Abstract
Sulfur hexafluoride (SF6), the strongest greenhouse gas, has great challenges in degradation because of its stable structure, posing significant environmental concerns. Photocatalysis offers an environmentally friendly, low-energy solution, but the fluoride deposition on catalysts reduces their activity, thus limiting their large-scale application. To prevent catalyst fluoride poisoning, we report a thin-layer graphitic carbon nitride (CN) material loaded with MoOx (CNM) that resists fluoride deposition for long-term SF6 degradation. By combining molecular structure design and nanostructure regulation, we construct a photocatalyst with enhanced charge carrier mobility and reduced transport distances. We find that the CNM exhibits a high specific surface area, increased contact between reactants and active sites, and efficient electron–hole separation due to the Mo-N bonds, achieving an SF6 degradation efficiency of 1.73 mmol/g after one day due to the prolonged catalytic durability of the catalyst, which is eight times higher than pristine g-C3N4 (0.21 mmol/g). We demonstrate the potential of CNMs for low-energy, high-efficiency SF6 degradation, offering a new approach to mitigate the environmental impact of this potent greenhouse gas. We envision that this study will inspire further research into advanced photocatalytic materials for environmental remediation, contributing to global efforts in combating climate change.
1. Introduction
Sulfur hexafluoride (SF6) plays a crucial role in various fields such as the electrical and aerospace industries [1,2,3,4,5,6]. However, it is also an extremely potent greenhouse gas [7], with a global warming potential (GWP) 24,300 times that of an equivalent amount of CO2 [8]. Therefore, the degradation of SF6 has become an urgent task. The stable structure of SF6 makes it difficult to degrade [9,10,11]. Photocatalysis, which utilizes solar energy to degrade pollutants, is an environmentally friendly and low-energy-consumption method for degrading stable SF6 [12,13]. Although there have been reports of improvements in the reaction system, the large-scale development of photocatalytic technology has been limited by the lack of efficient photocatalysts [14,15,16,17]. Hence, the development of photocatalysts is the focal point of photocatalytic technology advancements.
For photocatalytic degradation of sulfur hexafluoride (SF6), although our previous work has shown that the gas–liquid–solid three-phase system can improve the performance of the catalyst, the photocatalyst still faces the issue of deactivation in 6 h due to fluorine poisoning [12]. Thus, the reaction between the photocatalyst and inorganic F ions should be inhibited to develop photocatalyst performance. Organic polymers are less likely to be covered by inorganic fluoride in the solution and thus can keep their activity, due to their different hydrophobic/hydrophilic properties. Recently, two-dimensional conjugated polymer semiconductors, particularly covalently bonded carbon nitride materials (g-C3N4), have emerged as highly promising and representative materials [18,19,20,21,22,23]. Owing to their high chemical and thermal stability, visible-light response, and simple preparation methods, they have garnered global attention in artificial photosynthesis. Compared with traditional bulk materials, thin-layer carbon nanosheets offer significant advantages in enhancing photocatalytic activity; for instance, they possess larger surface areas, more exposed active sites, and shorter charge carrier transport distances from the bulk to surface-active sites. Moreover, forming porous structures on two-dimensional carbon nanosheets can further significantly improve charge and mass transport during the photocatalytic process [24].
However, g-C3N4, due to the absence of metal, lacks efficient active sites for the adsorption and activation of sulfur hexafluoride. Despite this, it not only facilitates the adsorption of organic reactants but also boasts a large specific surface area. Additionally, molybdenum fluoride exhibits a higher susceptibility to hydrolysis into MoOx in solution due to thermodynamic factors [25]. So, when MoOx, which contains variable-valence metals, acts as active centers, it is less likely to be poisoned and deactivated by fluoride ions [17,26]. In addition, Mo-based inorganic nanomaterials have demonstrated excellent catalytic performance and favorable characteristics for electron and hole transport, showing great application potential in photocatalysis. Research has shown that MoO2 has extensive applications in photocatalysis, including selective oxidation of lactic acid, CO2 photocatalytic reduction, and photocatalytic ammonia synthesis [27,28,29,30]. Combining high specific surface area materials that favor organic reactants with Mo-based inorganic nanocatalytic materials with variable metal valences is expected to provide an efficient pathway for the efficient adsorption and degradation of SF6.
Here, we synthesized a MoOx-loaded thin-layer carbon nitride composite (CNM), which enhanced the photocatalyst’s specific surface area, facilitating reactant contact with MoO2 active sites and boosting SF6 degradation. The organophilic g-C3N4 substrate improved SF6 mass transfer in organic solvents and sacrificial agent binding. Adsorption tests, DFT calculations, and in situ IR confirmed MoO2’s role in SF6 adsorption/activation. XPS revealed Mo-N bonds between MoOx and C3N4, establishing an electron transport channel that significantly enhanced charge separation. This design achieved SF6 degradation efficiency >10× higher than previously reported and surpassed our prior work due to prolonged catalyst durability. The findings provide a novel strategy for low-energy, stable, and efficient SF6 degradation.
2. Results and Discussion
2.1. Synthesis and Structure Characterization of Layered CNM Samples
As depicted in Figure 1a, layered CNM samples were synthesized via a straightforward two-step high-temperature calcination process. Initially, melamine underwent high-temperature polymerization to yield bulk g-C3N4. Chemically active (NH4)2MoO4 was employed as the Mo source, serving as the metal site for the adsorption and activation of sulfur hexafluoride (SF6). Subsequently, through additional calcination of the mixture of g-C3N4 and (NH4)2MoO4 under varying gas atmospheres, g-C3N4 underwent re-polymerization and functionalization with MoOx groups, culminating in the target photocatalyst CNM. Nitrogen adsorption–desorption experiments indicated that the pore volumes of HCNM3, NCNM3, and ACNM3 reach up to 0.26 cm3/g, 0.23 cm3/g, and 0.10 cm3/g, respectively, surpassing that of g-C3N4 (0.06 cm3/g) (Figure 1b). The specific surface areas of HCNM3 and NCNM3 are 34.5 m2/g and 30.7 m2/g, respectively, which are greater than that of g-C3N4 (10.86 m2/g), attributable to the gas etching effect and the high-temperature corrosion effect of the molybdenum salt. Unfortunately, the specific surface area of ACNM3 calcined in air is relatively small (4.60 m2/g). Moreover, the morphology of HCNM3 was characterized by scanning electron microscope (SEM, Figure S1) and transmission electron microscope (TEM, Figure 1c,d) images. It can be observed that HCNM3 exhibits a thinner sheet-like structure compared to g-C3N4. From the SEM images, CNM is revealed to be a layered material. The surface of HCNM3 is no longer smooth, and in-plane holes can be discerned. The G-C3N4 sample displays stacked sheets with a transition from polycrystalline to quasi-crystalline (Figure S2). However, as shown in Figure S3, the apparent porous nanosheets with some wrinkles are observed for HCNM3. Particles of approximately 20–30 nm in size can be detected, possessing an estimated d-spacing of 0.34 nm, which is attributed to the interplanar distance between the (110) planes of monoclinic MoO2 and is in good agreement with our subsequent XRD results. The element mapping images (Figure S3c,d) reveal the aggregation of Mo and O on these small particles, further confirming that these nanoparticles are oxides of molybdenum. Clearly, the high surface area of CNMs can be primarily attributed to its abundant pores and unique ultrathin morphology. Such a high specific surface area is of great significance for enhancing the catalytic properties of the materials.
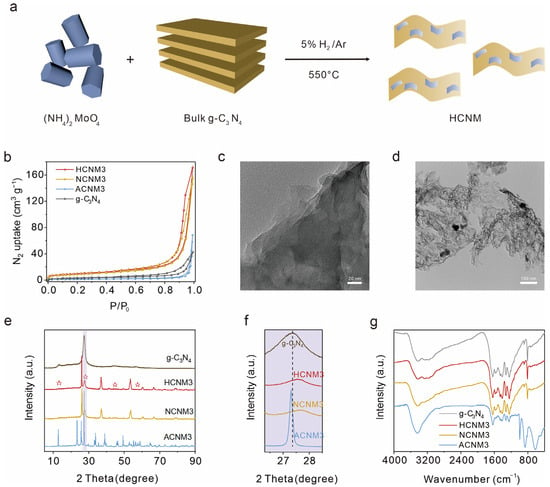
Figure 1.
Synthesis and structure characterization of g-C3N4 and layered CNM: (a) schematic illustration of HCNM nanocomposite synthesis by in situ growth of MoOx on the g-C3N4, (b) N2 sorption isotherms at 77 K, the transmission electron microscope (TEM) image of (c) g-C3N4 and (d) HCNM3, (e) X-ray diffraction (XRD), where ☆ represents g-C3N4, (f) zoom-in of the purple area in the total spectrum (e), and (g) FTIR spectra of g-C3N4 and CNM.
X-ray powder diffraction (XRD) patterns were utilized to ascertain the crystalline phases of the bulk g-C3N4 and CNM samples. As illustrated in Figure 1e,f and Figure S4, the HCNM and NCNM composites exhibit superimposed XRD patterns characteristic of g-C3N4 and MoO2 (JCPDS: 32-0671), while the ACNM samples additionally display a pronounced MoO3 diffraction peak (JCPDS: 50-0508), indicating the presence of heterostructures within the composites [31,32,33,34,35]. The peak around 27°, corresponding to the (002) π–π interlayer stacking of the conjugated aromatic system in g-C3N4, exhibits a positive shift and reduced intensity, which suggests that the interlayer stacking distance has been diminished due to enhanced van der Waals attraction between adjacent heptazine layers and the reduced thickness of the layered carbon nitride crystals [36,37].
The chemical structures of CNMs and g-C3N4 were confirmed through the combined application of Fourier transform infrared (FTIR) and Raman spectroscopy. In the FTIR spectra (Figure 1g and Figure S5), all samples exhibit a sharp peak at 812 cm−1, which corresponds to the characteristic breathing mode of the heptazine heterocyclic ring. The absorption band in the 1100–1700 cm−1 range is also attributed to the tri-s-triazine main structural unit. The broad peak located at 3000–3600 cm−1 is due to the stretching vibration of the –NHx groups of melamine. Additionally, the ACNM catalysts, which contain a higher proportion of Mo, display a new absorption peak at 985 cm−1 compared to g-C3N4, which is assigned to the symmetric vibration of the NC2 bond in the metal–NC2 group [38]. Peaks in the 400–900 cm−1 range were attributed to the intermediate bridging O–Mo–O bond, Mo=O, and Mo–O bonding types of MoO3 [39,40]. Raman spectra (Figure S6) similarly detect signal peaks for MoOx and g-C3N4 in the CNM samples [41,42,43,44,45,46,47]. The MoOx peaks in HCNMs are weaker, yet there are strong MoOx diffraction peaks in the XRD, which may be due to the metal particles obtained in the hydrogen–argon mixed gas being dispersed on the polymer substrate and coordinated with the polymer through Mo-N bonds.
2.2. Chemical Structural Analysis
CNMs obtained under different atmospheres may possess catalysts with different redox states. X-ray photoelectron spectra (XPS) were employed to characterize CNMs prepared under various atmospheres and at different ratios (Figure 2 and Figures S7–S10) to analyze the elemental valence state and chemical composition of CNM catalysts. The wide-scan XPS confirm the presence of Mo, O, N, and C in CNMs and O, N, and C in g-C3N4. All binding energies in the XPS spectra have been calibrated using the C 1s peak at 284.8 eV. As shown in Figure 2a,b, and Figure S7, for samples synthesized under H2/Ar and N2 gas, the Mo 3d core-level spectrum consists of a spin–orbit doublet with peaks at 232.5 and 229.3 eV, attributed to the 3d3/2 and 3d5/2 of Mo4+ species. Additionally, two peaks at 234.5 and 231.2 eV binding energies can be assigned to Mo5+ [40,48]. For the air-calcined sample ACNM3 (Figure 2c), peaks located at 235.8 and 232.7 eV correspond to Mo cations in high oxidation states (Mo6+), and other peaks at 234.5 and 231.4 eV are attributed to the Mo(V) oxidation state. The presence of Mo(V) could be due to the reduction by –NH2 groups of g-C3N4 during sample preparation.
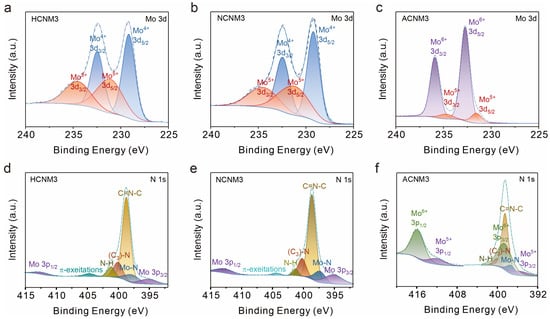
Figure 2.
X-ray photoelectron spectroscopy (XPS) analysis of HCNM3, NCNM3, and ACNM3: (a–c) Mo 3d of samples, (d–f) N 1s core level of samples.
The high-resolution XPS spectrum of the N 1s peak is shown in Figure 2d–f and Figure S8. Both CNMs and g-C3N4 include sp2-bonded nitrogen in C–N=C (398.7 eV), nitrogen in tertiary N–(C)3 groups (400.3 eV), and the presence of amino groups (C–N–H, 401.3 eV) caused by imperfect polymerization. Compared to g-C3N4, CNMs have a higher proportion of bridging nitrogen ((C)3–N), indicating the re-polymerization of g-C3N4 during the secondary calcination process. Moreover, in ACNM3, the N–H bonding content is significantly reduced, indicating that –NH2 has been oxidized. The analysis of the π-excitation peak at 404.4 eV in CNMs reveals that an excess of Mo leads to a weakening of π–π interactions within g-C3N4, with the peak at 404.4 eV being almost undetectable in the ACNM3 sample. It can be observed that the N 1s peak overlaps with the Mo 3p peak. The characteristic peak located at a binding energy of approximately 395.3 eV is attributed to Mo 3p3/2, which is about 17.2 eV lower than the peak position of 3p1/2 at around 412 eV. Additionally, CNMs show an extra peak corresponding to the Mo–N bond at 397.8 eV compared to g-C3N4 (Figure S8c), indicating the coordination of Mo to N within the g-C3N4 framework [49,50]. Notably, the deconvolution results of HCNM1 show a significant proportion of Mo–N at 397.8 eV [51,52] (Figure S8a), and HCNMs synthesized under H2/Ar atmosphere contain more Mo–N than NCNM3 synthesized under N2 gas, suggesting that MoOx particles are smaller and more uniformly dispersed on g-C3N4 under a hydrogen atmosphere, which also explains the stronger peaks belonging to Mo–O bonds observed in the Raman spectra of NCNM3 (Figure S6).
The deconvolution of C 1s for g-C3N4 exhibited three peaks at 284.8, 286.7, and 288.2 eV, corresponding to C–C/C=C, C–O, and C–NHx, respectively (Figure S9) [53]. The analysis of the C 1s spectra of HCNMs reveals that when the molybdenum content is low, the C–O peak is absent, indicating that the polymer substrate is reduced by H2/Ar. As the Mo content increases, a peak at 288.8 eV appears and gradually intensifies, attributed to C–OOH [54], indicating that an excess of molybdenum salts can oxidize the C-based substrate, even under reducing atmospheres. For samples HCNM3, NCNM3, and ACNM3 with the same proportion of molybdenum salts added, as the reducibility of the calcination atmosphere decreases and the oxidizing nature increases, a greater proportion of C is oxidized. Table 1 shows the C/N atomic ratios of CNMs obtained from the same precursors under different atmospheres. As the oxidizing nature of the secondary calcination atmosphere increases and the reducibility decreases, the C/N ratio gradually increases.

Table 1.
nC/nN of g-C3N4, HCNM3, NCNM3, and ACNM3 were obtained through simulation calculations, as depicted in Figure 2.
Figure S10 shows the O 1s spectra, with peaks attributable to oxides and chemisorbed oxygen visible. Although the assignments of oxygen species are not yet well established, all peak positions are in good agreement with literature values: the main component peak at 532.6 eV (yellow curve) corresponds to oxygen species dissolved in the metal or to adsorbed oxygen; the peak at 530.8 eV (blue curve) is attributed to Mo–O; the peak located at the lower binding energy of about 529 eV (purple curve) corresponds to (CₙNₘ)–O, and peaks with the higher binding energy at about 534 eV (green curve) are assigned to surface strongly adsorbed oxygen (OH− and O−) [55].
The XPS demonstrate that different molybdenum salt precursors and calcination atmospheres not only affect the oxidation state and concentration of MoOx loaded on the material but also impact the polymer substrate. They also confirm that the material is a composite of MoOx and C3N4 coordinated through Mo–N bonds.
2.3. Photocatalytic Performance Evaluation
We evaluated the photocatalytic performance of CNMs with different Mo loadings prepared under various atmospheres using an optimized system from our previous work for the degradation of SF6. As shown in Figure 3a, the photocatalytic activity of g-C3N4 for SF6 degradation was 0.21 mmol/g within one day, while the introduction of Mo species significantly enhanced the photocatalytic activity of CNMs. Overall, the HCNMs calcined under a hydrogen–argon atmosphere exhibited superior performance compared to the NCNMs obtained under nitrogen and the ACNMs obtained under air. Different Mo loadings affected the efficiency of catalytic SF6 degradation. The optimal CNM was HCNM3, achieving an SF6 degradation efficiency of 1.73 mmol/g, which is 8 to 9 times that of g-C3N4. Unfortunately, although HCNM3 demonstrates a longer operational duration compared to previous versions, it exhibits poor catalytic cycling stability (Figure S11). The temporal profile indicates that HCNM3 maintains relatively good stability within 12 h. However, after exceeding 12 h of operation, the degradation rate undergoes a significant decline, although it remains superior to C3N4 (Figure 3b). Based on previous findings where the catalyst undergoes fluoride poisoning-induced deactivation within 6 h in a three-phase system, we conducted cyclic experiments for a 6 h reaction (Figure 3c). The results reveal a partial activity loss in the third cycle, yet the catalytic activity remains largely preserved after three cycles and still surpasses that of untreated g-C3N4.
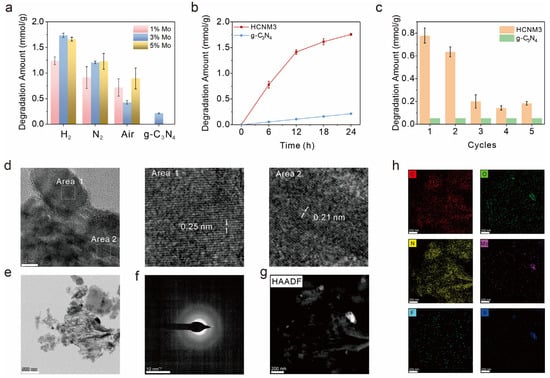
Figure 3.
Photocatalytic performance and cyclic stability evaluation: (a) SF6 degradation of different samples under LED irradiation (λ = 365 nm) at room temperature, (b) kinetic curves of photoreduction SF6 degradation of HCNM3 and g-C3N4, (c) SF6 degradation amount in the stability tests of the HCNM3 and g-C3N4 samples with the reaction hours of 6 h, (d) HRTEM, (e) TEM, (f) selected area electron diffraction (SAED), (g) HAADF-STEM (scanning transmission electron microscope) images, and (h) STEM-EDS maps of HCNM3 after 24 h reaction.
We collected and characterized the catalysts after the reaction; the IR and XRD signals showed no significant changes before and after the reaction (Figure S12). The morphology of the samples after the reaction was characterized by TEM/SEM (Figure 3d–g and Figure S13). No obvious morphological changes were observed in the SEM and TEM images. The high-resolution TEM images revealed lattice fringes of 0.21 nm and 0.25 nm, corresponding to the 1T-MoS2 and the (−211) lattice plane of MoO2, respectively [56,57]. MoS2 also serves as a photocatalyst. Although its incorporation reduces the catalytic degradation efficiency of HCNM3, it enhances the degradation of SF6 compared to C3N4. As shown in Figure 3h, the element mapping images revealed that the F element was uniformly distributed on the photocatalyst HCNM3 without significant aggregation, which may be attributed to the adsorption of fluorine species on the surface of the catalyst. The S element mainly accumulates on MoOx, indicating the interaction between MoOx and sulfur ions in the solution. We also compared the XPS before and after the reaction (Figure S14). The results showed increased COOH signals on the carbon nitride substrate and a higher content of Mo(V), indicating a charge transfer process between the catalyst and the reactants. Additionally, deposition signal peaks for the S and F elements were also detected.
2.4. Mechanistic Insights into Enhanced Photocatalysis
To gain insights into the mechanism behind the enhanced photocatalytic performance, the light absorption properties of the synthesized samples were examined using diffuse reflectance spectra (DRSs). As shown in Figure 4a, introducing Mo leads to a slight red-shift in the optical edges of the HCNM3 sample, demonstrating superior light absorption capabilities compared to the original g-C3N4. The color of g-C3N4 is yellow, while that of HCNM3 changes to a gray-green, which is consistent with the absorption spectra of the samples. The incorporation of Mo species also alters the band structure of the HCNM3 catalyst. The UV–vis DRS indicates that the corresponding bandgap energies (Egs) for the HCNM3 and g-C3N4 catalysts are 2.82 eV and 2.69 eV, respectively (Figure S15).
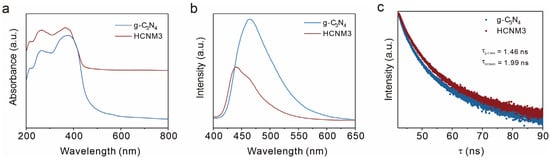
Figure 4.
Photocatalytic properties of the samples, including light absorption and carrier behavior. (a) UV–vis DRSs of g-C3N4 and HCNM3, (b) room temperature (298 K) steady-state PL spectra of the g-C3N4 and HCNM3 samples, and (c) time-resolved fluorescence kinetics monitored at the corresponding emission peaks.
The photoluminescence (PL) spectra were utilized to investigate the separation and recombination efficiency of charge carriers (Figure 4b). The PL intensity of the CNM composites was significantly weaker than that of g-C3N4. The quenching of PL essentially indicates faster interfacial charge transport, which may be attributed to structural and morphological optimization, as well as the more metallic nature of Mo species facilitating electron relocalization to impede charge recombination. To further explore the charge–carrier separation/recombination in HCNM3, time-resolved PL decay spectra were also examined. As depicted in Figure 4c, the average lifetime of photoexcited carriers for HCNM3 was prolonged to 1.99 ns compared to 1.46 ns for g-C3N4 [58,59]. This result further confirms the higher rate of photogenerated charge separation.
To further explore the mechanism of adsorption, DFT calculation is used to study the surface charge transfer and the absorption process between the MoO2, g-C3N4, CNM, and SF6 structures. After SF6 adsorption on the MoO2 or g-C3N4 interface, their adsorption energies are 0.17 eV and 0.50 eV, respectively, which means that the MoO2 and g-C3N4 interfaces do not have adsorption properties for SF6 (Figure 5a and Figure S16). However, as shown in Figure S17, electrons transfer from MoO2 to C3N4 after forming a heterostructure. It results in more positive charges on MoO2 to adsorb SF6. As a result, the adsorption energy of MoO2-C3N4 to SF6 is −0.53 eV (Figure 5b).
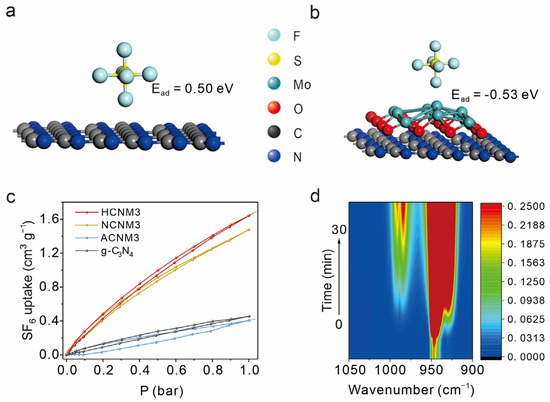
Figure 5.
The adsorption and activation of SF6. (a) The g-C3N4-SF6-ads structure with its adsorption energy by DFT calculation, (b) the MoO2-C3N4-SF6-ads structure with its adsorption energy by DFT calculation, (c) SF6 sorption isotherms at 273 K of g-C3N4 and CNMs, (d) In situ FT-IR spectra recorded after the adsorption of SF6.
To further substantiate the adsorption performance of the materials towards SF6, pressure swing SF6 adsorption/desorption and spectroscopic studies were conducted. Given the large porosity and the abundance of Mo atoms, which serve as adsorption sites for SF6, HCNM3 and NCNM3 act as more effective SF6 adsorbents with capacities of 1.6 cm3/g and 1.5 cm3/g, respectively, compared to g-C3N4 (0.45 cm3/g) at 273 K and 1 atm. Despite its smaller surface area, the modified ACNM3 exhibits an uptake of SF6 of 0.41 cm3/g at 273 K and 1 atm, which is comparable to g-C3N4, and a similar trend was also detected on the sulfur hexafluoride adsorption curve at 298 K 1 atm (Figure 5c and Figure S18).
In situ FT-IR further observed the adsorption behavior of SF6 on the g-C3N4 and HCNM3 catalysts (Figure 5d and Figure S19). The absorption peaks corresponding to SF6 adsorption are located in the 900–1000 cm−1 range. For ease of comparison, peaks in the range of 970–1000 cm−1 were analyzed. On HCNM3, the signal corresponding to SF6 showed a gradual increase over time accompanied by a red-shift, indicating the adsorption and activation of SF6 gas molecules on the HCNM3 surface. In contrast, g-C3N4 quickly reached adsorption saturation after gas introduction, with peak positions being more positive and peak intensities weaker than those of HCNM3. This semi-quantitative result indicates that, compared to g-C3N4, HCNM3 has a stronger adsorption and activation effect on SF6 and can adsorb a greater amount of SF6, which is consistent with the SF6 adsorption experiments and DFT calculation results.
We also conducted analyses on the substances present in the system after degradation. The signal peak at approximately −190 ppm in the nuclear magnetic resonance spectroscopy (NMR) spectrum revealed the generation of fluoride ions in the reaction solution (Figure S20), with no detection of C–F bond formation. F−, SO32−, and SO42− were detected via ion chromatography (IC) as the main reaction products in the solution (Figure S21). Gas chromatography did not detect any new substances. Thus, it can be inferred that the main products of the SF6 photoreduction are F−, SO32−, and SO42−. Based on these findings, we propose a photocatalytic reaction pathway for SF6 (Figure 6): The MoOx portion of the catalyst, which has a stronger adsorption affinity for SF6, initially adsorbs and activates the S–F bond in SF6. Upon light irradiation, the catalyst absorbs photons and separates into photoinduced electron–hole pairs. Subsequently, the electrons are transferred to SF6. Methanol acts as a sacrificial agent for the holes, as it is oxidized on the organophilic CN substrate, enabling the continuous separation of electron–hole pairs. Ultimately, the fluorine in SF6 is converted into F−/HF, while the sulfur element is transformed into SOx2− species.
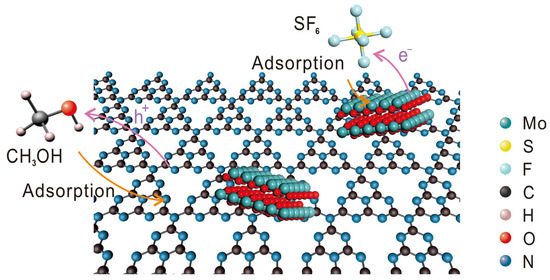
Figure 6.
Schematic illustration of the photocatalytic reaction pathway for SF6.
3. Materials and Methods
3.1. Materials
Methanol and acetonitrile were purchased from Aladdin Industrial Corporation (Beijing, China). Ammonium molybdate tetrahydrate ((NH4)6Mo7O24·4H2O) and melamine were sourced from Grat Company (Wuhan, China). SF6 was acquired from Shanghai Weichuang Standard Gas Company Limited (Shanghai, China). All reagents were used as received without further purification. Milli-Q water (Millipore Corp., Burlington, MA, USA, with a resistivity of 18.2 MΩ·cm) was employed for the experiments.
3.2. Preparation of g-C3N4
g-C3N4 was synthesized by heating melamine at 550 °C for 4 h with a heating rate of 2 °C/min in a muffle furnace.
3.3. Preparation of CNMs
The CNM samples were prepared by directly mixing and griding g-C3N4 powders (2 g) with different amounts (0.26, 0.77, and 1.29 g, respectively) of (NH4)6Mo7O24·4H2O powders. Then, they were placed in a crucible with a cover for re-polymerization. Next, they were placed in a quartz tube furnace with 5% H2/Ar and N2, followed by a muffle furnace at 550 °C for another 2 h, respectively. After being naturally cooled down, the collected samples were denoted as HCNMx, NCNMx, and ACNMx, correspondingly, where x represents the initial amount of (NH4)6Mo7O24·4H2O (x = 1, 3, and 5). The detailed information is shown in Table 2.

Table 2.
Detailed information of CNM preparation.
3.4. Photocatalytic SF6 Degradation Experiments
The photocatalytic reduction reactions of SF6 were conducted in a Schlenk flask reactor (40 mL). In the Schlenk flask, 30 mg of the catalyst was dispersed in a mixture containing 0.5 mL of methanol and 2.5 mL of solvent (acetonitrile: 2 mL; H2O: 0.5 mL). Prior to irradiation with a 50 W 365 nm UV-LED lamp (Epileds, Taiwan, China), the reaction vessel was repeatedly evacuated to completely remove air and then filled with SF6/Ar gas (1 atm, ~5% SF6). During the photocatalytic reaction, the reaction mixture was vigorously stirred at room temperature. For the recycling experiments, the reactor was refilled with the same gas mixture. After the reaction, the performance was assessed using gas chromatography.
3.5. Density Functional Theory (DFT) Computations
The DFT calculation was performed by using plane-wave basis sets on Materials Studio 2020 version. In order to optimize the MoO2, g-C3N4, CNM, and SF6 structures, the exchange correlation function, PBE, generalized gradient approximation with Koelling–Hamon relativistic treatment, and spin polarization assumptions were employed. Broyden–Fletcher–Goldfarb–Shanno geometry optimization is used for cell optimization. The interaction between valence electrons and the ionic core is described by using On-The-Fly-Generation ultra soft pseudo potential. The kinetic cutoff energy for the convergence test is 400 eV, and a k-point set mesh (1 × 1 × 1) parameter is used for Brillouin zone sampling. The threshold for self-consistent field iterations used is 5.0 × 10−6 eV atom−1. The convergence tolerance parameters of the optimized calculation are the tolerance for energy 2.0 × 10−5 eV atom−1, the maximum force of 0.05 eV Å−1, and the maximum displacement of 2 × 10−3 Å.
The adsorption performance of MoO2, g-C3N4, and CNM is investigated using Eads, where Eads is the adsorption energy of the SF6 molecule on the different structures. The Eads is calculated using the following equation:
where ESF6 molecule, Ematerial, and ESF6 molecule are the energies of the SF6 material, material, and SF6 molecule, respectively.
Eads = ESF6 molecule − Ematerial − ESF6 molecule
3.6. Characterization
The quantity of SF6 was ascertained utilizing gas chromatography (GC 7900, Techcomp Instrument Co., Ltd., Shanghai, China) equipped with a thermal conductivity detector (TCD). The operational temperatures for the column, injection port, and detector were maintained at 100, 100, and 150 °C, correspondingly. The specimen’s crystallographic information was captured via X-ray diffraction (XRD) on a Bruker D8 Advance diffractometer (Billerica, MA, USA) employing Cu-Kα radiation (λ = 1.5406 Å). Vibrational characteristics were probed with Raman spectroscopy on an inVia Qontor Renishaw instrument (Gloucestershire, UK). The physical configuration of the specimen was scrutinized using scanning electron microscopy (SEM, Nova NanoSEM 450, FEI, Hillsboro, OR, USA) operated at an accelerating voltage of 10 kV. The elemental distribution within the specimen was delineated via transmission electron microscopy (TEM, FEI Talos F200X) functioning at 200 kV in conjunction with Fischione model 2550 for specimen preparation. The specimen’s valence and electronic configurations were determined via X-ray photoelectron spectroscopy (XPS, AXIS Ultra DLD, Shimadzu, Kyoto, Japan), with Al Kα radiation serving as the excitation source. The optical absorption properties of the specimens were evaluated through UV/Vis diffuse reflectance spectroscopy on a Lamda 950 spectrophotometer (Perkin-Elmer, Waltham, MA, USA). Liquid samples were filtered for ion chromatography (IC) measurement on a Thermo Fisher ICS5000+ system (Waltham, MA, USA). For multivalent anions, an AS11/AG11 column set with NaOH eluent (1 mL/min) and conductivity detection was used; sulfate (SxOy2−) employed AS11-HC/AG11-HC columns with the EGC-generated KOH gradient (1 mL/min).
4. Conclusions
In summary, we have successfully developed a thin-layer CN material loaded with MoOx (CNM) for the efficient degradation of SF6, a potent greenhouse gas with significant environmental implications. The photocatalytic performance of the CNM was outstanding, achieving a degradation efficiency for SF6 of up to 1.73 mmol/g after one day of LED light irradiation at room temperature, which is notably more than eight times higher than that of pristine g-C3N4 (0.21 mmol/g). This remarkable enhancement can be attributed to the synergistic effects of the high specific surface area of the thin-layer CN, the introduction of MoO2 active sites, and the efficient electron transport facilitated by the Mo-N bonds. The combination of high specific surface area materials with Mo-based inorganic nanocatalytic materials presents a promising strategy for enhancing photocatalytic activity, which could be further explored for the degradation of other environmentally harmful substances. The findings of this study pave the way for low-energy, high-efficiency solutions to mitigate the impact of potent greenhouse gases like SF6, contributing to global efforts in combating climate change.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/molecules30071481/s1. References [60,61,62,63] are cited in the supplementary materials.
Author Contributions
Conceptualization, W.Z., J.Z. and X.Q.; methodology, W.Z., B.D. and J.Z.; software, W.Z. and B.D.; validation, W.Z., B.D., Z.S., Y.X., J.Z. and X.Q.; formal analysis, W.Z., B.D. and Y.X.; investigation, W.Z., B.D., Z.S., Y.X., Z.Z., Y.Z., C.S. and Z.L.; resources, W.Z., B.D., Z.S., Y.X., X.H., C.S. and Z.L.; data curation, W.Z., B.D., Y.X., Z.Z. and Y.Z.; writing—original draft preparation, W.Z. and B.D.; writing—review and editing, W.Z., B.D., X.H., C.S. and J.Z.; visualization, W.Z. and B.D.; supervision, J.Z. and X.Q.; project administration, W.Z., B.D., J.Z. and X.Q.; funding acquisition, J.Z. and X.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Research and application of zero emission recovery technology for sulfur hexafluoride based on catalytic degradation, Science and Technology Project of the State Grid Corporation of China (5200-202320487A-3-2-ZN).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available upon request.
Acknowledgments
The authors are grateful for X. Li et al. from the Instrumental Analysis Center of Shanghai Jiao Tong University for help and technical support.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CNM | carbon nitride material loaded with MoOx |
| GWP | global warming potential |
| SF6 | sulfur hexafluoride |
| in situ IR | in situ infrared spectroscopy |
| DFT | Density Functional Theory |
| XPS | X-ray photoelectron spectroscopy |
| XRD | X-ray diffraction |
| TEM | Transmission electron microscope |
| FTIR | Fourier transform infrared |
| DRS | diffuse reflectance spectra |
| SEM | scanning electron microscope |
| IC | ion chromatography |
| NMR | nuclear magnetic resonance spectroscopy |
| GC | gas chromatography |
| TCD | thermal conductivity detector |
| SAED | selected area electron diffraction |
References
- Eliyan, T.; Wadie, F. Evaluation of the efficacy of transient overvoltages suppression measures in different wind farm topologies using SF6 circuit breaker. Sci. Rep. 2023, 13, 13655. [Google Scholar] [CrossRef] [PubMed]
- Ko, M.K.W.; Sze, N.D.; Wang, W.C.; Shia, G.; Goldman, A.; Murcray, F.J.; Murcray, D.G.; Rinsland, C.P. Atmospheric Sulfur-Hexafluoride—Sources, Sinks and Greenhouse Warming. J. Geophys. Res.-Atmos. 1993, 98, 10499–10507. [Google Scholar]
- Hossain, A.A.; Wang, H.Z.; Catherall, D.S.; Leung, M.; Knoops, H.C.M.; Renzas, J.R.; Minnich, A.J. Isotropic plasma-thermal atomic layer etching of superconducting titanium nitride films using sequential exposures of molecular oxygen and SF6/H2 plasma. J. Vac. Sci. Technol. A 2023, 41, 2965. [Google Scholar] [CrossRef]
- Rombach, D.; Wagenknecht, H.A. Photoredox Catalytic α-Alkoxypentafluorosulfanylation of α-methyl- and α-phenylstyrene using SF6. Angew. Chem. Int. Ed. 2020, 59, 300–303. [Google Scholar]
- Xiao, S.; Shi, S.Y.; Li, Y.; Ye, F.C.; Li, Y.L.; Tian, S.S.; Tang, J.; Zhang, X.X. Review of decomposition characteristics of eco-friendly gas insulating medium for high-voltage gas-insulated equipment. J. Phys. D Appl. Phys. 2021, 54, 373002. [Google Scholar] [CrossRef]
- Liao, Q.B.; Ke, C.; Huang, X.; Wang, D.N.; Han, Q.W.; Zhang, Y.F.; Zhang, Y.Y.; Xi, K. A Versatile Method for Functionalization of Covalent Organic Frameworks via Suzuki-Miyaura Cross-Coupling. Angew. Chem. Int. Ed. 2021, 60, 1411–1416. [Google Scholar]
- Maiss, M.; Brenninkmeijer, C.A.M. Atmospheric SF6 Trends, sources, and prospects. Environ. Sci. Technol. 1998, 32, 3077–3086. [Google Scholar]
- Shi, S.Y.; Li, Y.; Cui, Z.L.; Yan, Y.X.; Zhang, X.X.; Tang, J.; Xiao, S. Recent advances in degradation of the most potent industrial greenhouse gas sulfur hexafluoride. Chem. Eng. J. 2023, 470, 144166. [Google Scholar]
- Case, J.R.; Nyman, F. Some Chemical Reactions of Sulphur Hexafluoride. Nature 1962, 193, 473. [Google Scholar]
- Holze, P.; Horn, B.; Limberg, C.; Matlachowski, C.; Mebs, S. The Activation of Sulfur Hexafluoride at Highly Reduced Low-Coordinate Nickel Dinitrogen Complexes. Angew. Chem. Int. Ed. 2014, 53, 2750–2753. [Google Scholar]
- Berg, C.; Braun, T.; Ahrens, M.; Wittwer, P.; Herrmann, R. Activation of SF6 at Platinum Complexes: Formation of SF6 Derivatives and Their Application in Deoxyfluorination Reactions. Angew. Chem. Int. Ed. 2017, 56, 4300–4304. [Google Scholar]
- Zhou, W.H.; Zhao, Y.; Dong, B.X.; Guo, H.R.; Si, Z.Q.; Ma, F.X.; Zhu, S.; Zai, J.T.; Qian, X.F. Efficient photocatalytic degradation of potent greenhouse gas SF6 at liquid-solid interface. Appl. Catal. B-Environ. Energy 2025, 363, 124773. [Google Scholar]
- Zhu, C.; Fang, Q.L.; Liu, R.L.; Dong, W.; Song, S.; Shen, Y. Insights into the Crucial Role of Electron and Spin Structures in Heteroatom-Doped Covalent Triazine Frameworks for Removing Organic Micropollutants. Environ. Sci. Technol. 2022, 56, 6699–6709. [Google Scholar]
- Yang, Z.P.; Shi, Y.B.; Li, H.; Mao, C.L.; Wang, X.B.; Liu, X.F.; Liu, X.; Zhang, L.Z. Oxygen and Chlorine Dual Vacancies Enable Photocatalytic O2 Dissociation into Monatomic Reactive Oxygen on BiOCl for Refractory Aromatic Pollutant Removal. Environ. Sci. Technol. 2022, 56, 3587–3595. [Google Scholar] [PubMed]
- Rasheed, H.M.; Aroosh, K.; Meng, D.P.; Ruan, X.W.; Akhter, M.; Cui, X.Q. A review on modified ZnO to address environmental challenges through photocatalysis: Photodegradation of organic pollutants. Mater. Today Energy 2025, 48, 101774. [Google Scholar]
- Jing, Y.N.; Yin, X.L.; Li, L.L. Modification strategies of TiO2-based nanocatalysts for CO2 reduction through photocatalysis: A mini review. Appl. Catal. A-Gen. 2025, 691, 120054. [Google Scholar]
- Kumar, E.V.; Soundarya, T.L.; Swamy, B.E.K.; Anitha; Nagaraju, G. Fabrication of CuS-MoO3 nanocomposite for high-performance photocatalysis and biosensing. J. Mol. Struct. 2025, 1324, 140823. [Google Scholar]
- Xiao, M.F.; Ren, X.L.; Ji, K.Y.; Chung, S.; Shi, X.Y.; Han, J.; Yao, Z.F.; Tao, X.D.; Zelewski, S.J.; Nikolka, M.; et al. Achieving ideal transistor characteristics in conjugated polymer semiconductors. Sci. Adv. 2023, 9, eadg8659. [Google Scholar]
- Li, J.G.; Zhang, G.Y.; Zyryanov, G.V.; Shabunina, O.V.; Guo, X.F.; Zhu, M.G.; Jin, Y.L.; Wang, Z. Nitric-Oxide-Enhanced Positively Charged Semiconductor Conjugated Polymer Composite Nanomaterials for Antibiofilm In Vivo. Adv. Funct. Mater. 2024, 35, 202415134. [Google Scholar]
- Zhou, Y.; Miao, C.Q.; Su, Z.H.; Gao, X.Y.; Wei, H.Y.; Liu, K.K.; Zhang, H.C. Varied π-Conjugated Extension Directions in B←N Coordinated Benzodipyrrolidone (BDP) Polymer Semiconductors: Effects on n-Type Organic Field-Effect Transistors (OFETs). Acs Mater. Lett. 2024, 6, 5093–5102. [Google Scholar]
- Li, X.H.; Wang, X.C.; Antonietti, M. Solvent-Free and Metal-Free Oxidation of Toluene Using O2 and g-C3N4 with Nanopores: Nanostructure Boosts the Catalytic Selectivity. Acs Catal. 2012, 2, 2082–2086. [Google Scholar] [CrossRef]
- Ming, H.B.; Bian, X.Q.; Cheng, J.J.; Yang, C.; Hou, Y.D.; Ding, K.N.; Zhang, J.S.; Anpo, M.; Wang, X.C. Carbon nitride with a tailored electronic structure toward peroxymonosulfate activation: A direct electron transfer mechanism for organic pollutant degradation. Appl. Catal. B-Environ. Energy 2024, 341, 123314. [Google Scholar] [CrossRef]
- Guo, H.S.; Zhao, W.F.; Chen, W.L.; Ge, Q.M.; Liu, M.; Cong, H.; Zhao, J.L. Thinned g-C3N4 nanosheets with microdopants of cucurbit[7]uril to improve photoelectrochemical water-splitting. J. Photochem. Photobiol. A 2025, 461, 116166. [Google Scholar] [CrossRef]
- Kocijan, M.; Curkovic, L.; Radosevic, T.; Podlogar, M. Enhanced Photocatalytic Activity of Hybrid rGO@TiO2/CN Nanocomposite for Organic Pollutant Degradation under Solar Light Irradiation. Catalysts 2021, 11, 1023. [Google Scholar] [CrossRef]
- Miller, A.P. Lange’s Handbook of Chemistry. J. Am. Soc. Nav. Eng. 1941, 53, 687–688. [Google Scholar] [CrossRef]
- Bao, F.X.; Liu, L.L.; Wang, X.L.; Xiao, B.Q.; Li, H.Y.; Yang, H.D.; Shen, K.; Mai, Y.H. Modification of the Se/MoO Rear Interface for Efficient Wide-Band-Gap Trigonal Selenium Solar Cells. Acs Appl. Mater. Inter. 2025, 17, 6222–6229. [Google Scholar] [CrossRef]
- Xi, Q.Y.; Liu, J.S.; Wu, Z.Y.; Bi, H.F.; Li, Z.Q.; Zhu, K.J.; Zhuang, J.J.; Chen, J.X.; Lu, S.L.; Huang, Y.F.; et al. In-situ fabrication of MoO3 nanobelts decorated with MoO2 nanoparticles and their enhanced photocatalytic performance. Appl. Surf. Sci. 2019, 480, 427–437. [Google Scholar] [CrossRef]
- Cai, W.; Liu, J.C.; Luo, Y.J.; Liao, Z.W.; Li, B.J.; Xiang, X.Y.; Fang, Y.X. Bifunctional CdS-MoO2 catalysts for selective oxidation of lactic acid coupled with photocatalytic H production. J. Colloid Interface Sci. 2024, 675, 836–847. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Yang, L.J.; Huang, M.; Li, Q.; Zhao, L.L.; Sang, Y.H.; Zhang, X.L.; Zhao, Z.H.; Liu, H.; Zhou, W.J. Oxygen vacancy-regulated metallic semiconductor MoO2 nanobelt photoelectron and hot electron self-coupling for photocatalytic CO2 reduction in pure water. Appl. Catal. B-Environ. Energy 2022, 319, 121887. [Google Scholar] [CrossRef]
- Xiao, C.L.; Wang, H.P.; Zhang, L.; Sun, S.M.; Wang, W.Z. Enhanced Photocatalytic Nitrogen Fixation on MoO2/BiOCl Composite. Chemcatchem 2019, 11, 6467–6472. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.C.; Antonietti, M. Polymeric Graphitic Carbon Nitride as a Heterogeneous Organocatalyst: From Photochemistry to Multipurpose Catalysis to Sustainable Chemistry. Angew. Chem. Int. Ed. 2012, 51, 68–89. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.J.; Zhou, W.J.; Hou, D.M.; Zhou, K.; Li, G.Q.; Tang, Z.H.; Li, L.G.; Chen, S.W. Porous metallic MoO2-supported MoS2 nanosheets for enhanced electrocatalytic activity in the hydrogen evolution reaction. Nanoscale 2015, 7, 5203–5208. [Google Scholar] [CrossRef]
- Qin, T.R.; Wang, Q.L.; Yue, D.H.; Liu, H.; Zheng, Y.J.; Han, Y.H.; Gao, C.X. The effect of pressure and temperature on the structure and electrical transport properties of MoO2. J. Alloy Compd. 2020, 814, 152336. [Google Scholar] [CrossRef]
- Sen, S.K.; Barman, U.C.; Manir, M.S.; Mondal, P.; Dutta, S.; Paul, M.; Chowdhury, M.A.M.; Hakim, M.A. X-ray peak profile analysis of pure and Dy-doped α-MoO3 nanobelts using Debye-Scherrer, Williamson-Hall and Halder-Wagner methods. Adv. Nat. Sci.-Nanosci. 2020, 11, 025004. [Google Scholar] [CrossRef]
- Zhang, X.H.; Tang, J.J.; Zhang, Q.; Liu, Q.Y.; Li, Y.P.; Chen, L.G.; Wang, C.G.; Ma, L.L. Hydrodeoxygenation of lignin-derived phenolic compounds into aromatic hydrocarbons under low hydrogen pressure using molybdenum oxide as catalyst. Catal. Today 2019, 319, 41–47. [Google Scholar] [CrossRef]
- Hu, E.M.; Chen, Q.; Gao, Q.; Fan, X.F.; Luo, X.J.; Wei, Y.; Wu, G.; Deng, H.B.; Xu, S.C.; Wang, P.; et al. Cyano-Functionalized Graphitic Carbon Nitride with Adsorption and Photoreduction Isosite Achieving Efficient Uranium Extraction from Seawater. Adv. Funct. Mater. 2024, 34, 202312215. [Google Scholar] [CrossRef]
- Ou, H.H.; Tang, C.; Zhang, Y.F.; Asiri, A.M.; Titirici, M.M.; Wang, X.C. Se-modified polymeric carbon nitride nanosheets with improved photocatalytic activities. J. Catal. 2019, 375, 104–112. [Google Scholar] [CrossRef]
- Pan, Z.M.; Zhang, G.G.; Zhang, X.R.; Xing, W.D.; Zheng, D.D.; Wang, S.B.; Hou, Y.D.; Wang, X.C. Unveiling the Key Obstacle in Photocatalytic Overall Water Splitting Reaction on Poly (heptazine imide) Semiconductors. Small 2025, 21, e2407307. [Google Scholar] [CrossRef]
- Mai, L.Q.; Hu, B.; Chen, W.; Qi, Y.Y.; Lao, C.S.; Yang, R.S.; Dai, Y.; Wang, Z.L. Lithiated MoO3 nanobelts with greatly improved performance for lithium batteries. Adv. Mater. 2007, 19, 3712. [Google Scholar] [CrossRef]
- Luo, Z.; Miao, R.; Huan, T.D.; Mosa, I.M.; Poyraz, A.S.; Zhong, W.; Cloud, J.E.; Kriz, D.A.; Thanneeru, S.; He, J.K.; et al. Mesoporous MoO3-x Material as an Efficient Electrocatalyst for Hydrogen Evolution Reactions. Adv. Energy Mater. 2016, 6, 100038. [Google Scholar] [CrossRef]
- Ma, W.L.; Alonso-González, P.; Li, S.J.; Nikitin, A.Y.; Yuan, J.; Martín-Sánchez, J.; Taboada-Gutiérrez, J.; Amenabar, I.; Li, P.N.; Vélez, S.; et al. In-plane anisotropic and ultra-low-loss polaritons in a natural van der Waals crystal. Nature 2018, 562, 557. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Sun, C.Y.; He, Q.; Liu, D.B.; Khalil, A.; Xiang, T.; Wu, Z.Y.; Wang, J.; Song, L. Ultrathin carbon layer coated MoO2 nanoparticles for high-performance near-infrared photothermal cancer therapy. Chem. Commun. 2015, 51, 10054–10057. [Google Scholar]
- Lai, K.; Yuan, K.; Ye, Q.; Chen, A.; Chen, D.; Chen, D.; Gu, C. Constructing the Mo2C@ MoOx heterostructure for improved SERS application. Biosensors 2022, 12, 50. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Chen, D.; Gu, C.; Jiang, T.; Zeng, S.; Wang, Y.Y.; Ni, Z.; Shen, X.; Zhou, J. Molybdenum oxide/tungsten oxide nano-heterojunction with improved surface-enhanced Raman scattering performance. ACS Appl. Mater. Interfaces 2021, 13, 33345–33353. [Google Scholar] [CrossRef]
- Yadav, A.A.; Kang, S.W.; Hunge, Y.M. Photocatalytic degradation of Rhodamine B using graphitic carbon nitride photocatalyst. J. Mater. Sci. Mater. Electron. 2021, 32, 15577–15585. [Google Scholar]
- Jiang, J.Z.; Lei, O.Y.; Zhu, L.H.; Zheng, A.M.; Zou, J.; Yi, X.F.; Tang, H.Q. Dependence of electronic structure of g-C3N4 on the layer number of its nanosheets: A study by Raman spectroscopy coupled with first-principles calculations. Carbon 2014, 80, 213–221. [Google Scholar]
- Yuan, Y.W.; Zhang, L.L.; Xing, J.; Utama, M.I.B.; Lu, X.; Du, K.Z.; Li, Y.M.; Hu, X.; Wang, S.J.; Genç, A.; et al. High-yield synthesis and optical properties of g-C3N4. Nanoscale 2015, 7, 12343–12350. [Google Scholar]
- Choi, J.G.; Thompson, L.T. XPS study of as-prepared and reduced molybdenum oxides. Appl. Surf. Sci. 1996, 93, 143–149. [Google Scholar] [CrossRef]
- Kou, M.P.; Liu, W.; Wang, Y.Y.; Huang, J.D.; Chen, Y.L.; Zhou, Y.; Chen, Y.; Ma, M.Z.; Lei, K.; Xie, H.Q.; et al. Photocatalytic CO2 conversion over single-atom MoN2 sites of covalent organic framework. Appl. Catal. B-Environ. 2021, 291, 120146. [Google Scholar]
- Wu, A.P.; Gu, Y.; Xie, Y.; Yan, H.J.; Jiao, Y.Q.; Wang, D.X.; Tian, C.G. Interfacial engineering of MoS2/MoN heterostructures as efficient electrocatalyst for pH-universal hydrogen evolution reaction. J. Alloy Compd. 2021, 867, 159066. [Google Scholar]
- Sun, Y.; Zhou, Y.L.; Zhu, Y.P.; Shen, Y.H.; Xie, A.J. In-Situ Synthesis of Petal-Like MoO2@MoN/NF Heterojunction As Both an Advanced Binder-Free Anode and an Electrocatalyst for Lithium Ion Batteries and Water Splitting. ACS Sustain. Chem. Eng. 2019, 7, 9153–9163. [Google Scholar]
- Bian, H.; Ji, Y.J.; Yan, J.Q.; Li, P.; Li, L.; Li, Y.Y.; Liu, S.Z. In Situ Synthesis of Few-Layered g-C3N4 with Vertically Aligned MoS2 Loading for Boosting Solar-to-Hydrogen Generation. Small 2018, 14, 201703003. [Google Scholar]
- Fang, Z.X.; Zhou, M.; Lin, Z.; Yang, C.; Hou, Y.D.; Yu, J.C.; Zhang, J.S.; Wang, X.C. Amide bonded polymeric carbon nitride for photocatalytic O2 activation and NO oxidation. Appl. Catal. B-Environ. Energy 2024, 353, 124022. [Google Scholar]
- Stobinski, L.; Lesiak, B.; Malolepszy, A.; Mazurkiewicz, M.; Mierzwa, B.; Zemek, J.; Jiricek, P.; Bieloshapka, I. Graphene oxide and reduced graphene oxide studied by the XRD, TEM and electron spectroscopy methods. J. Electron. Spectrosc. 2014, 195, 145–154. [Google Scholar] [CrossRef]
- Kim, D.; Kagwade, S.V.; Clayton, C.R. Identification of Mo(V) commonly observed in passive films formed on stainless steels. Surf. Interface Anal. 1998, 26, 155–159. [Google Scholar] [CrossRef]
- Yu, X.B.; Yan, F.; Zhao, Y.; Geng, B.; Ma, X.Z.; Wu, L.L.; Zhang, X.T.; Chen, Y.J. A heterostructure of interlayer-expanded 1T phase MoS2 and spherical MoO2 for efficient and stable hydrogen evolution. Appl. Catal. B-Environ. Energy 2024, 343, 123534. [Google Scholar] [CrossRef]
- Li, L.; Chen, J.; Xiao, Z.; Zhang, X.; Kwak, S.K.; Tian, D.; Lee, J.M. Coupled Lattice-Expanded Ni and MoO2 Array for Efficient Alkaline Hydrogen Evolution. Adv. Sustain. Syst. 2024, 2400587. [Google Scholar] [CrossRef]
- Berezin, M.Y.; Achilefu, S. Fluorescence Lifetime Measurements and Biological Imaging. Chem. Rev. 2010, 110, 2641–2684. [Google Scholar]
- Fang, Y.X.; Li, X.C.; Wang, Y.; Giordano, C.; Wang, X.C. Gradient sulfur doping along polymeric carbon nitride films as visible light photoanodes for the enhanced water oxidation. Appl. Catal. B-Environ. 2020, 268, 118398. [Google Scholar]
- Huang, L.; Gu, D.H.; Yang, L.Y.; Xia, L.Y.; Zhang, R.X.; Hou, H.Q. Photoreductive degradation of sulfur hexafluoride in the presence of styrene. J. Environ. Sci. 2008, 20, 183–188. [Google Scholar] [CrossRef]
- Song, X.X.; Liu, X.G.; Ye, Z.L.; He, J.C.; Zhang, R.X.; Hou, H.Q. Photodegradation of SF6 on polyisoprene surface: Implication on elimination of toxic byproducts. J. Hazard. Mater. 2009, 168, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Dong, W.B.; Zhang, R.X.; Hou, H.Q. Investigation of a new approach to decompose two potent greenhouse gases: Photoreduction of SF6 and SF5CF3 in the presence of acetone. Chemosphere 2007, 66, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Shen, Y.; Dong, W.B.; Zhang, R.X.; Zhang, J.L.; Hou, H.Q. A novel method to decompose two potent greenhouse gases: Photoreduction of SF6 and SF5CF3 in the presence of propene. J. Hazard. Mater. 2008, 151, 323–330. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).