A Novel Liquid Chromatographic Time-of-Flight Tandem Mass Spectrometric Method for the Determination of Secondary Metabolites in Functional Flours Produced from Grape Seed and Olive Stone Waste
Abstract
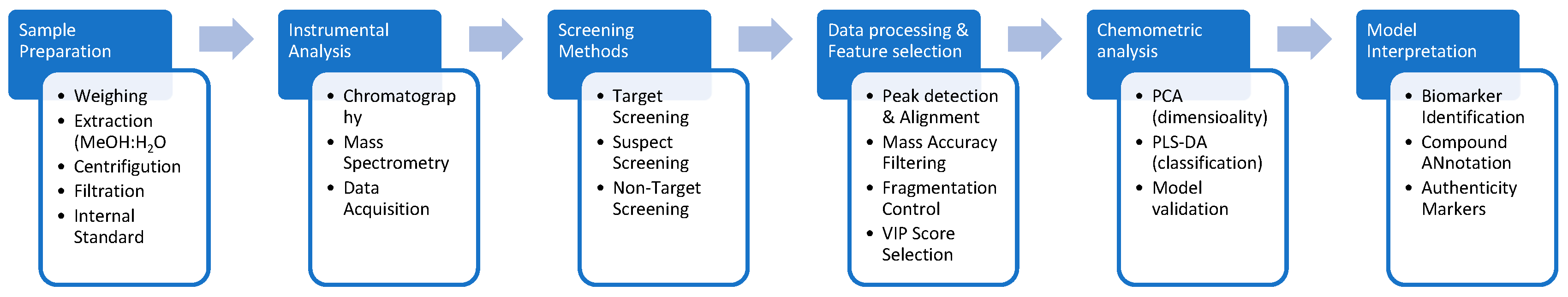
1. Introduction
2. Results and Discussion
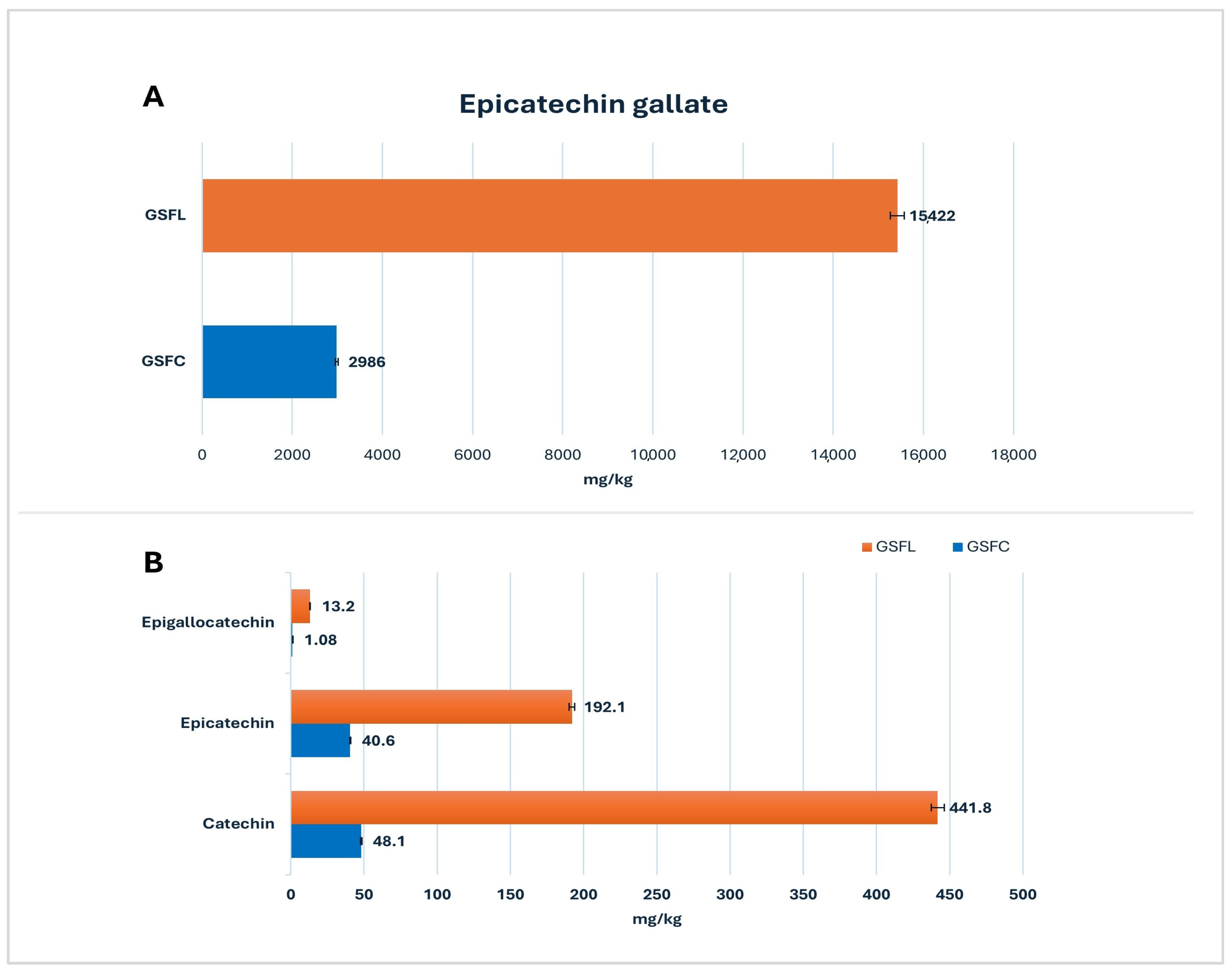
2.1. Grape Seed Flour (GSF)
2.1.1. Target Screening Results
2.1.2. Suspect Screening Results
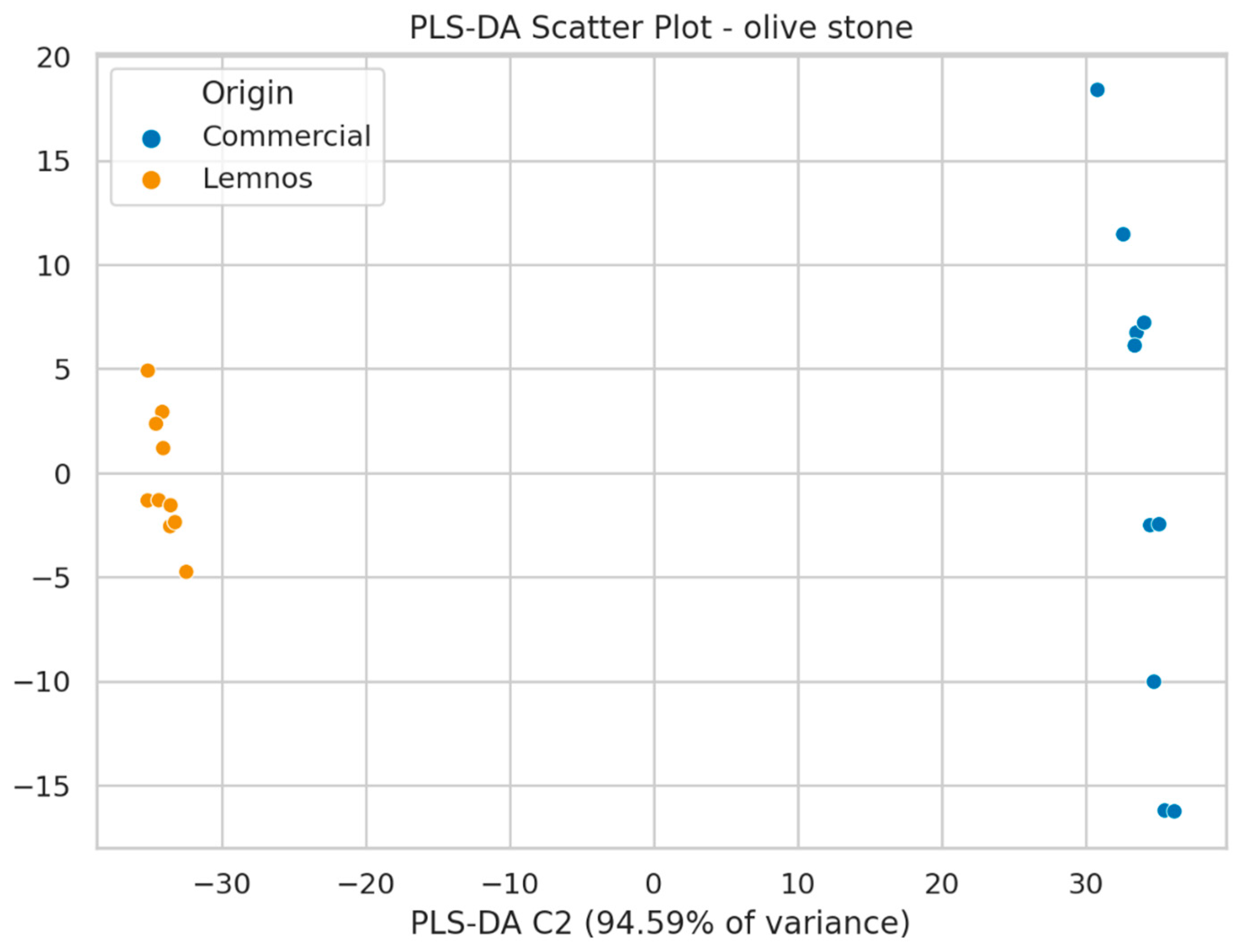
2.2. Olive Stone Flour (OSF)
2.2.1. Target Screening Results
2.2.2. Suspect Screening Results
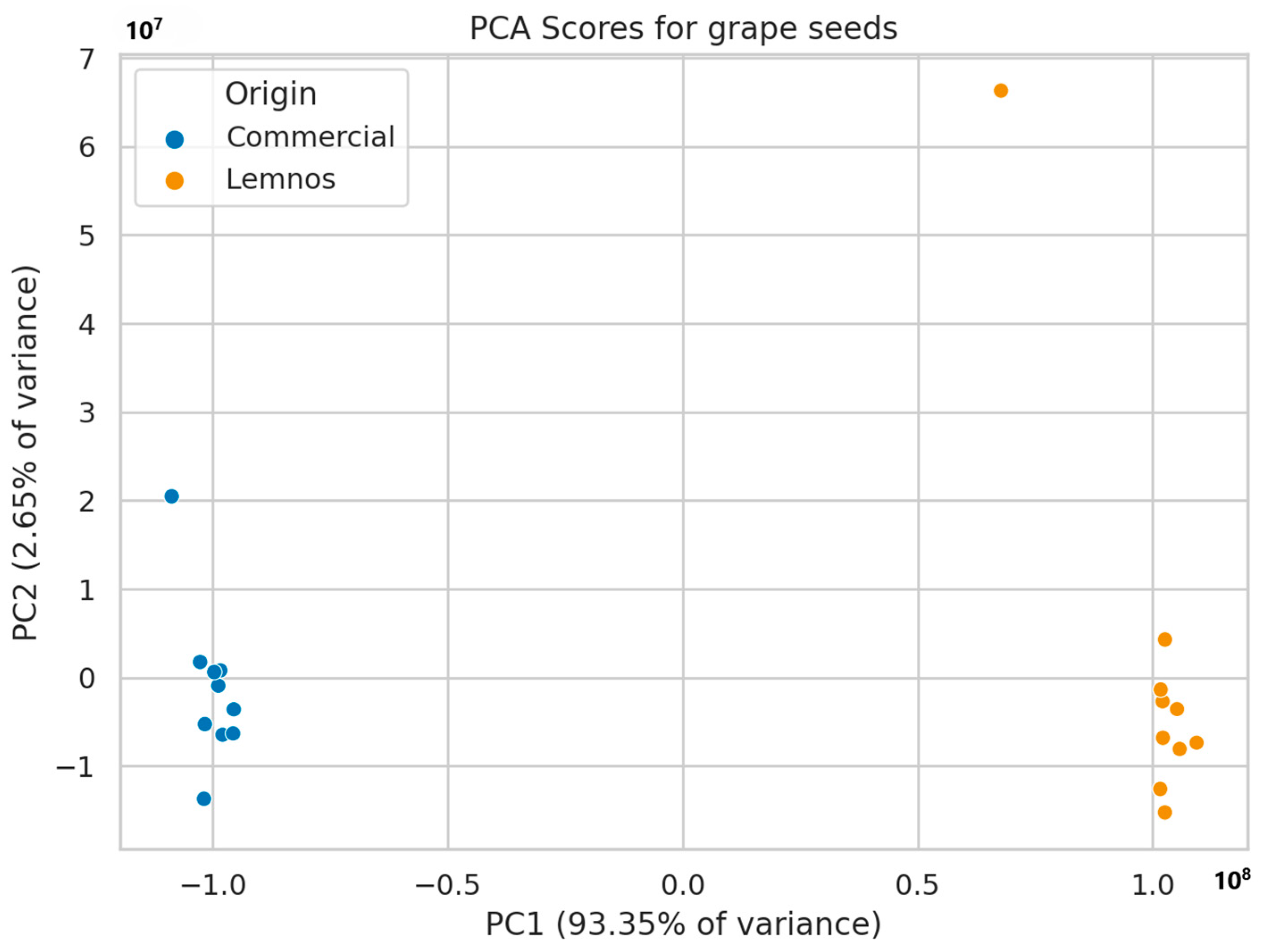
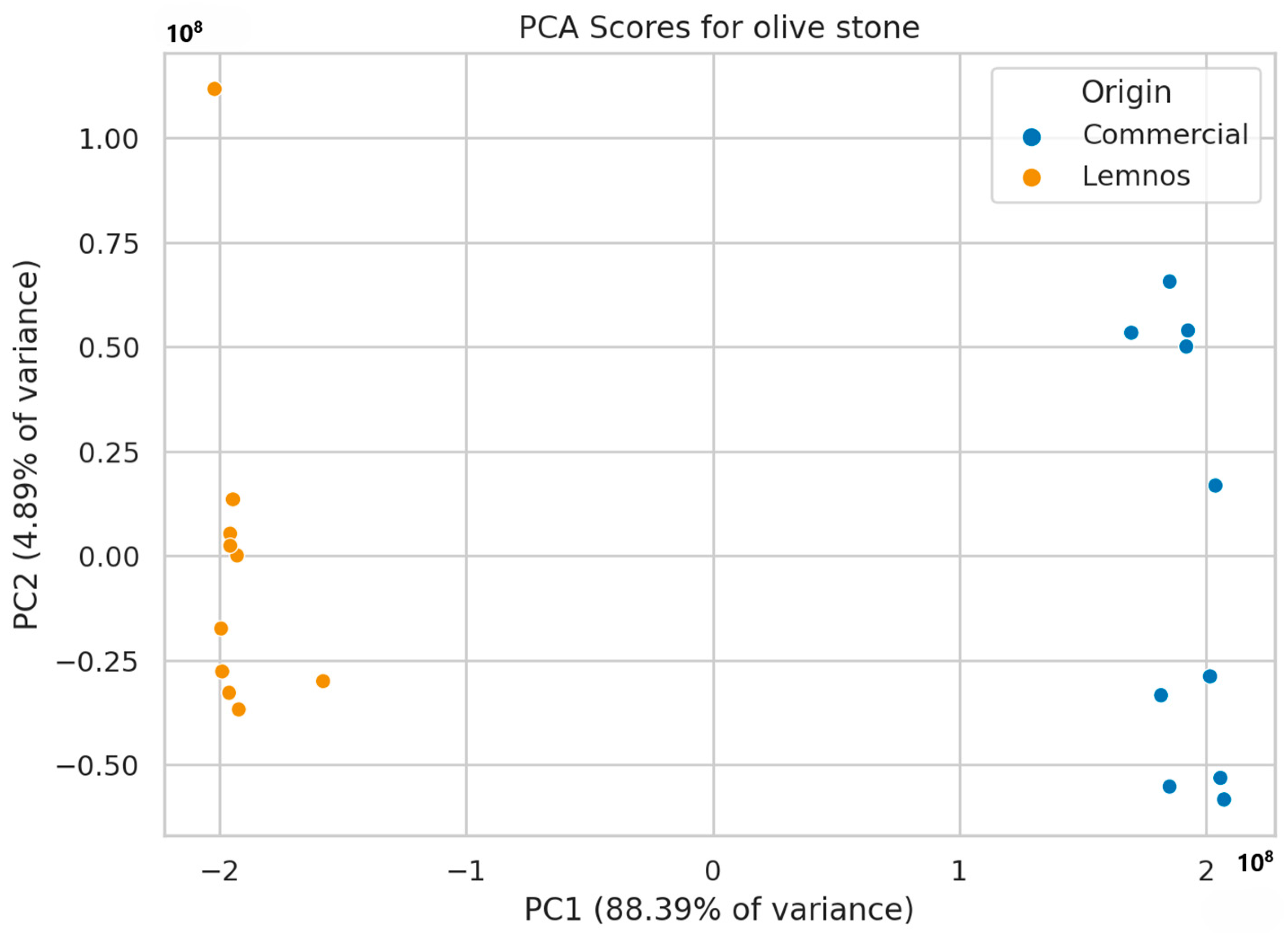
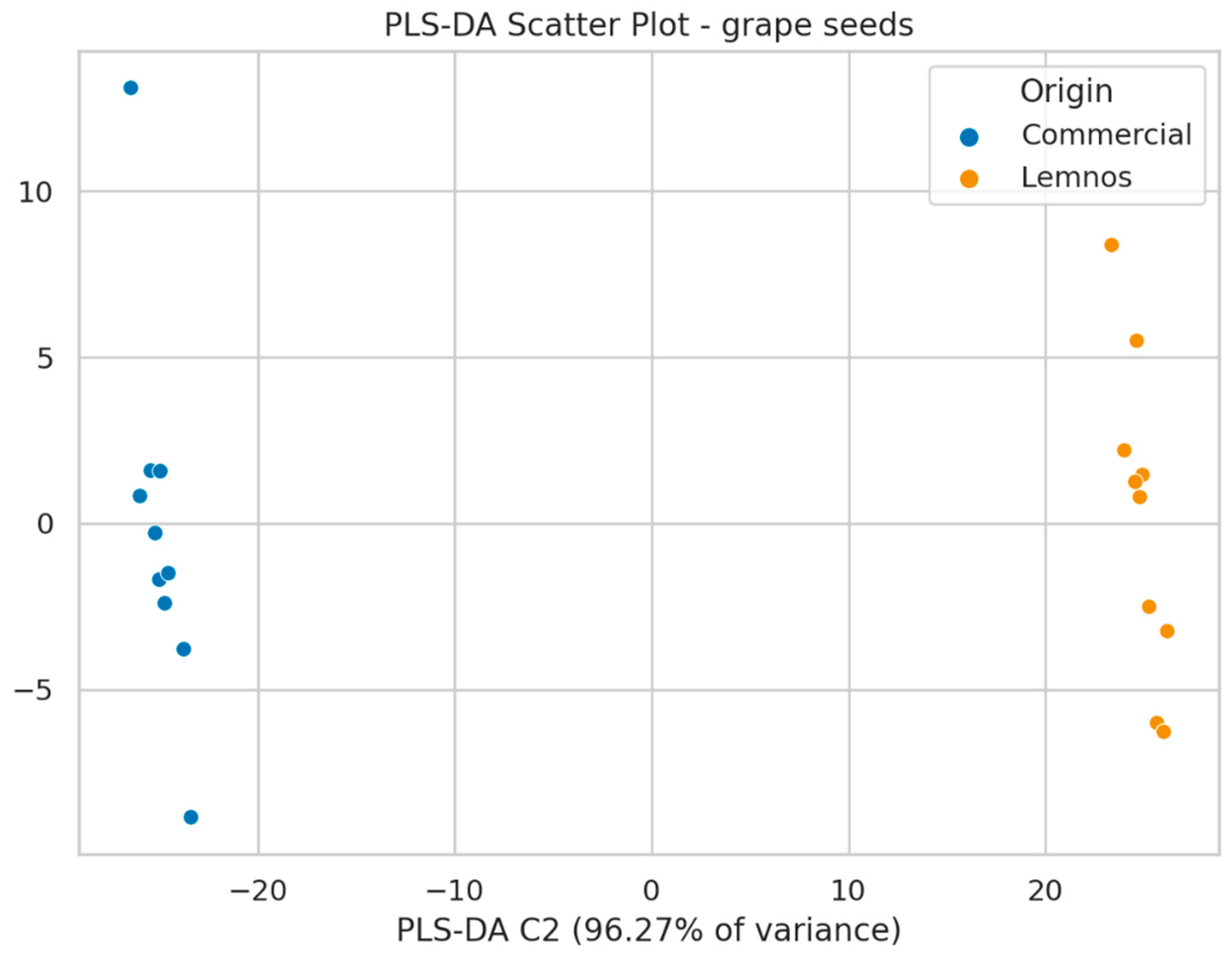
2.3. Non-Target Screening Results
| [M − H]−/RT | VIP | Plausible Molecular Formula | Tentative Analyte | Phytochemical Class | Formula Finder Score | MassBank ID/Reference |
|---|---|---|---|---|---|---|
| 109.0292/3.32 | 1.70990591 | C6H5O2 | 99 | |||
| 123.0449/3.61 | 3.88331912 | C7H8O2 | 99.9 | |||
| 153.0191/3.32 | 1.24806817 | C7H6O4 | 2,4-dihydroxybenzoic acid | Phenolic acid | 91.2 | BS003106 |
| 153.0554/3.61 | 4.87762151 | C8H10O3 | 94.6 | |||
| 171.1023/7.77 | 1.32240845 | C8H14O | 90 | |||
| 187.0966/7.65 | 2.20468526 | C9H16O4 | 82.76 | |||
| 199.0611/4.37 | 1.10983312 | C8H10O3 | Halleridone | Terpenoid | 90.7 | |
| 215.0556/4.16 | 2.44049438 | C9H12O6 | 90.8 | |||
| 215.0914/6.36 | 1.91315487 | C11H20S2 | 90.4 | |||
| 223.0612/5.43 | 2.02782415 | C12H16S2 | 89 | |||
| 229.0717/4.57 | 1.35870769 | C10H14O6 | Elenaic acid | Lipid | 85.1 | [60] |
| 241.1186/5.76 | 2.91752206 | C11H14O6 | Elenaic acid derivative | Lipid | 84.1 | |
| 253.2153/13.65 | 1.68171724 | C16H32O3 | 16-Hydroxy-hexadecanoic acid | Lipid | 91.1 | PR100487 |
| 265.0753/7.30 | 1.11639129 | C10H18O6S | 90.5 | |||
| 265.1462/13.56 | 5.82308316 | C12H26O4S | 90.9 | |||
| 266.1507/13.64 | 1.01144158 | C6H21N9OS | 91.2 | |||
| 267.1956/12.45 | 1.32783509 | C16H30O4 | Hexadecanedioate | Lipid derivative | 86.3 | JP001078 |
| 269.0445/9.02 | 2.64017779 | Apigenin (C15H10O6) | 98.9 | |||
| 275.1039/6.15 | 1.30711949 | |||||
| 279.2311/13.76 | 1.75842018 | C14H30N6O | 99.6 | |||
| 281.2468/13.99 | 3.41644995 | C18H36O3 | 77.7 | |||
| 282.2510/13.99 | 3.53055616 | |||||
| 283.2614/14.36 | 2.88130084 | C14H32N6 | 90.9 | |||
| 285.0386/8.36 | 9.07714971 | C15H10O6 | Luteolin | Flavone | 95.9 | PM000420 |
| 286.0441/8.50 | 1.61411498 | |||||
| 287.2212/10.64 | 5.0420702 | C16H32O4 | 90.6 | |||
| 293.2108/12.78 | 4.11240994 | |||||
| 294.2158/12.84 | 1.05069407 | C18H34O3 | ||||
| 295.2255/12.81 | 2.38947953 | C16H12O6 | ||||
| 297.2417/13.64 | 1.15555122 | 18-Hydroxyoleate | Lipid | 97 | [61] | |
| 299.0558/9.11 | 1.06125804 | C16H30O5 | Chrysoeriol | Flavone | 92.1 | BS003342 |
| 299.1136/4.50 | 2.42334052 | C5H10N10O6 | ||||
| 301.2009/10.45 | 2.5807432 | C18H30O4 | 92.3 | |||
| 305.0701/5.34 | 1.59445723 | C18H34O5 | 94.3 | |||
| 309.2069/12.08 | 1.20749047 | 13(S)-Hydroperoxylinolenic acid | Lipid derivative | 91.9 | EQ331602 | |
| 311.2220/12.14 | 1.00496969 | C14H20O8 | 90.2 | |||
| 313.2372/12.31 | 2.63234722 | C18H36O4 | ||||
| 315.1086/3.82 | 1.87543365 | C12H31N9O | Cornoside | Monoterpenoid | 82.2 | [62] |
| 315.2516/12.68 | 5.75432159 | C18H32O5 | (9R,10S)-Dihydroxystearate | Lipid | 83.8 | |
| 316.2560/12.68 | 1.87253721 | C18H34O6 | 99.6 | |||
| 327.2165/9.86 | 1.8513419 | C18H34O5 | 74.5 | |||
| 327.2170/10.58 | 1.27970038 | C18H34O5 | 89.4 | |||
| 329.2309/9.90 | 8.7666785 | C18H36O5 | 84.9 | |||
| 329.2312/10.46 | 15.8827924 | C18H36O5 | 85 | |||
| 331.2397/10.58 | 3.15270061 | C20H18O5 | 73.7 | |||
| 331.2471/10.32 | 4.29045396 | C15H24N10 | 9,10,18-Trihydroxyoctadecanoic acid | Lipid | 82.9 | CB000003 |
| 337.1071/7.59 | 1.13852175 | C14H20O7 | 98.7 | |||
| 343.2103/9.76 | 5.61777449 | C18H34O6 | 90.1 | |||
| 345.1194/4.45 | 1.59752959 | C21H38O5 | Salidroside | Phenol | 93.2 | [63] |
| 345.2267/10.14 | 2.43118178 | C14H30O4S | 94.7 | |||
| 351.2535/13.80 | 1.65705829 | 90.7 | ||||
| 353.1979/14.39 | 1.32301547 | C29H14N2 | 90.1 | |||
| 353.2683/14.03 | 3.8098374 | C24H40O4 | ||||
| 389.1082/5.26 | 2.22477443 | C30H16N2 | 98.7 | |||
| 391.2846/13.98 | 1.54222771 | C30H16N2 | 99.9 | |||
| 403.1231/5.43 | 11.927081 | C10H24N6O9S | 97.7 | |||
| 403.1241/6.21 | 1.51585553 | C27H19NO3 | 93 | |||
| 403.1248/4.90 | 1.70275032 | C22H22N2O6 | 91.9 | |||
| 404.1284/5.46 | 2.71837775 | C26H42O4 | 83.2 | |||
| 409.1404/9.40 | 1.02744581 | C25H36O6 | 94.9 | |||
| 417.2995/14.02 | 2.96165422 | C25H36O7 | 86 | |||
| 431.2417/12.15 | 4.06742772 | C30H48O3 | 98.4 | |||
| 447.2371/12.05 | 1.69705193 | 79.9 | ||||
| 455.3500/13.59 | 7.58565491 | C30H48O4 | 89.5 | |||
| 465.3191/13.91 | 2.81214421 | C21H46N8O4 | ||||
| 471.3445/13.17 | 7.15603201 | C30H46O5 | 88.3 | |||
| 473.3526/13.15 | 1.17624378 | C26H44N6O3 | 91.2 | |||
| 485.3251/12.40 | 1.38424034 | C22H24N10O6 | 94.9 | |||
| 487.3408/12.62 | 1.05541295 | C26H28N4O8 | 89.4 | |||
| 523.1812/7.95 | 2.2817832 | C22H24N10O7 | 92.5 | |||
| 523.1823/6.69 | 1.98662549 | C31H28N4S3 | 94.9 | |||
| 539.1758/7.35 | 4.19041331 | C30H62N6O4 | 93.2 | |||
| 551.1401/7.20 | 1.30540864 | C27H30N4O9 | 89.5 | |||
| 551.4651/14.77 | 1.80521386 | C27H34N4O9 | 94.5 | |||
| 553.1923/8.17 | 1.01121826 | C27H26N4O10 | 80.5 | |||
| 557.2215/9.09 | 1.91276674 | C34H64O6 | 92.1 | |||
| 565.1570/7.80 | 1.48905009 | C37H72S2 | 95.5 | |||
| 567.4602/14.38 | 1.17748434 | C32H46N4O2S2 | 91.2 | |||
| 579.4967/15.89 | 1.53743162 | C36H65O6 | 94 | |||
| 581.2984/15.37 | 1.15630577 | C38H46O4S | 94.9 | |||
| 593.4753/14.42 | 2.86762481 | C30H42N6O7 | 87.7 | |||
| 597.3028/14.89 | 1.26409343 | C26H28N10O9 | 91.7 | |||
| 597.3045/15.81 | 2.16408575 | C27H50N8O9 | 90.4 | |||
| 623.1956/6.31 | 6.10963709 | C36H38N4O8S | 93.1 | |||
| 629.3615/3.80 | 1.63848932 | C36H38N4O8S | 87.2 | |||
| 685.2318/6.70 | 11.0557952 | C37H34N8O4S | 94.3 | |||
| 685.2332/8.13 | 4.26228369 | C36H38N4O8S | 94.8 | |||
| 685.2340/7.49 | 2.87707627 | C34H44N2O9S2 | 94.4 | |||
| 685.2350/8.85 | 2.42497379 | C29H46N6O6S4 | 95.6 | |||
| 687.2405/6.71 | 2.44188861 | C37H40N4O9S | 94.7 | |||
| 701.2282/6.27 | 3.26078709 | C39H48O10S3 | 95.7 | |||
| 715.2448/6.86 | 2.14610112 | C33H42N9O7S3 | 95.2 | |||
| 771.2324/7.56 | 6.88415023 | C39H44N4O7S3 | 91.3 | |||
| 772.2375/7.58 | 3.06952732 | C40H46N4O7S3 | 90.7 | |||
| 775.2283/7.13 | 5.04216623 | C32H42N10O10S2 | 97.6 | |||
| 789.2423/6.92 | 5.89403491 | C38H74N8O10S | 94.3 | |||
| 789.2428/7.76 | 6.82049513 | C43H80N8S4 | 94.8 | |||
| 833.5162/13.97 | 1.10683778 | C6H5O2 | 99 | |||
| 835.5312/13.98 | 2.88542004 | C7H8O2 | 96 |
3. Materials and Methods
3.1. Flour Samples
3.2. Chemicals and Standards
3.3. Sample Preparation
3.4. Instrumental Analysis
3.5. Screening Methodology
3.5.1. Target Screening
3.5.2. Suspect Screening
3.5.3. Non Target Screening
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rekleitis, G.; Haralambous, K.-J.; Loizidou, M.; Aravossis, K. Utilization of agricultural and livestock waste in anaerobic digestion (A.D): Applying the biorefinery concept in a circular economy. Energies 2020, 13, 4428. [Google Scholar] [CrossRef]
- Zouboulis, A. Project: Best Practices for Agricultural Wastes (AW) Treatment and Reuse in the Mediterranean Countries. 2011. Available online: https://webgate.ec.europa.eu/life/publicWebsite/project/LIFE10-ENV-GR-000594/best-practices-for-agricultural-wastes-treatment-and-reuse-in-the-mediterranean-countries (accessed on 20 March 2025).
- Madureira, J.; Margaça, F.M.A.; Santos-Buelga, C.; Ferreira, I.C.F.R.; Verde, S.C.; Barros, L. Applications of bioactive compounds extracted from olive industry wastes: A review. Compr. Rev. Food Sci. Food Saf. 2021, 21, 453–476. [Google Scholar] [CrossRef]
- Amaya-Chantaca, D.; Flores-Gallegos, A.C.; Iliná, A.; Aguilar, C.N.; Verma, D.K.; Baranwal, D.; Chávez-González, M.L. Wine waste as a potential source of bioactive compounds. In Innovations in Fermentation and Phytopharmaceutical Technologies; Elsevier: Amsterdam, The Netherlands, 2022; pp. 361–380. [Google Scholar]
- Stefano, D.; Buzzanca, V.; Melilli, C.; Indelicato, S.; Mauro, M.; Vazzana, M.; Arizza, V.; Lucarini, M.; Durazzo, A.; Bongiorno, D. Polyphenol Characterization and Antioxidant Activity of Grape Seeds and Skins from Sicily: A Preliminary Study. Sustainability 2022, 14, 6702. [Google Scholar] [CrossRef]
- Jahanbakhshi, R.; Ansari, S. Physicochemical properties of sponge cake fortified by olive stone powder. J. Food Qual. 2020, 2020, 1493638. [Google Scholar] [CrossRef]
- Ben Mansour, A.; Porter, E.A.; Kite, G.C.; Simmonds, M.S.J.; Abdelhedi, R.; Bouaziz, M. Phenolic profile characterization of chemlali olive stones by liquid chromatography-ion trap mass spectrometry. J. Agric. Food Chem. 2015, 63, 1990–1995. [Google Scholar] [CrossRef]
- Marković, A.K.; Torić, J.; Barbarić, M.; Brala, C.J. Hydroxytyrosol, tyrosol and derivatives and their potential effects on human health. Molecules 2019, 24, 2001. [Google Scholar] [CrossRef]
- Maestri, D.; Barrionuevo, D.; Bodoira, R.; Zafra, A.; Jiménez-López, J.; Alché, J.d.D. Nutritional profile and nutraceutical components of olive (Olea europaea L.) seeds. J. Food Sci. Technol. 2019, 56, 4359–4370. [Google Scholar] [CrossRef]
- Ali, K.; Maltese, F.; Choi, Y.H.; Verpoorte, R. Metabolic constituents of grapevine and grape-derived products. Phytochem. Rev. 2009, 9, 357–378. [Google Scholar] [CrossRef] [PubMed]
- Santini, A.; Tenore, G.C.; Novellino, E. Nutraceuticals: A paradigm of proactive medicine. Eur. J. Pharm. Sci. 2017, 96, 53–61. [Google Scholar] [CrossRef]
- Hao, J.; Li, L.; Wolf, M.; Xu, M.; Brinsko, B.; Yanik, M.; Chen, S.; Binzer, L.; Green, S.; Hitz, C.; et al. Antioxidant properties and phenolic components of grape seeds. Funct. Plant Sci. Biotechnol. 2009, 3, 60–68. [Google Scholar]
- Kallithraka, S.; Garcia-Viguera, C.; Bridle, P.; Bakker, J. Survey of solvents for the extraction of grape seed phenolics. Phytochem. Anal. 1995, 6, 265–267. [Google Scholar] [CrossRef]
- Toop, T.A.; Ward, S.; Oldfield, T.; Hull, M.; Kirby, M.E.; Theodorou, M.K. AgroCycle—Developing a circular economy in agriculture. Energy Procedia 2017, 123, 76–80. [Google Scholar] [CrossRef]
- Valvez, S.; Maceiras, A.; Santos, P.; Reis, P.N.B. Olive stones as filler for polymer-based composites: A review. Materials 2021, 14, 845. [Google Scholar] [CrossRef]
- Bolek, S. Olive stone powder: A potential source of fiber and antioxidant and its effect on the rheological characteristics of biscuit dough and quality. Innov. Food Sci. Emerg. Technol. 2020, 64, 102423. [Google Scholar] [CrossRef]
- Oprea, O.B.; Popa, M.E.; Apostol, L.; Gaceu, L. Research on the potential use of grape seed flour in the bakery industry. Foods 2022, 11, 1589. [Google Scholar] [CrossRef]
- Aghamirzaei, M.; Peighambardoust, S.H.; Azadmard-Damirchi, S.; Majzoobi, M. Effects of Grape Seed Powder as a Functional Ingred Ient on Flour Physicochemical Characteristics and Dough Rheological Properties. J. Agric. Sci. Technol. 2018, 17, 365–373. [Google Scholar]
- Antonic, B.; Dordevic, D.; Jancikova, S.; Holeckova, D.; Tremlova, B.; Kulawik, P. Effect of grape seed flour on the antioxidant profile, textural and sensory properties of waffles. Processes 2021, 9, 131. [Google Scholar] [CrossRef]
- Soto, R.; Brown, M.U.; Ross, K. Antioxidant activity and consumer acceptance of grape seed flour-containing food products. Int. J. Food Sci. Technol. 2012, 47, 592–602. [Google Scholar]
- Hoye, J.C.; Ross, C.F. Total phenolic content, consumer acceptance, and instrumental analysis of bread made with grape seed flour. J. Food Sci. 2011, 76, S428–S436. [Google Scholar] [CrossRef]
- Fraga, H.; Moriondo, M.; Leolini, L.; Santos, J.A. Mediterranean olive orchards under climate change: A review of future impacts and adaptation strategies. Agronomy 2020, 11, 56. [Google Scholar] [CrossRef]
- Mousavi, S.; Stanzione, V.; Mariotti, R.; Mastio, V.; Azariadis, A.; Passeri, V.; Valeri, M.C.; Baldoni, L.; Bufacchi, M. Bioactive compound profiling of olive fruit: The contribution of genotype. Antioxidants 2022, 11, 672. [Google Scholar] [CrossRef] [PubMed]
- Ighbareyeh, J.M.; Carmona, E.C. Impact of environment conditions on grapevine (Vitis vinifera L.): To optimal production and sustainability, achieving food security and increasing the Palestinian economy. J. Geosci. Environ. Prot. 2018, 6, 62–73. [Google Scholar] [CrossRef]
- Tsarouhas, P.; Achillas, C.; Aidonis, D.; Folinas, D.; Maslis, V. Life Cycle Assessment of olive oil production in Greece. J. Clean. Prod. 2015, 93, 75–83. [Google Scholar] [CrossRef]
- Silva, A.; Silva, V.; Igrejas, G.; Aires, A.; Falco, V.; Valentão, P.; Poeta, P. Phenolic compounds classification and their distribution in winemaking by-products. Eur. Food Res. Technol. 2022, 249, 207–239. [Google Scholar] [CrossRef]
- Kapcsándi, V.; Lakatos, E.H.; Sik, B.; Linka, L.Á.; Székelyhidi, R. Antioxidant and polyphenol content of different Vitis vinifera seed cultivars and two facilities of production of a functional bakery product. Chem. Pap. 2021, 75, 5711–5717. [Google Scholar] [CrossRef]
- Juhaimi, A.; Özcan, F. Effect of cold press and soxhlet extraction systems on fatty acid, tocopherol contents, and phenolic compounds of various grape seed oils. J. Food Process. Preserv. 2018, 42, e13417. [Google Scholar]
- Yilmaz, Y.; Toledo, R.T. Major flavonoids in grape seeds and skins: Antioxidant capacity of catechin, epicatechin, and gallic acid. J. Agric. Food Chem. 2003, 52, 255–260. [Google Scholar] [CrossRef]
- Bucić-Kojić, A.; Planinić, M.; Tomas, S.; Jakobek, L.; Šeruga, M. Influence of solvent and temperature on extraction of phenolic compounds from grape seed, antioxidant activity and colour of extract. Int. J. Food Sci. Technol. 2009, 44, 2394–2401. [Google Scholar] [CrossRef]
- Gouvinhas, I.; Santos, R.; Queiroz, M.; Leal, C.; Saavedra, M.J.; Domínguez-Perles, R.; Rodrigues, M.A.M.; Barros, A. Monitoring the Antioxidant and Antimicrobial Power of Grape (Vitis vinifera L.) Stems Phenolics over Long-Term Storage. Ind. Crops Prod. 2018, 126, 83–91. [Google Scholar] [CrossRef]
- Perde-Schrepler, M.; Chereches, G.; Brie, I.; Tatomir, C.; Postescu, I.D.; Soran, L.; Filip, A. Grape seed extract as photochemopreventive agent against UVB-induced skin cancer. J. Photochem. Photobiol. B Biol. 2013, 118, 16–21. [Google Scholar] [CrossRef]
- Wolf, S.; Schmidt, S.; Müller-Hannemann, M.; Neumann, S. In silico fragmentation for computer assisted identification of metabolite mass spectra. BMC Bioinform. 2010, 11, 148. [Google Scholar] [CrossRef]
- Horai, H.; Arita, M.; Kanaya, S.; Nihei, Y.; Ikeda, T.; Suwa, K.; Ojima, Y.; Tanaka, K.; Tanaka, S.; Aoshima, K.; et al. MassBank: A public repository for sharing mass spectral data for life sciences. J. Mass Spectrom. 2010, 45, 703–714. [Google Scholar] [CrossRef]
- Deepika; Maurya, P.K. Health benefits of quercetin in age-related diseases. Molecules 2022, 27, 2498. [Google Scholar] [CrossRef] [PubMed]
- Hong, N.; Yaylayan, V.A.; Raghavan, G.S.V.; Paré, J.R.J.; Bélanger, J.M.R. Microwave-assisted extraction of phenolic compounds from grape seed. Nat. Prod. Lett. 2001, 15, 197–204. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhai, Y.; Wei, X.; Yang, X.; Deng, C.; Li, Q.; Wang, W.; Hao, R. Effects of grape seed procyanidins on the lipid metabolism of growing-finishing pigs based on transcriptomics and metabolomics analyses. Meat Sci. 2024, 213, 109504. [Google Scholar] [CrossRef] [PubMed]
- Unusan, N. Proanthocyanidins in grape seeds: An updated review of their health benefits and potential uses in the food industry. J. Funct. Foods 2020, 67, 103861. [Google Scholar] [CrossRef]
- Kang, I.; Buckner, T.; Shay, N.F.; Gu, L.; Chung, S. Improvements in metabolic health with consumption of ellagic acid and subsequent conversion into urolithins: Evidence and mechanisms. Adv. Nutr. Int. Rev. J. 2016, 7, 961–972. [Google Scholar] [CrossRef]
- Mateus, A.R.S.; Barros, S.C.; Cortegoso, S.M.; Sendón, R.; Barbosa-Pereira, L.; Khwaldia, K.; Pataro, G.; Ferrari, G.; Breniaux, M.; Ghidossi, R.; et al. Potential of fruit seeds: Exploring bioactives and ensuring food safety for sustainable management of food waste. Food Chem. X 2024, 23, 101718. [Google Scholar] [CrossRef]
- Barberis, A.; Deiana, M.; Spissu, Y.; Azara, E.; Fadda, A.; Serra, P.A.; D’Hallewin, G.; Pisano, M.; Serreli, G.; Orrù, G.; et al. Antioxidant, antimicrobial, and other biological properties of pompia juice. Molecules 2020, 25, 3186. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, X.; Tian, Y.; Zhai, S.; Liu, Y.; Xiong, Z.; Chu, S. An insight into novel therapeutic potentials of taxifolin. Front. Pharmacol. 2023, 14, 1173855. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA); Turck, D.; Bresson, J.L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.J.; et al. Scientific Opinion on taxifolin-rich extract from Dahurian Larch (Larix gmelinii). EFSA J. 2017, 15, e04682. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Frankel, E.; Bakhouche, A.; Lozano-Sánchez, J.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Literature review on production process to obtain extra virgin olive oil enriched in bioactive compounds. potential use of byproducts as alternative sources of polyphenols. J. Agric. Food Chem. 2013, 61, 5179–5188. [Google Scholar] [CrossRef]
- Servili, M.; Taticchi, A.; Esposto, S.; Urbani, S.; Selvaggini, R.; Montedoro, G.F. Effect of olive stoning on the volatile and phenolic composition of virgin olive oil. J. Agric. Food Chem. 2007, 55, 7028–7035. [Google Scholar] [CrossRef] [PubMed]
- Spizzirri, U.G.; Restuccia, D.; Chiricosta, S.; Parisi, O.I.; Cirillo, G.; Curcio, M.; Iemma, F.; Puoci, F.; Picci, N. Olive stones as a source of antioxidants for food industry. J. Food Nutr. Res. 2011, 50, 57–67. [Google Scholar]
- Fernández-Bolaɂos, J.; Felizón, B.; Brenes, M.; Guillén, R.; Heredia, A. Hydroxytyrosol and tyrosol as the main compounds found in the phenolic fraction of steam-exploded olive stones. J. Am. Oil Chem. Soc. 1998, 75, 1643–1649. [Google Scholar] [CrossRef]
- Barbaro, B.; Toietta, G.; Maggio, R.; Arciello, M.; Tarocchi, M.; Galli, A.; Balsano, C. Effects of the olive-derived polyphenol oleuropein on human health. Int. J. Mol. Sci. 2014, 15, 18508–18524. [Google Scholar] [CrossRef]
- Tuck, K.L.; Hayball, P.J. Major phenolic compounds in olive oil: Metabolism and health effects. Food Chem. X 2002, 13, 636–644. [Google Scholar] [CrossRef]
- Cardinali, A.; Pati, S.; Minervini, F.; D’antuono, I.; Linsalata, V.; Lattanzio, V. Verbascoside, isoverbascoside, and their derivatives recovered from olive mill wastewater as possible food antioxidants. J. Agric. Food Chem. 2012, 60, 1822–1829. [Google Scholar] [CrossRef]
- Castellano, J.M.; Ramos-Romero, S.; Perona, J.S. Oleanolic acid: Extraction, characterization and biological activity. Nutrients 2022, 14, 623. [Google Scholar] [CrossRef]
- Alam, M.Z.; Fristedt, R.; Landberg, R.; Kamal-Eldin, A. Soluble and hydrolyzable phenolic compounds in date fruits (Phoenix dactylifera L.) by UPLC-QTOF-MS/MS and UPLC-DAD. J. Food Compos. Anal. 2024, 132, 106354. [Google Scholar] [CrossRef]
- el Bouhaddani, S.; Houwing-Duistermaat, J.; Salo, P.; Perola, M.; Jongbloed, G.; Uh, H.-W. Evaluation of O2PLS in Omics data integration. BMC Bioinform. 2016, 17, S11. [Google Scholar] [CrossRef] [PubMed]
- Karadimou, C.; Petsa, E.; Ouroumi, N.; Papadakis, E.; Kontoudakis, N.; Theocharis, S.; Mourtzinos, I.; Menkissoglu-Spiroudi, U.; Kalogiouri, N.P.; Koundouras, S. Exploration of the anthocyanin and proanthocyanidin profile of Greek red grape skins belonging to Vradiano, Limnio, and Kotsifali cultivars, analyzed by a novel LC-QTOF-MS/MS method. Phytochem. Anal. 2024, 35, 1781–1793. [Google Scholar] [CrossRef] [PubMed]
- Mitsikaris, P.D.; Kostas, S.; Mourtzinos, I.; Menkissoglu-Spiroudi, U.; Papadopoulos, A.; Kalogiouri, N.P. Investigation of Rosa species by an optimized LC-QTOF-MS/MS method using targeted and non-targeted screening strategies combined with multivariate chemometrics. Phytochem. Anal. 2024, 35, 1100–1111. [Google Scholar] [CrossRef]
- Kalogiouri, N.P.; Aalizadeh, R.; Thomaidis, N.S. Application of an advanced and wide scope non-target screening workflow with LC-ESI-QTOF-MS and chemometrics for the classification of the Greek olive oil varieties. Food Chem. 2018, 256, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Kritikou, E.; Kalogiouri, N.P.; Kolyvira, L.; Thomaidis, N.S. Target and suspect HRMS metabolomics for the determination of functional ingredients in 13 varieties of olive leaves and drupes from Greece. Molecules 2020, 25, 4889. [Google Scholar] [CrossRef]
- Gouvinhas, I.; Garcia, J.; Granato, D.; Barros, A. Seed phytochemical profiling of three olive cultivars, antioxidant capacity, enzymatic inhibition, and effects on human neuroblastoma cells (SH-SY5Y). Molecules 2022, 27, 5057. [Google Scholar] [CrossRef]
- Bastoni, L.; Bianco, A.; Piccioni, F.; Uccella, N. Biophenolic profile in olives by nuclear magnetic resonance. Food Chem. 2001, 73, 145–151. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, N.; Joshi, R. Insights into the growth parameters, mineral composition, protein and UHPLC–QTOF–LC/MS-based non-targeted metabolomic fingerprints of wheatgrass as a function of photoperiod and temperature. Int. J. Food Sci. Technol. 2023, 58, 2423–2436. [Google Scholar]
- Luo, K.; Luo, D.; Liu, H. Determination of Metabolites in the Cerebellum of 2,3,7,8-Tetrachlorodibenzo-p-Dioxin Exposed Mice by Fourier Transform Ion Cyclotron Resonance Mass Spectrometry. Anal. Lett. 2014, 48, 594–604. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, C.; Zengin, G.; Segura-Carretero, A.; Lobine, D.; Mahomoodally, M.F. Chemical fingerprint and bioactivity evaluation of Globularia orientalis L. and Globularia trichosantha Fisch. & CA Mey. using non-targeted HPLC-ESI-QTOF-MS approach. Phytochem. Anal. 2019, 30, 237–252. [Google Scholar]
- Jiang, Y.; Mao, S.; Huang, W.; Lu, B.; Cai, Z.; Zhou, F.; Li, M.; Lou, T.; Zhao, Y. Phenylethanoid glycoside profiles and antioxidant activities of Osmanthus fragrans Lour. flowers by UPLC/PDA/MS and simulated digestion model. J. Agric. Food Chem. 2016, 64, 2459–2466. [Google Scholar]
- Li, J.; Chen, L.; Jiang, H.; Li, M.; Wang, L.; Li, J.X.; Wang, Y.Y.; Guo, Q.X. New Type of Tannins Identified from the Seeds of Cornus officinalis Sieb. et Zucc. by HPLC-ESI-MS/MS. Molecules 2023, 28, 2027. [Google Scholar] [CrossRef] [PubMed]
- Li, X.X.; Wang, L.; Liu, Y.L.; Zhao, Z.X.; Wang, X.L.; Lei, R.; Li, H.J. Comprehensive identification of Vitex trifolia fruit and its five adulterants by comparison of micromorphological, microscopic characteristics, and chemical profiles. Microsc. Res. Tech. 2020, 83, 1530–1543. [Google Scholar] [CrossRef]
- Wang, J.N.; Chen, Y.J.; Hano, Y.; Nomura, T.; Tan, R.X. Antioxidant activity of polyphenols from seeds of Vitis amurensis in vitro. Acta Pharmacol. Sin. 2000, 21, 633–636. [Google Scholar] [PubMed]
- Ziani, B.E.C.; Barros, L.; Boumehira, A.Z.; Bachari, K.; Heleno, S.A.; Alves, M.J.; Ferreira, I.C.F.R. Profiling polyphenol composition by HPLC-DAD-ESI/MSn and the antibacterial activity of infusion preparations obtained from four medicinal plants. Food Funct. 2017, 9, 149–159. [Google Scholar] [CrossRef]
- Krauss, M.; Singer, H.; Hollender, J. LC–high resolution MS in environmental analysis: From target screening to the identification of unknowns. Anal. Bioanal. Chem. 2010, 397, 943–951. [Google Scholar] [CrossRef]
- Harrington, R.A.; Adhikari, V.; Rayner, M.; Scarborough, P. Nutrient composition databases in the age of big data: foodDB, a comprehensive, real-time database infrastructure. BMJ Open 2019, 9, e026652. [Google Scholar] [CrossRef] [PubMed]
- Jolliffe, I. Principal component analysis for special types of data. In Principal Component Analysis; Springer: New York, NY, USA, 2002; pp. 338–372. [Google Scholar]
- Ruiz-Perez, D.; Guan, H.; Madhivanan, P.; Mathee, K.; Narasimhan, G. So you think you can PLS-DA? BMC Bioinform. 2020, 21, 2. [Google Scholar] [CrossRef]
- Gosselin, R.; Rodrigue, D.; Duchesne, C. A Bootstrap-VIP approach for selecting wavelength intervals in spectral imaging applications. Chemom. Intell. Lab. Syst. 2010, 100, 12–21. [Google Scholar] [CrossRef]
- Afanador, N.; Tran, T.; Buydens, L. Use of the bootstrap and permutation methods for a more robust variable importance in the projection metric for partial least squares regression. Anal. Chim. Acta 2013, 768, 49–56. [Google Scholar] [CrossRef]
- Peña-Herrera, J.; Montemurro, N.; Barceló, D.; Pérez, S. Combining quantitative and qualitative approaches using Sequential Window Acquisition of All Theoretical Fragment-Ion methodology for the detection of pharmaceuticals and related compounds in river fish extracted using a sample miniaturized method. J. Chromatogr. A 2020, 1620, 461009. [Google Scholar] [CrossRef] [PubMed]
| [M − H]−/RT | VIP | Plausible Molecular Formula | Tentative Compound | Phytochemical Class | Formula Finder Score | MassBank ID/Reference |
|---|---|---|---|---|---|---|
| 149.0088/0.99 | 1.908055 | C4H6O6 | Tartaric acid | Organic acids | 92.8 | KO001902 |
| 195.0504/0.98 | 4.165611 | C6H12O7 | Gluconic acid | Organic acids | 88.6 | KO000864 |
| 247.0249/5.13 | 1.542148 | C13H12OS2 | 87.9 | |||
| 255.2311/13.96 | 1.687796 | C16H32O2 | 65.6 | |||
| 265.1460/13.51 | 1.05701 | C12H26O4S | 79.3 | |||
| 265.1475/14.05 | 1.072462 | 88.4 | ||||
| 279.2309/13.79 | 6.86827 | 92.5 | ||||
| 281.2465/14.03 | 7.233686 | 91.4 | ||||
| 282.2507/14.01 | 2.547436 | 89.4 | ||||
| 283.2635/14.34 | 1.250575 | 78.5 | ||||
| 289.0702/5.24 | 12.24895 | 82.6 | ||||
| 289.0705/4.50 | 14.487 | C15H14O6 | Catechin | Flavonoid | 97.8 | BS003014 |
| 291.0146/5.12 | 3.056952 | C13H8O8 | Brevifolincarboxylic acid | Polyphenol | 92.5 | [64] |
| 293.1240/5.22 | 1.667305 | C12H22O8 | 89.9 | |||
| 293.1791/15.70 | 1.215117 | |||||
| 295.2263/13.06 | 5.327168 | 88.2 | ||||
| 297.2422/13.05 | 2.411256 | C18H34O3 | 91.4 | |||
| 300.9982/6.84 | 8.084582 | Ellagic acid | Polyphenol | 92.2 | NGA02837 | |
| 301.0345/8.09 | 1.688408 | Quercetin (C15H10O7) | Quercetin | Flavonoid | 93.5 | PR100233 |
| 309.1727/14.34 | 1.722773 | |||||
| 311.2221/11.84 | 1.983825 | |||||
| 313.2368/12.20 | 8.325131 | C18H36O5 | Phloionolic acid | Triterpenoid | 88.4 | |
| 314.2411/12.21 | 1.989586 | C12H29N9O | ||||
| 315.0875/7.06 | 1.83894 | C17H16O6 | Persicogenin | Flavonoid | 95.6 | [65] |
| 315.2528/12.65 | 1.635675 | |||||
| 329.2327/10.38 | 1.132457 | |||||
| 341.1077/0.98 | 4.904493 | |||||
| 341.1086/1.80 | 1.266851 | C13H26O6S2 | 82.2 | |||
| 366.1190/5.82 | 1.098081 | C17H21NO8 | 78.4 | |||
| 380.9556/6.29 | 1.721243 | C15H10O6S3 | 73.5 | |||
| 387.1144/0.96 | 1.194701 | |||||
| 409.2340/14.65 | 1.206799 | C21H34N2O6 | 78.8 | |||
| 433.0408/6.51 | 2.234584 | C20H10N4O8 | 75.5 | |||
| 433.2336/14.26 | 2.630344 | |||||
| 439.0650/6.75 | 4.659214 | C22H16O10 | 89.2 | |||
| 439.0656/7.42 | 5.428307 | C22H16O10 | Amurensisin | Flavonoid | 82.3 | [66] |
| 439.0841/0.99 | 1.663483 | C15H16N6O10 | 78.5 | |||
| 440.0712/7.56 | 1.214139 | C16H11N9O7 | 77.9 | |||
| 441.0808/5.73 | 8.944765 | C22H18O8 | Epicatechin gallate | Flavonoid | 81.3 | BS003900 |
| 442.0865/5.87 | 2.155587 | C16H12N9O7 | 82.2 | |||
| 453.0676/4.78 | 1.773836 | C20H14N4O9 | 83.4 | |||
| 455.3510/13.60 | 2.632012 | C30H48O3 | Oleanolic acid | Triterpenoid acid | 91.2 | TY000153 |
| 461.2655/15.86 | 2.204115 | C25H38N2O6 | 78.4 | |||
| 463.0515/5.80 | 1.35816 | C21H12N4O9 | 90.4 | |||
| 476.2760/13.28 | 1.116856 | C25H39N3O6 | 95.6 | |||
| 477.0664/6.62 | 1.626783 | C21H18O13 | Quercetin 3-O-glucuronide (Miquelianin) | Flavonoid glycoside | 97.7 | PR100978 |
| 479.0829/6.15 | 1.042092 | C21H20O13 | Myricetin-3-O-glucoside | Flavonoid glycoside | 92.2 | [67] |
| 571.2874/15.62 | 2.853407 | C40H36N4 | 83.5 | |||
| 577.1328/4.71 | 5.115305 | C27H17N10O6 | 87.4 | |||
| 577.1332/4.14 | 5.979778 | C27H18N10O6 | 86.5 | |||
| 577.1347/5.87 | 1.222666 | C23H26N6O10S | 88.2 | |||
| 578.1376/4.74 | 1.749601 | C24H21N9O9 | 92.1 | |||
| 578.1378/4.11 | 2.151917 | C24H21N9O9 | 86.4 | |||
| 595.2864/14.96 | 2.240486 | C41H40O4 | 98.1 | |||
| 729.1441/4.95 | 2.275241 | C31H42N2O8S5 | 84.2 | |||
| 865.1964/4.97 | 1.576048 | C47H46O8S4 | 83.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zalidis, A.P.; Kalogiouri, N.P.; Mourtzinos, I.; Sarris, D.; Gkatzionis, K. A Novel Liquid Chromatographic Time-of-Flight Tandem Mass Spectrometric Method for the Determination of Secondary Metabolites in Functional Flours Produced from Grape Seed and Olive Stone Waste. Molecules 2025, 30, 1527. https://doi.org/10.3390/molecules30071527
Zalidis AP, Kalogiouri NP, Mourtzinos I, Sarris D, Gkatzionis K. A Novel Liquid Chromatographic Time-of-Flight Tandem Mass Spectrometric Method for the Determination of Secondary Metabolites in Functional Flours Produced from Grape Seed and Olive Stone Waste. Molecules. 2025; 30(7):1527. https://doi.org/10.3390/molecules30071527
Chicago/Turabian StyleZalidis, Achilleas Panagiotis, Natasa P. Kalogiouri, Ioannis Mourtzinos, Dimitris Sarris, and Konstantinos Gkatzionis. 2025. "A Novel Liquid Chromatographic Time-of-Flight Tandem Mass Spectrometric Method for the Determination of Secondary Metabolites in Functional Flours Produced from Grape Seed and Olive Stone Waste" Molecules 30, no. 7: 1527. https://doi.org/10.3390/molecules30071527
APA StyleZalidis, A. P., Kalogiouri, N. P., Mourtzinos, I., Sarris, D., & Gkatzionis, K. (2025). A Novel Liquid Chromatographic Time-of-Flight Tandem Mass Spectrometric Method for the Determination of Secondary Metabolites in Functional Flours Produced from Grape Seed and Olive Stone Waste. Molecules, 30(7), 1527. https://doi.org/10.3390/molecules30071527









