A Bifunctional Fluorescence Probe for the Detection of Hypochlorous Acid and Viscosity in Living Cells and Zebrafish
Abstract
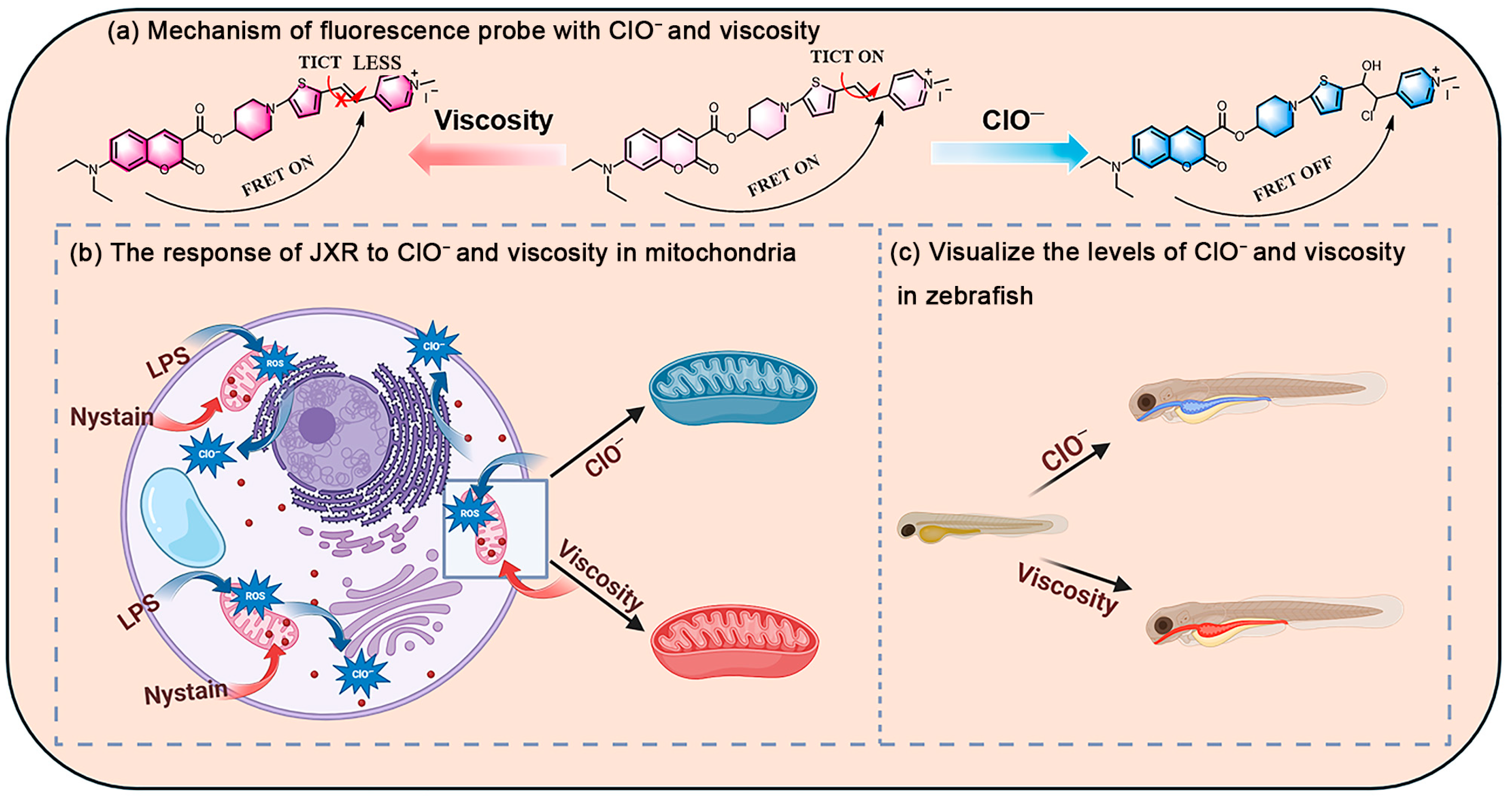
1. Introduction
2. Result and Discussion
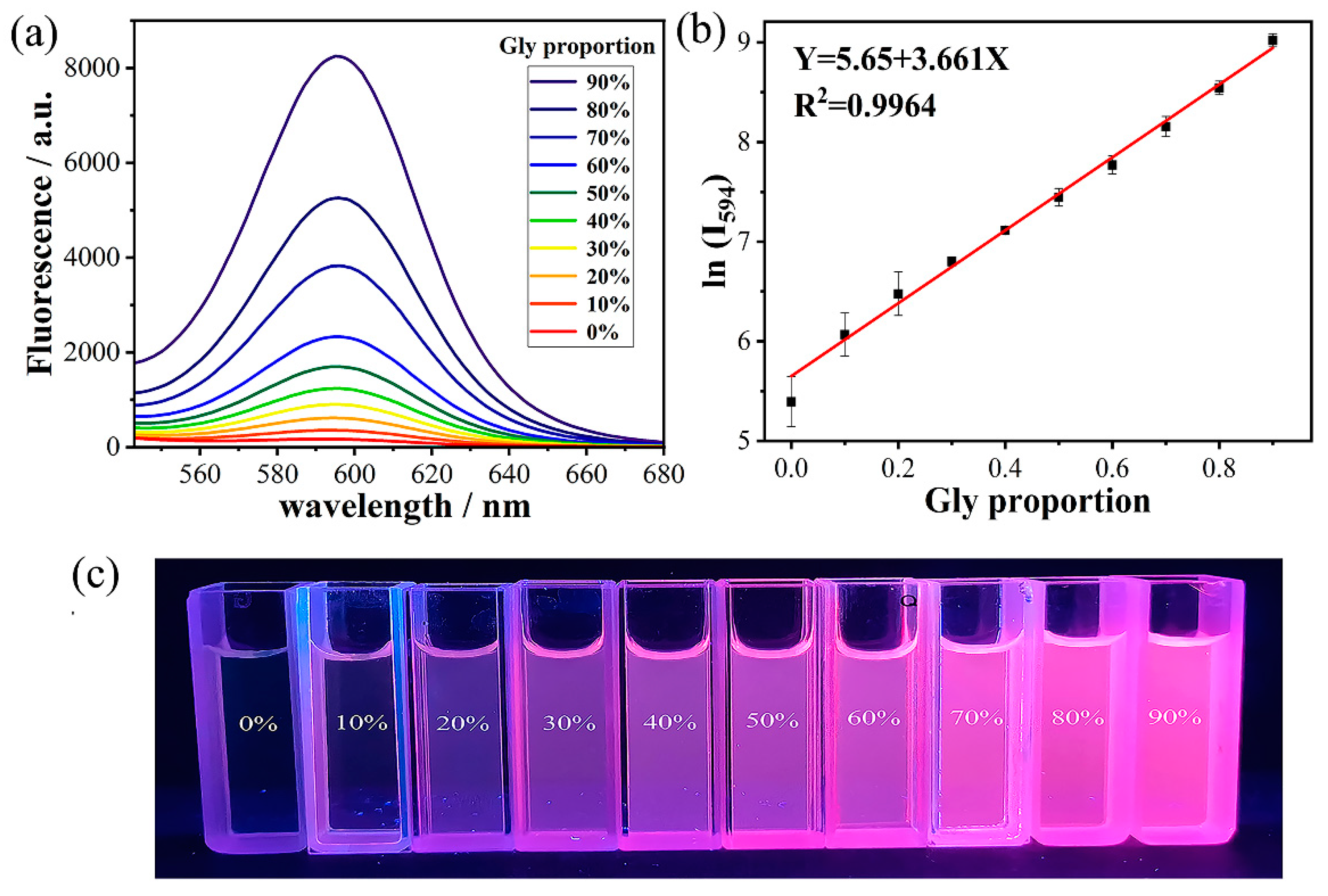
2.1. Response Properties
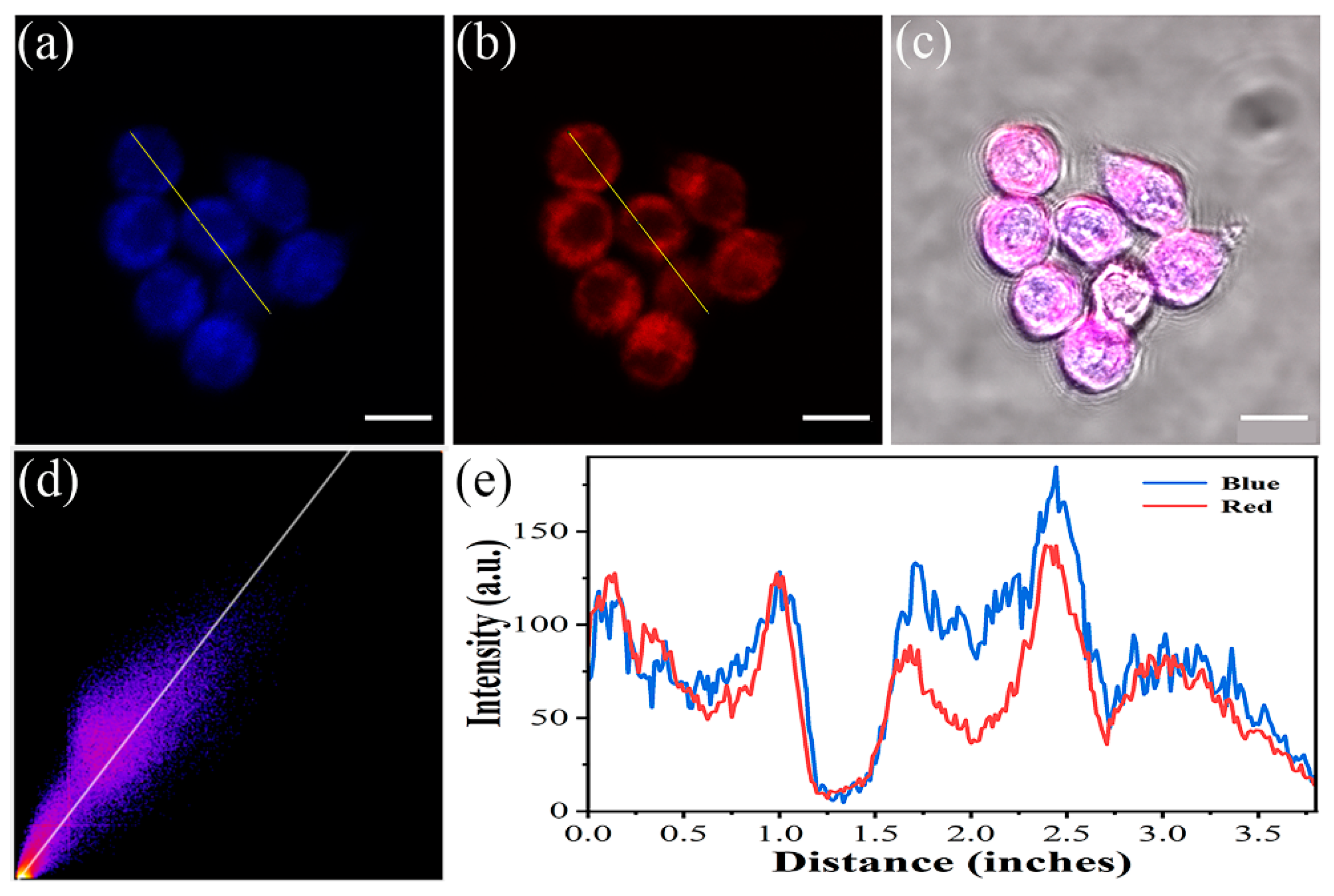
2.2. Fluorescence Imaging in Living Cells
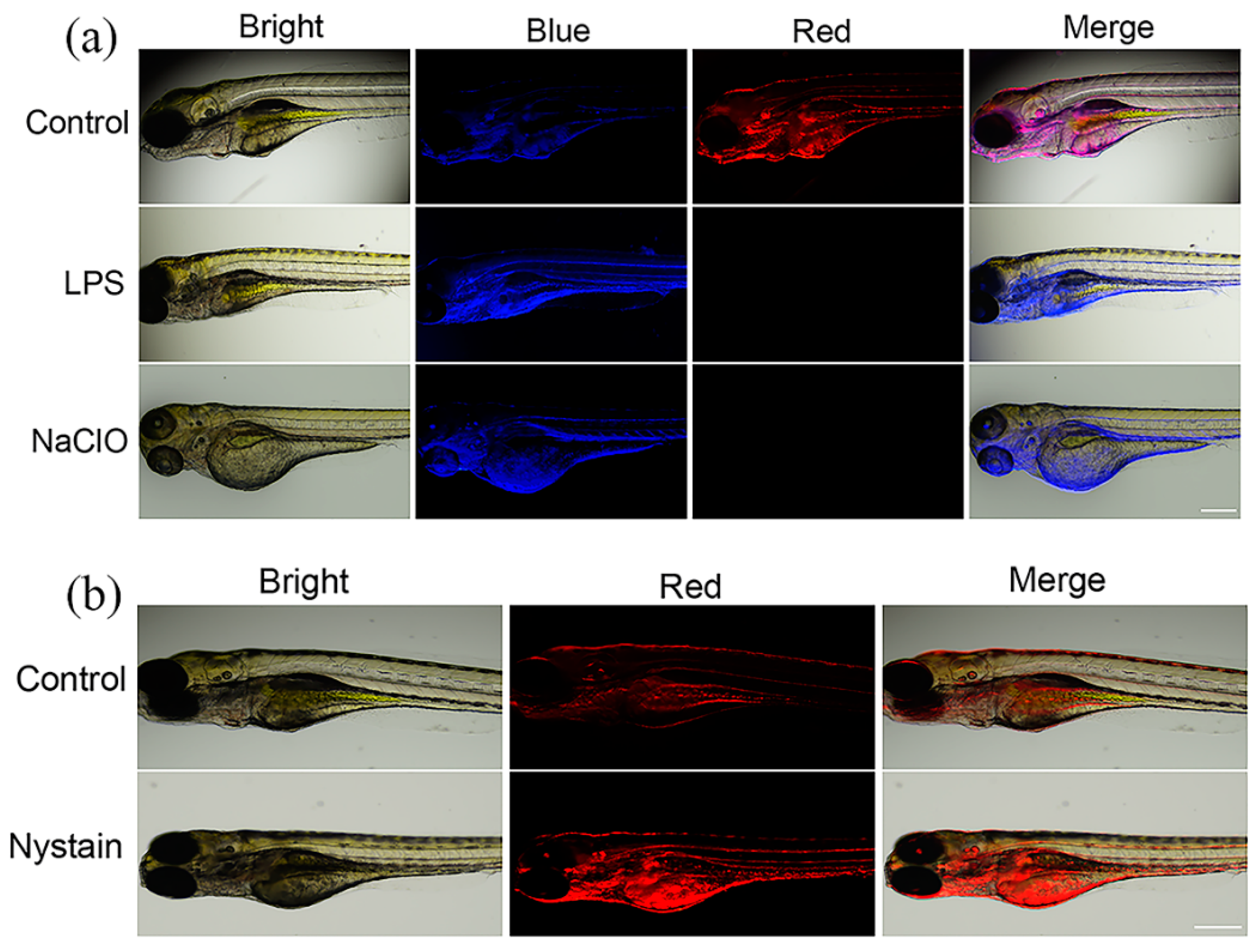
2.3. Bioimaging in Zebrafish
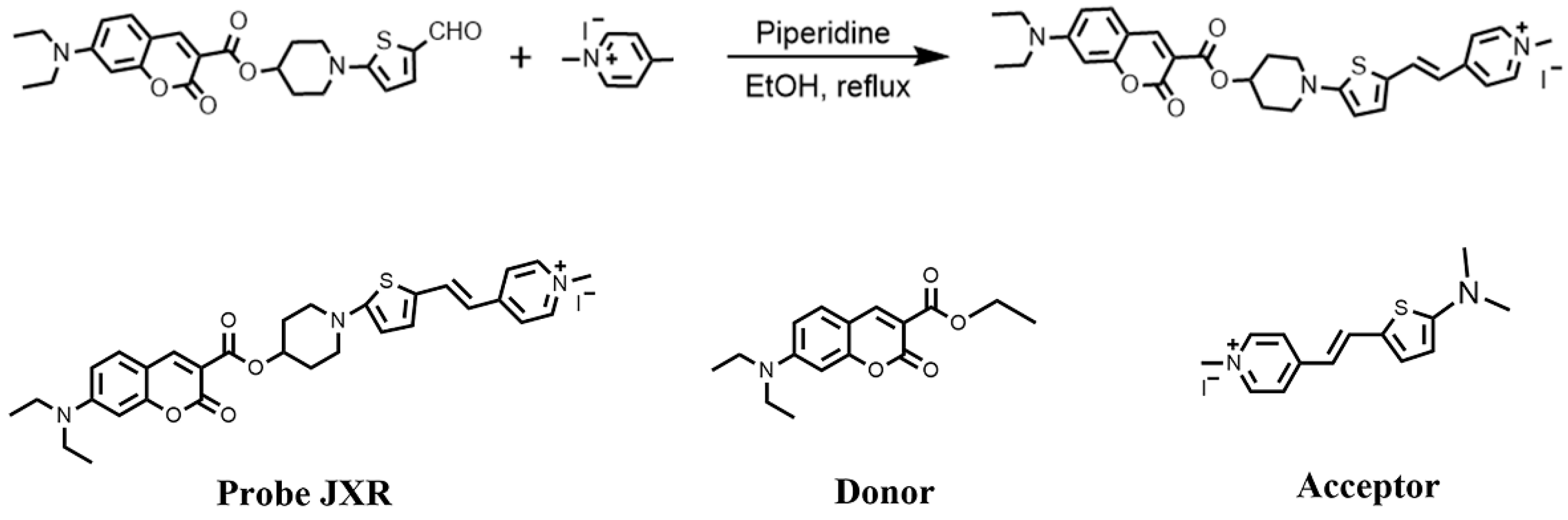
3. Experiments
3.1. Reagents and Instruments
3.2. Spectral Measurements and Cell Imaging
3.3. Synthesis of JXR
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tang, X.; Zhou, B.; Su, Z.; Wu, R.; Qiu, X.; Liu, L. A Cyanine Based Fluorescent Probe for Detecting Hypochlorite in Vitro and in Vivo. Spectrochim. Acta A 2024, 322, 124826. [Google Scholar] [CrossRef] [PubMed]
- Winterbourn, C.C. Reconciling the chemistry and biology of reactive oxygen species. Nat. Chem. Biol. 2008, 4, 278–286. [Google Scholar] [CrossRef]
- Liao, Y.; Yi, T.; Su, X.; Chen, S.; Lu, M.; Huang, X.; Yang, Y.; Qin, X.; Tang, C.; Zhao, Y.; et al. A Mitochondria-Targeted Fluorescence/Photoacoustic Dual-Modality Imaging Probe for Hypochlorous Acid-Related Inflammatory Responses in Vivo. J. Photochem. Photobiol. A Chem. 2025, 462, 116232. [Google Scholar] [CrossRef]
- Wang, Y.; Li, W.; Zhou, P. Sensing Mechanism of the Benzo-Bodipy Based Fluorescent Probe for Hypochlorous Acid Detection: Invalidity of Photoinduced Electron Transfer. Spectrochim. Acta A 2024, 323, 124923. [Google Scholar]
- Zhou, X.; Gao, S.; Yue, M.; Zhu, S.; Liu, Q.; Zhao, X. Recent Advances in Analytical Methods of Oxidative Stress Biomarkers Induced by Environmental Pollutant Exposure. TrAC Trend Anal. Chem. 2023, 160, 116978. [Google Scholar] [CrossRef]
- Xia, W.; Yuan, Y.; Shen, S.; Zhu, Y.; Wang, Y.; Hou, Y.; Huang, B.; Tian, M.; Feng, F. A Novel Diaminomaleonitrile-Based Fluorescent Probe for the Fast Detection of Hypochlorite in Water Samples and Foods. J. Food Compos. Anal. 2024, 128, 106053. [Google Scholar] [CrossRef]
- Chen, J.; Wang, F.; Yang, X.; Yuan, M.; Liu, H.; Xie, X.; Xu, K. A Novel Fluorescent Probe for Cascade Detection of Hydrogen Sulfide and Hypochlorous Acid and Its Application in Bioimaging. Talanta 2024, 270, 125649. [Google Scholar] [CrossRef]
- Liang, Y.; Zhang, C.; Meng, Z.; Gong, S.; Tian, J.; Li, R.; Wang, Z.; Wang, S. A Novel Lysosome-Targeting BODIPY-Based Fluorescent Probe with Two Near-Infrared Channel Signals for Ratiometric Detection of HClO and Its Application in Diabetes Mice Model. Sens. Actuators B Chem. 2024, 417, 136044. [Google Scholar] [CrossRef]
- Ye, Y.; Liu, C.; Wang, L.; Shen, X.; Chen, H. A Dual-Positive Charges Strategy for Sensitive and Quantitative Detection of Mitochondrial SO2 in Cancer Cells and Tumor Tissue. Talanta 2022, 249, 123699. [Google Scholar] [CrossRef]
- Liu, X.; Yan, M.; Chen, Z.; Zhang, B.; Yao, N.; Zhao, S.; Zhao, X.; Zhang, T.; Hai, G. A Dual-Site Multifunctional Fluorescent Probe for Selective Detection of Endogenous H2O2 and SO2 Derivatives Based on ICT Process and Its Bioimaging Application. Spectrochim. Acta A 2023, 286, 121955. [Google Scholar]
- Gyasi, Y.I.; Pang, Y.P.; Li, X.R.; Gu, J.X.; Cheng, X.J.; Liu, J.; Xu, T.; Liu, Y. Biological applications of near infrared fluorescence dye probes in monitoring Alzheimer’s disease. Eur. J. Med. Chem. 2020, 187, 111982. [Google Scholar]
- Liu, T.; Wang, S.; Xu, J.; Miao, J.; Zhao, B.; Lin, Z. FRET-Based Fluorescent Probe with Favorable Water Solubility for Simultaneous Detection of SO2 Derivatives and Viscosity. Talanta 2023, 256, 124302. [Google Scholar] [CrossRef]
- Bera, K.; Kiepas, A.; Godet, I.; Li, Y.; Mehta, P.; Ifemembi, B.; Paul, C.D.; Sen, A.; Serra, S.A.; Stoletov, K.; et al. Extracellular Fluid Viscosity Enhances Cell Migration and Cancer Dissemination. Nature 2022, 611, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Xing, H.; Meng, L.; Zhao, Y.; Zeng, Q.; Xiao, Q.; Li, N.; Xue, P.; Luo, H. Engineering HClO Ratiometric Fluorescent Probe by Inducing Molecular Aggregation to Suppress TICT Formation for Monitoring Drug-Induced Liver Injury. Anal. Chem. 2025, 97, 220–228. [Google Scholar]
- Mei, Y.; Hai, Z.; Li, Z.; Rong, K.; Tang, W.; Song, Q. Dual-Responsive Near-Infrared BODIPY-Based Fluorescent Probe for the Detection of F– and HClO in Organisms. Anal. Chem. 2024, 96, 3802–3809. [Google Scholar]
- Dou, K.; Huang, W.; Xiang, Y.; Li, S.; Liu, Z. Design of Activatable NIR-II Molecular Probe for In Vivo Elucidation of Disease-Related Viscosity Variations. Anal. Chem. 2020, 92, 4177–4181. [Google Scholar] [PubMed]
- Yu, X.; Huang, Y.; Tao, Y.; Fan, L.; Zhang, Y. Mitochondria-targetable small molecule fluorescent probes for the detection of cancer-associated biomarkers: A review. Anal. Chim. Acta. 2024, 1289, 342060. [Google Scholar] [PubMed]
- Deng, Y.; Feng, S.; Xia, Q.; Gong, S.; Feng, G. A Novel Reaction-Based Fluorescence Probe for Rapid Imaging of HClO in Live Cells, Animals, and Injured Liver Tissues. Talanta 2020, 215, 120901. [Google Scholar] [CrossRef]
- Ding, G.; Zuo, Y.; Gai, F.; Wang, X.; Gou, Z.; Lin, W. A POSS-Assisted Fluorescent Probe for the Rapid Detection of HClO in Mitochondria with a Large Emission Wavelength in Dual Channels. J. Mater. Chem. B 2021, 9, 6836–6843. [Google Scholar] [CrossRef]
- Fan, G.; Wang, N.; Zhang, J.; Ji, X.; Qin, S.; Tao, Y.; Zhao, W. BODIPY-Based near-Infrared Fluorescent Probe for Diagnosis Drug-Induced Liver Injury via Imaging of HClO in Cells and in Vivo. Dyes Pigm. 2022, 199, 110073. [Google Scholar] [CrossRef]
- Cao, Y.; Gu, J.; Chen, Z.; Gao, J.; Yang, J.; Wu, W.; Fang, M.; Li, Q.; Liu, B.; Li, Z. HClO-Activated Near-Infrared Chemiluminescent Probes with a Malononitrile Group for In-Vivo Imaging. Adv. Mater. 2024, 37, 2408941. [Google Scholar]
- Chen, J.; Wang, M.; Yang, X.; Yuan, M.; Liu, H.; Cheng, P.; Xu, K. Specific and Sequential Detection of Hydrogen Sulfide and Hypochlorous Acid Based on a Ring-Forming Reaction and Self-Assembly. Spectrochim. Acta A 2024, 405, 135313. [Google Scholar] [CrossRef]
- Cho, M.; Nguyen, V.N.; Yoon, J. Simultaneous Detection of Hypochlorite and Singlet Oxygen by a Thiocoumarin-Based Ratiometric Fluorescent Probe. ACS Meas. Sci. Au 2022, 2, 219–223. [Google Scholar]
- Sun, X.; Wang, J.; Shang, Z.; Wang, H.; Wang, Y.; Shuang, S. A triphenylamine-thiophene-based fluorescent probe for the dual-channel detection and imaging of hypochlorite and viscosity in live cells. J. Mol. Struct. 2024, 402, 124788. [Google Scholar]
- Cheng, Q.; Sun, J.; Wang, B.; Ding, A.; Sun, W.; Yang, F.; Zhang, J. Detecting mitochondrial hypochlorous acid and viscosity in atherosclerosis models via NIR fluorescent probes. Bioorg. Chem. 2025, 156, 108191. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, S.; Yan, J.; Xu, P.; Li, B.; Zhang, Y.; Li, J.; Wu, S. A dual-emission fluorescent probe for simultaneous detection of singlet oxygen and hypochlorous acid in lipid droplets. Sens. Actuators B Chem. 2024, 412, 135813. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, W.; Liu, Y.; Du, H.; Zhang, J.; Zhang, C.; Shuang, S.; Dong, C. Dual-response fluorescent probe for lipid droplets and hypochlorous acid and imaging study in inflammation and atheromatous plaques. J. Mol. Struct. 2025, 1321, 139994. [Google Scholar] [CrossRef]
- Huang, P.; Zhang, W.; Wang, J.; Huo, F.; Yin, C. Rapid and Specific Fluorescent Probe Visualizes Dynamic Correlation of Cys and HClO in OGD/R. Chin. Chem. Lett. 2025, 36, 109778. [Google Scholar]
- Shangguan, L.; Wang, J.; Qian, X.; Wu, Y.; Liu, Y. Mitochondria-targeted ratiometric chemdosimeter to detect hypochlorite acid for monitoring the drug-damaged liver and kidney. Anal. Chem. 2022, 94, 11881–11888. [Google Scholar]
- Yao, L.; Song, H.; Yin, C.; Huo, F. An ICT-Switched Fluorescent Probe for Visualizing Lipid and HClO in Lipid Droplets during Ferroptosis. Chem. Commun. 2024, 60, 835–838. [Google Scholar]
- Hao, T.; Yu, J.; Wu, Z.; Jiang, J.; Gong, L.; Wang, B.; Guo, H.; Zhao, H.; Lu, B.; Engelender, S.; et al. Hypoxia-Reprogramed Megamitochondrion Contacts and Engulfs Lysosome to Mediate Mitochondrial Self-Digestion. Nat. Commun. 2023, 14, 4105. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Xiao, J.; Li, F.; Liu, K.; Yang, M.; Liu, J. A PET Based Colorimetric/Fluorescent Dual-Signal Probe for Selective Detection of Hypochlorite in Real Water Samples. J. Mol. Struct. 2025, 1322, 140568. [Google Scholar]
- Li, X.; Wang, H.; Zhang, Y.; Cao, Q.; Chen, Y. A GSH-Responsive PET-Based Fluorescent Probe for Cancer Cells Imaging. Chin. Chem. Lett. 2021, 32, 1541–1544. [Google Scholar]
- Zhu, J.; Miao, C.; Wang, X. Designing a Turn-on Ultrasensitive Fluorescent Probe Based on ICT-FRET for Detection and Bioimaging of Hypochlorous Acid. Spectrochim. Acta A 2023, 294, 122546. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Yuan, Y.; Zhao, B. An Imidazo [1,5-α] Pyridines-Based Ratiometric Fluorescent Probe for Sensing Sulfur Dioxide Derivatives in Real Samples Based on a FRET Mechanism. Spectrochim. Acta A 2022, 282, 121694. [Google Scholar]
- Ren, H.; Huo, F.; Wu, X.; Liu, X.; Yin, C. An ESIPT-Induced NIR Fluorescent Probe to Visualize Mitochondrial Sulfur Dioxide during Oxidative Stress in Vivo. Chem. Commun. 2021, 57, 655–658. [Google Scholar]
- Zhao, J.; Dai, C.; Gu, B.; Wei, M. An ESIPT + AIE Based Dual-Response Fluorescent Probe for Continuous Detection of PhSH and HClO and Visualization of PhSH-Induced Oxidative Stress in Living Cells. Spectrochim. Acta A 2024, 320, 124664. [Google Scholar]
- Cao, D.; Zhu, L.; Liu, Z.; Lin, W. Through Bond Energy Transfer (TBET)-Based Fluorescent Chemosensors. J. Photochem. Photobiol. C Photochem. Rev. 2020, 44, 100371. [Google Scholar]
- Yang, X.; Qin, X.; Zhu, F.; Shi, W. A Through-Bond Energy Transfer-Based Ratiometric Fluorescent pH Probe: For Extreme Acidity and Extreme Alkaline Detection with Large Emission Shifts. Talanta 2019, 200, 350–356. [Google Scholar]
- Chen, C.; Zhou, C.; Yang, W.; Hu, Y. A FRET-Based Ratiometric Fluorescent Probe for SO32− Detection in Chinese Medicine and Living Cells. Spectrochim. Acta A 2023, 300, 122902. [Google Scholar]
- Zhang, W.; Huo, F.; Cheng, F.; Yin, C. Employing an ICT-FRET Integration Platform for the Real-Time Tracking of SO2 Metabolism in Cancer Cells and Tumor Models. J. Am. Chem. Soc. 2020, 142, 6324–6331. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Huo, F.; Chao, J.; Yin, C. Independent Bi-Reversible Reactions and Regulable FRET Efficiency Achieving Real-Time Visualization of Cys Metabolizing into SO2. Chem. Commun. 2020, 56, 11453–11456. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Zhang, K.; Gou, Z.; Yan, M. Polarity Responsive Polysiloxanes with Twisting Intramolecular Charge Transfer Effect for Monitoring Lipophagy Process and the Detection of Volatile Organic Compounds. J. Hazard. Mater. 2024, 465, 133106. [Google Scholar] [PubMed]
- Renno, G.; Cardano, F.; Volpi, G.; Barolo, C.; Viscardi, G.; Fin, A. Imidazo[1,5-a]pyridine-Based Fluorescent Probes: A Photophysical Investigation in Liposome Models. Molecules 2022, 27, 3856. [Google Scholar]
- Liu, F.; Wang, S.; Wang, Y.; Jiang, P.; Miao, J.; Zhao, B.; Lin, Z. A Near-Infrared Fluorescent Probe Based FRET for Ratiometric Sensing of H2O2 and Viscosity in Live Cells. Talanta 2024, 275, 126135. [Google Scholar] [CrossRef]
- Huang, X.; Luo, T.; Zhang, C.; Li, J.; Jia, Z.; Chen, X.; Hu, Y.; Huang, H. Dual-Ratiometric Fluorescence Probe for Viscosity and Hypochlorite Based on AIEgen with Mitochondria-Targeting Ability. Talanta 2022, 241, 123235. [Google Scholar]
- Cai, Y.; Hu, H.; Wu, Z.; Yu, C. A Dual-Lock-Controlled Mitochondria-Targeted Ratiometric Fluorescence Probe for Simultaneous Detection of Atherosclerosis-Related HClO and Viscosity. Spectrochim. Acta A 2023, 303, 123225. [Google Scholar]
- Gao, C.; Chen, D.; Zhang, L.; Ma, M.; Liu, H.; Cui, H. A Mitochondria-Targeting Fluorescent Probe for the Dual Sensing of Hypochlorite and Viscosity without Signal Crosstalk in Living Cells and Zebrafish. Molecules 2024, 29, 3059. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, D.; Ye, Y.; Zhao, Y. Recent Advances in Multifunctional Fluorescent Probes for Viscosity and Analytes. Coord. Chem. Rev. 2022, 453, 214336. [Google Scholar] [CrossRef]
- Liang, L.; Sun, Y.; Liu, C.; Jiao, X.; Shang, Y.; Zeng, X.; Zhao, L.; Zhao, J. Highly Selective Turn-on Fluorescent Probe for Hypochlorite and Viscosity Detection. J. Mol. Struct. 2021, 1227, 129523. [Google Scholar] [CrossRef]
- Ju, Q.; Qiao, Z.; Wei, N.; Zhang, Y. A Super-Fast Response Fluorescent Probe for Detecting Endogenous H2S in Living Cells. Dyes Pigm. 2023, 219, 111518. [Google Scholar]
- Liu, F.; Han, W.; Ren, H.; Wang, R.; Yang, W.; Miao, J.; Zhao, B.; Lin, Z. A Dicyanoisophorone-Quinolinium-Based near-Infrared-Emission Fluorescent Probe for Ratiometric Sensing of Bisulfite/Sulfite in Living Cells. Spectrochim. Acta A 2023, 380, 133305. [Google Scholar]
- Wang, T.; Zhang, X.; Huang, X.; Cao, X.; Shen, S. Rapid and Selective Visualization of Mitochondrial Hypochlorite by a Red Region Water-Soluble Fluorescence Probe. Spectrochim. Acta A 2021, 247, 119115. [Google Scholar]
- Pan, W.; Han, L.; Cao, X.; Shen, S.; Pang, X.; Zhu, Y. Dual-Response near-Infrared Fluorescent Probe for Detecting Cyanide and Mitochondrial Viscosity and Its Application in Bioimaging. Food Chem. 2023, 407, 135163. [Google Scholar]
- Tian, Y.; Liu, S.; Wu, W.; Zhao, X.; Wang, Y.; Fan, Y.; Xu, Z.; James, T.D. A Mitochondria-Targeting Fluorescent Probe for the Dual-Emission Fluorescence-Enhanced Detection of Hydrogen Sulfide and Turn-on Detection of Hydrazine. Spectrochim. Acta A 2024, 409, 135496. [Google Scholar]
- Ai, W.; Bu, Y.; Huang, H.; Wang, J.; Ren, M.; Deng, Y.; Zhu, Y.; Wang, S.; Yu, Z.; Zhou, H. Bifunctional Single-Molecular Fluorescent Probe: Visual Detection of Mitochondrial SO2 and Membrane Potential. Anal. Chem. 2023, 95, 6287–6294. [Google Scholar]
- Hanaoka, K.; Ikeno, T.; Iwaki, S.; Deguchi, S.; Takayama, K.; Mizuguchi, H.; Tao, F.; Kojima, N.; Ohno, H.; Sasaki, E.; et al. A General Fluorescence off/on Strategy for Fluorogenic Probes: Steric Repulsion-Induced Twisted Intramolecular Charge Transfer (Sr-TICT). Sci. Adv. 2024, 10, 8847. [Google Scholar]
- Zhai, S.; Hu, W.; Wang, W.; Chai, L.; An, Q.; Li, C.; Liu, Z. Tracking Autophagy Process with a through Bond Energy Transfer-Based Ratiometric Two-Photon Viscosity Probe. Biosens. Bioelectron. 2022, 213, 114484. [Google Scholar]
- Wu, Y.; Yin, C.; Zhang, W.; Zhang, Y.; Huo, F. Mitochondrial-targeting near-infrared fluorescent probe for visualizing viscosity in drug-induced cells and a fatty liver mouse model. Anal. Chem. 2022, 94, 5069–5074. [Google Scholar]
- Zhou, H.; Tang, J.; Zhang, J.; Chen, B.; Kan, J.; Zhang, W.; Zhou, J.; Ma, H. A Red Lysosome-Targeted Fluorescent Probe for Carboxylesterase Detection and Bioimaging. J. Mater. Chem. B 2019, 7, 2989–2996. [Google Scholar]
- Fang, J.; Li, X.; Gao, C.; Gao, S.; Li, W.; Seidu, M.A.; Zhou, H. A Unique Phenothiazine-Based Fluorescent Probe Using Benzothiazolium as a Reactivity Regulator for the Specific Detection of Hypochlorite in Drinking Water and Living Organisms. Talanta 2024, 268, 125299. [Google Scholar] [PubMed]
- Liu, F.; Li, N.; Chen, Y.; Yu, H.; Miao, J.; Zhao, B. A quinoline-coumarin near-infrared ratiometric fluorescent probe for detection of sulfur dioxide derivatives. Anal. Chim. Acta 2022, 1211, 339908. [Google Scholar] [PubMed]
- Seebacher, W.; Brun, R.; Kaiser, M.; Saf, R.; Weis, R. Synthesis and Evaluation of the Antitrypanosomal and Antiplasmodial Activities of New 4-Aminobicyclo[2.2.2]octane Derivatives. Eur. J. Med. Chem. 2005, 40, 888–896. [Google Scholar] [PubMed]
- He, G.; Guo, D.; He, C.; Zhang, X.; Zhao, X.; Duan, C. A Color-Tunable Europium Complex Emitting Three Primary Colors and White Light. Angew. Chem. Int. Ed. 2009, 48, 6132–6135. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Si, Y.; Chen, X.; Nie, X.; Zhang, Y.; Lin, L.; Yan, Y. A Bifunctional Fluorescence Probe for the Detection of Hypochlorous Acid and Viscosity in Living Cells and Zebrafish. Molecules 2025, 30, 1531. https://doi.org/10.3390/molecules30071531
Zhang X, Si Y, Chen X, Nie X, Zhang Y, Lin L, Yan Y. A Bifunctional Fluorescence Probe for the Detection of Hypochlorous Acid and Viscosity in Living Cells and Zebrafish. Molecules. 2025; 30(7):1531. https://doi.org/10.3390/molecules30071531
Chicago/Turabian StyleZhang, Xin, Yanmei Si, Xinpeng Chen, Xuqing Nie, Yiheng Zhang, Li Lin, and Yehao Yan. 2025. "A Bifunctional Fluorescence Probe for the Detection of Hypochlorous Acid and Viscosity in Living Cells and Zebrafish" Molecules 30, no. 7: 1531. https://doi.org/10.3390/molecules30071531
APA StyleZhang, X., Si, Y., Chen, X., Nie, X., Zhang, Y., Lin, L., & Yan, Y. (2025). A Bifunctional Fluorescence Probe for the Detection of Hypochlorous Acid and Viscosity in Living Cells and Zebrafish. Molecules, 30(7), 1531. https://doi.org/10.3390/molecules30071531





