Analysis of Chemical Composition and Biological Activities of Essential Oils from Different Parts of Alpinia uraiensis Hayata
Abstract
1. Introduction
2. Results and Discussion
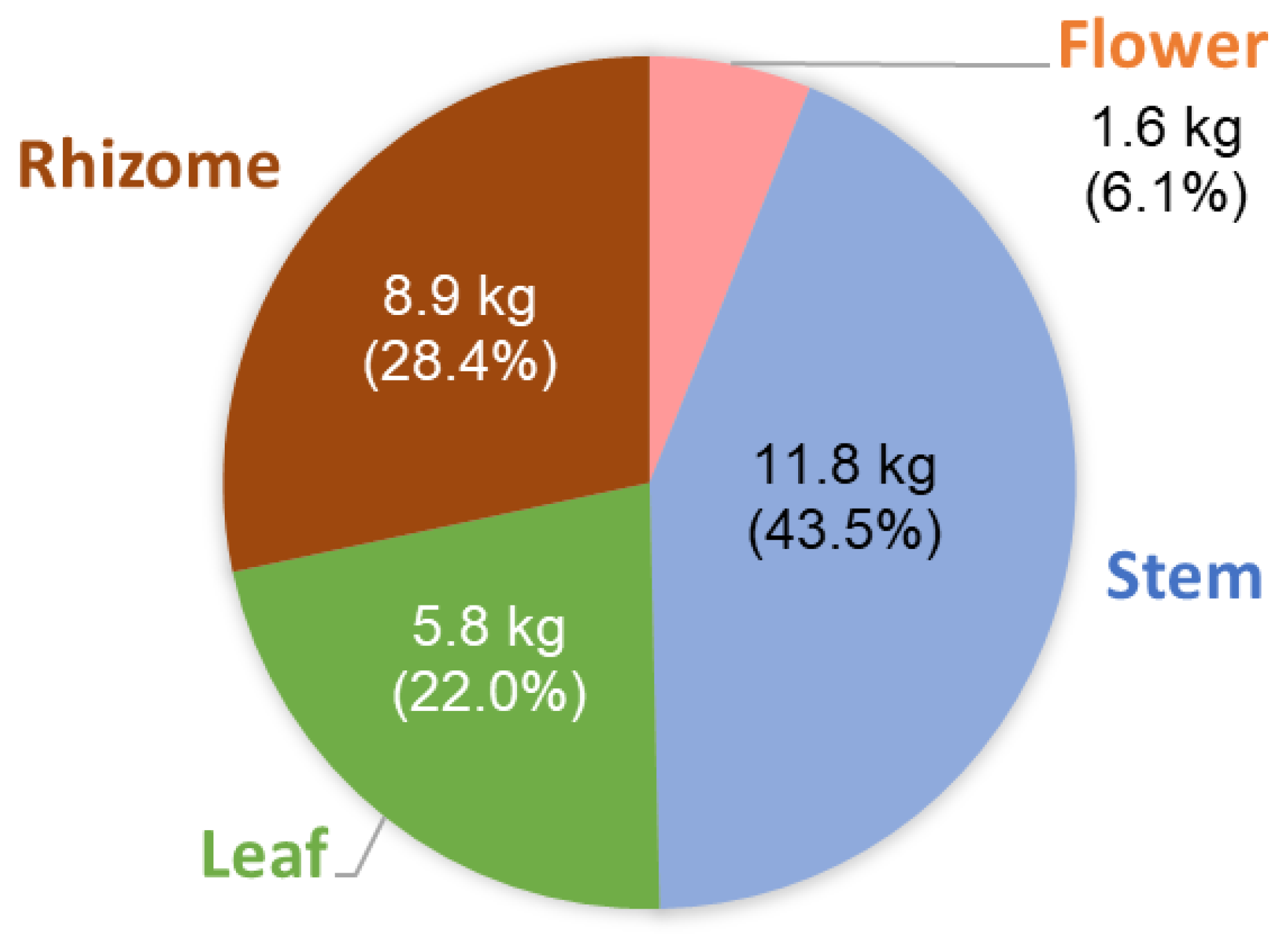
2.1. Yields of Essential Oils
2.2. Essential Oil Composition of Different Plant Parts
2.3. Comparative Analysis with Other Alpinia Species
2.3.1. Leaf Essential Oil
2.3.2. Rhizome Essential Oil
2.4. Antioxidant Activity of Essential Oils from Different Parts of A. uraiensis
2.5. Antifungal Activity of Essential Oils from Different Parts of A. uraiensis
3. Materials and Methods
3.1. Collection of Plant Material
3.2. Isolation of Essential Oils
3.3. Essential Oil Analysis
3.4. DPPH Free-Radical Scavenging Assay
3.5. Fungal Strain
3.6. Antifungal Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bown, D. New Encyclopedia of Herbs & Their Uses, 1st American ed.; DK Publishing: London, UK; New York, NY, USA, 2001. [Google Scholar]
- Kress, W.J.; Liu, A.Z.; Newman, M.; Li, Q.J. The molecular phylogeny of Alpinia (Zingiberaceae): A complex and polyphyletic genus of gingers. Am. J. Bot. 2005, 92, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.-W. Taxonomy of the Genus Alpinia (Zingiberaceae) in Taiwan and the Analyses of their Rootstock Essential Oils. Master’s Thesis, National Chiayi University, Chiayi, Taiwan, 2006. [Google Scholar]
- Kumar, K.J.S.; Wang, S.H.; Tseng, Y.H.; Tsao, N.W.; Kuo, Y.H.; Wang, S.Y. Trans-3-Methoxy-5-hydroxystilbene (MHS) from the rhizome of Alpinia nantonensis inhibits metastasis in human lung cancer cells. Phytomedicine 2018, 50, 223–230. [Google Scholar] [CrossRef]
- Hsu, C.L.; Yu, Y.S.; Yen, G.C. Anticancer effects of Alpinia pricei Hayata roots. J. Agric. Food Chem. 2010, 58, 2201–2208. [Google Scholar] [CrossRef]
- Chen, I.N.; Chang, C.C.; Ng, C.C.; Wang, C.Y.; Shyu, Y.T.; Chang, T.L. Antioxidant and antimicrobial activity of Zingiberaceae plants in Taiwan. Plant Food Hum. Nutr. 2008, 63, 15–20. [Google Scholar] [CrossRef]
- Youn, I.; Han, A.-R.; Piao, D.; Lee, H.; Kwak, H.; Lee, Y.; Nam, J.-W.; Seo, E.K. Phytochemical and pharmacological properties of the genus Alpinia from 2016 to 2023. Nat. Prod. Rep. 2024, 41, 1346–1367. [Google Scholar] [CrossRef]
- De Pooter, H.L.; Aboutabl, E.A.; El-Shabrawy, A.O. Chemical composition and antimicrobial activity of essential oil of leaf, stem and rhizome of Alpinia speciosa (JC Wendl.) K. Schum. grown in Egypt. Flavour Frag. J. 1995, 10, 63–67. [Google Scholar] [CrossRef]
- Nhan, N.T.; Lan, C.T.; Linh, L.; Huong, L.; Dai, D.; Ogunwande, I.A. Chemical compositions of essential oils and antimicrobial activity of Alpinia kwangsiensis from Vietnam. J. Essent. Oil Bear. Plants. 2021, 24, 714–723. [Google Scholar] [CrossRef]
- Zhou, C.Q.; Li, C.Z.; Siva, S.; Cui, H.Y.; Lin, L. Chemical composition, antibacterial activity and study of the interaction mechanisms of the main compounds present in the Alpinia galanga rhizomes essential oil. Ind. Crop. Prod. 2021, 165, 113441. [Google Scholar] [CrossRef]
- Ge, X.Z.; Liang, Q.H.; Long, Y.; Shen, H.S.; Zhang, Q.; Sun, Z.Z.; Li, W.H. Assessment of fresh Alpinia galanga (A. galanga) drying techniques for the chemical composition of essential oil and its antioxidant and biological activity. Food Chem. 2022, 392, 133314. [Google Scholar] [CrossRef]
- Salim, M.; Rajendran, R.; Nair, S.A.; Dan, M.; Baby, S. Chemical composition and biological activities of rhizome and fruit rind oils of Alpinia mutica from south India. J. Essent. Oil Res. 2016, 28, 428–435. [Google Scholar] [CrossRef]
- Tian, Y.F.; Jia, X.Y.; Wang, Q.Q.; Lu, T.Y.; Deng, G.D.; Tian, M.Y.; Zhou, Y. Antioxidant, antibacterial, enzyme inhibitory, and anticancer activities and chemical composition of Alpinia galanga flower essential oil. Pharmaceuticals 2022, 15, 1069. [Google Scholar] [CrossRef]
- Hong, Y.; Liu, X.L.; Wang, H.J.; Zhang, M.; Tian, M.Y. Chemical composition, anticancer activities and related mechanisms of the essential oil from Alpinia coriandriodora rhizome. Ind. Crop. Prod. 2022, 176, 114328. [Google Scholar] [CrossRef]
- Kumar, K.J.S.; Vani, M.G.; Wu, P.C.; Lee, H.J.; Tseng, Y.H.; Wang, S.Y. Essential oils of Alpinia nantoensis retard forskolin-induced melanogenesis via ERK1/2-mediated proteasomal degradation of MITF. Plants 2020, 9, 1672. [Google Scholar] [CrossRef]
- Lin, L.Y.; Shen, K.H.; Yeh, X.Y.; Huang, B.Y.; Wang, H.E.; Chen, K.C.; Peng, R.Y. Integrated process for production of galangal acetate, the wasabi-like spicy compound, and analysis of essential oils of Rhizoma Alpinia officinarum (Hance) farw. J. Food Sci. 2016, 81, H1565–H1575. [Google Scholar] [CrossRef]
- Padalia, R.C.; Chanotiya, C.S.; Sundaresan, V. Compositional variability in essential oil from different parts of Alpinia speciosa from India. Nat. Prod. Commun. 2010, 5, 279–282. [Google Scholar]
- Rout, P.K.; Sahoo, S.; Rath, S.P.; Rao, Y.R. Analysis of the leaf, rhizome and root oils of two accessions of Alpinia calcarata Rosc. cultivated at Bhubaneswar. J. Essent. Oil Res. 2005, 17, 398–400. [Google Scholar] [CrossRef]
- Huong, L.T.; Dai, D.N.; Chung, M.V.; Dun, D.M.; Ogunwande, I.A. Constituents of essential oils from the leaf, stem, root, fruit and flower of Alpinia macroura K. Schum. Bol. Latinoam. Y Del Caribe De Plantas Med. Y Aromat. 2017, 16, 26–33. [Google Scholar]
- Cheng, S.-S.; Lin, C.-Y.; Chung, M.-J.; Chen, Y.-J.; Chang, S.-T. Potential source of environmentally benign antifungal agents from Cinnamomum osmophloeum leaves against Phellinus noxius. Plant Prot. Sci. 2019, 55, 43–53. [Google Scholar]
- Almeida, N.A.; Freire, L.; Carnielli-Queiroz, L.; Bragotto, A.P.A.; Silva, N.C.C.; Rocha, L.O. Essential oils: An eco-friendly alternative for controlling toxigenic fungi in cereal grains. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13251. [Google Scholar] [CrossRef]
- Li, Y.X.; Erhunmwunsee, F.; Liu, M.; Yang, K.L.; Zheng, W.F.; Tian, J. Antimicrobial mechanisms of spice essential oils and application in food industry. Food Chem. 2022, 382, 132312. [Google Scholar] [CrossRef]
- Mukarram, M.; Choudhary, S.; Khan, M.A.; Poltronieri, P.; Khan, M.M.A.; Ali, J.; Kurjak, D.; Shahid, M. Lemongrass essential oil components with antimicrobial and anticancer activities. Antioxidants 2022, 11, 20. [Google Scholar] [CrossRef]
- Guo, Y.; Pizzol, R.; Gabbanini, S.; Baschieri, A.; Amorati, R.; Valgimigli, L. Absolute antioxidant activity of five phenol-rich essential oils. Molecules 2021, 26, 5237. [Google Scholar] [CrossRef]
- Zaman, F.Q.; Ridzuan, R.; Abdelmageed, A.H.A. Chemical composition, antioxidant and antimicrobial activities of the essential oils from rhizomes and leaves of Alpinia conchigera Griff. (Zingiberaceae). J. Essent. Oil Bear. Plants. 2021, 24, 1311–1322. [Google Scholar] [CrossRef]
- Tu, P.T.B.; Tawata, S. Anti-oxidant, anti-aging, and anti-melanogenic properties of the essential oils from two varieties of Alpinia zerumbet. Molecules 2015, 20, 16723–16740. [Google Scholar] [CrossRef]
- Ghosh, S.; Ozek, T.; Tabanca, N.; Ali, A.; Rehman, J.U.; Khan, I.A.; Rangan, L. Chemical composition and bioactivity studies of Alpinia nigra essential oils. Ind. Crop. Prod. 2014, 53, 111–119. [Google Scholar] [CrossRef]
- Huong, L.T.; Dai, D.N.; Thang, T.D.; Bach, T.T.; Ogunwande, I.A. Analysis of the volatile constituents of Alpinia pinnanensis. J. Essent. Oil Bear. Plants. 2017, 20, 264–271. [Google Scholar] [CrossRef]
- Jusoh, S.; Sirat, H.M.; Ahmad, F. Essential oils of Alpinia rafflesiana and their antimicrobial activities. Nat. Prod. Commun. 2013, 8, 1317–1320. [Google Scholar]
- Hung, N.D.; Huong, L.T.; Dai, D.N.; Hoi, T.M.; Ogunwande, I.A. Chemical composition of essential oils of Alpinia strobiliformis T. L. Wu & SJ Chen and Alpinia blepharocalyx K. Schum. from Vietnam. J. Essent. Oil Bear. Plants. 2018, 21, 1585–1593. [Google Scholar] [CrossRef]
- Huong, L.T.; Thang, T.D.; Ogunwande, I.A. Chemical constituents of essential oils from the leaves, stems, roots and fruits of Alpinia polyantha. Nat. Prod. Commun. 2015, 10, 367–368. [Google Scholar] [PubMed]
- Huong, L.T.; Dai, D.N.; Thang, T.D.; Bach, T.T.; Ogunwande, I.A. The essential oils of the leaf, pseudostem root and fruit of Alpinia mutica Roxb. J. Essent. Oil Bear. Plants 2016, 19, 2049–2055. [Google Scholar] [CrossRef]
- Huong, L.T.; Dai, D.N.; Chau, L.T.M.; Ogunwande, I.A. Analysis of essential oils from Alpinia napoensis. Chem. Nat. Compd. 2018, 54, 992–994. [Google Scholar] [CrossRef]
- Ho, J.C. Chemical composition and bioactivity of essential oil of seed and leaf from Alpinia speciosa grown in Taiwan. J. Chin. Chem. Soc. 2010, 57, 758–763. [Google Scholar] [CrossRef]
- Chen, Z.F.; He, B.; Zhou, J.; He, D.H.; Deng, J.D.; Zeng, R.H. Chemical compositions and antibacterial activities of essential oils extracted from Alpinia guilinensis against selected foodborne pathogens. Ind. Crop. Prod. 2016, 83, 607–613. [Google Scholar] [CrossRef]
- Lin, L.Y.; Peng, C.C.; Liang, Y.J.; Yeh, W.T.; Wang, H.E.; Yu, T.H.; Peng, R.Y. Alpinia zerumbet potentially elevates high-density lipoprotein cholesterol level in hamsters. J. Agric. Food Chem. 2008, 56, 4435–4443. [Google Scholar] [CrossRef]
- Sun, C.H.; Hsiao, W.F.; Wang, S.S. Compositional variability of functional ingredients from various parts of Alpinia uraiensis Hayata. J. Food Biochem. 2013, 37, 193–202. [Google Scholar] [CrossRef]
- Chen, W.; Vermaak, I.; Viljoen, A. Camphor—A fumigant during the black death and a coveted fragrant wood in ancient Egypt and Babylon—A review. Molecules 2013, 18, 5434–5454. [Google Scholar] [CrossRef]
- Mendes, F.R.S.; Silva, F.G.E.; Sousa, E.O.; Rodrigues, F.F.G.; Costa, J.G.M.; Monte, F.J.Q.; Lemos, T.L.G.; Assunçao, J.C.C. Essential oil of Alpinia zerumbet (Pers.) B.L. Burtt. & R.M. Sm. (Zingiberaceae): Chemical composition and modulation of the activity of aminoglycoside antibiotics. J. Essent. Oil Res. 2015, 27, 259–263. [Google Scholar] [CrossRef]
- Victório, C.P.; Leitao, S.G.; Lage, C.L.S. Chemical composition of the leaf oils of Alpinia zerumbet (Pers.) Burtt et Smith and A. purpurata (Vieill) K. Schum. from Rio de Janeiro, Brazil. J. Essent. Oil Res. 2010, 22, 52–54. [Google Scholar] [CrossRef]
- Zoghbi, M.D.B.; Andrade, E.H.A.; Maia, J.G.S. Volatile constituents from leaves and flowers of Alpinia speciosa K. Schum. and A. purpurata (Viell.) Schum. Flavour Frag. J. 1999, 14, 411–414. [Google Scholar] [CrossRef]
- Van, H.T.; Thang, T.D.; Luu, T.N.; Doan, V.D. An overview of the chemical composition and biological activities of essential oils from Alpinia genus (Zingiberaceae). RSC Adv 2021, 11, 37767–37783. [Google Scholar] [CrossRef]
- Lahlou, S.; Galindo, C.A.B.; Leal-Cardoso, J.H.; Fonteles, M.C.; Duarte, G.P. Cardiovascular effects of the essential oil of Alpinia zerumbet leaves and its main constituent, terpinen-4-ol, in rats:: Role of the autonomic nervous system. Planta Medica 2002, 68, 1097–1102. [Google Scholar] [CrossRef] [PubMed]
- Padalia, R.C.; Verma, R.S.; Sundaresan, V.; Chanotiya, C.S. Chemical diversity in the genus Alpinia (Zingiberaceae): Comparative composition of four Alpinia species grown in Northern India. Chem. Biodivers. 2010, 7, 2076–2087. [Google Scholar] [CrossRef] [PubMed]
- Indrayan, A.K.; Garg, S.N.; Rathi, A.K.; Sharma, V. Chemical composition and antimicrobial activity of the essential oil of Alpinia officinarum rhizome. Indian J. Chem. Sect B—Org. Chem. Incl. Med. Chem. 2007, 46, 2060–2063. [Google Scholar]
- Wu, Y.; Wang, Y.; Li, Z.H.; Wang, C.F.; Wei, J.Y.; Li, X.L.; Wang, P.J.; Zhou, Z.F.; Du, S.S.; Huang, D.Y.; et al. Composition of the essential oil from Alpinia galanga rhizomes and its bioactivity on Lasioderma serricorne. Bull. Insectol. 2014, 67, 247–254. [Google Scholar]
- Zhang, L.Y.; Pan, C.X.; Ou, Z.R.; Liang, X.X.; Shi, Y.H.; Chi, L.J.; Zhang, Z.J.; Zheng, X.; Li, C.L.; Xiang, H.P. Chemical profiling and bioactivity of essential oils from Alpinia officinarum Hance from ten localities in China. Ind. Crop. Prod. 2020, 153, 112583. [Google Scholar] [CrossRef]
- Prakash, O.; Joshi, S.; Pant, A.K.; Chanotiya, C.S.; Mathela, C.S. Volatile constituents of rhizomes and leaves of Alpinia allughas Roscoe. J. Essent. Oil Res. 2007, 19, 407–409. [Google Scholar] [CrossRef]
- de Sousa, D.P.; Damasceno, R.O.S.; Amorati, R.; Elshabrawy, H.A.; de Castro, R.D.; Bezerra, D.P.; Nunes, V.R.V.; Gomes, R.C.; Lima, T.C. Essential oils: Chemistry and pharmacological activities. Biomolecules 2023, 13, 1144. [Google Scholar] [CrossRef]
- Zengin, H.; Baysal, A.H. Antibacterial and antioxidant activity of essential oil terpenes against pathogenic and spoilage-forming bacteria and cell structure-activity relationships evaluated by SEM microscopy. Molecules 2014, 19, 17773–17798. [Google Scholar] [CrossRef] [PubMed]
- Guleria, S.; Tiku, A.K.; Koul, A.; Gupta, S.; Singh, G.; Razdan, V.K. Antioxidant and antimicrobial properties of the essential oil and extracts of Zanthoxylum alatum grown in north-western Himalaya. Sci. World J. 2013, 2013, 790580. [Google Scholar] [CrossRef]
- Chen, C.J.; Tong, Z.F.; Liao, D.K.; Li, Y.; Yang, G.; Li, M.F. Chemical composition and antimicrobial and DPPH scavenging activity of essential oil of Toona sinensis (A. Juss.) Roem from China. Bioresources 2014, 9, 5262–5278. [Google Scholar]
- Lin, C.W.; Yu, C.W.; Wu, S.C.; Yih, K.H. DPPH free-radical scavenging activity, total phenolic contents and chemical composition analysis of forty-two kinds of essential oils. J. Food Drug Anal. 2009, 17, 386–395. [Google Scholar]
- Chen, Y.H.; Lin, C.Y.; Yen, P.L.; Yeh, T.F.; Cheng, S.S.; Chang, S.T. Antifungal agents from heartwood extract of Taiwania cryptomerioides against brown root rot fungus Phellinus noxius. Wood Sci. Technol. 2017, 51, 639–651. [Google Scholar] [CrossRef]
- Hsiao, W.-W.; Lau, K.-M.; Chien, S.-C.; Chu, F.-H.; Chung, W.-H.; Wang, S.-Y. Antifungal Activity of Cedrol from Cunninghamia lanceolate var. konishii against Phellinus noxius and Its Mechanism. Plants 2024, 13, 321. [Google Scholar] [CrossRef]
- Cherrad, S.; Jaouadi, I.; Bouyahya, A.; Koursaoui, L.; Aouane, E.; Satrani, B.; Sultan, M.A.; Alotaibi, A.; Ullah, R.; Ghanmi, M.; et al. Phytochemical analysis and study of antioxidant and antimicrobial activities of two parts of Cupressus arizonica essential oils. J. Food Qual. 2022, 2022, 8629974. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography—Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Polatoglu, K.; Sen, A.; Kandemir, A.; Gören, N. Essential oil composition and DPPH scavenging activity of endemic Tanacetum mucroniferum Hub.–Mor. & Grierson from Turkey. J. Essent. Oil Bear. Plants. 2012, 15, 66–74. [Google Scholar] [CrossRef]
- Cheng, S.S.; Liu, J.Y.; Hsui, Y.R.; Chang, S.T. Chemical polymorphism and antifungal activity of essential oils from leaves of different provenances of indigenous cinnamon (Cinnamomum osmophloeum). Bioresour. Technol. 2006, 97, 306–312. [Google Scholar]
| Compounds | AI 1 | rAI 2 | Leaf | Flower | Root | Stem | Rhizome | Identification 3 |
|---|---|---|---|---|---|---|---|---|
| 2-Heptanol | 902 | 894 | 2.3 ± 0.7 a | 0.5 ± 0.5 b | n.d. 4 | 0.5 ± 0.5 b | 0.5 ± 0.2 b | MS, AI |
| Tricyclene | 924 | 921 | 0.0 ± 0.0 a | n.d. | n.d. | 0.0 ± 0.0 a | 0.0 ± 0.0 a | MS, AI |
| α-Thujene | 927 | 924 | 0.3 ± 0.1 c | 0.9 ± 0.2 b | n.d. | 1.2 ± 0.1 ab | 1.4 ± 0.3 a | MS, AI |
| α-Pinene | 934 | 932 | 3.1 ± 0.8 bc | 1.9 ± 1.0 cd | 0.7 ± 0.5 d | 4.9 ± 1.1 ab | 5.7 ± 0.8 a | MS, AI, ST |
| Camphene | 951 | 946 | 1.0 ± 1.0 a | 0.9 ± 0.9 a | 1.2 ± 0.7 a | 1.0 ± 0.9 a | 1.1 ± 1.2 a | MS, AI, ST |
| Sabinene | 974 | 969 | n.d. | 3.4 ± 0.1 a | n.d. | 1.0 ± 0.2 b | 1.2 ± 0.2 b | MS, AI, ST |
| β-Pinene | 979 | 974 | 2.3 ± 0.9 c | 7.6 ± 2.2 b | 0.4 ± 0.2 c | 15.0 ± 3.6 a | 17.9 ± 1.3 a | MS, AI, ST |
| Myrcene | 990 | 988 | 0.8 ± 0.1 b | 1.3 ± 0.3 a | 0.4 ± 0.2 c | 1.2 ± 0.1 a | 1.2 ± 0.0 a | MS, AI, ST |
| α-Phellandrene | 1008 | 1002 | 0.3 ± 0.1 bc | 2.3 ± 1.0 a | n.d. | 0.9 ± 0.4 bc | 1.4 ± 0.6 ab | MS, AI, ST |
| α-Terpinene | 1018 | 1014 | 3.3 ± 0.9 b | 9.5 ± 0.2 a | n.d. | 10.1 ± 2.9 a | 8.4 ± 1.7 a | MS, AI, ST |
| p-Cymene | 1026 | 1020 | 13.5 ± 0.3 a | 0.8 ± 0.0 d | 1.0 ± 0.0 d | 3.8 ± 0.3 c | 5.4 ± 0.0 b | MS, AI, ST |
| Limonene | 1030 | 1024 | 2.8 ± 0.3 a | 1.1 ± 0.2 b | 2.6 ± 0.1 a | 1.6 ± 0.3 b | 1.7 ± 0.2 b | MS, AI, ST |
| 1,8-Cineole | 1033 | 1026 | 16.8 ± 0.4 a | 8.8 ± 0.5 b | 2.9 ± 0.1 c | 8.5 ± 0.8 b | 8.7 ± 0.3 b | MS, AI, ST |
| 2-Heptyl acetate | 1038 | 1038 | n.d. | 0.9 ± 0.1 a | n.d. | n.d. | 0.1 ± 0.2 b | MS, AI |
| γ-Terpinene | 1059 | 1054 | 24.0 ± 3.6 a | 19.5 ± 0.9 a | 1.1 ± 0.0 b | 22.8 ± 3.3 a | 19.5 ± 3.6 a | MS, AI, ST |
| Terpinolene | 1088 | 1086 | 1.8 ± 0.6 b | 3.3 ± 0.1 a | 0.5 ± 0.0 c | 3.7 ± 0.5 a | 3.0 ± 0.6 a | MS, AI, ST |
| Fenchone | 1090 | 1083 | n.d. | n.d. | 1.2 ± 0.0 a | n.d. | n.d. | MS, AI |
| Linalool | 1100 | 1095 | 0.9 ± 0.1 c | 2.0 ± 0.1 a | n.d. | 0.3 ± 0.1 d | 1.3 ± 0.1 b | MS, AI, ST |
| Fenchol | 1120 | 1118 | n.d. | n.d. | 2.3 ± 0.0 a | n.d. | n.d. | MS, AI |
| Camphor | 1149 | 1141 | 2.2 ± 2.3 a | 3.5 ± 3.7 a | 0.4 ± 0.1 a | 2.2 ± 2.3 a | 2.7 ± 3.0 a | MS, AI, ST |
| Borneol | 1173 | 1165 | 0.0 ± 0.0 b | n.d. | 0.8 ± 0.1 a | 0.0 ± 0.0 b | 0.0 ± 0.0 b | MS, AI, ST |
| Terpinen-4-ol | 1181 | 1174 | 22.6 ± 0.9 ab | 28.9 ± 8.4 a | 0.3 ± 0.1 c | 20.0 ± 3.0 ab | 16.9 ± 1.3 b | MS, AI, ST |
| α-Terpineol | 1196 | 1186 | 1.0 ± 0.1 bc | 1.5 ± 0.3 a | 0.2 ± 0.1 d | 1.1 ± 0.0 b | 0.7 ± 0.2 c | MS, AI, ST |
| Fenchyl acetate | 1219 | 1218 | n.d. | n.d. | 72.2 ± 3.5 a | n.d. | n.d. | MS, AI, ST |
| Thymol methyl ether | 1229 | 1232 | n.d. | n.d. | 2.2 ± 0.1 a | n.d. | n.d. | MS, AI |
| Bornyl acetate | 1284 | 1284 | n.d. | n.d. | 0.3 ± 0.1 a | n.d. | n.d. | MS, AI, ST |
| α-Copaene | 1375 | 1374 | 0.1 ± 0.1 a | 0.2 ± 0.2 | n.d. | n.d. | 0.2 ± 0.2 a | MS, AI |
| β-Elemene | 1389 | 1389 | n.d. | n.d. | 0.2 ± 0.0 a | n.d. | n.d. | MS, AI, ST |
| (E)-Caryophyllene | 1419 | 1417 | 0.1 ± 0.0 bc | 0.2 ± 0.1 b | 0.8 ± 0.1 a | n.d. | 0.0 ± 0.0 bc | MS, AI, ST |
| α-Bergamotene | 1432 | 1432 | 0.4 ± 0.1 ab | 0.2 ± 0.1 ab | 0.3 ± 0.1 ab | 0.2 ± 0.1 b | 0.4 ± 0.1 a | MS, AI |
| α-Humulene | 1454 | 1452 | n.d. | n.d. | 0.4 ± 0.1 a | n.d. | n.d. | MS, AI, ST |
| 4,5-di-epi-Aristolochene | 1469 | 1471 | n.d. | n.d. | 0.6 ± 0.1 a | n.d. | n.d. | - |
| Germacrene D | 1488 | 1480 | 0.1 ± 0.1 a | n.d. | 0.2 ± 0.0 a | n.d. | 0.2 ± 0.0 a | MS, AI |
| Aristolochene | 1491 | 1487 | n.d. | n.d. | 0.2 ± 0.1 a | n.d. | n.d. | MS, AI |
| β-Selinene | 1495 | 1489 | n.d. | n.d. | 0.4 ± 0.1 a | n.d. | n.d. | MS, AI |
| β-Dihydro agarofuran | 1503 | 1503 | n.d. | n.d. | 0.2 ± 0.0 a | n.d. | n.d. | MS, AI |
| β-Bisabolene | 1508 | 1505 | n.d. | n.d. | 0.0 ± 0.0 a | n.d. | 0.0 ± 0.0 a | MS, AI |
| γ-Cadinene | 1511 | 1513 | n.d. | n.d. | 0.2 ± 0.1 a | n.d. | n.d. | MS, AI |
| 7-epi-α-Selinene | 1517 | 1520 | n.d. | n.d. | 0.4 ± 0.1 a | n.d. | n.d. | MS, AI |
| trans-Calamenene | 1520 | 1521 | n.d. | n.d. | 0.3 ± 0.0 a | n.d. | n.d. | MS, AI |
| Spathulenol | 1579 | 1577 | n.d. | n.d. | 0.3 ± 0.1 a | n.d. | n.d. | MS, AI |
| γ-Eudesmol | 1620 | 1630 | n.d. | n.d. | 0.2 ± 0.1 a | n.d. | n.d. | MS, AI |
| Monoterpene hydrocarbons | 53.3 ± 2.3 b | 52.7 ± 4.8 b | 8.0 ± 1.5 c | 67.2 ± 0.4 a | 68.0 ± 2.1 a | |||
| Oxygenated monoterpenes | 45.9 ± 2.3 b | 46.1 ± 5.0 b | 82.8 ± 3.3 a | 32.5 ± 0.4 c | 30.9 ± 1.9 c | |||
| Sesquiterpene hydrocarbons | 0.7 ± 0.1 bc | 0.6 ± 0.4 bc | 3.8 ± 0.9 a | 0.2 ± 0.1 c | 0.9 ± 0.2 b | |||
| Oxygenated sesquiterpenes | n.d. | n.d. | 0.7 ± 0.2 a | n.d. | n.d. |
| Concentration (mg/mL) | Leaf | Flower | Root | Stem | Rhizome | Ascorbic Acid b |
|---|---|---|---|---|---|---|
| 100 | 48.6 ± 1.4 a | 99.5 ± 0.5 | - | 75.0 ± 0.9 | 86.4 ± 0.1 | - |
| 50 | 34.2 ± 0.7 | 97.5 ± 0.1 | - | 46.8 ± 1.0 | 68.3 ± 0.7 | - |
| 10 | 24.3 ± 1.3 | 53.5 ± 1.3 | 44.3 ± 1.4 | 20.4 ± 1.9 | 32.9 ± 0.5 | - |
| 5 | 17.5 ± 0.9 | 33.3 ± 1.2 | 30.8 ± 0.7 | 14.3 ± 0.6 | 20.9 ± 0.5 | 96.5 ± 0.1 |
| 2.5 | 11.3 ± 0.4 | 13.9 ± 0.2 | 23.1 ± 0.8 | 10.6 ± 0.3 | 12.6 ± 0.6 | 96.3 ± 0.2 |
| 1.25 | 6.1 ± 2.4 | 9.8 ± 0.7 | 16.2 ± 1.3 | 7.0 ± 0.4 | 7.2 ± 0.4 | 95.8 ± 0.3 |
| IC50 (mg/mL) | 126.97 ± 9.58 | 8.76 ± 0.20 | 14.43 ± 1.34 | 52.49 ± 2.02 | 25.04 ± 0.21 | <1.25 |
| Concentration (μg/mL) | Antifungal Index (%) | ||||
|---|---|---|---|---|---|
| Leaf | Flower | Root b | Stem | Rhizome | |
| 800 | 33.3 ± 4.4 a | 56.0 ± 0.8 | 100.0 ± 0.0 | 28.6 ± 4.4 | 32.6 ± 4.6 |
| 400 | 26.4 ± 5.8 | 41.5 ± 3.0 | 100.0 ± 0.0 | 19.2 ± 6.8 | 23.9 ± 4.1 |
| 200 | 19.7 ± 5.2 | 29.6 ± 5.1 | 96.9 ± 5.4 | 17.2 ± 6.4 | 18.1 ± 1.7 |
| 100 | 16.1 ± 4.1 | 26.4 ± 3.6 | 71.8 ± 13.4 | 16.1 ± 4.7 | 16.1 ± 5.3 |
| 50 | 14.3 ± 5.0 | 16.5 ± 4.3 | 48.4 ± 15.5 | 11.4 ± 1.2 | 13.9 ± 1.6 |
| MIC (μg/mL) c | >800 | >800 | 200 | >800 | >800 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-J.; Chen, F.-H.; Liu, T.-Y.; Huang, Y.-M.; Chen, Y.-C.; Hsu, F.-L. Analysis of Chemical Composition and Biological Activities of Essential Oils from Different Parts of Alpinia uraiensis Hayata. Molecules 2025, 30, 1515. https://doi.org/10.3390/molecules30071515
Chen Y-J, Chen F-H, Liu T-Y, Huang Y-M, Chen Y-C, Hsu F-L. Analysis of Chemical Composition and Biological Activities of Essential Oils from Different Parts of Alpinia uraiensis Hayata. Molecules. 2025; 30(7):1515. https://doi.org/10.3390/molecules30071515
Chicago/Turabian StyleChen, Ying-Ju, Fen-Hui Chen, Tse-Yen Liu, Yao-Moan Huang, Yi-Chiann Chen, and Fu-Lan Hsu. 2025. "Analysis of Chemical Composition and Biological Activities of Essential Oils from Different Parts of Alpinia uraiensis Hayata" Molecules 30, no. 7: 1515. https://doi.org/10.3390/molecules30071515
APA StyleChen, Y.-J., Chen, F.-H., Liu, T.-Y., Huang, Y.-M., Chen, Y.-C., & Hsu, F.-L. (2025). Analysis of Chemical Composition and Biological Activities of Essential Oils from Different Parts of Alpinia uraiensis Hayata. Molecules, 30(7), 1515. https://doi.org/10.3390/molecules30071515







