation of methyl cellulose precipitable tannin assay and modified somers color assay to a rapid 96 well plate format. J. Agric. Food Chem. 2007, 55, 4651–4657. [Google Scholar] [CrossRef]
- Costa-Pérez, A.; Medina, S.; Sánchez-Bravo, P.; Domínguez-Perles, R.; García-Viguera, C. The (Poly)phenolic Profile of Separate Winery By-Products Reveals Potential Antioxidant Synergies. Molecules 2023, 28, 2081. [Google Scholar] [CrossRef]
| Samples | TPC (mg GAE/L) | ODC (mg GAE/L) | FC (mg CE/L) |
|---|---|---|---|
| 1 | 1009.01 ± 87.92 e | 3023.06± 81.29 b | 1607.25 ± 100.43 b,c |
| 2 | 1740.24 ± 131.82 c | 2263.20 ± 27.38 d | 1281.88 ± 93.82 d |
| 3 | 1818.32 ± 82.12 c | 2811.59 ± 23.87 b,c | 1715.94 ± 63.54 b |
| 4 | 1734.23 ± 193.05 c | 2837.87 ± 84.39 b,c | 1456.04 ± 71.49 c,d |
| 5 | 1029.28 ± 3.19 e | 3677.78 ± 166.7 a | 2115.22 ± 107.60 a |
| 6 | 1043.54 ± 78.68 e | 3505.74 ± 168.23 a | 2154.11 ± 116.58 a |
| 7 | 1464.72 ± 74.78 d | 2595.34 ± 65.7 c | 1692.75 ± 112.73 b,c |
| 8 | 2983.48 ± 227.93 a | 2816.37 ± 101.02 b,c | 2054.11 ± 156.32 a |
| 9 | 3308.56 ± 245.26 a | 518.28 ± 38.02 e | 730.44 ± 7.69 e |
| 10 | 2076.58 ± 66.36 b | 431.66 ± 27.38 e | 707.73 ± 41.64 e |
| Samples | Anthocyanins (Malv/L) | Tannins (EPICAT/L) |
|---|---|---|
| 1 | 175.60 ± 6.79 f | 97.28 ± 4.91 c |
| 2 | 91.40 ± 8.77 h | 79.43 ± 1.28 d,e |
| 3 | 94.40 ± 9.05 h | 157.79 ± 6.62 a |
| 4 | 440.00 ± 3.40 c | 67.05 ± 2.52 e,f |
| 5 | 556.00 ± 1.70 a | 67.90 ± 4.81 e,f |
| 6 | 497.20 ± 21.50 b | 77.23 ± 5.46 d,e,f |
| 7 | 441.20 ± 3.96 c | 65.84 ± 3.29 f |
| 8 | 138.00 ± 1.70 g | 124.92 ± 9.50 d |
| 9 | 228.60 ± 4.81 e | 93.85 ± 0.35 c |
| 10 | 246.20 ± 1.98 d | 85.23 ± 3.09 c,d |
| Rt | λ (nm) | [M–H]−, m/z | Identified Compounds | Quantification (mg/ L) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||||
| Stilbens | |||||||||||||
| 1.34 | 280 | 777 | Viniferin diglycoside | ND | 38.609 ± 0.240 c | 36.388 ± 0.646 c | ND | 75.836 ± 1.564 a | ND | 56.939 ± 0.760 b | ND | ND | ND |
| 15.13 | 320 | 905 | Resveratrol tetramer | ND | ND | ND | 1.197 ± 0.104 a | ND | ND | ND | ND | ND | ND |
| Total | ND | 38.609 ± 0.240 | 36.388 ± 0.646 | 1.197 ± 0.104 | 75.836 ± 1.564 | ND | 56.939 ± 0.760 | ND | ND | ND | |||
| Hydroxycinnamic acids | |||||||||||||
| 3.49 | 280 | 311 | Caftaric acid | ND | 1.901 ± 0.076 b,c | ND | 1.680 ± 0.021 c | 3.938 ± 0.117 b | 4.734 ± 0.096 a | ND | 1.909 ± 0.057 b | 4.631 ± 0.032 a | ND |
| 7.33 | 320 | 487 | Caftaric acid-glucuronide | ND | 0.934 ± 0.011 a | 0.831 ± 0.341 a | ND | ND | ND | ND | ND | ND | ND |
| 11.01 | 280 | 353 | Clorogenic acid | 0.683 ± 0.312 a | ND | ND | ND | ND | ND | ND | 0.868 ± 0.019 a | ND | ND |
| Total | 0.683 ± 0.312 | 2.835 ± 0.435 | 0.831 ± 0.341 | 1.680 ± 0.021 | 3.938 ± 0.117 | 4.734 ± 0.096 | ND | 2.777 ± 0.038 | 4.631 ± 0.032 | ND | |||
| Flavan-3-ols | |||||||||||||
| 2.37 | 280 | 879 | Procyanidin dimer digallate A-type isomer | ND | ND | ND | ND | 1.838 ± 0.442 | ND | ND | ND | ND | ND |
| 5.06 | 280 | 577 | Proanthocyanidin dimer | ND | ND | ND | ND | 3.370 ± 0.152 a | 3.391 ± 0.098 a | 3.057 ± 0.062 b | ND | ND | 2.694 ± 0.109 c |
| 5.81 | 280 | 1153 | Proanthocyanidin tetramer | ND | ND | ND | 4.956 ± 0.146 b | 8.762 ± 0.076 a | ND | ND | ND | ND | ND |
| 5.83 | 280 | 865 | Proanthocyanidin trimer | 8.548 ± 0.069 b | ND | 4.561 ± 0.136 d | ND | ND | 9.362 ± 0.151 a | 6.833 ± 0.228 c | 1.003 ± 0.044 e | ND | ND |
| 7.20 | 280 | 1017 | Proanthocyanidin trimer monogallate | ND | ND | ND | 3.404 ± 0.157 b | ND | 7.765 ± 0.073 a | ND | ND | ND | ND |
| 8.83 | 520 | 729 | (Epi)catechin-(epi)catechin gallate | ND | ND | ND | ND | ND | 2.387 ± 0.010 a | ND | ND | ND | ND |
| 10.40 | 280 | 613 | (Epi)catechin-3-O-dihexoside | ND | ND | ND | ND | ND | 7.240 ± 0.008 a | ND | ND | ND | ND |
| 10.40 | 280 | 745 | (Epi)catechin-(epi)gallocatechin gallate (EGCG) | ND | ND | 5.879 ± 0.016 a | ND | ND | ND | ND | ND | ND | ND |
| 11.62 | 520 | 593 | (Epi)gallocatechin-(epi)catechin | ND | ND | ND | ND | ND | ND | 0.484 ± 0.020 a | ND | ND | ND |
| Total | 8.548 ± 0.069 | ND | 10.44 ± 0.076 | 8.36 ± 0.151 | 13.97 ± 0.223 | 30.145 ±0.068 | 10.374 ± 0.31 | 1.003 ± 0.044 | ND | 2.694 ± 0.109 | |||
| Flavonols | |||||||||||||
| 9.21 | 280 | 479 | Myricetin-O-hexoside | ND | ND | 3.390 ± 0.034 a | ND | ND | ND | ND | ND | ND | ND |
| 10.90 | 320 | 1131 | Esterified quercetin II | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 14.75 | 360 | 301 | Esterified quercetin I | 2.028 ± 0.015 a | ND | ND | ND | ND | ND | 0.48 ± 0.00 b | ND | ND | ND |
| Total | 2.028 ± 0.015 | ND | 3.390 ± 0.034 | ND | ND | ND | 0.48 ± 0.00 | ND | ND | ND | |||
| Samples | FRAP (mmolTE/L) | ABTS (mmolTE/L) | DPPH (mmolTE/L) |
|---|---|---|---|
| 1 | 21.46 ± 0.30 b,c | 20.74 ± 0.62 b | 19.22 ± 0.78 c,d |
| 2 | 16.58 ± 0.48 d | 16.04 ± 0.32 c | 15.48 ± 0.22 e |
| 3 | 19.17 ± 1.06 c,d | 19.40 ± 0.82 b | 19.10 ± 1.27 c,d |
| 4 | 20.49 ± 0.62 c | 20.33 ± 0.96 b | 21.22 ± 0.44 b,c |
| 5 | 28.02 ± 1.32 a | 25.08 ± 0.51 a | 25.60 ± 1.82 a |
| 6 | 24.48 ± 1.99 a,b | 23.67 ± 1.79 a | 22.79 ± 1.72 a,b |
| 7 | 19.96 ± 1.60 c,d | 20.53 ± 1.19 b | 19.88 ± 1.64 b,c,d |
| 8 | 20.54 ± 1.70 c | 20.13 ± 1.44 b | 16.57 ± 0.88 d,e |
| 9 | 19.96 ± 1.60 c,d | 19.23 ± 0.06 b | 17.71 ± 1.28 d,e |
| 10 | 21.33 ± 0.42 b,c | 20.04 ± 0.37 b | 17.92 ± 0.77 c,d,e |
| Samples | Technical Characteristics | Potential Influencing Factors |
|---|---|---|
| 1 | Alcoholic fermentation in a “lagar”, with traditional treading | Traditional treading Aging for 12 months in wood barrel |
| The wine was placed in casks, where the malolactic fermentation occurred, and aging was performed in the same wood barrel for 12 months | ||
| 2 | Alcoholic fermentation in stainless tanks | Aging for 18 months in wood barrel |
| Aging for 18 months in wood barrel | ||
| 3 | Cold pre-fermentation | Maceration Aging in wood barrel new and neutral |
| Spontaneous alcoholic fermentation, with maceration for 32 days | ||
| 18 months in wood barrel (75% new, 25% neutral) | ||
| 4 | Alcoholic fermentation stainless tanks | Soft Pressing Aging for 24 months in wood barrel |
| After malolactic fermentation, soft pressing was performed | ||
| 24 months in wood barrel | ||
| 5 | Partially destemmed grapes | Soft pressing Aging for 36 months in wood barrel |
| Alcoholic fermentation in stainless tanks | ||
| After malolactic fermentation, soft pressing was performed | ||
| 36 months in wood barrel | ||
| 6 | Alcoholic fermentation in a “lagar”, with traditional treading | Aging for 36 months in wood barrel Aging for 24 months in bottle |
| After malolactic fermentation, soft pressing was performed | ||
| Malolactic fermentation in wood barrel | ||
| 36 months in wood barrel and 24 months in bottle | ||
| 7 | Alcoholic fermentation and malolactic fermentation simultaneously in stainless tanks for 12 days | Post-fermentation maceration Soft pressing Aging for 18 months in wood barrel Aging for 36 months in stainless tanks |
| Post-fermentation maceration for 30 days | ||
| Soft pressing | ||
| 18 months in wood barrel and 36 months stainless tanks | ||
| 8 | Maceration in “microlagares” (T: 26–28 °C) | Maceration Aging for 9 months in wood barrel |
| 9 months in wood barrel | ||
| 9 | Alcoholic fermentation in stainless tanks, for 8 days | Aging for 12 months in wood barrel |
| Malolactic fermentation in stainless tanks, for 3 weeks | ||
| 12 months in wood barrel | ||
| 10 | Grapes selection | Soft pressing Aging for 18 months in wood barrel |
| Alcoholic fermentation stainless tanks | ||
| After malolactic fermentation, soft pressing was performed | ||
| 18 months in wood barrel |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Branco, Z.; Baptista, F.; Paié-Ribeiro, J.; Gouvinhas, I.; Barros, A.N. Impact of Winemaking Techniques on the Phenolic Composition and Antioxidant Properties of Touriga Nacional Wines. Molecules 2025, 30, 1601. https://doi.org/10.3390/molecules30071601
Branco Z, Baptista F, Paié-Ribeiro J, Gouvinhas I, Barros AN. Impact of Winemaking Techniques on the Phenolic Composition and Antioxidant Properties of Touriga Nacional Wines. Molecules. 2025; 30(7):1601. https://doi.org/10.3390/molecules30071601
Chicago/Turabian StyleBranco, Zélia, Filipa Baptista, Jessica Paié-Ribeiro, Irene Gouvinhas, and Ana Novo Barros. 2025. "Impact of Winemaking Techniques on the Phenolic Composition and Antioxidant Properties of Touriga Nacional Wines" Molecules 30, no. 7: 1601. https://doi.org/10.3390/molecules30071601
APA StyleBranco, Z., Baptista, F., Paié-Ribeiro, J., Gouvinhas, I., & Barros, A. N. (2025). Impact of Winemaking Techniques on the Phenolic Composition and Antioxidant Properties of Touriga Nacional Wines. Molecules, 30(7), 1601. https://doi.org/10.3390/molecules30071601









