Abstract
Several CNN pincer Pd(II) complexes including chiral complexes 1a–e with 2-phenyl-6-(oxazolinyl)pyridines and achiral ones 2a–c with N-substituted-2-aminomethyl-6-phenylpyridines were prepared. In addition, the preparation of the achiral PCN pincer Pd(II) complexes 3a–e with aryl-based phosphinite–imine ligands and chiral 4a–c with aryl-based phosphinite–imidazoline ligands was also performed. Among them, the PCN Pd(II) pincers 3a–e were new complexes and were readily synthesized from commercially available materials in only two steps. The new complexes were characterized through elemental analyses, namely 1H NMR, 13C{1H} NMR, and 31P{1H} NMR spectroscopies. Furthermore, the molecular structure of complex 3a was determined via X-ray single-crystal diffraction analysis. In the presence of EtAlCl2, Et2AlCl, or methylaluminoxane (MAO), the CNN pincer Pd(II) complexes and PCN pincer Pd(II) complexes exhibited excellent activities and monomer conversion rates in norbornene addition polymerization. Surprisingly, the CNN pincer Pd(II) complexes exhibited a higher conversion rate (99.5%) with Et2AlCl as the cocatalyst, while the PCN pincer Pd(II) complexes showed a higher conversion rate (98.8%) with MAO.
1. Introduction
Polynorbornene (PNB) has been widely used in production and life since PNB shows numerous excellent properties, for instance beneficial UV resistance, outstanding transparency, high glass transition temperature, thermal stability, low dielectric loss, and optical birefringence [1,2,3]. The design and synthesis of highly efficient catalysts based on late-transition metals are a crucial strategy for the development of norbornene polymeric materials [4,5,6,7,8,9,10,11,12]. Late-transition metal catalysts bearing tridentate ligands have been extensively used in the field of metal-catalyzed olefin polymerization because of their better stability in air and great tolerance toward olefin polymerization [13,14,15,16,17,18,19,20]. In particular, the corresponding palladium(II) complexes are a type of outstanding catalysts and have been applied to ethylene polymerization [21], ethylene/10-undecen-1-ol copolymerization [22], norbornene (NB) polymerization [23,24,25,26,27,28,29,30,31,32,33,34], as well as the copolymerization of norbornene [35]. Among them, the palladium(II) complexes used to catalyze the polymerization of norbornene were the most reported and usually showed high activities. For example, the Jin group [25] reported that palladium(II) complexes (Chart 1, structure A) containing soft phosphorous or sulfur donors showed high activities with MAO for norbornene addition polymerization (up to 8.78 × 106 g of PNB (mol of Pd)−1h−1). Several PNN (Chart 1, structure B) [26] and PNP (Chart 1, structure C) [27] pincer palladium(II) complexes were synthesized by the Ganesan Mani group, which showed high activities up to 107 g of PNB (mol of Pd)−1h−1 in the presence of MMAO or EtAlCl2. Additionally, catalysts containing hard atoms (N and O) in the sidearm were investigated for their activities in NB polymerization. For instance, bis(oxazoline)-liganded NNN pincer palladium(II) (Chart 1, structure D) complexes have been proved to be effective for norbornene polymerization [30]. Recently, the Shi group [33] explored a family of Pd(II) complexes bearing oxazoline (Chart 1, structure E), which could be used as catalysts for norbornene polymerization, with catalytic activities up to 1.15 × 107 g of PNB (mol of Pd)−1h−1.
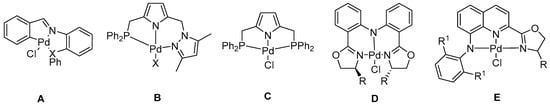
Chart 1.
Representative pincer palladium(II) catalysts for norbornene polymerization reported in the literature: (A) CNX pincer Pd(II) catalysts, (B) PNN pincer Pd(II) catalysts, (C) PNP pincer Pd(II) catalyst, (D,E) NNN pincer Pd(II) catalysts.
In recent decades, we have been working on the synthesis and applications of tridentate-coordinated pincer metal complexes [36,37,38,39,40,41,42,43,44,45]. For instance, chiral oxazoline-containing CNN Pd pincer 1 was found to be a stereoselective catalyst for the asymmetric allylation of isatins with allyltributyltin (up to 86% ee) and the Suzuki–Miyaura reaction (up to 68% ee) [36]. While the related achiral CNN pincer Pd complex 2 with N-substituted-2-aminomethyl-6-phenylpyridines CNN pincer Pd(II) complexes (Chart 2, 2) exhibited good activities in the allylation of aldehydes as well as three-component allylation of aldehydes, arylamines, and allyltributyltin [37]. The achiral phosphinite–imine complexes were effective catalysts for the Suzuki–Miyaura and copper-free Sonogashira cross-coupling reactions [38]. In addition, it was proved that the chiral PCN pincer Pd complexes with aryl-based phosphinite–imidazoline ligand 4 were also applied to asymmetric Suzuki–Miyaura reaction, albeit with rather modest enantioselectivities (up to 32% ee) [39]. All of these Pd pincer complexes could be conveniently and readily synthesized, generally in 2–3 steps. However, the potential of these complexes in olefin polymerization has not been explored. In continuation of our research interest in pincer chemistry [36,37,38,39,40,41,42,43,44,45], herein, we synthesized a series of known and new pincer Pd(II) complexes including achiral and chiral CNN and PCN Pd(II) pincers 1–4 (Chart 2) and investigated their catalytic properties in NB polymerization in the presence of EtAlCl2, Et2AlCl, or MAO.
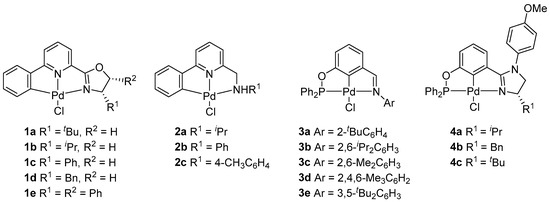
Chart 2.
CNN and PCN pincer palladium(II) complexes for norbornene polymerization.
2. Results and Discussion
2.1. Synthesis and Characterization of New Achiral PCN Pincer Pd (II) Complexes 3a–e
The new complexes with aryl-based phosphinite–imine ligands 3a–e were easily prepared when starting from commercially available 3-hydroxybenzaldehyde as they could be made in only two steps according to the method previously reported by us [38]. Firstly, 3-hydroxybenzaldehyde reacted separately with various substituted aromatic primary amines to afford the corresponding imines 3a′–e′ (Scheme 1). Subsequently, a one-pot phosphorylation/palladation reaction of PCN pincer preligands 3a′–e′ was carried out via treatment with Ph2PCl in the presence of the Et3N base in refluxing toluene for phosphorylation followed by in situ palladation with PdCl2, affording the complexes 3a–e with 30–70% yields. The new complexes 3a–e were fully characterized through 1H NMR, 13C{1H} NMR, and 31P{1H} NMR spectroscopies, as well as elemental analyses, which were consistent with the expected structures of these complexes. In addition, the molecular structure of complex 3a was determined via X-ray single-crystal diffraction analysis, which unambiguously confirmed the PCN tridentate coordination mode in the complex. The molecule is shown in Figure 1. The Pd(II) center adopts a typical distorted-square-planar geometry, and the bond lengths and angles around the Pd center are comparable to those in the homologous PCN Pd pincers [38].
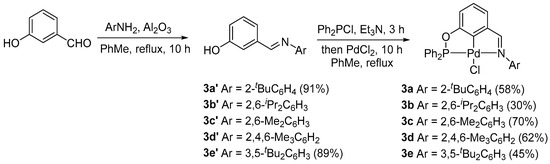
Scheme 1.
Synthesis of the achiral PCN pincer Pd(II) complexes 3a–e.
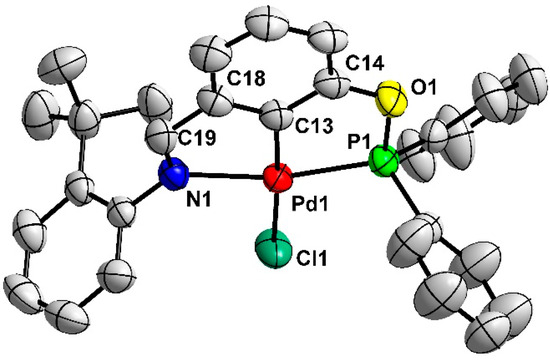
Figure 1.
Molecular structure of complex 3a with ellipsoids drawn at the 50% probability level. (Hydrogen atoms are omitted for clarity; one of the two independent molecules is shown.) Selected bond lengths (Å) and angles (deg) are as follows: Pd(1)-C(13) 1.951(4), Pd(1)-Cl(1) 2.3608(11), Pd(1)-N(1) 2.163(3), Pd(1)-P(1) 2.2016(11), C(13)-Pd(1)-Cl(1) 175.79(12), C(13)-Pd(1)-N(1) 79.14(15), C(13)-Pd(1)-P(1) 79.27(13), N(1)-Pd(1)-Cl(1) 98.05(9), N(1)-Pd(1)-P(1) 158.07(9), and P(1)-Pd(1)-Cl(1) 103.71(4).
2.2. Norbornene Polymerization
2.2.1. NB Polymerization Optimization with CNN Pd(II)/EtAlCl2
EtAlCl2 is a common and inexpensive cocatalyst; therefore, it was initially selected for the experiment, and the results are shown in Table 1. To understand the role of the catalysts, preliminary blank experiments were carried out with complex 1a. The control experiment using EtAlCl2 without 1a did not produce PNB (Table 1, entry 1). When the reaction was carried out with 1 µmol of pincer Pd(II) complex 1a and 2000 equiv. of EtAlCl2 in 1,2-dichlorobenzene at 40 °C for 30 min, a 72.9% monomer conversion rate and a moderate activity of 1.50 × 106 g of PNB (mol of Pd)−1h−1 were obtained (Table 1, entry 2). This result demonstrates that the pincer Pd(II) complex and cocatalyst synergistically catalyzed polymerization, which was consistent with the literature reports [1,12,20,22]. Elevating or reducing the Al/Pd ratio resulted in a slight decrease in the monomer conversion rates (Table 1, entries 3–4). In addition, the reaction temperature had a significant effect on the initiation and stability of the active species [31]. When the polymerization was conducted at 0 °C, the monomer conversion rate was reduced to 25.3% with an activity of 0.52 × 106 g of PNB (mol of Pd)−1h−1, compared to that achieved at the optimal temperature of 20 °C (a monomer conversion rate of 80.3% and an activity of 1.65 × 106 g of PNB (mol of Pd)−1h−1, Table 1, entries 5–6). Further increasing the polymerization temperature to 60 and 80 °C only slightly decreased the polymer activities (Table 1, entries 7–8), demonstrating the outstanding thermostability of the active species. The polymerization time had a significant influence on monomer conversion rates and catalytic activities. When the polymerization time was shortened from 30 min to 5 min, 1a exhibited the highest activity of 9.22 × 106 g of PNB (mol of Pd)−1h−1, and the monomer conversion rate was only 74.6% for NB polymerization (Table 1, entries 9–11). Considering the catalytic activity and monomer conversion rate, we chose 10 min as the optimized polymerization time. Then, when changing the amount of catalyst, the monomer conversion rate did not increase (Table 1, entries 12–13). Therefore, the optimum catalytic conditions were determined to be those of entry 10. With the optimal conditions, all of the other CNN pincer Pd(II) complexes 1b–e and 2a–c could catalyze norbornene polymerization with moderate monomer conversion rates (65.7–77.4%) and catalytic activities (4.06–4.78 × 106 g of PNB (mol of Pd)−1h−1) (Table 1, entries 14–20). We found that the catalytic activities order of CNN complexes 1a–e containing oxazoline coordination was 1a > 1b ≈ 1c ≈ 1d ≈ 1e; also, the catalytic activity was almost unaffected by the steric hindrance effect of the substituents on the oxazoline ring. We suspected that this might be due to the strong rigidity of this structure, which cannot rotate in the space [46]. The order of catalytic activities of complexes 2a–c was 2c > 2b > 2a. It might be that the rotation of the substituent on the amine group changed the steric hindrance of the catalyst’s active center, which resulted in different polymerization activities. According to previous reports, a possible polymerization mechanism had been speculated (Scheme S1) [30,32].

Table 1.
Vinyl polymerization of norbornene with CNN pincer Pd(II) complexes activated by EtAlCl2 a.
2.2.2. NB Polymerization Optimization with CNN Pd(II)/Et2AlCl
Owing to the unsatisfactory results of CNN pincer Pd(II) complexes with EtAlCl2 as the cocatalyst, for higher monomer conversion rates and activities, the cocatalyst Et2AlCl was applied to the experiment. We chose 1a as the pre-catalyst to investigate the optimized conditions, and the results are shown in Table 2. Upon activation with 2000 to 1500 equiv. of Et2AlCl, a monomer conversion rate of up to 95.9% was achieved for NB polymerization (Table 2, entries 1–2). When the Al/Pd ratio was reduced from 1500 to 500, relatively low monomer conversion rates were obtained (Table 2, entries 3–4). A near-quantitative monomer conversion rate of 99.5% and a higher activity of 7.69 × 106 g of PNB (mol of Pd)−1h−1 were obtained when the temperature was reduced to 20 °C (Table 2, entry 6). While elevating the temperature to 80 °C, the monomer conversion rate dramatically reduced to 5.2% (Table 2, entries 7–8). Subsequently, when the amount of catalyst was reduced to 0.8 μmol, the monomer conversion rate declined to 87.2% (Table 2, entry 9). When the reaction time was shortened to 6 min, the monomer conversion rate decreased to 93.2% (Table 2, entry 10). Therefore, the optimal conditions were determined to be an Al/Pd ratio of 1500, a temperature of 20 °C, a reaction time of 8 min, and 1 μmol of the pre-catalyst. Under the optimal conditions, the catalytic behaviors of the other CNN pincer Pd(II) complexes were further investigated. This study found that all CNN pincer Pd(II) complexes could perform efficient catalytic NB polymerization in the presence of Et2AlCl (Table 2, entries 12–18). Notably, the monomer conversion rates for NB polymerization catalyzed by 1b, 1c, and 2c exceeded 97.3% (Table 2, entries 12–13, 18).

Table 2.
Vinyl polymerization of norbornene with CNN pincer Pd(II) complexes activated by Et2AlCl a.
2.2.3. NB Polymerization Optimization with PCN Pd(II)/EtAlCl2
Next, PCN pincer Pd(II) complexes 3 and 4 were synthesized, and their potential applications in the polymerization of norbornene were investigated. We chose EtAlCl2 as the cocatalyst, and the results are shown in Table 3. First, we explored the effect of the Al/Pd ratio on the catalyst activities. When the Al/Pd ratio was 1000, a monomer conversion rate of 25.7% was obtained (Table 3, entry 1). Increasing the Al/Pd ratio to 2000 resulted in a higher monomer conversion rate of 68.9% (Table 3, entries 2–3). Next, we explored the effect of the polymerization temperature on catalyst activities. When elevating or lowering the reaction temperature, the monomer conversion rates had a significant reduction (Table 3, entries 5–8). Prolonging the polymerization time to 30 min with higher monomer conversion rates resulted in lower activities (Table 3, entry 9 vs. entry 3). When the polymerization time was further increased to 60 min, the monomer conversion rate was almost unchanged (Table 3, entry 10). Finally, when changing the amount of catalyst, the monomer conversion rates did not increase (Table 3, entries 11–12). Therefore, entry 9 was confirmed as the optimum catalytic conditions. Under the optimal conditions, all other PCN pincer Pd(II) complexes exhibited moderate monomer conversion rates and catalytic activities (Table 3, entries 13–19). It had been observed that the difference in the ligand skeleton of the pre-catalyst led to significant differences in catalytic activity (Table 1 vs. Table 3). The monomer conversion rates (65.7–79.2%) and catalytic activities (4.06–4.89 × 106 g of PNB (mol of Pd)−1h−1) of the polymer given by CNN pincer Pd(II) complexes 1 and 2 were greater than that of PCN pincer Pd(II) complexes 3 and 4 (monomer conversion rates: 25.9–85.3%; catalytic activities: 0.53–1.76 × 106 g of PNB (mol of Pd)−1h−1, respectively). We surmised that the decrease in activities with the PCN pincer Pd(II) complexes could be explained by more steric crowding around the active metal atom. The three-dimensional orientation of the -PPh2 substituents in the PCN pincer Pd(II) complexes effectively shields the active metal center, thereby hindering the polymerization of the sterically hindered norbornene monomers [26].

Table 3.
Vinyl polymerization of norbornene with PCN pincer Pd(II) complexes activated by EtAlCl2 a.
2.2.4. NB Polymerization Optimization with PCN Pd(II)/MAO
Although EtAlCl2 initiated NB polymerization, it resulted in only moderate monomer conversion rates and catalytic activities. The importance of the MAO in palladium-catalyzed norbornene polymerization is widely recognized [47]. Hence, we selected MAO as the cocatalyst, and the results are summarized in Table 4. The polymerization reaction was carried out with 1 µmol of pincer Pd(II) complex 3a and 5000 equiv. of MAO in 1,2-dichlorobenzene at 40 °C for 30 min, with a 98.7% monomer conversion rate and catalytic activities of 2.03 × 106 g of PNB (mol of Pd)−1h−1 (Table 4, entry 1). Initially, we reduced the Al/Pd molar ratio to 3000; the monomer conversion rate had an obvious reduction (Table 4, entries 2–3). Subsequently, we screened the polymerization temperature (Table 4, entries 4–6). To our delight, the monomer conversion rate increased to 97.9% when the polymerization temperature was at 80 °C, while the color of the NB polymer was slightly dark (Table 4, entry 6). Therefore, we chose 40 °C as the optimal reaction temperature. Next, by shortening the reaction time to 6 min, the monomer conversion rate obviously reduced (57.2%, Table 4, entries 7–8). Reducing the amount of pre-catalyst to 0.8 μmol caused the monomer conversion rate and activity to decrease significantly (Table 4, entry 9). Therefore, Table 4, entry 1 was confirmed to be the optimal conditions. Under these conditions, we continued to explore the catalytic performance of the other PCN pincer Pd(II) complexes (Table 4, entries 10–16). Significantly, complex 3c exhibited a superior monomer conversion rate (98.8%, Table 4, entry 11), whereas complexes 3d and 4b produced only trace amounts of polymer (Table 4, entries 12 and 15). Our investigation revealed that MAO, as a cocatalyst for norbornene polymerization, demonstrated superior catalytic activity and monomer conversion rates compared to EtAlCl2. However, the amount of MAO required was significantly higher than that of EtAlCl2.

Table 4.
Vinyl polymerization of norbornene with PCN pincer Pd(II) complexes activated by MAO a.
2.3. Polymer Characterization
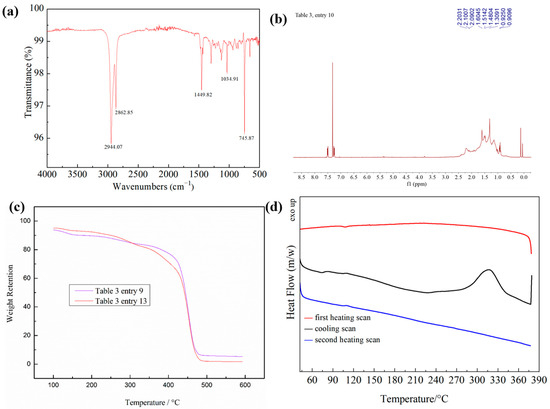
The synthesized polymers were characterized to determine their microstructure. First, the FT-IR spectrum of PNB revealed that there are no traces of (-C=C-) double bonds at 1600–1700 cm−1 (Figure 2a), and their 1H NMR spectra showed no peak around 5.0–6.0 ppm (Figure 2b), suggesting the presence of vinyl-type polynorbornene [19,48]. According to the thermogravimetric analysis (TGA) thermogram, the thermal stability of the polymer was as high as 400 °C (Figure 2c), and the polymer did not undergo a glass transition at 380 °C according to the analysis of the DSC diagram (Figure 2d). These results showed that the polymer had excellent thermal stability.
3. Materials and Methods
General Information: CNN pincer palladium(II) complexes 1 [36] and 2 [37], as well as PCN pincer palladium(II) complex 3 [38], were prepared according to the literature methods. All other chemicals were used as purchased. 1H NMR, 13C{1H} NMR, and 31P{1H} NMR spectra were recorded on a Bruker DPX-400 spectrometer with CDCl3 as the solvent and TMS as an internal standard (Bruker Corporation, Switzerland, Germany). TGA and DSC data were obtained via TGA-55 and DSC-Q2000 thermal analyzers, respectively (waters, Milford, MA USA). IR spectra were recorded using the Spectrum Two FT-IR Spectrometer (PerkinElmer, Waltham, MA, USA). Elemental analysis of catalysts was performed using Vario EL-III elemental analyzer (Elementar Company, Langenselbold, Germany).
3.1. Synthesis
3.1.1. Synthesis of m-Hydroxybenzaldimines 3a′–e′
Compounds 3a′–e′ were synthesized according to the procedure that we previously reported [38]. A mixture of m-hydroxybenzaldehyde (610.6 mg, 5.0 mmol) and primary amine (5.5 mmol) in toluene (20 mL) was refluxed for 10 h in the presence of activated Al2O3 (6.0 mmol) under an Ar atmosphere. After cooling, filtration, and evaporation, the residue was purified through flash column chromatography on a silica gel to afford the corresponding PCN pincer complexes 3a′–e′. The new compounds, 3a′ and 3e′, were characterized as follows.
- 3-(((2-(tert-butyl)phenyl)imino)methyl)phenol (3a′): Brown liquid (2.3 g, 10 mmol, and a 91% yield based on 2-(tert-butyl)aniline). 1H NMR (400 MHz, CDCl3): δ 8.18 (s, 1H, CH=N); 7.40–7.36 (m, 3H, and Ar-H); 7.26 (t, J = 7.8 Hz, 1H, and Ar-H); 7.22–7.13 (m, 2H, and Ar-H); 6.92–6.89 (m, 1H, and Ar-H); 6.80 (dd, J = 1.7, 7.4 Hz, 1H, and Ar-H); and 1.42 (s, 9H, and C(CH3)3) ppm. 13C{1H} NMR (100 MHz, CDCl3): δ 158.9, 156.2, 151.5, 143.0, 138.1, 130.3, 127.3, 126.3, 125.9, 122.2, 119.8, 118.9, 114.8, 35.8, and 30.7 ppm. HRMS (positive ESI): [M + H]+ calculated for C17H20NO+: 254.1539 was found to be 254.1543.
- 3-(((3,5-di-tert-butylphenyl)imino)methyl)phenol (3e′): Pale-brown solid (687.0 mg, 2.5 mmol, and a 89% yield based on 3,5-di-tert-butylaniline). mp 144–145 °C. 1H NMR (400 MHz, CDCl3): δ 8.42 (s, 1H, and CH=N); 7.46–7.45 (m, 1H, and Ar-H); 7.43–7.31 (m, 3H, and Ar-H); 7.05 (d, 2H, J = 1.7 Hz, and Ar-H); 6.99–6.96 (m, 1H, and Ar-H); and 1.35 (s, 18H, and C(CH3)3) ppm. 13C{1H} NMR (100 MHz, CDCl3): δ 160.0, 156.2, 151.9, 137.7, 130.1, 122.2, 120.4, 118.8, 115.2, 114.1, 110.1, 35.0, and 31.5 ppm.
3.1.2. Synthesis of PCN Pincer Palladium(II) Complexes 3a–e
Complexes 3a–e were synthesized according to the procedure that we previously reported [38]. To a stirred solution of 3a′–e′ (0.5 mmol) and triethylamine (85 μL, 0.55 mmol) in toluene (20 mL), diphenylchlorophosphine (121.3 mg, 0.55 mmol) under an Ar atmosphere was added. The mixture was refluxed for 3 h. PdCl2 (89 mg, 0.5 mmol) was then added, and the reaction mixture was refluxed for another 6 h. After cooling, filtration, and evaporation, the residue was purified via column chromatography on a silica gel with CH2Cl2/petroleum ether (10/1) to afford the corresponding PCN pincer complexes 3a–e. Complexes 3a–e were characterized as follows.
- 2-{N-(2-tert-butylphenyl)imino}-6-(diphenylphosphinoxy)phenylchloropalladium(II) (3a): Yellow solid (66.9 mg, 58% yield). mp 248–249 °C. 1H NMR (400 MHz, CDCl3): δ 8.16 (d, J = 4.9 Hz, 1H, and CH=N); 8.05–8.00 (m, 4H, and Ph-H); 7.50–7.47 (m, 7H, Ph-H, and Ar-H); 7.24–7.13 (m, 4H, and Ar-H); 7.04–6.97 (m, 2H, and Ar-H); 1.51 (s, 9H, and C(CH3)3) ppm. 13C{1H} NMR (100 MHz, CDCl3): δ 175.2 (d, J = 4.4 Hz); 162.2 (d, J = 9.4 Hz); 155.5, 148.4, 145.6, 141.3, and 133.0 (d, J = 55.2 Hz); 132.4, 132.2, 132.1, and 131.6 (d, J = 14.5 Hz); 128.9 (d, J = 11.8 Hz); 127.6, 126.9, 126.8, 126.5, 124.2, 123.2, and 115.5 (d, J = 17.6 Hz); 36.1; and 32.6 ppm. 31P{1H} NMR (162 MHz, CDCl3): δ 155.4 ppm. Anal. Calcd for C29H27ClNOPPd.0.5H2O: C, 59.30; H, 4.80; N, 2.38; Found: C, 59.05; H, 4.88; N, 2.15.
- 2-{N-(2,6-diisopropylphenyl)imino}-6-(diphenylphosphinoxy)phenylchloropalladium(II) (3b): Yellow solid (36.4 mg, 30% yield). mp 319–320 °C. 1H NMR (400 MHz, CDCl3): δ 8.09 (d, J = 5.4 Hz, 1H, and CH=N); 8.07–8.02 (m, 4H, and Ph-H); 7.50–7.44 (m, 6H, and Ph-H); 7.21–7.14 (m, 5H, and Ar-H); 7.04 (d, J = 7.6 Hz, 1H, and Ar-H); 3.30–3.23 (m, 2H, and CH(CH3)2); 1.36 (d, J = 6.8 Hz, 6H, and CH(CH3)2); and 1.17 (d, J = 6.9 Hz, 6H, and CH(CH3)2) ppm. 13C{1H} NMR (100 MHz, CDCl3): δ 176.1 (d, J = 4.4 Hz); 162.1 (d, J = 9.5 Hz); 155.6 (d, J = 2.3 Hz); 145.4, 144.3, 140.5, and 133.1 (d, J = 54.7 Hz); 132.2 (d, J = 2.3 Hz); 131.9 (d, J = 14.6 Hz); 128.9 (d, J = 12.4 Hz); 127.2, 127.0, 123.2, 123.1, and 115.6 (d, J = 17.5 Hz); 28.6; 24.2; and 23.1 ppm. 31P{1H} NMR (162 MHz, CDCl3): δ 154.8 ppm. Anal. Calcd for C31H31ClNOPPd.0.5H2O: C, 60.50; H, 5.24; N, 2.28. Found: C, 60.73; H, 5.23; N, 2.06.
- 2-{N-(2,6-dimethylphenyl)imino}-6-(diphenylphosphinoxy)phenylchloropalladium(II) (3c): Yellow solid (76.9 mg, 70% yield). mp 245–246 °C. 1H NMR (400 MHz, CDCl3): δ 8.09 (d, J = 5.4 Hz, 1H, and CH=N); 8.06–8.01 (m, 4H, and Ph-H); 7.49–7.44 (m, 6H, and Ph-H); 7.18–7.13 (m, 2H, and Ar-H); 7.09–7.03 (m, 4H, and Ar-H); and 2.33 (s, 6H, and CH3) ppm. 13C{1H} NMR (100 MHz, CDCl3): δ 176.8 (d, J = 4.6 Hz); 162.2 (d, J = 9.8 Hz); 155.3 (d, J = 2.2 Hz); 145.6, 144.5, 135.9, and 133.0 (d, J = 54.2 Hz); 132.2 (d, J = 2.5 Hz); 131.8 (d, J = 15.1 Hz); 129.6 and 128.9 (d, J = 11.8 Hz); 128.8, 127.0, 123.0, and 115.5 (d, J = 16.8 Hz); 21.0; and 19.0 ppm. 31P{1H} NMR (162 MHz, CDCl3): δ 154.5 ppm. Anal. Calcd for C27H23ClNOPPd: C, 58.93; H, 4.21; N, 2.55. Found: C, 58.66; H, 4.27; N, 2.33.
- 2-{N-(2,4,6-trimethylphenyl)imino}-6-(diphenylphosphinoxy)phenylchloropalladium(II) (3d): Yellow solid (69.8 mg, 62% yield). mp 319–320 °C. 1H NMR (400 MHz, CDCl3): δ 8.07 (d, J = 5.5 Hz, 1H, and CH=N); 8.06–8.01 (m, 4H, and Ph-H); 7.52–7.44 (m, 6H, and Ph-H); 7.17–7.12 (m, 2H, and Ar-H); 7.04–7.02 (m, 1H, and Ar-H); 6.91 (s, 2H, and Ar-H); and 2.30–2.29 (m, 9H, and CH3) ppm. 13C{1H} NMR (100 MHz, CDCl3): δ 176.9 (d, J = 4.4 Hz); 162.2 (d, J = 9.6 Hz); 155.3 (d, J = 1.7 Hz); 145.6, 144.5, 136.0, and 133.0 (d, J = 54.5 Hz); 132.2 (d, J = 2.4 Hz); 131.8 (d, J = 14.8 Hz); 129.7 and 128.9 (d, J = 11.9 Hz); 128.8, 127.0, 123.1, and 115.5 (d, J = 17.2 Hz); 21.0; and 19.0 ppm. 31P{1H} NMR (162 MHz, CDCl3): δ 154.4 ppm. Anal. Calcd for C28H25ClNOPPd.0.5H2O: C, 58.65; H, 4.57; N, 2.44. Found: C, 58.91; H, 4.52; N, 2.20.
- 2-{N-(3,5-di-tert-butylphenyl)imino}-6-(diphenylphosphinoxy)phenylchloropalladium(II) (3e): Yellow solid (57.0 mg, 45% yield). mp 247–248 °C. 1H NMR (400 MHz, CDCl3): δ 8.36 (d, J = 4.6 Hz, 1H, and CH=N); 8.08–8.03 (m, 4H, and Ph-H); 7.50–7.44 (m, 6H, and Ph-H); 7.40–7.39 (m, 3H, and Ar-H); 7.22 (d, J = 7.4 Hz, 1H, and Ar-H); 7.16–7.12 (m, 1H, and Ar-H); 7.00 (d, J = 8.0 Hz, 1H, and Ar-H); and 1.37 (s, 18H, and C(CH3)3) ppm. 13C{1H} NMR (100 MHz, CDCl3): δ 172.1 (d, J = 4.0 Hz); 162.2 (d, J = 9.4 Hz); 154.4, 151.5, 147.5, 146.2, and 132.8 (d, J = 55.6 Hz); 132.2 (d, J = 2.5 Hz); 131.8 (d, J = 14.4 Hz); 128.9 (d, J = 11.9 Hz); 127.0, 123.2, 122.1, 118.0, and 115.2 (d, J = 17.7 Hz); 35.2; and 31.5 ppm. 31P{1H} NMR (162 MHz, CDCl3): δ 153.7 ppm. Anal. Calcd for C33H35ClNOPPd.0.5H2O: C, 61.59; H, 5.64; N, 2.18. Found: C, 62.05; H, 5.65; N, 1.97.
3.2. General Procedure for Norbornene Polymerization
Under an argon atmosphere, norbornene (1.03 g) was dissolved in 5 mL of 1,2-dichlorobenzene; then, the exact amount of the EtAlCl2, Et2AlCl, or MAO solution was added. Next, to keep the mixture at the desired temperature for 3 min, the appropriate amount of the pincer Pd(II) complex dissolved in 1,2-dichlorobenzene was injected into the flask. The reaction mixture was stirred for 30 min, and 10% acidic ethanol was added to terminate the reaction. The PNB was washed with ethanol and dried at 80 °C in vacuo to a constant weight. In all the polymerization processes, the total reaction volume was 10 mL, which can be achieved by changing the amount of 1,2-dichlorobenzene added as necessary.
4. Conclusions
In summary, we synthesized a series of CNN and PCN pincer Pd(II) complexes, and these complexes were applied to the polymerization of norbornene. With the cocatalyst Et2AlCl, the CNN pincer Pd(II) complexes exhibited higher catalytic activities (9.6 × 106 g of PNB (mol of Pd)−1h−1) and a higher monomer conversion rate (99.5%) compared to EtAlCl2. In the presence of the cocatalyst MAO, the PCN pincer Pd(II) complexes exhibited higher catalytic activities (5.9 × 106 g of PNB (mol of Pd)−1h−1) and monomer conversion rates (98.8%) compared to the cocatalyst EtAlCl2. The monomer conversion rates (65.7–79.2%) and catalytic activities (4.06–4.89 × 106 g of PNB (mol of Pd)−1h−1) of the polymer given by the CNN pincer Pd(II) complexes were greater than those (monomer conversion rates: 25.9–85.3%; catalytic activities: 0.53–1.76 × 106 g of PNB (mol of Pd)−1h−1) given by the PCN pincer Pd(II) complexes.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/molecules30071530/s1, Table S1. Crystal Structure Determination for 3a. Scheme S1. Possible mechanism for EtAlCl2-activated norbornene polymerization. Excess EtAlCl2 first alkylates the S1a and then abstracts the ethyl group form cationic species. Due to the instability of cationic species, an asymmetric binuclear complex S1b with a neutral molecule was formed through the bridge forms. A norbornene molecule firstly coordinate to the Pd+ in S1c, and then the ethyl group attacks and inserts into the double bond of norbornene to generate S1d. After the reaction, the cation center transfers to the another Pd atom in S1d. A new norbornene molecule coordinate to the Pd+ in S1e, and then the norbornenyl group attacks and inserts into the double bond of norbornene to generate S1f. Continuous coordination and insertion of norbornene alternatively on the two Pd centers of binuclear complexes produce the resultant polynorbornene. Table S2. Comparison of Catalytic properties of the CNN and PCN pincer Pd(II) complexes with Previously Reported Pd(II) Complexes for Norbornene Polymerization. Figure S1. 1H NMR (400 MHz, CDCl3) spectrum of 3a′. Figure S2. 13C{1H}NMR (100 MHz, CDCl3) spectrum of 3a′. Figure S3. 1H NMR (400 MHz, CDCl3) spectrum of 3e′. Figure S4. 13C{1H}NMR (100 MHz, CDCl3) spectrum of 3e′. Figure S5. 1H NMR (400 MHz, CDCl3) spectrum of 3a. Figure S6. 13C{1H}NMR (100 MHz, CDCl3) spectrum of 3a. Figure S7. 31P{1H}NMR (162 MHz, CDCl3) spectrum of 3a. Figure S8. 1H NMR (400 MHz, CDCl3) spectrum of 3b. Figure S9. 13C{1H}NMR (100 MHz, CDCl3) spectrum of 3b. Figure S10. 31P{1H}NMR (162 MHz, CDCl3) spectrum of 3b. Figure S11. 1H NMR (400 MHz, CDCl3) spectrum of 3c. Figure S12. 13C{1H}NMR (100 MHz, CDCl3) spectrum of 3c. Figure S13. 31P{1H}NMR (162 MHz, CDCl3) spectrum of 3c. Figure S14. 1H NMR (400 MHz, CDCl3) spectrum of 3d. Figure S15. 13C{1H}NMR (100 MHz, CDCl3) spectrum of 3d. Figure S16. 31P{1H}NMR (162 MHz, CDCl3) spectrum of 3d. Figure S17. 1H NMR (400 MHz, CDCl3) spectrum of 3e. Figure S18. 13C{1H}NMR (100 MHz, CDCl3) spectrum of 3e. Figure S19. 31P{1H}NMR (162 MHz, CDCl3) spectrum of 3e. Figure S20. IR spectrum of PNB obtained by 1a/EtAlCl2 (Table 1, entry 9). Figure S21. IR spectrum of PNB obtained by 2c/EtAlCl2 (Table 1, entry 19). Figure S22. IR spectrum of PNB obtained by 1a/Et2AlCl (Table 2, entry 6). Figure S23. IR spectrum of PNB obtained by 1c/Et2AlCl (Table 2, entry 13). Figure S24. IR spectrum of PNB obtained by 4b/EtAlCl2 (Table 3 entry 18). Figure S25. IR spectrum of PNB obtained by 3a/MAO (Table 4, entry 1). Figure S26. IR spectrum of PNB obtained by 4c/MAO (Table 4, entry 16). Figure S27. DSC thermograms of PNB obtained by 1a/EtAlCl2 (Table 1, entry 9). Figure S28. DSC thermograms of PNB obtained by 2c/EtAlCl2 (Table 1, entry 19). Figure S29. DSC thermograms of PNB obtained by 1a/Et2AlCl (Table 2, entry 6). Figure S30. DSC thermograms of PNB obtained by 1c/Et2AlCl (Table 2, entry 13). Figure S31. DSC thermograms of PNB obtained by 4c/EtAlCl2 (Table 3, entry 19). Figure S32. DSC thermograms of PNB obtained by 3a/MAO (Table 4, entry 1). Figure S33. DSC thermograms of PNB obtained by 4c/MAO (Table 4, entry 16). Figure S34. TGA of PNB. Figure S35. TGA of PNB. Figure S36. TGA of PNB.
Author Contributions
This manuscript was written through contributions of all authors. H.W. carried out laboratory research. J.-K.L. wrote the manuscript draft. Y.-D.W. conducted the investigation and visualization and helped with some experiments. X.-Q.H., M.-P.S., H.J. and J.-F.G. handled the supervision, project administration, and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Nos. 22471247, U1904212).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhu, Y.-Z.; Liu, J.-Y.; Li, Y.-S.; Tong, Y.-J. Synthesis, structure and norbornene polymerization behavior of nickel complexes bearing two β-ketoiminato chelate ligands. J. Organomet. Chem. 2004, 689, 1295–1303. [Google Scholar] [CrossRef]
- Blank, F.; Scherer, H.; Ruiz, J.; Rodríguez, V.; Janiak, C. Palladium(II) complexes with pentafluorophenyl ligands: Structures, C6F5 fluxionality by 2D-NMR studies and pre-catalysts for the vinyl addition polymerization of norbornene. Dalton Trans. 2010, 39, 3609–3619. [Google Scholar] [PubMed]
- Li, M.; Song, H.; Wang, B. Synthesis, structures, and norbornene polymerization behavior of palladium methyl complexes bearing N-heterocyclic carbene-sulfonate ligands. J. Organomet. Chem. 2016, 804, 118–122. [Google Scholar]
- Bermesheva, E.V.; Medentseva, E.I.; Khrychikova, A.P.; Wozniak, A.I.; Guseva, M.A.; Nazarov, I.V.; Morontsev, A.A.; Karpov, G.O.; Topchiy, M.A.; Asachenko, A.F.; et al. Air-Stable Single-Component Pd-Catalysts for Vinyl-Addition Polymerization of Functionalized Norbornenes. ACS Catal. 2022, 12, 15076–15090. [Google Scholar] [CrossRef]
- Wang, W.; Qu, S.; Li, X.; Chen, J.; Guo, Z.; Sun, W.-H. Transition Metal Complex Catalysts Promoting Copolymers of Cycloolefin with Propylene/higher Olefins. Coord. Chem. Rev. 2023, 494, 215351. [Google Scholar]
- Li, M.; Fang, Y.; Cai, Z.; Eisen, M.S. Nickel- and Palladium-Catalyzed Copolymerizations of Norbornene with Polar α-Olefifins. ChemCatChem 2024, 16, e202301731. [Google Scholar] [CrossRef]
- Bermesheva, E.V.; Bermeshev, M.V. Single-Component Catalysts for the Vinyl-Addition Polymerization of Norbornene and its Derivatives. ChemCatChem 2023, 15, e202300818. [Google Scholar] [CrossRef]
- Lunin, A.O.; Andreyanov, F.A.; Makarov, I.S.; Bermeshev, M.V. Vinyl-Addition Homopolymeization of Norbornenes with Bromoalkyl Groups. Polymers 2023, 15, 4444. [Google Scholar] [CrossRef]
- Margaret Powell, E.; Mimna, R.A.; Day, C.S.; Day, V.W.; Long, B.K.; Rhodes, L.F. Polymerization of Ester-Functionalized Norbornenes Using Neutral Nickel Catalysts. Organometallics 2024, 43, 2565–2573. [Google Scholar] [CrossRef]
- Li, M.; Cai, Z.; Eisen, M.S. Rational Design of Aldimine Imidazolidin-2-imine/guanidine Nickel Catalysts for Norbornene (Co)polymerizations with Enhanced Catalytic Performance. J. Catal. 2023, 420, 58–67. [Google Scholar] [CrossRef]
- Li, D.; Zheng, H.; Gao, H.; Wang, X.; Gao, H. Ion Pair Effects of Zwitterionic Ni/Pd Allyl Complexes Bearing an α-Sulfonate-β-diimine Ligand by Binding of B(C6F5)3 on Norbornene Polymerization. Organometallics 2024, 43, 2527–2536. [Google Scholar] [CrossRef]
- Birajdar, R.S.; Gupta, P.; Gonnade, R.G.; Chikkali, S.H. Synthesis of Imine-Phenoxy Ligated Palladium Complexes for Norbornene Homopolymerization. Inorg. Chem. 2025, 64, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Pei, L.; Du, W.; Xiao, X.; Gao, H.; Zheng, H.; Gao, H. Synthesis of Branched Cyclo-Olefin Copolymers Using Neutral α-Sulfonate-β-Diimine Nickel Catalyst. Molecules 2025, 30, 157. [Google Scholar] [CrossRef] [PubMed]
- Ittel, S.D.; Johnson, L.K.; Brookhart, M. Late-Metal Catalysts for Ethylene Homo- and Copolymerization. Chem. Rev. 2000, 100, 1169–1203. [Google Scholar] [CrossRef]
- Dίez-González, S.; Marion, N.; Nolan, S.P. N-Heterocyclic Carbenes in Late Transition Metal Catalysis. Chem. Rev. 2009, 109, 3612–3676. [Google Scholar] [CrossRef]
- Bianchini, C.; Giambastiani, G.; Luconi, L.; Meli, A. Olefin oligomerization, homopolymerization and copolymerization by late transition metals supported by (imino)pyridine ligands. Coord. Chem. Rev. 2010, 254, 431–455. [Google Scholar] [CrossRef]
- Yao, Z.-J.; Jin, G.-X. Transition metal complexes based on carboranyl ligands containing N, P, and S donors: Synthesis, reactivity and applications. Coord. Chem. Rev. 2013, 257, 2522–2535. [Google Scholar] [CrossRef]
- Mu, H.; Pan, L.; Song, D.; Li, Y. Neutral Nickel Catalysts for Olefin Homo- and Copolymerization: Relationships between Catalyst Structures and Catalytic Properties. Chem. Rev. 2015, 115, 12091–12137. [Google Scholar] [CrossRef]
- Zhuang, R.; Liu, H.; Guo, J.; Dong, B.; Zhao, W.; Hu, Y.; Zhang, X. Highly active nickel(II) and palladium(II) complexes bearing N,N,P tridentate ligand for vinyl addition polymerization of norbornene. Eur. Polym. J. 2017, 93, 358–367. [Google Scholar] [CrossRef]
- Yang, D.; Dong, J.; Wang, B. Homo- and copolymerization of norbornene with tridentate nickel complexes bearing o-aryloxide-N-heterocyclic carbene ligands. Dalton Trans. 2018, 47, 180–189. [Google Scholar] [CrossRef]
- Santi, R.; Romano, A.M.; Sommazzi, A.; Grande, M.; Bianchini, C.; Mantovani, G. Catalytic polymerisation of ethylene with tris(pyrazolyl)borate complexes of late transition metals. J. Mol. Catal. A Chem. 2005, 229, 191–197. [Google Scholar] [CrossRef]
- Ortega-Jiménez, F.; López-Cortés, J.G.; Ortega-Alfaro, M.C.; Penieres-Carrillo, J.C.; Quijada, R.; Alvarez-Toledano, C. Evaluation of catalytic activity in ethylene polymerization and ethylene/10-undecen-1-ol copolymerization of new orthopalladated complexes derived from tridentade ligands [C,N,S]. Appl. Catal. A Gen. 2012, 417–418, 1–5. [Google Scholar] [CrossRef]
- Long, J.; Gao, H.; Song, K.; Liu, F.; Hu, H.; Zhang, L.; Zhu, F.; Wu, Q. Synthesis and Characterization of NiII and PdII Complexes Bearing N,N,S Tridentate Ligands and Their Catalytic Properties for Norbornene Polymerization. Eur. J. Inorg. Chem. 2008, 2008, 4296–4305. [Google Scholar] [CrossRef]
- Han, F.-B.; Zhang, Y.-L.; Sun, X.-L.; Li, B.-G.; Guo, Y.-H.; Tang, Y. Synthesis and Characterization of Pyrrole-imine [N-NP] Nickel(II) and Palladium(II) Complexes and Their Applications to Norbornene Polymerization. Organometallics 2008, 27, 1924–1928. [Google Scholar]
- Qiao, Y.-L.; Jin, G.-X. Nickel(II) and Palladium(II) Complexes with Tridentate [C,N,S] and [C,N,P] Ligands: Syntheses, Characterization, and Catalytic Norbornene Polymerization. Organometallics 2013, 32, 1932–1937. [Google Scholar] [CrossRef]
- Das, S.; Subramaniyan, V.; Mani, G. Nickel(II) and Palladium(II) Complexes Bearing an Unsymmetrical Pyrrole-Based PNN Pincer and Their Norbornene Polymerization Behaviors versus the Symmetrical NNN and PNP Pincers. Inorg. Chem. 2019, 58, 3444–3456. [Google Scholar] [CrossRef]
- Kumar, S.; Mani, G.; Mondal, S.; Chattaraj, P.K. Pyrrole-Based New Diphosphines: Pd and Ni Complexes Bearing the PNP Pincer Ligand. Inorg. Chem. 2012, 51, 12527–12539. [Google Scholar]
- Ghorai, D.; Kumar, S.; Mani, G. Mononuclear, helical binuclear palladium and lithium complexes bearing a new pyrrole-based NNN-pincer ligand: Fluxional property. Dalton Trans. 2012, 41, 9503–9512. [Google Scholar]
- Huang, Y.; He, J.; Liu, Z.; Cai, G.; Zhang, S.; Li, X. A highly active chiral (S,S)-bis(oxazoline) Pd(II) alkyl complex/activator catalytic system for vinyl polymerization of norbornene in air and water. Polym. Chem. 2017, 8, 1217–1222. [Google Scholar] [CrossRef]
- He, J.; Liu, Z.; Du, G.; Fu, Y.; Zhang, S.; Li, X. Chiral Palladium(II) and Nickel(II) Complexes with C2-Symmetrical Tridentate Bis(oxazoline) Ligands: Synthesis, Characterization, and Catalytic Norbornene Polymerization. Organometallics 2014, 33, 6103–6112. [Google Scholar]
- You, F.; Liu, H.; Luo, G.; Shi, X. Tridentate diarylamido-based pincer complexes of nickel and palladium: Sidearm effects in the polymerization of norbornene. Dalton Trans. 2019, 48, 12219–12227. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yuan, H.; Shi, X. Synthesis of nickel and palladium complexes with diarylamido-based unsymmetrical pincer ligands and application for norbornene polymerization. Dalton Trans. 2019, 48, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Liu, H.; You, F.; Shi, X. Synthesis of palladium complexes with quinolino-based tridentate ligands and their applications for norbornene polymerization. Inorg. Chem. Commun. 2020, 119, 108139. [Google Scholar] [CrossRef]
- Yang, D.; Tang, Y.; Song, H.; Wang, B. Synthesis, Structures, and Norbornene Polymerization Behavior of Palladium Complexes Bearing Tridentate o-Aryloxide-N-heterocyclic Carbene Ligands. Organometallics 2016, 35, 1392–1398. [Google Scholar] [CrossRef]
- Dong, J.; Wang, B. Homo- and copolymerization of norbornene using tridentate IzQO palladium catalysts with dimethylaminoethyl as a side arm. Polym. Chem. 2021, 12, 4736–4747. [Google Scholar] [CrossRef]
- Wang, T.; Hao, X.-Q.; Zhang, X.-X.; Gong, J.-F.; Song, M.-P. Synthesis, structure and catalytic properties of CNN pincer palladium(II) and ruthenium(II) complexes with N-substituted-2-aminomethyl-6-phenylpyridines. Dalton Trans. 2011, 40, 8964–8976. [Google Scholar] [CrossRef]
- Wang, T.; Hao, X.-Q.; Huang, J.-J.; Wang, K.; Gong, J.-F.; Song, M.-P. Chiral CNN Pincer Palladium(II) Complexes with 2-Aryl-6-(oxazolinyl)pyridine Ligands: Synthesis, Characterization, and Application to Enantioselective Allylation of Isatins and Suzuki-Miyaura Coupling Reaction. Organometallics 2014, 33, 194–205. [Google Scholar] [CrossRef]
- Zhang, B.-S.; Wang, W.; Shao, D.-D.; Hao, X.-Q.; Gong, J.-F.; Song, M.-P. Unsymmetrical Chiral PCN Pincer Palladium(II) and Nickel(II) Complexes of (Imidazolinyl)aryl Phosphinite Ligands: Synthesis via Ligand C-H Activation, Crystal Structures, and Catalytic Studies. Organometallics 2010, 29, 2579–2587. [Google Scholar] [CrossRef]
- Zhang, B.-S.; Wang, C.; Gong, J.-F.; Song, M.-P. Facile synthesis of achiral and chiral PCN pincer palladium(II) complexes and their application in the Suzuki and copper-free Sonogashira cross-coupling reactions. J. Organomet. Chem. 2009, 694, 2555–2561. [Google Scholar] [CrossRef]
- Liu, J.-K.; Gong, J.-F.; Song, M.-P. Chiral palladium pincer complexes for asymmetric catalytic reactions. Org. Biomol. Chem. 2019, 17, 6069–6098. [Google Scholar] [CrossRef]
- Huang, J.-J.; Zhang, X.-Q.; Yang, J.-J.; Gong, J.-F.; Song, M.-P. Chiral (phosphine)-(imidazoline) PCN pincer palladium(II) complexes: Synthesis and application in asymmetric hydrophosphination of 2-alkenoylpyridines with diphenylphosphine. Dalton Trans. 2022, 51, 8350–8367. [Google Scholar] [PubMed]
- Qu, J.-J.; Shi, L.-L.; Wang, Y.-B.; Yan, J.; Shao, T.; Hao, X.-Q.; Wang, J.-X.; Zhang, H.-Y.; Gong, J.-F.; Song, B. The Novel Function of Unsymmetrical Chiral CCN Pincer Nickel Complexes as Chemotherapeutic Agents Targeting Prostate Cancer Cells. Molecules 2022, 27, 3106. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Yang, X.; Zhu, Y.; Wang, F.; Gong, J.; Song, M. Pincer iridium(III)-catalyzed enantioselective C(sp3)-H functionalization via carbenoid C–H insertion of 3-diazooxindoles with 1,4-cyclohexadiene. Chin. Chem. Lett. 2022, 33, 2437–2441. [Google Scholar]
- Jiang, H.; Zhang, C.-Y.; Liu, J.-K.; Song, M.-P.; Gong, J.-F. Rhodium-Catalyzed Direct Enantioselective Alkynylation of Trifluoropyruvates with Terminal 1,3-Diynes. Adv. Synth. Catal. 2023, 365, 3967–3972. [Google Scholar] [CrossRef]
- Zhang, X.-Q.; Wang, H.-J.; Jiang, H.; Song, M.-P.; Gong, J.-F. Chiral bis(imidazoline) NCN pincer iridium(III)-catalyzed enantioselective alkynylation of trifluoropyruvates with terminal alkynes. Green Synth. Catal. 2024; in press. [Google Scholar] [CrossRef]
- Wang, X.; Dong, B.; Yang, Q.; Liu, H.; Hu, Y.; Zhang, X. Boosting the Thermal Stability of α-Diimine Palladium Complexes in Norbornene Polymerization from Construction of Intraligand Hydrogen Bonding and Simultaneous Increasing Axial/Equatorial Bulkiness. Inorg. Chem. 2021, 60, 2347–2361. [Google Scholar]
- Kaminsky, W. The Discovery of Metallocene Catalysts and Their Present State of the Art. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 3911–3921. [Google Scholar]
- Dong, J.; Yang, D.; Wang, B. Homo- and Copolymerization of Norbornene with Allyl Palladium and Nickel Complexes Bearing Imidazo[1,5-a]pyridine Sulfonate Ligands. Eur. J. Inorg. Chem. 2021, 2021, 4661–4668. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).