Isotopic Transient Kinetic Analysis of Soot Oxidation on Mn3O4, Mn3O4-CeO2, and CeO2 Catalysts in Tight Contact Conditions
Abstract
1. Introduction
2. Results and Discussion
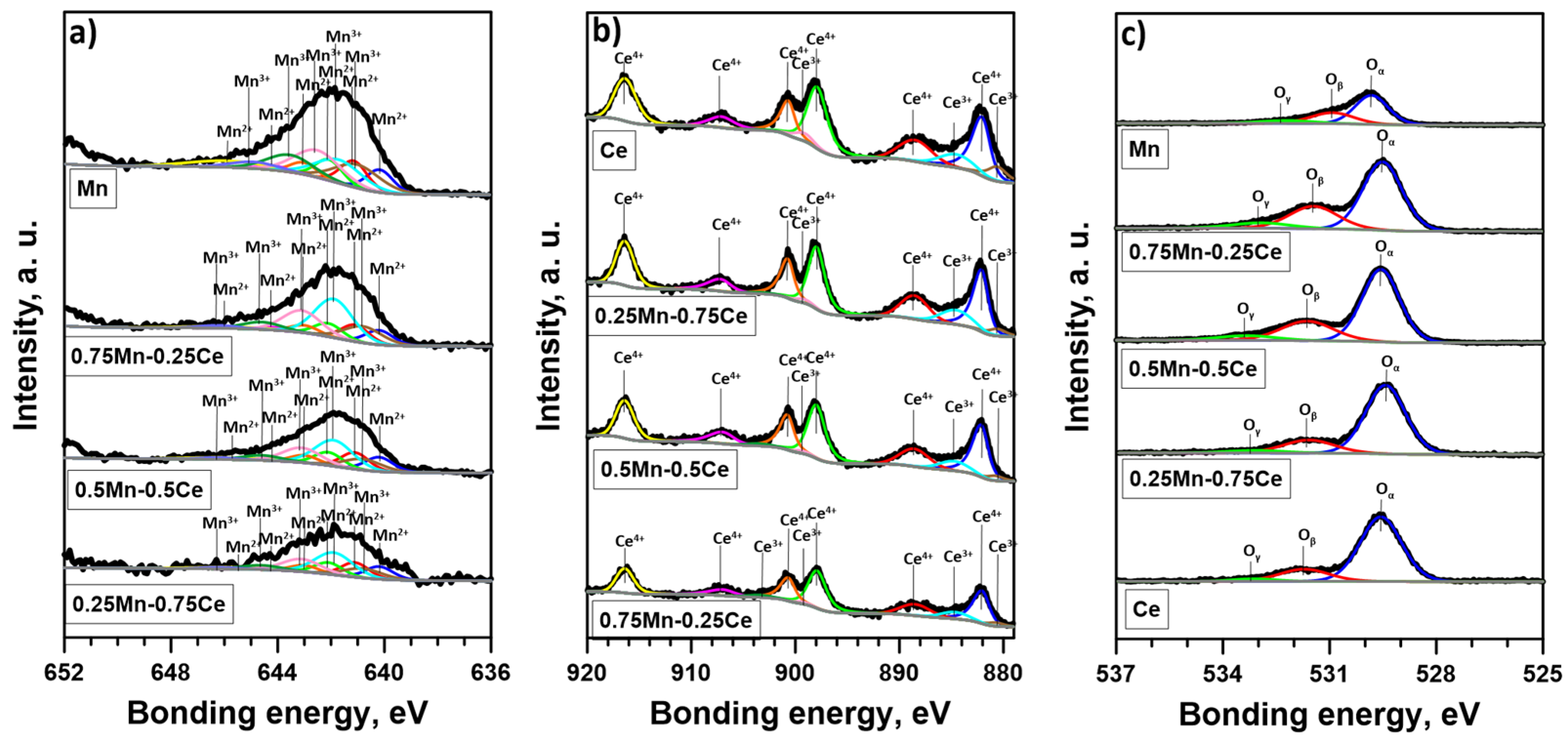
2.1. Catalysts’ Physicochemical Properties
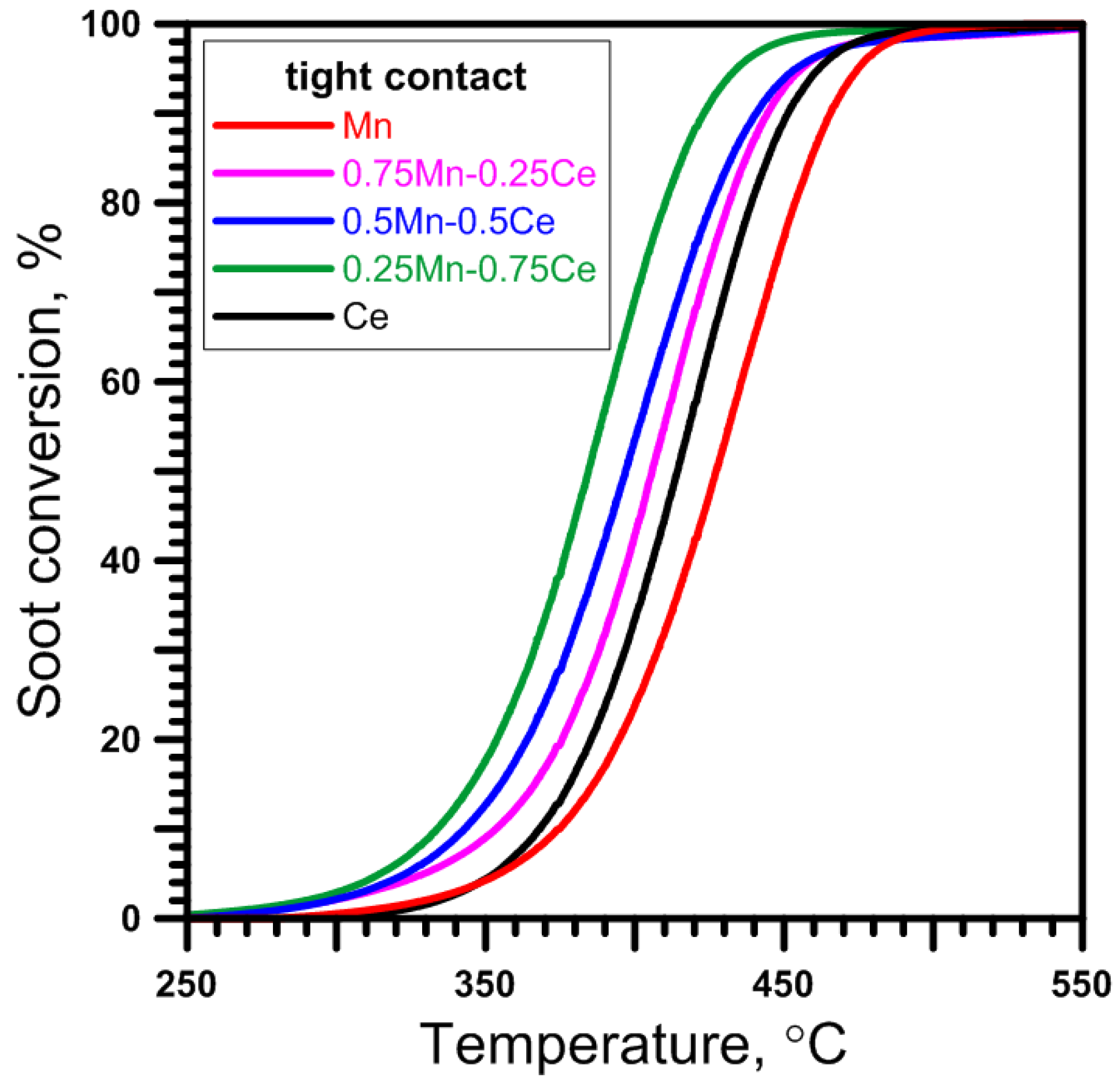
2.2. Catalytic Performance
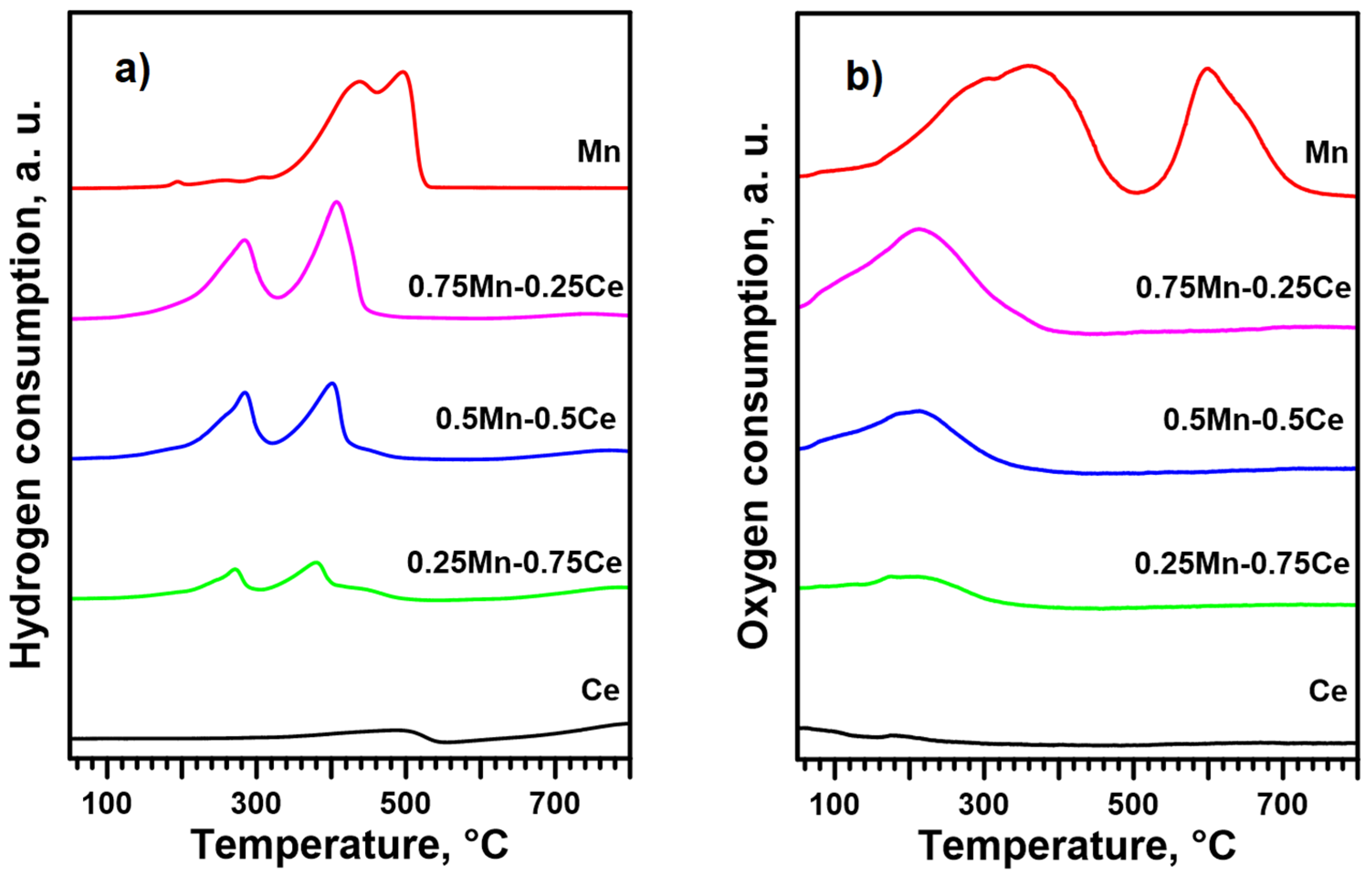
2.3. Oxidizability and Reducibility of the Catalytic Phase
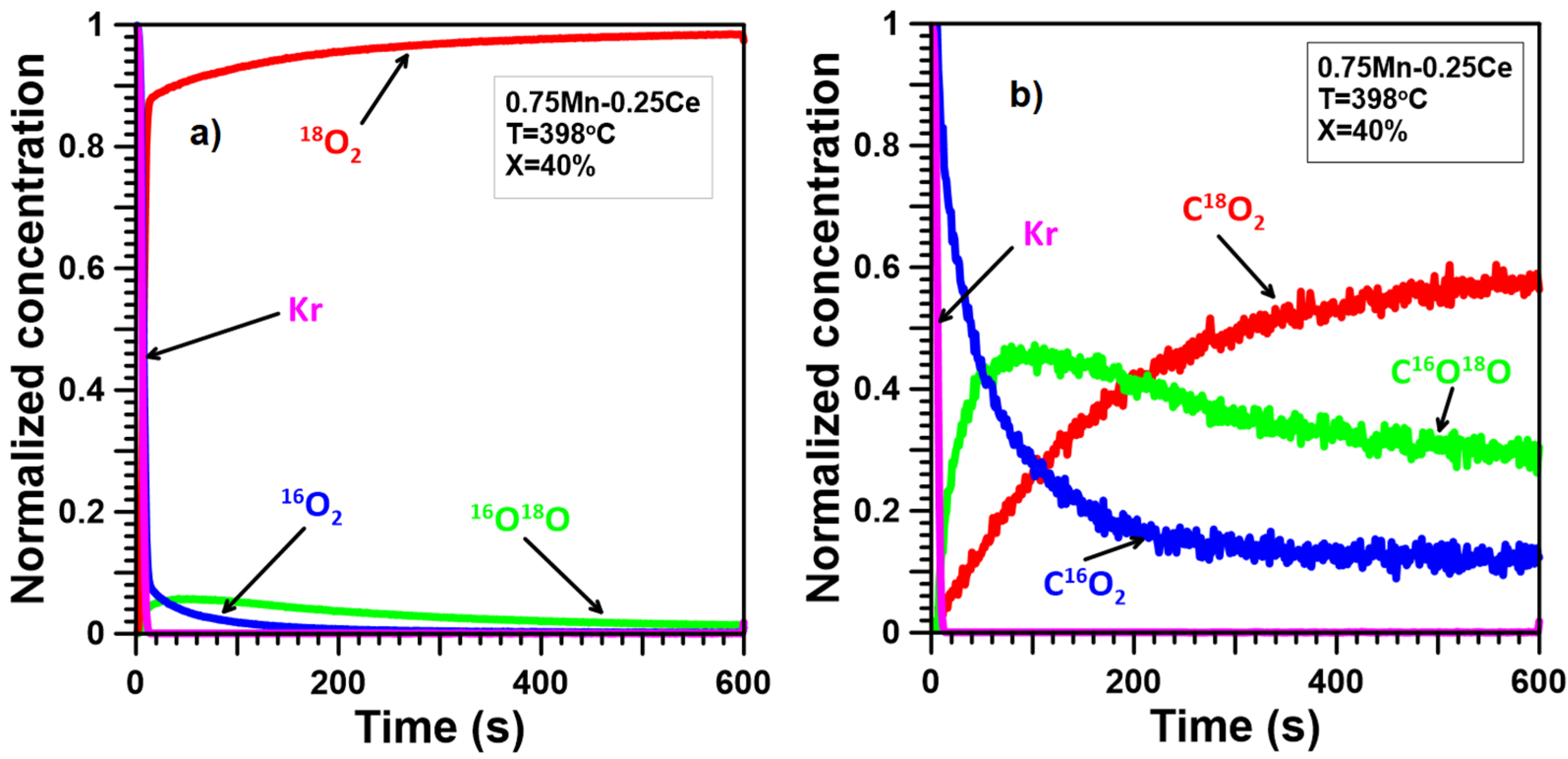
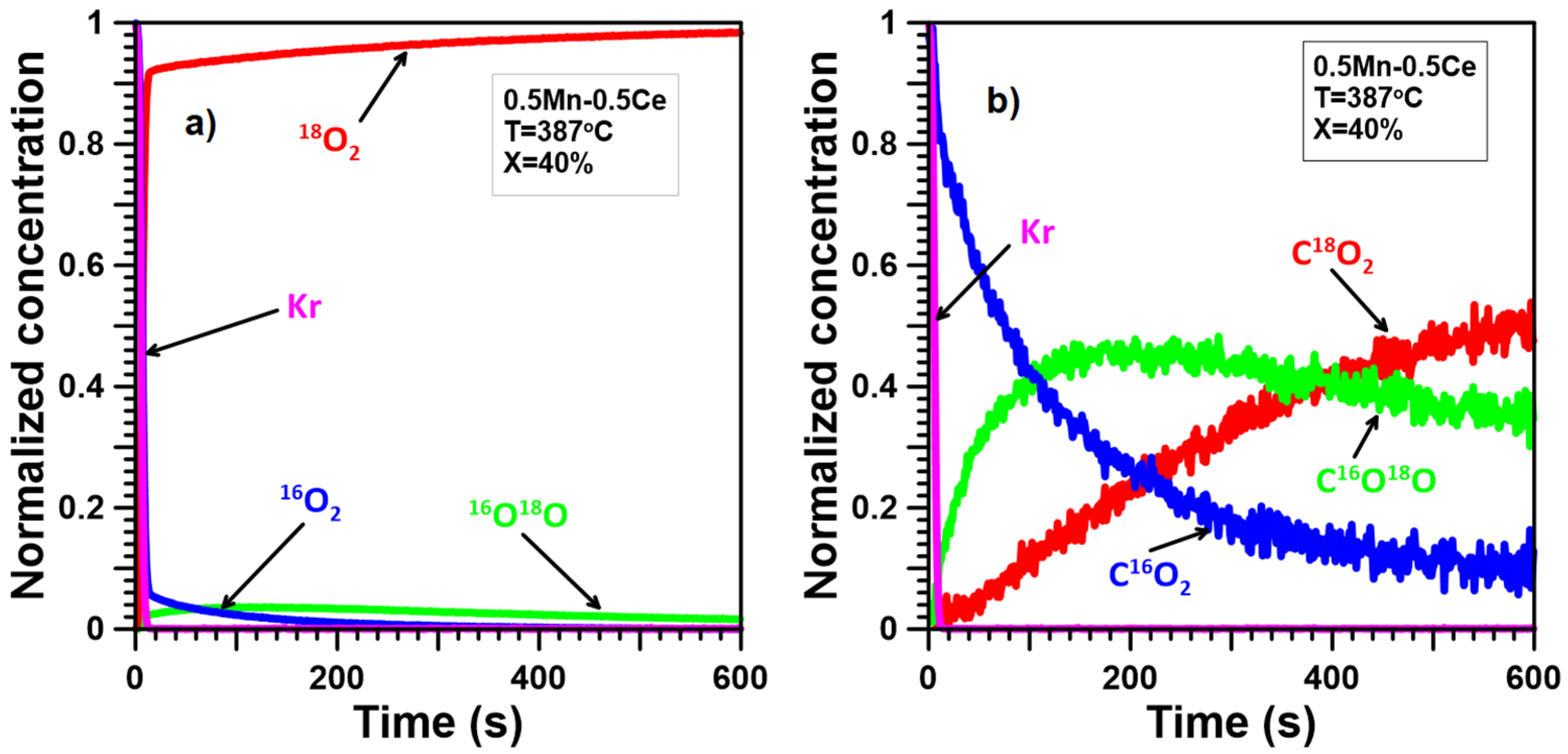
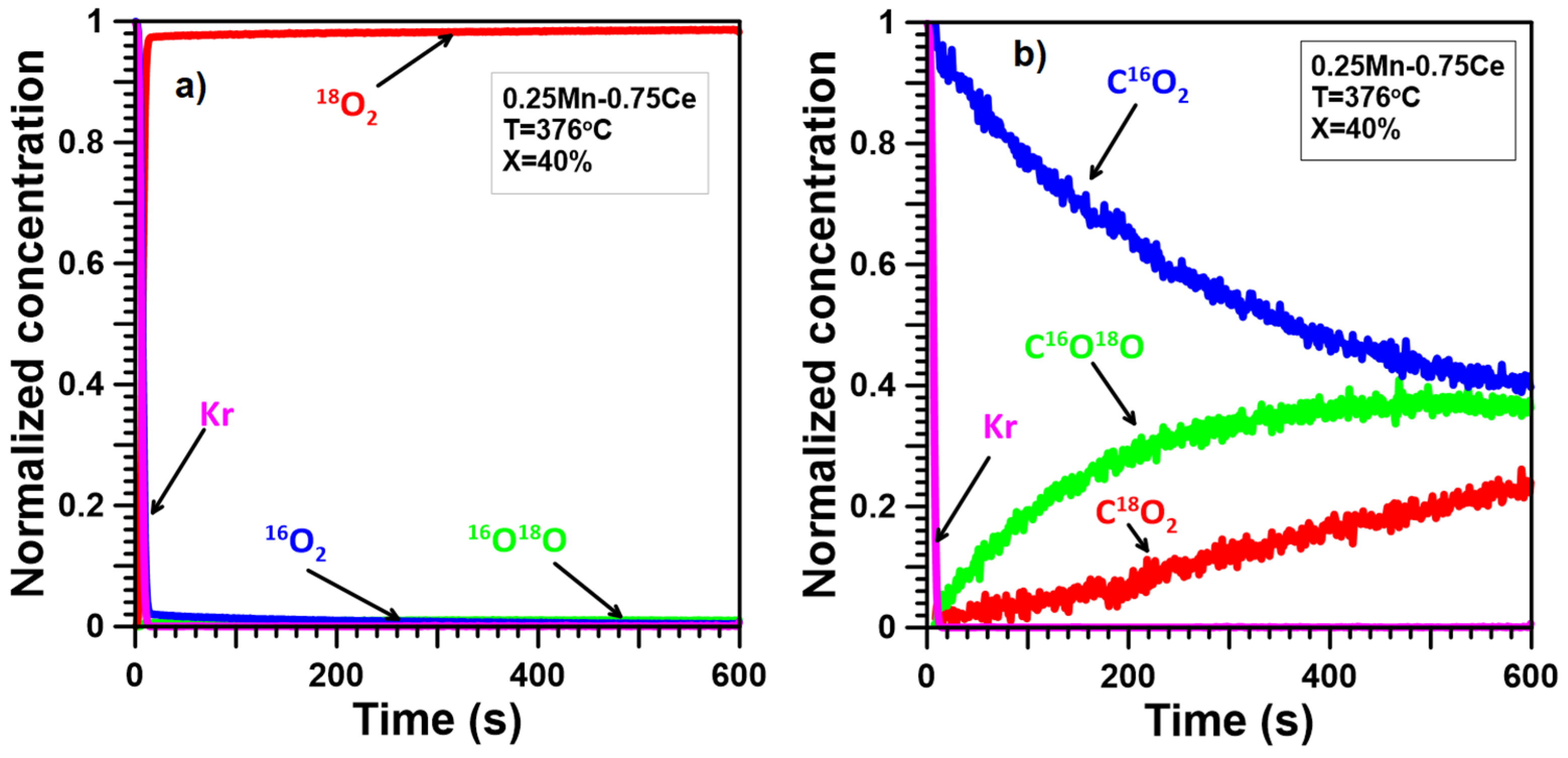
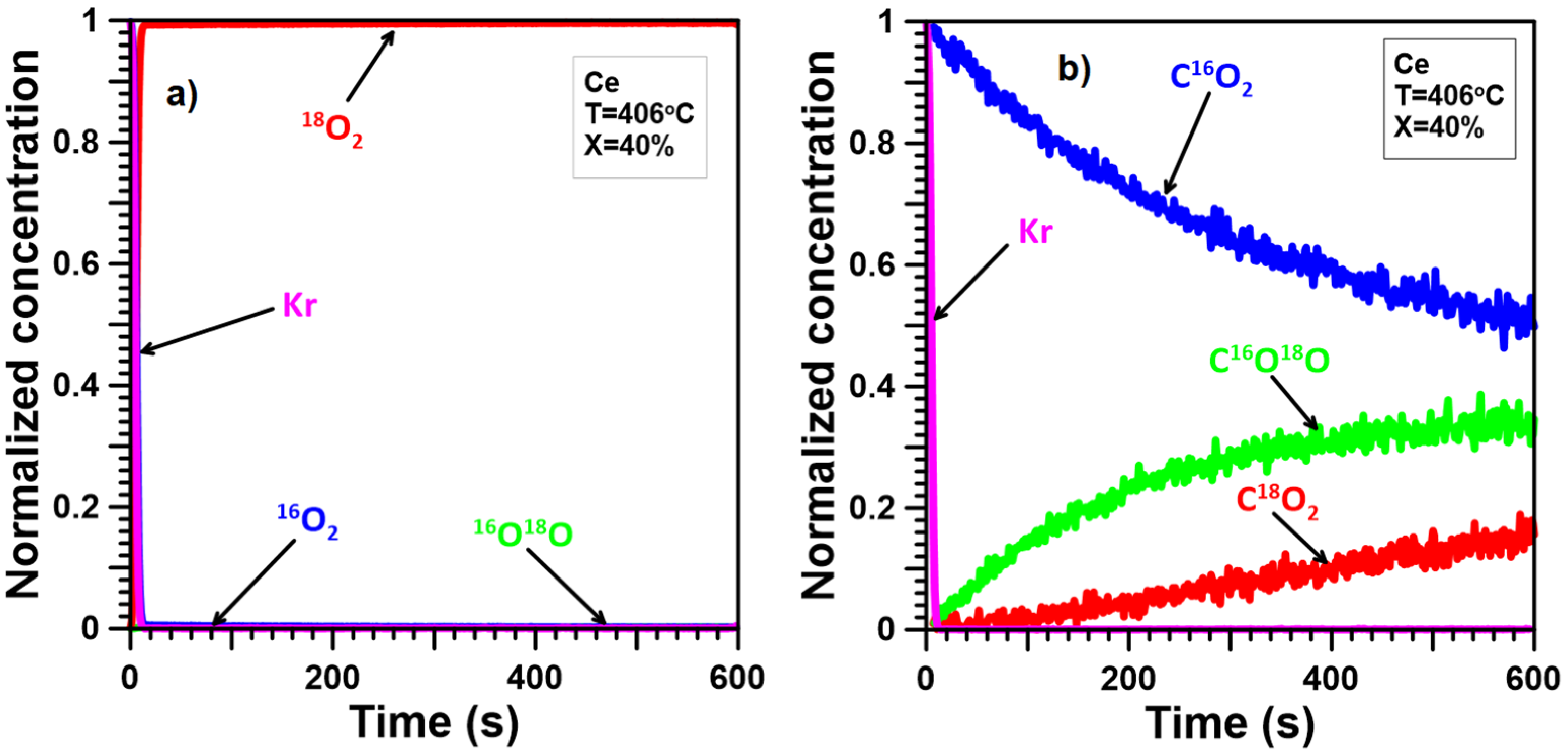
2.4. Oxygen Mobility
2.5. Reaction Mechanism
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Physicochemical Characteristics
3.3. Catalytic Studies
3.4. Temperature-Programmed Reduction/Oxidation
3.5. Isotopic Transient Kinetic Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Z.; Dong, R.; Lan, G.; Yuan, T.; Tan, D. Diesel particulate filter regeneration mechanism of modern automobile engines and methods of reducing PM emissions: A review. Environ. Sci. Pollut. Res. 2023, 30, 39338–39376. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, H.; Liu, Z.; Zhang, Z.; Pan, Y.; Liang, X.; Wu, S.; Xu, H.; Xu, S.; Jiang, C. A review of regeneration mechanism and methods for reducing soot emissions from diesel particulate filter in diesel engine. Environ. Sci. Pollut. Res. 2023, 30, 86556–86597. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Jing, M.; Wei, Y.; Zhao, Z.; Zhang, X.; Xiong, J.; Liu, J.; Song, W.; Li, J. High-efficient catalysts of core-shell structured Pt@transition metal oxides (TMOs) supported on 3DOM-Al2O3 for soot oxidation: The effect of strong Pt-TMO interaction. Appl. Catal. B-Environ. 2019, 244, 628–640. [Google Scholar] [CrossRef]
- Liu, S.; Luo, S.; Wu, H.; Wang, T.; Ran, R.; Weng, D.; Si, Z.; Liu, S. Application of silica-alumina as hydrothermally stable supports for Pt catalysts for acid-assisted soot oxidation. Rare Met. 2023, 42, 1614–1623. [Google Scholar] [CrossRef]
- Liu, S.; Wu, X.; Weng, D.; Li, M.; Ran, R. Roles of acid sites on Pt/H-ZSM5 catalyst in catalytic oxidation of diesel soot. ACS Catal. 2015, 5, 909–919. [Google Scholar] [CrossRef]
- Wei, J.; Fan, C.; Zhuang, Y.; Fu, Z.; Guan, Z.; Li, H.; Li, D.; Qian, Y. Diesel soot combustion over ceria catalyst: Evolution of functional groups on soot surfaces. Fuel 2023, 338, 127391. [Google Scholar] [CrossRef]
- Huang, J.; Wang, S.; Wang, X.; Gao, J.; Wang, Y.; Tian, G. Study on the differences between non-catalytic and catalytic oxidation of soot based on catalyst CeO2. J. Energy Inst. 2024, 113, 101506. [Google Scholar] [CrossRef]
- Bensaid, S.; Russo, N.; Fino, D. CeO2 catalysts with fibrous morphology for soot oxidation: The importance of the soot-catalyst contact conditions. Catal. Today 2013, 216, 57–63. [Google Scholar] [CrossRef]
- Machida, M.; Murata, Y.; Kishikawa, K.; Zhang, D.; Ikeue, K. On the reasons for high activity of CeO2 catalyst for soot oxidation. Chem. Mater. 2008, 20, 4489–4494. [Google Scholar] [CrossRef]
- Zouaoui, N.; Issa, M.; Kehrli, D.; Jeguirim, M. CeO2 catalytic activity for soot oxidation under NO/O2 in loose and tight contact. Catal. Today 2012, 189, 65–69. [Google Scholar] [CrossRef]
- Krishna, K.; Bueno- López, A.; Makkee, M.; Moulijn, J.A. Potential rare earth modified CeO2 catalysts for soot oxidation: I. Characterization and catalytic activity with O2. Appl. Catal. B-Environ. 2007, 75, 189–200. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, M.J.; Lee, E.J.; Lee, D.-W.; Kim, C.H.; Lee, K.-Y. Promoting effect of Rh-impregnation on Ag/CeO2 catalyst for soot oxidation. Appl. Surf. Sci. 2022, 572, 151504. [Google Scholar] [CrossRef]
- Gross, M.S.; Ulla, M.A.; Querini, C.A. Catalytic oxidation of diesel soot: New characterization and kinetic evidence related to the reaction mechanism on K/CeO2 catalyst. Appl. Catal. A-Gen. 2009, 360, 81–88. [Google Scholar] [CrossRef]
- Martínez-Munuera, J.C.; Cortés-Reyes, M.; García-García, A. An isotopic study on oxygen uptake/exchange over ceria-praseodymia mixed oxides with pulse experiments using 18O2: Implications on soot combustion activities in the GDI (Gasoline Direct Injection) context. Appl. Catal. B-Environ. 2023, 329, 122525. [Google Scholar] [CrossRef]
- Yamazaki, K.; Kayama, T.; Dong, F.; Shinjoh, H. A mechanistic study on soot oxidation over CeO2-Ag catalyst with ‘rice-ball’ morphology. J. Catal. 2011, 282, 289–298. [Google Scholar] [CrossRef]
- Medina, J.C.; Rodil, S.E.; Zanella, R. Synthesis of a CeO2/Co3O4 catalyst with a remarkable performance for the soot oxidation reaction. Catal. Sci. Technol. 2020, 10, 853–863. [Google Scholar] [CrossRef]
- Stegmayer, M.A.; Irusta, S.; Miró, E.E.; Milt, V.G.; Electrospinning synthesis and characterization of nanofibers of Co, Ce and mixed Co-Ce oxides. Their application to oxidation reactions of diesel soot and CO. Catal. Today 2022, 383, 266–276. [Google Scholar] [CrossRef]
- Wang, H.; Jin, B.; Wang, H.; Ma, N.; Liu, W.; Weng, D.; Wu, X.; Liu, S. Study of Ag promoted Fe2O3@CeO2 as superior soot oxidation catalysts: The role of Fe2O3 crystal plane and tandem oxygen delivery. Appl. Catal. B-Environ. 2018, 237, 251–262. [Google Scholar] [CrossRef]
- Jampaiah, D.; Velisoju, V.K.; Devaiah, D.; Singh, M.; Mayes, E.L.H.; Coyle, V.E.; Reddy, B.M.; Bansal, V.; Bhargava, S.K. Flower-like Mn3O4/CeO2 microspheres as an efficient catalyst for diesel soot and CO oxidation: Synergistic effects for enhanced catalytic performance. Appl. Surf. Sci. 2019, 473, 209–221. [Google Scholar] [CrossRef]
- Rotko, M.; Karpińska-Wlizło, K.; Dubińska, N.; Gac, W. Soot oxidation on Mn3O4-CeO2 catalysts. Przem. Chem. 2023, 102, 583–588. [Google Scholar]
- Wagloehner, S.; Nitzer-Noski, M.; Kureti, S. Oxidation of soot on manganese oxide catalysts. Chem. Eng. J. 2015, 259, 492–504. [Google Scholar] [CrossRef]
- Singer, C.; Kureti, S. Soot oxidation in diesel exhaust on manganese oxide catalyst prepared by flame spray pyrolysis. Appl. Catal. B-Environ. 2020, 272, 118961. [Google Scholar] [CrossRef]
- Rotko, M.; Machocki, A. Evaluation of activity of catalytic materials used for soot oxidation in exhaust gases from Diesel engines. Przem. Chem. 2014, 93, 1846–1849. [Google Scholar]
- Gao, Y.; Wang, Z.; Cui, C.; Wang, B.; Liu, W.; Liu, W.; Wang, L. Amorphous manganese oxide as highly active catalyst for soot oxidation. Environ. Sci. Pollut. Res. 2020, 27, 13488–13500. [Google Scholar] [CrossRef]
- Atribak, I.; Bueno-López, A.; García-García, A.; Navarro, P.; Frías, D.; Montes, M. Catalytic activity for soot combustion of birnessite and cryptomelane. Appl. Catal. B-Environ. 2010, 93, 267–273. [Google Scholar] [CrossRef]
- Wasalathanthri, N.D.; SantaMaria, T.M.; Kriz, D.A.; Dissanayakea, S.L.; Kuoa, C.-H.; Biswas, S.; Suiba, S.L. Mesoporous manganese oxides for NO2 assisted catalytic soot oxidation. Appl. Catal. B-Environ. 2017, 201, 543–551. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Kato, G.; Fujibayashi, A.; Mori, K.; Yamashita, H. Diesel soot combustion over Mn2O3 catalysts with different morphologies: Elucidating the role of active oxygen species in soot combustion. Chem. Asian. J. 2020, 15, 2005–2014. [Google Scholar] [CrossRef] [PubMed]
- Bueno-López, A.; Krishna, K.; Makkee, M.; Moulijn, J.A. Active oxygen from CeO2 and its role in catalysed soot oxidation. Catal. Lett. 2005, 99, 203–205. [Google Scholar] [CrossRef]
- Bueno-López, A. Diesel soot combustion ceria catalysts. Appl. Catal. B-Environ. 2014, 146, 1–11. [Google Scholar] [CrossRef]
- Rotko, M. The use of 18O2 to investigate the soot oxidation process on Co3O4, Co3O4-CeO2 and CeO2 catalysts in tight contact conditions. Catal. Commun. 2023, 177, 106644. [Google Scholar] [CrossRef]
- Gross, M.S.; Ulla, M.A.; Querini, C.A. Diesel particulate matter combustion with CeO2 as catalyst. Part I: System characterization and reaction mechanism. J. Mol. Catal. A-Chem. 2012, 352, 86–94. [Google Scholar] [CrossRef]
- Mao, X.; Liu, S.; Liu, W.; Wu, X.; Liu, S. A simple model catalyst study to distinguish the roles of different oxygen species in propane and soot combustion. Appl. Catal. B-Environ. 2022, 310, 121331. [Google Scholar] [CrossRef]
- Shannon, S.L.; Goodwin, J.G., Jr. Characterization of catalytic surfaces by isotopic-transient kinetics during steady-state reaction. Chem. Rev. 1995, 95, 677–695. [Google Scholar] [CrossRef]
- Ledesma, C.; Yang, J.; Chen, D.; Holmen, A. Recent approaches in mechanistic and kinetic studies of catalytic reactions using SSITKA technique. ACS Catal. 2014, 4, 4527–4547. [Google Scholar] [CrossRef]
- Jabłońska, M. An application of steady-state isotopic-transient kinetic analysis (SSITKA) in DeNOx process. ChemCatChem. 2021, 13, 818–827. [Google Scholar] [CrossRef]
- Holmen, A.; Yang, J.; Chen, D. Steady-state isotopic transient kinetic analysis (SSITKA). In Springer Handbook of Advanced Catalyst Characterization; Wachs, I.E., Bañares, M.A., Eds.; Springer: New York, NY, USA, 2023. [Google Scholar]
- Rotko, M.; Zawadzki, W.; Redko, V. Steady state isotopic transient kinetic analysis of the combustion of CH4 over Co-Mn-O catalysts. Catal. Commun. 2019, 125, 32–36. [Google Scholar] [CrossRef]
- Yang, Z.; Luo, L.; Tang, L.; Zhou, Z.; Zhou, Z.; Li, Y. Trimesic acid assisted synthesis of cerium-manganese oxide for catalytic diesel soot elimination: Enhancement of thermal aging resistibility. Fuel 2020, 278, 118369. [Google Scholar] [CrossRef]
- Lin, X.; Li, S.; He, H.; Wu, Z.; Wu, J.; Chen, L.; Ye, D. Evolution of oxygen vacancies in MnOx-CeO2 mixed oxides for soot oxidation. Appl. Catal. B-Environ. 2018, 223, 91–102. [Google Scholar] [CrossRef]
- Sacco, N.A.; Bortolozzi, J.P.; Milt, V.G.; Miró, E.E.; Banús, E.D. One step citric acid-assisted synthesis of Mn-Ce mixed oxides and their application to diesel soot combustion. Fuel 2022, 322, 124201. [Google Scholar] [CrossRef]
- Huang, H.; Liu, J.; Sun, P.; Ye, S.; Liu, B. Effects of Mn-doped ceria oxygen-storage material on oxidation activity of diesel soot. RSC Adv. 2017, 7, 7406–7412. [Google Scholar] [CrossRef]
- Wang, J.; Yang, S.; Sun, H.; Qiu, J.; Men, Y. Highly improved soot combustion performance over synergetic MnxCe1− xO2 solid solutions within mesoporous nanosheets. J. Colloid Interf. Sci. 2020, 577, 355–367. [Google Scholar] [CrossRef]
- Zhu, M.; Wen, Y.; Shi, L.; Tan, Z.; Shen, Y.; Yin, K.; Sun, L. Revealing the promoting effect of multiple Mn valences on the catalytic activity of CeO2 nanorods toward soot oxidation. Nanoscale 2022, 14, 11963–11971. [Google Scholar] [CrossRef] [PubMed]
- Sacco, N.A.; Miró, E.E.; Milt, V.G.; Banús, E.D.; Bortolozzi, J.P. Kinetic, stability and characterization studies of Ce, Mn and Mn-doped ceria paper catalysts towards soot combustion under different reaction conditions. Top. Catal. 2022, 65, 1262–1272. [Google Scholar] [CrossRef]
- Liu, Y.; Li, S.; Liu, X.; Tang, J.; Quan, W.; Cheng, H.; Chen, Z.; Wei, X.; Zhuang, J. Enhanced catalytic oxidation of toluene over heterostructured CeO2-CuO-Mn3O4 hollow nanocomposites. Colloids Surf. A Physicochem. Eng. Asp. 2023, 658, 130671. [Google Scholar] [CrossRef]
- Soldatova, A.V.; Balakrishnan, G.; Oyerinde, Q.F.; Romano, C.A.; Tebo, B.M.; Spiro, T.G. Biogenic and synthetic MnO2 nanoparticles: Size and growth probed with absorption and Raman spectroscopies and dynamic light scattering. Environ. Sci. Technol. 2019, 53, 4185–4197. [Google Scholar] [CrossRef]
- Thareja, S.; Kumar, A. One-pot in situ synthesis of Mn3O4/N-rGO nanohybrids for the fabrication of high cell voltage aqueous symmetric supercapacitors: An analysis of redox activity of Mn3O4 toward stabilizing the high potential window in salt-in-water and water-in-salt electrolytes. Energy Fuels 2022, 36, 15177–15187. [Google Scholar]
- Feng, N.; Zhu, Z.; Zhao, P.; Wang, L.; Wan, H.; Guan, G. Facile fabrication of trepang-like CeO2@MnO2 nanocomposite with high catalytic activity for soot removal. Appl. Surf. Sci. 2020, 515, 146013. [Google Scholar] [CrossRef]
- Tang, Q.; Du, J.; Xie, B.; Yang, Y.; Yu, W.; Tao, C. Rare earth metal modified three dimensionally ordered macroporous MnOx-CeO2 catalyst for diesel soot combustion. J. Rare Earths 2018, 36, 64–71. [Google Scholar] [CrossRef]
- Colman-Lerner, E.; Peluso, A.M.; Sambeth, J.; Thomas, H. Cerium, manganese and cerium/manganese ceramic monolithic catalysts. Study of VOCs and PM removal. J. Rare Earths 2016, 34, 675–682. [Google Scholar] [CrossRef]
- Jin, B.; Wu, X.; Weng, D.; Liu, S.; Yu, T.; Zhao, Z.; Wei, Y. Roles of cobalt and cerium species in three-dimensionally ordered macroporous CoxCe1-xOδ catalysts for the catalytic oxidation of diesel soot. J. Colloid Interface Sci. 2018, 532, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Piumetti, M.; Fino, D.; Russo, N. Mesoporous manganese oxides prepared by solution combustion synthesis as catalysts for the total oxidation of VOCs. Appl. Catal. B 2015, 163, 277–287. [Google Scholar] [CrossRef]


| Catalyst | Metal Content (% wt.) | Molar Ratio (Mn:Ce) | |
|---|---|---|---|
| Mn | Ce | ||
| Mn | 71.0 | 0.0 | 1.00:0.00 |
| 0.75Mn-0.25Ce | 40.2 | 31.7 | 0.76:0.24 |
| 0.5Mn-0.5Ce | 22.6 | 53.1 | 0.52:0.48 |
| 0.25Mn-0.75Ce | 9.6 | 70.6 | 0.26:0.74 |
| Ce | 0.0 | 81.2 | 0.00:1.00 |
| Catalyst | Total BET Surface Area (m2/g) | Average Pore Diameter (nm) | Volume of Pores (cm³/g) |
|---|---|---|---|
| Mn | 7.5 | 30.94 | 0.05 |
| 0.75Mn-0.25Ce | 76.5 | 7.77 | 0.14 |
| 0.5Mn-0.5Ce | 101.6 | 5.81 | 0.10 |
| 0.25Mn-0.75Ce | 102.0 | 6.11 | 0.07 |
| Ce | 85.9 | 5.51 | 0.04 |
| Catalyst | Crystallographic Phases | Mean Size of Crystallites (nm) | ||
|---|---|---|---|---|
| Chemical Formula | Content (%) | Crystallite Size (nm) | ||
| Mn | Mn3O4 | 95 | 70.8 | 69.7 |
| Mn2O3 | 5 | 49.8 | ||
| 0.75Mn-0.25Ce | Mn3O4 | 34 | 29.8 | 21.9 |
| CeO2 | 66 | 17.8 | ||
| 0.5Mn-0.5Ce | Mn3O4 | 8 | 18.3 | 11.5 |
| CeO2 | 92 | 10.9 | ||
| 0.25Mn-0.75Ce | Mn3O4 | 2 | 7.2 | 13.1 |
| CeO2 | 98 | 13.3 | ||
| Ce | CeO2 | 100 | 16.4 | 16.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rotko, M.; Karpińska-Wlizło, K. Isotopic Transient Kinetic Analysis of Soot Oxidation on Mn3O4, Mn3O4-CeO2, and CeO2 Catalysts in Tight Contact Conditions. Molecules 2025, 30, 343. https://doi.org/10.3390/molecules30020343
Rotko M, Karpińska-Wlizło K. Isotopic Transient Kinetic Analysis of Soot Oxidation on Mn3O4, Mn3O4-CeO2, and CeO2 Catalysts in Tight Contact Conditions. Molecules. 2025; 30(2):343. https://doi.org/10.3390/molecules30020343
Chicago/Turabian StyleRotko, Marek, and Karolina Karpińska-Wlizło. 2025. "Isotopic Transient Kinetic Analysis of Soot Oxidation on Mn3O4, Mn3O4-CeO2, and CeO2 Catalysts in Tight Contact Conditions" Molecules 30, no. 2: 343. https://doi.org/10.3390/molecules30020343
APA StyleRotko, M., & Karpińska-Wlizło, K. (2025). Isotopic Transient Kinetic Analysis of Soot Oxidation on Mn3O4, Mn3O4-CeO2, and CeO2 Catalysts in Tight Contact Conditions. Molecules, 30(2), 343. https://doi.org/10.3390/molecules30020343






