Advances in Antimicrobial Peptides: Mechanisms, Design Innovations, and Biomedical Potential
Abstract
1. Introduction
2. Classification of AMPs
2.1. Classification Based on Biological Sources
2.1.1. Animal-Derived AMPs
2.1.2. Plant-Derived AMPs
2.1.3. Microbial-Derived AMPs
| Name | Source | Bactericidal Activity | Mechanism of Action | Amino Acid Sequence | 3D Structure (a,b) |
|---|---|---|---|---|---|
| LysAB2 P3 [26] | Acinetobacter baumannii phage | A. baumannii | Degradation of peptidoglycan cell wall and subsequent decomposition of bacterial cells | NPEKALEKLIAIQKAIKGMLNGWFTGVGFRRKR | α-helix, β-sheet a |
| PK34 [27] | Mycobacterium phage | Mycobacterium tuberculosis | Inactivation of MAPK and PKB signaling reduces inflammatory cytokines secretion | LPRVIETKVHGREVTGLARNVSEENVDRLAKRWIK | α-helix, β-sheet a |
| Human β-defensin 3 [28] | Homo sapiens | Staphylococcus epidermidis | Downregulation of genes responsible for biofilm formation | GIINTLQKYYCRVRGGRCAVLSCLPKEEQIGKCSTRGRKCCRRKK | α-helix, β-sheet a |
| Microcin J25 [29] | E. coli | G− bacteria | Bind to RNA polymerase and inhibit the activity of RNA polymerase | GGAGHVPEYFVGIGTPISFYG | β-sheet a |
| Satanin 1 [30] | Scarabaeidae | G− bacteria | Inhibits the release of pro-inflammatory cytokines such as tumor necrosis factor-α | RSKKWRKIEKRVKKIFEKTKEALPVIQGVATIVGAVGR | α-helix b |
| Bac-7 [31] | Bos taurus | E. coli, Salmonella typhimurium | Inhibit 70S ribosome protein synthesis and DnaK activity | RRIRPRPPRLPRPRPRPFPRPGPRPRPRFPLPFP | β-sheet a |
| LL-37 [32] | Homo sapiens | Severe Acute Respiratory Syndrome Coronavirus-2 | Inhibit bacterial adhesion; disruption of cell signaling system | LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES | α-helix a |
| TO17 [33] | Sciaenops ocellatus | Infectious spleen and kidney necrosis virus | Induce degradation of genomic DNA and total RNA | KCRRRKVHGPMIRIRKK | β-sheet a |
| Buforin 2 [34] | Sphaenorhynchus lacteus | S. Aureus, E. coli | Bind with nucleic acids and finally inhibit the synthesis of DNA, RNA, and proteins | TRSSRAGLQFPVGRVHRLLRK | α-helix a |
| Pseudin-T2 [35] | Pseudis paradoxa | G− bacteria | Binds to DNA and forms pores on the cell membrane surface | LNALKKVFQKIHEAIKLI | α-helix a |
| Andropin [36] | Androctonus australis | G+ bacteria | Reducing the tissue levels of pro-inflammatory cytokines to inhibit inflammation | VFIDILDKVENAIHNAAQVGIGFAKPFEKLINPK | α-helix b |
| LactoferricinB [37] | Bos taurus | G+ bacteria, G− bacteria | Cause damage to the cell membrane | FKCRRWQWRMKKLGAPSITCVRRAF | β-sheet a |
| Bactericidin B-3 [38] | Manduca sexta | G+ bacteria, G− bacteria | * | WNPFKELERAGQRVRDAIISAGPAVATVGQAAAIARG | α-helix b |
| Bombinin H4 [39] | Bombina variegata | G+ bacteria, G− bacteria | Causes significant damage to the cell membrane, leading to protein leakage | ILGPVLGLVGSALGGLLKKI | α-helix a |
| Maximin 5 [40] | Bombina maxima | G+ bacteria, G− bacteria | Membranolytic mechanisms promoted by anionic lipid | SIGAKILGGVKTFFKGALKELASTYLQ | α-helix a |
| Brevinin-1 [41] | Rana brevipoda porsa | S. aureus, Klebsiella Pneumoniae | Lipopolysaccharide-neutralizing and anti-inflammatory activities | FLPVLAGIAAKVVPALFCKITKKC | α-helix, β-sheet b |
| Esculentin-1 [42] | Rana esculenta | E. coli, S. Aureus, P. aeruginosa | Disrupts membrane integrity, hydrolyzes DNA, and activates pro-inflammatory cytokines | GIFSKLGRKKIKNLLISGLKNVGKEVGMDVVRTGIDIAGCKIKGEC | α-helix a |
| Rugosin A [43] | Rana rugosa | S. aureus, Bacillus subtilis | Anti-inflammatory, promotes insulin secretion | GLLNTFKDWAISIAKGAGKGVLTTLSCKLDKSC | α-helix b |
| Thanatin [11] | Podisus maculiventris | G+ bacteria, G− bacteria | Competes with Ca2+ and Mg2+ for lipopolysaccharide, disrupts membrane integrity, inhibits NDM-1 activity | GSKKPVPIIYCNRRTGKCQRM | β-sheet a |
| AalCecA [44] | Aedes albopictus | G− bacteria | * | GGLKKLGKKLEGVGKRVFKASEKALPVAVGIKALG | α-helix b |
| Cecropin [45] | Antheraea pernyi | G+ bacteria, G− bacteria | Penetrates cardiolipin-containing bilayers, causing lipid instability. | WNPFKELERAGQRVRDAIISAGPAVATVAQATALAK | α-helix b |
| Melittin [46] | Apis mellifera | G+ bacteria, G− bacteria | Membrane permeabilization, ROS-mediated apoptosis, and (1,3)-β-D-glucan synthase inhibition | GIGAVLKVLTTGLPALISWIKRKRQQ | α-helix a |
| Indolicidin [47] | Bos taurus | G+ bacteria, G− bacteria | Indole derivatives increase membrane permeability, disrupting osmotic balance and causing cell rupture | ILPWKWPWWPWRR | Non-αβ a |
| Tritrpticin [48] | Sus scrofa | G+ bacteria, G− bacteria | Permeabilization of the cytoplasmic membrane | VRRFPWWWPFLRR | Non-αβ a |
| BTD-1 [49] | Papio anubis | E. coli, S. aureus | Cell permeabilization, intracellular accumulation of reactive oxygen species | GFCRCVCRRGVCRCVCTR | β-sheet a |
| Androctonin [50] | Androctonus australis | G+ bacteria, G− bacteria | High affinity for the postsynaptic acetylcholine receptor | RSVCRQIKICRRRGGCYYKCTNRPY | β-sheet a |
| Dermaseptin-S1 [51] | Phyllomedusa sauvagii | G+ bacteria, G− bacteria | Decreases expression of hyphal wall protein 1 and aspartic proteases genes | ALWKTMLKKLGTMALHAGKAALGAAADTISQGTQ | α-helix a |
| Pyrrhocoricin [52] | Pyrrhocoris apterus | G+ bacteria, G− bacteria | Inhibits the translation process in the protein synthesis system | VDKGSYLPRPTPPRPIYNRN | Non-αβ a |
| Drosocin [53] | Drosophila melanogaster | G+ bacteria, G− bacteria | Inhibits protein synthesis by blocking 50S ribosomal subunit assembly | GKPRPYSPRPTSHPRPIRV | Non-αβ a |
| Gomesin [54] | Acanthoscurria gomesiana | G+ bacteria, G− bacteria | L-type calcium channel influx, MAPK/ERK, PKC, and PI3K signaling, and ROS generation | QCRRLCYKQRCVTYCRGR | β-sheet a |
| Enbocin [55] | Bombyx mori | G+ bacteria, G− bacteria | * | PWNIFKEIERAVARTRDAVISAGPAVRTVAAATSVAS | α-helix b |
2.2. Classification Based on Three-Dimensional Structures
2.2.1. α-Helix Structure Peptides
2.2.2. β-Sheet Structure Peptides
2.2.3. αβ Structure Peptides
2.2.4. Non-αβ Structure Peptides
2.3. Classification Based on Molecular Targets
2.3.1. Cell-Penetrating Peptides
2.3.2. Cell Interior-Targeting Peptides
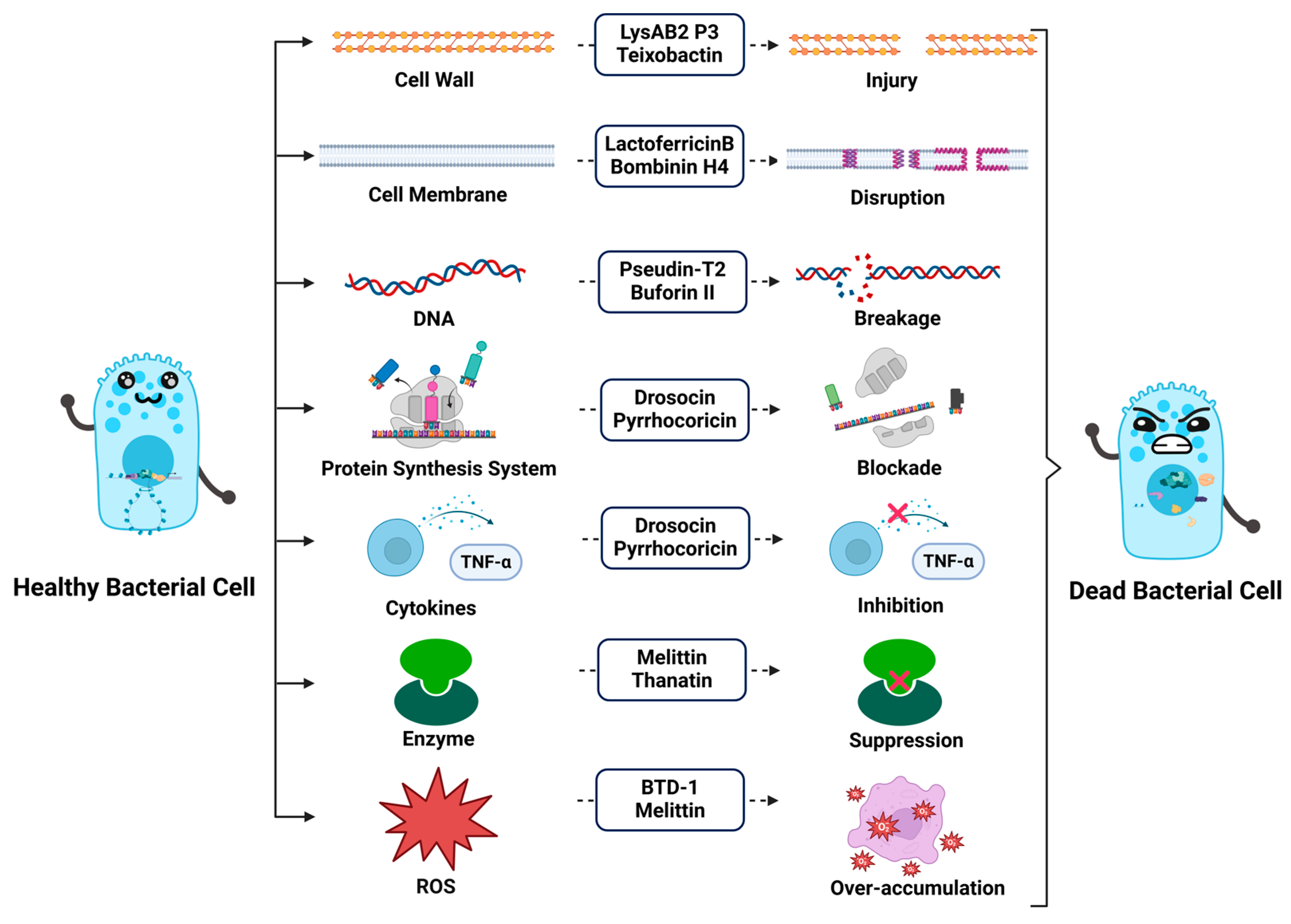
3. Mechanisms of Action of Antimicrobial Peptides
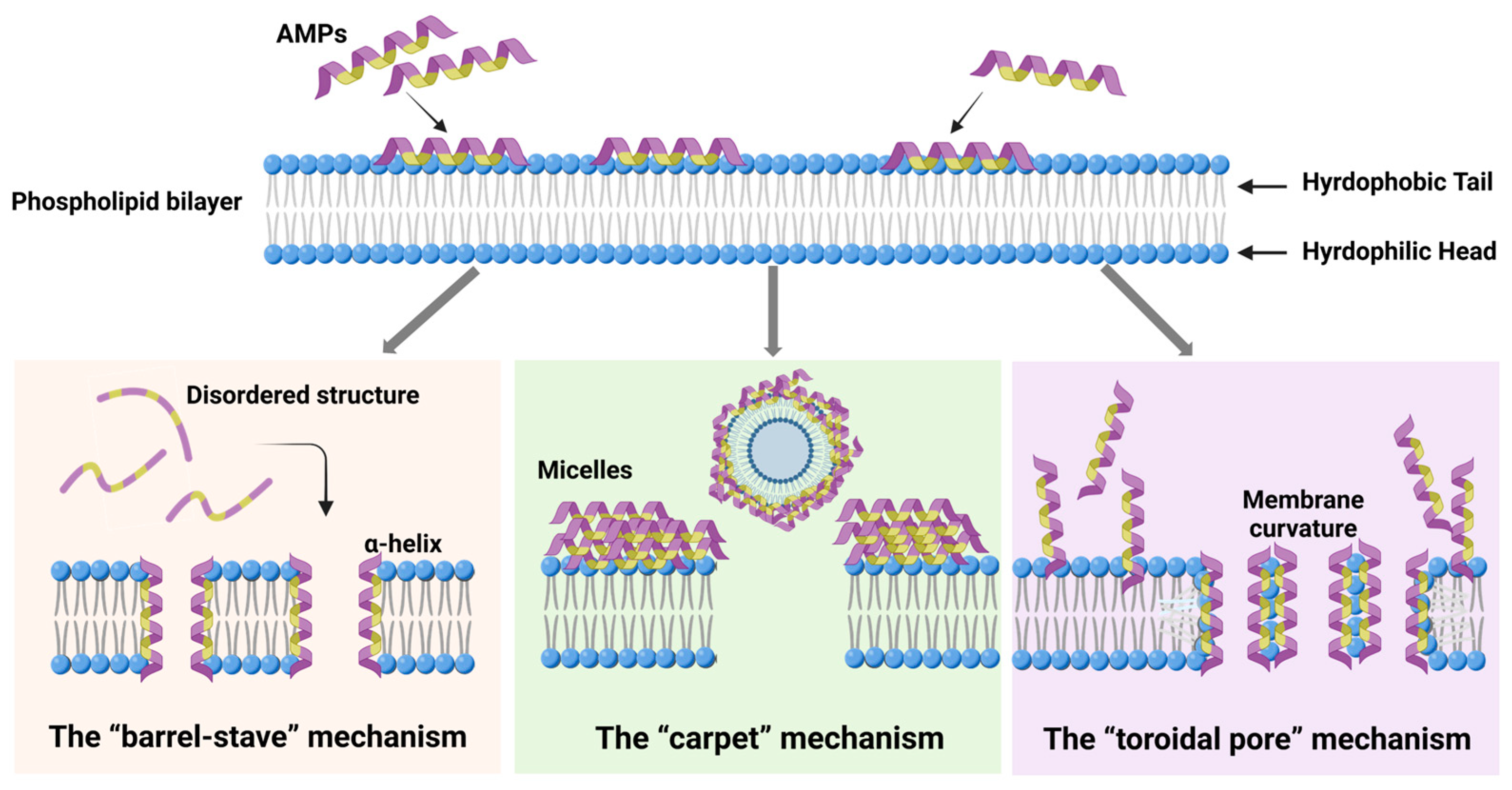
3.1. Direct Disruption of Microbial Cell Membranes
- (1)
- The “barrel-stave” mechanism: In this mechanism, AMPs insert into the phospholipid bilayer, undergoing lateral diffusion across the membrane. Once inserted, they undergo a conformational change, folding into an α-helix, which facilitates the formation of a barrel-like structure across the membrane. For example, Melittin, an AMP derived from bees, is a cationic linear peptide composed of 26 amino acid residues. Upon binding to the lipid bilayer, its structure changes from a disordered structure into an amphipathic α-helical structure [59]. In this way, the hydrophilic faces of the peptides orient toward the channel, while their hydrophobic faces interact with the interior of the phospholipid bilayer. This alignment induces the aggregation of lipids around the peptides, destabilizing the membrane and causing leakage of membrane components such as ions and small molecules, leading to a loss of membrane integrity and cell death [77,78,79].
- (2)
- The “carpet” mechanism: Unlike the barrel-stave model, AMPs in the carpet mechanism do not penetrate the bilayer directly. Instead, they align parallel to the membrane surface, binding to the lipid head groups and covering the membrane like a carpet. As the concentration of AMPs increases, they accumulate on the membrane surface, and the peptides aggregate, forming a dense layer. This accumulation leads to a detergent-like effect, disrupting the membrane’s structural integrity by disturbing the packing of lipids. Eventually, this results in the formation of micelles, with the membrane destabilized and substances leaking out of the cell, compromising its physiological functions [80,81].
- (3)
- The “toroidal pore” mechanism: This mechanism shares similarities with the carpet model; AMPs remain largely parallel to the membrane surface [82]. However, in the toroidal pore model, the peptides are oriented vertically and interact with the lipid heads at the membrane–water interface. This interaction induces a curvature in the membrane, leading to the formation of a pore with a water-filled core at the center. The hydrophilic residues of the peptides interact with the lipid heads, while the hydrophobic regions penetrate the bilayer. This structural change results in a distortion of the bilayer, where the lipid monolayer bends around the pore, forming a toroidal shape. The resulting pores allow the passage of ions and small molecules, disrupting the cell’s internal balance and contributing to cell death [83,84,85].
3.2. Impact on Cell Wall Formation
3.3. Interference with Intracellular Metabolism
3.4. Induction of Immune Responses
4. Biomedical Applications of Antimicrobial Peptides
4.1. Synthetic Polymers
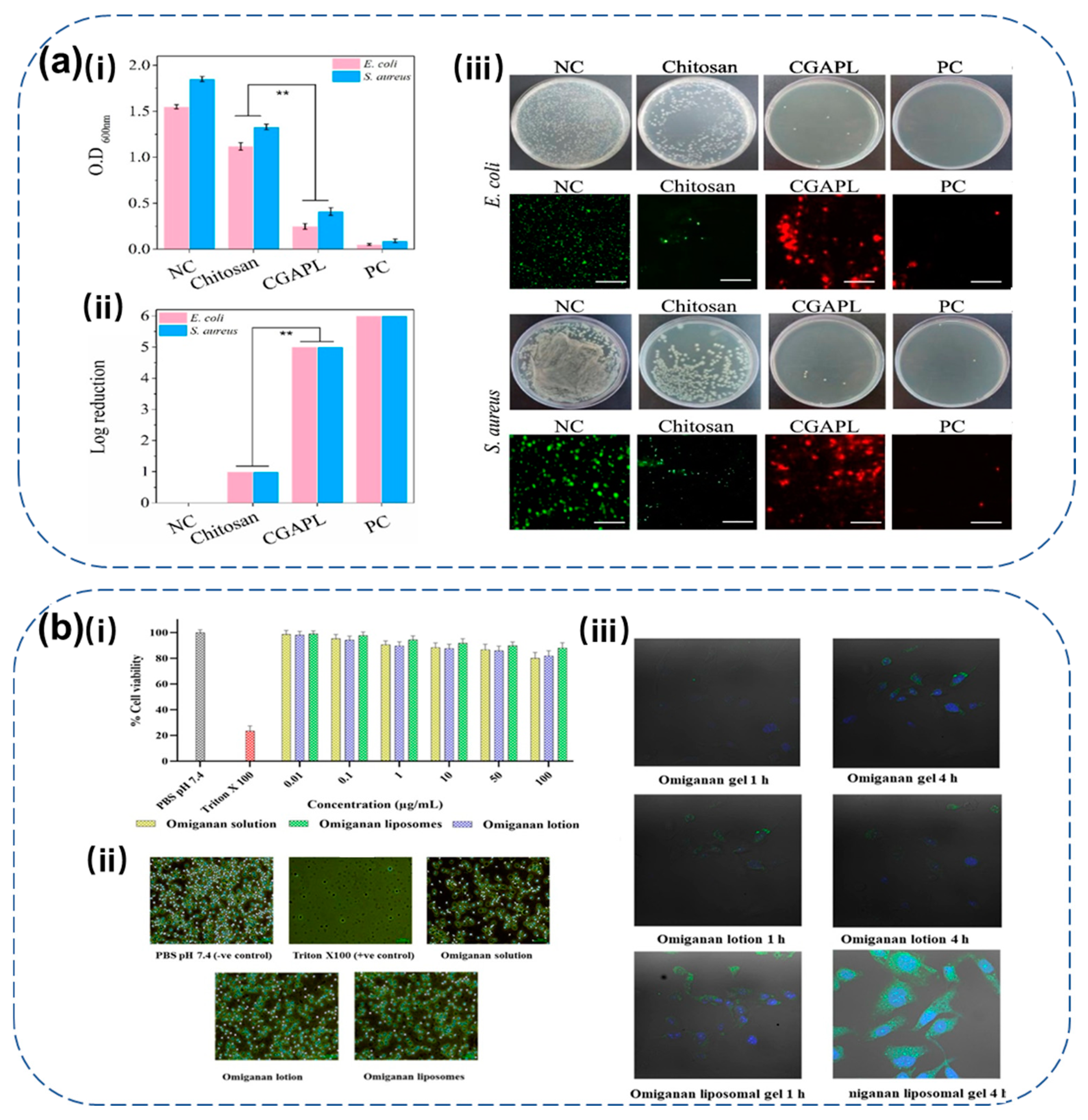
4.2. Liposomes
4.3. Hydrogels
4.4. Metal Nanoparticles
4.5. Combination with Multiple Biomaterials
5. Design and Optimization of Antimicrobial Peptides
5.1. Structure-Based Optimization of Antimicrobial Peptides
5.1.1. Peptide Truncation and Amino Acid Substitution
5.1.2. Cyclization and Modifications
5.2. Computer-Aided Design of Antimicrobial Peptides
| ID | Amino Acid Sequence | Activity | Target Site | 3D Structure |
|---|---|---|---|---|
| Magainin-2 [F16W] | GIGKFLHSAKKFGKAWVGEIMNS | G+ bacteria, G− bacteria, Mammalian Cell | Lipid Bilayer | β-sheet |
| Dermaseptin S4 (1–13) AMD [M4K] | ALWKTLLKKVLKA | G+ bacteria, G− bacteria, Parasite, Fungus, Mammalian Cell | Lipid Bilayer | β-sheet |
| Dermaseptin S4 (1–16) [M4K] | ALWKTLLKKVLKAAAK | G+ bacteria, G− bacteria, Virus, Parasite, Fungus, Mammalian Cell | Lipid Bilayer, Virus entry | β-sheet |
| Dermaseptin S4 [M4K] [N20K] | ALWKTLLKKVLKAAAKAALKAVLVGANA | G+ bacteria, G− bacteria, Virus, Parasite, Cancer, Fungus, Mammalian Cell, Biofilm | Lipid Bilayer, Virus entry | β-sheet |
| Dermaseptin S4 (5–15) AMD | TLLKKVLKAAA | G+ bacteria, G− bacteria | Lipid Bilayer | β-sheet |
| Dermaseptin S4 (4–15) AMD [M4K] | KTLLKKVLKAAA | G+ bacteria, G− bacteria | Lipid Bilayer | β-sheet |
| Magainin-2 [S8K,A9V,K11S,A15S,F16W,V17I] | GIGHFLHKVKSFGKSWIGEIMNS | G+ bacteria, G− bacteria, Mammalian Cell | Lipid Bilayer | β-sheet |
| Magainin-2 [L6G,H7K,S8A,K10A,K11H,A15K,F16W] | GIAKFGKAAAHFGKKWVGELMNS | G+ bacteria, G− bacteria, Mammalian Cell | Lipid Bilayer | β-sheet |
| CAP7 (1–20) [L6K,I13K] | GLRKRKRKFRNKKKEKLKKI | G+ bacteria, G− bacteria | Lipid Bilayer | β-sheet |
| CAP7 (1–20) [R5A,K16A] | GLRKALRKFRNKIKEALKKI | G+ bacteria, G− bacteria | Lipid Bilayer | β-sheet |
| LL-37 fragment KR-12 | KRIVQRIKDFLR | G+ bacteria, G− bacteria, Virus, Cancer, Fungus, Mammalian Cell, Biofilm | Lipid Bilayer, Virus replication | β-sheet |
| LL-37 (13–32) [I13G,G14I,E16Q] | GIKQFKRIVQRIKDFLRNLV | Virus, Mammalian Cell | Virus replication | β-sheet |
| Tritrpticin [V1R,W7VC]-R | RRRFPWVCWPFLRRR | Fungus | Lipid Bilayer | β-sheet |
| Cathelicidin-6 (1–15) | GRFKRFRKKFKKLFK | Virus, Mammalian Cell | Virus replication | β-sheet |
| Cathelicidin-6 (1–18) [F6I,F10L, L17I] | GRFKRIRKKLKKLFKKIS | Virus, Mammalian Cell | Virus replication | β-sheet |
| CP26, MBI 26 | KWKSFIKKLTSAAKKVVTTAKPLISS | G+ bacteria, G− bacteria, Fungus, Mammalian Cell | Lipid Bilayer | β-sheet |
| CEME | KWKLFKKIGIGAVLKVLTTGLPALIS | G+ bacteria, G− bacteria, Fungus | Lipid Bilayer | β-sheet |
| CEMA, MBI-28 | KWKLFKKIGIGAVLKVLTTGLPALKLTK | G+ bacteria, G− bacteria, Mammalian Cell | Lipid Bilayer | β-sheet |
| CP29, MBI 29 | KWKSFIKKLTTAVKKVLTTGLPALIS | G+ bacteria, G− bacteria, Fungus, Mammalian Cell | Lipid Bilayer | β-sheet |
| BP100 | KKLFKKILKYL | G+ bacteria, G− bacteria, Cancer, Fungus, Mammalian Cell | Lipid Bilayer | β-sheet |
| P18 | KWKLFKKIPKFLHLAKKF | G+, G−, Cancer, Fungus, Mammalian Cell | Lipid Bilayer | β-sheet |
| Loop region of human lactoferricin | FQWQRNMRKVRGPPVS | G+ bacteria, G− bacteria | Lipid Bilayer | β-sheet |
| [RW]5 | RWRWRWRWRW | G+ bacteria, G− bacteria, Fungus, Mammalian Cell | Lipid Bilayer | β-sheet |
| Amphipathic-1l, K6L9 | LKLLKKLLKKLLKLL | G+ bacteria, G− bacteria, Mammalian Cell | Lipid Bilayer | β-sheet |
| (RW)3 | RWRWRW | G+, G−, Cancer, Fungus, Mammalian Cell | Lipid Bilayer | β-sheet |
| B-38 | IKQLLHFFQRF | G+ bacteria, G− bacteria | Lipid Bilayer | Mixed-αβ |
| Thanatin (8–21) | IIYCNRRTGKCQRM | G+ bacteria, G− bacteria, Fungus | Lipid Bilayer | β-sheet |
| 50S ribosomal protein L1 HP (2–20) [Q16W] | AKKVFKRLEKLFSKIWNDK | G+ bacteria, G− bacteria, Fungus | Lipid Bilayer | β-sheet |
| Esculentin (1–21) | GIFSKLAGKKIKNLLISGLKG | G+ bacteria, G− bacteria, Cancer, Fungus, Mammalian Cell, Biofilm | Lipid Bilayer, DNA/RNA | β-sheet |
| Ovispirin-3 -OH | KNLRRIIRKIIHIIKKYG | G+ bacteria, G− bacteria, Cancer, Fungus, Mammalian Cell | Lipid Bilayer | β-sheet |
| Cecropin A (1–7) + Melittine (2–9), CM15 | KWKLFKKIGAVLKVL | G+ bacteria, G− bacteria, Mammalian Cell, Biofilm | Lipid Bilayer | β-sheet |
| gp41w | KWASLWNWFNITNWLWYIK | G+ bacteria, G− bacteria, Mammalian Cell | Lipid Bilayer | Mixed-αβ |
| Anoplin [R5W] | GLLKWIKTLL | G+ bacteria, G− bacteria, Fungus, Mammalian Cell | Lipid Bilayer | β-sheet |
| Database | URL |
|---|---|
| APD3 | https://aps.unmc.edu/home (accessed on 20 March 2025) |
| Cybase | https://www.cybase.org.au/ (accessed on 20 March 2025) |
| BACTIBASE | http://gec.u-picardie.fr/adaptable/ (accessed on 20 March 2025) |
| PhytAMP | http://gec.u-picardie.fr/adaptable/ (accessed on 20 March 2025) |
| CAMP | https://camp.bicnirrh.res.in/ (accessed on 20 March 2025) |
| DADP | http://split4.pmfst.hr/dadp/ (accessed on 20 March 2025) |
| DBAASP v3 | https://www.dbaasp.org/home (accessed on 20 March 2025) |
| DRAMP | http://dramp.cpu-bioinfor.org/ (accessed on 20 March 2025) |
| Prediction Tool | URL |
|---|---|
| APD3 | https://aps.unmc.edu/home (accessed on 20 March 2025) |
| BAGEL | http://bagel.molgenrug.nl/ (accessed on 20 March 2025) |
| antiSMASH | https://antismash.secondarymetabolites.org/#!/start (accessed on 20 March 2025) |
| AMPA | https://tcoffee.crg.eu/apps/ampa/do (accessed on 20 March 2025) |
| AMP_Scanner | https://www.dveltri.com/ascan/ (accessed on 20 March 2025) |
| CyPred | http://biomine.cs.vcu.edu/servers/CyPred/ (accessed on 20 March 2025) |
| AVPpred | http://crdd.osdd.net/servers/avppred (accessed on 20 March 2025) |
| AntiBP3 | https://webs.iiitd.edu.in/raghava/antibp3/ (accessed on 20 March 2025) |
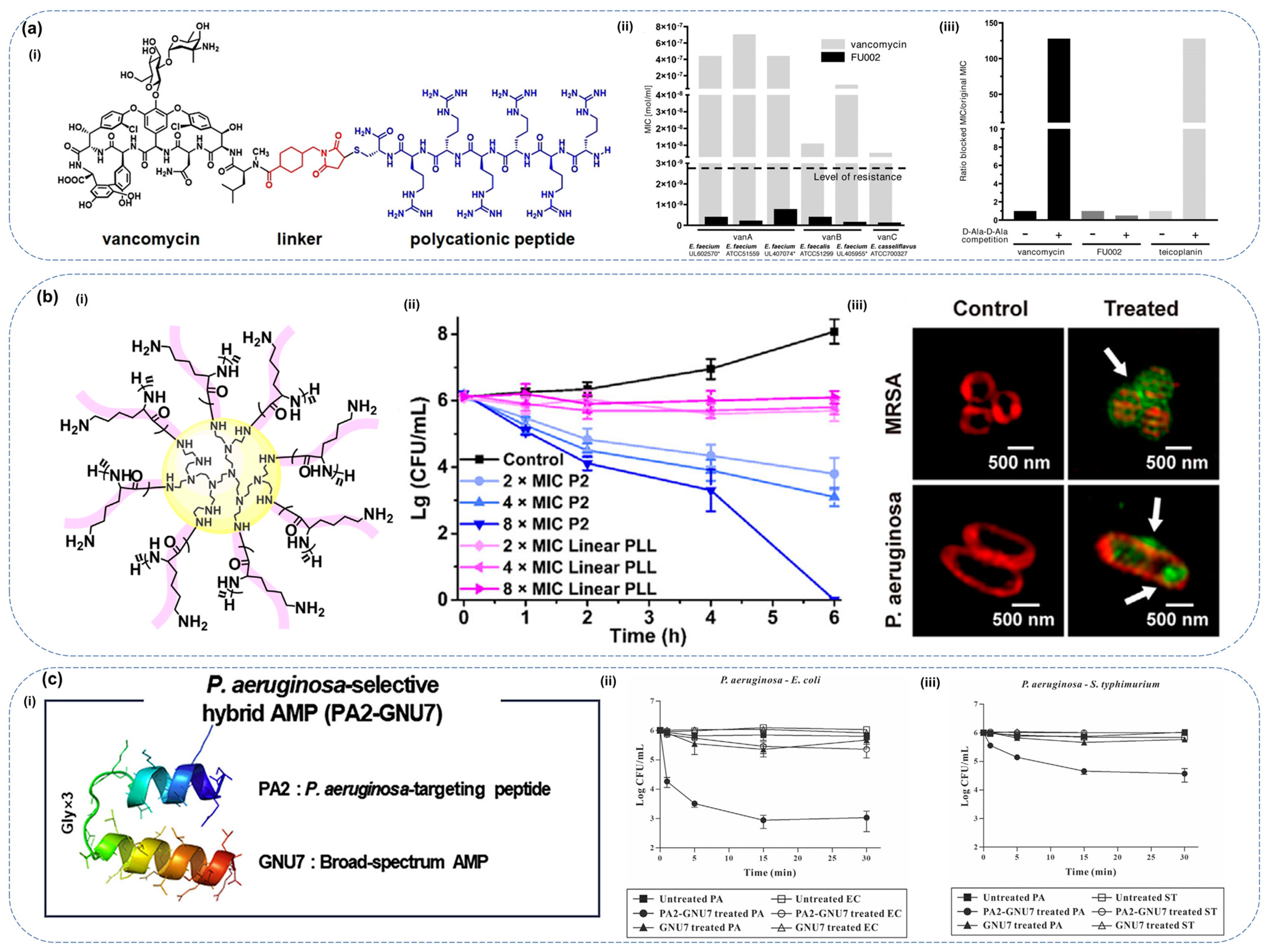
5.3. Conjugation of Antimicrobial Peptides
6. Challenges and Opportunities
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMPs | antimicrobial peptides |
| NMR | nuclear magnetic resonance |
| CD | circular dichroism |
| MIC | minimum inhibitory concentration |
| PKA | protein kinase A |
| RhoG | Ras homolog family member G |
| LPS | lipopolysaccharide |
| FDA | Food and Drug Administration |
| PLGA | poly (lactic-co-glycolic acid) |
| PEG | polyethylene glycol |
| PVA | polyvinyl alcohol |
| CS | chitosan |
| MIC | minimal inhibit concentration |
| GRAS | generally recognized as safe |
| GMP | glycomacropeptide |
| MBC | minimum bactericidal concentration |
| LNPs | lipid nanoparticles |
| HSPC | L-α-phosphatidylcholine |
| DPPG | 1,2-Dipalmitoyl-sn-glycero-3-phosphoglycerol |
| GMO | glyceryl monooleate |
| AZT | azithromycin |
| MRSA | methicillin-resistant Staphylococcus aureus |
| FBS | fetal bovine serum |
| PAO | plasma amine oxidase |
| ODEX | oxidized dextran |
| PRP | platelet-rich plasma |
| SBMA | sulfonate betaine ester |
| AAc | acrylic acid |
| MNPs | metal-based nanoparticles |
| AuNPs | gold nanoparticles |
| AgNPs | silver nanoparticles |
| OA1 | Odorranain-A-OA1 |
| CMCS/SA | chitosan/sodium alginate |
| R2AW | ranatuerin-2-AW |
| CAD | computer-aided design |
| SVM | Support Vector Machines |
| MDR | multidrug-resistant |
| VRE | vancomycin-resistant Enterococcus |
| HA | hydroxyapatite |
| hBD-3 | β-defensin-3 |
| hBD-4 | β-defensin-4 |
| PEI | polyethyleneimine |
| PLL | poly (L-lysine) |
| AMR | antimicrobial resistance |
| G+ | Gram-positive |
| G− | Gram-negative |
References
- Molineri, A.I.; Camussone, C.; Zbrun, M.V.; Archilla, G.S.; Cristiani, M.; Neder, V.; Calvinho, L.; Signorini, M. Antimicrobial Resistance of Staphylococcus Aureus Isolated from Bovine Mastitis: Systematic Review and Meta-Analysis. Prev. Vet. Med. 2021, 188, 105261. [Google Scholar] [CrossRef]
- van Eijk, M.; Boerefijn, S.; Cen, L.; Rosa, M.; Morren, M.J.H.; van der Ent, C.K.; Kraak, B.; Dijksterhuis, J.; Valdes, I.D.; Haagsman, H.P.; et al. Cathelicidin-Inspired Antimicrobial Peptides as Novel Antifungal Compounds. Med. Mycol. 2020, 58, 1073–1084. [Google Scholar] [CrossRef] [PubMed]
- Moretta, A.; Scieuzo, C.; Petrone, A.M.; Salvia, R.; Manniello, M.D.; Franco, A.; Lucchetti, D.; Vassallo, A.; Vogel, H.; Sgambato, A.; et al. Antimicrobial Peptides: A New Hope in Biomedical and Pharmaceutical Fields. Front. Cell. Infect. Microbiol. 2021, 11, 668632. [Google Scholar] [CrossRef]
- Monteiro, J.M.C.; Oliveira, M.D.; Dias, R.S.; Nacif-Marçal, L.; Feio, R.N.; Ferreira, S.O.; Oliveira, L.L.; Silva, C.C.; Paula, S.O. The Antimicrobial Peptide HS-1 Inhibits Dengue Virus Infecstion. Virology 2018, 514, 79–87. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, C.; Hansen, S.A.; Mitchell, W.J.; Zhang, M.Z.; Zhang, S. Antimicrobial Efficacy and Toxicity of Novel CAMPs against P. aeruginosa Infection in a Murine Skin Wound Infection Model. BMC Microbiol. 2019, 19, 293. [Google Scholar] [CrossRef]
- Nicolas, P.; Mor, A. Peptides as Weapons against Microorganisms in the Chemical Defense System of Vertebrates. Annu. Rev. Microbiol. 1995, 49, 277–304. [Google Scholar] [CrossRef]
- Magana, M.; Pushpanathan, M.; Santos, A.L.; Leanse, L.; Fernandez, M.; Ioannidis, A.; Giulianotti, M.A.; Apidianakis, Y.; Bradfute, S.; Ferguson, A.L.; et al. The Value of Antimicrobial Peptides in the Age of Resistance. Lancet Infect. Dis. 2020, 20, e216–e230. [Google Scholar] [CrossRef]
- Kapoor, G.; Saigal, S.; Elongavan, A. Action and Resistance Mechanisms of Antibiotics: A Guide for Clinicians. J. Anaesthesiol. Clin. Pharmacol. 2017, 33, 300–305. [Google Scholar] [CrossRef]
- Baran, A.; Kwiatkowska, A.; Potocki, L. Antibiotics and Bacterial Resistance—A Short Story of an Endless Arms Race. Int. J. Mol. Sci. 2023, 24, 5777. [Google Scholar] [CrossRef]
- Bucataru, C.; Ciobanasu, C. Antimicrobial Peptides: Opportunities and Challenges in Overcoming Resistance. Microbiol. Res. 2024, 286, 127822. [Google Scholar] [CrossRef]
- Ma, B.; Fang, C.; Lu, L.; Wang, M.; Xue, X.; Zhou, Y.; Li, M.; Hu, Y.; Luo, X.; Hou, Z. The Antimicrobial Peptide Thanatin Disrupts the Bacterial Outer Membrane and Inactivates the NDM-1 Metallo-β-Lactamase. Nat. Commun. 2019, 10, 3517. [Google Scholar] [CrossRef] [PubMed]
- Baharin, N.H.Z.; Mokhtar, N.F.K.; Desa, M.N.M.; Gopalsamy, B.; Zaki, N.N.M.; Yuswan, M.H.; Muthanna, A.; Dzaraly, N.D.; Abbasiliasi, S.; Hashim, A.M.; et al. The Characteristics and Roles of Antimicrobial Peptides as Potential Treatment for Antibiotic-Resistant Pathogens: A Review. PeerJ 2021, 9, e12193. [Google Scholar] [CrossRef] [PubMed]
- Meena, D.K.; Jayanthi, M. Monitoring Antibiotic Use in Public Health Care Facilities of South Indian Union Territory: A Step to Promote Rational Use of Antibiotics. Cureus 2021, 13, e18431. [Google Scholar] [CrossRef] [PubMed]
- Santos-Júnior, C.D.; Torres, M.D.T.; Duan, Y.; del Río, Á.R.; Schmidt, T.S.B.; Chong, H.; Fullam, A.; Kuhn, M.; Zhu, C.; Houseman, A.; et al. Discovery of Antimicrobial Peptides in the Global Microbiome with Machine Learning. Cell 2024, 187, 3761–3778.e16. [Google Scholar] [CrossRef]
- Sabin, A.B. A Study Of The Therapeutic Mechanism Of Antipneumococcic Serum On The Experimental Dermal Pneumococcus Infection In Rabbits. J. Exp. Med. 1933, 57, 139–163. [Google Scholar] [CrossRef]
- Dömer, D.; Walther, T.; Möller, S.; Behnen, M.; Laskay, T. Neutrophil Extracellular Traps Activate Proinflammatory Functions of Human Neutrophils. Front. Immunol. 2021, 12, 636954. [Google Scholar] [CrossRef]
- Berkowitz, B.A.; Bevins, C.L.; Zasloff, M.A. Magainins: A New Family of Membrane-Active Host Defense Peptides. Biochem. Pharmacol. 1990, 39, 625–629. [Google Scholar] [CrossRef]
- Brady, D.; Grapputo, A.; Romoli, O.; Sandrelli, F. Insect Cecropins, Antimicrobial Peptides with Potential Therapeutic Applications. Int. J. Mol. Sci. 2019, 20, 5862. [Google Scholar] [CrossRef]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial Peptides: Classification, Design, Application and Research Progress in Multiple Fields. Front. Microbiol. 2020, 11, 582779. [Google Scholar] [CrossRef]
- Kościuczuk, E.M.; Lisowski, P.; Jarczak, J.; Strzałkowska, N.; Jóźwik, A.; Horbańczuk, J.; Krzyżewski, J.; Zwierzchowski, L.; Bagnicka, E. Cathelicidins: Family of Antimicrobial Peptides. A Review. Mol. Biol. Rep. 2012, 39, 10957–10970. [Google Scholar] [CrossRef]
- Zhai, Y.J.; Feng, Y.; Ma, X.; Ma, F. Defensins: Defenders of Human Reproductive Health. Hum. Reprod. Update 2023, 29, 126–154. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Kaur, J.; Sharma, G.; Kashyap, P. Plant Derived Antimicrobial Peptides: Mechanism of Target, Isolation Techniques, Sources and Pharmaceutical Applications. J. Food Biochem. 2022, 46, e14348. [Google Scholar] [CrossRef] [PubMed]
- de Veer, S.J.; Kan, M.W.; Craik, D.J. Cyclotides: From Structure to Function. Chem. Rev. 2019, 119, 12375–12421. [Google Scholar] [CrossRef] [PubMed]
- Kamimori, H.; Hall, K.; Craik, D.J.; Aguilar, M.I. Studies on the Membrane Interactions of the Cyclotides Kalata B1 and Kalata B6 on Model Membrane Systems by Surface Plasmon Resonance. Anal. Biochem. 2005, 337, 149–153. [Google Scholar] [CrossRef]
- Wu, J.; Zang, M.; Wang, S.; Zhao, B.; Bai, J.; Xu, C.; Shi, Y.; Qiao, X. Nisin: From a Structural and Meat Preservation Perspective. Food Microbiol. 2023, 111, 104207. [Google Scholar] [CrossRef]
- Peng, S.Y.; You, R.I.; Lai, M.J.; Lin, N.T.; Chen, L.K.; Chang, K.C. Highly Potent Antimicrobial Modified Peptides Derived from the Acinetobacter Baumannii Phage Endolysin LysAB2. Sci. Rep. 2017, 7, 11477. [Google Scholar] [CrossRef]
- Wei, L.; Wu, J.; Liu, H.; Yang, H.; Rong, M.; Li, D.; Zhang, P.; Han, J.; Lai, R. A Mycobacteriophage-derived Trehalose-6,6′-dimycolate-binding Peptide Containing Both Antimycobacterial and Anti-inflammatory Abilities. FASEB J. 2013, 27, 3067–3077. [Google Scholar] [CrossRef]
- Sutton, J.M.; Pritts, T.A. Human Beta-Defensin 3: A Novel Inhibitor of Staphylococcus-Produced Biofilm Production. Commentary on “Human β-Defensin 3 Inhibits Antibiotic-Resistant Staphylococcus Biofilm Formation”. J. Surg. Res. 2014, 186, 99–100. [Google Scholar] [CrossRef]
- Braffman, N.R.; Piscotta, F.J.; Hauver, J.; Campbell, E.A.; Link, A.J.; Darst, S.A. Structural Mechanism of Transcription Inhibition by Lasso Peptides Microcin J25 and Capistruin. Proc. Natl. Acad. Sci. USA 2019, 116, 1273–1278. [Google Scholar] [CrossRef]
- Arias, D.C.H.; Toro, L.J.; Ramirez, G.A.T.; Osorio-Méndez, J.F.; Rodríguez-Carlos, A.; Valle, J.; Marín-Luevano, S.P.; Rivas-Santiago, B.; Andreu, D.; Osorio, J.C.C. Novel Antimicrobial Cecropins Derived from O. Curvicornis and D. Satanas Dung Beetles. Peptides 2021, 145, 170626. [Google Scholar] [CrossRef]
- Cardoso, M.H.; Meneguetti, B.T.; Costa, B.O.; Buccini, D.F.; Oshiro, K.G.N.; Preza, S.L.E.; Carvalho, C.M.E.; Migliolo, L.; Franco, O.L. Non-Lytic Antibacterial Peptides That Translocate Through Bacterial Membranes to Act on Intracellular Targets. Int. J. Mol. Sci. 2019, 20, 4877. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, S.; Li, D.; Chen, P.; Han, S.; Zhao, G.; Chen, Y.; Zhao, J.; Xiong, J.; Qiu, J.; et al. Human Cathelicidin Inhibits SARS-CoV-2 Infection: Killing Two Birds with One Stone. ACS Infect. Dis. 2021, 7, 1545–1554. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Wang, G.; Yue, B.; Zhou, S.; Zhang, M. TO17: A Teleost Antimicrobial Peptide That Induces Degradation of Bacterial Nucleic Acids and Inhibits Bacterial Infection in Red Drum, Sciaenops Ocellatus. Fish. Shellfish. Immunol. 2018, 72, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Camargo, C.; Salazar, V.A.; Barrero-Guevara, L.; Camargo, S.; Mosquera, A.; Groot, H.; Boix, E. Unveiling the Multifaceted Mechanisms of Antibacterial Activity of Buforin II and Frenatin 2.3S Peptides from Skin Micro-Organs of the Orinoco Lime Treefrog (Sphaenorhynchus Lacteus). Int. J. Mol. Sci. 2018, 19, 2170. [Google Scholar] [CrossRef]
- Kang, H.K.; Seo, C.H.; Luchian, T.; Park, Y. Pse-T2, an Antimicrobial Peptide with High-Level, Broad-Spectrum Antimicrobial Potency and Skin Biocompatibility against Multidrug-Resistant Pseudomonas Aeruginosa Infection. Antimicrob. Agents Chemother. 2018, 62, e01493-18. [Google Scholar] [CrossRef]
- Samakovlis, C.; Kylsten, P.; Kimbrell, D.A.; Engström, A.; Hultmark, D. The Andropin Gene and Its Product, a Male-Specific Antibacterial Peptide in Drosophila melanogaster. EMBO J. 1991, 10, 163–169. [Google Scholar] [CrossRef]
- Hilchie, A.L.; Vale, R.; Zemlak, T.S.; Hoskin, D.W. Generation of a Hematologic Malignancy-Selective Membranolytic Peptide from the Antimicrobial Core (RRWQWR) of Bovine Lactoferricin. Exp. Mol. Pathol. 2013, 95, 192–198. [Google Scholar] [CrossRef]
- Dickinson, L.; Russell, V.; Dunn, P.E. A Family of Bacteria-Regulated, Cecropin D-like Peptides from Manduca sexta. J. Biol. Chem. 1988, 263, 19424–19429. [Google Scholar] [CrossRef]
- Simmaco, M.; Kreil, G.; Barra, D. Bombinins, Antimicrobial Peptides from Bombina Species. BBA-Biomembr. 2009, 1788, 1551–1555. [Google Scholar] [CrossRef]
- Dennison, S.R.; Harris, F.; Phoenix, D.A. Investigations into the Potential Anticancer Activity of Maximin H5. Biochimie 2017, 137, 29–34. [Google Scholar] [CrossRef]
- Tian, M.; Wang, K.; Liang, Y.; Chai, J.; Wu, J.; Zhang, H.; Huang, X.; Chen, X.; Xu, X. The First Brevinin-1 Antimicrobial Peptide with LPS-Neutralizing and Anti-Inflammatory Activities in Vitro and in Vivo. Front. Microbiol. 2023, 14, 1102576. [Google Scholar] [CrossRef]
- Chen, J.; Yu, C.G.; Zhou, M.M.; Zhang, G.J.; Su, H.L.; Ding, G.H.; Wei, L.; Lin, Z.H.; Ma, L. An Esculentin-1 Homolog from a Dark-Spotted Frog (Pelophylax nigromaculatus) Possesses Antibacterial and Immunoregulatory Properties. BMC Veter. Res. 2024, 20, 164. [Google Scholar] [CrossRef]
- Kato, E.; Uenishi, Y.; Inagaki, Y.; Kurokawa, M.; Kawabata, J. Isolation of Rugosin A, B and Related Compounds as Dipeptidyl Peptidase-IV Inhibitors from Rose Bud Extract Powder. Biosci. Biotechnol. Biochem. 2016, 80, 2087–2092. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sun, D.; Eccleston, E.D.; Fallon, A.M. Cloning and Expression of Three Cecropin CDNAs from a Mosquito Cell Line. FEBS Lett. 1999, 454, 147–151. [Google Scholar] [CrossRef]
- Ramos-Martín, F.; Herrera-León, C.; D’Amelio, N. Bombyx Mori Cecropin D Could Trigger Cancer Cell Apoptosis by Interacting with Mitochondrial Cardiolipin. Biochim. Biophys. Acta Biomembr. 2022, 1864, 184003. [Google Scholar] [CrossRef]
- Memariani, H.; Memariani, M. Anti-Fungal Properties and Mechanisms of Melittin. Appl. Microbiol. Biotechnol. 2020, 104, 6513–6526. [Google Scholar] [CrossRef]
- Rokitskaya, T.I.; Kolodkin, N.I.; Kotova, E.A.; Antonenko, Y.N. Indolicidin Action on Membrane Permeability: Carrier Mechanism versus Pore Formation. Biochim. Biophys. Acta Biomembr. 2011, 1808, 91–97. [Google Scholar] [CrossRef]
- Arias, M.; Haney, E.F.; Hilchie, A.L.; Corcoran, J.A.; Hyndman, M.E.; Hancock, R.E.W.; Vogel, H.J. Selective Anticancer Activity of Synthetic Peptides Derived from the Host Defence Peptide Tritrpticin. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183228. [Google Scholar] [CrossRef]
- Basso, V.; Garcia, A.; Tran, D.Q.; Schaal, J.B.; Tran, P.; Ngole, D.; Aqeel, Y.; Tongaonkar, P.; Ouellette, A.J.; Selsted, M.E. Fungicidal Potency and Mechanisms of θ-Defensins against Multidrug-Resistant Candida Species. Antimicrob. Agents Chemother. 2018, 62, e00111-18. [Google Scholar] [CrossRef]
- Goyffon, M.; Saul, F.; Faure, G. Relationships between venomous function and innate immune function. Biol. Aujourdhui 2015, 209, 195–210. [Google Scholar] [CrossRef]
- Belmadani, A.; Semlali, A.; Rouabhia, M. Dermaseptin-S1 Decreases Candida Albicans Growth, Biofilm Formation and the Expression of Hyphal Wall Protein 1 and Aspartic Protease Genes. J. Appl. Microbiol. 2018, 125, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, M.; Ochiai, A.; Kondo, H.; Fukuda, S.; Ishiyama, Y.; Saitoh, E.; Kato, T.; Tanaka, T. Pyrrhocoricin, a Proline-Rich Antimicrobial Peptide Derived from Insect, Inhibits the Translation Process in the Cell-Free Escherichia coli Protein Synthesis System. J. Biosci. Bioeng. 2016, 121, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Krizsan, A.; Prahl, C.; Goldbach, T.; Knappe, D.; Hoffmann, R. Short Proline-Rich Antimicrobial Peptides Inhibit Either the Bacterial 70S Ribosome or the Assembly of Its Large 50S Subunit. ChemBioChem 2015, 16, 2304–2308. [Google Scholar] [CrossRef] [PubMed]
- Soletti, R.C.; del Barrio, L.; Daffre, S.; Miranda, A.; Borges, H.L.; Moura-Neto, V.; Lopez, M.G.; Gabilan, N.H. Peptide Gomesin Triggers Cell Death through L-Type Channel Calcium Influx, MAPK/ERK, PKC and PI3K Signaling and Generation of Reactive Oxygen Species. Chem. Biol. Interact. 2010, 186, 135–143. [Google Scholar] [CrossRef]
- Kaneko, Y.; Furukawa, S.; Tanaka, H.; Yamakawa, M. Expression of Antimicrobial Peptide Genes Encoding Enbocin and Gloverin Isoforms in the Silkworm, Bombyx mori. Biosci. Biotechnol. Biochem. 2007, 71, 2233–2241. [Google Scholar] [CrossRef]
- Wang, G. Structures of Human Host Defense Cathelicidin LL-37 and Its Smallest Antimicrobial Peptide KR-12 in Lipid Micelles. J. Biol. Chem. 2008, 283, 32637–32643. [Google Scholar] [CrossRef]
- Huang, D.; Hudson, B.C.; Gao, Y.; Roberts, E.K.; Paravastu, A.K. Solid-State NMR Structural Characterization of Self-Assembled Peptides with Selective 13C and 15N Isotopic Labels. Methods Mol. Biol. 2018, 1777, 23–68. [Google Scholar] [CrossRef]
- Powers, J.P.S.; Hancock, R.E.W. The Relationship between Peptide Structure and Antibacterial Activity. Peptides 2003, 24, 1681–1691. [Google Scholar] [CrossRef]
- Guha, S.; Ferrie, R.P.; Ghimire, J.; Ventura, C.R.; Wu, E.; Sun, L.; Kim, S.Y.; Wiedman, G.R.; Hristova, K.; Wimley, W.C. Applications and Evolution of Melittin, the Quintessential Membrane Active Peptide. Biochem. Pharmacol. 2021, 193, 114769. [Google Scholar] [CrossRef]
- Semeraro, E.F.; Pajtinka, P.; Marx, L.; Kabelka, I.; Leber, R.; Lohner, K.; Vácha, R.; Pabst, G. Magainin 2 and PGLa in Bacterial Membrane Mimics IV: Membrane Curvature and Partitioning. Biophys. J. 2022, 121, 4689–4701. [Google Scholar] [CrossRef]
- Dash, R.; Bhattacharjya, S. Thanatin: An Emerging Host Defense Antimicrobial Peptide with Multiple Modes of Action. Int. J. Mol. Sci. 2021, 22, 1522. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, S.; Silva, P.M.; Felício, M.R.; de Medeiros, L.N.; Kurtenbach, E.; Santos, N.C. Psd1 Effects on Candida Albicans Planktonic Cells and Biofilms. Front. Cell. Infect. Microbiol. 2017, 7, 249. [Google Scholar] [CrossRef]
- Aumer, T.; Voisin, S.N.; Knobloch, T.; Landon, C.; Bulet, P. Impact of an Antifungal Insect Defensin on the Proteome of the Phytopathogenic Fungus Botrytis cinerea. J. Proteome Res. 2020, 19, 1131–1146. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yao, Y.; Wei, P.; Song, C.; Wan, S.; Yang, S.; Zhu, G.M.; Liu, H.M. Housefly Phormicin Inhibits Staphylococcus Aureus and MRSA by Disrupting Biofilm Formation and Altering Gene Expression in Vitro and in Vivo. Int. J. Biol. Macromol. 2021, 167, 1424–1434. [Google Scholar] [CrossRef]
- Mangano, K.; Klepacki, D.; Ohanmu, I.; Baliga, C.; Huang, W.; Brakel, A.; Krizsan, A.; Polikanov, Y.S.; Hoffmann, R.; Vázquez-Laslop, N.; et al. Inhibition of Translation Termination by the Antimicrobial Peptide Drosocin. Nat. Chem. Biol. 2023, 19, 1082–1090. [Google Scholar] [CrossRef]
- Viel, J.H.; Jaarsma, A.H.; Kuipers, O.P. Heterologous Expression of Mersacidin in Escherichia coli Elucidates the Mode of Leader Processing. ACS Synth. Biol. 2021, 10, 600–608. [Google Scholar] [CrossRef]
- Li, J.F.; Zhang, J.X.; Li, G.; Xu, Y.Y.; Lu, K.; Wang, Z.G.; Liu, J.P. Antimicrobial Activity and Mechanism of Peptide CM4 against Pseudomonas aeruginosa. Food Funct. 2020, 11, 7245–7254. [Google Scholar] [CrossRef]
- Rádis-Baptista, G. Cell-Penetrating Peptides Derived from Animal Venoms and Toxins. Toxins 2021, 13, 147. [Google Scholar] [CrossRef]
- Frimodt-Møller, J.; Campion, C.; Nielsen, P.E.; Løbner-Olesen, A. Translocation of Non-Lytic Antimicrobial Peptides and Bacteria Penetrating Peptides across the Inner Membrane of the Bacterial Envelope. Curr. Genet. 2022, 68, 83–90. [Google Scholar] [CrossRef]
- Shah, P.; Hsiao, F.S.; Ho, Y.; Chen, C. The Proteome Targets of Intracellular Targeting Antimicrobial Peptides. Proteomics 2016, 16, 1225–1237. [Google Scholar] [CrossRef]
- Lohner, K. New Strategies for Novel Antibiotics: Peptides Targeting Bacterial Cell Membranes. Gen. Physiol. Biophys. 2009, 28, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, K. Why and How Are Peptide–Lipid Interactions Utilized for Self-Defense? Magainins and Tachyplesins as Archetypes. Biochim. Biophys. Acta Biomembr. 1999, 1462, 1–10. [Google Scholar] [CrossRef]
- Fuertes, G.; Giménez, D.; Esteban-Martín, S.; Sánchez-Muñoz, O.L.; Salgado, J. A Lipocentric View of Peptide-Induced Pores. Eur. Biophys. J. Biophy 2011, 40, 399–415. [Google Scholar] [CrossRef]
- Brogden, K.A. Antimicrobial Peptides: Pore Formers or Metabolic Inhibitors in Bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef]
- Zasloff, M. Antimicrobial Peptides of Multicellular Organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef]
- Bin Hafeez, A.; Jiang, X.; Bergen, P.J.; Zhu, Y. Antimicrobial Peptides: An Update on Classifications and Databases. Int. J. Mol. Sci. 2021, 22, 11691. [Google Scholar] [CrossRef]
- Ehrenstein, G.; Lecar, H. Electrically Gated Ionic Channels in Lipid Bilayers. Q. Rev. Biophys. 1977, 10, 1–34. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Shioyama, T.; Okamura, E.; Umemura, J.; Takenaka, T.; Takaishi, Y.; Fujita, T.; Miyajima, K. A Comparative Study on Interactions of α-Aminoisobutyric Acid Containing Antibiotic Peptides, Trichopolyn I and Hypelcin A with Phosphatidylcholine Bilayers. Biochim. Biophys. Acta Biomembr. 1991, 1070, 419–428. [Google Scholar] [CrossRef]
- Baumann, G.; Mueller, P. A Molecular Model of Membrane Excitability. J. Supramol. Struct. 1974, 2, 538–557. [Google Scholar] [CrossRef]
- Pouny, Y.; Rapaport, D.; Mor, A.; Nicolas, P.; Shai, Y. Interaction of Antimicrobial Dermaseptin and Its Fluorescently Labeled Analogs with Phospholipid Membranes. Biochemistry 1992, 31, 12416–12423. [Google Scholar] [CrossRef]
- Hancock, R. The Bacterial Outer Membrane as a Drug Barrier. Trends Microbiol. 1997, 5, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Leontiadou, H.; Mark, A.E.; Marrink, S.J. Antimicrobial Peptides in Action. J. Am. Chem. Soc. 2006, 128, 12156–12161. [Google Scholar] [CrossRef] [PubMed]
- Ludtke, S.J.; He, K.; Heller, W.T.; Harroun, T.A.; Yang, L.; Huang, H.W. Membrane Pores Induced by Magainin. Biochemistry 1996, 35, 13723–13728. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, K.; Murase, O.; Fujii, N.; Miyajima, K. An Antimicrobial Peptide, Magainin 2, Induced Rapid Flip-Flop of Phospholipids Coupled with Pore Formation and Peptide Translocation. Biochemistry 1996, 35, 11361–11368. [Google Scholar] [CrossRef]
- Bechinger, B.; Gorr, S.U. Antimicrobial Peptides: Mechanisms of Action and Resistance. J. Dent. Res. 2017, 96, 254–260. [Google Scholar] [CrossRef]
- Choi, H.; Rangarajan, N.; Weisshaar, J.C. Lights, Camera, Action! Antimicrobial Peptide Mechanisms Imaged in Space and Time. Trends Microbiol. 2016, 24, 111–122. [Google Scholar] [CrossRef]
- Rashid, R.; Veleba, M.; Kline, K.A. Focal Targeting of the Bacterial Envelope by Antimicrobial Peptides. Front. Cell. Dev. Biol. 2016, 4, 55. [Google Scholar] [CrossRef]
- Chen, X.; Hirt, H.; Li, Y.; Gorr, S.U.; Aparicio, C. Antimicrobial GL13K Peptide Coatings Killed and Ruptured the Wall of Streptococcus Gordonii and Prevented Formation and Growth of Biofilms. PLoS ONE 2014, 9, e111579. [Google Scholar] [CrossRef]
- Abdolhosseini, M.; Nandula, S.R.; Song, J.; Hirt, H.; Gorr, S.U. Lysine Substitutions Convert a Bacterial-Agglutinating Peptide into a Bactericidal Peptide That Retains Anti-Lipopolysaccharide Activity and Low Hemolytic Activity. Peptides 2012, 35, 231–238. [Google Scholar] [CrossRef]
- Shi, J.; Ross, C.R.; Chengappa, M.M.; Sylte, M.J.; McVey, D.S.; Blecha, F. Antibacterial Activity of a Synthetic Peptide (PR-26) Derived from PR-39, a Proline-Arginine-Rich Neutrophil Antimicrobial Peptide. Antimicrob. Agents Chemother. 1996, 40, 115–121. [Google Scholar] [CrossRef]
- Nicolas, P. Multifunctional Host Defense Peptides: Intracellular-targeting Antimicrobial Peptides. FEBS J. 2009, 276, 6483–6496. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Song, Y. Mechanism of Antimicrobial Peptides: Antimicrobial, Anti-Inflammatory and Antibiofilm Activities. Int. J. Mol. Sci. 2021, 22, 11401. [Google Scholar] [CrossRef] [PubMed]
- Kragol, G.; Hoffmann, R.; Chattergoon, M.A.; Lovas, S.; Cudic, M.; Bulet, P.; Condie, B.A.; Rosengren, K.J.; Montaner, L.J.; Otvos, L. Identification of Crucial Residues for the Antibacterial Activity of the Proline-rich Peptide, Pyrrhocoricin. Eur. J. Biochem. 2002, 269, 4226–4237. [Google Scholar] [CrossRef]
- Bamgbola, O. Review of Vancomycin-Induced Renal Toxicity: An Update. Ther. Adv. Endocrinol. Metab. 2016, 7, 136–147. [Google Scholar] [CrossRef]
- Otvos, L.; Snyder, C.; Condie, B.; Bulet, P.; Wade, J.D. Chimeric Antimicrobial Peptides Exhibit Multiple Modes of Action. Int. J. Pept. Res. Ther. 2005, 11, 29–42. [Google Scholar] [CrossRef]
- Otvos, J.L. The Short Proline-Rich Antibacterial Peptide Family. Cell. Mol. Life Sci. 2002, 59, 1138–1150. [Google Scholar] [CrossRef]
- Podda, E.; Benincasa, M.; Pacor, S.; Micali, F.; Mattiuzzo, M.; Gennaro, R.; Scocchi, M. Dual Mode of Action of Bac7, a Proline-Rich Antibacterial Peptide. Biochim. Biophys. Acta Gen. Subj. 2006, 1760, 1732–1740. [Google Scholar] [CrossRef]
- Li, X.; Zuo, S.; Wang, B.; Zhang, K.; Wang, Y. Antimicrobial Mechanisms and Clinical Application Prospects of Antimicrobial Peptides. Molecules 2022, 27, 2675. [Google Scholar] [CrossRef]
- Marchand, C.; Krajewski, K.; Lee, H.F.; Antony, S.; Johnson, A.A.; Amin, R.; Roller, P.; Kvaratskhelia, M.; Pommier, Y. Covalent Binding of the Natural Antimicrobial Peptide Indolicidin to DNA Abasic Sites. Nucleic Acids Res. 2006, 34, 5157–5165. [Google Scholar] [CrossRef]
- Röhrl, J.; Yang, D.; Oppenheim, J.J.; Hehlgans, T. Human β-Defensin 2 and 3 and Their Mouse Orthologs Induce Chemotaxis through Interaction with CCR2. J. Immunol. 2010, 184, 6688–6694. [Google Scholar] [CrossRef]
- Semple, F.; MacPherson, H.; Webb, S.; Cox, S.L.; Mallin, L.J.; Tyrrell, C.; Grimes, G.R.; Semple, C.A.; Nix, M.A.; Millhauser, G.L.; et al. Human Β-defensin 3 Affects the Activity of Pro-inflammatory Pathways Associated with MyD88 and TRIF. Eur. J. Immunol. 2011, 41, 3291–3300. [Google Scholar] [CrossRef] [PubMed]
- Pahar, B.; Madonna, S.; Das, A.; Albanesi, C.; Girolomoni, G. Immunomodulatory Role of the Antimicrobial LL-37 Peptide in Autoimmune Diseases and Viral Infections. Vaccines 2020, 8, 517. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhang, C.; Zhang, X.; Zhang, M.Z.; Rottinghaus, G.E.; Zhang, S. Structure-Function Analysis of Avian β-Defensin-6 and β-Defensin-12: Role of Charge and Disulfide Bridges. BMC Microbiol. 2016, 16, 210. [Google Scholar] [CrossRef]
- Marenah, L.; Flatt, P.R.; Orr, D.F.; Shaw, C.; Abdel-Wahab, Y.H.A. Skin Secretions of Rana Saharica Frogs Reveal Antimicrobial Peptides Esculentins-1 and -1B and Brevinins-1E and -2EC with Novel Insulin Releasing Activity. J. Endocrinol. 2006, 188, 1–9. [Google Scholar] [CrossRef]
- Vasu, S.; Ojo, O.O.; Moffett, R.C.; Conlon, J.M.; Flatt, P.R.; Abdel-Wahab, Y.H.A. Anti-Diabetic Actions of Esculentin-2CHa(1–30) and Its Stable Analogues in a Diet-Induced Model of Obesity-Diabetes. Amino Acids 2017, 49, 1705–1717. [Google Scholar] [CrossRef]
- Conlon, J.M.; Power, G.J.; Abdel-Wahab, Y.H.A.; Flatt, P.R.; Jiansheng, H.; Coquet, L.; Leprince, J.; Jouenne, T.; Vaudry, H. A Potent, Non-Toxic Insulin-Releasing Peptide Isolated from an Extract of the Skin of the Asian Frog, Hylarana Guntheri (Anura:Ranidae). Regul. Pept. 2008, 151, 153–159. [Google Scholar] [CrossRef]
- Marenah, L.; Flatt, P.R.; Orr, D.F.; Shaw, C.; Abdel-Wahab, Y.H.A. Characterization of Naturally Occurring Peptides in the Skin Secretion of Rana Pipiens Frog Reveal Pipinin-1 as the Novel Insulin-releasing Agent. Int. J. Pept. Res. Ther. 2005, 66, 204–210. [Google Scholar] [CrossRef]
- Mechkarska, M.; Ojo, O.O.; Meetani, M.A.; Coquet, L.; Jouenne, T.; Abdel-Wahab, Y.H.A.; Flatt, P.R.; King, J.D.; Conlon, J.M. Peptidomic Analysis of Skin Secretions from the Bullfrog Lithobates Catesbeianus (Ranidae) Identifies Multiple Peptides with Potent Insulin-Releasing Activity. Peptides 2011, 32, 203–208. [Google Scholar] [CrossRef]
- Soltaninejad, H.; Zare-Zardini, H.; Ordooei, M.; Ghelmani, Y.; Ghadiri-Anari, A.; Mojahedi, S.; Hamidieh, A.A. Antimicrobial Peptides from Amphibian Innate Immune System as Potent Antidiabetic Agents: A Literature Review and Bioinformatics Analysis. J. Diabetes Res. 2021, 2021, 2894722. [Google Scholar] [CrossRef]
- Huang, H.W. Daptomycin, Its Membrane-Active Mechanism vs. That of Other Antimicrobial Peptides. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183395. [Google Scholar] [CrossRef]
- Djayanti, K.; Maharjan, P.; Cho, K.H.; Jeong, S.; Kim, M.S.; Shin, M.C.; Min, K.A. Mesoporous Silica Nanoparticles as a Potential Nanoplatform: Therapeutic Applications and Considerations. Int. J. Mol. Sci. 2023, 24, 6349. [Google Scholar] [CrossRef] [PubMed]
- Nordström, R.; Malmsten, M. Delivery Systems for Antimicrobial Peptides. Adv. Colloid. Interface Sci. 2017, 242, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Water, J.J.; Smart, S.; Franzyk, H.; Foged, C.; Nielsen, H.M. Nanoparticle-Mediated Delivery of the Antimicrobial Peptide Plectasin against Staphylococcus Aureus in Infected Epithelial Cells. Eur. J. Pharm. Biopharm. 2015, 92, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Yang, N.; Mao, R.; Hao, Y.; Teng, D.; Huang, Y.; Wang, J. Polylactic Glycolic Acid-Mediated Delivery of Plectasin Derivative NZ2114 in Staphylococcus epidermidis Biofilms. Antibiotics 2024, 13, 228. [Google Scholar] [CrossRef]
- Deng, X.; Wang, H.; Fang, C.; Xu, M.; Chu, Z.; Li, M.; Hou, Z.; Qin, H. Hyaluronic Acid Based Nanoparticles That Mediate Sustained Thanatin Release Protect against NDM-1–Resistant Bacterial Infections in a Murine Model. Nanomedicine 2025, 63, 102796. [Google Scholar] [CrossRef]
- Sreekumar, S.; Goycoolea, F.M.; Moerschbacher, B.M.; Rivera-Rodriguez, G.R. Parameters Influencing the Size of Chitosan-TPP Nano- and Microparticles. Sci. Rep. 2018, 8, 4695. [Google Scholar] [CrossRef]
- Matica, M.A.; Aachmann, F.L.; Tøndervik, A.; Sletta, H.; Ostafe, V. Chitosan as a Wound Dressing Starting Material: Antimicrobial Properties and Mode of Action. Int. J. Mol. Sci. 2019, 20, 5889. [Google Scholar] [CrossRef]
- Rishi, P.; Bhogal, A.; Arora, S.; Pandey, S.K.; Verma, I.; Kaur, I.P. Improved Oral Therapeutic Potential of Nanoencapsulated Cryptdin Formulation against Salmonella Infection. Eur. J. Pharm. Sci. 2015, 72, 27–33. [Google Scholar] [CrossRef]
- Rivera, M.C.; Pinheiro, A.C.; Bourbon, A.I.; Cerqueira, M.A.; Vicente, A.A. Hollow Chitosan/Alginate Nanocapsules for Bioactive Compound Delivery. Int. J. Biol. Macromol. 2015, 79, 95–102. [Google Scholar] [CrossRef]
- Roshanak, S.; Yarabbi, H.; Movaffagh, J.; Shahidi, F. Fabrication and Characterization of Buforin I-Loaded Electrospun Chitosan/Polyethylene Oxide Nanofibrous Membranes with Antimicrobial Activity for Food Packing Applications. Polymers 2025, 17, 549. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, Preparation, and Applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- da Silveira, S.A.; Shorr, A.F. Critical Parameters for the Development of Novel Therapies for Severe and Resistant Infections—A Case Study on CAL02, a Non-Traditional Broad-Spectrum Anti-Virulence Drug. Antibiotics 2020, 9, 94. [Google Scholar] [CrossRef] [PubMed]
- Flühmann, B.; Ntai, I.; Borchard, G.; Simoens, S.; Mühlebach, S. Nanomedicines: The Magic Bullets Reaching Their Target? Eur. J. Pharm. Sci. 2019, 128, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Mu, L.M.; Ju, R.J.; Liu, R.; Bu, Y.Z.; Zhang, J.Y.; Li, X.Q.; Zeng, F.; Lu, W.L. Dual-Functional Drug Liposomes in Treatment of Resistant Cancers. Adv. Drug Deliv. Rev. 2017, 115, 46–56. [Google Scholar] [CrossRef]
- Herrera, C.V.; O’Connor, P.M.; Ratrey, P.; Ross, R.P.; Hill, C.; Hudson, S.P. Anionic Liposome Formulation for Oral Delivery of Thuricin CD, a Potential Antimicrobial Peptide Therapeutic. Int. J. Pharm. 2024, 654, 123918. [Google Scholar] [CrossRef]
- Hong, L.; Gontsarik, M.; Amenitsch, H.; Salentinig, S. Human Antimicrobial Peptide Triggered Colloidal Transformations in Bacteria Membrane Lipopolysaccharides. Small 2022, 18, e2104211. [Google Scholar] [CrossRef]
- Utterström, J.; Barriga, H.M.G.; Holme, M.N.; Selegård, R.; Stevens, M.M.; Aili, D. Peptide-Folding Triggered Phase Separation and Lipid Membrane Destabilization in Cholesterol-Rich Lipid Vesicles. Bioconjugate Chem. 2022, 33, 736–746. [Google Scholar] [CrossRef]
- Liu, X.; Li, Z.; Wang, X.; Chen, Y.; Wu, F.; Men, K.; Xu, T.; Luo, Y.; Yang, L. Novel Antimicrobial Peptide-Modified Azithromycin-Loaded Liposomes against Methicillin-Resistant Staphylococcus aureus. Int. J. Nanomed. 2016, 11, 6781–6794. [Google Scholar] [CrossRef]
- Kumar, A.; Kolar, S.S.; Zao, M.; McDermott, A.M.; Cai, C. Localization of Antimicrobial Peptides on Polymerized Liposomes Leading to Their Enhanced Efficacy against Pseudomonas aeruginosa. Mol. Biosyst. 2011, 7, 711–713. [Google Scholar] [CrossRef]
- Copling, A.; Akantibila, M.; Kumaresan, R.; Fleischer, G.; Cortes, D.; Tripathi, R.S.; Carabetta, V.J.; Vega, S.L. Recent Advances in Antimicrobial Peptide Hydrogels. Int. J. Mol. Sci. 2023, 24, 7563. [Google Scholar] [CrossRef]
- Hu, J.; Quan, Y.; Lai, Y.; Zheng, Z.; Hu, Z.; Wang, X.; Dai, T.; Zhang, Q.; Cheng, Y. A Smart Aminoglycoside Hydrogel with Tunable Gel Degradation, on-Demand Drug Release, and High Antibacterial Activity. J. Control. Release 2017, 247, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Kamali, A.; Moshiri, A.; Baharvand, H.; Daemi, H. Chemical Crosslinking of Biopolymeric Scaffolds: Current Knowledge and Future Directions of Crosslinked Engineered Bone Scaffolds. Int. J. Biol. Macromol. 2018, 107, 678–688. [Google Scholar] [CrossRef]
- Wei, J.; Wei, G.; Shang, Y.; Zhou, J.; Wu, C.; Wang, Q. Dissolution–Crystallization Transition within a Polymer Hydrogel for a Processable Ultratough Electrolyte. Adv. Mater. 2019, 31, e1900248. [Google Scholar] [CrossRef]
- Chen, C.; Hu, J.; Zeng, P.; Chen, Y.; Xu, H.; Lu, J.R. High Cell Selectivity and Low-Level Antibacterial Resistance of Designed Amphiphilic Peptide G(IIKK)3I-NH2. ACS Appl. Mater. Interfaces 2014, 6, 16529–16536. [Google Scholar] [CrossRef] [PubMed]
- Makovitzki, A.; Baram, J.; Shai, Y. Antimicrobial Lipopolypeptides Composed of Palmitoyl Di- and Tricationic Peptides: In Vitro and in Vivo Activities, Self-Assembly to Nanostructures, and a Plausible Mode of Action. Biochemistry 2008, 47, 10630–10636. [Google Scholar] [CrossRef]
- Chen, C.; Hu, J.; Zhang, S.; Zhou, P.; Zhao, X.; Xu, H.; Zhao, X.; Yaseen, M.; Lu, J.R. Molecular Mechanisms of Antibacterial and Antitumor Actions of Designed Surfactant-like Peptides. Biomaterials 2012, 33, 592–603. [Google Scholar] [CrossRef]
- Chen, C.; Pan, F.; Zhang, S.; Hu, J.; Cao, M.; Wang, J.; Xu, H.; Zhao, X.; Lu, J.R. Antibacterial Activities of Short Designer Peptides: A Link between Propensity for Nanostructuring and Capacity for Membrane Destabilization. Biomacromolecules 2010, 11, 402–411. [Google Scholar] [CrossRef]
- Bai, J.; Chen, C.; Wang, J.; Zhang, Y.; Cox, H.; Zhang, J.; Wang, Y.; Penny, J.; Waigh, T.; Lu, J.R.; et al. Enzymatic Regulation of Self-Assembling Peptide A9K2 Nanostructures and Hydrogelation with Highly Selective Antibacterial Activities. ACS Appl. Mater. Interfaces 2016, 8, 15093–15102. [Google Scholar] [CrossRef]
- Wei, S.; Xu, P.; Yao, Z.; Cui, X.; Lei, X.; Li, L.; Dong, Y.; Zhu, W.; Guo, R.; Cheng, B. A Composite Hydrogel with Co-Delivery of Antimicrobial Peptides and Platelet-Rich Plasma to Enhance Healing of Infected Wounds in Diabetes. Acta Biomater. 2021, 124, 205–218. [Google Scholar] [CrossRef]
- Qi, C.; Sun, Q.; Xiao, D.; Zhang, M.; Gao, S.; Guo, B.; Lin, Y. Tetrahedral Framework Nucleic Acids/Hyaluronic Acid-Methacrylic Anhydride Hybrid Hydrogel with Antimicrobial and Anti-Inflammatory Properties for Infected Wound Healing. Int. J. Oral. Sci. 2024, 16, 30. [Google Scholar] [CrossRef]
- Chen, H.; Song, Y.; Peng, Y.; Wang, M.; Dessie, W.; Duns, G.J.; Xu, L.; Luo, X.; Qin, Z. Hydrogel Complex Containing the Antimicrobial Peptide HX-12C Accelerates Healing of Infected Wounds. Macromol. Biosci. 2023, 23, e2200514. [Google Scholar] [CrossRef] [PubMed]
- Huan, Y.; Kong, Q.; Tang, Q.; Wang, Y.; Mou, H.; Ying, R.; Li, C. Antimicrobial Peptides/Ciprofloxacin-Loaded O-Carboxymethyl Chitosan/Self-Assembling Peptides Hydrogel Dressing with Sustained-Release Effect for Enhanced Anti-Bacterial Infection and Wound Healing. Carbohydr. Polym. 2022, 280, 119033. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhang, F.; Wei, Y.; Hu, Q.; Luo, Q.; Chen, C.; Wang, J.; Yang, L.; Luo, R.; Wang, Y. Dressing Blood-Contacting Materials by a Stable Hydrogel Coating with Embedded Antimicrobial Peptides for Robust Antibacterial and Antithrombus Properties. ACS Appl. Mater. Interfaces 2021, 13, 38947–38958. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, M.A. Some Interesting Properties of Metals Confined in Time and Nanometer Space of Different Shapes. Accounts. Chem. Res. 2001, 34, 257–264. [Google Scholar] [CrossRef]
- Moyano, D.F.; Rotello, V.M. Nano Meets Biology: Structure and Function at the Nanoparticle Interface. Langmuir 2011, 27, 10376–10385. [Google Scholar] [CrossRef]
- Kitov, P.I.; Bundle, D.R. On the Nature of the Multivalency Effect: A Thermodynamic Model. J. Am. Chem. Soc. 2003, 125, 16271–16284. [Google Scholar] [CrossRef]
- Yun, H.M.; Ahn, S.J.; Park, K.R.; Kim, M.J.; Kim, J.J.; Jin, G.Z.; Kim, H.W.; Kim, E.C. Magnetic Nanocomposite Scaffolds Combined with Static Magnetic Field in the Stimulation of Osteoblastic Differentiation and Bone Formation. Biomaterials 2016, 85, 88–98. [Google Scholar] [CrossRef]
- Lucky, S.S.; Soo, K.C.; Zhang, Y. Nanoparticles in Photodynamic Therapy. Chem. Rev. 2015, 115, 1990–2042. [Google Scholar] [CrossRef]
- Baptista, P.V.; McCusker, M.P.; Carvalho, A.; Ferreira, D.A.; Mohan, N.M.; Martins, M.; Fernandes, A.R. Nano-Strategies to Fight Multidrug Resistant Bacteria—“A Battle of the Titans”. Front. Microbiol 2018, 9, 1441. [Google Scholar] [CrossRef]
- Shamaila, S.; Zafar, N.; Riaz, S.; Sharif, R.; Nazir, J.; Naseem, S. Gold Nanoparticles: An Efficient Antimicrobial Agent against Enteric Bacterial Human Pathogen. Nanomaterials 2016, 6, 71. [Google Scholar] [CrossRef]
- Park, J.; Shin, E.; Yeom, J.H.; Choi, Y.; Joo, M.; Lee, M.; Kim, J.H.; Bae, J.; Lee, K. Gold Nanoparticle-DNA Aptamer-Assisted Delivery of Antimicrobial Peptide Effectively Inhibits Acinetobacter Baumannii Infection in Mice. J. Microbiol. 2022, 60, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.; Ilyas, H.; Ghosh, A.; Ali, H.; Ghorai, A.; Midya, A.; Jana, N.R.; Das, S.; Bhunia, A. Multivalent Gold Nanoparticle–Peptide Conjugates for Targeting Intracellular Bacterial Infections. Nanoscale 2017, 9, 14074–14093. [Google Scholar] [CrossRef] [PubMed]
- Comune, M.; Rai, A.; Chereddy, K.K.; Pinto, S.; Aday, S.; Ferreira, A.F.; Zonari, A.; Blersch, J.; Cunha, R.; Rodrigues, R.; et al. Antimicrobial Peptide-Gold Nanoscale Therapeutic Formulation with High Skin Regenerative Potential. J. Control. Release 2017, 262, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Khalandi, B.; Asadi, N.; Milani, M.; Davaran, S.; Abadi, A.; Abasi, E.; Akbarzadeh, A. A Review on Potential Role of Silver Nanoparticles and Possible Mechanisms of Their Actions on Bacteria. Drug Res. 2016, 67, 70–76. [Google Scholar] [CrossRef]
- Brahmkhatri, V.P.; Chandra, K.; Dubey, A.; Atreya, H.S. An Ultrastable Conjugate of Silver Nanoparticles and Protein Formed through Weak Interactions. Nanoscale 2015, 7, 12921–12931. [Google Scholar] [CrossRef]
- Pal, I.; Brahmkhatri, V.P.; Bera, S.; Bhattacharyya, D.; Quirishi, Y.; Bhunia, A.; Atreya, H.S. Enhanced Stability and Activity of an Antimicrobial Peptide in Conjugation with Silver Nanoparticle. J. Colloid. Interface Sci. 2016, 483, 385–393. [Google Scholar] [CrossRef]
- Li, R.; Mao, J.; Zheng, P.; Wang, R.; Yang, Z.; Qian, S. Improving the Biocompatibility and Antibacterial Efficacy of Silver Nanoparticles Functionalized with (LLRR)3 Antimicrobial Peptide. World J. Microbiol. Biotechnol. 2024, 40, 1. [Google Scholar] [CrossRef]
- Choudhary, P.; Ramalingam, B.; Das, S.K. Rational Design of Antimicrobial Peptide Conjugated Graphene-Silver Nanoparticle Loaded Chitosan Wound Dressing. Int. J. Biol. Macromol. 2023, 246, 125347. [Google Scholar] [CrossRef]
- Liu, H.; Zhan, J.; Lin, R.; Yin, Y.; Ren, L. Strong Infiltrative HHC36 Antimicrobial Peptide/Silver Nanoparticles-Loaded Carboxymethyl Chitosan/Sodium Alginate Hydrogel for Acne Vulgaris Therapy. Nanotechnology 2023, 34, 495101. [Google Scholar] [CrossRef]
- Cantor, S.; Vargas, L.; Rojas, A.O.E.; Yarce, C.J.; Salamanca, C.H.; Oñate-Garzón, J. Evaluation of the Antimicrobial Activity of Cationic Peptides Loaded in Surface-Modified Nanoliposomes against Foodborne Bacteria. Int. J. Mol. Sci. 2019, 20, 680. [Google Scholar] [CrossRef]
- Javia, A.; Misra, A.; Thakkar, H. Liposomes Encapsulating Novel Antimicrobial Peptide Omiganan: Characterization and Its Pharmacodynamic Evaluation in Atopic Dermatitis and Psoriasis Mice Model. Int. J. Pharm. 2022, 624, 122045. [Google Scholar] [CrossRef] [PubMed]
- Maria-Neto, S.; de Almeida, K.C.; Macedo, M.L.R.; Franco, O.L. Understanding Bacterial Resistance to Antimicrobial Peptides: From the Surface to Deep Inside. Biochim. Biophys. Acta Biomembr. 2015, 1848, 3078–3088. [Google Scholar] [CrossRef]
- Fleitas, O.; Franco, O.L. Induced Bacterial Cross-Resistance toward Host Antimicrobial Peptides: A Worrying Phenomenon. Front. Microbiol. 2016, 7, 381. [Google Scholar] [CrossRef]
- Lennard, P.R.; Hiemstra, P.S.; Nibbering, P.H. Complementary Activities of Host Defence Peptides and Antibiotics in Combating Antimicrobial Resistant Bacteria. Antibiotics 2023, 12, 1518. [Google Scholar] [CrossRef]
- Gagat, P.; Ostrówka, M.; Duda-Madej, A.; Mackiewicz, P. Enhancing Antimicrobial Peptide Activity through Modifications of Charge, Hydrophobicity, and Structure. Int. J. Mol. Sci. 2024, 25, 10821. [Google Scholar] [CrossRef]
- Ye, Z.; Xu, Z.; Ouyang, J.; Shi, W.; Li, S.; Wang, X.; Lu, B.; Wang, K.; Wang, Y. Improving the Stability and Anti-Infective Activity of Sea Turtle AMPs Using Multiple Structural Modification Strategies. J. Med. Chem. 2024, 67, 22104–22123. [Google Scholar] [CrossRef]
- Muhammad, T.; Strömstedt, A.A.; Gunasekera, S.; Göransson, U. Transforming Cross-Linked Cyclic Dimers of KR-12 into Stable and Potent Antimicrobial Drug Leads. Biomedicines 2023, 11, 504. [Google Scholar] [CrossRef]
- Yao, A.; Ma, Y.; Sun, R.; Zou, W.; Chen, X.; Zhou, M.; Ma, C.; Chen, T.; Shaw, C.; Wang, L. A Designed Analog of an Antimicrobial Peptide, Crabrolin, Exhibits Enhanced Anti-Proliferative and In Vivo Antimicrobial Activity. Int. J. Mol. Sci. 2023, 24, 14472. [Google Scholar] [CrossRef]
- Yao, A.; Liu, T.; Cai, Y.; Zhou, S.; Chen, X.; Zhou, M.; Ma, C.; Chen, T.; Shaw, C.; Wang, L. Progressive Design of a Ranatuerin-2 Peptide from Amolops Wuyiensis: Enhancement of Bioactivity and In Vivo Efficacy. Antibiotics 2023, 13, 5. [Google Scholar] [CrossRef]
- Wang, G.; Li, X.; Wang, Z. APD3: The Antimicrobial Peptide Database as a Tool for Research and Education. Nucleic Acids Res. 2016, 44, D1087–D1093. [Google Scholar] [CrossRef]
- Jhong, J.-H.; Yao, L.; Pang, Y.; Li, Z.; Chung, C.R.; Wang, R.; Li, S.; Li, W.; Luo, M.; Ma, R.; et al. DbAMP 2.0: Updated Resource for Antimicrobial Peptides with an Enhanced Scanning Method for Genomic and Proteomic Data. Nucleic Acids Res. 2022, 50, D460–D470. [Google Scholar] [CrossRef] [PubMed]
- Lertampaiporn, S.; Vorapreeda, T.; Hongsthong, A.; Thammarongtham, C. Ensemble-AMPPred: Robust AMP Prediction and Recognition Using the Ensemble Learning Method with a New Hybrid Feature for Differentiating AMPs. Genes 2021, 12, 137. [Google Scholar] [CrossRef]
- Okella, H.; Georrge, J.J.; Ochwo, S.; Ndekezi, C.; Koffi, K.T.; Aber, J.; Ajayi, C.O.; Fofana, F.G.; Ikiriza, H.; Mtewa, A.G.; et al. New Putative Antimicrobial Candidates: In Silico Design of Fish-Derived Antibacterial Peptide-Motifs. Front. Bioeng. Biotechnol. 2020, 8, 604041. [Google Scholar] [CrossRef]
- Yin, Q.; Wu, S.; Wu, L.; Wang, Z.; Mu, Y.; Zhang, R.; Dong, C.; Zhou, B.; Zhao, B.; Zheng, J.; et al. A Novel in Silico Antimicrobial Peptide DP7 Combats MDR Pseudomonas Aeruginosa and Related Biofilm Infections. J. Antimicrob. Chemother. 2020, 75, 3248–3259. [Google Scholar] [CrossRef]
- Porto, W.F.; Fensterseifer, I.C.M.; Ribeiro, S.M.; Franco, O.L. Joker: An Algorithm to Insert Patterns into Sequences for Designing Antimicrobial Peptides. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 2043–2052. [Google Scholar] [CrossRef]
- Porto, W.F.; Irazazabal, L.N.; Humblot, V.; Haney, E.F.; Ribeiro, S.M.; Hancock, R.E.W.; Ladram, A.; Franco, O.L. EcDBS1R6: A Novel Cationic Antimicrobial Peptide Derived from a Signal Peptide Sequence. Biochim. Biophys. Acta Gen. Subj. 2020, 1864, 129633. [Google Scholar] [CrossRef]
- Pirtskhalava, M.; Amstrong, A.A.; Grigolava, M.; Chubinidze, M.; Alimbarashvili, E.; Vishnepolsky, B.; Gabrielian, A.; Rosenthal, A.; Hurt, D.E.; Tartakovsky, M. DBAASP v3: Database of Antimicrobial/Cytotoxic Activity and Structure of Peptides as a Resource for Development of New Therapeutics. Nucleic Acids Res. 2021, 49, D288–D297. [Google Scholar] [CrossRef]
- Umstätter, F.; Domhan, C.; Hertlein, T.; Ohlsen, K.; Mühlberg, E.; Kleist, C.; Zimmermann, S.; Beijer, B.; Klika, K.D.; Haberkorn, U.; et al. Vancomycin Resistance Is Overcome by Conjugation of Polycationic Peptides. Angew. Chem. Int. 2020, 59, 8823–8827. [Google Scholar] [CrossRef]
- Ptaszyńska, N.; Gucwa, K.; Olkiewicz, K.; Łȩgowska, A.; Okońska, J.; Ruczyński, J.; Gitlin-Domagalska, A.; Dȩbowski, D.; Milewski, S.; Rolka, K.; et al. Antibiotic-Based Conjugates Containing Antimicrobial HLopt2 Peptide: Design, Synthesis, Antimicrobial and Cytotoxic Activities. ACS Chem. Biol. 2019, 14, 2233–2242. [Google Scholar] [CrossRef]
- Hirt, H.; Gorr, S.U. Antimicrobial Peptide GL13K Is Effective in Reducing Biofilms of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2013, 57, 4903–4910. [Google Scholar] [CrossRef]
- Sakoulas, G.; Bayer, A.S.; Pogliano, J.; Tsuji, B.T.; Yang, S.-J.; Mishra, N.N.; Nizet, V.; Yeaman, M.R.; Moise, P.A. Ampicillin Enhances Daptomycin- and Cationic Host Defense Peptide-Mediated Killing of Ampicillin- and Vancomycin-Resistant Enterococcus faecium. Antimicrob. Agents Chemother. 2012, 56, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xia, L.; Haapasalo, M.; Wei, W.; Zhang, D.; Ma, J.; Shen, Y. A Novel Hydroxyapatite-Binding Antimicrobial Peptide against Oral Biofilms. Clin. Oral Investig. 2019, 23, 2705–2712. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Quan, G.; Su, M.; Nimmagadda, A.; Chen, W.; Pan, M.; Teng, P.; Yu, F.; Liu, X.; Jiang, L.; et al. Molecular Architecture and Charging Effects Enhance the In Vitro and In Vivo Performance of Multi-Arm Antimicrobial Agents Based on Star-Shaped Poly(L-lysine). Adv. Ther. 2019, 2, 1900147. [Google Scholar] [CrossRef]
- Kim, H.; Jang, J.H.; Kim, S.C.; Cho, J.H. Development of a Novel Hybrid Antimicrobial Peptide for Targeted Killing of Pseudomonas aeruginosa. Eur. J. Med. Chem. 2020, 185, 111814. [Google Scholar] [CrossRef]
- Yu, W.; Ning, N.; Xue, Y.; Huang, Y.; Guo, F.; Li, T.; Yang, B.; Luo, D.; Sun, Y.; Li, Z.; et al. A Chimeric Cationic Peptide Composed of Human β-Defensin 3 and Human β-Defensin 4 Exhibits Improved Antibacterial Activity and Salt Resistance. Front. Microbiol. 2021, 12, 663151. [Google Scholar] [CrossRef]
- Pogue, J.M.; Ortwine, J.K.; Kaye, K.S. Clinical Considerations for Optimal Use of the Polymyxins: A Focus on Agent Selection and Dosing. Clin. Microbiol. Infect. 2017, 23, 229–233. [Google Scholar] [CrossRef]
- Kelesidis, T.; Falagas, M.E. The Safety of Polymyxin Antibiotics. Expert. Opin. Drug Saf. 2015, 14, 1687–1701. [Google Scholar] [CrossRef]
- Zavascki, A.P.; Nation, R.L. Nephrotoxicity of Polymyxins: Is There Any Difference between Colistimethate and Polymyxin B? Antimicrob. Agents Chemother. 2017, 61, e02319-16. [Google Scholar] [CrossRef]
- Maturana, P.; Martinez, M.; Noguera, M.E.; Santos, N.C.; Disalvo, E.A.; Semorile, L.; Maffia, P.C.; Hollmann, A. Lipid Selectivity in Novel Antimicrobial Peptides: Implication on Antimicrobial and Hemolytic Activity. Colloids Surf. B Biointerfaces 2017, 153, 152–159. [Google Scholar] [CrossRef]
- Edwards, I.A.; Elliott, A.G.; Kavanagh, A.M.; Blaskovich, M.A.T.; Cooper, M.A. Structure–Activity and −Toxicity Relationships of the Antimicrobial Peptide Tachyplesin-1. ACS Infect. Dis. 2017, 3, 917–926. [Google Scholar] [CrossRef]
- Olusanya, T.; Haj Ahmad, R.; Ibegbu, D.; Smith, J.; Elkordy, A. Liposomal Drug Delivery Systems and Anticancer Drugs. Molecules 2018, 23, 907. [Google Scholar] [CrossRef] [PubMed]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Lv, J.; Ma, Z.; Ma, J.; Chen, J. Advances in Antimicrobial Peptides: Mechanisms, Design Innovations, and Biomedical Potential. Molecules 2025, 30, 1529. https://doi.org/10.3390/molecules30071529
Zhang H, Lv J, Ma Z, Ma J, Chen J. Advances in Antimicrobial Peptides: Mechanisms, Design Innovations, and Biomedical Potential. Molecules. 2025; 30(7):1529. https://doi.org/10.3390/molecules30071529
Chicago/Turabian StyleZhang, He, Jiaxun Lv, Zhili Ma, Junfeng Ma, and Jing Chen. 2025. "Advances in Antimicrobial Peptides: Mechanisms, Design Innovations, and Biomedical Potential" Molecules 30, no. 7: 1529. https://doi.org/10.3390/molecules30071529
APA StyleZhang, H., Lv, J., Ma, Z., Ma, J., & Chen, J. (2025). Advances in Antimicrobial Peptides: Mechanisms, Design Innovations, and Biomedical Potential. Molecules, 30(7), 1529. https://doi.org/10.3390/molecules30071529






