A Correlation-Based Approach for Predicting Humic Substance Bioactivity from Direct Compost Characterization
Abstract
1. Introduction
2. Results and Discussion
2.1. Physico-Chemical and Spectroscopic Characterization of Compost Extracts
2.1.1. Dissolved Organic Matter (DOM) Extract
- Physico-chemical characterization
- Spectroscopic Characterization
2.1.2. Humic-like Substances
- Physico-Chemical Characterization
- Spectroscopic Characterization
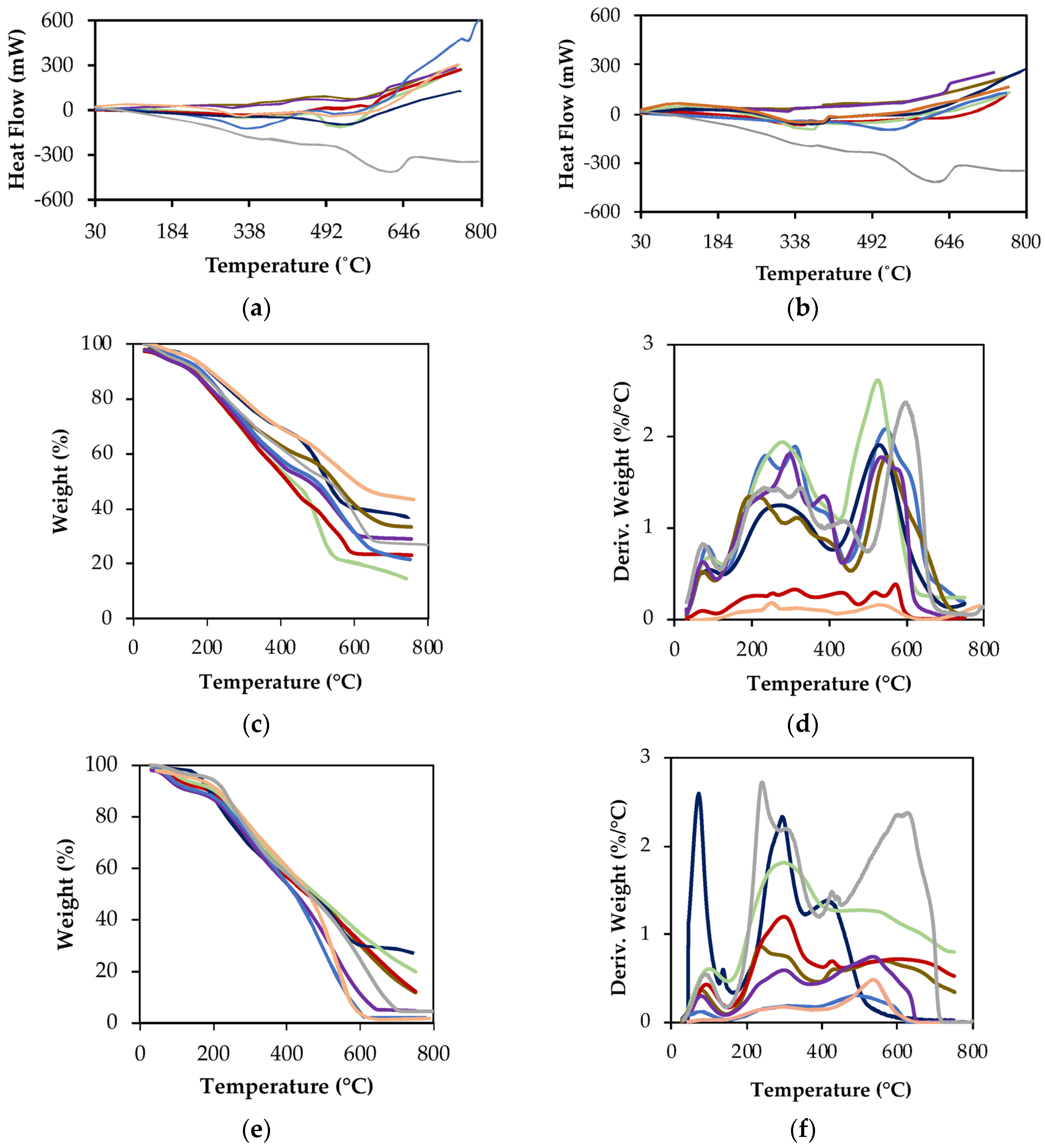
- Thermal Characterization
2.2. Correlations Between Physico-Chemical and Reactivity Parameters
- -
- The abundance of acidic functional groups (Mi) and protonation constants (Ki), derived from acid-base titrations [29].
- -
- The extent of Cd2+ binding by organic matter in the extracts, assessed at two metal concentrations—low (cML,L, 10−8 mol L−1) and high (cML,H, 10−6 mol L−1)—to distinguish between the strongest binding sites and the overall complexation capacity, evaluated through metal titrations [9].
- -
- Elemental composition (C, Coxi, N, C/N, Coxi/C, S, CEC, Na, K, Fe, As, Ca, Mg, Al, Cu, Mn);
- -
- Cation exchange capacity (CEC), which is a measure of the compost’s ability to hold positively charged ions;
- -
- Spectroscopic features from UV-vis (namely ε280) and ATR-FTIR (namely I1630/2925, I1630/2850 which serves as an aromaticity index, concerning the degree of aromatic condensation in humic structures;
- -
- Thermal properties assessed using Differential Scanning Calorimetry (DSC) (H1, H2, H3) and Thermogravimetric Analysis (TGA) (WL1, WL2, WL3, WL4) [30]:
- ▪
- H1 and WL1 (30–177 °C), correspond to dehydration and desorption processes, providing insight into the affinity for low molecular weight substances;
- ▪
- H2 and WL2 (177–400 °C) reflect the decomposition of recalcitrant organic matter;
- ▪
- WL3 (400–620 °C) + WL4 (620–800 °C) and H3 are related to the breakdown of extra-recalcitrant structures and carbonate decomposition.
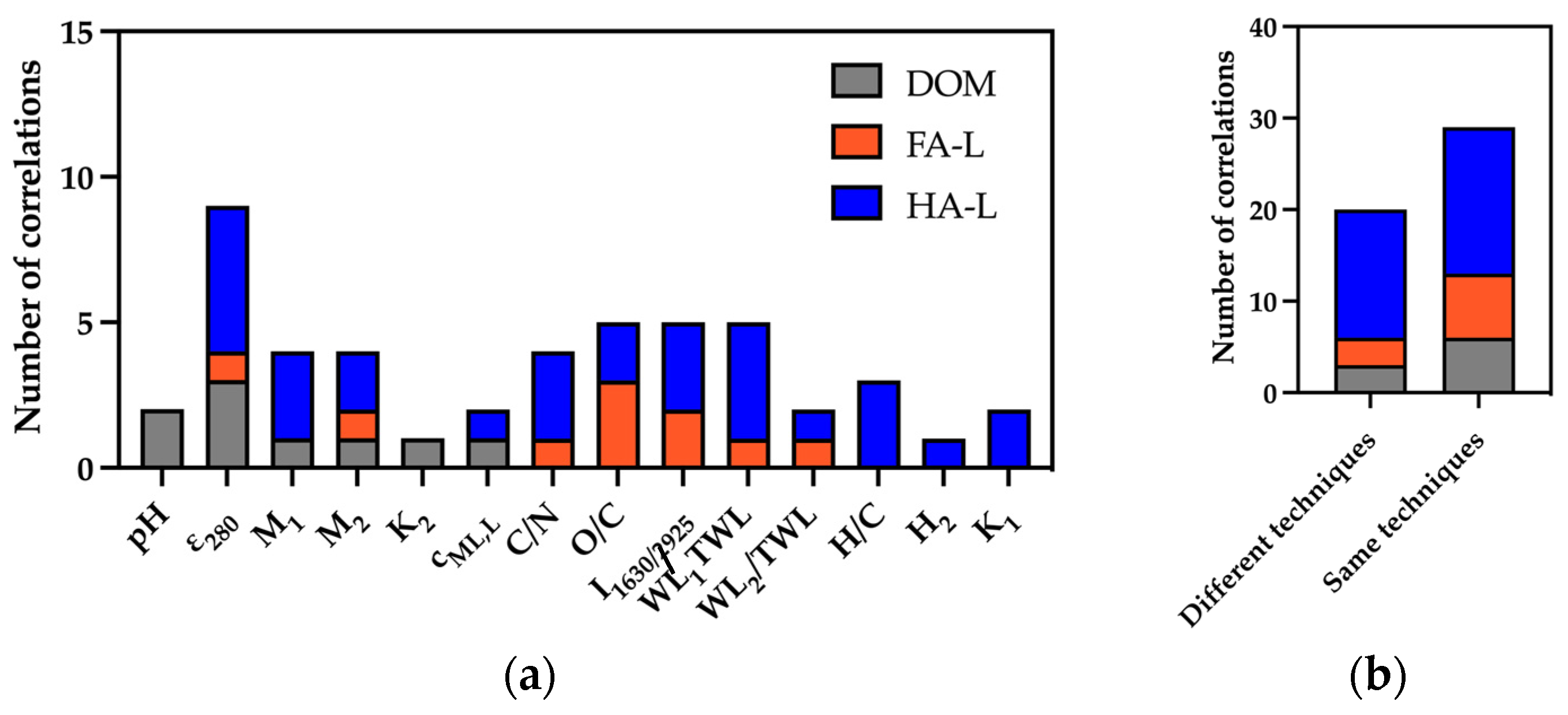
2.2.1. Correlation Between the Chemical Composition and Reactivity of Extracts
- General Trends
- FA-L Correlations
- (a)
- Aromaticity and Interaction with Low Molecular Weight Compounds: ε280 and I1630/2925 positively correlate with H1 and WL1/TWL, suggesting that more aromatic FA-L fractions exhibit stronger interactions with low molecular weight substances. While WL1 (30–177 °C) is primarily associated with moisture loss and volatile compound desorption, the observed correlation with aromaticity parameters may indicate that highly aromatic FA-L fractions provide a greater number of weakly bound interaction sites, leading to an increased release of volatile organic compounds during the first thermal stage. This suggests that, although WL1 does not directly quantify adsorption capacity, it may reflect the degree to which small molecules interact with and are retained by FA-L structures before being thermally desorbed.
- (b)
- Oxygen Content and Affinity Distributions: O/C correlates positively with K1, indicating that samples with a higher oxygen content tend to have carboxyl groups with stronger protonation constants (higher K1 values). This suggests that oxygen-rich FA-L fractions contain a greater proportion of carboxyl functional groups that exhibit a stronger affinity for protons (H+), likely due to increased acidity or structural differences in the organic matrix. The enhanced presence of oxygen-containing functional groups may contribute to greater reactivity in acid-base interactions, influencing the overall buffering capacity and metal-binding properties of FA-L.
- (c)
- Cd2+ Binding and Mass Loss: cML,H correlates with WL2/TWL, suggesting that the organic structures responsible for Cd2+ binding are also involved in the thermal degradation of biodegradable aromatic moieties. Specifically, this implies that carboxyl- and phenol-rich functional groups, which strongly complex Cd2+ at high concentrations (cML,H), may be associated with organic matter components that decompose in the 177–400 °C range (WL2). These structures likely include aromatic moieties linked to carbohydrates or aliphatic chains, which are more thermally labile than fully condensed aromatic domains. The correlation indicates that Cd2+ binding sites may be partially lost during thermal degradation, reflecting the reactivity of these functional groups in organic matter transformation processes.
- (d)
- Oxygen Content and Aromatic Stability: O/C negatively correlates with WL2/TWL and M2, suggesting that higher oxygen content is associated with a decrease in biodegradable aromatic structures and phenolic groups. This may indicate oxidation-driven conversion of aromatic moieties into more recalcitrant structures, possibly through phenolic-to-carboxyl transformations or mineralization to carbonates or CO2.
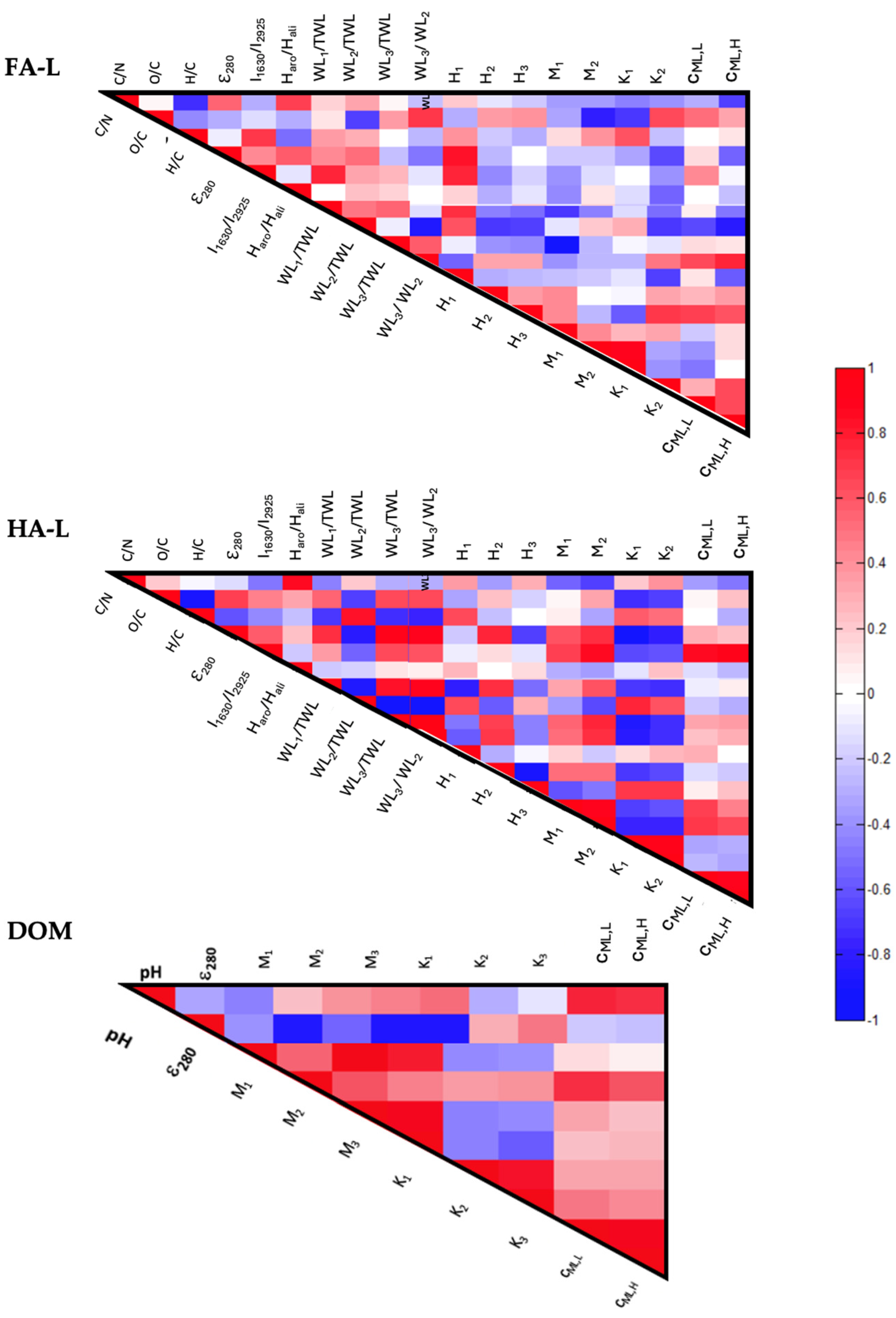
- HA-L Correlations
- (a)
- Aromaticity and Nitrogen Content: a positive correlation between C/N and Haro/Hali suggests that higher aromaticity (as assessed by 1H-NMR) is associated with lower nitrogen content in HA-L. This implies that as humic structures become more condensed and aromatic, nitrogen incorporation decreases, likely because nitrogen-rich compounds (e.g., proteins, peptides) degrade more readily during humification and are preferentially retained in less aromatic fractions.
- (b)
- Condensation and Degradability: The positive correlation between H/C with WL2/TWL indicates that higher H/C ratios (signifying less condensed structures) are associated with a greater abundance of biodegradable aromatic moieties. This suggests that less condensed HA-L fractions retain more aliphatic and labile aromatic components, which decompose in the 177–400 °C range (WL2), whereas highly condensed structures are more resistant to thermal degradation.
- (c)
- Aromaticity, Thermal Stability, and Interaction with Small Molecules: The positive correlation between ε280 and WL1/TWL, WL3/TWL, and H2 suggests that highly aromatic HA-L fractions are more thermally stable and exhibit enhanced interactions with small organic molecules. The correlation with WL1 (30–177 °C) indicates that aromatic HA-L structures may retain weakly bound volatile compounds, which are released at lower temperatures. Similarly, the correlation with WL3 (400–620 °C) implies that more condensed aromatic structures contribute to the retention of thermally stable components, which decompose only at higher temperatures. The association with H2 (177–400 °C) further suggests that aromatic HA-L fractions participate in exothermic reactions within this temperature range, possibly due to the controlled degradation of structurally stable organic moieties.
- (d)
- Aromaticity, Phenolic Groups, and Cd2+ Binding: I1630/2925 and ε280 correlate positively with M2, cML,L, and cML,H, indicating that higher aromaticity is linked to a greater abundance of phenolic functional groups and stronger metal-binding capacity. Since phenolic groups are known to contribute significantly to metal complexation, this correlation suggests that aromaticity enhances the density of reactive sites for metal interaction, increasing the binding affinity for Cd2+.
- (e)
- Cd2+ Binding and Carboxyl Group Reactivity: The positive correlation between K1 and cML,L demonstrates that Cd2+ affinity is closely related to carboxyl group reactivity. This suggests that carboxyl sites with stronger acid dissociation constants (higher K1 values) contribute more significantly to Cd2+ complexation, particularly at low metal concentrations.
- (f)
- Aromatic Condensation and Molecular Interaction Capacity: The negative correlation between H/C and WL3/TWL suggests that as HA-L becomes more condensed and aromatic (lower H/C), it contains a higher proportion of recalcitrant structures that degrade at higher temperatures (WL3). Additionally, the negative correlation between H/C and WL1/TWL indicates that these condensed domains may also retain small organic molecules, contributing to mass loss at lower temperatures (WL1). This suggests that strong π–π interactions and hydrophobic forces within highly aromatic HA-L fractions promote both structural stability and the retention of thermolabile compounds.
- (g)
- Aromaticity and Oxidation: The negative correlation between O/C and WL2/TWL suggests that higher oxidation levels reduce the abundance of easily biodegradable aromatic moieties. This implies that as HA-L undergoes oxidation, it becomes more chemically stable and less prone to thermal degradation in the 177–400 °C range (WL2), meaning that fewer labile aromatic structures remain in the extract.
- (h)
- Aromaticity and Recalcitrance: The negative correlation between ε280 and WL2/TWL indicates that more aromatic HA-L fractions contain fewer recalcitrant structures. This suggests that samples with higher aromaticity have undergone greater structural transformation, leading to a reduction in less-condensed, thermally labile components that degrade in the 177–400 °C range.
- (i)
- Nitrogen Content and Acidic Functional Group Reactivity: The negative correlation between C/N and K1 and K2 suggests that higher nitrogen content enhances the reactivity of both carboxylic (K1) and phenolic (K2) groups. This implies that as nitrogen-rich structures are incorporated into HA-L, they contribute to more reactive acidic functional groups, increasing their ability to participate in protonation and metal-binding interactions.
- DOM Correlations
- (a)
- Aromaticity and Carboxyl Reactivity: The positive correlation between ε280 and K1 suggests that more aromatic DOM fractions contain carboxyl groups with stronger acidity (lower protonation constants), making them more reactive. This implies that aromaticity is linked to the presence of highly dissociable carboxyl functional groups, enhancing proton exchange capacity in DOM.
- (b)
- pH and Cd2+ Binding: The positive correlation between pH and cML,L, cML,H, indicates that higher pH enhances the ability of DOM to bind Cd2+, likely due to the increased deprotonation of acidic functional groups. As pH rises, more carboxyl and phenolic sites lose protons, increasing their negative charge and strengthening metal complexation interactions.
- (c)
- Carboxylic Group Distribution and Cd2+ Binding: The positive correlation between M2 (carboxyl groups in organic acids) and cML,L suggests that organic acid-derived carboxyl groups are key contributors to Cd2+ binding in DOM, particularly at low metal concentrations. This implies that these functional groups provide effective coordination sites for Cd2+ complexation, enhancing the metal-binding reactivity of DOM.
- (d)
- Aromaticity and Functional Groups: The negative correlation between ε280 correlates and M1, M3 suggests that highly aromatic DOM fractions contain fewer amino acid-associated carboxyl groups (M1) and phenolic structures (M3). This implies that as DOM becomes more structurally condensed and aromatic, it may undergo selective depletion of phenolic functionalities, potentially altering its overall reactivity.
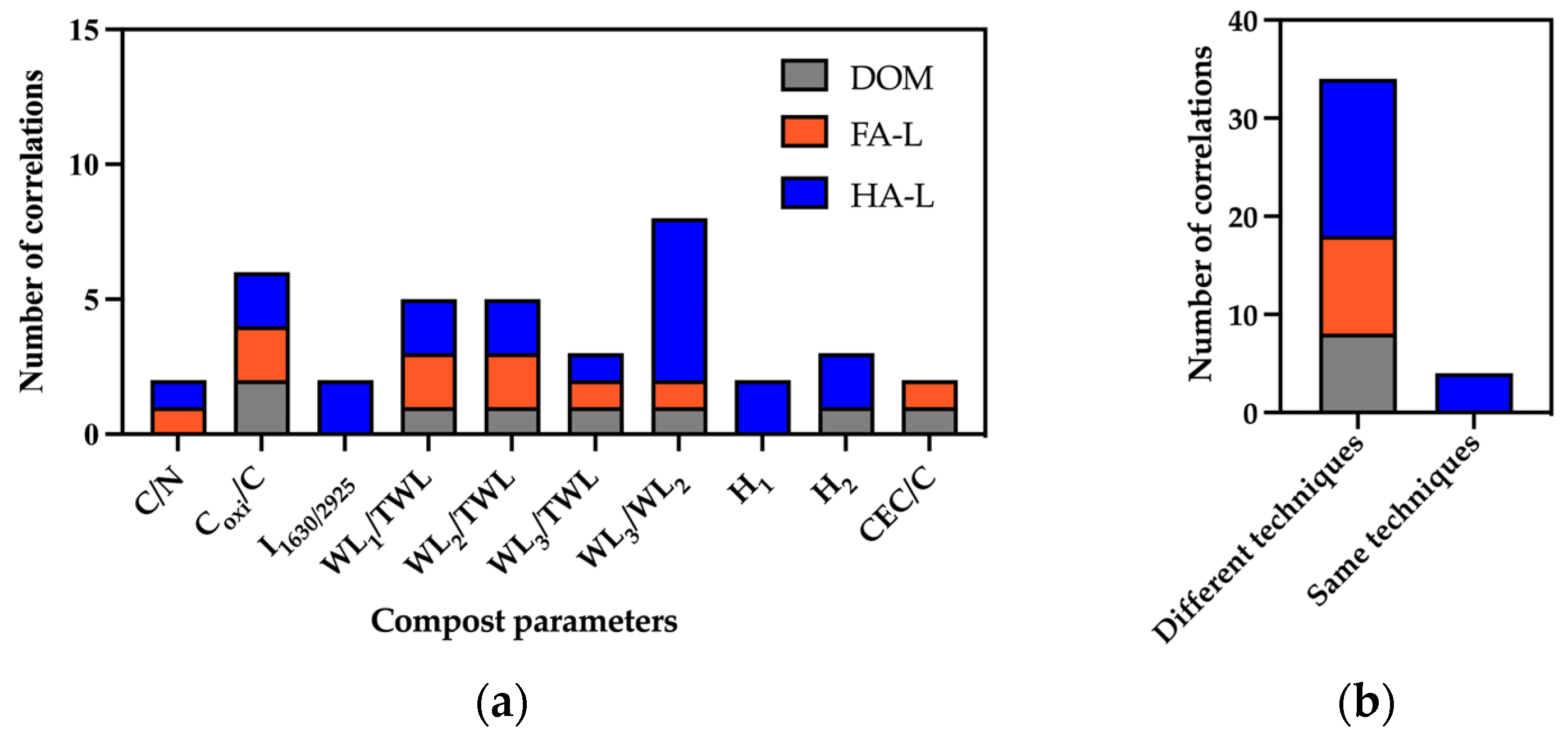
2.2.2. Correlation Between the Composition of the Extracts and the Original Compost
- DOM vs. Compost
- -
- Sulfur (S) in compost and SO42− and NH4+ in equilibrium solutions: This correlation suggests a strong association between compost sulfur content and sulfate and ammonium concentrations in solution. While the correlation between S and SO42− is expected, the link between S and NH4+ is less straightforward and requires further investigation.
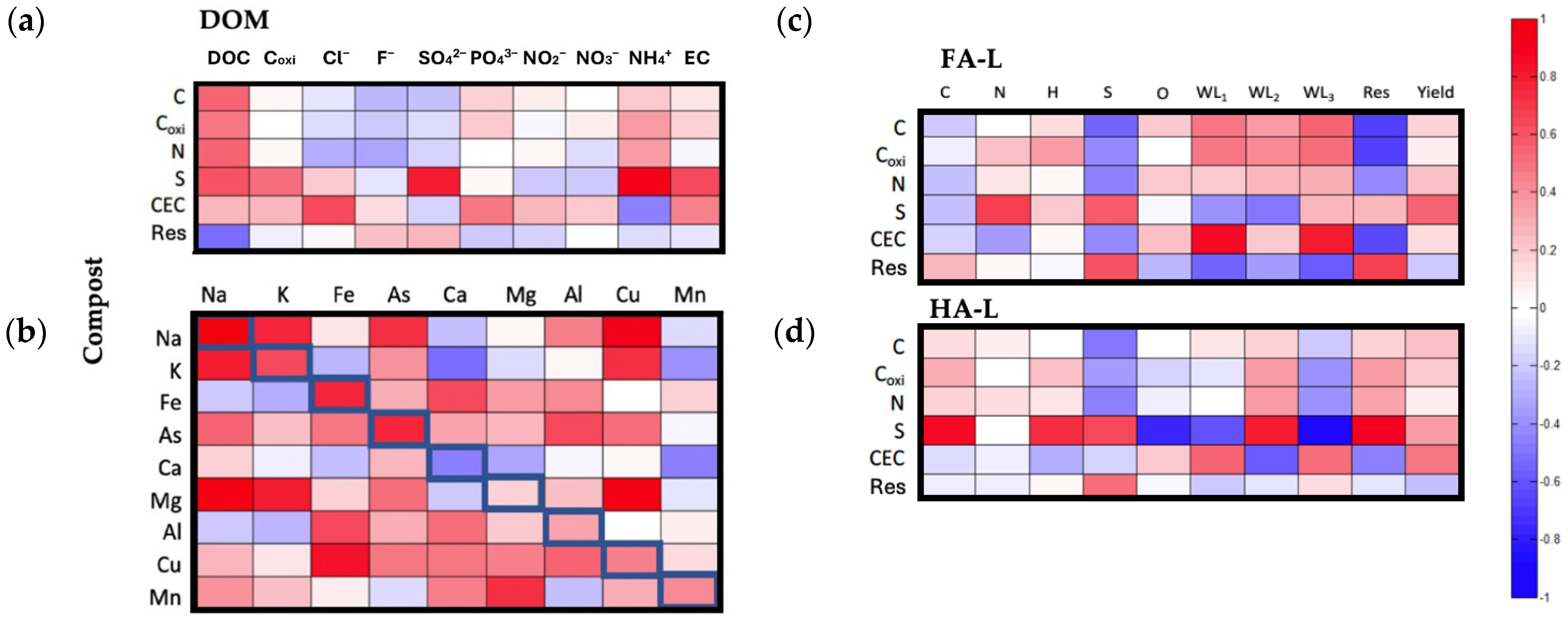
- HS-L vs. Compost
- (a)
- Cation Exchange Capacity and Thermal Stability of FA-L: The positive correlation between CEC and WL3 suggests that composts with higher cation exchange capacity (CEC) contain FA-L fractions enriched in extra-recalcitrant moieties, which may contribute to long-term soil fertility and metal-binding properties. Additionally, the correlation between CEC and WL1 indicates that composts with higher CEC tend to produce FA-L fractions with a greater capacity to retain and interact with low molecular weight substances, possibly due to the presence of reactive acidic functional groups.
- (a)
- Sulfur (S) in compost and HA-L Composition and Thermal Stability: The positive correlation between compost S and HA-L carbon (C), hydrogen (H), WL2, and residue (Res) in TGA suggests that sulfur-rich composts yield HA-L fractions with greater organic carbon and hydrogen content, a higher proportion of recalcitrant structures, and increased thermal stability. This could indicate that sulfur incorporation during composting enhances the structural integrity and stability of humic-like substances.
- (b)
- Sulfur (S) in compost and Oxygen Content and Recalcitrance in HA-L: The negative correlation between S in compost and O in HA-L suggests that higher sulfur content is associated with a reduced presence of oxygen-containing functional groups in HA-L. Simultaneously, the negative correlation between S and WL3 indicates that sulfur-rich composts yield HA-L fractions with fewer extra-recalcitrant structures. This suggests a structural modification of humic-like substances, where sulfur incorporation may alter oxidation pathways, potentially influencing the composition and long-term stability of HA-L.
2.2.3. Correlation Between Structural and Reactivity Features of Extracts and Compost Molecular Structure
- DOM vs. Compost
- (a)
- Carboxyl and Phenolic Group Reactivity in DOM and Readily Oxidizable Carbon: The positive correlation between K2 and K3 vs. Coxi/C suggests that highly reactive carboxyl and phenolic groups in DOM are associated with composts that contain a higher proportion of readily oxidizable carbon relative to total carbon. This indicates that oxidizable carbon plays a key role in enhancing the functional reactivity of DOM.
- (b)
- Amino Acid-Associated Carboxyl Groups and Phenolic Reactivity in DOM and Compost Thermal Stability: The positive correlation between M1 and (WL1/TWL) suggests that DOM fractions with a higher abundance of amino acid–associated carboxyl groups (M1) originate from composts with an increased ability to adsorb low molecular weight substances. Similarly, the correlation between K3 and WL2/TWL indicates that highly reactive phenolic groups (K3) are more prevalent in DOM extracted from composts with a greater proportion of recalcitrant structures.
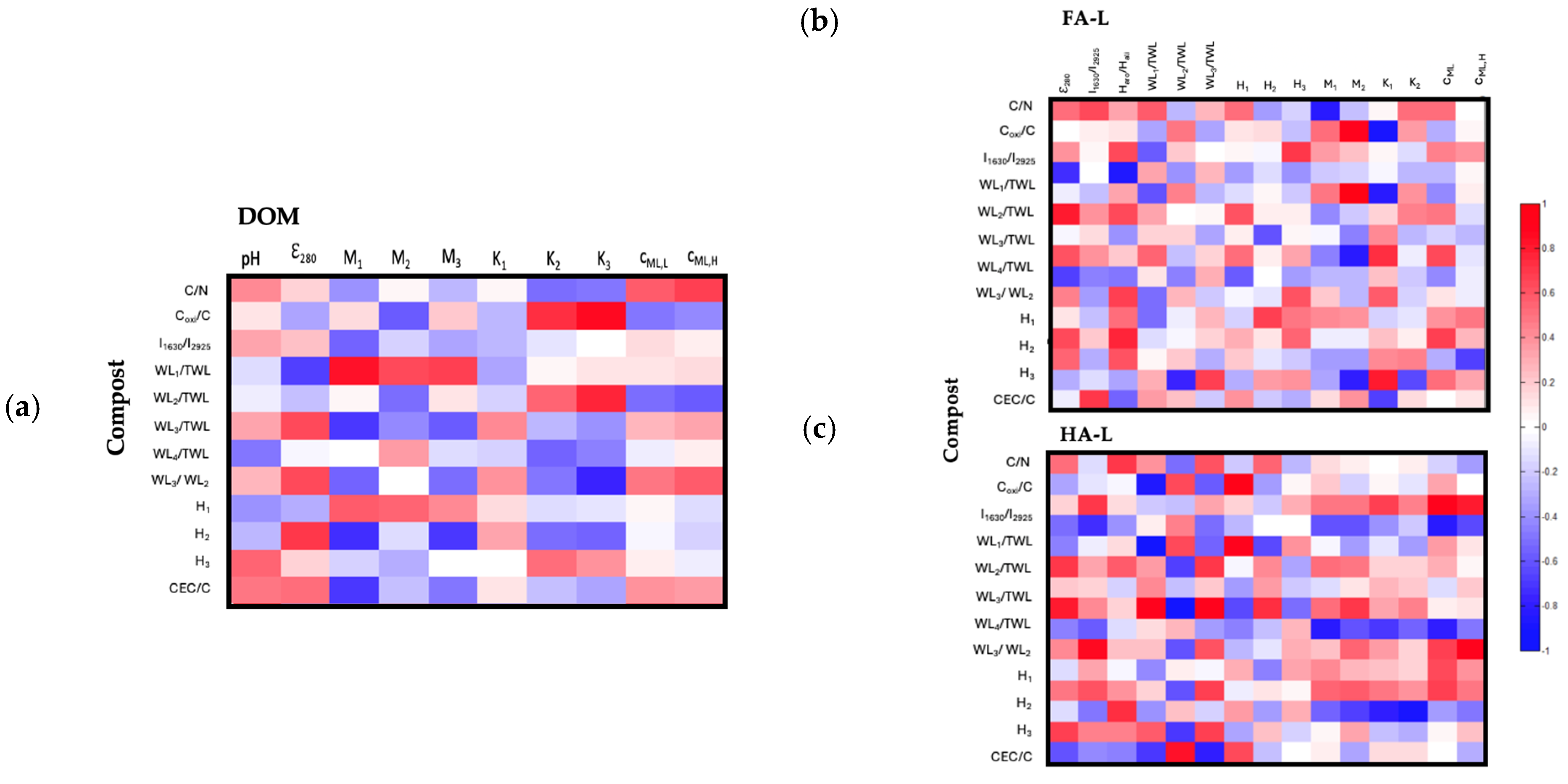
- (c)
- Amino Acid-Associated Carboxyl Groups and Compost Maturity Indicators: The negative correlations between M1 and WL3/TWL, H2, and CEC/C suggest that DOM fractions with a higher abundance of amino acid–associated carboxyl groups (M1) originate from composts with lower maturation indices (i.e., lower CEC/C, WL3/TWL and H2). This indicates that as compost matures, the contribution of amino acid–associated functional groups in DOM decreases.
- (d)
- Phenolic Group Reactivity and Compost Maturation Degree: The negative correlation between K3 and WL3/WL2 suggests that DOM fractions enriched in highly reactive phenolic groups (K3) are more prevalent in composts with lower maturation degrees. This implies that as compost matures, phenolic structures in DOM undergo transformations, reducing their reactivity.
- HS-L vs. Compost
- (a)
- Phenolic Group Abundance and Oxidizable Carbon and Thermal Stability of Compost: The positive correlation between M2 and Coxi/C, WL2/TWL suggests that FA-L fractions rich in phenolic groups originate from composts with higher readily oxidizable carbon content, which decomposes between 177 and 400 °C.
- (b)
- Aromaticity and Recalcitrant Structures of Compost: The positive correlation between ε280 and WL3/TWL indicates that more aromatic FA-L fractions (evaluated by ε280) are derived from composts with a greater abundance of extra-recalcitrant structures.
- (c)
- Aromaticity and Cation Exchange Capacity of Compost: The positive correlation between Haro/Hali and CEC/C demonstrates that the aromaticity of FA-L (assessed by 1H-NMR) is linked to composts with a greater ability to exchange cations per unit of carbon.
- (d)
- Carboxyl Group Content and Compost C/N Ratio of Compost: The negative correlation between M1 and C/N suggests that FA-L fractions with a higher content of carboxyl groups (M1) originate from composts with a greater nitrogen content relative to carbon.
- (e)
- Carboxyl Group Reactivity and Oxidizable Carbon of Compost: The negative correlation between K1 and Coxi/C, WL2/TWL suggests that more reactive carboxyl groups in FA-L are found in composts with lower readily oxidizable carbon content.
- (f)
- Aromaticity and Compost Adsorption of Low Molecular Weight substances: The negative correlations between ε280 and WL1/TWL, as well as Haro/Hali and WL1/TWL, indicate that FA-L fractions with higher aromaticity contain fewer adsorbed low molecular weight substances.
- (g)
- Phenolic Group Abundance and Compost Maturity: The negative correlation between M2 and WL3/WL2 suggests that phenolic-rich FA-L fractions are more prevalent in composts with lower maturation degrees.
- (a)
- Aromaticity and Nitrogen Content in Compost: The positive correlation between Haro/Hali and C/N indicates that aromaticity in HA-L (evaluated by 1H-NMR) is higher in composts with lower nitrogen content relative to carbon.
- (b)
- Low Molecular Weight Adsorption in HA-L and Readily Oxidizable Carbon in Compost: The positive correlations between H1 and Coxi/C, WL2/TWL suggest that HA-L fractions with a higher adsorption capacity for low molecular weight substances originate from composts with a greater content of readily oxidizable carbon.
- (c)
- Aromaticity and Compost Maturity: The positive correlations between ε280 and WL3/TWL, WL3/WL2, and I1630/2925 and H2 indicate that aromaticity in HA-L (evaluated by UV-vis) is greater in composts with a higher proportion of extra-recalcitrant structures and a greater degree of maturation.
- (d)
- Thermal Stability of Biodegradable Aromatic Structures and Phenolic Group Abundance, and Compost Maturation: The positive correlations between H2 and WL3/WL2, and M2 and WL3/WL2 suggest that HA-L fractions with higher thermal stability of biodegradable aromatic structures (H2) and a greater abundance of phenolic groups (M2) are associated with composts that have undergone a higher degree of maturation.
- (e)
- Cd2+ Binding and Aromaticity: The positive correlations between cML,H and H2, cML,L and I1630/2925, and cML,H and I1630/2925 indicate that HA-L fractions with a stronger capacity to bind Cd2+ are linked to composts with a higher proportion of aromatic structures (I1630/2925) and a greater thermal stability of recalcitrant materials (H2).
- (f)
- Low-Temperature Mass Loss and Readily Oxidizable Carbon in Compost: The negative correlation between WL1/TWL and Coxi/C suggests that HA-L fractions from composts with lower readily oxidizable carbon (Coxi/C,) exhibit reduced low-temperature weight loss (WL1/TWL). This indicates that as composts mature and lose their labile, easily oxidized carbon, the resulting HA-L fractions contain fewer volatile, weakly bound organic compounds that desorb at lower temperatures.
- (g)
- Aromaticity and Low Molecular Weight Adsorption: The negative correlation between I1630/2925 and WL1/TWL reinforces that aromaticity in HA-L is higher in composts with a lower adsorption capacity for low molecular weight substances.
- (h)
- Carboxyl Group Content, Cd2+ Binding, and Adsorption Capacity: The negative correlations between M1 and H1, cML,L and H1, and cML,L and WL1/TWL indicate that HA-L fractions with a greater abundance of carboxyl groups and stronger Cd2+ binding sites originate from composts with a lower capacity to adsorb low molecular weight substances.
- (a)
- Compost oxidation state (Coxi/C) correlates with HS-L aromaticity and stability, indicating that compost maturity predicts humic quality.
- (b)
- Thermal stability parameters (WL3/WL2) reflect the recalcitrance of organic matter, which influences the long-term persistence of HA-L and FA-L in soils.
- (c)
- Cation exchange capacity (CEC) is linked to FA-L composition, meaning that composts with higher CEC yield FA-L fractions with stronger adsorption potential for nutrients and organics.
- (d)
- Sulfur content in compost correlates with HA-L elemental composition, implying that basic compost elemental analysis could reveal humic structural properties.
- (e)
- Aromaticity indices (ε280, I1630/2925, Haro/Hali) strongly correlate with HS-L reactivity and metal-binding potential, reinforcing their importance for soil fertility applications.
3. Materials and Methods
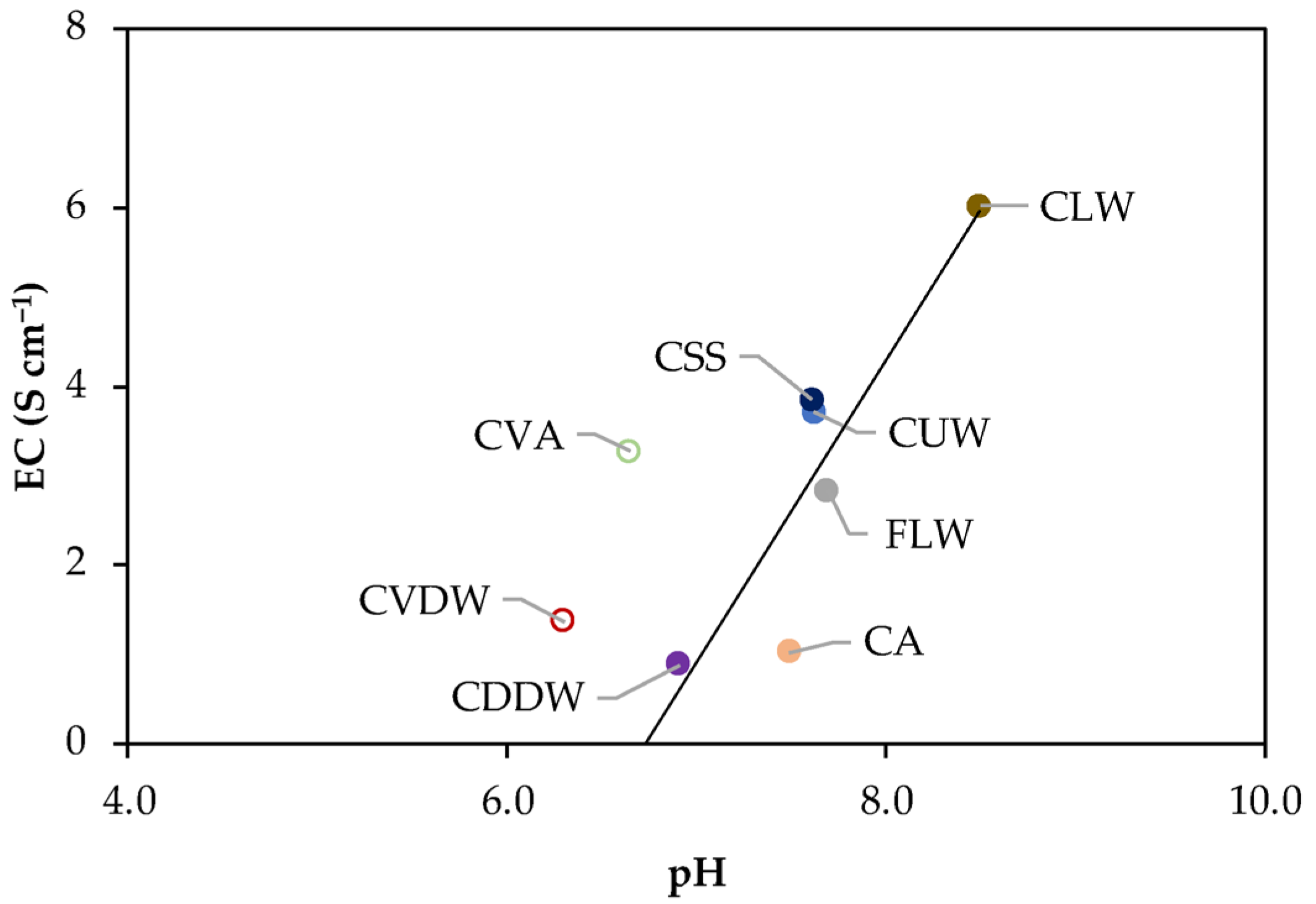
3.1. Compost Samples and Extraction Procedures
Extraction of Organic Matter (OM) Fractions
- DOM was extracted following the protocol in López et al. (2021) [29]. Briefly, 2.50 g of compost was mixed with 50 mL of ultrapure water at natural pH in an open system and equilibrated for 5 days. The supernatant was collected by centrifugation (6000 rpm, 20 min) and stored for analysis.
- HS-L (FA-L and HA-L) were extracted using the International Humic Substances Society (IHSS) protocol, as detailed in Silva et al. (2022) [30]. Compost samples were mixed with 0.1 M NaOH under a N2 atmosphere at a 10:1 (v/w) ratio. The extract was acidified to pH 1 with 6 M HCl, precipitating HA-L while leaving FA-L in solution. The HA-L purification was achieved through the washing of the precipitate with HCl/HF solution to remove mineral impurities and was then dialyzed. The supernatant was purified using XAD-8 resin, followed by cation exchange resin treatment to obtain the FA-L fraction [31].
3.2. DOM and HS-L Characterization
- ATR-FTIR spectra of FA-L and HA-L (Figure S2) were acquired using a Jasco FT/IR-4100 Spectrometer to identify aromatic, carboxyl, and phenolic groups, following the methodology described in Silva et al. (2022) [5]. These spectra were used to determine the relative intensity ratios of key functional groups (e.g., I1630/2925, I1630/2845) assessing the relative amounts of carboxyl, phenolic, and aromatic groups.
- 1H-NMR Spectroscopy was performed using a 400 MHz Bruker Avance II NMR spectrometer for the structural characterization of FA-L and HA-L. Chemical shifts (δ) are given in parts per million (ppm), downfield from tetramethylsilane (TMS), and coupling constants (J) in hertz (Hz). The HS-L solutions were obtained by dissolving 5 mg of the sample in 800 µL of deuterated water (Merck) and the pH was adjusted to 12 with 40% NaOD (Sigma-Aldrich), following Silva et al. (2022) [5]. This analysis was used to define aromatic-to-aliphatic proton ratio (Haro/Hali), assessing structural composition of HS-L fractions.
3.3. Compost Characterization
3.4. Statistical and Correlation Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Donn, S.; Wheatley, R.E.; McKenzie, B.M.; Loades, K.W.; Hallett, P.D. Improved soil fertility from compost amendment increases root growth and reinforcement of surface soil on slopes. Ecol. Eng. 2014, 71, 458–465. [Google Scholar] [CrossRef]
- Guo, X.; Liu, H.; Wu, S. Humic substances developed during organic waste composting: Formation mechanisms, structural properties, and agronomic functions. Sci. Total Environ. 2019, 662, 501–510. [Google Scholar] [CrossRef]
- Bertoldi, M.; Sequi, P.; Lemmes, B.; Papi, T. The Science of Composting; Springer: Dordrecht, The Netherlands, 1996; ISBN 978-94-009-1569-5. [Google Scholar]
- Spaccini, R.; Piccolo, A. Molecular characteristics of humic acids extracted from compost at increasing maturity stages. Soil Biol. Biochem. 2009, 41, 1164–1172. [Google Scholar] [CrossRef]
- Silva, A.C.; Rocha, P.; Antelo, J.; Valderrama, P.; López, R.; Geraldo, D.; Proença, M.F.; Pinheiro, J.P.; Fiol, S.; Bento, F. Comparison of a variety of physico-chemical techniques in the chronological characterization of a compost from municipal wastes. Process Saf. Environ. Prot. 2022, 164, 781–793. [Google Scholar] [CrossRef]
- El Ouaqoudi, F.Z.; El Fels, L.; Winterton, P.; Lemée, L.; Amblès, A.; Hafidi, M. Study of humic acids during composting of ligno-cellulose waste by infra-red spectroscopic and thermogravimetric/thermal differential analysis. Compost Sci. Util. 2014, 22, 188–198. [Google Scholar] [CrossRef]
- Chang Chien, S.W.; Wang, M.C.; Huang, C.C. Reactions of compost-derived humic substances with lead, copper, cadmium, and zinc. Chemosphere 2006, 64, 1353–1361. [Google Scholar] [CrossRef]
- Liu, H.; Wang, L.; Zhong, R.; Bao, M.; Guo, H.; Xie, Z. Binding characteristics of humic substances with Cu and Zn in response to inorganic mineral additives during swine manure composting. J. Environ. Manag. 2022, 305, 114387. [Google Scholar] [CrossRef]
- Silva, A.C.; Rocha, P.; Geraldo, D.; Cunha, A.; Antelo, J.; Pinheiro, J.P.; Fiol, S.; Bento, F. Developing a compost quality index (CQI) based on the electrochemical quantification of Cd (HA) reactivity. Molecules 2023, 28, 1503. [Google Scholar] [CrossRef]
- Muniz, D.H.F.; Oliveira-Filho, E.C. Multivariate statistical analysis for water quality assessment: A review of research published between 2001 and 2020. Hydrology 2023, 10, 196. [Google Scholar] [CrossRef]
- Balabanova, B.; Stafilov, T.; Šajn, R. use of multivariate statistical techniques to determine the source apportionment of heavy metals in soils and sediments. In Heavy Metals in the Environment; Elsevier: Amsterdam, The Netherlands, 2021; pp. 119–141. ISBN 978-0-12-821656-9. [Google Scholar]
- Shrestha, S.; Kazama, F. Assessment of surface water quality using multivariate statistical techniques: A case study of the Fuji river basin, Japan. Environ. Model. Softw. 2007, 22, 464–475. [Google Scholar] [CrossRef]
- Gad, M.; Khomami, N.T.S.; Krieg, R.; Schor, J.; Philippe, A.; Lechtenfeld, O.J. Environmental drivers of dissolved organic matter composition across central european aquatic systems: A novel correlation-based machine learning and FT-ICR MS approach. Water Res. 2025, 273, 123018. [Google Scholar] [CrossRef] [PubMed]
- Tassano, M.; Montañez, A.; Nuñez, L.; Trasante, T.; González, J.; Irigoyen, J.; Cabral, P.; Cabrera, M. Spatial cross-correlation between physicochemical and microbiological variables at superficial soil with different levels of degradation. CATENA 2021, 198, 105000. [Google Scholar] [CrossRef]
- Zhang, J.; Feng, Y.; Wu, M.; Chen, R.; Li, Z.; Lin, X.; Zhu, Y.; Delgado-Baquerizo, M. Evaluation of microbe-driven soil organic matter quantity and quality by thermodynamic theory. mBio 2021, 12, e03252-20. [Google Scholar] [CrossRef]
- Lasaridi, K.; Protopapa, I.; Kotsou, M.; Pilidis, G.; Manios, T.; Kyriacou, A. Quality assessment of composts in the Greek market: The need for standards and quality assurance. J. Environ. Manag. 2006, 80, 58–65. [Google Scholar] [CrossRef]
- Kucbel, M.; Raclavská, H.; Růžičková, J.; Švédová, B.; Sassmanová, V.; Drozdová, J.; Raclavský, K.; Juchelková, D. Properties of composts from household food waste produced in automatic composters. J. Environ. Manag. 2019, 236, 657–666. [Google Scholar] [CrossRef]
- Chin, Y.-P.; Aiken, G.; O’Loughlin, E. Molecular weight, polydispersity, and spectroscopic properties of aquatic humic substances. Environ. Sci. Technol. 1994, 28, 1853–1858. [Google Scholar] [CrossRef]
- Jamroz, E.; Bekier, J.; Medynska-Juraszek, A.; Kaluza-Haladyn, A.; Cwielag-Piasecka, I.; Bednik, M. The contribution of water extractable forms of plant nutrients to evaluate MSW compost maturity: A case study. Sci. Rep. 2020, 10, 12842. [Google Scholar] [CrossRef]
- Giovanela, M.; Crespo, J.S.; Antunes, M.; Adamatti, D.S.; Fernandes, A.N.; Barison, A.; Da Silva, C.W.P.; Guégan, R.; Motelica-Heino, M.; Sierra, M.M.D. Chemical and spectroscopic characterization of humic acids extracted from the bottom sediments of a Brazilian subtropical microbasin. J. Mol. Struct. 2010, 981, 111–119. [Google Scholar] [CrossRef]
- See, J.H.; Bronk, D.A. Changes in C:N ratios and chemical structures of estuarine humic substances during aging. Mar. Chem. 2005, 97, 334–346. [Google Scholar] [CrossRef]
- Fuentes, M.; Baigorri, R.; González-Gaitano, G.; García-Mina, J.M. The complementary use of 1H NMR, 13C NMR, FTIR and size exclusion chromatography to investigate the principal structural changes associated with composting of organic materials with diverse origin. Org. Geochem. 2007, 38, 2012–2023. [Google Scholar] [CrossRef]
- Pertusatti, J.; Prado, A.G.S. Buffer capacity of humic acid: Thermodynamic approach. J. Colloid Interface Sci. 2007, 314, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Amir, S.; Jouraiphy, A.; Meddich, A.; El Gharous, M.; Winterton, P.; Hafidi, M. Structural study of humic acids during composting of activated sludge-green waste: Elemental analysis, FTIR and 13C NMR. J. Hazard. Mater. 2010, 177, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Ait Baddi, G.; Hafidi, M.; Cegarra, J.; Alburquerque, J.A.; Gonzálvez, J.; Gilard, V.; Revel, J.-C. Characterization of fulvic acids by elemental and spectroscopic (FTIR and 13C-NMR) analyses during composting of olive mill wastes plus straw. Bioresour. Technol. 2004, 93, 285–290. [Google Scholar] [CrossRef]
- Silva, M.E.F.; Lemos, L.T.; Bastos, M.M.S.M.; Nunes, O.C.; Cunha-Queda, A.C. Recovery of humic-like susbtances from low quality composts. Bioresour. Technol. 2013, 128, 624–632. [Google Scholar] [CrossRef]
- Hanc, A.; Enev, V.; Hrebeckova, T.; Klucakova, M.; Pekar, M. Characterization of humic acids in a continuous-feeding vermicomposting system with horse manure. Waste Manag. 2019, 99, 1–11. [Google Scholar] [CrossRef] [PubMed]
- González Pérez, M.; Martin-Neto, L.; Saab, S.C.; Novotny, E.H.; Milori, D.M.B.P.; Bagnato, V.S.; Colnago, L.A.; Melo, W.J.; Knicker, H. Characterization of humic acids from a brazilian oxisol under different tillage systems by EPR, 13C NMR, FTIR and fluorescence spectroscopy. Geoderma 2004, 118, 181–190. [Google Scholar] [CrossRef]
- López, R.; Antelo, J.; Silva, A.C.; Bento, F.; Fiol, S. Factors That affect physicochemical and acid-base properties of compost and vermicompost and its potential use as a soil amendment. J. Environ. Manag. 2021, 300, 113702. [Google Scholar] [CrossRef]
- Silva, A.C.; Teixeira, A.; Antelo, J.; Valderrama, P.; Oliveira, R.; Cunha, A.; Gley, R.; Pinheiro, J.P.; Fiol, S.; Bento, F. Distinctive features of composts of different origin: A thorough examination of the characterization results. Sustainability 2022, 14, 7449. [Google Scholar] [CrossRef]
- Zhao, S.; Guo, Y.; Sheng, Q.; Shyr, Y. Advanced heat map and clustering analysis using heatmap3. BioMed Res. Int. 2014, 2014, 986048. [Google Scholar] [CrossRef]
- Leenheer, J.A. Comprehensive approach to preparative isolation and fractionation of dissolved organic carbon from natural waters and wastewaters. Environ. Sci. Technol. 1981, 15, 578–587. [Google Scholar] [CrossRef]
- Smidt, E.; Tintner, J. Application of differential scanning calorimetry (DSC) to evaluate the quality of compost organic matter. Thermochim. Acta 2007, 459, 87–93. [Google Scholar] [CrossRef]
- Díaz, M.J.; Ruiz-Montoya, M.; Palma, A.; de-Paz, M.-V. Thermogravimetry Applicability in compost and composting research: A review. Appl. Sci. 2021, 11, 1692. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, A.C.; Rocha, P.; Valderrama, P.; Antelo, J.; Geraldo, D.; Proença, M.F.; Fiol, S.; Bento, F. A Correlation-Based Approach for Predicting Humic Substance Bioactivity from Direct Compost Characterization. Molecules 2025, 30, 1511. https://doi.org/10.3390/molecules30071511
Silva AC, Rocha P, Valderrama P, Antelo J, Geraldo D, Proença MF, Fiol S, Bento F. A Correlation-Based Approach for Predicting Humic Substance Bioactivity from Direct Compost Characterization. Molecules. 2025; 30(7):1511. https://doi.org/10.3390/molecules30071511
Chicago/Turabian StyleSilva, Ana Catarina, Pedro Rocha, Patrícia Valderrama, Juan Antelo, Dulce Geraldo, Maria Fernanda Proença, Sarah Fiol, and Fátima Bento. 2025. "A Correlation-Based Approach for Predicting Humic Substance Bioactivity from Direct Compost Characterization" Molecules 30, no. 7: 1511. https://doi.org/10.3390/molecules30071511
APA StyleSilva, A. C., Rocha, P., Valderrama, P., Antelo, J., Geraldo, D., Proença, M. F., Fiol, S., & Bento, F. (2025). A Correlation-Based Approach for Predicting Humic Substance Bioactivity from Direct Compost Characterization. Molecules, 30(7), 1511. https://doi.org/10.3390/molecules30071511








