Abstract
Compounds with thiosemicarbazide and semicarbazide scaffolds are among the most promising structures in medicinal chemistry due to the possibility of forming multiple hydrogen bonds. Therefore, six new derivatives of 4-fluorophenoxyacetylthiosemicarbazide and 4-fluorophenoxyacetylthiosemicarbazide were designed to compare their physicochemical properties, biological activity, and in silico pharmacokinetic parameters. All compounds were characterized by 1H, 13C NMR, 19F, IR spectra. For selected derivatives (AB2 and AB5), X-ray studies were performed to confirm their synthetic route and identify the tautomeric forms and intra- and intermolecular interactions occurring in the crystalline state. In the in silico pharmacokinetic study, a clear difference in lipophilicity was observed between thiosemicarbazide and semicarbazide derivatives. In vitro biological studies have shown the promising activity of thiosemicarbazides against prostate cancer cell line LNCaP, with a higher safety profile than semicarbazides. The most active compound AB2 showed IC50 = 108.14 μM against LNCaP. Based on biological studies, topoisomerase IIα was proposed as a potential molecular target, which was confirmed by molecular docking studies.
1. Introduction
Thiosemicarbazide and semicarbazide systems are promising structural fragments in medicinal chemistry. They owe their unique properties to the ability to form hydrogen bonds, which are the most important interactions of a potential drug with a biological target [1,2]. In fact, they are an important element for various drugs and bioactive compounds endowed with a wide range of therapeutic and pharmacological properties. Semicarbazide derivatives exhibit anticancer [3,4,5,6,7,8], antimicrobial [9,10], antioxidant [11,12,13], anticonvulsant [14,15,16,17,18], antiviral [19], antituberculosis [9,20], antimalarial [21], analgesic [22], and anti-inflammatory [22,23] activity. In turn, thiosemicarbazide derivatives exhibit anticancer [24,25,26], antimicrobial [9,27,28,29], antiviral [30,31], antioxidant [32], anticonvulsant [15,33,34], antitubercular [9,20,29,35], analgesic [28], anti-inflammatory [28] and antifungal [29] activity. Structurally, thio/semicarbazide derivatives differ only in the presence of sulfur or oxygen. This is a phenomenon called isosteric in medicinal chemistry, and the exchanged groups are isosteric groups. Replacing sulfur atom with an oxygen atom results in a change in physicochemical properties, molecular size, polarity, and electron distribution. This is crucial in creating a hydrogen bond network that stabilizes ligand-receptor interactions and recognizes bioactive sites. All these elements undoubtedly affect both the biological activity of compounds and their toxicity. Parks et al. reported that semicarbazide is about 1/20 as toxic as thiosemicarbazide [36]. Szopa et al. mentioned that replacing sulfur in thiosemicarbazide derivatives with an oxygen atom increases antitumor activity but also toxicity in zebrafish embryos [8]. On the other hand, 1-(4-nitrobenzoyl)-4-ethylsemicarbazide and 1-[(4-nitrophenyl)acetyl]-4-hexylsemicarbazide do not cause morphological abnormalities, including cardiotoxicity [8]. Moreover, the presence of a fluorine atom in the molecular structure of biologically active compounds may have a significant effect on their physicochemical properties. The introduction of a fluorine atom to the parent compound causes significant changes in the properties due to differences in the energy levels of HOMO and LUMO [37]. Morgenthaler et al. reported that the introduction of fluorine atom to drugs containing amino groups increases their bioavailability by regulating the pKa value [38]. It is worth mentioning that fluorine can be used as bioisostere of hydroxyl groups (-OH), because it acts as a weak hydrogen bond acceptor [39]. Moreover, the introduction of fluorine substituent, due to the high stability of C-F bond and electronegativity of fluorine [37,40,41], provides an increase in the metabolic stability of the drug [39,42,43]. Fluoridation also increases bioavailability by increasing the lipophilicity of the compound and thus increases the potency of the drug [37,44]. Fluorine atoms add inter- and intramolecular spaces that provide appropriate strong and selective interactions with enzymes and receptors [45,46]. Currently, 20% of pharmaceutical products contain fluorine compounds [37]. Figure 1 shows selected FDA-approved drugs that have fluorine atom in their structure.
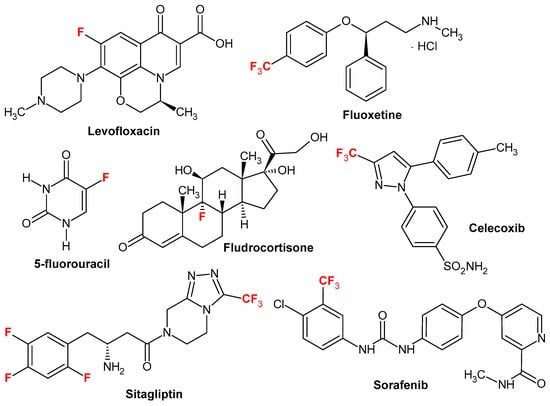
Figure 1.
Selected FDA-approved drugs bearing fluorine atom.
This indicates a further need for optimization of thio/semicarbazide derivatives structure in order to optimize biological activity and toxicity profile. For this purpose, new thiosemicarbazide and semicarbazide derivatives containing 4-fluorophenoxyl fragment were designed. Their anticancer potential and cytotoxicity of obtained derivatives toward normal BJ fibroblast cells were investigated. Analysis of gene expression related to antioxidant defense, cell cycle regulation, DNA damage detection and repair, and apoptosis was performed. Molecular docking was performed to determine potential molecular target.
2. Results and Discussion
2.1. Chemistry
The phenoxy group is a privileged scaffold in medicinal chemistry [47]. In our studies and previous works, we have shown promising anticancer activity of thiosemicarbazide derivatives with 2,4-dichlorophenoxy [25,26], phenoxy [24] and 4-fluorophenoxy [48] groups. Considering the valuable role of fluorine as well as reports on the activity and toxicological profile of thiosemicarbazide and semicarbazide, we designed new 1,4-disubstituted thiosemicarbazide (AB1–AB6) and semicarbazide (AB7–AB12) derivatives. The title compounds were obtained by the reaction of 4-fluorophenoxyacetic acid hydrazide with the following isothiocyanates: phenyl (AB1), 2-chlorophenyl (AB2), 3-chlorophenyl (AB3), 4-chlorophenyl (AB4), 2,4-dichlorophenyl (AB5), 3,4-dichlorophenyl (AB6) and with the following isocyanates: phenyl (AB7), 2-chlorophenyl (AB8), 3-chlorophenyl (AB9), 4-chlorophenyl (AB10), 2,4-dichlorophenyl (AB11), 3,4-dichlorophenyl (AB12). The reactions were carried out at the boiling point of methyl alcohol. The yield for thiosemicarbazide derivatives was in the range of 62–80%, while for semicarbazide derivatives it was 26–58%. Scheme 1 shows the synthetic path of the mentioned phenoxyacetyl(thio)semicarbazide derivatives.
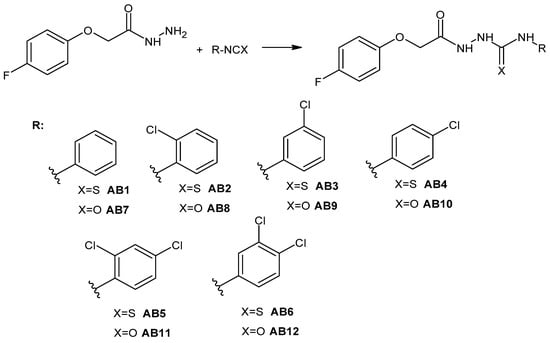
Scheme 1.
Synthesis pathway of 4-fluorophenoxy(thio)semicarbazide derivatives AB1–AB12.
The structures of the obtained compounds were confirmed by NMR and MS spectra. In the 1H NMR spectra, characteristic signals were observed for NH groups in the range of 9.56–10.36 ppm (for thiosemicarbazide derivatives) and 8.27–10.00 ppm, respectively, for semicarbazide derivatives. The presence of the carbonyl group (C=O) is confirmed by peaks in the range of about 181–182 ppm and the thion group (C=S) in the range of 167–168 ppm for thiosemicarbazides. For semicarbazide derivatives, a signal from the C=O group is visible in the range of 172 ppm. The presence of the above functional groups was also confirmed by 19F and infrared spectra. The purity of the obtained compounds was confirmed by MS spectrometry.
2.2. X-Ray Structure Determination
X-ray analysis was carried out for AB2 and AB5 in order to confirm the synthetic route, the assumed their molecular structures, including the observed tautomeric form in the crystalline state, and to examine the crystal structure for the identification of intra- and intermolecular interactions. The molecular structures of AB2 and AB5 in the conformation observed in the crystal is shown in Figure 2.
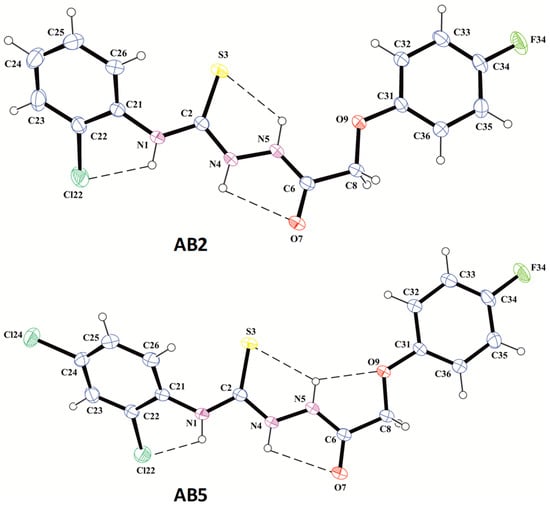
Figure 2.
The molecular structures of compounds AB2 and AB5 in conformation observed in the crystal with atom labeling and displacement ellipsoids drawing at the 30% probability level. The dashed lines show the intramolecular hydrogen bonds.
X-ray analysis revealed that the compounds AB2 and AB5 exist in N1-amino/S3-thione/N4-amino/N5-amino/O9-keto tautomeric form with the position of the hydrogen atoms on the differential electron density maps in the immediate vicinity of the N atoms and bond lengths C2–S3 of 1.666(3) and C6–O7 of 1.237(3) Å in AB2 and 1.668(3) and 1.236(3) Å in AB5, respectively. These bond lengths are typical for the thione (1.671(24) Å) and carbonyl (1.234(12) Å in amides) groups [49].
In both molecules, the acethylthiosemicarbazide chain connecting terminal phenyl substituents adopts almost planar conformation, and both benzene rings are arranged coplanar with respect to the plane of their chain linker. The appropriate torsion angles are presented in Table S1 (Supplementary Material). The conformations of molecules are stabilized by the intramolecular hydrogen bonds N1–H1…Cl22, N4–H4…O7 and N5–H5…S3 in AB2 and N1–H1…Cl22, N4–H4…O7, N5–H5…S3 and N5–H5…O9 in AB5 (Figure 3, Table S2 Supplementary Material) creating systems of five-membered rings designated as S(5) in graph set notation [50].
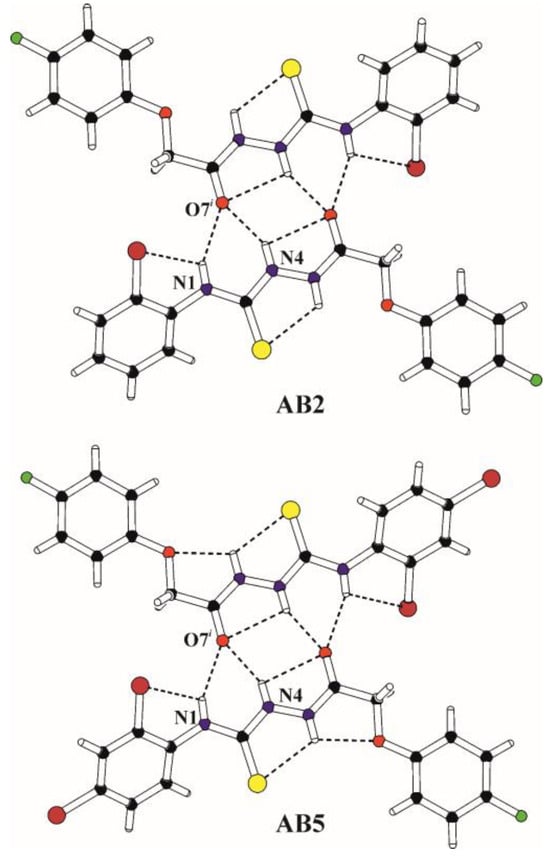
Figure 3.
The structural motif (molecular dimer) formed by inversion-related molecules in the crystals of AB2 (i = −x, 1−y, −z) and AB5 (i = −x, 1−y, −z). The intra- and intermolecular hydrogen bonds are marked with dashed lines.
The molecular packing in the crystals of AB2 and AB5 is influenced by the bifurcated intermolecular N1–H1…O7 and N4–H4…O7 and hydrogen bonds with the amino N–H groups as proton donors and the carbonyl O atom as the protons acceptor strictly connected with the tautomeric form observed in the crystalline state (Figure 3, Table S2 Supplementary Material). Inversion-related molecules form molecular dimers via these hydrogen bonds in which the S(4) ring and two S(6) rings are conjugated to rings formed by intramolecular hydrogen bonds. The molecular dimers observed in the crystal structures of AB2 and AB5 are shown in Figure 3.
A detailed characterization of intermolecular interactions was performed in crystal structures of AB2 and AB5 by the Hirshfeld method using CrystalExplorer Version 17 software [51]. This method included analysis of Hirshfeld surface and two-dimensional fingerprint plots presented in Figure 4a,b, where the distances shorter than the sum of the van der Waals radii of the nearest atoms are shown in red while the blue color indicates the low-intensity contacts.
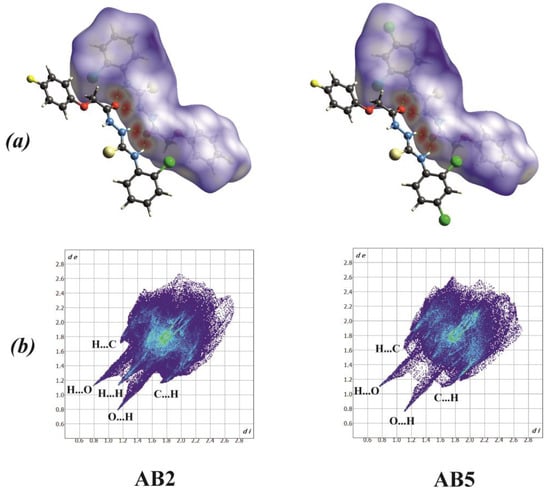
Figure 4.
(a) Hirshfeld surfaces plotted over dnorm; the hydrogen bonds are indicated by the red spots, (b) fingerprint plots of the nearest internal distance di versus the nearest external distance de.
The only similarity for AB2 and AB5 fingerprints is this presence of sharp H…O/O…H contacts with values of 8.9% and 8.3%, respectively. Fingerprint AB2 has more distinct H…C/C…H (16.00%) contacts, as compared to AB5, where they are quite blurred and rounded and have a slightly higher value of 16.70%. Moreover, for the AB2 fingerprint there is a clear sharp H…H contact (30.50%), which is poorly visible for AB5 (22.90%).
It is worth mentioning that H…H interactions contributed the most for both structures but are associated with rather repulsive interactions. A graph comparing the percentage of short contacts for AB2 and AB5 is presented in Figure 5.
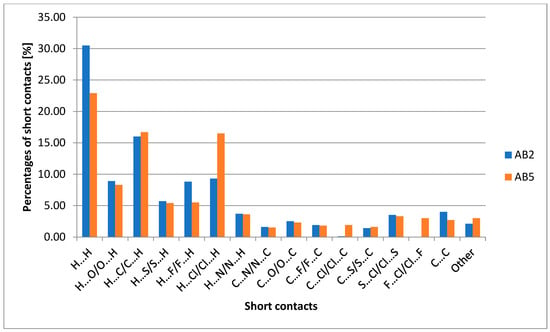
Figure 5.
Chart comparing the percentage of short contacts for AB2 and AB5.
2.3. ADMET Analysis
High therapeutic effectiveness is ensured by the properties of ADMET. Therefore, predictive characterization of Absorption, Distribution, Metabolism, Elimination, and toxicity (ADMET) was performed. Guan et al. reported that both high effectiveness in relation to the therapeutic target and appropriate properties of ADMET characterize a good drug candidate [52]. The BOILED-Egg diagram indicated that both thiosemicarbazides (AB1–AB6) and semicarbazides (AB7–AB12) are passively absorbed in the gastrointestinal tract (Figure 6). This property makes them potentially effective drugs. None of the designed compounds cross the blood–brain barrier. The compounds obtained by us are not substrates for P-glycoprotein (P-gp). It should be mentioned that not all anticancer drugs are substrates for Pgp [53].
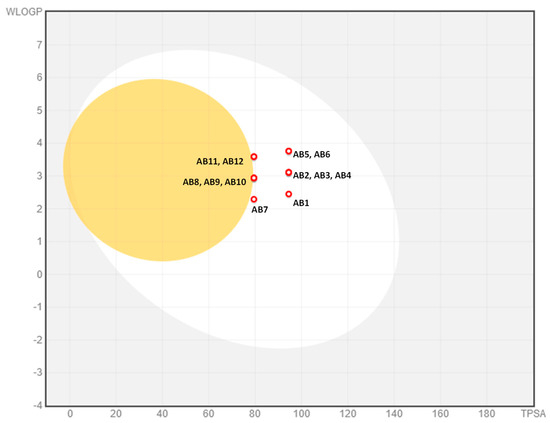
Figure 6.
Dependence of logp (WLOGP) on the topological polar surface (TPSA). BOILED − Egg diagram for AB1–AB12.
The Figure 6 shows the dependence of logp (WLOGP) on topological polar surface area (TPSA). The grey zone indicates compounds practically not absorbed by the gastrointestinal tract, the white sphere indicates compounds passively absorbed by the gastrointestinal tract, and the yellow sphere indicates compounds penetrating the blood–brain barrier.
A bioavailability radar for the mentioned compounds was presented in Figure 7. As a result of an insufficient ratio of carbon atoms in sp3 hybridization in relation to the total number of carbon atoms in the molecule, all compounds are characterized by an exceeded saturation parameter. However, the remaining physicochemical parameters are adequate for oral bioavailability. All of the compounds were found to have a good bioavailability score (0.55) [54]. Druglikeness compliance analysis showed that the designed compounds were consistent with the Lipiński [55], Ghose [56], Egan [57], Veber [58] and Muegge rule [59]. Additionally, we present the putative prediction drug–drug interactions via cytochrome P450 (CYP) inhibition (Table 1). There is a clear difference in potential interactions with CYP1A2, where all semicarbazides do not have inhibitory properties, and for thiosemicarbazides, most of them have inhibitory properties, except for AB1 and AB2. Similarly, this is the case for CYP2C9, where all thiosemicarbazides have inhibitory properties, and in the case of semicarbazides, only AB8, AB11 and AB12. In the case of CYP2C19, only AB7 does not have inhibitory properties. AB6 has inhibitory properties for CYP2D6, and AB4, AB5, and AB6 have inhibitory properties for CYP3A4 among the series of designed derivatives.
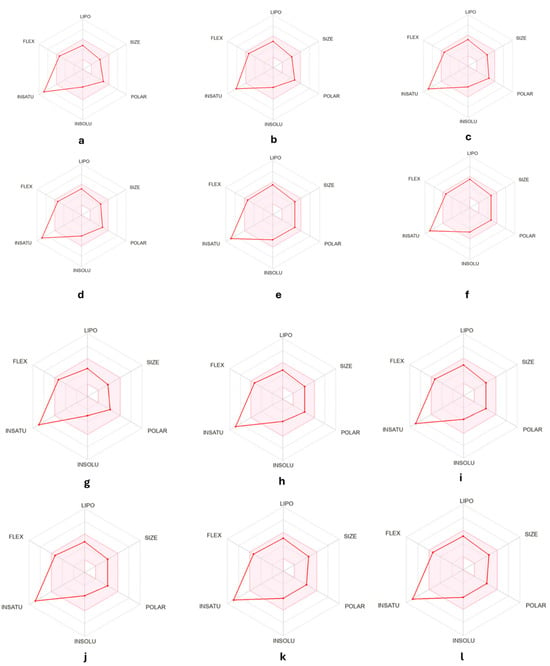
Figure 7.
Bioavailability radar for (a). AB1, (b). AB2, (c). AB3, (d). AB4, (e). AB5, 208 (f). AB6, (g). AB7, (h). AB8, (i). AB9, (j). AB10, (k). AB11, (l). AB12. (lipophility (LIPO) are within the range 0.7 < XlogP3 < +5.0; molecular weight (SIZE) is 150 g mol 1 < MW < 500 g mol 1; polarity (POLAR) is 20 Å2 < Topological Polar Surface (TPSA) < 130 Å2; insolubility (INSOLU) is 0 < logS < 6; insaturation (INSATU) are 0.25 < Fraction Csp3 < 1; and Flexibility (FLEX) are 0 < Num. rotatable bonds < 9).

Table 1.
The putative prediction drug–drug interactions via cytochrome P450 (CYP) for given compounds.
In order to confirm the safety profile of the AB1–AB12, prediction of toxicity was performed using the in silico method. Pro Tox-II classified AB1–AB6 and AB12 to toxicity class 4 (predicted lethal dose [LD50] = 500 mg/kg for AB1–AB6 and [LD50] = 850 mg/kg for AB12) and AB7–AB11 to class 5 (predicted [LD50] = 2500 mg/kg). This indicates that the designed semicarbazides have a substantially lower toxicity, as compared to thiosemicarbazides. Moreover, all compounds showed no immunotoxic activity.
2.4. Biological Activity
2.4.1. Cytotoxicity Assessment
The biological activity of thiosemicarbazide derivatives (AB1–AB6) and semicarbazide derivatives (AB7–AB12) was evaluated in a panel of human cell lines, including non-cancerous BJ fibroblasts as normal cells and cancer cell lines representing different types of malignancies. These cancer cell lines comprised prostate cancer (PC-3, DU-145, LNCaP), melanoma (SK-MEL28, A375, G-361), breast cancer (MCF-7, MDA-MB-231), and lung cancer (NCI-H1563). The MTT assay was carried out across a wide range of concentrations for 48 h. Thiosemicarbazide derivatives were tested at concentrations ranging from 500 to 100 μM, whereas semicarbazide derivatives were evaluated at a more limited range of 100 μM to 1 μM due to their low solubility. The semicarbazides assessed at the maximum concentration of 100 µM did not achieve IC50 values in any of the tested cancer cell lines and showed a low level of cytotoxicity toward cancer cells, which led to their exclusion from further studies.
To determine the selectivity of the thiosemicarbazide derivatives (AB1–AB6), toxicity to normal cells (BJ fibroblasts) was carried out. Among all tested derivatives, IC50 values ranged from 497.71 µM (AB1) to below 100 µM (AB6), indicating varying levels of toxicity toward healthy cells (Table 2). Compound AB6 exhibited the highest level of toxicity to BJ cells, with an IC50 value of less than 100 μM, suggesting the possibility of off-target effects. In contrast, the remaining thiosemicarbazide derivatives showed moderate to low toxicity, with IC50 values ranging from 302.68 μM (AB5) to 497.71 μM (AB1), which was close to the maximum tested concentration. The toxicity of thiosemicarbazides toward healthy BJ fibroblasts increases depending on the chlorine atom’s location in the chlorophenyl substituent and the quantity of chlorine atoms. The cytotoxicity is augmented as chlorine atoms occupy various locations on the phenyl ring. Furthermore, an augmentation in the quantity of chlorine atoms in the chlorophenyl moiety is associated with an increased level of toxicity. This indicates that both the spatial configuration and the extent of halogenation substantially affect the biological activity of these chemicals.

Table 2.
IC50 values were obtained for evaluated thiosemicarbazides (AB1–AB6) based on MTT test results after 48 h treatment of BJ fibroblasts, prostate cancer cell lines, melanoma cancer cell lines, breast cancer cell lines, and lung cancer cell line.
The anti-cancer activity screening of thiosemicarbazides revealed that AB1 showed the lowest activity, with IC50 values exceeding the highest evaluated concentration of 500 µM in seven out of the nine tested cancer cell lines. The only two cell lines that achieved an IC50 value in the tested concentration range were LNCaP (252.14 µM) and G-361 (369.37 µM). AB2 exhibited lower IC50 values, with the greatest activity also in LNCaP (108.14 μM) and G-361 (222.74 μM) cell lines. Similar results were obtained with the other investigated thiosemicarbazides (AB3–AB6), again showing the lowest IC50 and therefore the greatest anti-tumor activity in the LNCaP and G-361 lines. Despite the fact that the lowest IC50 values for the above-mentioned cell lines were observed in the case of AB6, this compound was excluded from further studies due to the low selectivity index (SI) (<1), indicating high toxicity of AB6 against normal cells. The selectivity index (SI) is a measure of a compound’s ability to target cancer cells over normal cells, calculated by dividing the IC50 value for normal cells (such as fibroblasts) by the IC50 value for cancer cells, with higher SI values indicating greater selectivity for cancer cells. Out of all investigated thiosemicarbazides, the highest SI values were obtained for AB2 in the G-361 melanoma cell line (2.04) and LNCaP prostate cancer cell line (4.19). Therefore, the above-mentioned compound and cell lines were selected for further in-depth studies.
This study investigated the antitumor efficacy of chlorophenyl-containing thiosemicarbazides, which have demonstrated activity across several cancer cell lines, especially G-361 melanoma cells and LNCaP prostate cancer cells. Our team’s previously published research papers have demonstrated the anticancer potential of thiosemicarbazides in various cancer types, including the MKN74 gastric cancer cell line [26] and G-361 malignant melanoma cells [25]. Research conducted by Malki et al. demonstrated that 4-(4-chlorophenyl)thiosemicarbazides displayed significant anticancer activity against MCF-7 breast cancer cells [60]. Many other studies have shown that compounds possessing a privileged thiosemicarbazide scaffold are potential anticancer agents i.a., acridine thiosemicarbazides derivatives exhibited significant anticancer activity against acute lymphoblastic leukemia MT-4 cells [61] and chalcone thiosemicarbazide derivatives have antiproliferative activity against HepG2 human hepatocellular liver carcinoma [62]. Therefore, thiosemicarbazides require additional investigation about their anticancer potential.
2.4.2. Cell Cycle Analysis and Apoptosis Detection
To further evaluate the anticancer activity of thiosemicarbazide derivative AB2 in G-361 and LNCaP cell lines, cell cycle analysis by image cytometry and detection of apoptosis using annexin V and propidium iodide was performed. Analyses of the action of AB2 on the distribution of cells in different phases of the cell cycle indicated a significant increase in the population of cells in the sub-G1 phase compared to the control cultures, in both tested prostate and melanoma cells. This population increased from 7% and 5% in control cells to 26% and 27% after exposure to AB2 in LNCaP and G-361 cells, respectively (Figure 8A). The increase in the cell population in the sub-G1 phase indicates the presence of apoptosis or necrosis in the investigated cells and not a significant inhibition of cell proliferation, i.e., a cytostatic effect. In another study evaluated by our research team, thiosemicarbazide derivatives PK2, PK6, and PK9 also caused an increase in a population of cells in the sub-G1 phase on G-361 melanoma cells [25]. Surprisingly, another research established that the other thiosemicarbazides derivatives compounds 1 and 2 caused the arrest of the cell cycle in the S and G2 phases on the MKN74 gastric cell line [26].
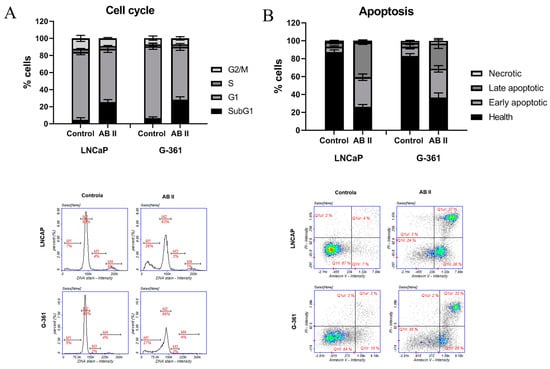
Figure 8.
(A) Analysis of cell cycle of LNCaP prostate cancer cell line and G-361 melanoma cell line. The cells were treated for 48 h with AB2 in concentration approximate to IC50 value. Results of three independent experiments are shown as mean ± SD. Representative LNCaP and G-361 cell cycle histograms (M1—subG1, M2—G1, M3—S, M4—G2/M phase) are presented below the graph. (B) Cell apoptosis/necrosis of LNCaP prostate cancer cell line and G-361 melanoma cell line treated for 48 h with AB2 in concentration approximate to IC50 value. Results of three independent experiments are shown as mean ± SD. Representative LNCaP and G-361 cell cycle histograms (Q1II: live cells; Q1Ir: early apoptotic cells; Q1ur: late apoptotic cells; Q1uI: necrotic cells) are presented below the graph.
Following treatment with AB2, a significant increase in the population of cells in both early and late apoptosis was observed in both the LNCaP and G-361 cell lines. In prostate cancer cells, the percentage of early apoptotic cells substantially elevated after AB2 treatment compared with the control group, indicating the initial activation of the apoptotic pathway. A similar trend was seen in the melanoma cell line, where the proportion of early apoptotic cells also rose significantly. The percentage of cells in the early phase of apoptosis is approximately 36% and 28% in LNCaP and G-361 cells, respectively (Figure 8B). At the same time, the population of cells in late apoptosis increased in both cell lines, implying a progression of the apoptotic process after AB2 exposure. This increase in late apoptotic cells was particularly pronounced in LNCaP cells (37%).
2.4.3. Analysis of Gene Expression Related to Antioxidant Defense, Cell Cycle Regulation, DNA Damage Sensing and Repair and Apoptosis
An analysis was conducted to examine the impact of the thiosemicarbazide AB2 on LNCaP prostate cancer cells and G-361 melanoma cells, focusing on the expression of genes associated with oxidative stress, cell cycle regulation, DNA repair, and apoptosis. Superoxide dismutase (SOD2), catalase (CAT), and glutathione peroxidase 1 (GPX1) are critical antioxidant enzymes that protect cells from oxidative stress by neutralizing reactive oxygen species (ROS) and mitigating potential cellular damage [63]. The expression of the SOD2 gene increased significantly following AB2 exposure in both cell lines, with G-361 cells exhibiting over a 100-fold rise and LNCaP cells showing more than a 20-fold increase compared to control cultures, indicating a robust activation of antioxidant defense mechanisms (Figure 9A). AB2 significantly increased the expression of CAT and GPX1 in both tested cell lines. CAT showed an approximately 8-fold increase in G-361 and a 6-fold increase in LNCaP, while GPX1 exhibited a moderate but significant rise in both cell lines. In the next group of analyzed genes, AB2 treatment resulted in a significant downregulation of TP53 expression in both tested cell lines, suggesting a potential suppression of the p53-mediated DNA damage response pathway [64]. CDKN1A (p21) expression was significantly upregulated in assessed melanoma and prostate cancer cells after AB2 treatment. This indicates heightened activation of cell cycle arrest mechanisms, given that p21 acts as an important inhibitor of cyclin-dependent kinases essential for cell cycle progression [65]. Conversely, CYCD (cyclin D), which facilitates the transition from G1 to S phase, was notably downregulated in both cell lines. This suggests that AB2 may impede cell proliferation by inhibiting cyclin D-mediated progression through the cell cycle. The contrasting alterations in CDKN1A and CYCD indicate a transition toward cell cycle arrest in response to AB2 (Figure 9B). The next assessed panel of genes includes those involved in DNA damage sensing and repair, specifically ATM and ATR. ATM and ATR are fundamental components of the DNA damage response, responsible for the detection and repair of double-strand and single-strand DNA breaks, respectively [66]. Both of the evaluated genes’ expression is downregulated after AB2 treatment in both tested cell lines, but a decrease in expression of the above-mentioned genes is stronger in the G-361 cell line (Figure 9C). The decrease in their expression indicates that AB2 could interfere with the ability of cells to accurately detect and repair DNA damage.
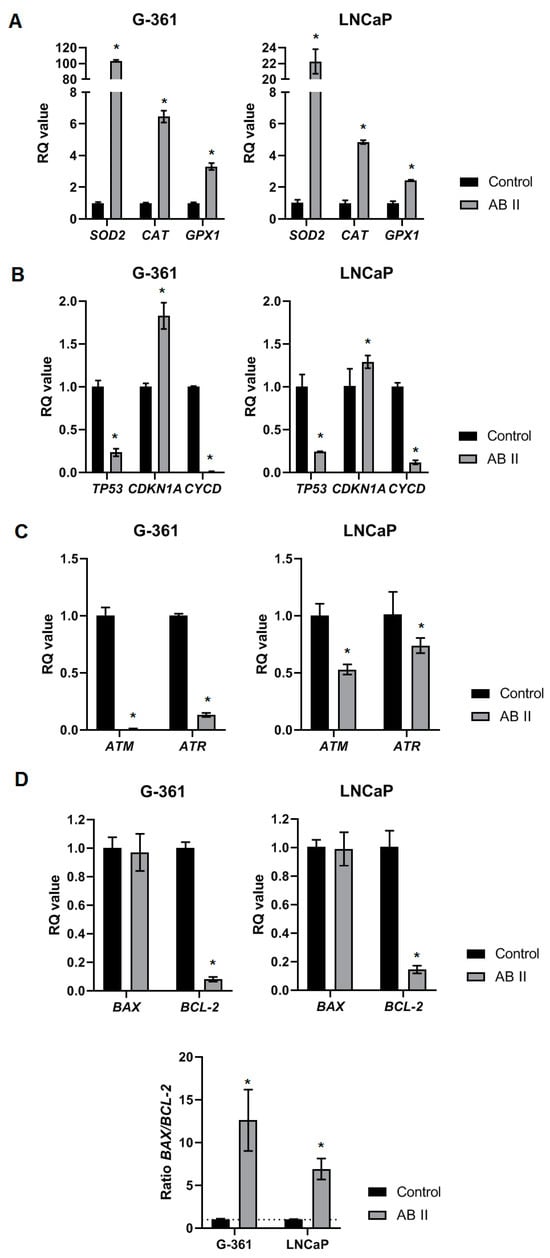
Figure 9.
Relative mRNA expression level of genes related to (A) antioxidant defense, (B) cell cycle regulation, (C) DNA repair and (D) apoptosis in G-361 melanoma cell line and LNCaP prostate cancer cell line after 24h treatment with AB2 in concentration approximate to IC50 value. BACT and RNA18SN5 were used as reference genes. Results have been determined as RQ values and are expressed as means ± SDs. Compound vs. Control * p < 0.05.
The last assessed panel of genes includes genes involved in the process of apoptosis. BAX is a protein that enhances apoptosis, a natural to eliminate damaged or cancer cells. On the other hand, BCL-2 prevents apoptosis, helping cancer cells survive and resist therapy [67]. After AB2 treatment, the expression levels of the pro-apoptotic gene BAX did not differ from the control cultures in both G-361 and LNCaP cell lines. The expression of the anti-apoptotic gene BCL-2 was significantly downregulated in both cell lines. The decrease in BCL-2 expression, along with stable BAX levels, led to a higher BAX/BCL-2 ratio in comparison to the control. The increased BAX/BCL-2 ratio indicates a transition toward pro-apoptotic signaling, suggesting that AB2 may augment apoptotic potential in these cell lines by diminishing the protective role of BCL-2, hence promoting cell death.
Gene expression analysis enhances the understanding of the possible mechanism of action of thiosemicarbazide AB2 regarding cancer cells. The elevated expression of CAT, SOD2, and GPX1 genes indicates the occurrence of oxidative alterations in cancer cells induced by the AB2. Oxidative stress induced by Reactive Oxygen Species (ROS) results in DNA damage, requiring an efficient functioning of the DNA damage repair mechanism (DDR) within the cell. In response to oxidative stress, key DNA damage response regulators, including ATM and ATR, should be activated [68,69]. The experiment reveals a notable reduction in ATM and ATR expression, indicating that the AB2 disrupts the DDR pathway. The decreased expression of ATM and ATR likely results in an accumulation of DNA damage, contributing to heightened genomic instability and possibly playing an essential part in the pro-apoptotic effects of AB2 [70]. The presence of apoptotic cells in the Sub-G1 phase in cell cycle analysis may be a consequence of irreversible DNA damage that triggered cell cycle arrest and subsequent activation of apoptotic pathways in response to the inability of cells to repair this damage. This investigation confirmed the presence of apoptotic cells in response to AB2, revealing an increased population of both early and late apoptotic cells in both of the investigated cell lines. In summary, biological studies suggest a possible mechanism of action for thiosemicarbazide AB2 in LNCaP prostate cancer cells and G-361 melanoma cells, associated with the concurrent induction of oxidative stress and disruption of DNA repair, leading to the initiation of apoptosis in cancer cells. Nonetheless, additional comprehensive research is required to identify the precise molecular target of the AB2.
2.5. Molecular Docking
Taking into account the results of biological activity obtained in in vitro tests, it is highly probable that a potential molecular target may be topoisomerase IIα, which is responsible for the control of the topological state of the DNA strand [71]. Topoisomerase II occurs in two main isoforms: alpha (IIα) and beta (IIβ), but only isoform α is crucial for cell proliferation [72]. Moreover, the expression of topoisomerase IIα has been associated with the progression of prostate cancer [71,73]. Therefore, it was selected by our team as a potential target for molecular docking. The crystal structure of human topoisomerase II alpha in complex with DNA and etoposide as a molecular target for investigated ligands was downloaded from Protein Data Bank (PDB ID: 5GWK [74]).
For the validation of the docking method, redocking of the native ligand was performed, which gave the best match to the binding site with a ChemScore value of 112.58 and RMS value of 1.022 Å, indicating the correctness of the docking method (Figure 10).
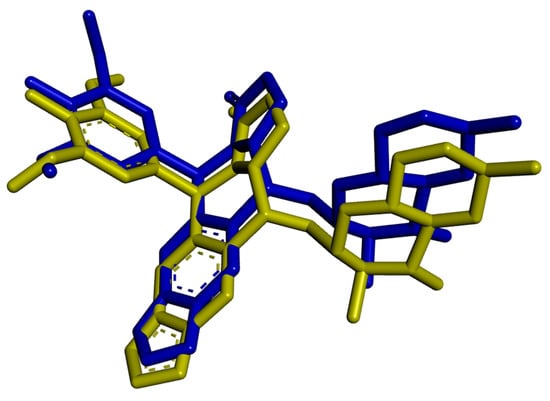
Figure 10.
Superimpose of native ligand from protein (PDB ID: 5GWK) [74] (yellow) and redox (blue) with RMS = 1.022 Å. (Only non-hydrogen atoms are shown).
Molecular docking was performed only for thiosemicarbazide derivatives, due to the low cytotoxicity toward cancer cells of semicarbazide derivatives. Molecular docking study showed that all tested ligands bind to the active site of the indicated molecular target. The ChemScore scoring function values and amino acids involved in hydrogen bonds are presented in Table 3.

Table 3.
The results of the molecular docking of ligands AB1–AB6 to topoisomerase IIα.
The ChemScore values for all designed ligands are within a narrow range, with the highest value of 75.42 obtained for AB2 and the lowest value of 70.91 estimated for AB1. These values are significantly lower than those obtained for the native ligand (112.58). However, etoposide is an officially approved chemotherapeutic drug and eukaryotic topoisomerase II poison [72]. Therefore the best result in molecular docking is a confirmation of its high potential.
It is worth noting that the highest ChemScore value was obtained for compound AB2, which correlates with the highest activity obtained in biological studies for this compound. Figure 11 shows the interactions of AB2 with the active site of topoisomerase IIα (interactions for other ligands are presented in Figures S25–S29, Supplementary Materials).
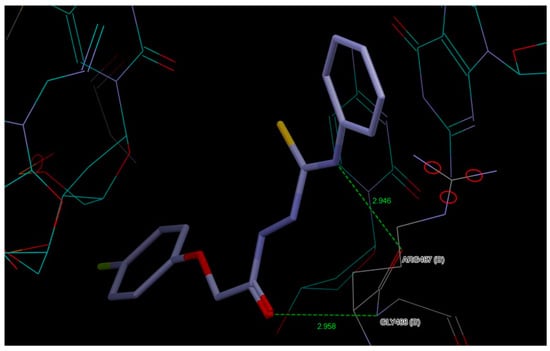
Figure 11.
Interactions of AB2 with the active site of topoisomerase IIα.
The most active ligand AB2 forms a hydrogen bond between the aromatic hydrogen atom of the 4-fluorophenoxyacetyl moiety and the nitrogen atom of the DA12(D) DNA chain and this hydrogen bond is also present for AB3, AB4 and AB6. AB5 is the only compound that interacts with the molecular target via two chlorine atoms of the phenyl ring derived from isothiocyanate.
However, the AB1 compound, unlike others, interacts with the target molecule via hydrogen bonds formed by the oxygen of the carbonyl group and the nitrogen of the amino group of the thiosemicarbazide chain. For the AB4 compound, the formation of one hydrogen bond is observed by the amino group of the thiosemicarbazide chain, while for the AB3 and AB6 compounds, the thione group from the thiosemicarbazide chain participates in the formation of the hydrogen bond.
3. Experimental
3.1. Chemical Reagents and General Method
The chemicals and reagents were commercially available and were purchased from Sigma-Aldrich (Sigma-Aldrich, Saint Louis, MO, USA), AlfaAesar (Haverhill, MA, USA), POCH (Haverhill, MA, USA), Fluorochem (Fluorochem, GBR) and used without further purification. The crude compounds were purified by crystallization from methanol. 1H and 13C NMR spectra were performed on a Bruker Avance 600 spectrometer (Bruker BioSpin GmbH, Rheinstetten, Germany). TMS (tetramethylsilane) was used as an internal standard and DMSO-d6 was used to dissolve the samples. The chemical shifts (d) are indicated in ppm. For accurate mass measurements, an Agilent Technologies 1290 liquid chromatograph coupled to an Agilent Technologies 6550 iFunnel Q-TOF LC/MS was employed. Flow injection analyses utilized a mobile phase of 0.1% formic acid in water:acetonitrile (50:50 v/v) with a flow rate of 0.5 mL/min. Compounds were ionized by electrospray and detected in positive polarity. Mass correction using two reference ions of m/z 121.0509 and 922.0098 was enabled. Instrument control, data acquisition, and analysis were conducted using Agilent MassHunter Workstation B.07 software. For all compounds single-charged protonated ions were observed.
3.2. General Procedure for the Synthesis of 1-(4-Fluorophenoxyacetyl)-4-substituted Thiosemicarbazide (AB1–AB6)
2-(4-Fluorophenoxy)acetohydrazide (0.5 g, 2.71 mmol) and the appropriate isothiocyanate (dissolved in approximately 15–20 mL of methanol) were mixed in a round-bottomed flask.
The solution was heated for an hour and then cooled to precipitate. The precipitate was filtered off, recrystallized from methanol. Figure 12 shows the general formula of 4-fluorophenoxyacetylthiosemicarbazides AB1–AB6. Spectroscopic data of the obtained compounds can be found in the Supplementary Material.
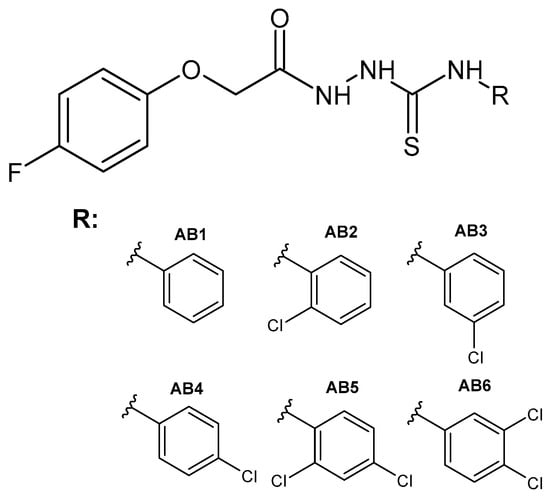
Figure 12.
The general formula of the designed thiosemicarbazides AB1–AB6.
1-(4-Fluorophenoxy)-4-(phenyl)acetylthiosemicarbazide (AB1)
Molecular formula: C15H14FN3O2S (319.36g/mol), white solid, yield 80% (0.69g); m.p. 179–181 °C. 1H NMR (600 MHz, DMSO-d6): 4.62 (s, 2H, CH2); 7.03–7.42 (m, 9H, CHar), 9.68 (s, 2H, NH); 10.28 (s, 1H, NH). 13C NMR (300 MHz, DMSO): 67, 116, 116, 125.70–126.51 (1JC–F = 243 Hz), 129, 140, 1554, 1564, 158, 168, 181. 19F NMR (400 MHz, CFCL3-d8): −122.8 (m, 1F). IR γmax (cm−1): 3227 (NH), 3099 (NH), 2934 (NH), 1701 (C=O), 1201 (C=S). LC-QTOF MS (m/z): calculated monoisotopic mass: 319.0790, measured monoisotopic mass: 320.0862.
1-(2-Chlorophenyl)-4-(4-fluorophenoxy)acetylthiosemicarbazide (AB2)
Molecular formula: C15H13ClFN3O2S (353.79 g/mol), white solid yield 73% (0.70 g); m.p. 166–168 °C. 1H NMR (600 MHz, DMSO-d6): 4.63 (s, 2H, CH2); 6.96–7.50 (m, 8H, CHar); 9.56 (s, 1H, NH); 9.82 (s, 1H, NH); 10.36 (s, 1H, NH) ppm. 13C NMR (300 MHz, DMSO): 67, 116, 116, 128, 129, 130, 131.54–131.99 (1JC–F = 135 Hz), 137, 1554, 156, 158, 167, 182. 19F NMR (400 MHz, CFCL3-d8): −122.9 (m, 1F). IR γmax (cm−1): 3290 (NH), 3228 (NH), 3130 (NH), 1664 (C=O), 1217 (C=S). LC-QTOF MS (m/z): calculated monoisotopic mass: 353.0401, measured monoisotopic mass: 354.0472.
1-(3-Chlorophenyl)-4-(4-fluorophenoxy)acetylthiosemicarbazide (AB3)
Molecular formula: C15H11ClFN3O2S (353.79 g/mol), white solid yield 53% (0.51 g), m.p. 163–165 °C. 1H NMR (600 MHz, DMSO-d6): 4.63 (s, 2H, CH2); 7.04–7.60 (m, 8H, CHar); 9.80 (s, 2H, 2xNH); 10.32 (s, 1H, NH) ppm. 13C NMR (300 MHz, DMSO): 67, 116, 116, 124.66–125.37 (1JC–F = 213 Hz), 126, 130, 133, 141, 154, 156, 158, 168, 181. 19F NMR (400 MHz, CFCL3-d8): −122.8 (m, 1F). IR γmax (cm−1): 3265 (NH), 3186 (NH), 3020 (NH), 1684 (C=O), 1210 (C=S). LC-QTOF MS (m/z): calculated monoisotopic mass: 353.0401, measured monoisotopic mass: 354.0473.
1-(4-Chlorophenyl)-4-(4-fluorophenoxy)acetylthiosemicarbazide (AB4)
Molecular formula: C15H13ClFN3O2S (353.79 g/mol), white solid yield 42% (0.40 g), m.p. 168–169 °C. 1H NMR (600 MHz, DMSO-d6): 4.62 (s, 2H, CH2); 7.05–7.47 (m, 8H, CHar); 9.79 (s, 1H, NH); 9.77 (s, 1H, NH); 10.30 (s, 1H, NH) ppm. 13C NMR (300 MHz, DMSO): 67, 116, 116, 128.07–128.46 (1JC–F = 117 Hz), 129, 138, 154, 155, 158, 167, 181. 19F NMR (400 MHz, CFCL3-d8): −122.8 (m, 1F). LC-QTOF MS (m/z): calculated monoisotopic mass: 353.0401, measured monoisotopic mass: 354.0470.
1-(2,4-Dichlorophenyl)-4-(4-fluorophenoxy)acetylthiosemicarbazide (AB5)
Molecular formula: C15H12Cl2FN3O2S (388.24 g/mol), white solid yiled 63% (0.66 g), m.p. 181–183 °C. 1H NMR (600 MHz, DMSO-d6): 4.62 (s, 2H, CH2); 7.02–7.70 (m, 7H, CHar); 9.59 (s, 1H, NH); 9.91 (s, 1H, NH); 10.37 (s, 1H, NH) ppm.13C NMR (300 MHz, DMSO): 67, 116, 116, 128, 129, 131, 132.12–132.86 (1JC–F = 222 Hz), 136, 1554, 157, 158, 168, 182. 19F NMR (400 MHz, CFCL3-d8): −122.8 (m, 1F). IR γmax (cm−1): 3285 (NH), 3235 (NH), 3116 (NH), 1667 (C=O), 1204 (C=S). LC-QTOF MS (m/z): calculated monoisotopic mass: 387.0011, measured monoisotopic mass: 3.88.0082.
1-(3,4-Dichlorophenyl)-4-(4-fluorophenoxy)acetylthiosemicarbazide (AB6)
Molecural formula: C15H12Cl2FN3O2S (388.24 g/mol), white solid yield 76% (0.80 g), m.p. 176–178 °C. 1H NMR (600 MHz, DMSO-d6): 4.63 (s, 2H, CH2); 7.03–7.81 (m, 7H, CHar); 9.87 (s, 2H, 2xNH); 10.33 (s, 1H, NH) ppm. 13C NMR (300 MHz, DMSO): 67, 116, 116, 126, 127, 130.35–131.06 (1JC–F = 213 Hz), 139, 154, 155, 158, 168, 181. 19F NMR (400 MHz, CFCL3-d8): −122.7 (m, 1F). IR γmax (cm−1): 3287 (NH), 3252 (NH), 3102 (NH), 1666 (C=O), 1212 (C=S). LC-QTOF MS (m/z): calculated monoisotopic mass: 387.0011, measured monoisotopic mass: 388.0082.
3.3. General Procedure for the Synthesis of 1-(4-Fluorophenoxyacetyl)-4-substituted Semicarbazide (AB7–AB12)
2-(4-Fluorophenoxy)acetohydrazide (0.5 g, 2.71 mmol) and the appropriate isocyanate (dissolved in approximately 15–20 mL of ethanol) were mixed in a round-bottomed flask.
The solution was heated for an hour and then cooled to precipitate. The precipitate was filtered off and recrystallized from ethanol. Figure 13 shows the general formula of 4-fluorophenoxyacetylsemicarbazides AB7–AB12. Spectroscopic data of the obtained compounds can be found in the Supplementary Material.
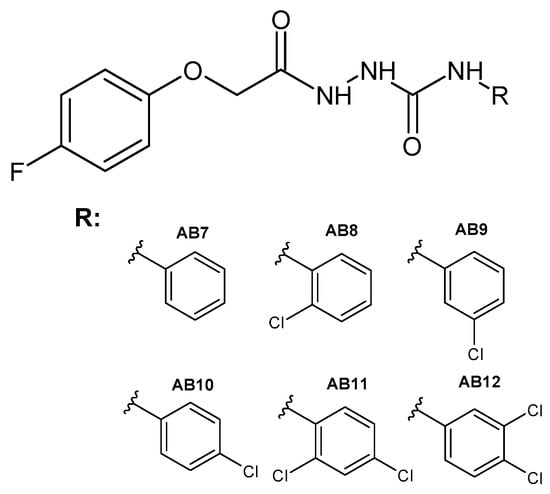
Figure 13.
The general formula of the designed semicarbazides AB7–AB12.
1-(4-Fluorophenoxy)-4-(phenyl)acetylsemicarbazide (AB7)
Summary formula: C15H14FN3O3 (303.29 g/mol), white solid yield 37% (0.30 g), m.p. 222–223 °C. 1H NMR (600 MHz, DMSO-d6): 4.60 (s, 2H, CH2); 6.96–7.44 (m, 9H, CHar); 8.14 (s, 1H, NH); 8.75 (s, 1H, NH); 10.00 (s, 1H, NH) ppm. 13C NMR (300 MHz, DMSO): 67, 116, 119.05–119.72 (1JC–F = 231 Hz), 122, 129, 140, 154, 156, 159, 168, 172. 19F NMR (400 MHz, CFCL3-d8): −122.8 (m, 1F). IR γmax (cm−1): 3268 (NH), 3020 (NH), 2920 (NH), 1682 (C=O), 1644 (C=O). LC-QTOF MS (m/z): calculated monoisotopic mass: 303.1019, measured monoisotopic mass: 304.1085.
1-(2-Chlorophenyl)-4-(4-fluorophenoxy)acetylsemicarbazide (AB8)
Summary formula: C15H13ClFN3O3 (337.73 g/mol), white solid yield 26% (0.23 g), m.p. 225–227 °C. 1H NMR (600 MHz, DMSO-d6): 4.61 (s, 2H, CH2); 7.00–8.05 (m, 8H, CHar); 8.27 (s, 1H, NH); 8.83 (s, 1H, NH); 10.134 (s, 1H, NH) ppm. 13C NMR (300 MHz, DMSO): 67, 116, 121.42–122.24 (1JC–F = 246 Hz), 124, 128, 129, 136, 154, 155, 156, 158, 168, 172. 19F NMR (400 MHz, CFCL3-d8): −122.7 (m, 1F). IR γmax (cm−1): 3308 (NH), 3258 (NH), 2913 (NH), 1679 (C=O), 1657 (C=O). LC-QTOF MS (m/z): calculated monoisotopic mass: 337.0629, measured monoisotopic mass: 338.0696.
1-(3-Chlorophenyl)-4-(4-fluorophenoxy)acetylsemicarbazide (AB9)
Summary formula: C15H13ClFN3O3 (337.73 g/mol), white solid yield 28% (0.25 g), m.p. 212–214 °C. 1H NMR (600 MHz, DMSO-d6): 4.60 (s, 2H, CH2); 7.01–7.67 (m, 8H, CHar); 8.32 (s, 1H, NH); 8.99 (s, 1H, NH); 10.01 (s, 1H, NH) ppm. 13C NMR (300 MHz, DMSO): 67, 116, 116.99–117.94 (1JC–F =285) 122, 130, 133, 141, 154, 155, 156, 158, 168, 172. 19F NMR (400 MHz, CFCL3-d8): −122.8 (m, 1F). IR γmax (cm−1): 3236 (NH), 3060 (NH), 2909 (NH), 1679 (C=O), 1642 (C=O). LC-QTOF MS (m/z): calculated monoisotopic mass: 337.0629, measured monoisotopic mass: 338.0695.
1-(4-Chlorophenyl)-4-(4-fluorophenoxy)acetylsemicarbazide (AB10)
Summary formula: C15H13ClFN3O3 (337.73 g/mol), white solid yield 34% (0.31 g), m.p. 212–214 °C. 1H NMR (600 MHz, DMSO-d6): 4.59 (s, 2H, CH2); 7.00–7.50 (m, 8H, CHar); 8.24 (s, 1H, NH); 8.92 (s, 1H, NH); 10.00 (s, 1H, NH) ppm. 13C NMR (300 MHz, DMSO): 67, 116, 120, 125.50–126.30 (1JC–F =240), 128, 139, 154, 156, 158, 168, 172. 19F NMR (400 MHz, CFCL3-d8): −122.8 (m, 1F). IR γmax (cm−1): 3265 (NH), 3005 (NH), 2910 (NH), 1677 (C=O), 1648 (C=O). LC-QTOF MS (m/z): calculated monoisotopic mass: 337.0629, measured monoisotopic mass: 338.0696.
1-(2,4-Dichlorophenyl)-4-(4-fluorophenoxy)acetylsemicarbazide (AB11)
Summary formula: C15H12Cl2FN3O3 (372.18 g/mol), white solid yield 58% (0.52 g), m.p. 211–213 °C. 1H NMR (600 MHz, DMSO-d6): 4.61 (s, 2H, CH2); 6.9–8.08 (m, 7H, CHar); 8.36 (s, 1H, NH); 8.87 (s, 1H, NH); 10.14 (s, 1H, NH) ppm. 13C NMR (300 MHz, DMSO): 67, 116, 122.11–122.95 (1JC–F =252), 126, 128, 129, 135, 154, 1554, 156, 158, 168, 172. 19F NMR (400 MHz, CFCL3-d8): −122.7 (m, 1F). IR γmax (cm−1): 3308 (NH), 3251 (NH), 2914 (NH), 1681 (C=O), 1655 (C=O). LC-QTOF MS (m/z): calculated monoisotopic mass: 371.0239, measured monoisotopic mass: 372.0306.
1-(3,4-Dichlorophenyl)-4-(4-fluorophenoxy)acetylsemicarbazide (AB12)
Summary formula: C15H12Cl2FN3O3 (372.18 g/mol), white solid yield 40% (0.39 g), m.p. 232–234 °C. 1H NMR (600 MHz, DMSO-d6): 4.59 (s, 2H, CH2); 7.01–7.86 (m, 7H, CHar); 8.42 (s, 1H, NH); 9.11 (s, 1H, NH); 10.02 (s, 1H, NH) ppm. 13C NMR (300 MHz, DMSO): 67, 116, 118.68–119.68 (1JC–F =300), 123, 130, 131, 140, 154, 155, 156, 158, 168, 172. 19F NMR (400 MHz, CFCL3-d8): −122.8 (m, 1F). IR γmax (cm−1): 3256 (NH), 3025 (NH), 2910 (NH), 1679 (C=O), 1645 (C=O). LC-QTOF MS (m/z): calculated monoisotopic mass: 371.0239, measured monoisotopic mass: 372.0304.
3.4. X-Ray Structure Determinations
The experimental and crystal data of AB2 and AB5 are presented in Table 4.

Table 4.
Experimental details of AB2 and AB5 crystals.
Both structures were solved using direct methods with SHELXS-2013/1 [76] and refined by full matrix least squares with SHELXL-2014/7 [76]. The H atoms connected to N atoms were located on differential electron density maps and refined freely. The remaining H atoms positioned geometrically were treated as riding on their parent C atoms with C-H distances of 0.93 Å (aromatic) and 0.97 Å (CH2). All H atoms were refined with isotropic displacement parameters taken as Uiso(H) = 1.5Ueq(C,N). All calculations were performed using WINGX version 2014.1 package [77]. CCDC- 2423249 (AB2) and CCDC- 2423250 (AB5) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge at www.ccdc.cam.ac.uk/conts/retrieving.html (URL accessed on 27 March 2025) [or from the Cambridge Crystallographic Data Centre (CCDC), 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44(0) 1223 336,033; email: deposit@ccdc.cam.ac.uk].
3.5. ADMET Analysis
BOILED-Egg [78] and SwissADME service (Swiss Institute of Bioinformatics 2021): A free web tool to evaluate pharmacokinetics, drug likeness, and the medicinal chemistry friendliness of small molecules [79] was used to predict absorption in the gastrointestinal tract and ADME analysis, respectively. Potential toxicity was assessed using the ProTOX II service [80].
3.6. Biological Evaluation
3.6.1. Cell Culture and Treatment
The research was conducted on one primary cell line: BJ (fibroblast) and four types of cancerous ones: three prostate cancer cell lines (PC-3, DU-145, LNCaP); 3 Melanoma cell lines (MEL-28, A375, G-361); 2 breast cancer cell lines (MCF-7, MDA-MB-23) and one lung cancer cell line (NCI-H1563). All cell lines were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) and were grown in media (Corning, New York, NY, USA) according to ATCC guidelines. The culture media was enriched with 10% heat-inactivated fetal bovine serum (Life Technologies, Carlsbad, CA, USA) and antibiotics: penicillin (100 units) and streptomycin (100 μg/mL) from Sigma-Aldrich (St. Louis, MO, USA). The cells were incubated at 37 °C with 5% CO2 in air.
The cytotoxic analysis of thiosemicarbazides (TSC) (AB1–AB6) and semicarbazides (SC) (AB7–AB12) was conducted in various concentrations. In the case of TSC, it was from 500 to 100 µM, while for SC, it was from 100 to 1 µM (due to its low solubility). The cells were treated with compounds or DMSO as a vehicle in control cultures for 48h. IC50 values were determined using the AAT Bioquest IC50 calculator (AAT Bioquest, Inc., Pleasanton, CA, USA, Quest Graph™ EC50 Calculator. https://www.aatbio.com/tools/ec50-calculator) (accessed on 7 October 2024). According to the lowest obtained IC50 value and the highest Selective Index of tested compounds on selected cancer cell lines, the AB2 (thiosemicarbazide) was chosen for further depth research on LNCaP and G-361 cell lines.
3.6.2. MTT Assay
The cytotoxic activity of the thiosemicarbazides and semicarbazides was measured using the MTT colorimetric assay (MTT Cell Proliferation Assay Kit—Invitrogen, Waltham, MA, USA). The cells were seeded in the 96-well plates at a density of 1.5 × 105 cells/mL. After reaching 70–80% of confluence, the tested compounds in a wide range of concentrations were added. Plates were incubated for 48 h at 37 °C in 5% CO2 air. Then, orange tetrazolium salt (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide)—MTT reagent (0.5 mg/mL) dissolved in PBS was added to each well for 4 h. The MTT solution was then removed from above the water-insoluble purple formazan crystals, which were then dissolved in DMSO (dimethyl sulfoxide, 200 mL/well; POCH, Gliwice, Poland). The absorbance value of obtained solutions was measured using a PowerWave ™ microplate spectrophotometer (Bio-Tek Instruments, Winooski, VT, USA) at a wavelength of 570 nm. Each cytotoxicity assay was repeated three times in triplicates.
3.6.3. Cell Cycle Analysis
Cell cycle analysis was assessed with the NucleoCounter NC-3000 (ChemoMetec, Allerod, Denmark) following the 2-Step Cell Cycle Assay protocol recommended by the manufacturer. The research was performed after 48 h incubation of prostate cancer cells (LNCaP) and melanoma cells (G-361) with AB2 compound in a concentration corresponding to IC50 value. Analysis was performed in line with the previously described methodology.
3.6.4. Apoptosis Detection
Detection of apoptosis in tested cells was conducted with the NucleoCounter NC-3000 (ChemoMetec, Allerod, Denmark) following the Annexin V Apoptosis Assay recommended by the manufacturer. The research was performed after 48 h incubation of prostate cancer cells (LNCaP) and melanoma cells (G-361) with AB2 compound in a concentration corresponding to IC50 value. Analysis was performed in line with the previously described methodology.
3.6.5. Quantitative Real-Time PCR Analysis (qRT-PCR)
The relative gene expression was quantitatively assessed using real-time PCR. The cells were seeded in 6-well plates after reaching 70–80% of confluency the compound AB2 in a concentration corresponding to IC50 was added for 24 h. Next, for cell lysis the 1 mL of TRIzol™ reagent (Invitrogen, Carlsbad, CA, USA) was added. RNA was isolated according to the Chomczynski and Sacchi method [81]. The RNA was reverse transcribed using NG dART RT-PCR reagents (EURx, Gdańsk, Poland) according to the manufacturer’s instructions. Following the manufacturer’s recommendation, the qPCR reaction was conducted in triplicate in a 7500 fast real-time PCR machine (ThermoFisher, Waltham, MA, USA) using Fast SG/ROX qPCR Master Mix (2×) (EURx, Gdansk, Poland). 18SRNA and BACT were used as reference genes. Gene expression was evaluated using qRT-PCR and ΔΔCt. Statistical analysis was conducted using RQ values (RQ = 2∆ΔCt).
The information on the genes and primer sequences used in the research are summarized in Table 5.

Table 5.
Genes and primer sequences used in the research.
3.6.6. Statistical Analysis
Statistical analysis was performed using STATISTICA 13 software (StatSoft, Kraków, Poland). Data were compared using one-way analysis of variance (ANOVA), followed by post hoc multiple comparisons with Tukey’s honest significant difference test (Tukey’s HSD test). The results are presented as mean ± SD. Statistical significance was set at a p-value of <0.05.
3.7. Molecular Docking
The crystal structure of human topoisomerase IIα in complex with DNA and etoposide was downloaded from Protein Data Bank (PDB ID: 5GWK; resolution 3.15 Å) [74]. GOLD Suite v.5.6.3 was used to perform molecular docking analysis [82]. Receptor preparation including addition of hydrogen atoms, extraction of the original ligand, and removal of water molecules from the active site of the protein was performed using GOLD according to default settings. Binding site was determined using the position of native ligand (etoposide). Redocking of native ligand (etoposide) to its binding site with RMSD values of 1.022 Å for ChemScore confirmed the correctness of the selected molecular docking parameters. In docking, it was assumed that ligands are flexible and amino acid residues of the receptor binding site are rigid. In case of simulation runs, default parameter values were used. The selection of active site atoms within 6 Å of the original ligands was chosen as default. ChemScore was chosen as the scoring function to rank the compounds to be studied. Hermes v1.8.2 was used to analyze protein–ligand interactions [82]. The superimpose of redocked etoposide with crystal ligand coordinates from the protein (PDB ID: 5GWK) [74] was analyzed by Discovery Studio Visualizer [83].
4. Conclusions
In this study, six new derivatives of 4-fluorophenoxyacetylthiosemicarbazide and 4-fluorophenoxyacetylsemicarbazide were obtained. X-ray structural analysis was performed for AB2 and AB5, which confirmed the assumed molecular structures and the synthetic route. The compounds studied occurred in the N1-amino/S3-thione/N4-amino/N5-amino/O9-keto tautomeric form and were characterized by the occurrence of inter- and intramolecular hydrogen bonds closely related to the presented tautomeric form in the crystalline state. Hirshfeld analysis showed high similarity of fingerprints and short interactions for AB2 and AB5. Clear differences are observed for the percentage of short contacts H…H and H…Cl/Cl…H. In silico analysis of pharmacokinetic parameters showed that all obtained compounds are absorbed from the gastrointestinal tract and do not cross the blood–brain barrier. There is a visible difference in lipophilicity between the group of thiosemicarbazide and semicarbazide derivatives. Furthermore, semicarbazide in in silico studies had lower toxicity than thiosemicarbazide. All designed compounds meet the main rules of druglikeness. Biological studies showed clear differences in the activity profile of thiosemicarbazide and semicarbazide. The semicarbazide were poorly soluble and had low cytotoxicity to cancer cells, which is why they were omitted from further studies. For thiosemicarbazide derivatives, activity was determined against nine cancer cell lines: prostate cancer (PC-3, DU-145, LNCaP), melanoma (SK-MEL28, A375, G-361), breast cancer (MCF-7, MDA-MB-231), and lung cancer (NCI-H1563). All tested thiosemicarbazides were characterized by moderate activity against the prostate cancer line LNCaP, the highest activity was possessed by AB2 (IC50 = 108.14 μM), bearing a 2-chlorophenyl group. A large population of early apoptotic cells was observed in the LNCaP cell line treated with AB2. Moreover, gene expression analysis showed that AB2 induced an increase in the level of SOD2, CAT, GPX1, CDKN1A and a decrease in the level of TP53, CYCD, ATM, ATR, and BCL-2, which indicates that the tested compound induces oxidative DNA damage and impairs the DNA repair mechanism, which leads to apoptosis. This point to the tested compounds may be potential topoisomerase IIα inhibitors, which was evaluated in molecular docking analysis. Molecular docking analysis confirmed the promising character of AB2, which showed the highest ChemScore value among the thiosemicarbazide tested. Molecular docking indicated that for AB2, the hydrogen bond formed with topoisomerase IIα is created by the hydrogen atom of the 4-fluorophenoxyacetyl group, which highlights the key role of this moiety for biological activity. It is also important to emphasize that replacing the sulfur atom with an oxygen atom in our compounds reduces the cytotoxicity toward cancer cell lines, which is a valuable clue for further potential optimization of the scaffold.
Supplementary Materials
The following supporting information can be downloaded at: www.mdpi.com/article/10.3390/molecules30071576/s1.
Author Contributions
P.K.: investigation, methodology, formal analysis, writing—original draft, writing—review and editing; E.H.: methodology, writing—original draft; Z.K.: formal analysis, writing—original draft; A.B.: methodology, formal analysis; G.A.: investigation; A.K.-P.: investigation, methodology; W.W.: methodology, formal analysis; M.P.: conceptualization, methodology, supervision, project administration, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. These studies were carried out as part of the Statutory Activity DS 16 of the Medical University of Lublin.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available on request.
Acknowledgments
The authors thank Agata Sumara (Department of Bioanalytics, Medical University of Lublin, Poland) for LC/QTOF data acquisition and analysis.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Patel, J.J.; Modh, R.P.; Asamdi, M.; Chikhalia, K.H. Comparative Biological Study between Quinazolinyl–Triazinyl Semicarbazide and Thiosemicarbazide Hybrid Derivatives. Mol. Divers. 2021, 25, 2271–2287. [Google Scholar] [CrossRef] [PubMed]
- Zarrinzadeh, G.; Tajbakhsh, M.; Hosseinzadeh, R.; Khalilzadeh, M.A.; Hosseinzadeh, M. Biological Evaluation and Molecular Docking Study of Euparin and Its Maleic Anhydride and Semicarbazide Derivatives. Polycycl. Aromat. Compd. 2023, 43, 409–420. [Google Scholar] [CrossRef]
- Çelik, B.; Buran Uğur, S.; Baran, M.; Gündüz, M.G.; Keskin, S.; Önder, G.Ö.; Bitgen, N.; Kaya, S.; Doğan, Ş.D. Semicarbazides Carrying Indole Core: Synthesis, Cytotoxicity Evaluation against Human Breast Cancer Cell Lines, and Molecular Modeling Studies. Chem. Biodivers. 2023, 20, e202300609. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Ling, Y.; Li, B.; Li, Y.; Rui, C.; Cui, J.; Shi, Y.; Yang, X. Synthesis and Bioactivity of N-Benzoyl-N’-[5-(2’-Substituted Phenyl)-2-Furoyl] Semicarbazide Derivatives. Molecules 2010, 15, 4267–4282. [Google Scholar] [CrossRef]
- Pavić, K.; Perković, I.; Cindrić, M.; Pranjić, M.; Martin-Kleiner, I.; Kralj, M.; Schols, D.; Hadjipavlou-Litina, D.; Katsori, A.-M.; Zorc, B. Novel Semicarbazides and Ureas of Primaquine with Bulky Aryl or Hydroxyalkyl Substituents: Synthesis, Cytostatic and Antioxidative Activity. Eur. J. Med. Chem. 2014, 86, 502–514. [Google Scholar] [CrossRef]
- Perković, I.; Tršinar, S.; Žanetić, J.; Kralj, M.; Martin-Kleiner, I.; Balzarini, J.; Hadjipavlou-Litina, D.; Katsori, A.M.; Zorc, B. Novel 1-Acyl-4-Substituted Semicarbazide Derivatives of Primaquine-Synthesis, Cytostatic, Antiviral and Antioxidative Studies. J. Enzym. Inhib. Med. Chem. 2013, 28, 601–610. [Google Scholar] [CrossRef]
- Perković, I.; Butula, I.; Kralj, M.; Martin-Kleiner, I.; Balzarini, J.; Hadjipavlou-Litina, D.; Katsori, A.-M.; Zorc, B. Novel NSAID 1-Acyl-4-Cycloalkyl/Arylsemicarbazides and 1-Acyl-5-Benzyloxy/Hydroxy Carbamoylcarbazides as Potential Anticancer Agents and Antioxidants. Eur. J. Med. Chem. 2012, 51, 227–238. [Google Scholar] [CrossRef]
- Szopa, A.; Herbet, M.; Pachuta-Stec, A.; Lachowicz, J.; Pawłowski, K.; Iwan, M.; Jarecka-Florek, D.; Krasińska, O.; Serefko, A.; Poleszak, E.; et al. Evaluation of Developmental Toxicity in Zebrafish Embryos and Antiproliferative Potential against Human Tumor Cell Lines of New Derivatives Containing 4-Nitrophenyl Group. Toxicol. Appl. Pharmacol. 2023, 458, 116325. [Google Scholar] [CrossRef]
- Rane, R.A.; Naphade, S.S.; Bangalore, P.K.; Palkar, M.B.; Shaikh, M.S.; Karpoormath, R. Synthesis of Novel 4-Nitropyrrole-Based Semicarbazide and Thiosemicarbazide Hybrids with Antimicrobial and Anti-Tubercular Activity. Bioorg. Med. Chem. Lett. 2014, 24, 3079–3083. [Google Scholar] [CrossRef]
- Song, M.; Wang, S.; Wang, Z.; Fu, Z.; Zhou, S.; Cheng, H.; Liang, Z.; Deng, X. Synthesis, Antimicrobial and Cytotoxic Activities, and Molecular Docking Studies of N-Arylsulfonylindoles Containing an Aminoguanidine, a Semicarbazide, and a Thiosemicarbazide Moiety. Eur. J. Med. Chem. 2019, 166, 108–118. [Google Scholar] [CrossRef]
- Liu, Y.-H.; Liang, W.-L.; Lee, C.-C.; Tsai, Y.-F.; Hou, W.-C. Antioxidant and Semicarbazide-Sensitive Amine Oxidase Inhibitory Activities of Glucuronic Acid Hydroxamate. Food Chem. 2011, 129, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Manaf, A.; Khan, M.; Zaman, K.; Ali, M.; Alam, F.; Ali, K.M.K.; Ali, B. Synthesis, Characterization and Antioxidant Activities of Semicarbazide and Thiosemicarbazide Derivatives. JCSP 2021, 43, 475. [Google Scholar]
- Sazeli, S.; Nath, A.R.; Ahmad, M.H.; Zulkifli, N.W.M.; Johan, M.R.; Yehye, W.A.; Voon, L.H. Semicarbazide and Thiosemicarbazide Containing Butylated Hydroxytoluene Moiety: New Potential Antioxidant Additives for Synthetic Lubricating Oil. RSC Adv. 2021, 11, 7138–7145. [Google Scholar] [CrossRef] [PubMed]
- Azam, F.; Alkskas, I.A.; Khokra, S.L.; Prakash, O. Synthesis of Some Novel N4-(Naphtha[1,2-d]Thiazol-2-Yl)Semicarbazides as Potential Anticonvulsants. Eur. J. Med. Chem. 2009, 44, 203–211. [Google Scholar] [CrossRef]
- Jain, J.; Kumar, Y.; Stables, J.; Sinha, R. Menthone Semicarbazides and Thiosemicarbazides as Anticonvulsant Agents. Med. Chem. 2010, 6, 44–50. [Google Scholar] [CrossRef]
- Nie, Y.; Zhong, M.; Jiang, Z.; Sun, J.; Gao, Y.; Ding, F.; Li, H.; Zhang, Y.; He, X. Synthesis and Potential Anticonvulsant Activity of New Aryl Sulfonyl Semicarbazide Derivatives. Med. Chem. Res. 2016, 25, 1425–1432. [Google Scholar] [CrossRef]
- Sharma, C.S.; Verma, T.; Singh, H.P.; Kumar, N. Synthesis, Characterization and Preliminary Anticonvulsant Evaluation of Some Flavanone Incorporated Semicarbazides. Med. Chem. Res. 2014, 23, 4814–4824. [Google Scholar] [CrossRef]
- Yogeeswari, P.; Sriram, D.; Saraswat, V.; Ragavendran, J.V.; Kumar, M.M.; Murugesan, S.; Thirumurugan, R.; Stables, J.P. Synthesis and Anticonvulsant and Neurotoxicity Evaluation of N4-Phthalimido Phenyl (Thio) Semicarbazides. Eur. J. Pharm. Sci. 2003, 20, 341–346. [Google Scholar] [CrossRef]
- Pavić, K.; Perković, I.; Gilja, P.; Kozlina, F.; Ester, K.; Kralj, M.; Schols, D.; Hadjipavlou-Litina, D.; Pontiki, E.; Zorc, B. Design, Synthesis and Biological Evaluation of Novel Primaquine-Cinnamic Acid Conjugates of the Amide and Acylsemicarbazide Type. Molecules 2016, 28, 1629. [Google Scholar] [CrossRef]
- Pitucha, M.; Karczmarzyk, Z.; Swatko-Ossor, M.; Wysocki, W.; Wos, M.; Chudzik, K.; Ginalska, G.; Fruzinski, A. Synthesis, In Vitro Screening and Docking Studies of New Thiosemicarbazide Derivatives as Antitubercular Agents. Molecules 2019, 24, 251. [Google Scholar] [CrossRef]
- Kedzierska, E.; Orzelska, J.; Perković, I.; Knežević, D.; Fidecka, S.; Kaiser, M.; Zorc, B. Pharmacological Effects of Primaquine Ureas and Semicarbazides on the Central Nervous System in Mice and Antimalarial Activity in Vitro. Fundam. Clin. Pharmacol. 2016, 30, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Horváth, Á.; Menghis, A.; Botz, B.; Borbély, É.; Kemény, Á.; Tékus, V.; Csepregi, J.Z.; Mócsai, A.; Juhász, T.; Zákány, R.; et al. Analgesic and Anti-Inflammatory Effects of the Novel Semicarbazide-Sensitive Amine-Oxidase Inhibitor SzV-1287 in Chronic Arthritis Models of the Mouse. Sci. Rep. 2017, 7, 39863. [Google Scholar] [CrossRef]
- Wang, E.Y.; Gao, H.; Salter-Cid, L.; Zhang, J.; Huang, L.; Podar, E.M.; Miller, A.; Zhao, J.; O’Rourk, A.; Linnik, M.D. Design, Synthesis, and Biological Evaluation of Semicarbazide-Sensitive Amine Oxidase (SSAO) Inhibitors with Anti-Inflammatory Activity. J. Med. Chem. 2006, 49, 2166–2173. [Google Scholar] [CrossRef]
- Kozyra, P.; Adamczuk, G.; Karczmarzyk, Z.; Matysiak, J.; Podkościelna, B.; Humeniuk, E.; Wysocki, W.; Korga-Plewko, A.; Senczyna, B.; Pitucha, M. Novel Phenoxyacetylthiosemicarbazide Derivatives as Novel Ligands in Cancer Diseases. Toxicol. Appl. Pharmacol. 2023, 475, 116634. [Google Scholar] [CrossRef]
- Kozyra, P.; Korga-Plewko, A.; Karczmarzyk, Z.; Hawrył, A.; Wysocki, W.; Człapski, M.; Iwan, M.; Ostrowska-Leśko, M.; Fornal, E.; Pitucha, M. Potential Anticancer Agents against Melanoma Cells Based on an As-Synthesized Thiosemicarbazide Derivative. Biomolecules 2022, 12, 151. [Google Scholar] [CrossRef]
- Pitucha, M.; Korga-Plewko, A.; Kozyra, P.; Iwan, M.; Kaczor, A.A. 2,4-Dichlorophenoxyacetic Thiosemicarbazides as a New Class of Compounds against Stomach Cancer Potentially Intercalating with DNA. Biomolecules 2020, 10, 296. [Google Scholar] [CrossRef]
- Altalhi, A.A.; Hashem, H.E.; Negm, N.A.; Mohamed, E.A.; Azmy, E.M. Synthesis, Characterization, Computational Study, and Screening of Novel 1-Phenyl-4-(2-Phenylacetyl)-Thiosemicarbazide Derivatives for Their Antioxidant and Antimicrobial Activities. J. Mol. Liq. 2021, 333, 115977. [Google Scholar] [CrossRef]
- Lahari, K.; Sundararajan, R. Design and Synthesis of Novel Isatin Derivatives as Potent Analgesic, Anti-Inflammatory and Antimicrobial Agents. J. Chem. Sci. 2020, 132, 94. [Google Scholar] [CrossRef]
- Özcan, E.; Vagolu, S.K.; Gündüz, M.G.; Stevanovic, M.; Kökbudak, Z.; Tønjum, T.; Nikodinovic-Runic, J.; Çetinkaya, Y.; Doğan, Ş.D. Novel Quinoline-Based Thiosemicarbazide Derivatives: Synthesis, DFT Calculations, and Investigation of Antitubercular, Antibacterial, and Antifungal Activities. ACS Omega 2023, 8, 40140–40152. [Google Scholar] [CrossRef]
- Cihan-Üstündağ, G.; Gürsoy, E.; Naesens, L.; Ulusoy-Güzeldemirci, N.; Çapan, G. Synthesis and Antiviral Properties of Novel Indole-Based Thiosemicarbazides and 4-Thiazolidinones. Bioorg. Med. Chem. 2016, 24, 240–246. [Google Scholar] [CrossRef]
- Moharana, A.K.; Dash, R.N.; Subudhi, B.B. Thiosemicarbazides: Updates on Antivirals Strategy. Mini Rev. Med. Chem. 2020, 20, 2135–2152. [Google Scholar] [CrossRef] [PubMed]
- Bulut, N.; Kocyigit, U.M.; Gecibesler, I.H.; Dastan, T.; Karci, H.; Taslimi, P.; Durna Dastan, S.; Gulcin, I.; Cetin, A. Synthesis of Some Novel Pyridine Compounds Containing Bis-1,2,4-Triazole/Thiosemicarbazide Moiety and Investigation of Their Antioxidant Properties, Carbonic Anhydrase, and Acetylcholinesterase Enzymes Inhibition Profiles. J. Biochem. Mol. Toxicol. 2018, 32, e22006. [Google Scholar] [CrossRef]
- Kulandasamy, R.; Adhikari, A.V.; Taranalli, A.; Venkataswamy, T. New Hydrazides and Thiosemicarbazides Derived from Ethylenedioxythiophene as Potential Anticonvulsants. Phosphorus Sulfur Silicon Relat. Elem. 2010, 185, 1358–1368. [Google Scholar] [CrossRef]
- Nevagi, R.J.; Dhake, A.S.; Narkhede, H.I.; Kaur, P. Design, Synthesis and Biological Evaluation of Novel Thiosemicarbazide Analogues as Potent Anticonvulsant Agents. Bioorg. Chem. 2014, 54, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Abhale, Y.K.; Shinde, A.; Deshmukh, K.K.; Nawale, L.; Sarkar, D.; Mhaske, P.C. Synthesis, Antitubercular and Antimicrobial Potential of Some New Thiazole Substituted Thiosemicarbazide Derivatives. Med. Chem. Res. 2017, 26, 2557–2567. [Google Scholar] [CrossRef]
- Parks, R.E.; Kidder, G.W.; Dewey, V.C. Thiosemicarbazide Toxicity in Mice. Proc. Soc. Exp. Biol. Med. 1952, 79, 287–289. [Google Scholar] [CrossRef]
- Chandra, G.; Singh, D.V.; Mahato, G.K.; Patel, S. Fluorine-a Small Magic Bullet Atom in the Drug Development: Perspective to FDA Approved and COVID-19 Recommended Drugs. Chem. Zvesti 2023, 77, 4085–4106. [Google Scholar] [CrossRef]
- Morgenthaler, M.; Schweizer, E.; Hoffmann-Röder, A.; Benini, F.; Martin, R.E.; Jaeschke, G.; Wagner, B.; Fischer, H.; Bendels, S.; Zimmerli, D.; et al. Predicting and Tuning Physicochemical Properties in Lead Optimization: Amine Basicities. ChemMedChem 2007, 2, 1100–1115. [Google Scholar] [CrossRef]
- Inoue, M.; Sumii, Y.; Shibata, N. Contribution of Organofluorine Compounds to Pharmaceuticals. ACS Omega 2020, 5, 10633–10640. [Google Scholar] [CrossRef]
- Pal, S.; Chandra, G.; Patel, S.; Singh, S. Fluorinated Nucleosides: Synthesis, Modulation in Conformation and Therapeutic Application. Chem. Rec. 2022, 22, e202100335. [Google Scholar] [CrossRef]
- Shet, H.; Sahu, R.; Sanghvi, Y.S.; Kapdi, A.R. Strategies for the Synthesis of Fluorinated Nucleosides, Nucleotides and Oligonucleotides. Chem. Rec. 2022, 22, e202200066. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.M.; Shu, Y.-Z.; Zhuo, X.; Meanwell, N.A. Metabolic and Pharmaceutical Aspects of Fluorinated Compounds. J. Med. Chem. 2020, 63, 6315–6386. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, C.; Amata, S.; Pibiri, I.; Pace, A.; Buscemi, S.; Palumbo Piccionello, A. FDA-Approved Fluorinated Heterocyclic Drugs from 2016 to 2022. Int. J. Mol. Sci. 2023, 24, 7728. [Google Scholar] [CrossRef]
- Gillis, E.P.; Eastman, K.J.; Hill, M.D.; Donnelly, D.J.; Meanwell, N.A. Applications of Fluorine in Medicinal Chemistry. J. Med. Chem. 2015, 58, 8315–8359. [Google Scholar] [CrossRef]
- Purser, S.; Moore, P.R.; Swallow, S.; Gouverneur, V. Fluorine in Medicinal Chemistry. Chem. Soc. Rev. 2008, 37, 320–330. [Google Scholar] [CrossRef]
- Zhang, C. Fluorine in Medicinal Chemistry: In Perspective to COVID-19. ACS Omega 2022, 7, 18206–18212. [Google Scholar] [CrossRef]
- Kozyra, P.; Pitucha, M. Terminal Phenoxy Group as a Privileged Moiety of the Drug Scaffold—A Short Review of Most Recent Studies 2013–2022. Int. J. Mol. Sci. 2022, 23, 8874. [Google Scholar] [CrossRef]
- Kozyra, P.; Wysocki, W.; Walczak, Ł.J.; Herbet, M.; Karczmarzyk, Z.; Pitucha, M. Application of in Silico Methods to Assess the Anticancer Potential of New Derivatives of 4-Fluorophenoxythiosemicarbazide Derivatives. J. Mol. Struct. 2025, 1326, 141090. [Google Scholar] [CrossRef]
- Allen, F.H.; Kennard, O.; Watson, D.G.; Brammer, L.; Orpen, A.G.; Taylor, R. Tables of Bond Lengths Determined by X-Ray and Neutron Diffraction. Part 1. Bond Lengths in Organic Compounds. J. Chem. Soc. Perkin Trans. 2 1987, 12, S1–S19. [Google Scholar] [CrossRef]
- Bernstein, J.; Davis, R.E.; Shimoni, L.; Chang, N.-L. Patterns in Hydrogen Bonding: Functionality and Graph Set Analysis in Crystals. Angew. Chem. Int. Ed. Engl. 1995, 34, 1555–1573. [Google Scholar] [CrossRef]
- Turner, M.J.; Mckinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer; Version 17; University of Western Australia: Crawley, Australia, 2017; Available online: https://scholar.google.com/scholar_lookup?hl=en&publication_year=2017&author=M.+J.+Turner&author=J.+J.+McKinnon&author=S.+K.+Wolff&author=D.+J.+Grimwood&author=P.+R.+Spackman&author=D.+Jayatilaka&author=M.+A.+Spackman&title=CrystalExplorer17 (accessed on 27 March 2025).
- Guan, L.; Yang, H.; Cai, Y.; Sun, L.; Di, P.; Li, W.; Liu, G.; Tang, Y. ADMET-Score—A Comprehensive Scoring Function for Evaluation of Chemical Drug-Likeness. Medchemcomm 2018, 10, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Czylkowska, A.; Lanka, S.; Szczesio, M.; Czarnecka, K.; Szymański, P.; Pitucha, M.; Drabińska, A.; Camargo, B.C.; Szczytko, J. New Derivatives of 5-((1-Methyl-Pyrrol-2-Yl) Methyl)-4-(Naphthalen-1-Yl)-1,2,4-Triazoline-3-Thione and Its Coordination Compounds with Anticancer Activity. Int. J. Mol. Sci. 2022, 23, 9162. [Google Scholar] [CrossRef] [PubMed]
- Martin, Y.C. A Bioavailability Score. J. Med. Chem. 2005, 48, 3164–3170. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings. Adv. Drug Deliv. Rev. 2012, 64, 4–17. [Google Scholar] [CrossRef]
- Ghose, A.K.; Viswanadhan, V.N.; Wendoloski, J.J. A Knowledge-Based Approach in Designing Combinatorial or Medicinal Chemistry Libraries for Drug Discovery. 1. A Qualitative and Quantitative Characterization of Known Drug Databases. J. Comb. Chem. 1999, 1, 55–68. [Google Scholar] [CrossRef]
- Egan, W.J.; Merz, K.M.; Baldwin, J.J. Prediction of Drug Absorption Using Multivariate Statistics. J. Med. Chem. 2000, 43, 3867–3877. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular Properties That Influence the Oral Bioavailability of Drug Candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Muegge, I.; Heald, S.L.; Brittelli, D. Simple Selection Criteria for Drug-like Chemical Matter. J. Med. Chem. 2001, 44, 1841–1846. [Google Scholar] [CrossRef]
- Malki, A.; Elbayaa, R.Y.; Ashour, H.M.A.; Loffredo, C.A.; Youssef, A.M. Novel Thiosemicarbazides Induced Apoptosis in Human MCF-7 Breast Cancer Cells via JNK Signaling. J. Enzyme Inhib. Med. Chem. 2015, 30, 786–795. [Google Scholar] [CrossRef]
- Chen, R.; Huo, L.; Jaiswal, Y.; Huang, J.; Zhong, Z.; Zhong, J.; Williams, L.; Xia, X.; Liang, Y.; Yan, Z. Design, Synthesis, Antimicrobial, and Anticancer Activities of Acridine Thiosemicarbazides Derivatives. Molecules 2019, 24, 2065. [Google Scholar] [CrossRef]
- Zhang, H.-J.; Qian, Y.; Zhu, D.-D.; Yang, X.-G.; Zhu, H.-L. Synthesis, Molecular Modeling and Biological Evaluation of Chalcone Thiosemicarbazide Derivatives as Novel Anticancer Agents. Eur. J. Med. Chem. 2011, 46, 4702–4708. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, O.M.; Akinloye, O.A. First Line Defence Antioxidants-Superoxide Dismutase (SOD), Catalase (CAT) and Glutathione Peroxidase (GPX): Their Fundamental Role in the Entire Antioxidant Defence Grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Abuetabh, Y.; Wu, H.H.; Chai, C.; Al Yousef, H.; Persad, S.; Sergi, C.M.; Leng, R. DNA Damage Response Revisited: The P53 Family and Its Regulators Provide Endless Cancer Therapy Opportunities. Exp. Mol. Med. 2022, 54, 1658–1669. [Google Scholar] [CrossRef]
- Abbas, T.; Dutta, A. P21 in Cancer: Intricate Networks and Multiple Activities. Nat. Rev. Cancer 2009, 9, 400–414. [Google Scholar] [CrossRef]
- Foo, T.K.; Vincelli, G.; Huselid, E.; Her, J.; Zheng, H.; Simhadri, S.; Wang, M.; Huo, Y.; Li, T.; Yu, X.; et al. ATR/ATM-Mediated Phosphorylation of BRCA1 T1394 Promotes Homologous Recombinational Repair and G2-M Checkpoint Maintenance. Cancer Res. 2021, 81, 4676–4684. [Google Scholar] [CrossRef]
- Qian, S.; Wei, Z.; Yang, W.; Huang, J.; Yang, Y.; Wang, J. The Role of BCL-2 Family Proteins in Regulating Apoptosis and Cancer Therapy. Front. Oncol. 2022, 12, 985363. [Google Scholar] [CrossRef]
- Davalli, P.; Marverti, G.; Lauriola, A.; D’Arca, D. Targeting Oxidatively Induced DNA Damage Response in Cancer: Opportunities for Novel Cancer Therapies. Oxidative Med. Cell. Longev. 2018, 2018, 2389523. [Google Scholar] [CrossRef]
- Barzilai, A.; Yamamoto, K.-I. DNA Damage Responses to Oxidative Stress. DNA Repair 2004, 3, 1109–1115. [Google Scholar] [CrossRef]
- Maynard, S.; Schurman, S.H.; Harboe, C.; de Souza-Pinto, N.C.; Bohr, V.A. Base Excision Repair of Oxidative DNA Damage and Association with Cancer and Aging. Carcinogenesis 2009, 30, 2–10. [Google Scholar] [CrossRef]
- Hongo, H.; Kosaka, T.; Suzuki, Y.; Mikami, S.; Fukada, J.; Oya, M. Topoisomerase II Alpha Inhibition Can Overcome Taxane-Resistant Prostate Cancer through DNA Repair Pathways. Sci. Rep. 2021, 11, 22284. [Google Scholar] [CrossRef]
- Linka, R.M.; Porter, A.C.G.; Volkov, A.; Mielke, C.; Boege, F.; Christensen, M.O. C-Terminal Regions of Topoisomerase II α and II β Determine Isoform-Specific Functioning of the Enzymes in Vivo. Nucleic Acids Res. 2007, 35, 3810–3822. [Google Scholar] [CrossRef] [PubMed]
- Schaefer-Klein, J.L.; Murphy, S.J.; Johnson, S.H.; Vasmatzis, G.; Kovtun, I.V. Topoisomerase 2 Alpha Cooperates with Androgen Receptor to Contribute to Prostate Cancer Progression. PLoS ONE 2015, 10, e0142327. [Google Scholar] [CrossRef]
- Wang, Y.-R.; Chen, S.-F.; Wu, C.-C.; Liao, Y.-W.; Lin, T.-S.; Liu, K.-T.; Chen, Y.-S.; Li, T.-K.; Chien, T.-C.; Chan, N.-L. Producing Irreversible Topoisomerase II-Mediated DNA Breaks by Site-Specific Pt(II)-Methionine Coordination Chemistry. Nucleic Acids Res. 2017, 45, 10861–10871. [Google Scholar] [CrossRef] [PubMed]
- CrysAlisPro. Agilent Technologies. Version 1.171.37.35h, 2015 (Release 09-02-2015 CrysAlis171.NET), (Compiled Feb 9 2015, 16: 28: 20); Agilent Technologies: Oxfordshire, UK, 2015. [Google Scholar]
- Sheldrick, G.M. A Short History of SHELX. Acta Cryst. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for Windows: An Update. J. Appl. Cryst. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Daina, A.; Zoete, V. A BOILED-Egg To Predict Gastrointestinal Absorption and Brain Penetration of Small Molecules. ChemMedChem 2016, 11, 1117–1121. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Banerjee, P.; Eckert, A.O.; Schrey, A.K.; Preissner, R. ProTox-II: A Webserver for the Prediction of Toxicity of Chemicals. Nucleic Acids Res. 2018, 46, W257–W263. [Google Scholar] [CrossRef]
- Chomczynski, P.; Sacchi, N. Single-Step Method of RNA Isolation by Acid Guanidinium Thiocyanate-Phenol-Chloroform Extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- Jones, G.; Willett, P.; Glen, R.C.; Leach, A.R.; Taylor, R. Development and Validation of a Genetic Algorithm for Flexible Docking1. J. Mol. Biol. 1997, 267, 727–748. [Google Scholar] [CrossRef]
- BIOVIA. Dassault Systèmes, Discovery Studio Visualizer, V21. 1.0.20298; Dassault Systèmes: San Diego, CA, USA, 2021. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).